Summary
Determining the functional attributes of pancreatic transcription factors is essential to understand how the pancreas is specified distinct from other endodermal organs, such as liver, stomach and duodenum, and to direct the differentiation of other cell types into pancreas. Previously, we demonstrated that Pdx1-VP16 was sufficient to convert liver to pancreas. In this paper we characterize the functional ability of another pancreatic transcription factor, Ptf1a, in promoting ectopic pancreatic fates at early stages throughout the endoderm and later in during organogenesis. Using the transthyretin promoter to drive expression in the early liver region/bud of transgenic Xenopus tadpoles, we find that Ptf1a-VP16 is able to convert liver to pancreas. Overexpression of the unmodified Ptf1a on the other hand, has no effect in liver, but is able to convert stomach and duodenum to pancreas. When overexpressed at earlier embryonic stages throughout the endoderm, Ptf1a activity is similarly limited, whereas Ptf1a-VP16 has increased activity. Interestingly, in all instances we find that Ptf1a-VP16 is only capable of promoting acinar cell fates, whereas Ptf1a promotes both acinar and endocrine fates. Lastly, we demonstrate that, similar to mouse and zebrafish, Xenopus Ptf1a is essential for the initial specification of both endocrine and exocrine cells during normal pancreas development.
Keywords: Xenopus, pancreas, Ptf1a, specification, transdifferentiation, endocrine, exocrine, organogenesis
Introduction
The vertebrate pancreas has its embryological origin as two endodermal buds developing on the dorsal and ventral side of the duodenum (Edlund, 2002; Kim and MacDonald, 2002). The dorsal bud arises just below the notochord, while the ventral bud develops adjacent to the hepatic diverticulum (Slack, 1995). The fusion of the two buds gives rise to a single mixed gland composed of exocrine and endocrine cells. The exocrine pancreas is a lobulated branched tissue, which includes acinar and ductal cells that secrete and transport digestive enzymes into the duodenum. The endocrine cells are grouped into islets of Langerhans composed of five principal cell types, α, β, δ, ε and PP that secrete glucagon, insulin, somatostatin, ghrelin and pancreatic polypeptide hormones into the bloodstream.
In the amphibian Xenopus laevis, development of the pancreas proceeds in an almost identical manner to that seen in mammals (Kelly and Melton, 2000). The dorsal bud is the first to appear from a region just below the notochord at stage 35/36. The ventral pancreas derives from two ventral buds adjacent to the liver that fuse at stage 37/38. At stage 39 morphogenetic movements of the gastrointestinal tract reposition the pancreatic rudiments leading to their fusion and formation of a single organ. Signals from the mesoderm are required for pancreatic differentiation, and development of exocrine and endocrine cells occurs in a spatially and temporally distinct manner (Horb and Slack, 2001; Horb and Slack, 2002; Kelly and Melton, 2000). Exocrine cells are initially specified in the ventral pancreas, and endocrine cells in the dorsal. Amylase is first detected at stage 40 only in the ventral pancreas, and expression subsequently spreads to the dorsal pancreas such that by stage 45 it is present throughout the entire pancreas (Horb and Slack, 2002). On the other hand, insulin is first expressed at stage 32 in the dorsal pancreatic endoderm; expression in the ventral pancreas is only detected at stage 47 (Horb and Slack, 2002; Kelly and Melton, 2000). In contrast, the other endocrine markers glucagon and somatostatin are not detected in the pancreas until stage 45; though expression is present in the stomach at earlier stages.
Several classes of transcription factors are involved in the specification and differentiation of both endocrine and exocrine lineages (Habener et al., 2005). Although the number of these transcription factors is significant, their precise roles in the pancreatic transcriptional cascade and the downstream targets they regulate remain unknown. Two of the earliest acting transcription factors are Pdx1 and Ptf1a. Pdx1 is a ParaHox gene that is expressed at the earliest stages in the dorsal and ventral pancreatic buds as well as in the duodenum (Wright et al., 1989); at later stages it is highly expressed in beta cells, with lower levels also found in acinar cells and all rostral duodenal cells (Jonsson et al., 1995). Mice lacking Pdx1 do not develop a pancreas (Jonsson et al., 1994; Offield et al., 1996) and mutations in the human homologue, Ipf1, are associated with pancreatic agenesis (Stoffers et al., 1997).
Ptf1a is a bHLH gene that is expressed in early pancreatic progenitors (dorsal and ventral buds) (Kawaguchi et al., 2002), but in adults is only expressed in acinar cells (Krapp et al., 1996). The early induction of Ptf1a in the dorsal pancreas was shown to require interactions with endothelial cells (Yoshitomi and Zaret, 2004), while Fgf10 is necessary to maintain this dorsal expression (Jacquemin et al., 2006). Loss-of-function studies in mice have demonstrated that Ptf1a is essential for acinar cell development and plays an important role in endocrine cell development as well (Kawaguchi et al., 2002; Krapp et al., 1998); in humans, PTF1A gene mutations are associated with pancreatic and cerebellar agenesis (Sellick et al., 2004). Similarly, morpholino knockdown studies in zebrafish and more recently in Xenopus have shown that Ptf1a is required for development of all acinar cells and a subset of endocrine cells (Afelik et al., 2006; Lin et al., 2004). It is however, only expressed in a subset of pancreatic progenitors in the left ventrolateral endoderm, and not in the dorsal posterior endoderm (Lin et al., 2004; Zecchin et al., 2004). Several reports have suggested that Ptf1a may function as a master regulator of pancreatic cell fate. For example, pancreatic cells lacking Ptf1a switch their fate and become duodenal (Kawaguchi et al., 2002), and loss of Hes1 leads to the generation of ectopic Ptf1a expression in stomach, duodenum and bile duct resulting in ectopic pancreas formation (Fukuda et al., 2006). Similarly, overexpression of Ptf1a in Xenopus embryos leads to an expansion of the pancreatic region, but only within the Pdx1-expression domain, whereas combined overexpression of Pdx1 and Ptf1a is sufficient to promote acinar cell fates in posterior endoderm (Afelik et al., 2006). Altogether, these results suggest that Ptf1a plays a central role in the decision to become stomach, duodenum, bile duct or pancreas.
Transdifferentiation is the conversion of one differentiated cell type to another (Okada, 1991; Slack and Tosh, 2001). At the molecular level transdifferentiation is associated with a change in the expression of master regulatory genes (Tosh and Slack, 2002), of which Pdx1 is considered to be the pancreatic master gene. Although it is expressed in and required for the development of an endodermal domain broader than the pancreas alone, Pdx1 can be considered to be part of the transcription factor program associated with the adoption of pancreas fate because it is expressed very early in the outgrowth of the anlagen of this organ, and because there is such an early abrogation of pancreas development in Pdx1-/- mutants. In agreement with this, Pdx1 has been shown to convert liver to pancreas (Meivar-Levy and Ferber, 2006). Overexpression of Pdx1 in liver activates expression of pancreatic endocrine and exocrine markers (Ferber et al., 2000; Kojima et al., 2003; Miyatsuka et al., 2003; Tang et al., 2006b), and is sufficient to prevent STZ induced hyperglycemia (Ber et al., 2003; Ferber et al., 2000; Sapir et al., 2005; Zalzman et al., 2003). We also showed that expression of a super-active form of Pdx1 (Pdx1-VP16) in both human HepG2 cells and Xenopus transgenics converts liver into pancreatic tissue containing both exocrine and endocrine cell types (Horb et al., 2003; Li et al., 2005). As seen with Pdx1, these Pdx1-VP16 expressing liver cells are capable of functioning as beta cells to restore euglycemia in diabetic mice (Cao et al., 2004; Imai et al., 2005; Kaneto et al., 2005; Tang et al., 2006a). Apart from NeuroD, which was shown to induce islet neogenesis in the liver (Kojima et al., 2003), the functional ability of other pancreatic transcription factors to convert liver to pancreas has not been fully explored.
We now report our results examining whether Ptf1a has similar ability to convert liver to pancreas. Indeed, we find that Ptf1a is able to promote ectopic pancreas formation in the endoderm. We demonstrate that Ptf1a and Ptf1a-VP16 have differential activities in converting endodermal organs to pancreas, dependant on the timing of overexpression. Furthermore, we find that Ptf1a-VP16 is only capable of promoting an acinar cell fate, while the unmodified Ptf1a promotes both acinar and endocrine cell fates. Last, we investigate the role of Ptf1a in normal Xenopus pancreas development and find that large-scale knock-down of Ptf1a function affects the initial specification of both exocrine and endocrine cells. Taken together, our results establish Ptf1a as being both necessary and sufficient for endocrine and exocrine pancreatic cell fate.
Materials and Methods
Xenopus transgenics and transgene construction
All Xenopus transgenic constructs were cloned into the TTR-VP16:Elas-GFP vector that was derived from TTR-Xlhbox8-VP16:Elas-GFP. Each transgene was constructed as follows and confirmed by sequencing. TTR-VP16-mPtf1a:Elas-GFP-mPtf1a cut EcoRI (mung bean nuclease) and cloned into TTR-VP16:Elas-GFP cut XhoI (blunt). TTR-mPtf1a:Elas-GFP- mPtf1a cut XbaI (blunt)-XhoI and cloned into TTR-Xlhbox8-VP16:Elas-GFP cut StuI-XhoI. IFABP-mPtf1a:Elas-GFP- TTR-mPtf1a:Elas-GFP cut KpnI (blunt) and ligated into IFABP-GFP cut BamHI (blunt). Same procedure followed for IFABP-VP16-mPtf1a:Elas-GFP. TTR-Xptf1a:Elas-GFP- Xptf1a in pCR-Script cut NotI-ClaI (blunt) and cloned into the TTR-Xlhbox8-VP16:Elas-GFP vector cut ClaI-XhoI (blunt). Xptf1a-VP16- Xptf1a isolated as above and cloned into VP16-N vector cut ClaI (blunt). TTR-Xptf1a-VP16:Elas-GFP- Xptf1a-VP16 cut NcoI-XbaI (blunt) cloned into TTR-Xlhbox8-VP16:Elas-GFP as above. Xenopus laevis transgenics were produced as previously described (Horb et al. 2003). In later stages of our work we switched the protocol for creating transgenics to the I-SceI meganuclease method, with no change in activity (Pan et al., 2006; Thermes et al., 2002). All functional portions (TTR-GeneX;Elas-GFP) of the transgenes described above were cloned in between two I-SceI sites.
