Abstract
Atrial fibrillation (AF) has long been associated with a heightened risk of ischemic stroke and systemic thromboembolism, but recent data require a re-evaluation of our understanding of the nature of this relationship. New findings about the temporal connection between AF and stroke, alongside evidence linking markers of left atrial abnormalities with stroke in the absence of apparent AF, suggest that left atrial thromboembolism may occur even without AF. These observations undermine the hypothesis that the dysrhythmia that defines AF is necessary and sufficient to cause thromboembolism. In this commentary, we instead suggest that the substrate for thromboembolism may often be the anatomic and physiological atrial derangements associated with AF. Therefore, our understanding of cardioembolic stroke may be more complete if we shift our representation of its origin from AF to the concept of atrial cardiopathy.
Keywords: Arrhythmia, atrial abnormality, atrial cardiopathy, atrial fibrillation, atrial substrate, cardiac embolism, cerebrovascular disease, embolic stroke, stroke
Background
AF affects approximately 33 million people worldwide, making it the most common sustained cardiac arrhythmia.1 Its incidence rises steeply with age, and given increasing life expectancy and the anticipated aging of the population, the next several decades may see a doubling in the burden of AF;2 as of now, more than 10% of people over the age of 80 years have AF.2 The incidence of AF-related stroke in some high-income countries has nearly tripled in the past 3 decades and may triple again by 2050.3 Strokes associated with AF cost more and result in greater disability and mortality than other types of stroke, including other causes of cardiac embolism.4, 5 Partly as a result of its thromboembolic complications, AF imposes a substantial societal cost; for example, it accounts for approximately 1% of the entire budget of the U.K. National Health Service.6 Recent data from globally representative surveys support the impression of an increasing burden of AF worldwide.1
The thromboembolic risk associated with AF, which represents a major component of the overall burden of AF, can be substantially reduced with the use of anticoagulant therapies. Numerous randomized clinical trials have demonstrated that anticoagulation with vitamin-K antagonists reduces the relative risk of stroke and systemic embolism by approximately 50% as compared with aspirin.7 Over the past few years, several novel oral anticoagulant drugs have been approved that provide fixed dosing regimens and fewer drug-drug interactions than vitamin-K antagonists. In a meta-analysis of randomized trials, these new drugs resulted in similar rates of ischemic stroke, higher rates of gastrointestinal bleeding, lower rates of intracranial hemorrhage, and lower mortality than warfarin.8 In a randomized comparison against aspirin in patients with AF, one of these novel oral anticoagulant drugs reduced the risk of ischemic stroke by more than half without a substantial increase in bleeding risk.9
Taken together, these observations indicate that AF is an increasingly common condition associated with a disabling complication whose risk can be substantially reduced with appropriate use of existing medications. This conclusion underscores the importance of AF as a target for primary and secondary stroke prevention.
Atrial Fibrillation and Cryptogenic Stroke
One of the challenges of effectively preventing AF-related stroke is that AF does not always come to light before a patient has had a stroke. A substantial proportion of patients with AF-related stroke have not received a diagnosis of AF prior to the stroke,3 thereby precluding opportunities to institute anticoagulant therapy for primary stroke prevention. Given the often paroxysmal nature of AF, it has long been recognized that a patient with underlying AF may be in normal sinus rhythm at the time of presentation with stroke. Although cardioembolic stroke often presents with specific clinical features,10 the transient nature of AF often leads to a failure to recognize AF as the cause of stroke. Highlighting the challenges of identifying AF as a cause of stroke, a study of stroke patients with pacemakers indicated that these patients manifested AF for fewer than 2 hours per day and had no AF at all on more than 90% of days.11 Furthermore, a prospective study of patients with recently implanted pacemakers or defibrillators demonstrated that even a single 6-minute episode of asymptomatic AF was associated with a more than 2-fold higher risk of stroke,12 which supports the hypothesis that brief and difficult-to-diagnose episodes of AF may be responsible for some proportion of cryptogenic strokes.
The possibility of undetected AF in stroke patients has important implications, because one-third of ischemic strokes are of unknown cause even in the modern era of neuroimaging.13 Based on the radiographic pattern of brain infarction, many of these cryptogenic strokes appear to have arisen from a distant embolism; this clinical impression is so strong that a recent paper suggested labeling these as “embolic strokes of undetermined source.”14 The high prevalence of cryptogenic stroke presents an important target for better stroke preventions strategies, because optimal control of stroke risk factors is difficult in the face of unknown stroke etiologies; we cannot be sure that we are maximally treating potential causes of stroke when we are unsure of what all the causes are.
