Abstract
The intracellular protein p120 catenin aids in maintenance of cell-cell adhesion by regulating E-cadherin stability in epithelial cells. In an effort to understand the biology of p120 catenin in pancreas development, we ablated p120 catenin in mouse pancreatic progenitor cells, which resulted in deletion of p120 catenin in all epithelial lineages of the developing mouse pancreas: islet, acinar, centroacinar, and ductal. Loss of p120 catenin resulted in formation of dilated epithelial tubules, expansion of ductal epithelia, loss of acinar cells, and the induction of pancreatic inflammation. Aberrant branching morphogenesis and tubulogenesis were also observed. Throughout development, the phenotype became more severe, ultimately resulting in an abnormal pancreas comprised primarily of duct-like epithelium expressing early progenitor markers. In pancreatic tissue lacking p120 catenin, overall epithelial architecture remained intact; however, actin cytoskeleton organization was disrupted, an observation associated with increased cytoplasmic PKCζ. Although we observed reduced expression of adherens junction proteins E-cadherin, β-catenin, and α-catenin, p120 catenin family members p0071, ARVCF, and δ-catenin remained present at cell membranes in homozygous p120f/f pancreases, potentially providing stability for maintenance of epithelial integrity during development. Adult mice homozygous for deletion of p120 catenin displayed dilated main pancreatic ducts, chronic pancreatitis, acinar to ductal metaplasia (ADM), and mucinous metaplasia that resembles PanIN1a. Taken together, our data demonstrate an essential role for p120 catenin in pancreas development.
Keywords: p120 catenin, pancreas development, adherens junction, tubulogenesis, branching morphogenesis, PKCζ, pancreatitis
INTRODUCTION
Pancreatic development proceeds from a cluster of endodermal epithelial cells that give rise to a highly specialized, heterogeneous endocrine and exocrine organ. The early pancreatic bud is enveloped by mesenchyme, which is required for pancreas development and is thought to provide inductive signals for the specification of various cell types (Golosow and Grobstein, 1962; Landsman et al., 2011). Beginning at E13.5, the mouse pancreas changes rapidly during the ‘secondary transition,’ which is marked by dramatic increases in endocrine cell numbers and acinar cell differentiation (Rutter et al., 1968). Endocrine cells delaminate from the embryonic epithelia, coalesce into early islets, and migrate throughout the tissue (Pictet et al., 1972). The developing pancreas arborizes to generate a highly branched network of exocrine tissue consisting of acini capping the tips of terminal ducts and extending to the main pancreatic duct (Puri and Hebrok, 2007; Villasenor et al., 2010).
Epithelial integrity is essential for proper organogenesis during development. Adherens junctions are an integral part of the maintenance of tight cell-cell adhesion in epithelial tissues. Adherens junction proteins serve roles in tissue homeostasis, embryonic development, tissue morphogenesis, and tumorigenesis (Hartsock and Nelson, 2008; Perez-Moreno and Fuchs, 2006). The core of the adherens junction in epithelial tissues is comprised of E-cadherin, β-catenin, p120 catenin, and α-catenin. Through homophilic, Ca2+-dependent interactions, extracellular E-cadherin associates with E-cadherin molecules of adjacent cells (Shapiro and Weis, 2009). β-catenin binds to the catenin-binding domain of intracellular E-cadherin and α-catenin, which associates with the actin cytoskeleton. p120 catenin stabilizes epithelial cell adherens junctions through its interaction with the juxtamembrane domain of E-cadherin molecules (Ishiyama et al., 2010). Cadherin-catenin complexes are rapidly turned over in the absence of p120 catenin, demonstrating a crucial role for p120 catenin in cadherin stability (Davis et al., 2003). p120 catenin in the cytoplasm modulates the activities of small Rho family GTPases by inhibiting RhoA and activating Rac1 and Cdc42, which together influence cytoskeletal dynamics and cell migration (Anastasiadis et al., 2000; Noren et al., 2000).
p120 catenin is a member of the larger catenin gene family that is comprised of three subfamilies; p120, beta, and alpha. The p120 subfamily contains 7 members which include p120 catenin, ARVCF, δ-catenin, p0071, and plakophilins 1–3 (Zhao et al., 2011). Like p120 catenin, ARVCF, δ-catenin, and p0071 are capable of binding to the juxtamembrane domain of cadherin molecules in adherens junctions through their central Armadillo repeat domains, while plakophilins 1–3 primarily function in linking the intermediate filaments of cells through desmosomes. p120 catenin, ARVCF, and p0071 are expressed ubiquitously and as multiple isoforms. Expression of δ-catenin is thought to be restricted almost entirely to the nervous system (Mariner et al., 2000; Pieters et al., 2012). The biological interplay between p120 catenin and its family members is incompletely understood, especially during development.
A number of studies have revealed critical yet diverse roles for p120 catenin in different organ systems (Bartlett et al., 2010; Davis and Reynolds, 2006; Elia et al., 2006; Kurley et al., 2012; Marciano et al., 2011; Oas et al., 2010; Perez-Moreno et al., 2006; Perez-Moreno et al., 2008; Schackmann et al., 2013; Smalley-Freed et al., 2010; Smalley-Freed et al., 2011; Stairs et al., 2011; Tian et al., 2012). In p120 catenin conditional deletion studies, the results are highly tissue-specific and unpredictable. In terms of tissue-specific effects, the biological contribution of p120 catenin to pancreas development has not been studied. Here, we ablated p120 catenin selectively in developing pancreatic epithelium using an early Pdx1:Cre (hereafter CPdx1) driver. Expression of Cre in this line is ubiquitous in multipotent pancreatic progenitor cells as early as E8.5, and thus targets all epithelial lineages of the developing pancreas – acinar, islet, duct, and centroacinar (Gu et al., 2002). CPdx1; p120f/f pancreases displayed striking developmental anomalies. Loss of p120 catenin early in pancreatic development resulted in aberrant tubulogenesis, expansion of tubular epithelia, loss of acinar cell mass, disruption of islet localization, and inflammation within the embryonic pancreas. The expanded tubular epithelium was accompanied by the continued expression of Sox9 and Aldh1, suggesting a possible block in progenitor differentiation. In expanded ductal epithelia of CPdx1; p120f/f pancreata, actin cytoskeleton organization was disrupted, which was accompanied by an increase in cytoplasmic PKCζ, a modulator of cytoskeletal dynamics, suggesting a connection between p120 catenin loss and actin cytoskeleton organization mediated by PKCζ. Despite the loss of p120 catenin, epithelial cell-cell junctions appeared normal and defects in cell adhesion were not observed. Although epithelium lacking p120 catenin displayed a reduction in adherens junction members E-cadherin, β-catenin, and α-catenin, we show that p120 catenin family members ARVCF, δ-catenin, and p0071 are present at cell membranes in p120 catenin-null epithelia, suggesting that these proteins might serve to stabilize adherens junctions in the absence of p120 catenin. Adult homozygous p120f/f animals displayed dilated main pancreatic ducts, chronic pancreatitis, acinar to ductal metaplasia (ADM), and mucinous metaplasia that resembled Pancreatic Intraepithelial Neoplasia 1a (PanIN1a). Taken together, our data suggest a crucial role for p120 catenin in proper tubulogenesis and lineage specification during pancreatic development.
MATERIALS AND METHODS
Mice
All animal studies were approved by the Animal Care and Use Committee at Johns Hopkins University. Mouse strains used in this study were Tg(Pdx1-cre)89.1Dam (MGI ID: 2684317) (Gu et al., 2002), Ctnnd1tm1Abre (MGI ID: 3617486) (Davis and Reynolds, 2006), and ROSAmT/mG (MGI ID: 3716464) (Muzumdar et al., 2007). The mice were housed under a 14/10 hour light/dark cycle with free access to food and water.
Genotyping
Ctnnd1tm1Abre, Pdx1-cre, and ROSAmT/mG alleles were maintained by breeding heterozygous mice to C57BL/6J mice. Genotyping was accomplished by PCR or Transnetyx. Primers used to genotype for the Ctnnd1tm1Abre allele were p120 FP (5′-TTTTAGAGCCTCCCACATACAAGC-′) and p120 RP (5′-TCAGCACCCACACAAAGGTTG-3′) as previously described (Davis and Reynolds, 2006). Primers used to genotype for the Pdx1-cre allele were Pdx1-FP (5′-GAACTGGGGAGGAAAAGGAG-3′) and Cre2-RP (5′-GATGAAGCATGTTTAGCTGG-3′). Primers used to genotype for the ROSAmT/mG allele were Rosa26r FP (5′-CTCTGCTGCCTCCTGGCTTCT-3′), Rosa26r RP (5′-CGAGGCGGATCACAAGCAATA-3′), and mTmG RP (5′-TCAATGGGCGGGGGTCGTT-3′). Primers used to determine the sex of neonatal mice were designed to amplify the ZFX and ZFY genes as previously described (Valer Carstea et al., 2007).
Histology/Immunofluorescence
Mouse pancreata were fixed in 10% Neutral Buffered Formalin or 4% Paraformaldehyde at 4°C and embedded in paraffin for sectioning. Five micron sections were prepared for hematoxylin and eosin staining, alcian blue staining, immunofluorescence, and immunohistochemistry. Primary antibodies and other immunofluorescent reagents used in this study are listed in Table S1. Secondary antibodies were used at 1:250 and were from Jackson ImmunoResearch.
