Abstract
Inflammation and oxidative stress in the brain are major causes of HIV-associated neurocognitive disorders. Previously we have reported high content of glutathione (GSH) in the thalamus of rats with F344 genetic background. In this study, we investigated the changes of GSH metabolism and GSH-dependent antioxidant enzymes in the rat thalamus in response to HIV-1 transgenesis, and their associations with oxidative stress, inflammation, and neuronal development. Male HIV-1 transgenic (HIV-1Tg) rats and wild type F344 rats at 10 months were used in this study, with 5 rats in each group. Parameters measured in this study included: total and oxidized GSH, glutathione peroxidase (GPx), glutathione-S-transferase (GST), gamma-glutamylcysteine synthetase (GCS), gamma-glutamyl transferase (GGT), cysteine/cystine transporters, 4-hydroxynonenal (HNE), interleukin 12 (IL12), neuronal nuclei (NeuN), microtubule-associated protein (MAP2), and glia fibrillary acidic protein (GFAP). The levels of total GSH, oxidized GSH (GSSG) and MAP2 protein, and enzymatic activities of GCS, GPx and GST were significantly higher in HIV-1Tg rats compared with F344 rats, but the ratio of GSSG/GSH, activity of GGT and levels of HNE, NeuN protein and GFAP protein did not change. HIV-1Tg rats showed a lower level of IL12 protein. GSH positively correlated with GCS, GST and MAP2, GSSG/GSH ratio positively correlated with HNE and IL12, the activities of GPx, GST and GCS positively correlated with each other, and negatively correlated with HNE. These findings suggest an important role of the GSH-centered system in reducing oxidative stress and neuroinflammation, and enhancing neuron differentiation in the thalamus of HIV-1Tg rats.
Keywords: HIV, thalamus, glutathione, antioxidant, oxidative stress, inflammation
INTRODUCTION
Glutathione (GSH) is the most abundant antioxidant in the brain and plays important roles in antioxidant defense, cell proliferation, and neuronal differentiation (Aoyama et al. 2008). HIV-induced GSH depletion has been reported in both human (Buhl et al. 1989; Staal et al. 1992) and animals (Price et al. 2006), and HIV viral proteins were found to decrease the concentration of GSH in cultured cells in a duration- and dose-dependent manner (Price et al. 2005; Toborek et al. 2003). Previously we documented high GSH concentration in the thalamus of Fisher 344 (F344) rats, and observed that HIV-1 transgenesis in these rats caused GSH depletion in this brain region, which was associated with compensatory transcriptional upregulation of enzymes involved in GSH metabolism (Pang et al. 2013). These findings led us to hypothesize that (1) GSH may have an important protective role in thalamus, especially during the exposure to HIV viral proteins, and that (2) the transcriptional upregulation of GSH metabolic enzymes in response to HIV transgenesis in an early age (3 months) may lead to increases of GSH level at later ages and thus effectively exert the protective effects.
In the present study, we carried out a systematic comparison between the HIV-1transgenic (HIV-1Tg) rats and the wild type F344 rats at 10 months in contrast to 3 months in the previous publication (Pang et al. 2013), with emphasis on GSH, GSH metabolism, GSH-dependent antioxidant enzymes, oxidative stress, inflammation, and neuron development in the thalamus.
METHODS
Animals and Dietary Treatment
Five (5) one-month old HIV-1 NL4-3 gag/pol HIV-1Tg rats and 5 genetic background control F344 (Control) rats were purchased from Harlan Inc. (Indianapolis, IN) and housed at the Laboratory Animal Service facility of the University of Hawaii. The rats were maintained on a 12-hour light/dark schedule. Food and water were accessible to the animals ad libitum. The experimental procedures were approved by the Institutional Animal Care and Use Committees (IACUC) of the University of Hawaii.
Sample preparation
At age 10 months, the rats were euthanized in a CO2 induction chamber. Thalamus was dissected from the brain on ice and stored at −80°C. The thalamus tissue was then powderized on dry ice. An aliquot of the powder was sonicated in PBS (except for samples prepared for western blot, see below), centrifuged at 18,000 × g for 10 min at 4°C, and the supernatant was collected. The protein concentration of supernatant was measured using Bradford assay (BioRad, Hercules, CA). The samples were stored at −80°C until assayed.
Chemicals and instruments
All chemicals used in this study were purchased from Sigma (St. Louis, MO) unless otherwise noted. Centrifuges used in this study were Microfuge 22R (Beckman, Brea, CA) and Sorvall ST 40 (Thermo, Waltham, MA). The spectrophotometers used were SpectraMax 340 (for GSH, GCS, and HNE-His assays) and SpectraMax M3 (for GGT, GPx, and GST assays), both from Molecular Devices, Sunnyvale, CA. Protein electrophoresis system (BioRad, Hercules, CA) and Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE) were used in western blot. Real-time PCR was carried out using a Lightcycler 480 II (Roche Applied Science, Indianapolis, IN).
Concentrations of GSH and HNE-His, and activities of GCS, GGT, GPx, and GST
Details of these assay procedures have been published in our previous report (Pang et al. 2013). All samples were measured in triplicates.