The notion that occult paroxysmal AF may prove to be the source of many cryptogenic strokes is particularly appealing given the therapeutic implications outlined above; namely, the identification of AF would result in a change to anticoagulant therapy instead of the antiplatelet drugs that are standard for secondary stroke prevention.15 This therapeutic implication has driven the increasingly common practice of performing prolonged heart-rhythm monitoring in patients with otherwise unexplained strokes. Dozens of observational studies over the past several decades have reported that this practice leads to a new diagnosis of AF in about 10% of stroke patients.16 These findings were corroborated in two recent randomized clinical trials comparing routine clinical follow-up versus a strategy of at least several weeks of continuous heart-rhythm monitoring. Together, these trials indicated that one previously undiagnosed case of AF will be detected for every 10 cryptogenic stroke patients screened with monitoring.17, 18
On the basis of these studies, ambulatory heart-rhythm monitoring is likely to be used increasingly in the evaluation of patients with cryptogenic stroke. However, long-term follow-up of one of these two studies found that only 30% of cryptogenic stroke patients manifested any AF even after 3 years of continuous heart-rhythm monitoring via an implantable loop recorder.18 Therefore, AF cannot explain the majority of cryptogenic strokes, suggesting that other sources of thromboembolism are at play. Furthermore, a recent analysis of the temporal link between AF and stroke found that 31% of patients with both AF and stroke did not have any AF prior to their stroke and only manifested AF for the first time afterward, despite undergoing a median 8 months of continuous heart-rhythm monitoring before the stroke.19 As the authors of this analysis point out in their discussion, this finding undermines the cause-effect interpretation of the long-established association between AF and stroke and indicates that the association must partly be explained by factors other than the dysrhythmia itself. While some of the association between AF and stroke may be explained by residual confounding from shared risk factors such as hypertension and atherosclerosis, a cardiac process is still likely to play a substantial role in the association between AF and stroke because of the clearly embolic-appearing character of most cryptogenic strokes. Often this occult cardiac process may involve an establish but difficult-to-prove source such as paradoxical embolism through a patent foramen ovale, but many patients with cryptogenic stroke lack evidence of any such established sources. What then are potential sources of currently unrecognized cardiac thromboembolism?
Evidence for Left Atrial Thromboembolism in the Absence of Atrial Fibrillation
Several emerging lines of evidence suggest that left atrial thromboembolism can occur even in the absence of AF. In other words, the dysrhythmia that defines AF may not be a necessary step in the pathogenesis of left atrial thromboembolism. AF has been associated with several atrial derangements including endothelial dysfunction,20 fibrosis,21 impaired myocyte function,22 and chamber dilatation.23 Possibly these other atrial derangements are responsible for left atrial thromboembolism, not just the dysrhythmia itself.
In support of this hypothesis, other atrial dysrhythmias besides AF have been associated with stroke. In a population-based, prospective study, frequent premature atrial contractions during 48-hour Holter monitoring were associated with a heightened risk of subsequent stroke, even after censoring patients at the time of AF diagnoses.24 Furthermore, we found an association between paroxysmal supraventricular tachycardia and subsequent stroke in a population of patients without apparent AF.25 Lastly, frequent premature atrial contractions occur more commonly in patients with cryptogenic stroke than in those with non-cardioembolic stroke subtypes such as large-artery atherosclerosis or small-vessel occlusion.26 These findings support the hypothesis that underlying atrial derangements are implicated in the pathogenesis of many cases of cryptogenic stroke, although it should be noted that these atrial electrical derangements simply serve as risk factors for occult AF.
Other investigations have linked the presence of left atrial abnormalities with heightened stroke risk even in the absence of any overt atrial dysrhythmia at all. P-wave terminal force in electrocardiogram (ECG) lead V1 (PTFV1)—a long established marker of left atrial pathophysiological processes such as fibrosis, elevated filling pressures, and dilatation27–30—has been associated with incident ischemic stroke31, 32 and neuroimaging evidence of vascular brain injury.33 One apparent explanation for this finding is that PTFV1 simply predicts the future occurrence of AF,34 but in two studies there was a notable absence of any change in the association between PTFV1 and stroke or vascular brain injury regardless of whether the models adjusted for the presence of AF.31, 33 Since the inclusion of a mediator, even one imperfectly detected, should attenuate the relationship between a predictor variable and the outcome, the absence of attenuation in these models suggests that PTFV1 signals left atrial pathophysiological processes that form a substrate for thromboembolism via pathways other than the dysrhythmia that characterizes AF.
These results are supported by findings of associations between other markers of atrial dysfunction and stroke risk (Table 1). Left atrial size as assessed by echocardiography has been associated with a heightened risk of ischemic stroke, even in patients without AF35, 36 or after adjusting for the presence of AF.37, 38 Left atrial size and contractile function have also been linked with the radiographic burden of vascular brain injury, again independently of AF.39 Additionally, serum levels of N-terminal pro-brain natriuretic peptide (NT-proBNP) have been associated with ischemic stroke even after adjustment for AF.40, 41
Table 1.