Embryonic tissues prepared for frozen sections were fixed in 4% Paraformaldehyde at 4°C, subsequently incubated in 30% sucrose at 4°C for cryoprotection, and embedded in OCT (Sakura Finetek 4583 CRYO-OCT Compound). Frozen sections were used for Phalloidin, CD49f, and Muc1 staining in Fig. 5A and α-catenin and p120 catenin staining in Fig. 6B. A citrate-based Antigen Unmasking Solution from Vector Laboratories (H-3300) was used for antigen retrieval for all immunofluorescent staining except in OCT embedded sections. Primary antibody epitopes were retrieved with a heat-mediated microwave antigen retrieval method. All sections were blocked in 10% FBS with 0.2% Triton-X 100 in PBS. Primary antibodies were incubated overnight in blocking buffer at 4°C. Subsequently, secondary antibodies were incubated at RT for 2 hours. Immunofluorescent images were visualized on a Nikon A1 confocal microscope system. Histology of adult CPdx1; p120f/f males was examined by a pathologist.
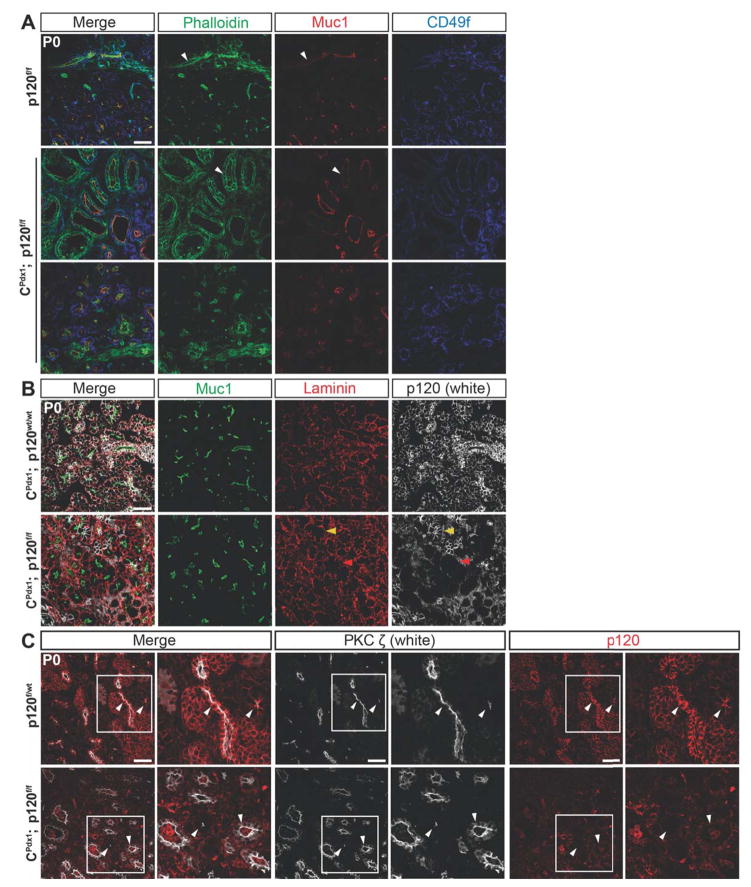
Figure 5. Changes in cytoskeletal architecture in pancreatic epithelium lacking p120 catenin.
Immunofluorescent staining of P0 pancreas showing the apical markers Muc1, Phalloidin, and PKCζ as well as the basal markers CD49f and Laminin in homozygous p120f/f and wild-type control pancreases. (A) Phalloidin was localized apically in wild-type ducts and apically, laterally, and basally in homozygous p120f/f ducts, indicating an alteration in cytoskeletal organization. Phalloidin was localized apically in the acini of both homozygous p120f/f and wild-type control pancreases. (A–B) Muc1 was localized apically in both wild-type and homozygous p120f/f pancreatic epithelium. White arrows point to Phalloidin and Muc1 staining in both wild-type and homozygous p120f/f ducts. Both basal markers CD49f and Laminin have comparable basal localization in wild-type and CPdx1; p120f/f pancreatic epithelium. (B) A section with unusually high mosaicism for p120 catenin was intentionally chosen to allow comparison of Muc1 and Laminin staining in p120 catenin-expressing and p120 catenin-deleted tissue in the same section. Yellow arrows point to a p120 catenin-expressing acinus and red arrows show a p120 catenin-deleted acinus. There was no difference in Laminin or Muc1 staining between p120 catenin-expressing and p120 catenin-deleted tissue. (C) In the wild-type panel, white arrows point to apical localization of PKCζ in ductal epithelium (left arrow) and in an acinus (right arrow). In the CPdx1; p120f/f panel, white arrows point to apical localization of PKCζ in an acinus (left arrow) and both apical and increased cytoplasmic localization of PKCζ in expanded ductal epithelium (right arrow). Higher magnification images are shown for each panel. Scale bars are 50μm.
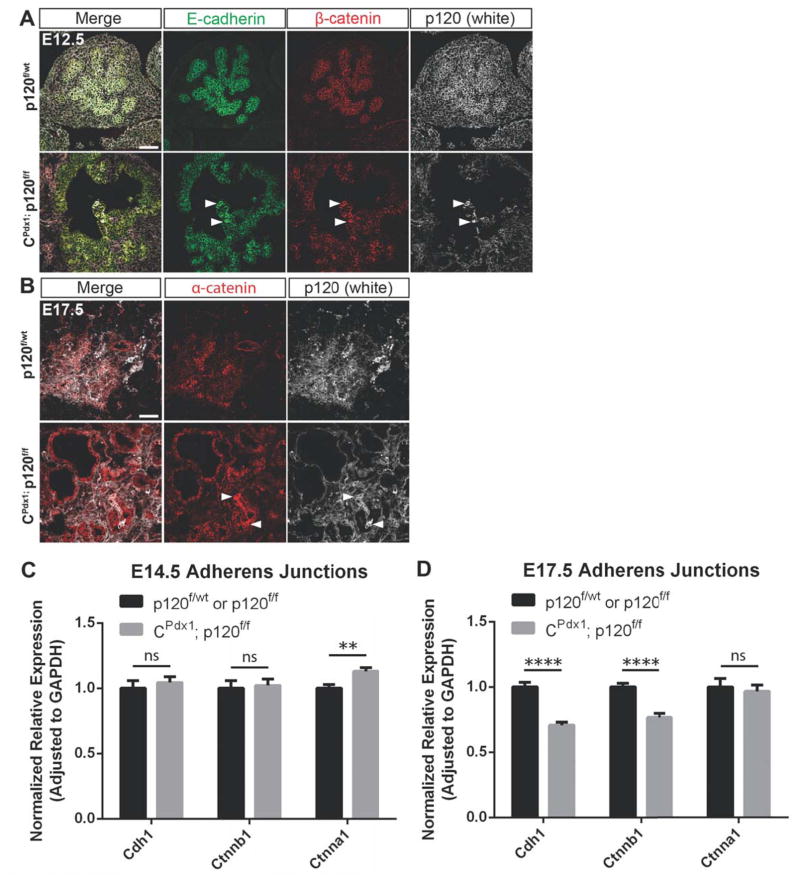
Figure 6. Adherens junction components are retained in p120 catenin null epithelia during development.
(A–B) Comparison of immunofluorescent staining of p120 catenin, E-cadherin, β-catenin, and α-catenin using embryonic tissues selected for the presence of mosaic p120 catenin-expressing epithelial cells revealed reduced levels but persistent presence of adherens junction components at cell membranes of epithelial cells lacking p120 catenin. White arrows point to a few p120 catenin-expressing epithelial cells in a largely p120 catenin-deleted pancreatic epithelium. Scale bars are 50μm. (C–D) Comparison of gene expression of Cdh1, Ctnnb1, and Ctnna1 between wild-type and homozygous p120f/f pancreata at E14.5 and E17.5 using qPCR. Note that expression of Cdh1 and Ctnnb1 was not significantly different at E14.5 but was reduced at E17.5 in homozygous p120f/f pancreases when compared to controls. Wild-type control targets were normalized to 1. For all genotypes and all genes at both E14.5 and E17.5 time points, n=7, and reactions were run in quadruplicate. For interpretation of statistical results from Student’s t test, ns = not significant and p value > 0.05, * = p value ≤ 0.05, ** = p value ≤ 0.01, *** = p value ≤ 0.001, and **** = p value ≤ 0.0001.
Immunohistochemistry
Antigen retrieval for Aldh1 immunohistochemistry was accomplished using Retrievit 6 (BS-1006-00, BioGenex). For Cytokeratin 19 immunolabeling, antigen retrieval was done by digesting sections with 250 μg/mL proteinase K in 2.5 mmol/L CaCl2 and 10mmol/L Tris-HCl (pH 7.5) for 6 minutes at room temperature. For NF-kB immunohistochemistry, antigen retrieval was accomplished using R-Buffer A (62706-10, Electron Microscopy Sciences). Antigen retrieval for CD45 was accomplished using a citrate based antigen retrieval buffer and a heat-mediated microwave method. Immunohistochemistry staining was visualized on an Olympus BX40 light microscope.
