Abstract
Background
Phosphate binders are the cornerstone of hyperphosphatemia management in dialysis patients. Ferric citrate is an iron-based oral phosphate binder that effectively lowers serum phosphorus levels.
Study Design
52-week, open-label, phase 3, randomized, controlled trial for safety-profile assessment.
Setting & Participants
Maintenance dialysis patients with serum phosphorus levels ≥6.0 mg/dL after washout of prior phosphate binders.
Intervention
2:1 randomization to ferric citrate or active control (sevelamer carbonate and/or calcium acetate).
Outcomes
Changes in mineral bone disease, protein-energy wasting/inflammation, and occurrence of adverse events after 1 year.
Measurements
Serum calcium, intact parathyroid hormone, phosphorus, aluminum, white blood cell count, percentage of lymphocytes, serum urea nitrogen, and bicarbonate.
Results
There were 292 participants randomly assigned to ferric citrate, and 149, to active control. Groups were well matched. For mean changes from baseline, phosphorus levels decreased similarly in the ferric citrate and active control groups (−2.04 ± 1.99 [SD] vs −2.18 ± 2.25 mg/dL, respectively; P = 0.9); serum calcium levels increased similarly in the ferric citrate and active control groups (0.22 ± 0.90 vs 0.31 ± 0.95 mg/dL; P = 0.2). Hypercalcemia occurred in 4 participants receiving calcium acetate. Parathyroid hormone levels decreased similarly in the ferric citrate and active control groups (−167.1 ± 399.8 vs −152.7 ± 392.1 pg/mL; P = 0.8). Serum albumin, bicarbonate, serum urea nitrogen, white blood cell count and percentage of lymphocytes, and aluminum values were similar between ferric citrate and active control. Total and low-density lipoprotein cholesterol levels were lower in participants receiving sevelamer than those receiving ferric citrate and calcium acetate. Fewer participants randomly assigned to ferric citrate had serious adverse events compared with active control.
Limitations
Open-label study, few peritoneal dialysis patients.
Conclusions
Ferric citrate was associated with similar phosphorus control compared to active control, with similar effects on markers of bone and mineral metabolism in dialysis patients. There was no evidence of protein-energy wasting/inflammation or aluminum toxicity, and fewer participants randomly assigned to ferric citrate had serious adverse events. Ferric citrate is an effective phosphate binder with a safety profile comparable to sevelamer and calcium acetate.
Keywords: Hemodialysis, hyperphosphatemia, ferric citrate, sevelamer carbonate, calcium acetate, phosphate binder, mineral bone disease, protein-energy wasting (PEW)/inflammation, adverse events, safety, end-stage renal disease (ESRD)
Hyperphosphatemia occurs frequently in patients with end-stage renal disease (ESRD), contributing to secondary hyperparathyroidism and associated skeletal and vascular complications.1 Thrice-weekly dialysis insufficiently removes dietary phosphorus even with phosphorus-restricted diets. Current ESRD guidelines recommend using phosphate binders with meals to manage hyperphosphatemia when dietary restriction fails.2 The optimal binder regimen likely depends on the side-effect profiles of the available drugs and the concurrent metabolic bone disorder status of the individual patient.
Beyond pill burden, there are potential undesired side effects for all phosphate binders. Aluminum-based binders may result in skeletal, neurologic, and other potential associated toxicity.3-5 Calcium-containing binders can produce positive calcium balance and might increase the risk for hypercalcemia and vascular calcification.6,7 Other binders, including newer non—calcium-containing ones, are associated with gastrointestinal symptoms.8
Ferric citrate is an iron-based compound that binds phosphorus in the intestine and lowers serum phosphorus levels.9,10 Recently, the principal results were reported from a randomized clinical trial with a 52-week active control period comparing ferric citrate to active control with a subsequent 4-week placebo-control period.11 Active control consisted of sevelamer carbonate and/or calcium acetate based on physician preference to replicate the treatment options commonly used in current clinical practice. That initial report demonstrated that ferric citrate effectively controlled phosphorus levels while concurrently increasing iron stores and reducing intravenous iron and erythropoietin-stimulating agent use, while also maintaining hemoglobin levels compared to active control.11 In this prespecified secondary analysis, we report the effects of ferric citrate compared to active control on components of mineral and bone metabolism, protein-energy wasting (PEW)/inflammation, and overall adverse-event profile. This includes assessment of aluminum levels because older studies demonstrated increased aluminum absorption when citrate and aluminum-based binders were used concomitantly.12
METHODS
Study Overview
This phase 3, sequential, randomized, open-label trial was conducted at 60 sites in the United States and Israel from December 2010 through November 2012. The protocol was written by the Collaborative Study Group, whose independent statisticians performed analyses. The rationale and study design were published previously.13 The trial was approved by a local or central institutional review board for each site, including the Clinical Coordinating Center. Participants provided written informed consent prior to study procedures. The trial was registered with ClinicalTrials.gov (study number: NCT01191255) and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. This trial was conducted under a Special Protocol Assessment agreement with the US Food and Drug Administration.