F0 Elas-GFP transgenics were generated using the Elas-GFP transgene as described (Beck and Slack, 1999). F1 offspring were generated by crossing F0 a female Elas-GFP adult with a wild type male, whereas F2 offspring were generated by fertilizing F1 female transgenic eggs with F1 transgenic sperm in vitro. Germline transmission of the Elas-GFP transgene from these F1 adults is found in 75% of the offspring when transgenic eggs are fertilized with F1 transgenic male sperm (n>300). When transgenic sperm is used to fertilize wild type female eggs, only 45% of the embryos are transgenic.
Isolation of Xenopus ptf1a
Degenerate PCR primers used to amplify a partial fragment of the bHLH region of Xptf1a were based on the following peptides: PTLPYEKR, 5′-TCCCCACCCTGCCCtaygaraarmg-3′ for the forward primer and ENEPPFEFV, 5′-CACGAACTCGAAAGGGggytcrttytc-3′ for the reverse primer. The PCR product was cloned into the pCR-Script vector, and the sequence was used to identify a Ptf1a open reading frame from the X. tropicalis genome assembly. Based on the X. tropicalis Ptf1a sequence the following primers were designed to amplify the 5′ end of X. laevis Ptf1a from stage 42 whole gut cDNA: forward 5′-CCGGCACCATGGAAACGGT-3′ and reverse primer 5′-ATCCTCAGGAGTCCACACT-3′. The PCR product was cloned into the pCR-Script vector and ligated to the bHLH region of Xptf1a previously isolated by cloning into the Not1-Bsu36I sites. The 5′ UTR of Xptf1a was cloned using 5′ RACE (BD Biosciences). 5′ ready cDNA was prepared from stage 42 Xenopus laevis whole tadpoles (gift of G. Andelfinger). Two different reverse primers were designed: the first primer was designed 720bp from the start site 5′-ATCCTCAGGAGTCCACACT-3′ and the second 500bp from the start site 5′-TGAGGAAGTTAATGTAGC-3′. The PCR product was cloned into the pCRII vector (Invitrogen).
For cloning the X. laevis intron we designed the following primers 60bp upstream and downstream of the predicted site of the intron: forward 5′-GTACAGTCCGATCTGCCGCT-3′ and reverse 5′-CTCAGTTGCTTCTCATCAGT-3′. We expected the X. laevis intron to be approximately 500bp in size since the X. tropicalis and mouse introns are 477bp and 330bp in length, respectively. We amplified the X. laevis intron from stage 42 whole gut cDNA; this can be accomplished since X. laevis cDNA frequently contains intronic sequence. A single band of 832bp was amplified and cloned into the pCRII vector and sequenced; the intron being 712bp long. Interestingly, when compared to the X. tropicalis intron only one region of 43bp was similar in sequence, showing 84% nucleotide identity (data not shown). The accession number for the complete cDNA sequence including 5′ UTR is AY372268.
Embryological assays and whole mount In situ hybridization
Antisense morpholino oligonucleotides were designed by Gene Tools, LLC. MO1 5′-CAACTGCTCCAGGACCGTTTCCATG-3′ targets the initiation codon of Xptf1a. MO2 5′-ACGTTGGACTTACTTGTGCCCCGG-3′ targets the exon-intron boundary. Synthetic mRNA transcripts were synthesized by SP6 in vitro transcription (mMessage machine, Ambion). Whole-mount in situ hybridizations with single probes were performed as described using BM Purple (Horb et al., 2003). Antisense digoxigenin probe for Xptf1a was synthesized from Xptf1a in pCR-Script linearized with SacII and transcribed with T7 RNA polymerase. Probes for other pancreatic markers were prepared as previously described (Horb et al., 2003).
Real time PCR
The Mx3005® multiplex quantitative PCR Thermal Cycler system from Stratagene was used to monitor the real-time experiments and generated data were collected and analyzed using the MxPro software provided with the system. RNA was extracted from whole embryos and explants using Trizol, cDNA prepared using Superscript reverse transcriptase (Invitrogen). Primers were designed based on sequences available in Unigene and quality tested for accuracy. Real-time PCR reactions were prepared using the QuantiTect SYBER Green PCR kit from Qiagen. 10μl of 2× QuantiTect SYBER Green PCR Master Mix was used for each reaction and primer concentration was optimized for 0.8μM. PCR conditions were as follows 15 min activation at 95 degree, 30 sec denaturation at 94 degree, 1 min annealing at 58 degree and 30 sec extension at 72 degree for 40 cycles. Real time PCR values were normalized according to EF1α.
Results
Previously, we demonstrated that Pdx1, when fused to VP16, was sufficient to convert liver to pancreas (Horb et al., 2003), but whether this attribute was a general characteristic of every pancreatic transcription factor or limited to those that function as master regulators of early cell fate was not explored. To address this we examined the transdifferentiation activity of several pancreatic transcription factors using Xenopus transgenics. Both unmodified and VP16 fusions of seven different pancreatic transcription factors, Hlxb9, NeuroD, Islet1, Pax4, Pax6, Nkx2.2 and Ptf1a, were expressed in Xenopus tadpole liver (Horb, unpublished). We created transgenic tadpoles overexpressing each of these pancreatic transcription factors using the double transgene construct previously described with Pdx1-VP16 (Horb et al., 2003). Briefly, each gene was cloned down stream of the liver transthyretin (TTR) promoter and the result ant transgenic tadpoles examined for ectopic Elas-GFP expression. In Xenopus, the TTR promoter directs expression to the liver, stomach, and duodenum after stage 44 as we previously demonstrated (Fig. 4I,J in (Horb et al., 2003)); this is after organ bud formation and cell fate specification, but prior to complete maturation (Yan et al., 1990). The pancreas-specific elastase promoter controls GFP expression providing a real-time marker of pancreatic cell fate and showing that the transgene is not silenced due to integration-site effects (Beck and Slack, 1999; Horb et al., 2003). Though elastase is an acinar-specific gene product, the elastase promoter element (A+B+C) used here is the 213 bp fragment of the complete promoter that is active in both endocrine and exocrine cells as it lacks the endocrine repressive element (Kruse et al., 1993). We defined positive transdifferentiation activity as ectopic expression of GFP in transgenic tadpole liver and/or stomach/duodenum (Horb et al., 2003); ectopic GFP expression representing a generic pancreatic fate. Of these fourteen constructs (VP16 fusions and unmodified) we found only two, Ptf1a-VP16 and Ptf1a, were able to promote ectopic activation of Elas-GFP.
In transgenic TTR-mPtf1a-VP16:Elas-GFP tadpoles ectopic GFP fluorescence is detected in the liver after stage 45 (Fig. 1C), whereas in TTR-mPtf1a:Elas-GFP transgenics ectopic activation of Elas-GFP is detected in the stomach and duodenum and not in the liver (Fig. 1B). Prior to this stage Elas-GFP expression is only found in the pancreas, and initial development of liver, pancreas, stomach and duodenum occurs normally in both Ptf1a and Ptf1a-VP16 transgenics. In Ptf1a-VP16 transgenic tadpoles we find only partial conversion of the liver, similar to that seen in Pdx1-VP16 transgenics (Horb et al., 2003), whereas in Ptf1a transgenics we find complete conversion of the stomach and duodenum (see below). The proportion of Ptf1a (n=77) and Ptf1a-VP16 (n=36) transgenic tadpoles showing ectopic GFP expression was 27% and 44%, respectively. This was quite different from the results we previously obtained with Pdx1-VP16, where we obtained 61% transgenics with ectopic pancreas (Horb et al., 2003). The reason for these differences is unclear, but may reflect the fact that the Pdx1 acts earlier than Ptf1a in pancreas development. These results demonstrate that the ability to bring about transdifferentiation of liver to pancreas is specific to Pdx1-VP16 and Ptf1a-VP16 and does not arise simply from the use of the strong transactivator VP16 with any pancreatic transcription factor. Furthermore, it demonstrates that the ability to convert other organs to pancreas is not a general characteristic of every pancreatic transcription factor, but is limited to those that act during initial stages of cell fate specification (perhaps as master regulatory genes).
Figure 1.
