Abstract
Background
Few studies have directly assessed associations between craving and subsequent opioid use among treated patients. Our objective was to prospectively evaluate the relative utility of two craving questionnaires to predict opioid use among opioid dependent patients in treatment.
Method
Opioid dependent patients (n=147) initiating buprenorphine treatment were assessed for three months. Craving was measured using: 1) the Desires for Drug Questionnaire (DDQ) and 2) the Penn Alcohol-Craving Scale adapted for opioid craving (PCS) for this study. Multi-level logistic regression models estimated the effects of craving on the likelihood of opioid use after adjusting for gender, age, ethnicity, education, opioid of choice, frequency of use, pain and depression. In these analyses craving assessed at time t was entered as a time-varying predictor of opioid use at time t+1.
Results
In adjusted regression models, a 1-point increase in PCS scores (on a 7-point scale) was associated with a significant increase in the odds of opioid use at the subsequent assessment (OR = 1.27, 95% CI 1.08; 1.49, p < .01). The odds of opioid use at the subsequent follow-up assessment increased significantly as DDQ desire and intention scores increased (OR = 1.25, 95%CI 1.03; 1.51, p< .05), but was not associated significantly with DDQ negative reinforcement (OR = 1.01, 95%CI 0.88; 1.17, p > .05) or DDQ control (OR = 0.97, 95%CI 0.85; 1.11, p > .05) scores.
Conclusion
Self-reported craving for opioids was associated with subsequent lapse to opioid use among a cohort of patients treated with buprenorphine.
Keywords: Opioid dependence, Craving, Buprenorphine
1. Introduction
Based on 2008 national data, nearly 2 million individuals in the U.S. report opioid abuse or dependence (1). Morbidity and mortality related to opioid abuse and dependence, and their economic consequences, are substantial (2–4). Buprenorphine is a safe and effective office-based treatment for opioid dependence (5). It has partial agonist properties and, like methadone, it is considered to be an “anti-craving” medication (6). Craving is a subjective phenomenon conceptualized as an individual’s desire or urge to use a previously experienced drug (6–8), which is endorsed as an important research outcome in treatment studies (9). However, research to confirm the relationship of craving to substance use outcomes (particularly for non-nicotine drugs) is still needed (8).
Although buprenorphine has been demonstrated to reduce subjective craving (6), a substantial percentage of patients treated with buprenorphine will still experience cravings and be unable to refrain from using drugs (10). While declines in craving have been shown to parallel changes in opioid use in clinical studies, evidence from prospective studies that more definitively establish causality are needed, as cravings can occur in the setting of acute withdrawal. Furthermore, longitudinal studies that have evaluated the effects of buprenorphine have often used a single Visual Analogue Scale question to measure craving (6). Multicomponent questionnaires for craving may provide more detailed and nuanced information about patients’ subjective craving experiences, but have not been routinely applied to opioid-using cohorts initiating treatment. Craving is a complex construct and differences in conceptualizations have generated questionnaires that vary in emphasis on craving phenomena (7, 11). Understanding the predictive value of craving measures has practical value for clinicians, and may contribute to a better scientific understanding of the salient features of opioid craving.
Our primary objective was to evaluate the relative utility of two craving questionnaires in predicting subsequent opioid use during a 3-month period following the initiation of buprenorphine treatment in a cohort of opioid dependent patients with depressive symptoms. Specifically, we hypothesized that after controlling for demographic characteristics, baseline measures of substance use, and depression assessed at time of follow-up, higher craving would be associated with a higher likelihood of opioid use, as defined by positive urine toxicology or self-report, at the subsequent visit. Craving questionnaires include the validated Desires for Drug Questionnaire (DDQ) (12) which has subscales assessing current desire and intention to use, negative reinforcement, and control of drug use. Additionally, a secondary aim was to evaluate the predictive utility of a version of the Penn Alcohol-Craving Scale (PACS) (13) adapted to assess opioid craving.
2. Materials and methods
2.1 Study Sample and Design
This study used longitudinal data from a randomized controlled trial that evaluated whether treatment with escitalopram increased retention among opioid dependent patients with depressive symptoms who were initiating buprenorphine/naloxone (14). Participants were recruited through community advertising, physician referrals and word-of-mouth. Study inclusion criteria included: age 18–65, a DSM-IV diagnosis of opioid dependence, a score on the Modified Hamilton Depression Revised Scale (MHDRS) greater than 14 (15), the absence of significant suicidal ideation, willingness and ability to complete a 3-month treatment with buprenorphine, no history of severe mental illness (bipolar disorder, schizophrenia, schizo-affective, or paranoid disorder), not currently prescribed medications for depression (participants were not excluded if they were taking a tricyclic anti-depressant for pain), and the ability to complete the study assessment in English. All patients provided informed consent and the study was approved by the Rhode Island Hospital and Butler Hospital Institutional Review Boards.