Western blot
Thalamus tissue powder was sonicated in 1M Tris (pH 7.5) membrane lysis buffer containing 1M NaCl, 1% Trition X-100, 5mM EDTA, proteinase inhibitor and phosphatase inhibitor. Supernatant was collected after 10 min centrifugation at 18,000 × g, 4°C. Protein concentration was measured by Bradford assay. The western blot procedure was the same as previously published (Pang et al. 2013). Briefly, proteins were separated on 4–20% criterion TGX gels (BioRad, 567–1094) and transferred overnight to Immobilon PVDF transfer membranes (Millipore, Billerica, MA, IPFL00010). Membranes were blocked over night at 4°C with primary antibodies and blocked 1 hr at room temperature with secondary antibodies. Primary antibodies purchased from Abcam (Cambridge, MA) included rabbit anti-GFAP (ab7260, 1:50,000), rabbit anti-GGT7 (ab80903, 1:1000), mouse anti-MAP2 (ab11267, 1:4000), mouse anti-MRP1 (ab32574, 1:500), mouse anti-CD68 (ab31630, 1:500). Mouse anti-NeuN was from Millipore (Billerica, MA, MAB377, 1:1000) and mouse anti-β-actin was from Santa Cruz (Santa Cruz, CA, sc-8432, 1:1000). Rabbit anti-GPx4 antibody was purchased from Cayman (Ann Arbor, MI, 10005258, 1:300) and goat anti-GPx1 was from R&D system (Minneapolis, MN, AF3798, 1:300). Secondary antibodies were from Li-Cor (Lincoln, NE).
Quantitative real-time PCR
Total RNA was extracted from thalamus using Trizol from Invitrogin (Grand Island, NY) and cleaned up using RNeasy mini kit (Qiagen, Valencia, CA). The reverse transcription kit for cDNA synthesis was from Applied Biosystems (Foster City, CA). SABiosciences syber® Green (PA-010-24) kits were used for quantitative PCR. The sequences of primers were: HPRT forward: ctcctcagaccgcttttcc, reverse: tcataacctggttcatcatcactaa; GusB forward: ctctggtggccttacctgat, reverse: cagactcaggtgttgtcatcg; GCS-HC forward: cgatgttcttgaaactctgcaa, reverse: ctggtctccagagggttgg; GCS-LC forward: ctgactcacaatgacccaaaag, reverse: gatgctttcttgaagagcttcct; GGT7 forward: tggcccaataggactgctaa, reverse: tccctggctgtaccgagtt; GGT6 forward: gccctgtggatcttccag, reverse: cagcgctgtggtggtaca; GGT 5 forward: gcatcctcctcaacaacga, reverse: accgttgaacctggcttgt; GGT 1 forward: tggttcgggtatgatgtgaa, reverse: ggcaaaagctggttgtgaag; GPx1 forward: cgacatcgaacccgatataga, reverse: atgccttaggggttgctagg; GPx4 forward: tgggaaatgccatcaaatg, reverse: cggcaggtccttctctatca; GSTα4 forward: tgaaccaggagtcatggaagt, reverse:actccagctgtagccagca; MRP1 forward: cacgagaactcatgacatttgaa, reverse: caggagcgaatgaactggtat.; xCT forward: tccatgaacggtggtgtgt, reverse: cccttctcgagatgcaacat; EAAC1 forward: gaactgcaaccacctattctca, reverse: actgcgtatcacaatcacagaga; HES1 forward: aacacgacaccggacaaac, reverse: cgcctcttctccatgatagg; Notch1 forward: ctggaccccatggacatc, reverse: actgtacacactgccggttg; Mash1 forward: ctgggaatggactttggaag, reverse: tgacgtcgttgtcaagaaaca. Other real-time PCR procedures were the same as previously reported (Pang et al. 2013). All reactions were carried out in quadruplicates.
Cytokine measurements
A cytokine screening test was carried out using a Multi-Analyte ELISArray Kit (SABiosciences, Valencia, CA). Same amount of protein was collected from the tissue lysate of each rat, and the samples from the same group were pooled, and measured in duplicates.
The change of IL12 as a representative cytokine was further confirmed using a Rat IL-12 +p40 ELISA kit (Invitrogen, Grand Island, NV). Tissue lysate from each individual rat was measured in duplicates.
Statistical analysis
Prism 5 (GraphPad Software Inc., La Jolla, CA) was used for statistical analysis. Differences among the means of GSH, enzyme activities, and cytokine levels were analyzed using Student t-test; differences among the means of qPCR and western blot were analyzed using Mann Whitney test. Pearson correlation was used to test correlation among studied parameters. p< 0.05 was considered statistically significant.
RESULTS
GSH concentration
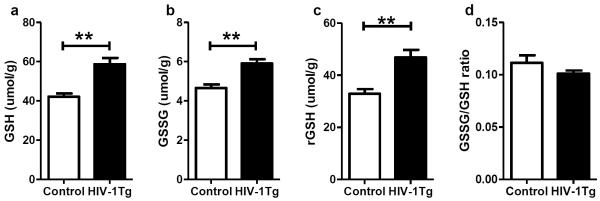
In contrast to previously reported GSH depletion in the thalamus of 3-month HIV-1Tg rats (Pang et al. 2013), the present study shows that at 10 months, HIV-1Tg rats had significantly higher GSH concentration in the thalamus than the control rats (+39%, p=0.0018, Fig 1a). This is a result of increases of both GSSG and rGSH (+27% for GSSG, and +42% for rGSH, p=0.0032 for both, Fig 1b and c) in the HIV-1Tg group, and the ratio of GSSG/GSH remained the same in both groups (Fig 1d).
Figure 1. Glutathione status in the thalamus of F344 (control) and HIV-1Tg rats.
(a) Concentration of total GSH. (b) Concentration of oxidized GSH (GSSG). (c) Concentration of reduced GSH (rGSH). (d) Ratio of GSSG/GSH. ** p<0.01, t-test.