Summary of Studies Demonstrating an Association between Markers of Left Atrial Abnormality and Incident Stroke Independently of Atrial Fibrillation
| Marker | Authors | Year | Outcome | Association | |
|---|---|---|---|---|---|
| Not Adjusted for AF | Adjusted for AF | ||||
| ECG markers | |||||
| Frequent PACs | Binici et al24 | 2010 | Stroke | 1.79 (1.14–2.81)a | 1.73 (1.09–2.75)a |
| PSVT | Kamel et al25 | 2013 | Stroke | N/A | 2.10 (1.69–2.62)b |
| PTFV1 | Kamel et al31 | 2014 | Stroke | 1.22 (1.03–1.45)c | 1.21 (1.02–1.44)c |
| PTFV1 | Kamel et al33 | 2015 | SBI | 1.09 (1.04–1.16)d | 1.09 (1.04–1.15)d |
| PTFV1 | Kamel et al33 | 2015 | WMH | 0.05 (0–0.10)e | 0.05 (0–0.10)e |
| Echocardiographic markers | |||||
| Left atrial size | Benjamin et al38 | 1995 | Stroke | N/A | 2.4 (1.6–3.7)f |
| Left atrial size | Di Tullio et al37 | 1999 | Stroke | N/A | 1.47 (1.03–2.11)g |
| Left atrial volume | Barnes et al36 | 2004 | Stroke | N/A | 1.63 (1.08–2.46)h |
| Left atrial size | Karas et al35 | 2012 | Stroke | N/A | 1.35 (1.12–1.62)i |
| Left atrial size | Russo et al39 | 2013 | SBI | N/A | 1.37 (1.04–1.80)j |
| Left atrial size | Russo et al39 | 2013 | WMH | N/A | 0.12k |
| Left atrial function | Russo et al39 | 2013 | SBI | N/A | 0.67 (0.50–0.90)l |
| Left atrial function | Russo et al39 | 2013 | WMH | N/A | −0.09m |
| Serum markers | |||||
| NT-proBNP | Folsom et al40 | 2013 | Stroke | N/A | 2.92 (2.04–4.17)n |
| NT-proBNP | Cushman et al41 | 2014 | Stroke | N/A | 2.9 (1.8–4.5)o |
Abbreviations: AF, atrial fibrillation; ECG, electrocardiographic; NT-proBNP, N-terminal pro-brain natriuretic peptide; PSVT, paroxysmal supraventricular tachycardia; PACs, premature atrial contractions; PTFV1, P-wave terminal force in lead V1; SBI, silent brain infarction on magnetic resonance imaging; WMH, white matter hyperintensities on magnetic resonance imaging.
Data represent the hazard ratio (HR) and 95% confidence interval (CI) for the primary outcome of death or stroke.
Data represent the HR (95% CI).
Data represent the HR (95% CI) per 1-standard deviation (SD) increase in PTFV1.
Data represent the HR (95% CI) per 1-SD increase in PTFV1.
Data represent the increase on a 10-point scale of white matter disease severity per 1-SD increase in PTFV1.
Data represent the HR (95% CI) per 10-mm increase in left atrial size in men. The association was not significant in women (HR, 1.4; 95% CI, 0.9–2.1).
Data represent the odds ratio (OR) and 95% CI per 10 mm/1.7 m2 increase in the left atrial diameter divided by body surface area (left atrial index).
Data represent the HR (95% CI) for left atrial volume ≥32 ml/m2.
Data represent the HR (95% CI) per 1-SD increase in left atrial diameter.
Data represent the OR (95% CI) for each 1-SD increase in the left atrial minimum volume.
Data represent the standardized parameter estimate (P < 0.01) for the correlation between left atrial minimum volume and the log of WMH volume.
Data represent the OR (95% CI) for each 1-SD increase in the left atrial ejection fraction.
Data represent the standardized parameter estimate (P < 0.05) for the correlation between left atrial fraction and the log of WMH volume.
Data represent the HR (95% CI) for the highest quintile of NT-proBNP.
Data represent the HR (95% CI) for the highest quartile of NT-proBNP.