2.2 Study Procedures
Between November 2006 and May 2009, 932 individuals were screened by telephone, 394 appeared eligible and were invited for an in-person screening visit. Of the 226 who attended, 147 met criteria and agreed to enroll. Participants completed a baseline interview and were randomized to escitalopram versus placebo. Approximately 5–7 days after beginning the study medication, participants returned to the research office for buprenorphine (buprenorphine/naloxone) induction. The majority of participants were maintained on the standard dose of 16 mg/day during the study; however, some participants chose lower doses based on past experience with buprenorphine. One person briefly received 20 mg during the first two weeks of the protocol after reporting continuing withdrawal symptoms.
At each biweekly follow-up appointment (which coincided with research interviews), participants were provided with exactly enough study medication and buprenorphine to last until the next appointment. Participants had follow-up interviews at weeks 1, 2, 4, 6, 8, 10 and 12 post-enrollment, assessing drug use (including opioids), study medication use, presence of side effects and depressive symptoms. At each interview, participants were asked to provide a specimen for urine toxicological testing using the Screeners® Dip Drug Test with the Integrated Screeners® Autosplit® KO12B™ Test Cup, a test which utilizes screening cut-off for opiate positive specimens set by the Substance Abuse and Mental Health Services Administration (SAMHSA) and which reports >99% positive and >90% negative agreement when comparing to gas chromatography/mass spectrometry. Urine testing was monitored by research staff; all specimens were evaluated for appropriate appearance and temperature. Participants were not discharged for continued use of drugs, nor was their frequency of follow-up medical visits changed by positive results.
2.3 Measures
The primary outcome was opioid use, defined as either testing positive for opiates on urine toxicology screening or self-reported opioid use between assessments. This outcome was assessed at 2 week intervals post induction (weeks 2 through 12). Participants lost to follow-up were assumed opioid positive. Measures of craving included the Desires for Drug Questionnaire (DDQ) which we modified to ask about craving for heroin or prescription opioids (12). The DDQ has subscales assessing 3 domains of current craving: 7 items assessing desire and intent to use opioids, 4 items assessing negative reinforcement expectations of opioid use (i.e. relief of negative states by using opioids), and 2 items assessing perceived ability to control opioid use. In addition, we modified the Penn Alcohol Craving Scale (PACS) (13) to assess craving for opioids. The PACS was designed as a brief (5-item) unidimensional measure of alcohol craving. Items assess craving in the past week with response categories ranging from 0 to 6, and scale scores defined as the mean item score. In the context of craving for alcohol, the initial psychometric assessment found the instrument to have high internal reliability and good convergent and discriminant validity (13). Furthermore, it was predictive of alcohol relapse in treatment seeking samples (16) and had strong correlations with other published measures of alcohol craving (17). Depression was measured using the Beck Depression Inventory II (BDI II) (18). Measures of craving and depression were assessed at baseline and at all follow-up assessments.
Time-invariant covariates assessed at baseline included age, gender, race/ethnicity, educational status, primary illicit opioid used, intervention assignment, number of days used opioids in the past month, cocaine use in the past month, and pain interference. Pain interference was assessed using the mean of the 7-item subscale from the Brief Pain Inventory Short Form (BPI) (19).
2.4 Statistical Analysis
Descriptive statistics are presented to summarize sample characteristics. We present line graphs and estimated unconditional latent growth models to evaluate overall change in the likelihood of being positive for opioids between weeks 2–12. Latent growth models evaluated linear change, quadratic change, and unconstrained change in which test rates were free to vary at all time points. We also used graphical techniques to describe changes in craving between baseline and the 12-week final assessment. We used confirmatory factor analysis (CFA) to test the hypothesis that the Penn Craving Scale (PCS), adapted for opioids, was unidimensional. Items were defined as ordered categorical and the default WLSMV estimator in Mplus 5.1 (20) was used to estimate the CFA model. We also report Cronbach’s alpha as a measure of internal consistency reliability for the 5-item summated measure. Convergent validity was assessed by examining the correlation between the PCS and the DDQ subscales. We used multi-level logistic regression models to estimate the adjusted effects of craving on subsequent opioid use. In these analyses craving assessed at time t was entered as a time-varying predictor of opioid use at time t+1. More specifically, in these analyses, time varying predictors assessed at weeks 1, 2, 4, 6, 8, & 10 predicted opioid use measured at weeks 2, 4, 6, 8, 10, & 12, respectively Models were estimated by maximum likelihood which has desirable statistical properties when data are missing at random or missing completely at random. We also report the AIC (21) and BIC (22) statistics to compare models using the alternative measures of craving. These measures can be used to compare both nested and non-nested models. All models were estimated using Stata 10.1 (23).