Dissection and in vitro culturing of pancreatic bud explants
Pregnant female mice were sacked on E11.5, and embryos were removed. Pancreatic anlagen including surrounding mesenchyme, caudal stomach, and proximal duodenum were dissected from each embryo and cultured as previously described for up to 7 days (Puri and Hebrok, 2007). Day 0 is defined as the day of dissection. For timed breeding, noon of the day when vaginal plugs were first observed was considered 0.5 days post conception (dpc).
RNA isolation, quantitative RT-PCR, and statistical analysis
RNA was isolated from whole mouse pancreas (epithelium + mesenchyme) at various stages of embryonic development and at a postnatal stage using the RNeasy Mini kit (Qiagen). Reverse transcription was accomplished using QuantiTect Reverse Transcription kit (Qiagen). Complementary DNA was amplified using a 7900HT Fast Real-Time PCR System and TaqMan Gene Expression Assays (Life Technologies). Statistical calculations were performed using GraphPad Prism (GraphPad software) or Microsoft Excel (Microsoft Office 2013), and data are presented as mean ± SEM. Data were compared between groups using an unpaired Student’s t test. Significance was accepted at a p value ≤ 0.05.
RESULTS
Characterization of p120 catenin expression in mouse pancreas
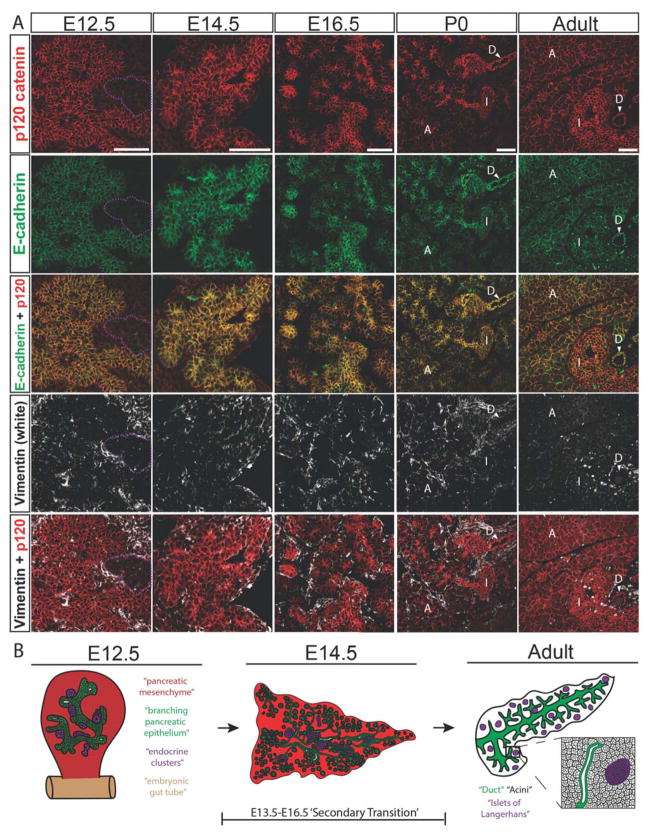
We first sought to determine baseline expression of p120 catenin in the embryonic, neonatal, and adult mouse pancreas. To accomplish this, we used immunofluorescence with an antibody that recognizes isoforms 1–4 of p120 catenin and also an antibody specific for isoforms 1–3 of p120 catenin. Immunolabeling with an antibody that recognizes isoforms 1–4 of p120 catenin, E-cadherin, and Vimentin showed ubiquitous expression of p120 catenin in both the pancreatic epithelium and the pancreatic mesenchyme throughout development, and in the neonatal and adult pancreatic epithelium (Fig. 1A). At E12.5 and E14.5, p120 catenin labeling was more highly enriched in pancreatic epithelium over pancreatic mesenchyme, except in the endocrine clusters at E12.5, which displayed low levels of adherens junction proteins E-cadherin and p120 catenin. Immunostaining using an antibody specific for isoforms 1–3 of p120 catenin showed a labeling pattern indistinguishable from the isoforms 1–4 specific antibody (Fig. S1). A schematic of pancreas development is depicted in Fig. 1B.
Figure 1. p120 catenin is expressed ubiquitously in the embryonic, neonatal, and adult mouse pancreas.
(A) Immunostaining showing p120 catenin expression using an antibody specific for isoforms 1–4, E-cadherin, and Vimentin in developing, neonatal, and adult mouse pancreas. For E12.5, purple dotted lines show examples of early endocrine clusters. For neonatal and adult pancreas, white arrows point to cells indicated with a “D” for ducts, “I” indicates islets of Langerhans, and “A” points out acinar cells. (B) Schematic depiction of branching pancreatic epithelium at E12.5 with emerging endocrine clusters (left), arborization of the pancreatic epithelium at E14.5 with accompanying endocrine cluster migration (middle), and an illustration of the adult pancreas containing duct, islet, and endocrine cells (right). For E12.5 and E14.5, various cell types of the developing pancreas are color coded, with red indicating mesenchymal cells, green indicating tubular epithelium, and purple indicating endocrine clusters. The time frame for the ‘secondary transition’ during pancreatic development is also depicted. For the adult illustration, green represents ducts, purple represents islets of Langerhans, and black indicates differentiated acinar cells. Scale bars are 50μm.
Loss of p120 catenin during development disrupts pancreatic tissue architecture
CPdx1; p120f/wt mice were bred with p120f/f mice, irrespective of gender, to generate CPdx1; p120f/f homozygotes. p120f/f mice harbor floxed p120 catenin alleles with loxP sites flanking all four known translational start sites; thus, genetic recombination should prevent expression of all isoforms of p120 catenin (Davis and Reynolds, 2006). Offspring from crosses were born in Mendelian ratios, but 100% of female CPdx1; p120f/f homozygotes died during the early post-natal period while male CPdx1; p120f/f homozygotes survived to adulthood.
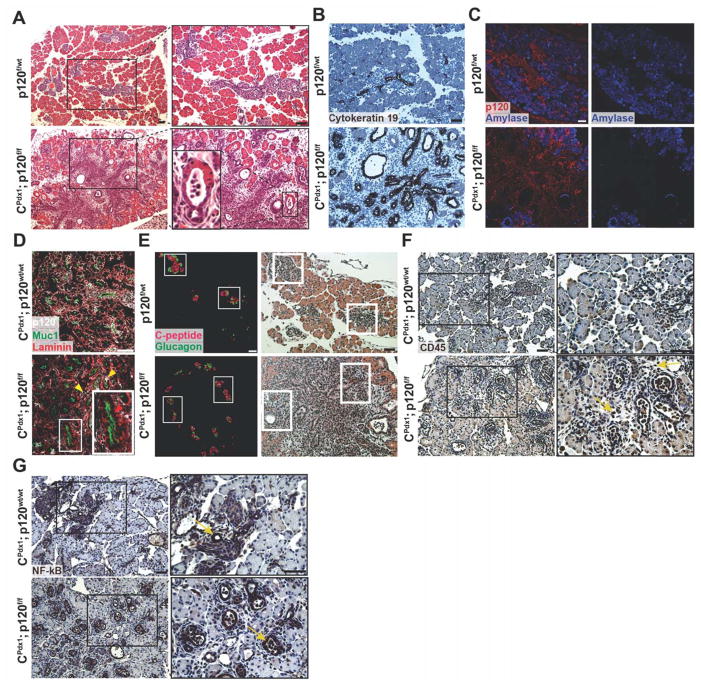
Pancreatic histology of CPdx1; p120f/f and wild-type littermate controls was initially examined at P0, revealing profound changes in pancreatic epithelial architecture in homozygous p120f/f pancreata (Fig. 2A). CPdx1; p120f/f pancreases displayed a dramatic expansion of tubular epithelium, which was positive for the epithelial marker Cytokeratin 19 (Fig. 2A,B). A substantial number of Vimentin+ cells were centrally located in the pancreata of homozygous p120f/f animals (Fig. S1). Amylase immunofluorescence demonstrated an overall defect in mature acinar cell differentiation and distribution in homozygous p120f/f pancreases when compared to controls (Fig. 2C). Acinar units in homozygous p120f/f animals were peripherally located in early pancreatic lobules and often displayed expanded lumens. Transitional structures with both acinar and duct morphologies were also evident (Fig. 2A,B). The abnormal epithelial tubules and transitional structures morphologically resembled areas of ADM, which occur in the human pancreas in the setting of either pancreatitis or early pancreatic cancer.
Figure 2. Characterization of p120 catenin deletion in neonatal P0 mice.
(A) Hematoxylin and eosin staining showed disruption of pancreatic tissue architecture in homozygous p120f/f animals compared to wild-type controls. A magnified image of an abnormal transitional structure is shown. (B) Immunohistochemistry demonstrated that expanded ductal epithelium in homozygous p120f/f pancreases is Cytokeratin 19 positive. (C) Amylase immunofluorescence showed aberrant acinar cell differentiation in CPdx1; p120f/f pancreases. (D) Immunofluorescent imaging documented epithelial-specific p120 catenin deletion. In the CPdx1; p120f/f figure, the top right yellow arrow points to a rare p120 catenin-expressing epithelial cell. The left yellow arrow points to a p120 catenin-expressing non-epithelial cell. The red arrow in the inset shows epithelia in CPdx1; p120f/f pancreas that lack p120 catenin. Note that p120 catenin staining is nearly completely absent in CPdx1; p120f/f epithelial pancreas, with very few cells mosaic for p120 catenin expression. Muc1 and Laminin provide clear boundaries for distinction of apical and basal regions of the pancreatic epithelium. (E) Immunofluorescence of C-peptide and Glucagon demonstrated abnormal islet distribution in homozygous p120f/f pancreas. Identical sections were stained with hematoxylin and eosin to demonstrate tissue context, and white boxes show corresponding islets. (F) CD45 immunohistochemistry revealed the recruitment of an inflammatory infiltrate in CPdx1; p120f/f pancreas, but not in wild-type controls. In the CPdx1; p120f/f bottom panel, yellow arrows point to CD45+ cells. (G) NF-kB signaling was detected in the ductal epithelium of both wild-type and homozygous p120f/f pancreas, which is indicated by yellow arrows. Scale bars are 50μm.