The Concept of Atrial Cardiopathy as a Cause of Thromboembolism
Although AF undoubtedly worsens atrial tissue pathology through a variety of mechanisms, certain instances of genetic disorders provide compelling evidence that underlying atrial tissue abnormalities can be the cause of AF rather than just the effect. For example, several cases have been reported of young patients with primary muscular dystrophy whose initial manifestation was AF,42 and atrial muscle involvement has been recognized as a frequent initial manifestation of muscular dystrophy.43 AF often arises as a consequence of underlying cardiac abnormalities,44 and then worsens these abnormalities through a variety of mechanisms such as atrial remodeling, leading to the acknowledgment that AF is both a cause and a consequence of atrial cardiopathy.45, 46 A recent study provides important proof for this concept: patients who underwent intensive vascular risk factor management after catheter ablation of AF had a significant reduction in left atrial size and a lower rate of AF recurrence than patients whose risk factors were not managed as intensively.47 In fact, it is likely that a disease characterized by sustained AF as a purely electrical phenomenon is vanishingly rare.21
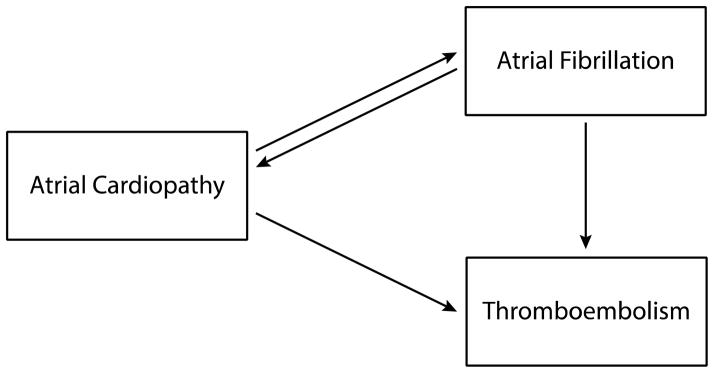
We suggest that this basic insight about the pathogenesis of AF can be used to fruitfully reframe our conceptual understanding of AF-related thromboembolism. If AF is sometimes the consequence of atrial cardiopathy, then it is conceivable that such an atrial cardiopathy might result in thromboembolism before it results in AF. Rather than viewing AF as the necessary and sufficient cause of the thromboembolic risk seen in patients with AF, it may be more helpful to view both AF and thromboembolism as common manifestations of an underlying atrial cardiopathy. In this formulation, the driving force of thromboembolism is not simply the dysrhythmia but rather a host of underlying pathological tissue changes. The credibility of such a scenario is supported by the recent discovery of a homozygous mutation of the natriuretic peptide precursor A gene that results in adult-onset atrial dilatation and eventual atrial electrophysiological standstill. In a group of patients with this disorder, thromboembolic complications were common even though AF was not evident on invasive electrophysiological studies,48 indicating that progressive atrial tissue pathology resulted in thromboembolism even in the absence of AF, which supports the clinical findings detailed above about the relationship of different markers of atrial abnormality with stroke risk even in the absence of AF.31, 38, 40 Nevertheless, AF remains an important component of thromboembolic risk in our formulation because it likely signals a more severe or later-stage form of atrial cardiopathy, and because the dysrhythmia feeds back to both worsen the tissue changes and worsen left atrial contractile function, thereby increasing the risk of thromboembolism even further (Figure 1).
Figure 1.
Atrial cardiopathy as a stroke risk factor. In this formulation, the driving force of thromboembolism is not simply atrial fibrillation but rather underlying atrial tissue changes, with the dysrhythmia feeding back to both worsen the tissue changes and worsen left atrial contractile function, thereby increasing the risk of thromboembolism even further.
Atrial Cardiopathy May Explain Several Paradoxes About Atrial Fibrillation and Stroke
The formulation of atrial cardiopathy as a stroke risk factor helps to explain several otherwise puzzling observations about AF and stroke (Table 2). First, young and otherwise healthy men with clinically apparent AF do not appear to face a significantly higher risk of stroke than AF-free controls,49 while even several minutes of subclinical AF in older patients with vascular risk factors markedly increases their relative risk of stroke.12 In patients with AF, the remarkable degree of risk modification imparted by vascular comorbidities50 supports the hypothesis that stroke risk is driven by the underlying tissue substrate rather than the electrophysiological status of the patient. Second, the concept of atrial cardiopathy helps to explain why a recent meta-analysis of eight randomized clinical trials found no evidence of any reduction in stroke risk with rhythm-control strategies as compared to rate-control strategies (odds ratio, 0.99; 95% confidence interval, 0.76–1.30) despite a substantial increase in restoration of sinus rhythm (odds ratio, 4.39; 95% confidence interval, 2.84–6.78).51 If dysrhythmia alone causes stroke, then restoration of normal rhythm should reduce stroke risk, whereas if dysrhythmia is a manifestation of underlying tissue pathology, then treatment of the dysrhythmia alone may not suffice to reduce stroke risk. Third, the concept of atrial cardiopathy helps to explain why cases of stroke have long been noted to cluster at the onset of AF diagnosis.52 Such a finding would be surprising if the dysrhythmia by itself were sufficient to cause thromboembolism, but would not be surprising if thromboembolism and dysrhythmia both developed in parallel as part of the progression of an underlying atrial cardiopathy. These considerations would also help to explain why almost one-third of AF patients manifested AF for the first time only after a stroke despite undergoing 8 months of continuous heart-rhythm monitoring beforehand.19 Fourth, the concept of atrial cardiopathy would help to explain the puzzle of cryptogenic stroke, which constitutes one-third of ischemic strokes even in the modern era.13 Many cryptogenic strokes are suspected to result from occult AF, but while almost half of these patients have ECG evidence of left atrial abnormality at the time of stroke,53 only a minority manifest AF even after several years of continuous cardiac monitoring.18 Several different markers of atrial disease have been associated with stroke (Table 1),31, 38, 40 but the traditional conception of AF as a necessary requirement for left atrial thromboembolism prevents the recognition that many cases of apparently cryptogenic stroke may originate in the left atrium despite the apparent absence of AF.
Table 2.