3. Results
Table 1 describes the baseline characteristics of the study sample. The majority of the sample were middle-aged Caucasian men. On average participants used opioids on 22.7 (± 9.2) of the 30 days prior to baseline, and 93 (63.7%) reported heroin was their opioid of choice. At baseline, 46 (31.7%), 53 (36.1%), and 43 (29.3%) tested positive for cocaine, cannabinoids, and benzodiazepines, respectively on urine toxicity screening. The mean score on the DDQ scales were 4.9 (± 1.5), 4.9 (± 1.6), and 5.8 (± 1.5) on the desire and intention to use, negative reinforcement, and control subscales, respectively. The mean PCS score at baseline was 4.7 (± 1.4). One hundred thirty five (91.8%) of participants were observed at 1 or more follow-up assessments; 131 (89.1%), 135 (91.8%), 128 (87.1%), 114 (77.6%), 104 (70.1%), 89 (60.5%), and 90 (61.2%) participants were observed at the 1-, 2-, 4-, 6-, 8-, 10-, & 12-week assessments, respectively. Urine toxicology tests for opiate use were available for 128 (87.8%), 121 (82.3%), 106 (72.1%), 100 (68.0%), 82 (55.6%), and 87 (59.2%) participants at the 2-, 4-, 6-, 8-, 10-, and 12-week follow-ups, respectively. During the course of the study, 92.3% of urine toxicology tests were positive for buprenorphine, demonstrating good adherence to this medication.
Table 1.
ARISE Baseline Characteristics and Rates of Toxicology Testing at Post Induction Follow-Ups (n = 147).
| Mean (SD) | n (%) | |
|---|---|---|
| Age (Yrs) | 37.5 (±9.9) | |
| Gender (Male) | 112 (76.2%) | |
| Ethnicity | ||
| Caucasian | 117 (80.1%) | |
| African-American | 7 (4.8%) | |
| Hispanic | 14 (9.6%) | |
| Other | 8 (5.5%) | |
| Days Used Opioids | 22.7 (±9.2) | |
| Heroin Opiate of Choice (Yes) | 93 (63.7%) | |
| Opiate Positive (Yes) | 93 (63.7%) | |
| Cocaine Positive (Yes) | 46 (31.7%) | |
| Beck Depression Inventory | 28.4 (±9.7) | |
| Pain Interference | 4.1 (± 3.0) | |
| Penn Craving Scale | 4.7 (± 1.4) | |
| DDQ Desire and Intent | 4.9 (± 1.5) | |
| DDQ Negative Reinforcement | 4.9 (± 1.6) | |
| DDQ Control | 5.8 (± 1.5) |
3.1 Dimensionality and Internal Consistency Reliability of the PCS
We specified a 1-factor CFA model using Mplus 5.1 (20). The 1-factor model provided acceptable statistical fit with the observed data (χ2 = 6.81, df = 5, p = .235). Incremental fit indices (CFI = .991, TLI = .984) indicated excellent model fit. All items loaded significantly (p < .001) on a common factor and fully standardized loadings ranged from .58 to .93. Internal consistency reliability for the summated scale was .88 at baseline.
3.2 Convergent Validity of the PCS
At baseline the PCS was strongly correlated (r = .66, p < .01) with the DDQ desire and intention scale, and moderately correlated (r = .44, p < .01) with the DDQ negative reinforcement scale (Table 2). The DDQ control scale was only weakly correlated with the PCS (r = .17, p < .05), the DDQ desire and intention scale (r = .09, p < .05), and the DDQ negative reinforcement scale (r = .21, p < .05). A principal components factor analysis of these 4 scales indicated substantial overlap among scales: the first and second principal components accounted for 55.7% and 27.7% of the total variance in the correlation matrix. Table 2 also gives principal component factor loadings after promax rotation. The PCS, DDQ desire and intention, and DDQ negative reinforcement scales load strongly (> .80) on a common factor suggesting that these scales substantially assess a common construct. The DDQ Control scale was the only craving scale to load on the second principal component factor and was not reliably related to the other craving scales. Using time-varying data across all follow-up assessments (not reported here) the observed associations between the PCS, the DDQ desire and intention, and DDQ negative reinforcement scales were stronger than those reported using only the baseline data. The substantial intercorrelations among these measures suggest that their simultaneous inclusion in a common model will result in interpretational confounding. Therefore, we estimated 4 models in which the adjusted effect of each of these measures on the likelihood of using opioids was assessed independently.