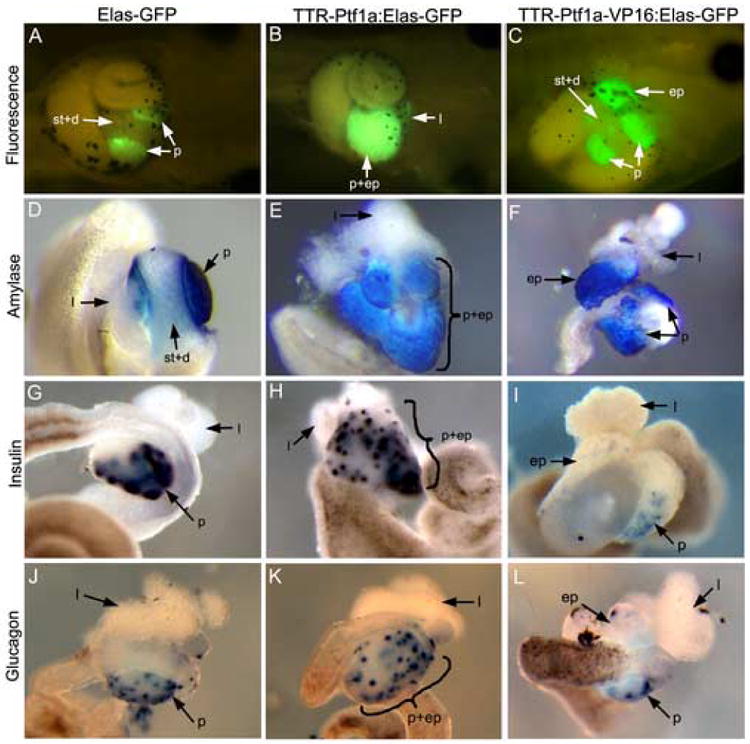
Transgenic overexpression of Ptf1a and Ptf1a-VP16 promotes ectopic pancreas formation. (A) Control St.44 Elas-GFP transgenic tadpole showing GFP expression only in the pancreas (p). No expression is seen in the stomach and duodenum (st+d). (B) St.45 TTR-Ptf1a:Elas-GFP transgenic tadpole. No stomach or duodenum is evident and instead ectopic GFP is detected throughout this region (p+ep). The liver (l) is normal. (C) St.45 TTR-Ptf1a-VP16:Elas-GFP transgenic tadpole. Ectopic expression (ep) of Elas-GFP is seen opposite the pancreas in the liver. Stomach and duodenum are normal. (The punctate GFP expression in between the ectopic and normal pancreas does not represent epithelial cells in the stomach or duodenum, but are cells released from the ectopic pancreas due to mechanical abrasion during processing of samples for photography.) (D-F) Amylase RNA expression in stage 46/47 whole guts. (D) In control whole guts amylase is only detected in the pancreas. (E) In TTR-Ptf1a transgenics ectopic amylase expression is now seen in the stomach and duodenum that is fused with the normal pancreas (p+ep) and not in the liver. (F) Ectopic expression (ep) is seen in the livers of TTR-Ptf1a-VP16 tadpoles. (G-I) Insulin RNA expression in stage 46/47 whole guts. (G) In controls, insulin is found expressed in a punctate fashion within the pancreas. (H) In TTR-Ptf1a transgenics insulin expression is detected in the ectopic pancreatic tissue encompassing the stomach, duodenum and pancreas (p+ep). The normal pancreas is seen bulging out to the left, while the ectopic pancreas is fused with it just to the right. The shape of this ectopic pancreatic tissue resembles the normal stomach. The liver (l) is normal. (I) Expression of insulin is only seen in the pancreas of control and TTR-Ptf1a-VP16 transgenics. No expression is seen in the ectopic pancreas (ep). (J-L) Glucagon RNA expression in stage 46/47 whole guts. (J) In controls, glucagon is expressed in a punctate fashion much like insulin. (K) In TTR-Ptf1a transgenics, ectopic glucagon expression is detected in the stomach and duodenum. (L) In TTR-Ptf1a-VP16 transgenics, no ectopic glucagon expression is detected.
The unmodified Ptf1a converts duodenum and stomach to pancreas
Upon dissection of whole guts from TTR-Ptf1a:Elas-GFP transgenic tadpoles after stage 46 we found no evidence of stomach or duodenum, while the esophagus, intestine and liver appeared normal. Instead we found a large region of pancreatic tissue in the posterior foregut resulting from fusion of the normal pancreas with ectopic pancreatic tissue generated from the stomach and duodenum (Fig. 1B). We examined TTR-Ptf1a transgenics for ectopically expressed pancreatic differentiation markers. Interestingly, we detected expression of both endocrine and exocrine markers, amylase, insulin and glucagon, within the ectopic pancreatic tissue emanating from the region of the stomach, duodenum and pancreas (Fig. 1E,H,K). This is in contrast to that seen in Ptf1a-VP16 transgenics, where only exocrine markers were detected in ectopic pancreatic tissue. These results demonstrate that overexpression of the unmodified Ptf1a after organogenesis of stomach, duodenum and liver is sufficient to promote both ectopic pancreatic endocrine and exocrine cell fates in the stomach/duodenum, but not in the liver.
To confirm transdifferentiation of stomach and duodenum to pancreas we examined whether there was loss of the stomach/duodenum marker frp5 in Ptf1a transgenic guts. We found an almost complete absence of frp5 expression in whole guts isolated from TTR-Ptf1a transgenics as compared to control and TTR-Ptf1a-VP16 transgenics (Fig. 2E). We did detect a small amount of frp5 staining at the position corresponding to reappearance of the gut tube (“d”), at the posterior end of the ectopic pancreatic tissue (Fig. 2E). We next examined whether liver differentiation occurred normally, and found normal expression of the liver differentiation marker transthyretin in Ptf1a transgenics (Fig. 2B). This was in contrast to the reduced expression found in Ptf1a-VP16 transgenics (Fig. 2C), but in agreement with the lack of ectopic Elas-GFP expression in the livers of Ptf1a transgenics (Fig. 1C). Serial histological analysis of whole guts isolated from TTR-Ptf1a transgenics confirmed the loss of stomach and duodenum, and replacement with pancreatic tissue (Fig. 3C-F). In anterior sections the esophagus is evident, whereas in more posterior sections no gut tube is present, and the entire region is replaced with pancreatic tissue (Fig. 3E). In anterior sections, both the liver and pancreas can be seen clearly (Fig. 3C); in more posterior sections the pancreas expands to encompass the whole region of the gut tube that should have developed as stomach and duodenum (Fig. 3D,E). We have determined that the ectopic pancreatic tissue replaces the stomach and duodenal tube for a distance of 168μm (Fig. 3C-F). In conclusion, these results confirm that unmodified Ptf1a is sufficient to cause transdifferentiation of stomach/duodenum to pancreas, but has no effect in liver cells.
Figure 2.
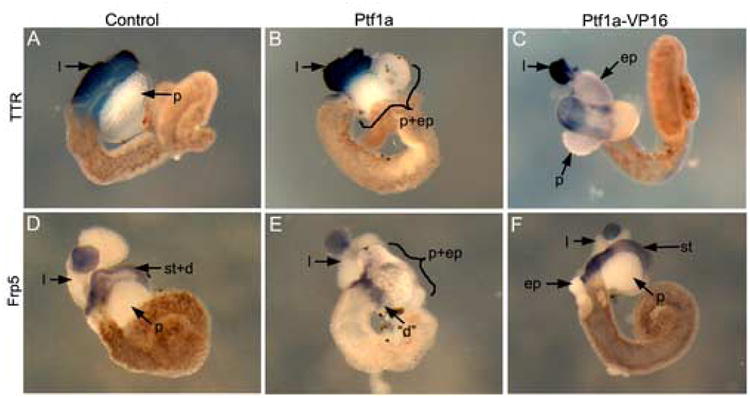
Effects of transgenic overexpression of Ptf1a-VP16 and Ptf1a on liver, stomach and duodenum. (A-C) Liver differentiation marker, transthyretin expression in stage 46/47 whole guts. (A) Control showing normal domain of TTR expression. Notice the size of the liver. (B) In Ptf1a transgenics transthyretin expression is normal. (C) Ptf1a-VP16 transgenic whole gut. Only a small domain of transthyretin expression is evident, located adjacent to the ectopic pancreas. (D-F) Expression of the stomach differentiation marker frp5 in stage 46/47 whole guts. (D) Frp5 expression in control whole guts extends from the duodenum through the stomach region (st+d), (E) Ptf1a transgenic whole gut. Almost no frp5 expression is detected. A small region at the posterior end of the pancreas and ectopic pancreas does express frp5 and may be marking a small remnant of the duodenum (“d”). (F) Ptf1a-VP16 transgenic whole gut-frp5 expression is normal.
Figure 3.
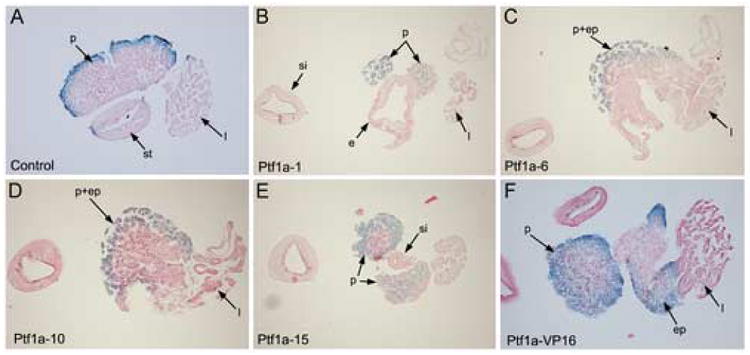
Histological analysis of Ptf1a and Ptf1a-VP16 transgenic whole guts. (A) Control whole gut section stained for amylase showing normal stomach (st), pancreas (p) and liver (l). (B-E) Single whole gut from TTR-Ptf1a:Elas-GFP transgenic tadpole was processed for histology after staining for amylase expression. 12μm serial sections numbers correlate to specific section, with number 1 corresponding to the first section obtained from the isolated whole gut. The posterior small intestine (si) is evident in every section on the left side for orientation. (B) Section #5- esophagus (e), pancreas (p) and liver (l) are all present. (C) Section #11- the pancreas has almost completely replaced the stomach and duodenum (p+st/d). A small portion of remaining stomach tube (“st”) is evident, but contains no recognizable stomach cells and the tube is open. Part of the liver (l) can still be seen. (D) Section #18- no gut tube is evident, and a large ectopic pancreas (p+st/d) is found in place of the stomach and duodenum. (E) Section #24- the small intestine (si) first reappears, with pancreatic tissue and liver still present. We measured the lack of stomach and duodenum from section 10-24 to be 168μm in total. (F) Section from Ptf1a-VP16 transgenic whole gut stained with amylase. The ectopic pancreas (ep) is seen fused with the liver, and the normal pancreas adjacent but separate.