Study Population
Inclusion criteria were adult patients with ESRD receiving thrice-weekly in-center hemodialysis or peritoneal dialysis, dialysis vintage ≥ 3 months, prescription of 3 to 18 pills per day of commercially available phosphate binders, serum ferritin level < 1,000 ng/mL, serum transferrin saturation < 50%, and serum phosphorus level of 2.5 to 8.0 mg/dL at the screening visit. Exclusion criteria were parathyroidectomy within 6 months of screening, an absolute indication for oral iron or vitamin C, or prior intolerance to calcium acetate or sevelamer. Aluminum-containing phosphate binders were prohibited during this trial.
Study Design
This trial had 3 periods (Fig S1, available as online supplementary material). The results presented here reflect the 52-week active control period. Results from the 4-week placebo-controlled study that followed the 52-week active control period have been previously published.11 Prior to the active control period, all phosphate binders were discontinued during a 2-week washout period. Participants with a postwashout serum phosphorus level ≥ 6.0 mg/dL were randomly assigned 2:1 to ferric citrate or active control. Baseline values refer to the period following washout before randomization. Ferric citrate was supplied as 1-g tablets containing 210 mg of ferric iron and was titrated per specified protocol.10,14 Active control binders (calcium acetate, 667-mg capsules; sevelamer, 800-mg tablets) were prescribed, provided to participants by the study, and titrated according to US Food and Drug Administration—approved package inserts15,16; participants could be prescribed either or both active control drugs. Medication dosing and titration were determined by serum phosphorus levels obtained from a central laboratory. Participants were instructed to ingest binders with meals or within 1 hour of eating. Pill counts were conducted monthly to assess adherence. If serum calcium level was > 10.5 mg/dL despite conservative management, participants were considered to have had treatment failures and were switched to ferric citrate if they had been on calcium acetate treatment.
Blood specimens were obtained prior to dialysis through the individual's dialysis access, and analytes were measured within 24 to 48 hours of collection in a central laboratory. Intact parathyroid hormone (PTH) was measured in serum using an ADVIA Centaur iPTH assay, a 2-site sandwich immunoassay using direct chemiluminometric technology (reference range, 13.8-85.0 pg/mL; coefficient of variation, 4%).
Adverse events were recorded through the final study visit or 30 days after the last dose of study drug. A Collaborative Study Group medical monitoring committee reviewed and adjudicated all serious adverse events (SAEs) within 24 hours of a reported event. Frequencies and proportions of participants with adverse events were tabulated for participants who received at least one dose of study medication. Numbers of participants with adverse events were tabulated until study drug discontinuation for non-SAEs and until 30 days after discontinuation of study drug for SAEs.
Statistical Analysis
Continuous data were summarized using mean ± standard deviation or median with interquartile range as appropriate. Frequencies and percentages were used to summarize categorical data. Analysis of covariance (ANCOVA) models were applied during the 52-week active control period to compare mean changes in continuous variables from baseline with each follow-up assessment between the ferric citrate and active control groups, controlling for baseline values. The same analyses were performed to compare differences among all other groups, including subgroups of the active control group. Wilcoxon rank sum tests were used to confirm ANCOVA results for intact PTH due to its non-normal distribution.
After defining outcome measurements following study drug discontinuation as missing, all missing values were imputed using a strategy of last follow-up value carried forward within the active control period. Analyses of laboratory parameters using last follow-up value carried forward were confirmed using corresponding ANCOVAs on the basis of longitudinal mixed-effect models with unstructured covariance matrices to account for repeated measurements. For the prespecified secondary outcomes presented in this study, analyses are presented on a comparison-wise basis without adjustment for multiple comparisons. Percentages of participants in each group with at least one SAE were compared with a Fisher exact test. Statistical analyses were performed using SAS, versions 9.3 and 9.4 (SAS Institute Inc).