γ-Glutamycystein synthetase (GCS) gene and protein expression and activity
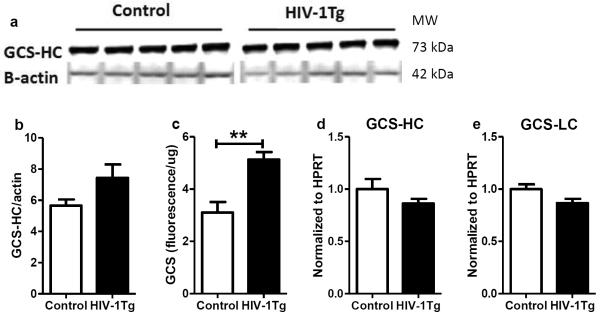
As the rate-limiting enzyme of GSH de novo synthesis, the gene expression, protein expression and activity of GCS were measured in the thalamus. As shown in Fig 2, the protein expression of GCS catalytic domain (GCS-HC) was similar between control and HIV-1Tg rats (Fig 2a). But a significantly higher GCS activity was found in the HIV-1Tg rats compared to the control rats (+65%, p=0.0035, Fig 2b). The mRNA levels of both GCS catalytic domain (Fig 2d) and modulatory domain (GCS-LC, Fig 2e) showed no difference between the two groups.
Figure 2. γ-Glutamylcysteine synthetase (GCS) expression and activity in the thalamus of F344 rats (control) and HIV-1Tg rats.
(a) Western blot image of protein expression of the catalytic domain of GCS (GCS-HC). (b) Relative quantification of GCS-HC protein expression. (c) GCS activity. (d) Relative quantification of gene expression of GCS-HC. (e) Relative quantification of gene expression of GCS light chain (GCS-LC). ** p<0.01, t-test.
γ-Glutamyl transferase (GGT) gene and protein expression and activity
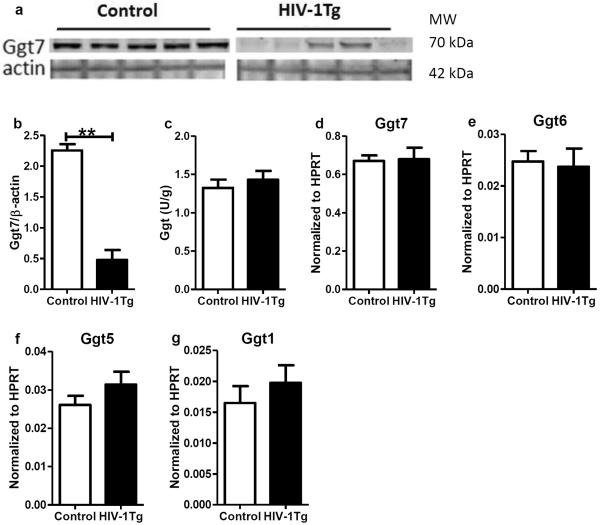
GGT limits the rate of extracellular GSH catabolism, and recycles cysteine for intracellular GSH synthesis. The gene and protein expression of selected GGT isoforms and the activity of GGT are shown in Fig 3. GGT7, the isoform with the highest gene expression, was selected for protein measurement, and its protein level was much lower in the HIV-1Tg rats (−79%, p=0.008, Fig 3 a and b), but the GGT activity remained the same in the two groups (Fig 5c). The gene expression of GGT7 and other GGT isoforms did not change between the two groups (Fig 3d–g).
Figure 3. γ-Glutamyl transferase (GGT) expression and activity in the thalamus of F344 rats (control) and HIV-1Tg rats.
(a) Western blot image of protein expression of GGT isoform 7 (GGT7). (b) Relative quantification of GGT7 protein expression. (c) GGT activity. (d–g) Relative quantifications of gene expression of GGT isoforms 7, 6, 5 and 1, respectively. ** p<0.01, Mann Whitney test.
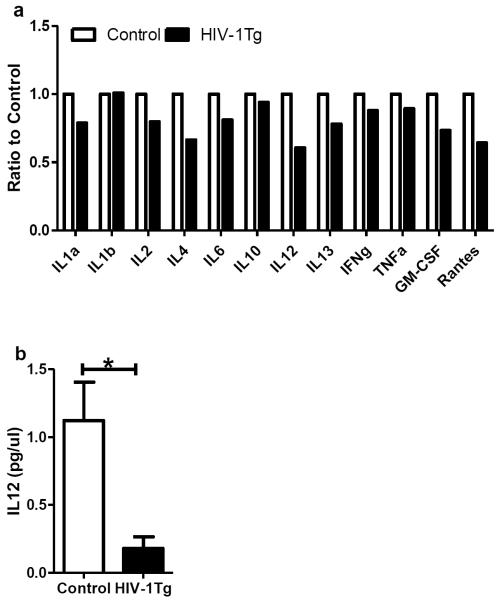
Figure 5. Cytokine expression in the thalamus of F344 (control) and HIV-1Tg rats.
(a) The expression of 12 cytokines. Five samples from each group were pooled for this screening test, and each column represents the average of duplicate measurements of the pooled samples. (b) Protein concentrations of interleukin 12 (IL12). Each column represents the average and SD of 5 individual samples. * p<0.05, t-test.
GSH, cysteine, and cystine transporters
GSH is synthesized in the cells, and transported to the extracellular space for decomposition. Multidrug resistance-associated proteins (MRPs) mediate efflux of GSH and GSSG. The protein and gene expression levels of MRP1 in the HIV-1Tg group were comparable to those in the Control group (Supplemental Fig 1a–c). The gene expression of MRP2 did not change between the two groups (Supplemental Fig 1d).
Cysteine is the limiting substrate for GSH synthesis, and it can be transported into the cells by excitatory amino acid carrier 1 (EAAC1), or by system xc– in the form of cystine. The functional domain of system xc– is xCT. The mRNA levels of EAAC1 and xCT were found similar between HIV-1Tg and control (Supplemental Fig 1e and f).