Summary of Key Arguments
| Arguments Against Atrial Fibrillation as the Root Cause of Left Atrial Thromboembolism |
|
| Arguments For Atrial Cardiopathy as the Root Cause of Left Atrial Thromboembolism |
|
Diagnostic Implications of Atrial Cardiopathy as a Stroke Risk Factor
Reframing thromboembolic risk as the result of atrial cardiopathy rather than the result of dysrhythmia holds the promise of substantially simplifying the identification of patients at risk for stroke. In the current conception of AF as the direct cause of stroke, a reliable approach to stratifying risk and instituting prophylactic therapy requires prolonged periods of continuous heart-rhythm monitoring to fully rule out even brief episodes of dysrhythmia. Such monitoring is expensive54 and inconvenient for patients.55 Even more limited step-wise screening approaches to AF detection56 have not resulted in recommendations for widespread adoption because of concerns about high rates of false-negative findings.57 As a result, many cases of potentially avoidable stroke occur in patients whose AF is not recognized until after their presentation with stroke.3 More importantly, many cases of left atrial thromboembolism are likely not recognized as such at all because of an absence of AF, and the stroke is instead labeled cryptogenic. Given these challenges, the concept of atrial cardiopathy may substantially advance our ability to determine stroke risk by providing risk markers that are not only more convenient and less costly than prolonged heart-rhythm monitoring but also more likely to identify those at risk. A combination of ECG measures such as PTFV1,31 echocardiographic measurements such as left atrial size38 and function,39 and serum biomarkers such as NT-proBNP40 may allow at a single point in time a more complete assessment of a patient’s thromboembolic risk based on a global view of the left atrium. Future studies will be needed to compare the predictive utility of various atrial cardiopathy markers both in combination with and as an alternative to diagnoses of AF.
Therapeutic Implications of Atrial Cardiopathy as a Stroke Risk Factor
As outlined above, numerous randomized trials have proven that vitamin-K antagonists7 and novel oral anticoagulant drugs8 substantially reduce the risk of thromboembolism in patients with AF. Furthermore, the effectiveness and tolerability of new oral anticoagulant drugs has been highlighted in trials such as AVERROES, in which apixaban significantly reduced stroke risk in patients with AF without increasing bleeding risk compared to aspirin.9 If thromboembolism in AF patients is driven by an underlying atrial cardiopathy and not just the dysrhythmia, and if atrial cardiopathy can exist and cause thromboembolism even in the absence of AF, then anticoagulant therapies may well be of benefit for stroke prevention in a wider population of patients than just those with AF. Preliminary evidence to this effect can be seen in an analysis of the WARSS trial, in which a subgroup of patients with elevated NT-proBNP had a substantially lower risk of recurrent stroke or death when treated with warfarin rather than aspirin.58 Therefore, future studies may be warranted to compare different antithrombotic strategies in patients at high risk even if they have no apparent AF. Randomized trials are underway to compare anticoagulant versus antiplatelet drugs for prevention of recurrent stroke in patients with cryptogenic stroke regardless of the presence of atrial cardiopathy, but the results of such trials would not shed much light on optimal strategies for primary prevention of stroke.
Future Directions
Future research should continue to assess the relationship between left atrial abnormality and stroke while thoroughly ascertaining AF. Furthermore, different markers of left atrial abnormality (e.g., biomarkers versus imaging markers) should be compared and perhaps combined in order to optimize prediction of stroke risk. Ultimately, establishing the validity of atrial cardiopathy as a therapeutic target will require randomized clinical trials of stroke prevention therapies such as antithrombotic drugs. Given that stroke accounts for 10% of deaths worldwide and imposes a substantial burden of disability,59 these types of further investigations of atrial cardiopathy as an under-appreciated and modifiable stroke risk factor may prove to have a substantial impact on public health.
Executive Summary.
Burden of Atrial Fibrillation
Atrial fibrillation (AF) affects 33 million people worldwide and is associated with an increased risk of ischemic stroke.
Atrial Fibrillation May Not Account for All Cases of Left Atrial Thromboembolism
Given its paroxysmal nature, AF may not be recognized as the cause of stroke because a patient may be in normal sinus rhythm upon presentation with stroke. Prolonged continuous heart-rhythm monitoring after stroke of undetermined cause may often reveal paroxysmal AF. However, most patients with cryptogenic stroke will not manifest AF, indicating that other embolic sources are likely.
Left Atrial Thromboembolism in the Absence of Atrial Fibrillation
Recent evidence links other markers of left atrial abnormality to an increased risk of stroke. ECG-defined left atrial abnormality, left atrial enlargement on echocardiogram, and elevated NT-proBNP have all been associated with stroke independently of AF. This suggests that left atrial thromboembolism may occur even in the absence of AF.
The Concept of Atrial Cardiopathy as the Source of Thromboembolism
We suggest that the concept of atrial cardiopathy, rather than AF, may better explain the phenomenon of left atrial embolisation. Rather than viewing AF as the necessary and sufficient cause of the thromboembolic risk seen in patients with AF, it may be more helpful to view both AF and thromboembolism as common manifestations of an underlying atrial cardiopathy. In this formulation, the driving force of thromboembolism is not the dysrhythmia but rather a host of underlying pathological tissue changes.