RESULTS
Baseline Characteristics
Four hundred forty-one participants were randomly assigned, with 292 assigned to ferric citrate and 149 assigned to active control. Baseline characteristics of randomly assigned participants are listed in Table 1. Among active control participants, 78 initially received sevelamer only; 41, calcium acetate only; and 30, a combination of these agents.
Table 1.
Baseline Characteristics of Study Population Prior to Randomization
| Ferric Citrate (n = 292) | Active Control (n = 149) | |
|---|---|---|
| Age (y) | 54.9 ± 13.4 | 53.7 ± 13.0 |
| Male sex | 183 (62.7) | 87 (58.4) |
| Race | ||
| Black/African American | 154 (52.7) | 78 (52.4) |
| White/Caucasian | 124 (42.5) | 62 (41.6) |
| Other/unknown | 14 (4.8) | 9 (6.0) |
| Hispanic or Latino ethnicity | 43 (14.8) | 23 (15.4) |
| Cause of ESRD | ||
| Diabetic nephropathy | 120 (41.1) | 65 (43.6) |
| Hypertensive nephrosclerosis | 89 (30.5) | 45 (30.2) |
| Glomerular disease or other | 83 (28.4) | 39 (26.2) |
| Peritoneal dialysis | 11 (2.5) | 3 (0.7) |
| Vitamin D/vitamin D analogue use | 223 (76.4) | 126 (84.6) |
| Phosphate-binder use at screening | ||
| Sevelamer | 170 (58.2) | 96 (64.4) |
| Calcium acetate | 104 (36.0) | 57 (38.3) |
| Lanthanum carbonate | 28 (9.6) | 12 (8.1) |
| Calcium carbonate | 15 (5.2) | 13 (8.7) |
| Other | 5 (1.7) | 1 (0.7) |
| Parathyroidectomy | 19 (6.6) | 4 (2.7) |
| Calcium (mg/dL) | 9.00 [8.40-9.45] | 9.00 [8.50-9.58] |
| Phosphorus (mg/dL) | 7.20 [6.30-8.30] | 7.40 [6.20-8.50] |
| Calcium-phosphorus product (mg2/dL2) | 63.51 [54.60-75.57] | 66.18 [56.15-75.84] |
| Intact PTH (pg/mL) | 514 [331-794] | 479 [278-755] |
| Serum bicarbonate (mmol/L) | 24 [22.0-26.0] | 24 [22.0-26.0] |
| SUN (mg/dL) | 55 [45.0-65.5] | 56 [44.5-68.5] |
| Serum albumin (g/dL) | 4.0 [3.8-4.2] | 4.0 [3.8-4.2] |
| Total cholesterol (mg/dL) | 151.0 [128.0-180.0] | 148.0 [125.5-176.0] |
| LDL cholesterol (mg/dL) | 79.0 [62-103.0] | 80.0 [64.0-99.0] |
| White blood cell count (×103) | 6.54 [5.31-7.9] | 6.36 [5.06-7.88] |
| Lymphocytes (%) | 21.3 [16.1-26.6] | 20.2 [16.8-27.4] |
Note: Values for categorical variables are given as number (percentage); values for continuous variables, as mean ± standard deviation or median [interquartile range]. Conversion factors for units: calcium in mg/dL to mmol/L, ×0.2495; cholesterol in mg/dL to mmol/L, ×0.02586; phosphorus in mg/dL to mmol/L, ×0.3229; SUN in mg/dL to mmol/L, ×0.357.
Abbreviations: ESRD, end-stage renal disease; LDL, low-density lipoprotein; PTH, parathyroid hormone; SUN, serum urea nitrogen.
Phosphorus, Calcium, and PTH
Baseline serum phosphorus levels were similar in the ferric citrate and active control groups (Table 1). As reported previously, there was a significant reduction in phosphorus levels in both the ferric citrate and active control groups during the trial11 (mean changes from baseline, −2.04 ± 1.99 and −2.18 ± 2.25 mg/dL, respectively). This change was similar between the ferric citrate and active control groups (P = 0.9), as well as between ferric citrate and individual active control subgroups (Table 2). There were no differences in the proportion of participants from the ferric citrate or active control group that achieved the recommended serum phosphorus target (3.5-5.5 mg/dL). Following dose-titration protocols that achieved nearly identical serum phosphorus levels, mean number of pills taken per day in the ferric citrate group was 8.1 ± 2.4, which was statistically similar to that in the calcium acetate—only group (7.6 ± 2.5; P = 0.3) but significantly less than that in the sevelamer-only group (8.7 ± 2.8; P = 0.03).