GSH-dependent antioxidant enzymes and Lipid peroxidation
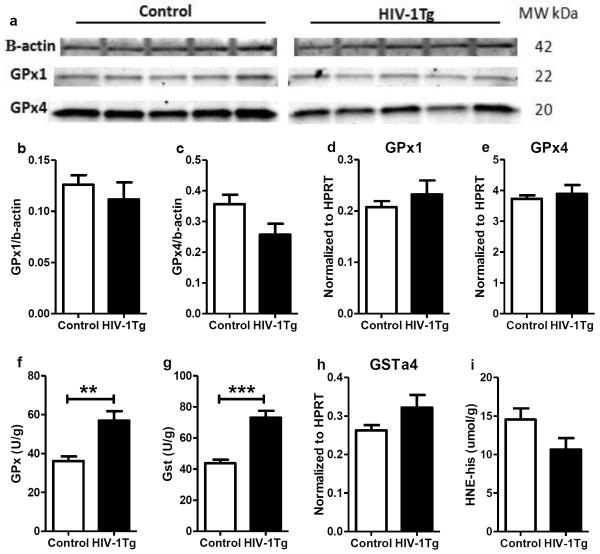
Glutathione peroxidase (GPx) and glutathione-S-transferase (GST) are two antioxidants using GSH as a co-factor. The protein and mRNA levels of GPx1 and GPx4, two major GPx isoforms in the brain, were similar between the control and HIV-1Tg rats (Fig 4a– 4e). However, the GPx activity was significantly increased in the HIV-1Tg rats (+56%, p=0.005, Fig 4f). GST activity was also increased in the thalamus of HIV-1Tg rats (+67%, p=0.0004, Fig 4g), but the mRNA level of GSTα4 (a major isoform of GST conjugating GSH and HNE) did not change (Fig 5h). The concentrations of HNE-His were similar between the two groups (Fig 4i).
Figure 4. The gene and protein expression and enzymatic activity of glutathione peroxidase (GPx) and glutathione-s-transferase (GST), and lipid peroxidation in the thalamus of F344 (control) and HIV-1TG rats.
(a) Protein expression of glutathione peroxidase 1 and 4 (GPx 1 and 4). (b–c) Relative quantification of GPx1 and GPx 4 protein expression. (d–e) mRNA expression of GPx1 and 4. (f) Total enzyme activity of GPx. (g) Enzyme activity of GST. (h) mRNA expression of GSTa4 isoform. (i) HNE-histidine concentration.**p<0.01, ***p<0.001, t-test.
Inflammatory status in the thalamus
We measured the protein levels of 12 cytokines simultaneously in the initial screening test, in which samples from the same group were pooled for the assay (see Methods). Fig 5a shows that most of the cytokines were lower in the HIV-1Tg rats compared to the F344 rats, with IL12 having the largest decrease. IL12 was then selected as a representative cytokine, and the protein level of IL12 of each individual rat was further measured using another ELISA kit. As shown in Fig 5b, the IL12 protein level of the HIV-1Tg rats was 5-fold lower than that in the control rats (p=0.01), confirming the change observed in the initial screening.
Neurons and glial cells
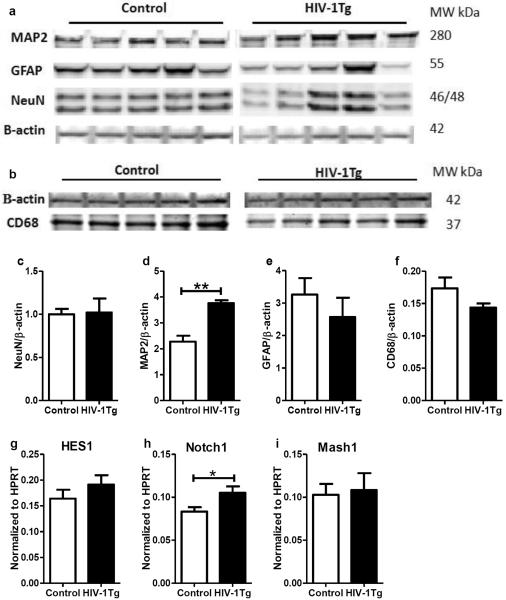
To evaluate the changes of neurons and glial cells in the thalamus, we examined the protein expression of neuronal nuclei (NeuN, neuron-specific nuclear protein), microtubule-associated protein 2 (MAP2, a neuron-specific cytoskeletal protein, related to the formation of neuronal dendrites), glia fibrillary acidic protein (GFAP, an astrocyte marker), and cluster of differentiation 68 (CD68, a microglia marker). As shown in Fig 6, no differences were found in the expression of NeuN, GFAP and CD68 between the two groups (Fig 6c, e and f), while a higher expression level of MAP2 was found in the thalamus of the HIV-1Tg rats (+65%, p=0.0079, panel d).
Figure 6. Expression of neuron and glial cell markers in the thalamus of F344 (control) and HIV-1Tg rats.
(a) The western blot image of astrocyte marker glia fibrillary acidic protein (GFAP), neuronal marker neuronal nuclei (NeuN) and microtubule-associated protein-2 (MAP2). (b) The western blot image of microglia marker CD68. (c–f) The relative quantification of protein expression in (a) and (b). (g–i) Gene expression levels of transcriptional factors HES1, Notch1, and Mash1, respectively. * p<0.05, ** p<0.01, Mann Whitney test.
The mRNA levels of several translational factors relevant to neuronal differentiation were measured using real-time PCR. These factors were: neuronal transcription factor hairy and enhancer of split 1 (HES1), MAP2 regulation factor Notch1, and GABAergic neuron transcription factor achaete-scute family bHLH transcription factor 1(Mash1). While the gene expression of HES1 and Mash1 did not change, Notch1 mRNA level was significantly higher in the HIV-1Tg group (+26%, p=0.032).