Implications of the Atrial Cardiopathy Model
The concept of atrial cardiopathy would explain several puzzling observations about AF and stroke: namely, the lack of a temporal or dose-response relationship between AF and stroke.
The concept of atrial cardiopathy may allow better screening for thromboembolic risk than the current approach of relying on the identification of AF, which can be difficult to document given its paroxysmal nature, and which may miss some patients with left atrial abnormality but no dysrhythmia.
The concept of atrial cardiopathy may also serve as a valid therapeutic target for anticoagulant drugs, and such a hypothesis will require testing in randomized clinical trials.
Acknowledgments
Funding Sources
This research was funded by grant K23NS082367 (Kamel) from the National Institute of Neurological Disorders and Stroke, as well as the Michael Goldberg Stroke Research Fund (Kamel).
Footnotes
Disclosures
Dr. Elkind reports serving as a consultant for BMS-Pfizer Partnership, Daiichi-Sankyo, Janssen Pharmaceuticals, and Boehringer-Ingelheim on the subject of antithrombotic therapy for AF; and for Biotelemetry on the subject of cardiac monitoring for paroxysmal AF. The other authors report no conflicts of interest.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Yiin GSC, Howard DPJ, Paul NLM, Li L, Luengo-Fernandez R, Bull LM, et al. Age-specific incidence, outcome, cost, and projected future burden of atrial fibrillation–related embolic vascular events: a population-based study. Circulation. 2014;130:1236–1244. doi: 10.1161/CIRCULATIONAHA.114.010942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin HJ, Wolf PA, Kelly-Hayes M, Beiser AS, Kase CS, Benjamin EJ, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27:1760–1764. doi: 10.1161/01.str.27.10.1760. [DOI] [PubMed] [Google Scholar]
- 5.Arboix A, Garcia-Eroles L, Massons JB, Oliveres M, Pujades R, Targa C. Atrial fibrillation and stroke: clinical presentation of cardioembolic versus atherothrombotic infarction. Int J Cardiol. 2000;73:33–42. doi: 10.1016/s0167-5273(99)00214-4. [DOI] [PubMed] [Google Scholar]
- 6.Stewart S, Murphy NF, Walker A, McGuire A, McMurray JJ. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart. 2004;90:286–292. doi: 10.1136/hrt.2002.008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007:CD006186. doi: 10.1002/14651858.CD006186.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 9.Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 10.Arboix A, Alió J. Cardioembolic stroke: clinical features, specific cardiac disorders and prognosis. Current Cardiology Reviews. 2010;6:150–161. doi: 10.2174/157340310791658730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziegler PD, Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, et al. Incidence of newly detected atrial arrhythmias via implantable devices in patients with a history of thromboembolic events. Stroke. 2010;41:256–260. doi: 10.1161/STROKEAHA.109.571455. [DOI] [PubMed] [Google Scholar]
- 12*.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. This landmark study found that even a single 6-minute episode of subclinical atrial was associated with a heightened risk of ischemic stroke. [DOI] [PubMed] [Google Scholar]
- 13.Marnane M, Duggan CA, Sheehan OC, Merwick A, Hannon N, Curtin D, et al. Stroke subtype classification to mechanism-specific and undetermined categories by TOAST, AS-C-O, and Causative Classification system. Stroke. 2010;41:1579–1586. doi: 10.1161/STROKEAHA.109.575373. [DOI] [PubMed] [Google Scholar]
- 14.Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O’Donnell MJ, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429–438. doi: 10.1016/S1474-4422(13)70310-7. [DOI] [PubMed] [Google Scholar]
- 15.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 16.Kishore A, Vail A, Majid A, Dawson J, Lees KR, Tyrrell PJ, et al. Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Stroke. 2014;45:520–526. doi: 10.1161/STROKEAHA.113.003433. [DOI] [PubMed] [Google Scholar]
- 17.Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–2477. doi: 10.1056/NEJMoa1311376. [DOI] [PubMed] [Google Scholar]
- 18.Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 19*.Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto C, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–2099. doi: 10.1161/CIRCULATIONAHA.113.007825. This analysis found that among patients with both subclinical atrial fibrillation and stroke, 31% had no AF during a median 8 months of continuous heart-rhythm monitoring before the stroke, and only manifested AF after the stroke. [DOI] [PubMed] [Google Scholar]