Table 2.
Changes in Serum Phosphorus, Calcium, and PTH Levels in Ferric Citrate and Active Control Participants Over 52-Week Active Control Study Period
| Mean Change From Baselinea |
Adjusted Mean Differences Between Treatmentsb |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| FC | AC | SC | CA | SC + CA | FC vs AC | FC vs SC | FC vs CA | FC vs SC + CA | |
| Phosphorus (mg/dL) | –2.04 ± 1.99 | –2.18 ± 2.25 | –2.11 ± 2.31 | –2.11 ± 1.92 | –2.46 ± 2.52 | 0.01 (–0.30 to 0.33) | 0.02 (–0.38 to 0.41) | 0.05 (–0.46 to 0.57) | –0.04 (–0.64 to 0.57) |
| n = 281; 189 | n = 146; 113 | n = 78; 63 | n = 39; 28 | n = 29; 22 | P = 0.9 | P = 0.9 | P = 0.8 | P = 0.9 | |
| Calcium (mg/dL) | 0.22 ± 0.90 | 0.31 ± 0.95 | 0.17 ± 0.81 | 0.49 ± 0.96 | 0.41 ± 1.24 | –0.12 (–0.28 to 0.04) | –0.08 (–0.27 to 0.12) | –0.25 (–0.52 to 0.02) | –0.04 (–0.35 to 0.27) |
| n = 281; 189 | n = 146; 113 | n = 78; 63 | n = 39; 28 | n = 29; 22 | P = 0.2 | P = 0.5 | P = 0.07 | P = 0.8 | |
| PTH (pg/dL) | –167.1 ± 399.8 | –146.5 ± 397.0 | –134.3 ± 419.4 | –98.9 ± 251.3 | –244.5 ± 482.8 | 4.3 (–61.5 to 70.0) | –43.5 (–128.5 to 41.6) | 58.1 (–53.4 to 169.6) | 59.6 (–68.9 to 188.1) |
| n = 247; 189 | n = 133; 108 | n = 72; 62 | n = 35; 26 | n = 26; 20 | P = 0.9 | P = 0.3 | P = 0.3 | P = 0.4 | |
Abbreviations: AC, active control; CA, calcium acetate; FC, ferric citrate; SC, sevelamer carbonate; SC + CA, combination of sevelamer carbonate and calcium acetate; PTH, parathyroid hormone.
Analyses performed using last-value-carried-forward imputation for missing measurements. Sample sizes indicate the number of patients with nonmissing baseline and follow-up data after the last-value-carried-forward imputation, followed by the sample size for patients with nonmissing baseline and 12-month follow-up measurements. Values present the mean change from baseline ± standard deviation.
Shown are the estimated mean differences (95% confidence interval) in change from baseline to 12 months between the indicated treatment groups after adjustment for baseline measurement of the outcome variable. All P values for comparisons of active control subgroups with each other (CA vs SC, CA vs SC + CA, SC vs SC + CA) were > 0.05.
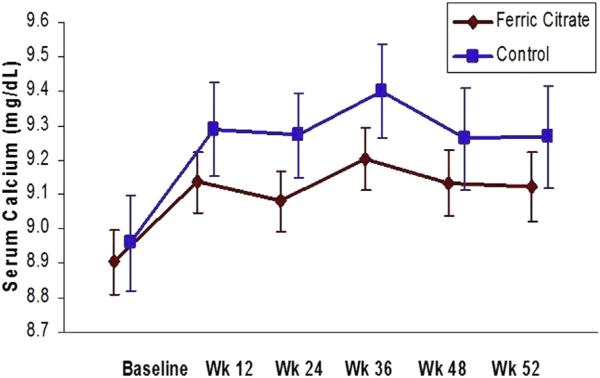
Following the washout, serum calcium levels were similar in the ferric citrate and active control groups (Table 1; P = 0.6). During the study, calcium levels increased in both the ferric citrate and active control groups (Fig 1). Following the active control period, serum calcium levels were 9.12 ± 0.86 mg/dL in the ferric citrate group and 9.27 ± 0.92 mg/dL in the active control group (P = 0.2 for between-group change). The difference in change between groups was not changed when controlling for PTH level. Active control participants receiving calcium acetate only had a mean serum calcium level of 8.84 ± 0.83 mg/dL following washout, which increased to 9.35 ± 1.06 mg/dL (P < 0.001 for within-group change; P = 0.07 for between-group change compared to ferric citrate; Table S1). Participants receiving sevelamer only had mean serum calcium levels that increased from 9.13 ± 0.70 to 9.30 ± 0.81 mg/dL (P = 0.06 for within-group change; P = 0.5 compared to ferric citrate). Hypercalcemia (serum calcium level persistently > 10.5 mg/dL) occurred in only 4 participants. All these participants were receiving calcium acetate only, and they were switched to ferric citrate per protocol.