Immunofluorescence demonstrated epithelial-specific loss of p120 catenin in homozygous p120f/f pancreata (Fig. 2D). As expected, expression of p120 catenin in the pancreatic mesenchyme was unaffected (Fig. 2D). We observed a small degree of mosaicism for recombination of the floxed p120 catenin allele during development. Epithelium retaining p120 catenin accounted for 6.07% ± 2.49% pancreatic cells during development (n=7) and 59.77% ± 3.31% pancreatic cells in CPdx1; p120f/f adult males (n=6). This suggests that there may be a selection bias for cells that retain expression of p120 catenin. Islets were abnormally distributed throughout the tissue in a pattern such that they surrounded the central Vimentin+ cells in homozygous p120f/f pancreases. The differentiation of α and β cells, marked by Glucagon and C-peptide, respectively, was unaffected throughout embryonic development and in the neonatal pancreas (Fig. 2E and data not shown). No difference was observed in the number of proliferating cells or cells undergoing apoptosis in the pancreatic epithelial or mesenchymal compartments when comparing wild-type and homozygous p120f/f animals, as examined by Cleaved Caspase-3 and Ki67 immunofluorescence (Fig. S3, S4). CPdx1; p120f/wt pancreata were histologically indistinguishable from wild-type pancreases both during development and in adulthood, suggesting that a single wild-type allele of p120 catenin is sufficient for normal pancreatic development and homeostasis (data not shown).
Loss of p120 catenin causes inflammation in the neonatal and adult pancreas
The overt phenotype observed at P0 resembled pancreatitis, with regions containing abundant Vimentin+ cells (Fig. S1), expanded epithelial tubules, and ADM-like transitional structures. Therefore, we next hypothesized that p120 catenin loss might induce inflammation in the neonatal pancreas. To test this hypothesis, we stained for CD45 in CPdx1; p120f/f and wild-type control pancreases. Abundant CD45+ cells were detected in CPdx1; p120f/f P0 pancreases but not wild-type controls, indicating the recruitment of an inflammatory infiltrate in CPdx1; p120f/f pancreases (Fig. 2F). In an effort to understand the mechanism of induction of inflammation in neonatal pancreas, we next stained for a known regulator of inflammation that has been previously associated with p120 catenin loss and states of pancreatic inflammation, NF-kB (Perez-Moreno et al., 2006; Rakonczay et al., 2008; Stairs et al., 2011). NF-kB was detected in ductal epithelium in wild-type pancreases as well as in expanded ductal epithelium in CPdx1; p120f/f pancreases at P0 (Fig. 2G). As incorrect regulation of NF-kB has been linked to dysregulation of the immune system and inflammation (Perez-Moreno et al., 2006; Stairs et al., 2011), widespread pancreatic upregulation of NF-kB in expanded ductal epithelium of CPdx1; p120f/f pancreases may represent a possible mechanism for the induction of inflammation.
We next sought to determine if p120 catenin also regulated inflammation in adult pancreas. We examined the pancreatic histology of cohorts of animals ranging in age from 1 to 12 months. At 1 month of age, 2/4 CPdx1; p120f/f males displayed frank pancreatitis (Fig. S2). Over time, these animals also developed dilated main pancreatic ducts, chronic pancreatitis, ADM, and mucinous metaplasia resembling PanIN1a, with incomplete penetrance (4 out of 7 mice) (Fig. S2). Remaining adult homozygous p120f/f pancreases were histologically normal. Adult CPdx1; p120f/f animals also displayed abundant CD45+ cells in regions characterized by pancreatitis (Fig. S2). Incomplete penetrance of the phenotype in adult pancreases might be influenced by the degree of mosaicism for loss of p120 catenin. Immunolabeling for p120 catenin revealed that ADM and mucinous metaplastic lesions lack p120 catenin, suggesting that these lesions may represent cell autonomous sequelae of p120 catenin deletion (Fig. S2).
p120 catenin is required for normal pancreatic tubulogenesis and branching morphogenesis
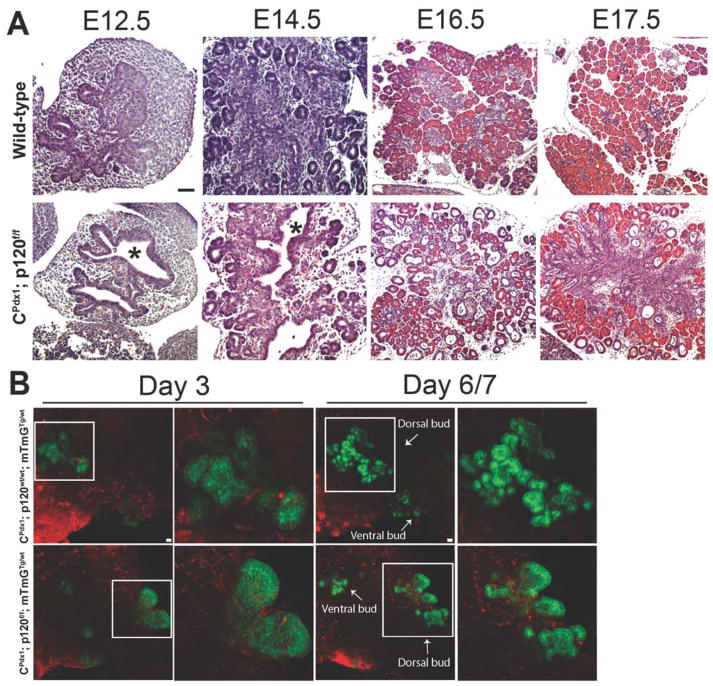
To examine the p120 catenin loss-of-function phenotype during development, we next undertook a histological examination of wild-type and homozygous p120f/f pancreata in a developmental series from E12.5 through E17.5. CPdx1; p120f/f pancreases displayed decreased ramification of branches and expanded epithelial tubule lumen diameter throughout development (Fig. 3A). Aberrant tubulogenesis became evident as early as E12.5 and epithelial tubules continued to show expanded lumens through E17.5. An increased proportion of duct-like epithelium became evident at E14.5 (Fig. S1) and was found distributed throughout the tissue by E16.5 (Fig. 3A). Proper acinar cell differentiation was impeded throughout development in homozygous p120f/f animals.
Figure 3. Developmental analysis of the p120 catenin loss-of-function phenotype.
Hematoxylin and eosin staining at various time points during pancreatic development demonstrated a manifest phenotype as early as E12.5 in CPdx1; p120f/f animals. (A) p120 catenin loss resulted in expansion of epithelial tubule lumen diameter, decreased ramification of branching tubules, and defects in distribution of all pancreatic cell types during development. Asterisks indicate dilated lumens in CPdx1; p120f/f pancreases. (B) CPdx1; p120f/f; mTmGTg/wt pancreatic bud explants dissected at E11.5 and placed in in vitro culture displayed aberrant tubulogenesis, evident as early as Day 3 of culture, suggesting that aberrant tubulogenesis occurred in the absence of associated inflammation. White boxes surround the dorsal pancreatic buds, which are displayed as higher magnification images. White arrows depict the ventral and dorsal pancreatic buds on Day 6/7. Scale bars are 50μm.
As we identified that homozygous deletion of p120 catenin resulted in the recruitment of an inflammatory environment during pancreatogenesis, we next asked if the aberrant tubulogenesis evident at E12.5 would still occur in the absence of an inflammatory infiltrate. To address this question, we employed a double fluorescent mTmG reporter allele (hereafter denoted as “mTmG”) to visualize the dynamics of pancreatic tubulogenesis in dissected pancreatic anlagen in an inflammation-free in vitro setting. CPdx1; p120f/wt; mTmGTg/Tg mice were bred with p120f/wt mice to generate CPdx1; p120wt/wt; mTmGTg/wt, CPdx1; p120f/wt; mTmGTg/wt, and CPdx1; p120f/f; mTmGTg/wt mice. Pancreatic rudiments including surrounding mesenchyme, caudal stomach, and proximal duodenum were dissected at E11.5 and cultured for up to 7 days as previously described (Puri and Hebrok, 2007). Aberrant pancreatic tubulogenesis similar to that observed in vivo was also found to occur in vitro in CPdx1; p120f/f; mTmGTg/wt mice and not in wild-type controls. This suggests that the aberrant pancreatic tubulogenesis observed in homozygous p120f/f mice occurred even in the absence of concomitant inflammation (Fig. 3B). As expected given their normal histological phenotype in vivo, pancreatic explants from CPdx1; p120f/wt; mTmGTg/wt mice recapitulated normal pancreatic tubulogenesis in vitro (data not shown).