Ptf1a-VP16 is sufficient to convert liver to pancreas
To characterize the ectopic pancreatic tissue in the livers of Ptf1a-VP16 transgenics we determined which pancreatic cell types (endocrine or exocrine) were present. Whole guts were isolated from transgenic tadpoles at stage 46-47 and the expression of both acinar and endocrine markers was examined by whole mount in situ hybridization. Abundant expression of amylase RNA was detected throughout the ectopic pancreas (Fig. 1F). We did not detect expression of insulin or glucagon, although occasionally a single positive cell was detected in the ectopic pancreatic tissue (Fig. 1I,L). To confirm that our results represented a transdifferentiation event, we examined Ptf1a-VP16 transgenics for loss of liver tissue. We found a large decrease in expression of the liver differentiation marker transthyretin (Fig. 2C). In the example shown only a small piece of liver tissue remains fused with the ectopic pancreas. In agreement with our previous results with Pdx1-VP16 we found different phenotypes of liver to pancreas transdifferentiation, from an almost complete loss of liver tissue to partial conversions (data not shown). Histological analysis confirmed ectopic pancreas fusion with liver in Ptf1a-VP16 transgenics (Fig. 3B). In conclusion, these results demonstrate that Ptf1a-VP16 is able to cause transdifferentiation of liver to pancreas, but only to acinar cells and not endocrine cells.
Since the mouse Ptf1a protein shares only 60% overall amino acid identity with Xenopus Ptf1a (see below) we decided to examine whether Xenopus Ptf1a had similar transdifferentiation activity. We therefore cloned Xenopus Ptf1a and created a Xenopus Ptf1a-VP16 fusion and overexpressed it in transgenic tadpole liver. Identical to that seen with mouse Ptf1a, Xenopus Ptf1a-VP16 was sufficient to promote pancreatic acinar, and not endocrine, fate in liver (data not shown). To determine whether the placement of VP16 is important we created both N- and C-terminal fusions with Ptf1a, but found no difference in activity (data not shown). These results establish that placement of the VP16 activation domain is not important, and that the mouse and Xenopus proteins behave similarly.
We next examined whether Ptf1a and Ptf1a-VP16 transdifferentiation activity is limited to stomach, duodenum and liver by testing their ability to convert more posterior intestinal cells into pancreas using the intestinal fatty acid binding (IFABP) promoter (Sweetser et al., 1988). In Xenopus transgenics, the IFABP promoter is active throughout the entire intestine posterior to the duodenum beginning at stage 44, but not in the colon (Beck and Slack, 1999). Transgenic tadpoles were generated bearing the transgenes IFABP-mPtf1a:Elas-GFP or IFABP-VP16-mPtf1a:Elas-GFP, and examined for ectopic intestinal GFP expression. However, at no time (up to stage 48/49) did we observe ectopic GFP fluorescence in the intestine (data not shown). These results demonstrate that the ability of Ptf1a and Ptf1a-VP16 to convert other cell types into pancreas is limited to the posterior foregut derivatives stomach/duodenum and liver, respectively.
Ptf1a and Ptf1a-VP16 have similar capabilities in embryonic endoderm, prior to organogenesis
To determine whether Ptf1a and Ptf1a-VP16 activity is dependant on the differentiation state of endodermal cells, we tested their ability to ectopically activate the Elas-GFP promoter at earlier embryonic stages, prior to organogenesis. Ptf1a mRNA was injected into the four vegetal blastomeres of 8 cell transgenic F2 Elas-GFP X. laevis embryos (see Materials and Methods). The Elas-GFP transgene is active from stage 32 and GFP fluorescence can be observed as early as stage 38 in all pancreatic cells (Beck and Slack, 1999; Horb et al., 2003). Overexpression of Ptf1a mRNA in vegetal blastomeres, which was expected to diffuse broadly throughout the endodermal progenitors, leads to ectopic activation of the Elas-GFP transgene only in the stomach and duodenum (Fig. 4C). No ectopic expression was detected either in the liver or posterior endoderm, similar to that seen in transgenics. We also observed a second milder phenotype of enlarged pancreas, which contained a recognizable stomach and duodenum of approximately normal size, in contrast to the absence of these tissues as noted above (Fig. 4B). To confirm that ectopic activation of the Elas-GFP transgene is indeed indicative of pancreatic differentiation we examined injected embryos for expression of elastase and insulin using whole mount in situ hybridization. In Ptf1a-injected embryos we found ectopic expression of both elastase and insulin in the stomach and duodenum (Fig. 4G,K). In the enlarged pancreata we also found increased expression of both insulin and elastase (Fig. 4F,J). We confirmed the expression level changes of insulin and amylase in Ptf1a-injected embryos using real time PCR. Four individual embryos (control and Ptf1a-injected) were processed for real time PCR. In agreement with our in situ results, we found a 1.4 fold increase in insulin expression and a 2.3 fold increase in amylase expression (Fig. 4M). The other endocrine markers glucagon and somatostatin are not expressed in the developing Xenopus pancreas until stage 45; prior to this stage however, both are expressed in stomach and duodenal endocrine cells. To examine glucagon and somatostatin expression in Ptf1a-injected embryos we collected whole guts at stage 45. Overall we found reduced levels of both genes, which may be indicative of the loss of stomach and duodenum (Fig. 5B,E). We did detect relatively normal levels of expression of glucagon and somatostatin in the ectopic pancreas, but unlike insulin expression we did not detect increased expression of these genes (Fig. 5). These results demonstrate that overexpression of Ptf1a in early endoderm is sufficient to promote pancreatic acinar and beta cell development, and repress stomach and duodenal endocrine cell development.
Figure 4.
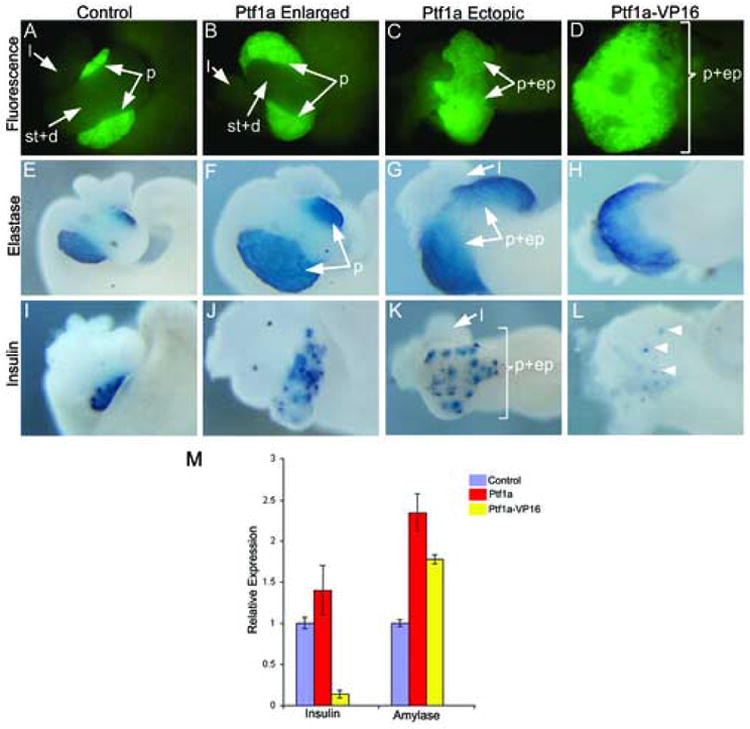
Overexpression of Ptf1a and Ptf1a-VP16 mRNA promotes ectopic and enlarged pancreas formation. Stage 42-44 dissected whole guts are shown in each image. (A) Control F2 Elas-GFP transgenic tadpole whole gut. (B) Ptf1a-injected embryos showing expanded GFP fluorescence. Stomach (st), duodenum (d) and liver (l) are normal, but the pancreas (p) is enlarged. (C) Ectopic pancreas formation in Ptf1a-injected embryo. The pancreas, stomach and duodenum (p+ep) form a large ectopic pancreas expressing Elas-GFP. (D) Ptf1a-VP16 injected embryo showing ectopic GFP fluorescence throughout the anterior endoderm. (E-H) In situ hybridization for elastase expression on isolated whole guts from (E) Control, (F,G) Ptf1a-injected embryos and (H) Ptf1a-VP16 injected embryos. Notice expanded and ectopic expression of elastase in Ptf1a and Ptf1a-VP16 injected embryos. (I-L) In situ hybridization for insulin in (I) Control, (J,K) Ptf1a-injected embryos and (L) Ptf1a-VP16 injected embryos. More insulin-expressing cells are detected in Ptf1a-injected whole guts, while there is a large decrease in insulin-expressing cells in Ptf1a-VP16 injected whole guts (arrowheads). (M) Real time PCR analysis of endocrine and exocrine markers in Ptf1a and Ptf1a-VP16 injected embryos for amylase and insulin expression in control, Ptf1a-injected and Ptf1a-VP16 injected whole embryos at stage 35. Each bar is an average of 4 individual whole tadpoles. Amylase expression is increased in both, while insulin expression is decreased in Ptf1a-VP16 and increased in Ptf1a-injected embryos. Purple-control tadpoles, red-Ptf1a mRNA injected tadpoles, yellow-Ptf1a-VP16 injected tadpoles.