Correlations
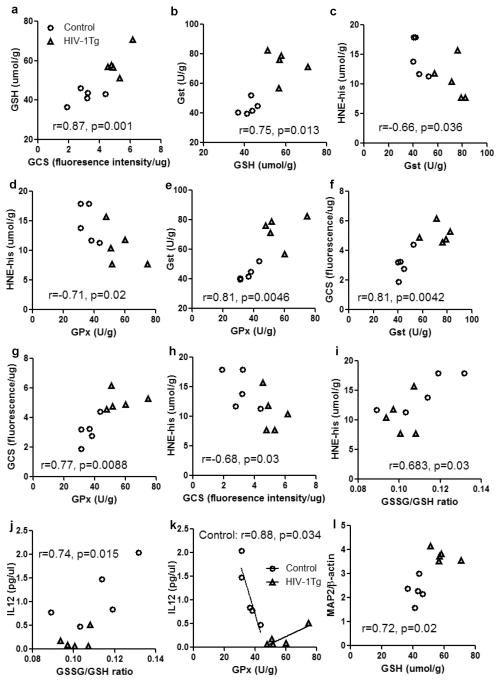
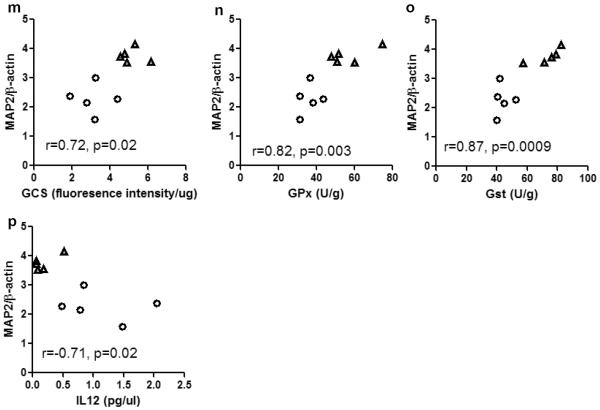
Correlations between the parameters are summarized in Fig 7. GSH concentration positively correlated with GCS activity (r=0.87, p=0.001, Fig 7a), suggesting the increased GCS activity is a major causative factor of GSH upregulation. GSH concentration also positively correlated with GST activity (r=0.75, p=0.01, Fig 7b), thus GST equipped with sufficient GSH can effectively execute antioxidant function, as evidenced by a negative correlation between GST and HNE (Fig 7c). Similar negative correlation was found between GPx and HNE (Fig 7d), suggesting a significant role of GPx in preventing lipid peroxidation in the thalamus. GST and GPx also showed a strong positive correlation (Fig 7e). GCS activity positively correlated with GST and GPx activities, and negatively correlated with HNE concentration (Fig 7f–h). Both HNE and IL12 positively correlated with GSSG/GSH ratio, suggesting they are responsive to the shifting of redox status in the thalamus. Interestingly, GPx seemed to have different functions in regulating IL12 production in the two groups of animals, i.e. it negatively correlated with IL12 in the control rats, but had a marginal (p=0.051) positive correlation with IL12 in the HIV-1Tg rats. The slopes of the regression lines of these two groups are significantly different (p=0.002). Furthermore, the protein expression of MAP2 positively correlated with GSH, GCS, GPx, and GST (Fig 7l–o), but negatively correlated with IL12 (Fig 7p), indicating the neuronal differentiation in the thalamus may be stimulated by GSH-centered antioxidants, but inhibited by neuroinflammation.
Figure 7. Correlations among parameters involved in GSH metabolism and antioxidant function, oxidative stress, neuroinflammation, and neuronal differentiation in the thalamus of F344 rats (control) and HIV-1Tg rats.
Control = open circle, HIV-1Tg = filled triangle. Pearson correlation test was used in the statistical analysis. Abbreviation: GSH, glutathione; GCS, γ-glutamylcysteine synthetase; GST, glutathione-S-transferase; GPx, glutathione peroxidase; HNE-his, 4-hydroxynonenal and histidine conjugates; IL12, interleukin 12; MAP2, microtubule-associated protein-2.
DISCUSSION
In conjunction with our previous study on younger HIV-1Tg rats (Pang et al. 2013), the present study demonstrated the age-dependent changes of the GSH-centered antioxidant system in the thalamus of these HIV-1Tg rats, and the significance of this system in preventing oxidative stress and regulating neuroinflammation and neuronal differentiation in this brain region.
GSH regulation in the thalamus
The thalamus remains a less visited brain region in the research field of HIV neuroscience. Our previous study showed that at 3 months, HIV-1Tg caused a 30% decrease of GSH content in the thalamus, without increase of lipid peroxidation (Pang et al. 2013). This suggests that GSH is in the front line of antioxidant defense, and consumption of GSH may have contributed to the neutralization of excessive reactive oxygen species (ROS) generated by HIV viral proteins. At 10 months, the GSSG concentration of the HIV-1Tg rats increased by 27%, implicating increased transfer of reducing power from GSH to GSH-dependent antioxidant enzymes, such as GPx and GST. The increase of GSSG in the HIV-1Tg rats was balanced by the increase of rGSH, and the GSSG/GSH ratio remained unchanged compared with the control rats.
The increase of GCS activity was a causative factor for GSH upregulation in the thalamus at 10 months. But the activity of GCS did not correlate with the gene or protein expression of its subunits, suggesting GCS activity in the thalamus is regulated post-translationally. Increased GCS activity also has been reported in the liver and muscle tissues of SIV-infected macaques (Gross et al. 1996), whereas lower gene and protein expression of GCS-LC has been reported in the liver of Tat transgenic mice (Choi et al. 2000). However none of the studies measured both gene/protein expression and enzyme activity of GCS, therefore whether HIV proteins can post-translationally regulate GCS in other tissues is not known.
Previously reported associations between HIV infection /HIV viral protein exposure and GSH depletion in the CSF (Castagna et al. 1995), hippocampus, posterior cerebral cortex (Tang et al. 2009), and brain microvascular endothelial cells (Price et al. 2005; Toborek et al. 2003) are in contrast to the findings in our present and previous studies (Pang et al. 2013) that collectively highlight biphasic changes of GSH concentration in the thalamus of the HIV-1Tg rats. Such differences may be explained by tissue specific regulatory mechanisms, and the time points selected in each study.