- 20.Cai H, Li Z, Goette A, Mera F, Honeycutt C, Feterik K, et al. Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: potential mechanisms for atrial thrombosis and stroke. Circulation. 2002;106:2854–2858. doi: 10.1161/01.cir.0000039327.11661.16. [DOI] [PubMed] [Google Scholar]
- 21.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 22.Mihm MJ, Yu F, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, et al. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–180. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- 23.Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724–730. doi: 10.1161/01.cir.89.2.724. [DOI] [PubMed] [Google Scholar]
- 24*.Binici Z, Intzilakis T, Nielsen OW, Kober L, Sajadieh A. Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation. 2010;121:1904–1911. doi: 10.1161/CIRCULATIONAHA.109.874982. This study demonstrated an association between premature atrial contractions and stroke risk independently of AF. [DOI] [PubMed] [Google Scholar]
- 25*.Kamel H, Elkind MS, Bhave PD, Navi BB, Okin PM, Iadecola C, et al. Paroxysmal supraventricular tachycardia and the risk of ischemic stroke. Stroke. 2013;44:1550–1554. doi: 10.1161/STROKEAHA.113.001118. This study demonstrated an association between PSVT and stroke independently of AF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Todo K, Moriwaki H, Saito K, Naritomi H. Frequent premature atrial contractions in stroke of undetermined etiology. Eur Neurol. 2009;61:285–288. doi: 10.1159/000206853. [DOI] [PubMed] [Google Scholar]
- 27.Morris JJ, Jr, Estes EH, Jr, Whalen RE, Thompson HK, Jr, McIntosh HD. P-wave analysis in valvular heart disease. Circulation. 1964;29:242–252. doi: 10.1161/01.cir.29.2.242. [DOI] [PubMed] [Google Scholar]
- 28.Alpert MA, Munuswamy K. Electrocardiographic diagnosis of left atrial enlargement. Arch Intern Med. 1989;149:1161–1165. [PubMed] [Google Scholar]
- 29.Goyal SB, Spodick DH. Electromechanical dysfunction of the left atrium associated with interatrial block. Am Heart J. 2001;142:823–827. doi: 10.1067/mhj.2001.118110. [DOI] [PubMed] [Google Scholar]
- 30.Scott CC, Leier CV, Kilman JW, Vasko JS, Unverferth DV. The effect of left atrial histology and dimension on P wave morphology. J Electrocardiol. 1983;16:363–366. doi: 10.1016/s0022-0736(83)80086-7. [DOI] [PubMed] [Google Scholar]
- 31*.Kamel H, Soliman EZ, Heckbert SR, Kronmal RA, Longstreth WT, Jr, Nazarian S, et al. P-wave morphology and the risk of incident ischemic stroke in the Multi-Ethnic Study of Atherosclerosis. Stroke. 2014;45:2786–2788. doi: 10.1161/STROKEAHA.114.006364. This study demonstrated an association between ECG-defined left atrial abnormality and stroke independently of AF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohsaka S, Sciacca RR, Sugioka K, Sacco RL, Homma S, Di Tullio MR. Electrocardiographic left atrial abnormalities and risk of ischemic stroke. Stroke. 2005;36:2481–2483. doi: 10.1161/01.STR.0000185682.09981.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamel H, Bartz TM, Longstreth WT, Jr, Okin PM, Thacker EL, Patton KK, et al. Association between left atrial abnormality on ECG and vascular brain injury on MRI in the Cardiovascular Health Study. Stroke. 2015;46:711–716. doi: 10.1161/STROKEAHA.114.007762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC., Jr Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40:1204–1211. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karas MG, Devereux RB, Wiebers DO, Whisnant JP, Best LG, Lee ET, et al. Incremental value of biochemical and echocardiographic measures in prediction of ischemic stroke: the Strong Heart Study. Stroke. 2012;43:720–726. doi: 10.1161/STROKEAHA.111.631168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnes ME, Miyasaka Y, Seward JB, Gersh BJ, Rosales AG, Bailey KR, et al. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin Proc. 2004;79:1008–1014. doi: 10.4065/79.8.1008. [DOI] [PubMed] [Google Scholar]
- 37.Di Tullio MR, Sacco RL, Sciacca RR, Homma S. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke. 1999;30:2019–2024. doi: 10.1161/01.str.30.10.2019. [DOI] [PubMed] [Google Scholar]
- 38*.Benjamin EJ, D’Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835–841. doi: 10.1161/01.cir.92.4.835. This study showed an association between left atrial size on echocardiogram and stroke risk independently of AF. [DOI] [PubMed] [Google Scholar]