Figure 5.
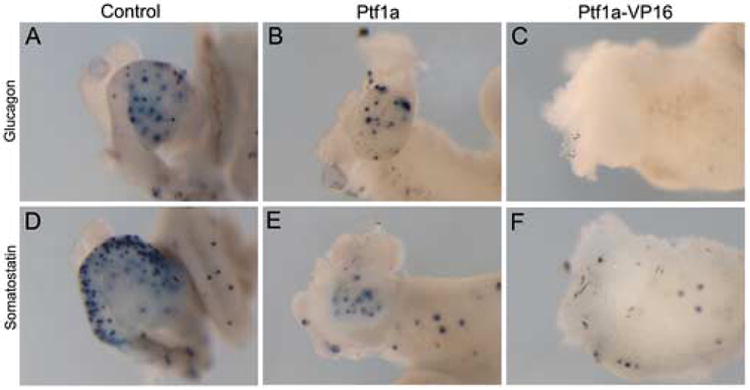
Development of glucagon and somatostatin expressing cells in Ptf1a and Ptf1a-VP16 injected embryos. (A-C) In situ hybridization for glucagon expression in control, Ptf1a and Ptf1a-VP16 injected embryos at stage 45. (B) Stomach and duodenal expression of glucagon is absent, while pancreatic glucagon expression appears slightly reduced in Ptf1a-injected ectopic pancreas phenotype. (C) No glucagon expression is detected in Ptf1a-VP16 injected embryos. (D-F) Somatostatin expression. (E) Overall somatostatin expression is reduced in Ptf1a-injected embryos. Pancreatic expression appears normal, while stomach and duodenal expression is completely absent. (F) Somatostatin expression is completely absent in Ptf1a-VP16 injected embryos.
To determine what effects Ptf1a has on development of adjacent endodermal organs, we examined Ptf1a-injected embryos for expression of Frp5 (Pilcher and Krieg, 2002) and Hex (Newman et al., 1997), markers of stomach/duodenum and liver respectively. In Ptf1a-injected embryos we found normal expression of Hex, but little to no frp5 expression was detected, indicating that stomach and duodenum did not develop properly, while liver development was normal (Fig. 6C,G). In the enlarged pancreas phenotype both frp5 and Hex expression was normal (Fig. 6B,F). We next examined Ptf1a-injected embryos for excessive histone H3 expression to determine if this activity is due to an overproliferation of pancreatic cells. However, we did not detect any extra histone H3 staining above control (data not shown). These results indicate that Ptf1a is sufficient to respecify early stomach/duodenal cells to pancreas, prior to their differentiation, but has no effect in the developing liver bud or posterior endoderm, identical to that seen in transgenics.
Figure 6.
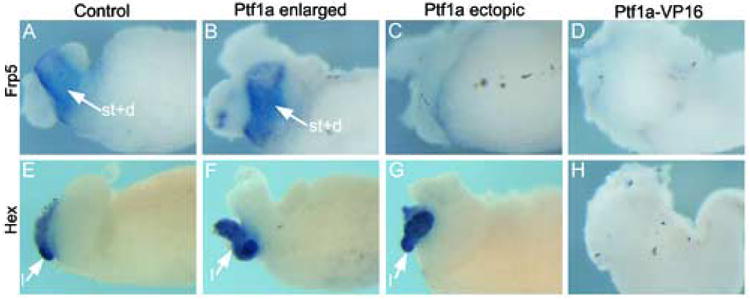
Effects of Ptf1a and Ptf1a-VP16 mRNA overexpression on organogenesis of liver, stomach and duodenum. (A-D) In situ hybridization for frp5 expression in control, Ptf1a-injected embryos and Ptf1a-VP16 injected embryos. (B) Normal expression is seen in the enlarged pancreas phenotype from Ptf1a-injected embryos, (C) whereas reduced expression is seen in the ectopic pancreas phenotype from Ptf1a-injected embryos. (D) No frp5 expression is detected in whole guts from Ptf1a-VP16 injected embryos. (E-H) In situ hybridization for Hex expression in Control, Ptf1a-injected embryos and Ptf1a-VP16 injected embryos. (F,G) Normal expression of Hex is detected in both enlarged and ectopic phenotypes from Ptf1a-injected embryos. (H) No Hex expression is detected in whole guts from Ptf1a-VP16 injected embryos.
We next examined whether Ptf1a-VP16 activity is similarly restricted in early endoderm as in transgenics. Similar to that seen with the unmodified Ptf1a, overexpression of Ptf1a-VP16 with in the entire endoderm resulted in ectopic Elas-GFP expression only in the anterior endoderm (Fig. 4D); no ectopic expression was detected in posterior endoderm in agreement with the results obtained in transgenic overexpression. In these embryos we found Elas-GFP expression to encompass a much greater area than that seen with Ptf1a, suggesting that Ptf1a-VP16 was now able to respecify liver, stomach and duodenum into pancreas (Fig. 4). We confirmed the loss of liver, stomach and duodenum by examination of Hex and frp5 expression (Fig. 6D,H). To determine which pancreatic cell types Ptf1a-VP16 promoted we examined endocrine and exocrine gene expression in stage 40 injected embryos. In agreement with the transgenic overexpression data, we found abundant expression of elastase throughout the Elas-GFP domain (Fig. 4H). However, to our surprise almost no insulin expression was detected (Fig. 4L). To determine more quantitatively the effects of Ptf1a-VP16 on insulin expression, we examined initial insulin expression at stage 35 using real time PCR. In agreement with the results seen in stage 40 whole guts, we found an 86% decrease in insulin expression in Ptf1a-VP16 injected embryos at early stage 35 when beta cells are first specified (Fig. 4M). In contrast, in Ptf1a-injected embryos we found a 40% increase in insulin expression at this same early stage. We also examined Ptf1a-injected embryos for activation of acinar gene products. By real time PCR we found a 1.8 fold increase in amylase expression in Ptf1a-VP16 injected embryos (Fig. 4M). In conclusion, these results demonstrate that Ptf1a-VP16 has greater activity in early endoderm than at later organogenesis stages, but that its capability is similarly restricted to promoting only acinar cell fate. In addition, the fact that insulin is down-regulated by Ptf1a-VP16 suggests that Ptf1a-VP16 alters the differentiation program of endocrine precursors, such that they differentiate along the acinar lineage.
Xenopus Ptf1a is essential for both exocrine and endocrine pancreas development
To examine the functional role of Ptf1a in normal Xenopus pancreas development, we isolated the X. laevis Ptf1a cDNA. The deduced protein sequence of Xenopus Ptf1a shares approximately 60% amino acid identity with other species, with highest similarity found in the bHLH DNA binding domain (data not shown). Similar to that seen by Afelik et al., we find Ptf1a to be first expressed at neurula stages in the midbrain-hindbrain region. Within the pancreatic endoderm Ptf1a is first detected in both dorsal and ventral pancreatic anlagen at stage 32 prior to overt morphogenesis and differentiation (data not shown). The expression of Ptf1a in both dorsal and ventral pancreatic buds prior to differentiation suggests that it may play a fundamental role in early cell fate specification of both endocrine and exocrine cells.
To determine if Ptf1a is essential for early pancreas development, we used antisense morpholinos to block translation and splicing of Ptf1a. A morpholino overlapping the translation start site of Ptf1a was designed (MO1) based on 5′ UTR sequence, which we cloned using 5′ rapid amplification of cDNA ends (see materials and methods). A second morpholino (MO2) was also designed to the exon-intron boundary to inhibit splicing of the Ptf1a RNA (see materials and methods). We examined the efficacy of this second morpholino by determining what effects it had on splicing of the Ptf1a transcript. Injection of MO2 prevented splicing of the primary Ptf1a transcript leading to inclusion of the single intron (Fig. 7R). We were still able to detect some of the proper splice transcript in MO2-injected embryos suggesting that the single morpholino was unable to completely inhibit Ptf1a translation; this may explain the reason for a more severe phenotype being found with o-injection of both morpholinos. To specifically target loss of Ptf1a to the region of the embryo where it is selectively expressed in the pancreatic endoderm, and avoid any indirect effects of reducing protein levels in neural tissue, another site of robust Ptf1a expression, we injected Ptf1a morpholinos into all four vegetal blastomeres of 8 cell Xenopus embryos. The antisense morpholinos are fluorescently labeled thus allowing us to identify rapidly whether the injection was properly targeted to the anterior endoderm. Embryos were then allowed to develop to tadpole stages and analyzed for any abnormalities.
Figure 7.