Pancreatic progenitor markers are retained in expanded tubular epithelium
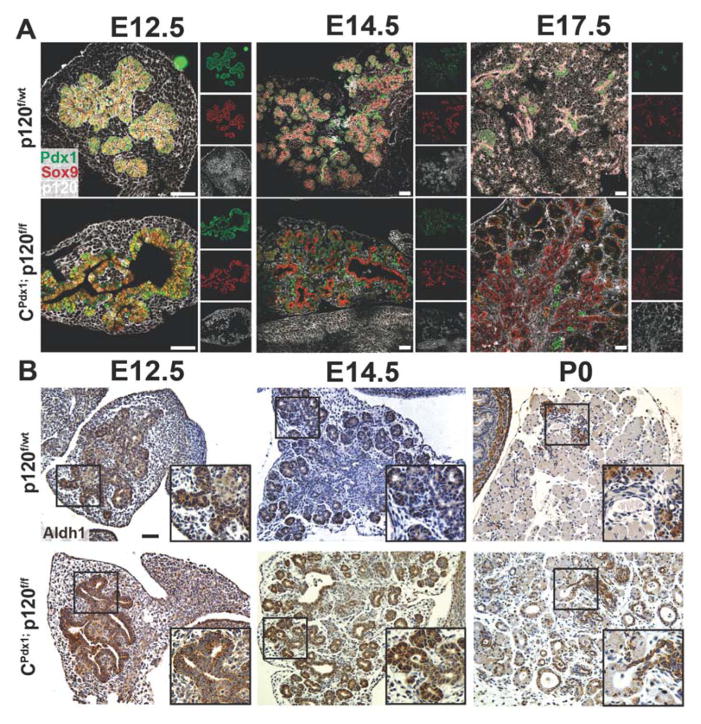
Because we identified an alteration in the relative abundance of specific pancreatic cell types in CPdx1; p120f/f animals, we next evaluated expression of known progenitor markers in developing pancreatic epithelia as a means to assess progenitor differentiation. We examined the pancreatic progenitor markers Sox9, Pdx1, and Aldh1 in a developmental series using immunofluorescence and immunohistochemistry. Pdx1 and Sox9 were expressed in the vast majority of pancreatic epithelial cells at E12.5. During development, Pdx1 expression gradually became restricted to endocrine cells, while Sox9 expression became restricted to duct-like epithelium and centroacinar cells (Fig. 4A). Pdx1 localization in homozygous p120f/f pancreases is unaltered in the developing pancreas when compared to wild-type controls (Fig 4A). Between E12.5 and E14.5, Aldh1 was expressed in the tips of normal branching pancreatic epithelia (Fig. 4B) (Rovira et al., 2010). By P0, wild-type Aldh1 expression was restricted to endocrine cells, a subset of ductal cells, and centroacinar cells (Fig. 4B). In the absence of p120 catenin, Aldh1 was expressed in the expanded tubular epithelium throughout development, as was Sox9 (Fig. 4A, 4B). Since the early pancreatic progenitor marker Aldh1 is expressed in a subset of ductal cells at P0 in wild-type pancreases, continued widespread expression of Aldh1 in expanded duct-like epithelium of homozygous p120f/f pancreases might represent a more underdeveloped, undifferentiated cellular state suggestive of disrupted progenitor differentiation, as has been previously reported for forced ongoing expression of the early pancreatic progenitor marker Sox9 (Seymour et al., 2007).
Figure 4. Expression of multipotent pancreatic progenitor markers Sox9, Pdx1, and Aldh1.
(A) Immunofluorescent images showed expression of early pancreatic progenitor markers Pdx1 and Sox9. The expression pattern of Pdx1 was comparable in both wild-type and homozygous p120f/f pancreases throughout development, while the normal downregulation of Sox9 expression failed to occur in the absence of p120 catenin. (B) Immunohistochemical labeling for Aldh1 demonstrated persistent expression in expanded tubular pancreatic epithelium lacking p120 catenin. Scale bars are 50μm.
Loss of p120 catenin alters cytoskeletal organization but not cell polarity
As an additional means to assess the p120 catenin loss-of-function phenotype, we next undertook an examination of apical and basal markers in CPdx1; p120f/f and control pancreata. MUC1 is a transmembrane glycoprotein with extensive O-glycosylation that is localized to the apical surface of pancreatic epithelial cells (Hollingsworth and Swanson, 2004; Liu et al., 2014). We examined localization of the apical marker MUC1 at P0 in CPdx1; p120f/f and littermate control pancreata by immunofluorescence and found that MUC1 was localized apically in both homozygous p120f/f animals and wild-type controls in both pancreatic ducts and acinar cells (Fig. 5A,B). Phalloidin binds specifically to f-actin filaments, and Phalloidin has been shown to be localized apically and laterally in pancreatic acinar cells (O’Konski and Pandol, 1990; Wulf et al., 1979). We found that Phalloidin localization was apical, lateral, and basal in ductal epithelia, and apical in the acini of CPdx1; p120f/f pancreata at P0, which differs from the apical localization of Phalloidin in ducts of wild-type pancreases (Fig. 5A).
In an effort to understand atypical actin cytoskeleton organization in expanded ductal epithelium of CPdx1; p120f/f pancreata, we next assessed localization of PKCζ, a known cytoplasmic regulator of actin cytoskeleton dynamics (Even-Faitelson and Ravid, 2006; Gomez et al., 1995; Guo et al., 2009; Liu et al., 2007; Uberall et al., 1999). PKCζ/Par6/Par3, a complex activated by Cdc42, is associated with the assembly of tight junctions, apical polarization of epithelial cells, and microtubule organization (Baluch and Capco, 2008; Etienne-Manneville and Hall, 2001; Izumi et al., 1998; Johansson et al., 2000; Lin et al., 2000; Qiu et al., 2000). PKCζ/Par6/Par3, when associated with tight junctions is located at the apical surface of epithelial cells (Ebnet et al., 2001). Since p120 catenin activates Cdc42, and Cdc42 activates PKCζ, we next hypothesized that assembly of the PKCζ/Par3/Par6 complex associated with tight junctions might be impaired in the expanded ductal epithelium of homozygous p120f/f pancreata. To address this, we immunostained for PKCζ, which revealed an increase in cytoplasmic PKCζ in expanded ductal epithelium of CPdx1; p120f/f pancreases when compared to controls (Fig. 5C). Therefore, these results suggest a connection between increased cytoplasmic availability of the cytoskeletal remodeler PKCζ and the observed defects in actin cytoskeletal rearrangement associated with p120 catenin loss.
We next assessed basal polarity by examination of Integrin alpha 6/CD49f using immunofluorescence. At P0, both CPdx1; p120f/f and littermate control pancreata had basal localization of CD49f in ductal and acinar epithelia (Fig. 5A). As another assessment of basal polarity, we immunostained for Laminin. Both CPdx1; p120f/f and littermate control pancreases had basal extracellular localization of Laminin (Fig. 5B). Taken together, these data suggest that basal polarity is maintained, but actin cytoskeleton organization is not completely intact in expanded ductal epithelium of homozygous p120f/f pancreases, an observation which might be mediated by increased cytoplasmic PKCζ.
p120 catenin-null pancreas retains adherens junctions during development but not in adulthood
It was striking to observe that epithelial integrity remained intact during development in CPdx1; p120f/f pancreases. This was surprising because biochemical studies have demonstrated that loss of p120 catenin results in a failure to retain E-cadherin at epithelial cell membranes (Ireton et al., 2002). This would predict a loss of adherens junction integrity and compromised epithelial cell-cell adhesion. In order to examine adherens junction integrity, we next evaluated localization of members of the adherens junction complex during pancreatic development.
p120 catenin-null pancreatic epithelia display an overall reduction in adherens junction components during development when compared to wild-type controls. However, despite the loss of p120 catenin in pancreatic epithelium, significant amounts of E-cadherin, β-catenin, and α-catenin remained detectable at the membrane throughout embryonic development as assessed by immunofluorescence (Fig. 6 A,B and data not shown). The reduction of adherens junction proteins at cell membranes is particularly evident in homozygous p120f/f animals containing mosaic tissue that display both p120 catenin-null and p120 catenin-expressing pancreatic epithelium in the same section (Fig. 6 A,B).
As an additional means to assess expression of adherens proteins, quantitative RT-PCR was used to compare expression of Cdh1, Ctnnb1, and Ctnna1 between wild-type and CPdx1; p120f/f pancreases at E14.5 and E17.5. At E14.5, there is no difference in expression of Cdh1 or Ctnnb1, but Ctnna1 expression is significantly increased in homozygous p120f/f pancreata when compared to controls (Fig. 6C). At E17.5, there is a very significant reduction in expression of Cdh1 and Ctnnb1 in CPdx1; p120f/f pancreases when compared to wild-type and no difference in expression of Ctnna1 (Fig. 6D). It is worth noting that although RNA was collected from whole pancreas (epithelium + mesenchyme), we did not observe any difference in the amount of p120 catenin-expressing mesenchyme in homozygous p120f/f animals vs. controls during pancreatic development, which allows us to attribute any differences observed in gene expression directly to pancreatic epithelium. Collectively, these results suggest that despite p120 catenin loss, there remains significant expression of Cdh1, Ctnnb1, and Ctnna1 in pancreatic epithelium during development.