HIV and GGT regulation
Increased GGT activity was found in the periphery of both HIV naïve patients and patients with antiretroviral therapy (Li et al. 2007; Vayà et al. 2012), and use of the antiretroviral drug nevirapine increased GGT activity in liver (Li et al. 2007). Region-specific elevation of GGT activity was found in the brain with Parkinson's disease (Sian et al. 1994). GGT activity was found increased in the thalamus of the HIV-1Tg rats at 3 months, which was consistent with GGT7 gene upregulation (Pang et al. 2013). No changes of GGT activity and GGT7 gene expression were observed at 10 months, but the protein expression of GGT7 was significantly decreased in the HIV-1Tg rats compared with the Control rats. GGT7 was found only expressed in the nervous system (Yamaguchi et al. 2000). Our pilot study showed the mRNA level of GGT7 was ~20-fold higher than the other GGT isoforms in the brain. As a major GGT isoform, the decrease of GGT7 protein expression in the thalamus of HIV-1Tg rats may contribute to compromised GSH metabolism as the rats continue to age.
Oxidative stress, neuroinflammation, and neuronal differentiation
HIV-induced lipid peroxidation in the brain has been reported to be region-specific (Sacktor et al. 2004) and associated with the severity of HAND (Bandaru et al. 2007). Exposing brain endothelial cells to Tat caused ROS generation dose-dependently (Toborek et al. 2003). In the present study the HNE-his concentration in the thalamus of the HIV-1Tg rats was similar to the control rats, and the level of lipid peroxidation negatively correlated with GPx and GST activities, demonstrating the effectiveness of the GSH-dependent antioxidant enzymes in the amelioration of oxidative stress during HIV transgenesis.
HIV seropositive patients showed decreased GPx activity in the CSF (Velazquez et al. 2009), and increased GPx activity in the plasma (Look et al. 1997; Ogunro et al. 2006; Stephensen et al. 2007; Velazquez et al. 2009). GPx activity decreased in brain endothelial cells exposed to gp120 or Tat (Price et al. 2005), in contrast, GPx activity increased in cortical cells after a 2-hour gp120 exposure, and normalized after 4 hours and maintained at the baseline after 24 hours (Brooke et al. 2002). Therefore the responses of GPx activity to HIV seem to be tissue- or cell type-specific. Our studies showed age-dependent changes of GPx activity in the thalamus of the HIV-1Tg rats, from no response at 3 months to upregulation at 10 months, and this upregulation in combination with higher availability of GSH rendered significant protection against lipid peroxidation. The discrepancy between GPx protein expressions and enzyme activity observed in this study may be due to post-translational regulation.
Lower GST activity was reported in the plasma of HIV patients (Deresz et al. 2010) and in the brain of patients with Alzheimer's disease (Lovell et al. 1998), and increased GST activity prevented neuron loss in an animal model of Parkinson's disease (Whitworth et al. 2005). Similar to the changes of GPx observed in our studies, thalamus GST showed age-dependent changes in the HIV-1Tg rats, and has a significant role in preventing oxidative stress.
Increased IL12 production can be induced by GSH in monocytes (Guerra et al. 2011), by GSH precursor in splenocytes of Tat-exposed mice (Fraternale et al. 2011), and by low dose GSH precursor in macrophages (Alam et al. 2010). IL12p40 knockout mice showed decreased GPx activity in lungs (Gutierrez et al. 2008). However, patients with systemic lupus erythematosus showed low GSH and GPx activity but high IL12 concentrations in erythrocyte hemolysate (Shah et al. 2010). These literatures seem to implicate interrelationship between IL12, GSH, and GPx, although the exact mechanism is not known. In line with these records, the concentration of IL12 in the thalamus of HIV-1Tg rats responded to the redox status of GSH and the activity of GPx; interestingly, HIV-1 transgenesis changed the direction of the correlation between IL12 and GPx (Fig 7k), suggesting the interactions between GPx and IL12 may be regulated by pathological conditions.
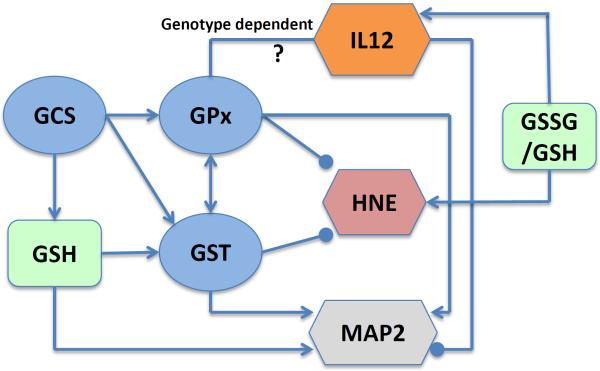
The protein expression of GFAP, CD68 and NeuN were similar in the thalamus of the F344 rats and the HIV-1Tg rats, suggesting the lack of glial activation and neuron loss in the transgenic rats at this time point. Several studies have shown decreased neuronal dendritic branches in the hippocampus of HIV-1 infected patients (Everall et al. 1999; Masliah et al. 1997; Sa et al. 2004), but our result showed increased MAP2 protein in the thalamus of the HIV-1Tg rats, implicating a higher level of neuronal differentiation in this brain region. Furthermore, the increase of Notch1 gene expression implicates possible enhancement of the MAP2 promoter activity (Bhat et al. 2006). Correlation analysis also indicates that MAP2 expression may be enhanced by GSH and the activities of GCS, GPx, and GST, but inhibited by IL12. The potential intricate links among GSH metabolism, oxidative stress, inflammation, and neuronal development in the rat thalamus suggested by this study are outlined in Fig 8.
Figure 8. Relationship among GSH concentration, GSH redox status (GSSG/GSH), GSH-centered enzymes (GCS, GPx, GST), and markers of oxidative stress (HNE), inflammation (IL12) and neuronal differentiation (MAP2) in the rat thalamus.