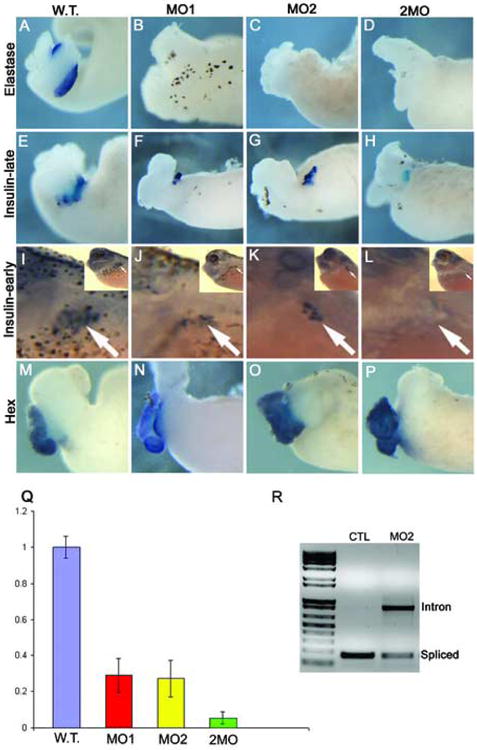
Elastase expression is absent in Ptf1a morphants, while insulin expression is reduced at late stages, but absent at early stages. (A-D) Whole mount in situ hybridization for elastase RNA expression in control and Ptf1a-MO dissected whole guts at stage 42. Elastase expression is not detected in either single or double morpholino injected guts. (E-H) Whole mount in situ hybridization for insulin RNA expression in control and Ptf1a-MO whole guts. (F,G) Insulin expression is decreased in both single morpholino injections. (H) In embryos injected with both morpholinos together insulin expression is also reduced, but still present. (I-L) Initial insulin expression is lacking in Ptf1a morphants. Insulin RNA expression by whole mount in situ hybridization in control and Ptf1a-MO injected embryos at stage 35. Inset in each panel is the low power view. (I) Control tadpole showing normal punctate insulin expression in the dorsal pancreas (arrow). (J,K) Insulin expression is reduced in single morpholino injected embryos, but (L) completely lacking in double morpholino injected embryos. (M-P) Expression of the liver marker Hex is normal in both single and double Ptf1a morpholino injected embryos. (Q) Real time PCR analysis of insulin expression in control and Ptf1a-MO injected embryos at stage 35 confirms insulin reduction in single and double morpholino injected embryos. Each bar is an average of four individual tadpoles. There is a 50-60% reduction in single morpholino tadpoles (40ng), but an almost complete absence in the double morpholino tadpoles (20ng each). (R) RT-PCR analysis of splicing in control and MO2-injected embryos showing the inhibition of splicing by MO2. Loading control was established with EF1α (not shown). Primers were designed flanking the single Ptf1a intron.
Embryos injected with 40ng of either MO1 or MO2 developed normally through gastrula, neurula and tail bud stages. However, at tadpole stages the gut developed abnormally with almost no recognizable pancreas (Fig. 7). At stage 45 approximately 50% of injected embryos have a small dorsal nub of tissue remaining, while the other 50% seem to completely lack any obvious pancreatic outgrowth (data not shown). Expression of acinar differentiation markers was completely lost (Fig. 7A-C), while insulin expression was reduced substantially (Fig. 7E-G). Interestingly, even in those embryos with no discernable pancreatic tissue, several insulin-expressing cells can be detected. These results would therefore seem to suggest that Ptf1a is not essential for endocrine cell development. Alternatively, it is possible that since we are looking at insulin expression four to five days after morpholino injection the presence of endocrine cells may be due to a dilution of the morpholino allowing newly generated beta cells to develop. To address this possibility, we examined the insulin expression at the earlier tadpole stage 32 when it is first detected. Similar to the results at later stages, we found insulin expression reduced, but not completely absent (Fig. 7Q), thus arguing against a dilution of the morpholino. It is possible that reduction of Ptf1a function more significantly affects exocrine (acinar) differentiation, and perhaps that lower levels still allow the derivation of endocrine cells from the uncommitted progenitor cells.
To determine if an incomplete block of Ptf1a was responsible for the partial inhibition in endocrine cell development we co-injected both morpholinos. To accurately compare our results, we injected the same total amount of both morpholinos (20ng each) used with each single morpholino. 20ng of MO1 and 20ng of MO2 (2MO) were co-injected into the vegetal blastomeres of eight cell embryos. Similar to that seen with each single morpholino, no pancreatic outgrowth was evident; elastase expression was absent (Fig. 7D), and insulin expression was reduced at stage 42 (Fig. 7H). Interestingly, in contrast to the single morpholino injections, expression of insulin was completely absent at early stage 32 demonstrating that Ptf1a function is essential for initial specification of both endocrine and exocrine cells (Fig. 7L). We confirmed insulin expression differences quantitatively by real-time PCR analysis of MO1-, MO2-, 2MO-injected and control embryos at stage 35. Four individual embryos were examined for each injection. There was an average decrease in insulin expression of 3.5 fold for single MO1- and MO2-injected embryos, whereas in 2MO-injected embryos there was an 18 fold reduction (Fig. 7Q). In all cases, single or double morpholino, we found liver development occurred normally as marked by expression of Xhex (Fig. 7M-P). The loss of insulin expression at early stages and subsequent re-expression at later stages suggests that in Xenopus Ptf1a is essential for the initial specification of both endocrine and exocrine cells, while its function is dispensable for the generation of the later-appearing insulin cells.
Mouse Ptf1a mRNA rescues pancreatic agenesis caused by Xptf1a morpholino
To determine if the phenotype caused by the Ptf1a morpholino is directly related to inhibition of endogenous Ptf1a, we attempted to rescue the loss of pancreatic tissue and acinar differentiation markers by co-expressing mouse Ptf1a mRNA. Injection of 25ng of MO2 leads to a complete loss of ventral pancreatic tissue and persistence of a dorsal nub in 50% of injected embryos (Fig. 8B). Co-injection of 300pg of mPtf1a mRNA rescued the loss of pancreatic tissue and acinar gene expression. 50% of rescued embryos developed a normal sized pancreas with elastase expression (Fig. 8C). The other 50% had a slightly enlarged pancreas with elastase expression, similar to the Ptf1a overexpression phenotype (Fig. 8D). These results clearly demonstrate that the Ptf1a morpholino phenotype is indeed due to inhibition of the endogenous Xenopus Ptf1a mRNA.
Figure 8.
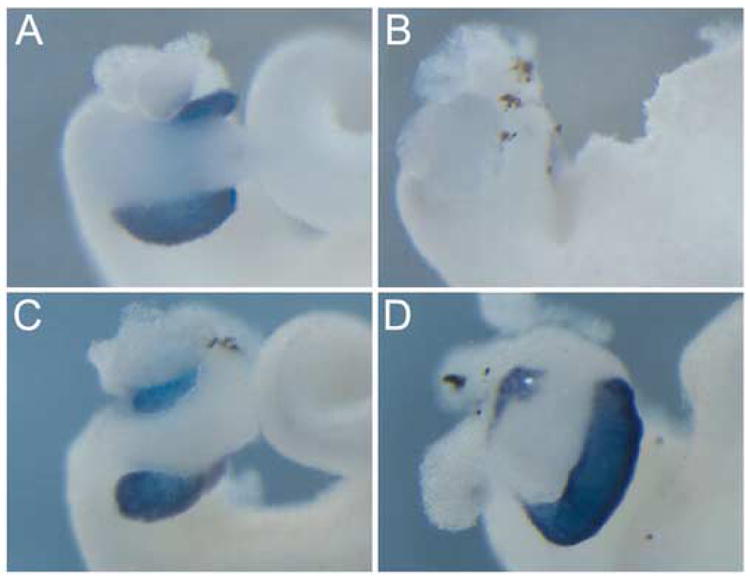
Mouse Ptf1a rescues the Ptf1a-morpholino induced phenotype. (A) Control whole gut showing elastase expression in the pancreas. (B) In Ptf1a-MO whole guts elastase is absent. (C,D) Pancreatic tissue and elastase expression is restored in Ptf1a-MO embryos co-injected with mouse Ptf1a.
Discussion
Despite the numerous studies pursuing loss-of-function studies with pancreatic transcription factors, few have addressed the sufficiency of these factors in activating the pancreatic differentiation program in other cell types. In this paper we have used Xenopus transgenics and mRNA overexpression to examine the functional ability of seven different pancreatic transcription factors in converting liver to pancreas, and found that only one of these, Ptf1a-VP16, is sufficient. We also show that, while the unmodified Ptf1a has no effect in liver, it is sufficient to reprogram stomach/duodenum to pancreas. Last, we have investigated the role of Xenopus laevis Ptf1a in early pancreatic cell fate specification and found that it is essential for proper development of both endocrine and exocrine pancreatic lineages. These results establish Ptf1a as a central player in directing pancreatic cell fate determination being both necessary and sufficient.
Ptf1a is a master regulator of pancreatic cell fate
Much of the previous work on converting other cell types to pancreas has focused on Pdx1 since in its absence the pancreas does not form. Indeed, we showed that Pdx1-VP16 is sufficient to convert liver to pancreas (Cao et al., 2004; Horb et al., 2003; Li et al., 2005). The ability of other PTFs to convert liver to pancreas has not been explored. Using our transgenic transdifferentiation assay we find that only one other transcription factor, Ptf1a, has any activity to ectopically activate the Elas-GFP transgene in Xenopus transgenics. The ability to convert liver to pancreas would therefore seem to be limited to only those transcription factors that function during the initial stages of pancreas development, prior to the division of endocrine and exocrine progenitors. The fact that loss of Ptf1a or Pdx1 leads to pancreas agenesis, while loss of the other PTFs affects only certain endocrine lineages supports this notion. We have not however thoroughly investigated every pancreatic transcription factor, and it will be of interest to determine if this concept holds true for other early acting lineage restricted transcription factors, such as ngn3. Furthermore, it is possible that a whole series of combinations of the various factors (twos, threes, etc.) might reveal interesting conversion phenotypes consistent with the ability of Ptf1a and Pdx1 to act early to engage these suites of transcription factors in the correct order/combinations.