In contrast to the maintenance of organized adherens junctions during development, adult CPdx1; p120f/f pancreases do not retain E-cadherin and β-catenin at cell membranes in epithelial cells lacking p120 catenin (Fig. S2). These data suggest that there are compensatory mechanisms present during pancreatic development, but not in adulthood, that permit retention of adherens junctions in the absence of p120 catenin. Nuclear labeling of β-catenin was minimal and comparable in both wild-type and homozygous p120f/f pancreases, as assessed by high resolution confocal microscopy, suggesting that decreased localization of β-catenin to the membrane was not accompanied by an increase in translocalization of β-catenin to the nucleus in homozygous p120f/f pancreases (data not shown).
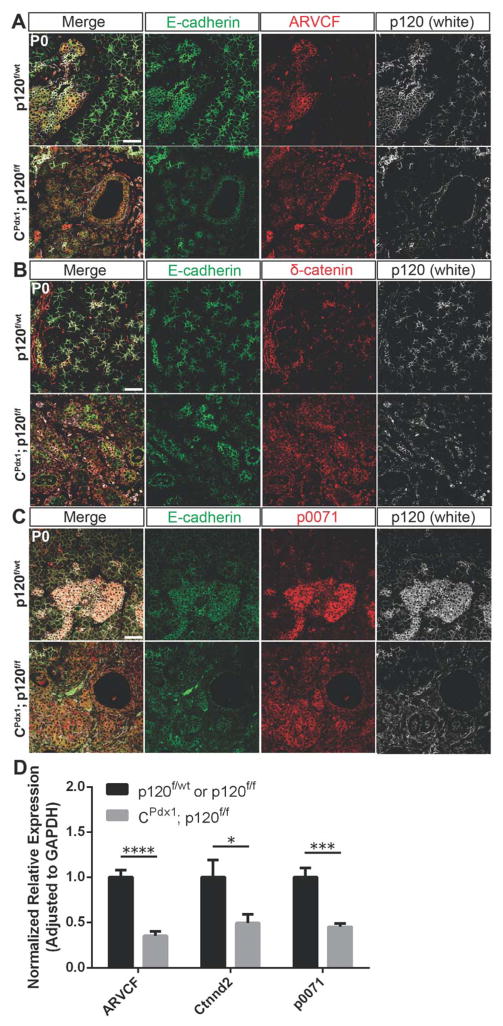
Because p120 catenin has been shown to play a crucial role in cadherin molecule stability and regulation of cadherin turnover at cell membranes (Davis et al., 2003; Ireton et al., 2002), we next sought to understand the retention of adherens junction members in p120 catenin-null epithelia in vivo. We examined the expression of related p120 catenin family members in normal and p120 catenin-deleted pancreas by immunofluorescence and qPCR. In wild-type pancreases, ARVCF, β-catenin, and p0071 were localized uniformly along cell membranes of islet and ductal cells, and at the apical surface of some acini (Fig. 7A,B,C). β-catenin was also expressed in some lateral acinar cell membranes in wild-type pancreas (Fig. 7B). We found that all of the p120 catenin family members examined were present at cell membranes in both p120 catenin-null ducts and acinar cells. Quantitative RT-PCR showed a significant reduction in expression of ARVCF, β-catenin, and p0071 in homozygous p120f/f pancreases when compared to controls (Fig. 7D). Retention of E-cadherin, which might be stabilized by substitution of ARVCF, β-catenin, and p0071 for p120 catenin, likely provides stability for the maintenance of other adherens junctions members and accompanying epithelial integrity during development in CPdx1; p120f/f animals.
Figure 7. p120 catenin family members localize to the membranes of epithelial cells in the absence of p120 catenin.
Immunofluorescent images demonstrating expression of p120 catenin family members in wild-type and homozygous p120f/f pancreas. (A) ARVCF is expressed uniformly in the membranes of duct and islet cells and apically in some acinar cells in wild-type pancreas. It is also localized to the membranes of expanded duct cells and residual acinar cells in homozygous p120f/f pancreas. (B) δ-catenin is expressed uniformly in the membranes of duct and islet cells and also in the apical and lateral membranes of some acinar cells in wild-type pancreas. In homozygous p120f/f pancreas, δ-catenin localized to the membranes of expanded ducts. (C) p0071 was expressed uniformly in the membranes of duct and islet cells in wild-type pancreas. Expression of p0071 was also seen uniformly in the membranes of duct cells and remaining acinar cells in homozygous p120f/f pancreas. Scale bars are 50μm. (D) Expression of ARVCF, δ-catenin, and p0071 in CPdx1; p120f/f and wild-type pancreases was compared using qPCR. A significant reduction in expression of ARVCF, δ-catenin, and p0071 was observed in homozygous p120f/f pancreases when compared to controls. Wild-type control targets were normalized to 1. For all genotypes and all genes, n=7, and reactions were run in quadruplicate. For interpretation of statistical results from Student’s t test, ns = not significant and p value > 0.05, * = p value ≤ 0.05, ** = p value ≤ 0.01, *** = p value ≤ 0.001, and **** = p value ≤ 0.0001.
DISCUSSION
The effects of conditional deletion of p120 catenin in different organ systems is highly variable, ranging from no observed phenotype (prostate and ureteric bud) to severe developmental defects (salivary gland, mammary gland, kidney, and other organs) and tumor formation (breast, esophagus, and other organs). We have used a mouse model of conditional p120 catenin deletion in epithelial pancreatic lineages to examine the role of p120 catenin during pancreatic development. Our data show that loss of p120 catenin results in overall defects in pancreatic tubulogenesis, branching morphogenesis, and acinar cell differentiation. We also observed differences in the abundance of specific pancreatic cell types and induction of inflammation within the neonatal pancreas. Strikingly, loss of p120 catenin induced changes in the embryonic pancreas that resembled ADM, a condition in the human pancreas that is present in the setting of either pancreatitis or early pancreatic cancer. p120 catenin functions as a regulator of innate anti-inflammatory responses in a growing number of tissues, which now include pancreas (Hu, 2012). Tissue-specific deletion of p120 catenin in both mouse skin and intestine resulted in endogenous recruitment of immune cells and the release of proinflammatory cytokines (Perez-Moreno et al., 2006; Perez-Moreno et al., 2008; Smalley-Freed et al., 2010; Smalley-Freed et al., 2011). In the epidermis, the immune response induced by p120 loss was mediated partly by upregulation of NF-kB signaling (Perez-Moreno et al., 2006). We show that NF-kB is upregulated in the pancreata of CPdx1; p120f/f animals, and thus might contribute to the recruitment of an inflammatory environment.
Aberrant tubulogenesis has been previously reported in a mouse model of loss of p120 catenin in the renal mesenchyme during glomerulogenesis (Marciano et al., 2011). In homozygous p120f/f animals, we observed aberrant tubulogenesis and an overall decrease in ramification of pancreatic branches at E12.5. The defects in branching morphogenesis may be a secondary effect of the extended epithelial tubule lumen diameter, or p120 catenin loss may have a direct effect on both branching morphogenesis and tubulogenesis. CPdx1; p120f/f animals also display abnormal localization of all epithelial pancreatic cell types during embryonic development when compared to wild-type controls. Islets fail to migrate throughout the tissue and instead surround a central cluster of Vimentin+ cells. Acinar cells are peripherally located while ductal epithelium is mostly centrally located, but can be found throughout the tissue. Homozygous p120f/f pancreases also show a decrease in normal acinar cell differentiation and an expansion of duct-like epithelium during development. A similar result has been previously reported upon deletion of p120 catenin in the mouse salivary gland, which showed blocked acinar cell differentiation and a corresponding increase in ductal epithelium (Davis and Reynolds, 2006). Aberrant actin cytoskeleton organization in expanded ductal epithelium of homozygous p120f/f pancreases was accompanied by increased cytoplasmic PKCζ, suggesting a connection between modulation of the actin cytoskeleton by PKCζ, specifically in the context of p120 catenin loss.
A reduction of adherens junction members at cell membranes upon conditional ablation of p120 catenin has been reported in many developing organ systems (dental enamel, salivary gland, mammary gland, kidney, skin, and vasculature). Despite a reduction, significant retention of adherens junction components are observed in many of these developing organ systems upon loss of p120 catenin. Collectively, these in vivo data differ from biochemical in vitro studies which have shown that loss of p120 catenin results in near complete loss of adherens junction components, thereby suggesting that compensatory mechanism(s) for stabilization of cadherin molecules in the absence of p120 catenin exist in vivo in some developing organ systems (Davis et al., 2003). For pancreas, retention of adherens junction components in the absence of p120 catenin is observed only during pancreatic development, and not in adults. We show that p120 catenin family members ARVCF, δ-catenin, and p0071 are present at cell membranes even following p120 catenin deletion; since these family members are also capable of stabilizing E-cadherin, they may partially compensate for p120 catenin loss. Relevant to our in vivo observations, ARVCF and δ-catenin have previously been shown to rescue E-cadherin stabilization in the absence of p120 catenin in vitro (Davis et al., 2003). However, to our knowledge, no in vivo studies to date have shown full functional redundancy for cadherin stabilization by substitution of ARVCF, δ-catenin, or p0071 for p120 catenin in p120 catenin-deleted tissues. Although p120 catenin family members might partially compensate for retention of adherens junctions with loss of p120 catenin, this compensation is not sufficient to guarantee normal pancreas development, and loss of p120 catenin results in a dramatic phenotype. Taken together, our data indicate a crucial role for p120 catenin in pancreatic tubulogenesis, branching morphogenesis, acinar cell differentiation, and regulation of inflammation in both the developing and adult pancreas.