Table 2.
Zero-Order Correlations and Principal-Component Factor Analysis of Craving Scales Measured at Baseline (n = 147).
| Product-Moment Correlations | PCFa | |||||
|---|---|---|---|---|---|---|
| 1. | 2. | 3. | 4. | F1 | F2 | |
| 1. Penn Craving Scale | 1.00 | .81 | .03 | |||
| 2. DDQ – Desire/Intention | .66** | 1.00 | .95 | −.10 | ||
| 3. DDQ – Negative Reinforcement | .44** | .69** | 1.00 | .81 | .09 | |
| 4. DDQ - Control | .17* | .09 | .21* | 1.00 | −.02 | 1.00 |
p < .05,
p < .01
Principal-component factor analysis with promax rotation.
3.3 Opioid Use Results by Assessment Week
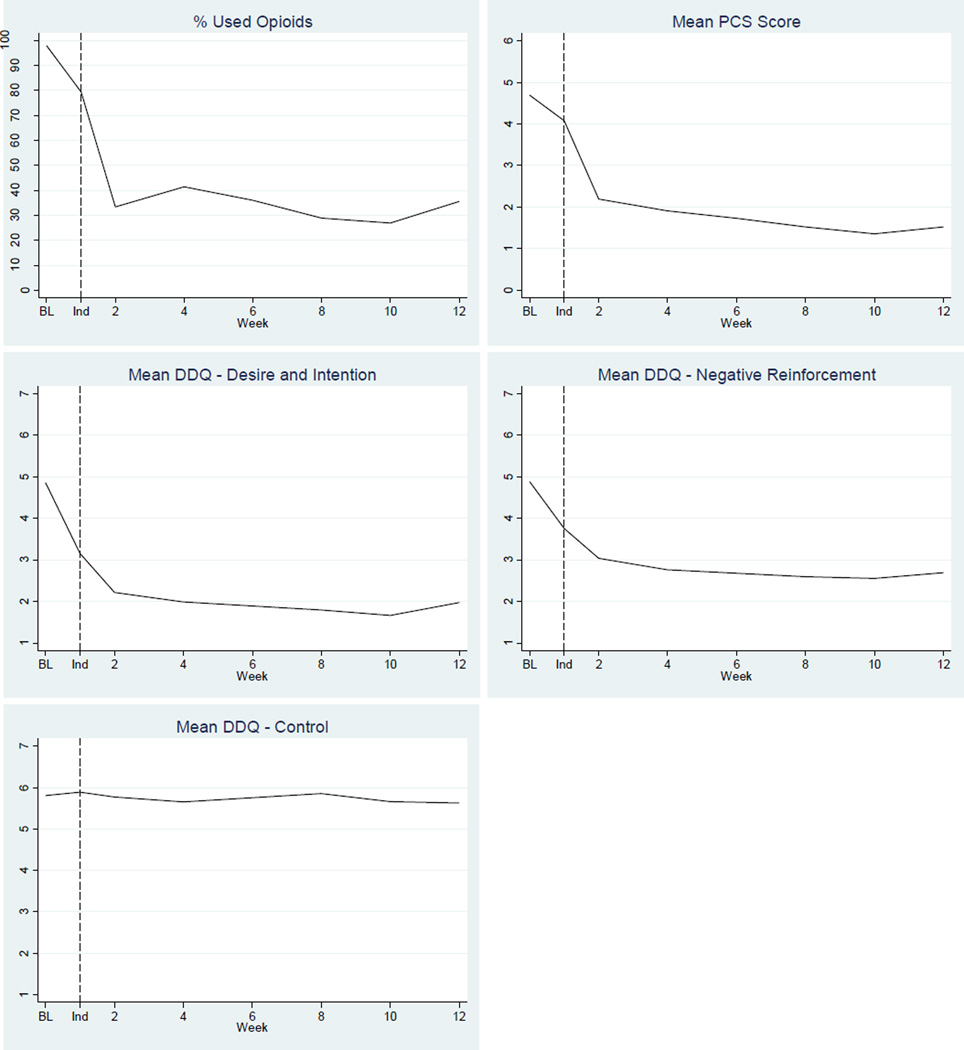
Rates of being positive for opioid use (by urine screening or self-report) at all assessments are shown in Figure 1. At 2-weeks about 33.3% of the participants were positive for opioids. Observed rates of having used opioids ranged from 41.1% at 4-weeks to 27.0% at 10-weeks. Unconditional growth models for linear time (Wald χ2 = 0.02, df = 1, p = .883), quadratic time (Wald χ2 = 0.55, df = 2, p = .758), and unconstrained time (Wald χ2 = 8.49, df = 5, p = .131) indicated that post-induction variations in rates of opioid use were not statistically significant. The linear effect of time is included in subsequent models as a covariate.