- 39.Russo C, Jin Z, Liu R, Iwata S, Tugcu A, Yoshita M, et al. LA volumes and reservoir function are associated with subclinical cerebrovascular disease: the CABL (Cardiovascular Abnormalities and Brain Lesions) study. JACC Cardiovasc Imaging. 2013;6:313–323. doi: 10.1016/j.jcmg.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40*.Folsom AR, Nambi V, Bell EJ, Oluleye OW, Gottesman RF, Lutsey PL, et al. Troponin T, N-terminal pro-B-type natriuretic peptide, and incidence of stroke: the atherosclerosis risk in communities study. Stroke. 2013;44:961–967. doi: 10.1161/STROKEAHA.111.000173. This study demonstrated an association between NT-proBNP and stroke risk independently of AF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cushman M, Judd SE, Howard VJ, Kissela B, Gutierrez OM, Jenny NS, et al. N-terminal pro-B-type natriuretic peptide and stroke risk: the reasons for geographic and racial differences in stroke cohort. Stroke. 2014;45:1646–1650. doi: 10.1161/STROKEAHA.114.004712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoyanov N, Winterfield J, Varma N, Gollob MH. Atrial arrhythmias in the young: early onset atrial arrhythmias preceding a diagnosis of a primary muscular dystrophy. Europace. 2014;16:1814–1820. doi: 10.1093/europace/euu141. [DOI] [PubMed] [Google Scholar]
- 43.Buckley AE, Dean J, Mahy IR. Cardiac involvement in Emery Dreifuss muscular dystrophy: a case series. Heart. 1999;82:105–108. doi: 10.1136/hrt.82.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453–1468. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 45.Gallagher MM, Obel OA, Camm JA. Tachycardia-induced atrial myopathy: an important mechanism in the pathophysiology of atrial fibrillation? J Cardiovasc Electrophysiol. 1997;8:1065–1074. doi: 10.1111/j.1540-8167.1997.tb00631.x. [DOI] [PubMed] [Google Scholar]
- 46.De Jong AM, Maass AH, Oberdorf-Maass SU, Van Veldhuisen DJ, Van Gilst WH, Van Gelder IC. Mechanisms of atrial structural changes caused by stretch occurring before and during early atrial fibrillation. Cardiovasc Res. 2011;89:754–765. doi: 10.1093/cvr/cvq357. [DOI] [PubMed] [Google Scholar]
- 47.Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. 2014;64:2222–2231. doi: 10.1016/j.jacc.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 48.Disertori M, Quintarelli S, Grasso M, Pilotto A, Narula N, Favalli V, et al. Autosomal recessive atrial dilated cardiomyopathy with standstill evolution associated with mutation of Natriuretic Peptide Precursor A. Circ Cardiovasc Genet. 2013;6:27–36. doi: 10.1161/CIRCGENETICS.112.963520. [DOI] [PubMed] [Google Scholar]
- 49.Chao TF, Liu CJ, Chen SJ, Wang KL, Lin YJ, Chang SL, et al. Atrial fibrillation and the risk of ischemic stroke: does it still matter in patients with a CHA2DS2-VASc score of 0 or 1? Stroke. 2012;43:2551–2555. doi: 10.1161/STROKEAHA.112.667865. [DOI] [PubMed] [Google Scholar]
- 50.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 51.Al-Khatib SM, Allen LaPointe NM, Chatterjee R, Crowley MJ, Dupre ME, Kong DF, et al. Rate- and rhythm-control therapies in patients with atrial fibrillation: a systematic review. Ann Intern Med. 2014;160:760–773. doi: 10.7326/M13-1467. [DOI] [PubMed] [Google Scholar]
- 52.Wolf PA, Kannel WB, McGee DL, Meeks SL, Bharucha NE, McNamara PM. Duration of atrial fibrillation and imminence of stroke: the Framingham study. Stroke. 1983;14:664–667. doi: 10.1161/01.str.14.5.664. [DOI] [PubMed] [Google Scholar]
- 53.Cotter PE, Martin PJ, Ring L, Warburton EA, Belham M, Pugh PJ. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology. 2013;80:1546–1550. doi: 10.1212/WNL.0b013e31828f1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamel H, Hegde M, Johnson DR, Gage BF, Johnston SC. Cost-effectiveness of outpatient cardiac monitoring to detect atrial fibrillation after ischemic stroke. Stroke. 2010;41:1514–1520. doi: 10.1161/STROKEAHA.110.582437. [DOI] [PubMed] [Google Scholar]
- 55.Kamel H, Navi BB, Elijovich L, Josephson SA, Yee AH, Fung G, et al. Pilot randomized trial of outpatient cardiac monitoring after cryptogenic stroke. Stroke. 2013;44:528–530. doi: 10.1161/STROKEAHA.112.679100. [DOI] [PubMed] [Google Scholar]
- 56.Engdahl J, Andersson L, Mirskaya M, Rosenqvist M. Stepwise screening of atrial fibrillation in a 75-year-old population: implications for stroke prevention. Circulation. 2013;127:930–937. doi: 10.1161/CIRCULATIONAHA.112.126656. [DOI] [PubMed] [Google Scholar]
- 57.Lovett KM, Liang BA. Direct-to-consumer cardiac screening and suspect risk evaluation. JAMA. 2011;305:2567–2568. doi: 10.1001/jama.2011.865. [DOI] [PubMed] [Google Scholar]
- 58*.Longstreth WT, Jr, Kronmal RA, Thompson JL, Christenson RH, Levine SR, Gross R, et al. Amino terminal pro-B-type natriuretic peptide, secondary stroke prevention, and choice of antithrombotic therapy. Stroke. 2013;44:714–719. doi: 10.1161/STROKEAHA.112.675942. This analysis of data from the WARSS trial demonstrated superiority of warfarin over aspirin for stroke prevention in cryptogenic stroke patients with high levels of NT-proBNP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]