Our examination of the sufficiency of Ptf1a in converting other endodermal cells into pancreas suggests that the addition of VP16 (though enhancing transcriptional activation) actually restricts Ptf1a activity, and has revealed differential responses to this transcription factor among distinct foregut tissues. Several lines of evidence support this contention: 1) the unmodified Ptf1a is sufficient to convert stomach/duodenum to pancreas at organogenesis stages, whereas Ptf1a-VP16 has no such ability; 2) Ptf1a-VP16 can only promote acinar cell fate, while the unmodified Ptf1a promotes both endocrine and acinar cell fates; and 3) even at earlier stages, prior to differentiation and organogenesis, Ptf1a-VP16 only promotes acinar cell fates. These ideas do not however address the fact that the unmodified Ptf1a is itself limited in function, such that it is unable to exert any phenotypic effect in liver cells at any stage. We simply suggest that the addition of VP16 restricts Ptf1a target specificity and/or protein interactions to only those promoting acinar and not endocrine differentiation. Therefore, the ability of Ptf1a to promote either endocrine or acinar cell fates seems to be determined by its partner protein interactions at different times during embryogenesis. This implies that the intrinsic activity of Ptf1a when expressed as a VP16 fusion is to convert liver, and endoderm-generally, to acinar fates, while a version that does not “bypass” its normal transcriptional complex build-up is able to promote both acinar and endocrine cell fates. The finding that Ptf1a can only convert posterior foregut tissues to pancreas would therefore suggest that other coactivators are essential for Ptf1a to bring about a fate change. Indeed, the fact that PTF1a is part of a heterotrimeric complex and that it is regulated differently depending on its interaction with partner proteins supports this argument (Beres et al., 2006; Esni et al., 2004).
Identification of the different protein partners and downstream targets of Ptf1a and Ptf1a-VP16 will help elucidate how pancreatic progenitors are directed down an endocrine or acinar lineage. The fact that Ptf1a-VP16 is able to promote acinar cell fate in early anterior endoderm and later liver cells (but not stomach/duodenum) suggests that the same protein partners are present throughout the anterior endoderm as in the liver, and not in the posterior endoderm or the stomach and duodenum. Of interest along these lines is the finding that neither Ptf1a nor Ptf1a-VP16 overexpression in early embryos has any effect on gastrulation and neurulation. In fact, we only see a phenotype after stage 30. Based on results with other transcription factors in Xenopus, one would have thought that overexpression of a VP16 fusion would lead to early developmental defects. The simplest interpretation is that the necessary cofactors for Ptf1a are only present after stage 30. However, since Ptf1a is a bHLH protein it should interact with other bHLH proteins at all stages and interfere with their function. Alternatively, since we are only injecting the mRNA into vegetal blastomeres, it may be that the early endoderm lacks the necessary bHLH cofactors. In seeming agreement with this, overexpression of Ptf1a in ectoderm inhibits neurogenesis at early stages (Obata et al., 2001).
Two pathways to generate endocrine cells: Ptf1a dependent and independent
Our results concur with previous data in zebrafish and mouse demonstrating that Ptf1a is an essential regulator of acinar cell development, whereas it is only required for the development of a subset of endocrine cells. In zebrafish Ptf1a morphants no exocrine pancreatic tissue develops, while early endocrine cell development is normal. The development of a late-appearing population of endocrine cells however does require Ptf1a (Lin et al., 2004). Similarly, in Ptf1a mutant mice there is a complete loss of acinar cells, with a dramatic reduction in all endocrine cell types (Kawaguchi et al., 2002; Krapp et al., 1998). Here we demonstrate that in Xenopus the initial specification of both exocrine and endocrine cells is inhibited in Ptf1a morphants, whereas a late-appearing population of insulin-positive cells is unaffected. Our results are however in conflict with the recent study examining Xenopus Ptf1a function during normal development (Afelik et al., 2006). In their study, they come to the opposite conclusion that Ptf1a function is not necessary for early insulin-expressing cells, but rather is essential for late-appearing endocrine cells. In agreement with their data, we also found normal development of early insulin-expressing cells in single Ptf1a morpholino injections. In contrast however, we found that early-appearing insulin cells did not develop in our double Ptf1a morphants. Furthermore, in both our single and double Ptf1a morphants the late-appearing insulin population always develops. The main difference between our studies and theirs is that we have used a combination of two morpholinos to knock-down Ptf1a function. In agreement with this our results demonstrating that the second splice morpholino effectively inhibits splicing of the Ptf1a transcript also revealed that splicing was not completely inhibited (see Fig. 7). This suggests that either single morpholino, whether inhibiting translation or splicing, is not completely effective in blocking Ptf1a and that a combination of both is more effective. This explains why we found effects on the early insulin-expressing population, whereas the previous study did not. In combination, the results from all three species would therefore suggest that there are two pathways to generate endocrine cells: a Ptf1a-dependent and a Ptf1a independent pathway. This is entirely consistent with the existence of a lower number of endocrine cells in the pancreatic rudiment formed in Ptf1a-null mouse embryos.
Acknowledgments
M.E.H. would like to thank the IRCM for help in setting up his lab, Jonathan M.W. Slack and David Tosh for their guidance and support in entering the Xenopus pancreas field, and Mona Nemer for her assistance throughout the project. We also thank Ira Blitz for his helpful comments on the manuscript, Frédéric Bourque for maintenance of the frogs, and Lori Dawn Horb for technical assistance. We also thank Violette Thermes, Jean-Stéphane Joly and Frédéric Sohm for the I-SceI plasmids. This work was supported in part by grants from the Canadian Institutes of Health Research MOP-69094 and the Canadian Cancer Society 016243 (M.E.H.) and from the National Institutes of Health DK-61215 (S.D.L.). M.E.H. is a Junior 2 Research Scholar of the Fonds de la recherche en santé du Québec (FRSQ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afelik S, Chen Y, Pieler T. Combined ectopic expression of Pdx1 and Ptf1a/p48 results in the stable conversion of posterior endoderm into endocrine and exocrine pancreatic tissue. Genes Dev. 2006;20:1441–1446. doi: 10.1101/gad.378706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CW, Slack JM. Gut specific expression using mammalian promoters in transgenic Xenopus laevis. Mech Dev. 1999;88:221–227. doi: 10.1016/s0925-4773(99)00217-8. [DOI] [PubMed] [Google Scholar]
- Ber I, Shternhall K, Perl S, Ohanuna Z, Goldberg I, Barshack I, Benvenisti-Zarum L, Meivar-Levy I, Ferber S. Functional, persistent, and extended liver to pancreas transdifferentiation. J Biol Chem. 2003;278:31950–31957. doi: 10.1074/jbc.M303127200. [DOI] [PubMed] [Google Scholar]
- Beres TM, Masui T, Swift GH, Shi L, Henke RM, MacDonald RJ. PTF1 is an organ-specific and Notch-independent basic helix-loop-helix complex containing the mammalian Suppressor of Hairless (RBP-J) or its paralogue, RBP-L. Mol Cell Biol. 2006;26:117–130. doi: 10.1128/MCB.26.1.117-130.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao LZ, Tang DQ, Horb ME, Li SW, Yang LJ. High glucose is necessary for complete maturation of Pdx1-VP16-expressing hepatic cells into functional insulin-producing cells. Diabetes. 2004;53:3168–3178. doi: 10.2337/diabetes.53.12.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund H. Pancreatic organogenesis--developmental mechanisms and implications for therapy. Nat Rev Genet. 2002;3:524–532. doi: 10.1038/nrg841. [DOI] [PubMed] [Google Scholar]
- Esni F, Ghosh B, Biankin AV, Lin JW, Albert MA, Yu X, MacDonald RJ, Civin CI, Real FX, Pack MA, Ball DW, Leach SD. Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development. 2004;131:4213–4224. doi: 10.1242/dev.01280. [DOI] [PubMed] [Google Scholar]
- Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N, Karasik A. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568–572. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- Fukuda A, Kawaguchi Y, Furuyama K, Kodama S, Horiguchi M, Kuhara T, Koizumi M, Boyer DF, Fujimoto K, Doi R, Kageyama R, Wright CV, Chiba T. Ectopic pancreas formation in Hes1 -knockout mice reveals plasticity of endodermal progenitors of the gut, bile duct, and pancreas. J Clin Invest. 2006;116:1484–1493. doi: 10.1172/JCI27704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habener JF, Kemp DM, Thomas MK. Minireview: transcriptional regulation in pancreatic development. Endocrinology. 2005;146:1025–1034. doi: 10.1210/en.2004-1576. [DOI] [PubMed] [Google Scholar]