Figure 1.
Percentage Used Opioids and Mean Scores on the PCS and DDQ Scale Scores by Week of Assessment.
3.4 Mean Craving by Week
Figure 1 also describes changes in PCS and DDQ scale scores by week of assessment. At baseline, participants exhibited high craving with a mean score on the PCS of 4.7 (± 1.4). Change in mean PCS, mean DDQ desire and intention, and DDQ negative reinforcement scale scores largely paralleled those observed for opioid use results. Baseline means on these 3 scales were high at baseline, decreased sharply between baseline and week 2, and then remained relatively steady from weeks 2 through 12. Mean DDQ control scale scores exhibited a distinctly different pattern; at baseline, the mean was 5.80 (± 1.52) on a scale that ranged from 1 (Strongly Agree) to 7 (Strongly Disagree). This score suggests average disagreement with self-reported ability to use opioids in a controlled way. Mean DDQ control scale scores exhibited little change over time.
3.5 Predictors of Using Opioids During Follow-Up
Model 1 in Table 3 gives the estimated adjusted lagged effect of the PCS on the likelihood of having used opioids at follow-up. A 1-point increase in PCS scores (on a 7-point scale) was associated with a significant increase in odds of opioid use at the subsequent assessment (OR = 1.27, 95% CI 1.08; 1.49, p < .01). The estimated odds of opioid use was also estimated to be significantly higher (OR = 2.88, 95% CI 1.62; 5.12, p < .01) among persons who reported that heroin was their opioid of choice, increased significantly as frequency of baseline opioid use increased (OR = 1.04, 95%CI 1.01; 1.07, p < .05), was significantly higher (OR = 2.36, 95%CI 1.31, 4.25, p < .01) among persons who reported cocaine use at baseline, and decreased significantly (OR = 0.97, 95%CI 0.94; 0.99) as age increased. The likelihood of having used opioids at follow-up was not associated significantly with other covariates included in the multilevel logistic regression model.
Table 3.
Multi-Level Logistic Regression Models Estimating the Adjusted Effects of Penn Craving Scale and Desires for Drug Questionnaire Subscales on the Odds of Opioid Use During the Subsequent Follow-Up Period (n = 138).
| MODEL 1 | MODEL 2 | MODEL 3 | MODEL 4 | |
|---|---|---|---|---|
| Fixed Effects | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Time-Invariant Baseline Covariates | ||||
| Years Age | 0.97* (0.94; 0.99) | 0.97* (0.94; 0.99) | 0.96** (0.94; 0.99) | 0.96** (0.94; 0.99) |
| Gender (Male) | 1.39 (0.73; 2.63) | 1.36 (0.71; 2.62) | 1.35 (0.69; 2.63) | 1.35 (0.6971; 2.63) |
| Ethnicity (Non-Hispanic Caucasian) | 0.70 (0.36; 1.33) | 0.71 (0.37; 1.38) | 0.70 (0.36; 1.38) | 0.71 (0.36; 1.40) |
| Years Education | 1.02 (0.88; 1.18) | 1.02 (0.88; 1.19) | 1.02 (0.88; 1.20) | 1.02 (0.88; 1.20) |
| Opiate of Choice (Heroin) | 2.88** (1.62; 5.12) | 3.14** (1.76; 5.62) | 3.31** (1.84; 6.07) | 3.37** (1.87; 6.09) |
| Days (0–30) Used Opioids | 1.04* (1.01; 1.07) | 1.05** (1.02; 1.08) | 1.05** (1.02; 1.08) | 1.05** (1.02; 1.08) |
| Cocaine+ at Baseline | 2.36* (1.31; 4.25) | 2.41** (1.32; 4.40) | 2.32** (1.25; 4.30) | 2.29** (1.25; 4.30) |
| BPI (Pain Interference) | 1.01 (0.92; 1.11) | 1.02 (0.92; 1.12) | 1.00 (0.91; 1.11) | 1.00 (0.91; 1.10) |
| Intervention | 0.70 (0.41; 1.20) | 0.66 (0.38; 1.14) | 0.65 (0.37; 1.14) | 0.65 (0.37; 1.14) |
| Time-Varying Predictors | ||||
| Linear Time | 1.37* (1.29; 1.47) | 1.35** (1.27; 1.44) | 1.34** (1.26; 1.43) | 1.34** (1.26; 1.43) |