Figure 1.
Serum calcium levels during the 52-week (Wk) active control period, demonstrating no significant difference between groups (P = 0.2). Serum calcium was measured in the ferric citrate group and active control group following the washout period (week 0 or baseline) and at weeks 12, 24, 36, 48, and 52. Bars reflect 95% confidence intervals.
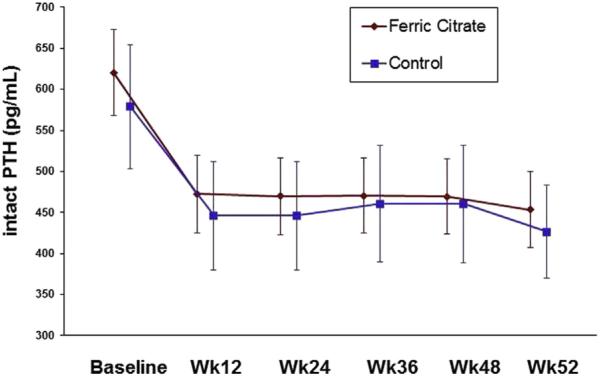
Following washout, serum intact PTH levels were similar in the ferric citrate and active control groups (P = 0.9; Table 1) and decreased in both groups during the active control period (P < 0.001 for within-group change for both groups; Fig 2). At the end of the active control period, mean intact PTH levels were 453.4 ± 369.1 and 431.5 ± 335.3 pg/mL in the ferric citrate and active control groups, respectively (P = 0.9 for between-group change from baseline). There were no significant differences in change in PTH levels between participants in the ferric citrate group compared with participants taking calcium acetate only or sevelamer only (Table 2). The number of participants concomitantly receiving vitamin D analogues at each quarter of the active control period was similar in the ferric citrate and active control groups (Table 3).
Figure 2.
Serum parathyroid hormone (PTH) measurements during the 52-week (Wk) active control period, demonstrating no significant difference between groups (P = 0.8). Bars reflect 95% confidence intervals.
Table 3.
Concomitant Use of Vitamin D Analogues During the Active Control Period
| FC (n = 281) | AC (n = 146) | SC (n = 78) | CA (n = 39) | SC + CA (n = 29) | |
|---|---|---|---|---|---|
| Week 12 | 224 (80) | 120 (82) | 67 (86) | 31 (79) | 22 (76) |
| Weeks 12-24 | 209 (74) | 111 (76) | 64 (82) | 26 (67) | 21 (72) |
| Weeks 24-36 | 182 (65) | 104 (71) | 59 (76) | 24 (62) | 21 (72) |
| Weeks 36-52 | 155 (55) | 85 (58) | 48 (62) | 22 (56) | 15 (52) |
Note: Values are given as number (percentage). Reported medications include alfacalcidol, calcitriol, doxercalciferol, and paricalcitol.
Abbreviations: AC, active control; CA, calcium acetate; FC, ferric citrate; SC, sevelamer carbonate; SC + CA, combination of sevelamer carbonate and calcium acetate.
Markers of PEW/Inflammation
Mean serum ferritin levels increased during the active control period in the ferric citrate group (593 ± 293 to 899 ± 488 ng/mL), while not changing significantly in the active control group (609 ± 307 to 628 ± 367 ng/mL; adjusted mean difference between groups, P < 0.001). There were no significant differences between groups in changes in several important markers of nutrition and inflammation, including serum albumin, white blood cell count, and percentage of white blood cells that were lymphocytes (Table 4). Serum urea nitrogen (SUN) levels decreased in both the ferric citrate and active control groups (P < 0.001 and P < 0.006, respectively; P = 0.6 for ferric citrate vs active control) with minimal change in serum creatinine levels. Serum bicarbonate levels increased in both groups (P < 0.001 for both ferric citrate and active control; P = 0.1 for between-group comparison). Total and low-density lipoprotein (LDL) cholesterol levels were similar at baseline in the ferric citrate and active control groups (Table 1). Upon completion of the 52-week active control period, there were greater reductions in both total and LDL cholesterol levels in the sevelamer-only group compared to either the ferric citrate or calcium acetate—only groups (Tables 4 and S2).