Lines end with an arrow indicate positive correlation, and lines end with a point indicate negative correlation. Abbreviation: GSH, glutathione; GCS, γ-glutamylcysteine synthetase; GST, glutathione-S-transferase; GPx, glutathione peroxidase; HNE, 4-hydroxynonenal; IL12, interleukin 12; MAP2, microtubule-associated protein-2.
CONCLUSION
In summary, this study systematically investigated the changes of GSH-centered antioxidant system in the thalamus of HIV-1Tg rats, highlighted the significance of this system in the prevention of oxidative stress, and in the regulation of neuroinflammation and neuronal differentiation. This study in conjunction with our previous report (Pang et al. 2013) showed age-dependent responses of the GSH system to HIV-1 transgenesis, implicating adaptive capacity of the thalamus to HIV-induced oxidative stress.
Supplementary Material
(a) Western blot image of multidrug resistance-associated proteins 1 (MRP1). (b) Relative quantification of MRP1 protein expression. (c–f) Relative gene expression levels of MRP isoforms 1 and 2, system xc-catalytic domain (xCT), and excitatory amino acid carrier 1 (EAAC1), respectively.
ACKNOWLEDGEMENT
We would like to thank Dr. Linda Chang (Department of Medicine, University of Hawaii) for her constructive review of this manuscript. This study was made possible by NIH grants 5P20RR016467-11 and 8P20GMl03466-11 (INBRE II), 5G12RR003061 and 8G12MD007601 (RCMI/BRIDGES), and R24 PAR09-011 (DIDARP). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
CONFLICT OF INTEREST The authors declare that there are no conflicts of interest.
REFERENCES
- Alam K, Ghousunnissa S, Nair S, Valluri VL, Mukhopadhyay S. Glutathione-redox balance regulates c-rel-driven IL-12 production in macrophages: possible implications in antituberculosis immunotherapy. J Immunol. 2010;184:2918–29. doi: 10.4049/jimmunol.0900439. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Watabe M, Nakaki T. Regulation of neuronal glutathione synthesis. J Pharmacol Sci. 2008;108:227–38. doi: 10.1254/jphs.08r01cr. [DOI] [PubMed] [Google Scholar]
- Bandaru VV, McArthur JC, Sacktor N, Cutler RG, Knapp EL, et al. Associative and predictive biomarkers of dementia in HIV-1-infected patients. Neurology. 2007;68:1481–7. doi: 10.1212/01.wnl.0000260610.79853.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat KM, Maddodi N, Shashikant C, Setaluri V. Transcriptional regulation of human MAP2 gene in melanoma: role of neuronal bHLH factors and Notch1 signaling. Nucleic Acids Res. 2006;34:3819–32. doi: 10.1093/nar/gkl476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke SM, McLaughlin JR, Cortopassi KM, Sapolsky RM. Effect of GP120 on glutathione peroxidase activity in cortical cultures and the interaction with steroid hormones. J Neurochem. 2002;81:277–84. doi: 10.1046/j.1471-4159.2002.00825.x. [DOI] [PubMed] [Google Scholar]
- Buhl R, Jaffe HA, Holroyd KJ, Wells FB, Mastrangeli A, et al. Systemic glutathione deficiency in symptom-free HIV-seropositive individuals. Lancet. 1989;2:1294–8. doi: 10.1016/s0140-6736(89)91909-0. [DOI] [PubMed] [Google Scholar]
- Castagna A, Le Grazie C, Accordini A, Giulidori P, Cavalli G, et al. Cerebrospinal fluid S-adenosylmethionine (SAMe) and glutathione concentrations in HIV infection: effect of parenteral treatment with SAMe. Neurology. 1995;45:1678–83. doi: 10.1212/wnl.45.9.1678. [DOI] [PubMed] [Google Scholar]
- Choi J, Liu RM, Kundu RK, Sangiorgi F, Wu W, et al. Molecular mechanism of decreased glutathione content in human immunodeficiency virus type 1 Tat-transgenic mice. J Biol Chem. 2000;275:3693–8. doi: 10.1074/jbc.275.5.3693. [DOI] [PubMed] [Google Scholar]
- Deresz LF, Sprinz E, Kramer AS, Cunha G, de Oliveira AR, et al. Regulation of oxidative stress in response to acute aerobic and resistance exercise in HIV-infected subjects: a case-control study. AIDS Care. 2010;22:1410–7. doi: 10.1080/09540121003758549. [DOI] [PubMed] [Google Scholar]
- Everall IP, Heaton RK, Marcotte TD, Ellis RJ, McCutchan JA, et al. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol. 1999;9:209–17. doi: 10.1111/j.1750-3639.1999.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraternale A, Paoletti MF, Dominici S, Buondelmonte C, Caputo A, et al. Modulation of Th1/Th2 immune responses to HIV-1 Tat by new pro-GSH molecules. Vaccine. 2011;29:6823–9. doi: 10.1016/j.vaccine.2011.07.101. [DOI] [PubMed] [Google Scholar]
- Gross A, Hack V, Stahl-Hennig C, Droge W. Elevated hepatic gamma-glutamylcysteine synthetase activity and abnormal sulfate levels in liver and muscle tissue may explain abnormal cysteine and glutathione levels in SIV-infected rhesus macaques. AIDS Res Hum Retroviruses. 1996;12:1639–41. doi: 10.1089/aid.1996.12.1639. [DOI] [PubMed] [Google Scholar]
- Guerra C, Morris D, Sipin A, Kung S, Franklin M, et al. Glutathione and adaptive immune responses against Mycobacterium tuberculosis infection in healthy and HIV infected individuals. PLoS One. 2011;6:e28378. doi: 10.1371/journal.pone.0028378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez JG, Valdez SR, Di Genaro S, Gomez NN. Interleukin-12p40 contributes to protection against lung injury after oral Yersinia enterocolitica infection. Inflamm Res. 2008;57:504–11. doi: 10.1007/s00011-008-7162-2. [DOI] [PubMed] [Google Scholar]