| Beck Depression Inventory | 1.00 (0.98; 1.03) | 1.01 (0.98; 1.03) | 1.02 (0.99; 1.04) | 1.02 (0.99; 1.04) |
| Penn Craving Scale | 1.27** (1.08; 1.49) | NA | NA | NA |
| DDQ – Desire & Intention | NA | 1.25* (1.03; 1.51) | NA | NA |
| DDQ – Negative Reinforcement | NA | NA | 1.01 (0.88; 1.17) | NA |
| DDQ - Control | NA | NA | NA | 0.97 (0.85; 1.11) |
| SD Random Intercept | 1.00 | 1.04 | 1.09 | 1.09 |
| −2 Log-Likelihood | 730.9 | 733.9 | 739.1 | 739.0 |
| AIC | 758.9 | 761.9 | 767.1 | 767.0 |
| BIC | 822.1 | 825.2 | 830.4 | 830.3 |
p < .05,
p < .01
Models 2, 3, and 4 (Table 3) give the results for parallel models assessing the adjusted effects of the DDQ desire and intention, DDQ negative reinforcement, and DDQ control subscales on the likelihood of using opioids at the subsequent follow-up assessment, respectively. The odds of opioid use at the subsequent follow-up assessment increased significantly as DDQ desire and intention scores increased (Model 2: OR = 1.25, 95%CI 1.03; 1.51), but was not associated significantly with DDQ negative reinforcement (Model 3: OR = 1.01, 95%CI 0.88; 1.17, p > .05) or DDQ control (Model 4: OR = 0.97, 95%CI 0.85; 1.11, p > .05) scores.
AIC and BIC statistics favor Models 1 and 2 (Table 3) over Models 3 and 4. The difference in BIC statistics for Models 1 and 2 provides positive evidence that the PCS may be a stronger predictor of subsequent opioid use than previously validated DDQ scales.
4. Discussion
In this study of opioid dependent patients who initiated treatment with buprenorphine, we found that after controlling for demographic characteristics, baseline measures of substance use, and depression, a greater degree of craving as measured by the desire/intention scale of the DDQ was associated with a higher likelihood of using opioids. In addition, this study found preliminary evidence for the reliability and validity of the Penn Craving Scale adapted for opioid use. Craving as measured by the PCS was also significantly associated with subsequent opioid use over time. These results reinforce the relationship between craving and opioid use, and support the measurement of craving as a research outcome with clinical significance.
The results of this study are consistent with clinical trials of buprenorphine that have measured craving using single-item measures. Those studies have demonstrated that craving is reduced by initiation of treatment with buprenorphine, and that buprenorphine is associated with less opioid use over time (10, 24). Our study extends those results by reporting direct associations between craving and opioid use, providing support for craving as an important pathway leading to opioid use in treated samples. Furthermore, our study results suggest that craving scores plateau at approximately 2 weeks after initiation of buprenorphine. Therefore, it may be especially important to assess craving for opioids a week or two after initiating buprenorphine to assess lapse risk. Patients who relate persistent cravings during that time may need additional clinical interventions or intensive monitoring to prevent treatment failure. Our study was also consistent with prior studies demonstrating that a substantial percentage of treated patients will continue to use opioids. This underlines the fact that successful treatment of opioid dependence is contingent on factors other than simple reductions in craving.