Supplementary Material
(A) Immunolabeling using an antibody specific for isoforms 1–3 of p120 catenin showed ubiquitous expression of p120 catenin in developing, neonatal, and adult mouse pancreas. For neonatal and adult pancreas, white arrows show cells indicated with a “D” for ducts, “I” indicates islets of Langerhans, and “A” designates acinar cells. (B) Expansion of a centralized Vimentin+ cluster of cells and DBA+ epithelium in homozygous p120f/f pancreas was evident as early as E14.5. Some expanded epithelial tubules lost expression of the ductal marker DBA at E17.5, but retained expression of Cytokeratin 19 at P0 (Fig. 2B). Scale bars are 50μm.
(A–B) Adult CPdx1; p120f/f males displayed chronic pancreatitis, dilated main pancreatic ducts, ADM, and mucinous metaplasia that resembled PanIN1a. (A) Pancreatitis and fatty replacement of acinar tissue was evident in CPdx1; p120f/f animals as early as 1 month. Immunohistochemistry showed CD45+ cells in CPdx1; p120f/f animals at 10 months of age in regions characterized by pancreatitis. Yellow arrows point to examples of CD45+ cells. (B) Hematoxylin and eosin and Alcian blue staining revealed the presence of mucinous metaplasia in CPdx1; p120f/f pancreata. Serial sections were used in the CPdx1; p120f/f panel for Hematoxylin and eosin and Alcian blue staining. (C) E-cadherin and p120 catenin immunostaining in animals 10–12 months of age demonstrated that ADM lesions lack p120 catenin in CPdx1; p120f/f pancreases, suggesting that these lesions may be forming in a cell autonomous manner. The bottom panel of images depicts mosaicism for p120 catenin expression in the main pancreatic duct of CPdx1; p120f/f animals. Yellow arrows point to p120 catenin-expressing cells while red arrows show p120 catenin-null cells. (D) Immunofluorescence demonstrated mosaicism for p120 catenin, E-cadherin, and β-catenin in adult CPdx1; p120f/f pancreases 10 months of age. Note that (C) and (D) collectively demonstrate that unlike embryonic pancreas, adult pancreatic epithelium lacking p120 catenin does not retain adherens junction proteins E-cadherin and β-catenin. Scale bars are 50μm.
No difference was detected in Cleaved Caspase-3 staining throughout development in CPdx1; p120f/f and littermate control pancreata. Scale bars are 50μm.
No difference was detected in Ki67 staining throughout development in CPdx1; p120f/f and littermate control pancreata. Scale bars are 50μm.
Highlights.
We examine the consequences of deletion of p120 cetenin in mouse pancreas development
p120 is required for proper pancreatic tubulogenesis and branching morphogenesis
p120 deletion results in induction of inflammation in developing and adult pancreas
Despite p120 loss, E-cadherin, β-catenin, and α-catenin are retained in development
Family members ARVCF, δ-catenin, and p0071 might partially compensate for p120 loss
Acknowledgments
We cite our colleagues in Table S1 references for their generous contribution of antibodies including ARVCF and p0071, and Pdx1 given by Ilse Hoffman and Chris Wright, respectively. The authors wish to thank Qingfeng Zhu, Anzer Habibulla, and Mara Swaim for their invaluable assistance in maintaining mouse colonies and genotyping and Katherine Wu for her expert technical assistance. The authors are extremely grateful for correspondences and technical guidance from Sapna Puri. We also give special thanks to Andrew Ewald for reading this manuscript. This study was supported by National Institutes of Health grant R01 DK56211 (S.D.L).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Audrey M. Hendley, Email: ahendle1@jhmi.edu.
Elayne Provost, Email: elayne.provost@gmail.com.
Jennifer M. Bailey, Email: Jennifer.M.Bailey@uth.tmc.edu.
Yue J. Wang, Email: tiwang03@gmail.com.
Megan H. Cleveland, Email: genetics85@gmail.com.
Danielle Blake, Email: arielturnip@gmail.com.
Ross W. Bittman, Email: rossbittman@gmail.com.
Jeffrey C. Roeser, Email: jroeser10@gmail.com.
Anirban Maitra, Email: amaitra@mdanderson.org.
Albert B. Reynolds, Email: al.reynolds@vanderbilt.edu.
Steven D. Leach, Email: leachs@mskcc.org.
References
- Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, Reynolds AB. Inhibition of RhoA by p120 catenin. Nature cell biology. 2000;2:637–644. doi: 10.1038/35023588. [DOI] [PubMed] [Google Scholar]
- Baluch DP, Capco DG. GSK3 beta mediates acentromeric spindle stabilization by activated PKC zeta. Developmental biology. 2008;317:46–58. doi: 10.1016/j.ydbio.2008.01.044. [DOI] [PubMed] [Google Scholar]
- Bartlett JD, Dobeck JM, Tye CE, Perez-Moreno M, Stokes N, Reynolds AB, Fuchs E, Skobe Z. Targeted p120-catenin ablation disrupts dental enamel development. PLoS One. 2010:5. doi: 10.1371/journal.pone.0012703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. The Journal of cell biology. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Reynolds AB. Blocked acinar development, E-cadherin reduction, and intraepithelial neoplasia upon ablation of p120-catenin in the mouse salivary gland. Dev Cell. 2006;10:21–31. doi: 10.1016/j.devcel.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Ebnet K, Suzuki A, Horikoshi Y, Hirose T, Meyer Zu Brickwedde MK, Ohno S, Vestweber D. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM) The EMBO journal. 2001;20:3738–3748. doi: 10.1093/emboj/20.14.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia LP, Yamamoto M, Zang K, Reichardt LF. p120 catenin regulates dendritic spine and synapse development through Rho-family GTPases and cadherins. Neuron. 2006;51:43–56. doi: 10.1016/j.neuron.2006.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Even-Faitelson L, Ravid S. PAK1 and aPKCzeta regulate myosin II-B phosphorylation: a novel signaling pathway regulating filament assembly. Mol Biol Cell. 2006;17:2869–2881. doi: 10.1091/mbc.E05-11-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golosow N, Grobstein C. Epitheliomesenchymal interaction in pancreatic morphogenesis. Developmental biology. 1962;4:242–255. doi: 10.1016/0012-1606(62)90042-8. [DOI] [PubMed] [Google Scholar]
- Gomez J, Martinez de Aragon A, Bonay P, Pitton C, Garcia A, Silva A, Fresno M, Alvarez F, Rebollo A. Physical association and functional relationship between protein kinase C zeta and the actin cytoskeleton. European journal of immunology. 1995;25:2673–2678. doi: 10.1002/eji.1830250941. [DOI] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Guo H, Gu F, Li W, Zhang B, Niu R, Fu L, Zhang N, Ma Y. Reduction of protein kinase C zeta inhibits migration and invasion of human glioblastoma cells. Journal of neurochemistry. 2009;109:203–213. doi: 10.1111/j.1471-4159.2009.05946.x. [DOI] [PubMed] [Google Scholar]
- Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochimica et biophysica acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nature reviews Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- Hu G. p120-Catenin: a novel regulator of innate immunity and inflammation. Critical reviews in immunology. 2012;32:127–138. doi: 10.1615/critrevimmunol.v32.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian L, Bundy LM, Sealy L, Gilbert B, van Roy F, Reynolds AB. A novel role for p120 catenin in E-cadherin function. The Journal of cell biology. 2002;159:465–476. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiyama N, Lee SH, Liu S, Li GY, Smith MJ, Reichardt LF, Ikura M. Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell-cell adhesion. Cell. 2010;141:117–128. doi: 10.1016/j.cell.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Hirose T, Tamai Y, Hirai S, Nagashima Y, Fujimoto T, Tabuse Y, Kemphues KJ, Ohno S. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. The Journal of cell biology. 1998;143:95–106. doi: 10.1083/jcb.143.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson A, Driessens M, Aspenstrom P. The mammalian homologue of the Caenorhabditis elegans polarity protein PAR-6 is a binding partner for the Rho GTPases Cdc42 and Rac1. J Cell Sci. 2000;113(Pt 18):3267–3275. doi: 10.1242/jcs.113.18.3267. [DOI] [PubMed] [Google Scholar]
- Kurley SJ, Bierie B, Carnahan RH, Lobdell NA, Davis MA, Hofmann I, Moses HL, Muller WJ, Reynolds AB. p120-catenin is essential for terminal end bud function and mammary morphogenesis. Development. 2012;139:1754–1764. doi: 10.1242/dev.072769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsman L, Nijagal A, Whitchurch TJ, Vanderlaan RL, Zimmer WE, Mackenzie TC, Hebrok M. Pancreatic mesenchyme regulates epithelial organogenesis throughout development. PLoS biology. 2011;9:e1001143. doi: 10.1371/journal.pbio.1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nature cell biology. 2000;2:540–547. doi: 10.1038/35019582. [DOI] [PubMed] [Google Scholar]
- Liu X, Yi C, Wen Y, Radhakrishnan P, Tremayne JR, Dao T, Johnson KR, Hollingsworth MA. Interactions between MUC1 and p120 catenin regulate dynamic features of cell adhesion, motility, and metastasis. Cancer research. 2014;74:1609–1620. doi: 10.1158/0008-5472.CAN-13-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XJ, Yang C, Gupta N, Zuo J, Chang YS, Fang FD. Protein kinase C-zeta regulation of GLUT4 translocation through actin remodeling in CHO cells. Journal of molecular medicine. 2007;85:851–861. doi: 10.1007/s00109-007-0232-z. [DOI] [PubMed] [Google Scholar]