- Horb ME, Shen CN, Tosh D, Slack JM. Experimental conversion of liver to pancreas. Curr Biol. 2003;13:105–115. doi: 10.1016/s0960-9822(02)01434-3. [DOI] [PubMed] [Google Scholar]
- Horb ME, Slack JM. Endoderm specification and differentiation in Xenopus embryos. Dev Biol. 2001;236:330–343. doi: 10.1006/dbio.2001.0347. [DOI] [PubMed] [Google Scholar]
- Horb ME, Slack JM. Expression of amylase and other pancreatic genes in Xenopus. Mech Dev. 2002;113:153–157. doi: 10.1016/s0925-4773(02)00019-9. [DOI] [PubMed] [Google Scholar]
- Imai J, Katagiri H, Yamada T, Ishigaki Y, Ogihara T, Uno K, Hasegawa Y, Gao J, Ishihara H, Sasano H, Mizuguchi H, Asano T, Oka Y. Constitutively active PDX1 induced efficient insulin production in adult murine liver. Biochem Biophys Res Commun. 2005;326:402–409. doi: 10.1016/j.bbrc.2004.11.047. [DOI] [PubMed] [Google Scholar]
- Jacquemin P, Yoshitomi H, Kashima Y, Rousseau GG, Lemaigre FP, Zaret KS. An endothelial-mesenchymal relay pathway regulates early phases of pancreas development. Dev Biol. 2006;290:189–199. doi: 10.1016/j.ydbio.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Jonsson J, Ahlgren U, Edlund T, Edlund H. IPF1, a homeodomain protein with a dual function in pancreas development. Int J Dev Biol. 1995;39:789–798. [PubMed] [Google Scholar]
- Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–9. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Nakatani Y, Miyatsuka T, Matsuoka TA, Matsuhisa M, Hori M, Yamasaki Y. PDX-1/VP16 fusion protein, together with NeuroD or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes. 2005;54:1009–1022. doi: 10.2337/diabetes.54.4.1009. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- Kelly OG, Melton DA. Development of the pancreas in Xenopus laevis. Dev Dyn. 2000;218:615–627. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1027>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Kim SK, MacDonald RJ. Signaling and transcriptional control of pancreatic organogenesis. Curr Opin Genet Dev. 2002;12:540–547. doi: 10.1016/s0959-437x(02)00338-6. [DOI] [PubMed] [Google Scholar]
- Kojima H, Fujimiya M, Matsumura K, Younan P, Imaeda H, Maeda M, Chan L. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med. 2003;9:596–603. doi: 10.1038/nm867. [DOI] [PubMed] [Google Scholar]
- Krapp A, Knofler M, Frutiger S, Hughes GJ, Hagenbuchle O, Wellauer PK. The p48 DNA-binding subunit of transcription factor PTF1 is a new exocrine pancreas-specific basic helix-loop-helix protein. EMBO J. 1996;15:4317–4329. [PMC free article] [PubMed] [Google Scholar]
- Krapp A, Knofler M, Ledermann B, Burki K, Berney C, Zoerkler N, Hagenbuchle O, Wellauer PK. The bHLH protein PTF1-p48 is essential for the formation of the exocrine and the correct spatial organization of the endocrine pancreas. Genes Dev. 1998;12:3752–3763. doi: 10.1101/gad.12.23.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse F, Rose SD, Swift GH, Hammer RE, MacDonald RJ. An endocrine-specific element is an integral component of an exocrine-specific pancreatic enhancer. Genes Dev. 1993;7:774–786. doi: 10.1101/gad.7.5.774. [DOI] [PubMed] [Google Scholar]
- Li WC, Horb ME, Tosh D, Slack JM. In vitro transdifferentiation of hepatoma cells into functional pancreatic cells. Mech Dev. 2005;122:835–847. doi: 10.1016/j.mod.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Lin JW, Biankin AV, Horb ME, Ghosh B, Prasad NB, Yee NS, Pack MA, Leach SD. Differential requirement for ptf1a in endocrine and exocrine lineages of developing zebrafish pancreas. Dev Biol. 2004;274:491–503. doi: 10.1016/j.ydbio.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Meivar-Levy I, Ferber S. Regenerative medicine: using liver to generate pancreas for treating diabetes. Isr Med Assoc J. 2006;8:430–434. [PubMed] [Google Scholar]
- Miyatsuka T, Kaneto H, Kajimoto Y, Hirota S, Arakawa Y, Fujitani Y, Umayahara Y, Watada H, Yamasaki Y, Magnuson MA, Miyazaki J, Hori M. Ectopically expressed PDX-1 in liver initiates endocrine and exocrine pancreas differentiation but causes dysmorphogenesis. Biochem Biophys Res Commun. 2003;310:1017–1025. doi: 10.1016/j.bbrc.2003.09.108. [DOI] [PubMed] [Google Scholar]
- Newman CS, Chia F, Krieg PA. The XHex homeobox gene is expressed during development of the vascular endothelium: overexpression leads to an increase in vascular endothelial cell number. Mech Dev. 1997;66:83–93. doi: 10.1016/s0925-4773(97)00092-0. [DOI] [PubMed] [Google Scholar]
- Obata J, Yano M, Mimura H, Goto T, Nakayama R, Mibu Y, Oka C, Kawaichi M. p48 subunit of mouse PTF1 binds to RBP-Jkappa/CBF-1, the intracellular mediator of Notch signalling, and is expressed in the neural tube of early stage embryos. Genes Cells. 2001;6:345–360. doi: 10.1046/j.1365-2443.2001.00422.x. [DOI] [PubMed] [Google Scholar]
- Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- Okada TS. Transdifferentiation: flexibility in cell differentiation. Clarendon Press; Oxford: 1991. [Google Scholar]
- Pan FC, Chen Y, Loeber J, Henningfeld K, Pieler T. I-SceI meganuclease-mediated transgenesis in Xenopus. Dev Dyn. 2006;235:247–52. doi: 10.1002/dvdy.20608. [DOI] [PubMed] [Google Scholar]
- Pilcher KE, Krieg PA. Expression of the Wnt inhibitor, sFRP5, in the gut endoderm of Xenopus. Gene Expr Patterns. 2002;2:369–372. doi: 10.1016/s1567-133x(02)00023-6. [DOI] [PubMed] [Google Scholar]
- Sapir T, Shternhall K, Meivar-Levy I, Blumenfeld T, Cohen H, Skutelsky E, Eventov-Friedman S, Barshack I, Goldberg I, Pri-Chen S, Ben Dor L, Polak-Charcon S, Karasik A, Shimon I, Mor E, Ferber S. Cell-replacement therapy for diabetes: Generating functional insulin-producing tissue from adult human liver cells. Proc Natl Acad Sci U S A. 2005;102:7964–7969. doi: 10.1073/pnas.0405277102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellick GS, Barker KT, Stolte-Dijkstra I, Fleischmann C, Coleman RJ, Garrett C, Gloyn AL, Edghill EL, Hattersley AT, Wellauer PK, Goodwin G, Houlston RS. Mutations in PTF1A cause pancreatic and cerebellar agenesis. Nat Genet. 2004;36:1301–1305. doi: 10.1038/ng1475. [DOI] [PubMed] [Google Scholar]
- Slack JM. Developmental biology of the pancreas. Development. 1995;121:1569–1580. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- Slack JM, Tosh D. Transdifferentiation and metaplasia--switching cell types. Curr Opin Genet Dev. 2001;11:581–586. doi: 10.1016/s0959-437x(00)00236-7. [DOI] [PubMed] [Google Scholar]
- Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet. 1997;15:106–110. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- Sweetser DA, Hauft SM, Hoppe PC, Birkenmeier EH, Gordon JI. Transgenic mice containing intestinal fatty acid-binding protein-human growth hormone fusion genes exhibit correct regional and cell-specific expression of the reporter gene in their small intestine. Proc Natl Acad Sci U S A. 1988;85:9611–9615. doi: 10.1073/pnas.85.24.9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DQ, Cao LZ, Chou W, Shun L, Farag C, Atkinson MA, Li SW, Chang LJ, Yang LJ. Role of Pax4 in Pdx1-VP16-mediated liver-to-endocrine pancreas transdifferentiation. Lab Invest. 2006a doi: 10.1038/labinvest.3700434. [DOI] [PubMed] [Google Scholar]
- Tang DQ, Lu S, Sun YP, Rodrigues E, Chou W, Yang C, Cao LZ, Chang LJ, Yang LJ. Reprogramming liver-stem WB cells into functional insulin-producing cells by persistent expression of Pdx1- and Pdx1-VP16 mediated by lentiviral vectors. Lab Invest. 2006b;86:83–93. doi: 10.1038/labinvest.3700368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thermes V, Grabher C, Ristoratore F, Bourrat F, Choulika A, Wittbrodt J, Joly JS. I-SceI meganuclease mediates highly efficient transgenesis in fish. Mech Dev. 2002;118:91–8. doi: 10.1016/s0925-4773(02)00218-6. [DOI] [PubMed] [Google Scholar]
- Tosh D, Slack JM. How cells change their phenotype. Nat Rev Mol Cell Biol. 2002;3:187–194. doi: 10.1038/nrm761. [DOI] [PubMed] [Google Scholar]
- Wright CV, Schnegelsberg P, De Robertis EM. XlHbox 8: a novel Xenopus homeo protein restricted to a narrow band of endoderm. Development. 1989;105:787–794. doi: 10.1242/dev.105.4.787. [DOI] [PubMed] [Google Scholar]
- Yan C, Costa RH, Darnell JE, Jr, Chen JD, Van Dyke TA. Distinct positive and negative elements control the limited hepatocyte and choroid plexus expression of transthyretin in transgenic mice. EMBO J. 1990;9:869–878. doi: 10.1002/j.1460-2075.1990.tb08184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitomi H, Zaret KS. Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development. 2004;131:807–817. doi: 10.1242/dev.00960. [DOI] [PubMed] [Google Scholar]
- Zalzman M, Gupta S, Giri RK, Berkovich I, Sappal BS, Karnieli O, Zern MA, Fleischer N, Efrat S. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proc Natl Acad Sci U S A. 2003;100:7253–7258. doi: 10.1073/pnas.1136854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecchin E, Mavropoulos A, Devos N, Filippi A, Tiso N, Meyer D, Peers B, Bortolussi M, Argenton F. Evolutionary conserved role of ptf1a in the specification of exocrine pancreatic fates. Dev Biol. 2004;268:174–184. doi: 10.1016/j.ydbio.2003.12.016. [DOI] [PubMed] [Google Scholar]