This is the first study that has adapted the Penn Craving Scale for measurement of opioid craving. The results suggest that the PCS is a valid measurement tool for evaluating opioid craving. Notably, only one of the DDQ scales (desire/intention) predicted opioid use. It would appear that the other two DDQ subscales which measure perceived ability to use opioids in a controlled way and expectations for relieving negatives states with opioid use do not have as great predictive utility. The PCS and DDQ desire/intention subscale were each significantly associated with opioid use, so it appears that they each represent salient aspects of craving experiences. The PCS targets the quantity and frequency of thoughts about the positive reward value of using and urges to control over the past week, whereas the DDQ subscale focuses on current desire to use. The fact that the PCS is relatively brief (5 questions) and was slightly more strongly related to opioid use may make it attractive for research and clinical use.
There are a number of limitations to this study. Although the study used a lagged predictor approach, we cannot definitively conclude that craving directly caused subjects to use opioids, as there was a two-week interval between study visits. Use of ecological momentary assessments, in which participants to make repeated, frequent “real-time” assessments of substance use and craving might shed better light on causality in future studies. A prior study of stress and craving in cocaine and heroin users that used ecological momentary assessment found that subjects’ reported craving spiked during the hour before use (25), supporting the suggestion that craving precedes lapse to drug use. Second, our study is focused on a relatively specific patient population, namely opioid dependent patients initiating buprenorphine with depressive symptoms, which may limit the generalizability of the findings. However, depression is common among opioid addicts: studies estimate that approximately a third to one half suffer from depression (26, 27). Furthermore, our sample appears to be similar to other populations of buprenorphine treated patients with regards to the proportion currently using heroin v. non-heroin opioids, supporting its overall general representativeness (28). Finally, our study did not include a single item assessment of craving, so we were unable to compare the relative value of the multi-item scales used to a single item measure.
In summary, this study of opioid dependent patients with depressive symptoms who were initiating buprenorphine found that opioid craving, as measured by the DDQ subscale for desire/intention to use and the adapted Penn Craving Scale, was significantly associated with subsequent opioid use. Craving scores appear to reach a steady state approximately 2 weeks after induction; therefore assessment may be clinically useful at that time to predict an individual’s risk for treatment failure/lapse to use. Based on this evidence, reducing craving for opioids is an important mechanism for reducing subsequent use among opioid dependent patients.
Acknowledgments
This study was funded by the National Institute on Drug Abuse DA022207 (Clinical Trial NCT# 00475878) and National Institute on Drug Abuse Mid-Career Award DA 000512. Dr. Tsui is funded by NIDA award K23DA027367. Study medication was provided by Reckitt Benckiser.
References
- 1.Substance Abuse and Mental Health Services Administration. Rockville, MD: 2009. Results from the 2008 National Survey on Drug Use and Health: National Findings. Office of Applied Studies, NSDUH Series H-36, HHS Publication No. SMA 09-4434. [Google Scholar]
- 2.Hulse GK, English DR, Milne E, Holman CD. The quantification of mortality resulting from the regular use of illicit opiates. Addiction. 1999;94(2):221–229. doi: 10.1046/j.1360-0443.1999.9422216.x. [DOI] [PubMed] [Google Scholar]
- 3.Mark TL, Woody GE, Juday T, Kleber HD. The economic costs of heroin addiction in the United States. Drug Alcohol Depend. 2001;61(2):195–206. doi: 10.1016/s0376-8716(00)00162-9. [DOI] [PubMed] [Google Scholar]
- 4.Stein MD. Medical consequences of substance abuse. Psychiatr Clin North Am. 1999;22(2):351–370. doi: 10.1016/s0193-953x(05)70081-2. [DOI] [PubMed] [Google Scholar]
- 5.Ling W, Wesson DR. Clinical efficacy of buprenorphine: comparisons to methadone and placebo. Drug Alcohol Depend. 2003;70(2 Suppl):S49–S57. doi: 10.1016/s0376-8716(03)00059-0. [DOI] [PubMed] [Google Scholar]
- 6.Fareed A, Vayalapalli S, Casarella J, Amar R, Drexler K. Heroin anticraving medications: a systematic review. Am J Drug Alcohol Abuse. 2010;36(6):332–341. doi: 10.3109/00952990.2010.505991. [DOI] [PubMed] [Google Scholar]