- Li H, Zheng YH, Shen Z, Liu M, Liu C, et al. Evaluation for two-year highly active antiretroviral therapy in Chinese HIV-1 infection patients. Zhonghua Yi Xue Za Zhi. 2007;87:2973–6. [PubMed] [Google Scholar]
- Look MP, Rockstroh JK, Rao GS, Kreuzer KA, Barton S, et al. Serum selenium, plasma glutathione (GSH) and erythrocyte glutathione peroxidase (GSH-Px)-levels in asymptomatic versus symptomatic human immunodeficiency virus-1 (HIV-1)-infection. Eur J Clin Nutr. 1997;51:266–72. doi: 10.1038/sj.ejcn.1600401. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Xie C, Markesbery WR. Decreased glutathione transferase activity in brain and ventricular fluid in Alzheimer's disease. Neurology. 1998;51:1562–6. doi: 10.1212/wnl.51.6.1562. [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42:963–72. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Ogunro PS, Ogungbamigbe TO, Elemie PO, Egbewale BE, Adewole TA. Plasma selenium concentration and glutathione peroxidase activity in HIV-1/AIDS infected patients: a correlation with the disease progression. Niger Postgrad Med J. 2006;13:1–5. [PubMed] [Google Scholar]
- Pang X, Panee J, Liu X, Berry MJ, Chang SL, Chang L. Regional Variations of Antioxidant Capacity and Oxidative Stress Responses in HIV-1 Transgenic Rats With and Without Methamphetamine Administration. J Neuroimmune Pharmacol. 2013;8:691–704. doi: 10.1007/s11481-013-9454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TO, Ercal N, Nakaoke R, Banks WA. HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res. 2005;1045:57–63. doi: 10.1016/j.brainres.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Price TO, Uras F, Banks WA, Ercal N. A novel antioxidant N-acetylcysteine amide prevents gp120- and Tat-induced oxidative stress in brain endothelial cells. Exp Neurol. 2006;201:193–202. doi: 10.1016/j.expneurol.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Sa MJ, Madeira MD, Ruela C, Volk B, Mota-Miranda A, Paula-Barbosa MM. Dendritic changes in the hippocampal formation of AIDS patients: a quantitative Golgi study. Acta Neuropathol. 2004;107:97–110. doi: 10.1007/s00401-003-0781-3. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Haughey N, Cutler R, Tamara A, Turchan J, et al. Novel markers of oxidative stress in actively progressive HIV dementia. J Neuroimmunol. 2004;157:176–84. doi: 10.1016/j.jneuroim.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Shah D, Kiran R, Wanchu A, Bhatnagar A. Oxidative stress in systemic lupus erythematosus: relationship to Th1 cytokine and disease activity. Immunol Lett. 2010;129:7–12. doi: 10.1016/j.imlet.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Sian J, Dexter DT, Lees AJ, Daniel S, Jenner P, Marsden CD. Glutathione-related enzymes in brain in Parkinson's disease. Ann Neurol. 1994;36:356–61. doi: 10.1002/ana.410360306. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Roederer M, Israelski DM, Bubp J, Mole LA, et al. Intracellular glutathione levels in T cell subsets decrease in HIV-infected individuals. AIDS Res Hum Retroviruses. 1992;8:305–11. doi: 10.1089/aid.1992.8.305. [DOI] [PubMed] [Google Scholar]
- Stephensen CB, Marquis GS, Douglas SD, Kruzich LA, Wilson CM. Glutathione, glutathione peroxidase, and selenium status in HIV-positive and HIV-negative adolescents and young adults. Am J Clin Nutr. 2007;85:173–81. doi: 10.1093/ajcn/85.1.173. [DOI] [PubMed] [Google Scholar]
- Tang H, Lu D, Pan R, Qin X, Xiong H, Dong J. Curcumin improves spatial memory impairment induced by human immunodeficiency virus type 1 glycoprotein 120 V3 loop peptide in rats. Life Sci. 2009;85:1–10. doi: 10.1016/j.lfs.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Toborek M, Lee YW, Pu H, Malecki A, Flora G, et al. HIV-Tat protein induces oxidative and inflammatory pathways in brain endothelium. J Neurochem. 2003;84:169–79. doi: 10.1046/j.1471-4159.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- Vayà A, De la Fuente J, Montero M, Perez R, Ricart J. Erythrocyte deformability in naÖve HIV-infected patients. Clinical hemorheology and microcirculation. 2012;51:235–41. doi: 10.3233/CH-2011-1529. [DOI] [PubMed] [Google Scholar]
- Velazquez I, Plaud M, Wojna V, Skolasky R, Laspiur JP, Melendez LM. Antioxidant enzyme dysfunction in monocytes and CSF of Hispanic women with HIV-associated cognitive impairment. J Neuroimmunol. 2009;206:106–11. doi: 10.1016/j.jneuroim.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth AJ, Theodore DA, Greene JC, Benes H, Wes PD, Pallanck LJ. Increased glutathione S-transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson's disease. Proc Natl Acad Sci U S A. 2005;102:8024–9. doi: 10.1073/pnas.0501078102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Takei N, Araki K, Ishii K, Nagano T, et al. Molecular characterization of a novel gamma-glutamyl transpeptidase homologue found in rat brain. J Biochem. 2000;128:101–6. doi: 10.1093/oxfordjournals.jbchem.a022718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Western blot image of multidrug resistance-associated proteins 1 (MRP1). (b) Relative quantification of MRP1 protein expression. (c–f) Relative gene expression levels of MRP isoforms 1 and 2, system xc-catalytic domain (xCT), and excitatory amino acid carrier 1 (EAAC1), respectively.