- Marciano DK, Brakeman PR, Lee CZ, Spivak N, Eastburn DJ, Bryant DM, Beaudoin GM, 3rd, Hofmann I, Mostov KE, Reichardt LF. p120 catenin is required for normal renal tubulogenesis and glomerulogenesis. Development. 2011;138:2099–2109. doi: 10.1242/dev.056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariner DJ, Wang J, Reynolds AB. ARVCF localizes to the nucleus and adherens junction and is mutually exclusive with p120(ctn) in E-cadherin complexes. J Cell Sci. 2000;113(Pt 8):1481–1490. doi: 10.1242/jcs.113.8.1481. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Noren NK, Liu BP, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. The Journal of cell biology. 2000;150:567–580. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Konski MS, Pandol SJ. Effects of caerulein on the apical cytoskeleton of the pancreatic acinar cell. The Journal of clinical investigation. 1990;86:1649–1657. doi: 10.1172/JCI114887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oas RG, Xiao K, Summers S, Wittich KB, Chiasson CM, Martin WD, Grossniklaus HE, Vincent PA, Reynolds AB, Kowalczyk AP. p120-Catenin is required for mouse vascular development. Circulation research. 2010;106:941–951. doi: 10.1161/CIRCRESAHA.109.207753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno M, Davis MA, Wong E, Pasolli HA, Reynolds AB, Fuchs E. p120-catenin mediates inflammatory responses in the skin. Cell. 2006;124:631–644. doi: 10.1016/j.cell.2005.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev Cell. 2006;11:601–612. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno M, Song W, Pasolli HA, Williams SE, Fuchs E. Loss of p120 catenin and links to mitotic alterations, inflammation, and skin cancer. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15399–15404. doi: 10.1073/pnas.0807301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pictet RL, Clark WR, Williams RH, Rutter WJ. An ultrastructural analysis of the developing embryonic pancreas. Developmental biology. 1972;29:436–467. doi: 10.1016/0012-1606(72)90083-8. [DOI] [PubMed] [Google Scholar]
- Pieters T, van Hengel J, van Roy F. Functions of p120ctn in development and disease. Frontiers in bioscience. 2012;17:760–783. doi: 10.2741/3956. [DOI] [PubMed] [Google Scholar]
- Puri S, Hebrok M. Dynamics of embryonic pancreas development using real-time imaging. Developmental biology. 2007;306:82–93. doi: 10.1016/j.ydbio.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu RG, Abo A, Steven Martin G. A human homolog of the C. elegans polarity determinant Par-6 links Rac and Cdc42 to PKCzeta signaling and cell transformation. Current biology : CB. 2000;10:697–707. doi: 10.1016/s0960-9822(00)00535-2. [DOI] [PubMed] [Google Scholar]
- Rakonczay Z, Jr, Hegyi P, Takacs T, McCarroll J, Saluja AK. The role of NF-kappaB activation in the pathogenesis of acute pancreatitis. Gut. 2008;57:259–267. doi: 10.1136/gut.2007.124115. [DOI] [PubMed] [Google Scholar]
- Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter WJ, Kemp JD, Bradshaw WS, Clark WR, Ronzio RA, Sanders TG. Regulation of specific protein synthesis in cytodifferentiation. Journal of cellular physiology. 1968;72(Suppl 1):1–18. doi: 10.1002/jcp.1040720403. [DOI] [PubMed] [Google Scholar]
- Schackmann RC, Klarenbeek S, Vlug EJ, Stelloo S, van Amersfoort M, Tenhagen M, Braumuller TM, Vermeulen JF, van der Groep P, Peeters T, van der Wall E, van Diest PJ, Jonkers J, Derksen PW. Loss of p120-catenin induces metastatic progression of breast cancer by inducing anoikis resistance and augmenting growth factor receptor signaling. Cancer research. 2013;73:4937–4949. doi: 10.1158/0008-5472.CAN-13-0180. [DOI] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L, Weis WI. Structure and biochemistry of cadherins and catenins. Cold Spring Harbor perspectives in biology. 2009;1:a003053. doi: 10.1101/cshperspect.a003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley-Freed WG, Efimov A, Burnett PE, Short SP, Davis MA, Gumucio DL, Washington MK, Coffey RJ, Reynolds AB. p120-catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. The Journal of clinical investigation. 2010;120:1824–1835. doi: 10.1172/JCI41414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley-Freed WG, Efimov A, Short SP, Jia P, Zhao Z, Washington MK, Robine S, Coffey RJ, Reynolds AB. Adenoma formation following limited ablation of p120-catenin in the mouse intestine. PLoS One. 2011;6:e19880. doi: 10.1371/journal.pone.0019880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stairs DB, Bayne LJ, Rhoades B, Vega ME, Waldron TJ, Kalabis J, Klein-Szanto A, Lee JS, Katz JP, Diehl JA, Reynolds AB, Vonderheide RH, Rustgi AK. Deletion of p120-catenin results in a tumor microenvironment with inflammation and cancer that establishes it as a tumor suppressor gene. Cancer Cell. 2011;19:470–483. doi: 10.1016/j.ccr.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Sanders E, Reynolds A, van Roy F, van Hengel J. Ocular anterior segment dysgenesis upon ablation of p120 catenin in neural crest cells. Investigative ophthalmology & visual science. 2012;53:5139–5153. doi: 10.1167/iovs.12-9472. [DOI] [PubMed] [Google Scholar]
- Uberall F, Hellbert K, Kampfer S, Maly K, Villunger A, Spitaler M, Mwanjewe J, Baier-Bitterlich G, Baier G, Grunicke HH. Evidence that atypical protein kinase C-lambda and atypical protein kinase C-zeta participate in Ras-mediated reorganization of the F-actin cytoskeleton. The Journal of cell biology. 1999;144:413–425. doi: 10.1083/jcb.144.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valer Carstea B, Catunda Lemos AP, Ilie ED, Varga L, Bodo S, Kovacs A, Bosze Z, Gocza E. Production of identical mouse twins and a triplet with predicted gender. Cloning and stem cells. 2007;9:247–256. doi: 10.1089/clo.2006.0055. [DOI] [PubMed] [Google Scholar]
- Villasenor A, Chong DC, Henkemeyer M, Cleaver O. Epithelial dynamics of pancreatic branching morphogenesis. Development. 2010;137:4295–4305. doi: 10.1242/dev.052993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf E, Deboben A, Bautz FA, Faulstich H, Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:4498–4502. doi: 10.1073/pnas.76.9.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZM, Reynolds AB, Gaucher EA. The evolutionary history of the catenin gene family during metazoan evolution. BMC evolutionary biology. 2011;11:198. doi: 10.1186/1471-2148-11-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Immunolabeling using an antibody specific for isoforms 1–3 of p120 catenin showed ubiquitous expression of p120 catenin in developing, neonatal, and adult mouse pancreas. For neonatal and adult pancreas, white arrows show cells indicated with a “D” for ducts, “I” indicates islets of Langerhans, and “A” designates acinar cells. (B) Expansion of a centralized Vimentin+ cluster of cells and DBA+ epithelium in homozygous p120f/f pancreas was evident as early as E14.5. Some expanded epithelial tubules lost expression of the ductal marker DBA at E17.5, but retained expression of Cytokeratin 19 at P0 (Fig. 2B). Scale bars are 50μm.
(A–B) Adult CPdx1; p120f/f males displayed chronic pancreatitis, dilated main pancreatic ducts, ADM, and mucinous metaplasia that resembled PanIN1a. (A) Pancreatitis and fatty replacement of acinar tissue was evident in CPdx1; p120f/f animals as early as 1 month. Immunohistochemistry showed CD45+ cells in CPdx1; p120f/f animals at 10 months of age in regions characterized by pancreatitis. Yellow arrows point to examples of CD45+ cells. (B) Hematoxylin and eosin and Alcian blue staining revealed the presence of mucinous metaplasia in CPdx1; p120f/f pancreata. Serial sections were used in the CPdx1; p120f/f panel for Hematoxylin and eosin and Alcian blue staining. (C) E-cadherin and p120 catenin immunostaining in animals 10–12 months of age demonstrated that ADM lesions lack p120 catenin in CPdx1; p120f/f pancreases, suggesting that these lesions may be forming in a cell autonomous manner. The bottom panel of images depicts mosaicism for p120 catenin expression in the main pancreatic duct of CPdx1; p120f/f animals. Yellow arrows point to p120 catenin-expressing cells while red arrows show p120 catenin-null cells. (D) Immunofluorescence demonstrated mosaicism for p120 catenin, E-cadherin, and β-catenin in adult CPdx1; p120f/f pancreases 10 months of age. Note that (C) and (D) collectively demonstrate that unlike embryonic pancreas, adult pancreatic epithelium lacking p120 catenin does not retain adherens junction proteins E-cadherin and β-catenin. Scale bars are 50μm.
No difference was detected in Cleaved Caspase-3 staining throughout development in CPdx1; p120f/f and littermate control pancreata. Scale bars are 50μm.
No difference was detected in Ki67 staining throughout development in CPdx1; p120f/f and littermate control pancreata. Scale bars are 50μm.