- 7.Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG. The measurement of drug craving. Addiction. 2000;95(Suppl 2):S189–S210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiffany ST, Wray JM. The clinical significance of drug craving. Ann N Y Acad Sci. 2011;1248:1–17. doi: 10.1111/j.1749-6632.2011.06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiffany ST, Friedman L, Greenfield SF, Hasin DS, Jackson R. Beyond drug use: a systematic consideration of other outcomes in evaluations of treatments for substance use disorders. Addiction. 2011;107(4):709–718. doi: 10.1111/j.1360-0443.2011.03581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ling W, Charuvastra C, Collins JF, Batki S, Brown LS, Jr, Kintaudi P, Wesson DR, McNicholas L, Tusel DJ, Malkerneker U, Renner JA, Jr, Santos E, Casadonte P, Fye C, Stine S, Wang RI, Segal D. Buprenorphine maintenance treatment of opiate dependence: a multicenter, randomized clinical trial. Addiction. 1998;93(4):475–486. doi: 10.1046/j.1360-0443.1998.9344753.x. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg H. Clinical and laboratory assessment of the subjective experience of drug craving. Clin Psychol Rev. 2009;29(6):519–534. doi: 10.1016/j.cpr.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Franken IH, Hendriksa VM, van den Brink W. Initial validation of two opiate craving questionnaires the obsessive compulsive drug use scale and the desires for drug questionnaire. Addict Behav. 2002;27(5):675–685. doi: 10.1016/s0306-4603(01)00201-5. [DOI] [PubMed] [Google Scholar]
- 13.Flannery BA, Volpicelli JR, Pettinati HM. Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res. 1999;23(8):1289–1295. [PubMed] [Google Scholar]
- 14.Stein MD, Herman DS, Kettavong M, Cioe PA, Friedmann PD, Tellioglu T, Anderson BJ. Antidepressant treatment does not improve buprenorphine retention among opioid-dependent persons. J Subst Abuse Treat. 2010;39(2):157–166. doi: 10.1016/j.jsat.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller I, Bishop S, Norman W, Maddever H. The modified Hamilton rating scale for depression: reliability and validity. Psych Res. 1985;14:131–142. doi: 10.1016/0165-1781(85)90057-5. [DOI] [PubMed] [Google Scholar]
- 16.Flannery BA, Poole SA, Gallop RJ, Volpicelli JR. Alcohol craving predicts drinking during treatment: an analysis of three assessment instruments. J Stud Alcohol. 2003;64(1):120–126. doi: 10.15288/jsa.2003.64.120. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg H, Mazzola J. Relationships among self-report assessments of craving in binge-drinking university students. Addict Behav. 2007;32(12):2811–2818. doi: 10.1016/j.addbeh.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Beck AT, Steer RA, Brown GK. Manual for the BDI-II. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 19.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 20.Muthén BO, Muthén BC. Mplus Version 5.1 Ed. Los Angeles, CA: Muthén & Muthén; 2008. [Google Scholar]
- 21.Akaike H. Information theory and an extension of the maximum likelihood principle. In: Petrov B, Csaki F, editors. Second International Symposium on Information Theory. Budapest: Akademiai Kiado; 1973. pp. 267–281. [Google Scholar]
- 22.Raftery AE. Bayesian model selection in social research. In: Marsden PV, editor. Sociological Methodology. Vol. 26. Oxford: Blackwell; 1996. [Google Scholar]
- 23.StataCorp. Stata Statistical Software: Release 10.1. College Station, TX: StataCorp LP; 2010. [Google Scholar]
- 24.Fudala PJ, Bridge TP, Herbert S, Williford WO, Chiang CN, Jones K, Collins J, Raisch D, Casadonte P, Goldsmith RJ, Ling W, Malkerneker U, McNicholas L, Renner J, Stine S, Tusel D. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949–958. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- 25.Preston KL, Epstein DH. Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology (Berl) 2011;218(1):29–37. doi: 10.1007/s00213-011-2183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brienza RS, Stein MD, Chen M, Gogineni A, Sobota M, Maksad J, Hu P, Clarke J. Depression among needle exchange program and methadone maintenance clients. J Subst Abuse Treat. 2000;18(4):331–337. doi: 10.1016/s0740-5472(99)00084-7. [DOI] [PubMed] [Google Scholar]
- 27.Rounsaville BJ, Weissman MM, Crits-Christoph K, Wilber C, Kleber H. Diagnosis and symptoms of depression in opiate addicts. Course and relationship to treatment outcome. Arch Gen Psychiatry. 1982;39(2):151–156. doi: 10.1001/archpsyc.1982.04290020021004. [DOI] [PubMed] [Google Scholar]
- 28.Stanton L, McLeod C, Luckey B, Kissin WB, Sonnenfeld LJ. SAMHSA/CSAT Evaluation of the Buprenorphine Waiver Program. [accessed 6/05/12];2006 http://buprenorphine.samhsa.gov/ASAM_06_Final_Results.pdf.