Table 4.
Changes in Markers of Nutrition and Inflammation Among Ferric Citrate and Active Control Participants
| Mean Change From Baselinea |
Adjusted Mean Differences in Mean Changes Between Treatmentsb |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome Variable | FC | AC | SC | CA | SC + CA | FC vs AC | FC vs SC | FC vs CA | FC vs SC + CA |
| Serum albumin (g/dL) | –0.05 ± 0.31 | –0.03 ± 0.33 | –0.03 ± 0.31 | 0.05 ± 0.34 | –0.14 ± 0.35 | –0.01 (–0.08 to 0.05) | –0.01 (–0.09 to 0.07) | –0.09 (–0.20 to 0.02) | 0.09 (–0.04 to 0.22) |
| n = 245; 183 | n = 126; 105 | n = 68; 58 | n = 35; 27 | n = 23; 20 | P = 0.7 | P = 0.7 | P = 0.1 | P = 0.2 | |
| Serum bicarbonate (mmol/L) | 0.88 ± 3.60 | 1.68 ± 4.62 | 1.61 ± 4.92 | 1.12 ± 3.77 | 2.71 ± 4.86 | –0.58 (–1.33 to 0.17) | –0.61 (–1.54 to 0.31) | –0.06 (–1.20 to 1.08) | –1.37 (–2.77 to 0.03) |
| n = 220; 162 | n = 121; 94 | n = 66; 51 | n = 33; 23 | n = 22; 20 | P = 0.1 | P = 0.2 | P = 0.9 | P = 0.06 | |
| SUN (mg/dL) | –4.03 ± 15.50 | –4.53 ± 18.18 | –5.34 ± 16.72 | –0.74 ± 17.36 | –7.91 ± 22.88 | –0.78 (–3.70 to 2.14) | 0.97 (–2.57 to 4.52) | –4.44 (–9.06 to 0.18) | –0.09 (–5.93 to 5.76) |
| n = 243; 182 | n = 126; 105 | n = 68; 58 | n = 35; 27 | n = 23; 20 | P = 0.6 | P = 0.6 | P = 0.06 | P = 0.9 | |
| Serum creatinine (mg/dL) | 0.13 ± 1.67 | –0.06 ± 1.89 | –0.03 ± 1.54 | 0.15 ± 2.53 | –0.45 ± 1.69 | 0.16 (–0.20 to 0.53) | 0.18 (–0.25 to 0.61) | –0.11 (–0.73 to 0.50) | 0.50 (–0.19 to 1.19) |
| n = 244; 183 | n = 126; 105 | n = 68; 58 | n = 35; 27 | n = 23; 20 | P = 0.4 | P = 0.4 | P = 0.7 | P = 0.2 | |
| WBC count (×103) | –0.07 ± 1.51 | –0.02 ± 2.11 | –0.11 ± 2.04 | 0.10 ± 2.37 | 0.09 ± 2.01 | 0.00 (–0.33 to 0.33) | 0.06 (–0.33 to 0.44) | –0.07 (–0.62 to 0.47) | –0.10 (–0.70 to 0.49) |
| n = 248; 188 | n = 132; 111 | n = 72; 62 | n = 35; 27 | n = 25; 22 | P = 0.9 | P = 0.8 | P = 0.8 | P = 0.7 | |
| Lymphocytes/WBCc (%) | –0.24 ± 6.45 | –0.62 ± 8.11 | –1.12 ± 7.42 | 0.25 ± 8.75 | –0.38 ± 9.25 | 0.37 (–0.97 to 1.71) | 0.93 (–0.66 to 2.51) | –0.65 (–2.87 to 1.57) | 0.24 (–2.29 to 2.76) |
| n = 248; 188 | n = 132; 111 | n = 72; 62 | n = 35; 27 | n = 25; 22 | P = 0.6 | P = 0.3 | P = 0.6 | P = 0.9 | |
| Total cholesterol (mg/dL)d | –0.82 ± 32.41 | –15.75 ± 32.99 | –19.78 ± 32.87 | –3.63 ± 34.49 | –22.30 ± 26.97 | 16.83 (10.45 to 23.21) | 21.42 (13.37 to 29.47) | 4.51 (–6.24 to 15.25) | 21.25 (8.50 to 33.99) |
| n = 243; 182 | n = 126; 103 | n = 68; 57 | n = 35; 26 | n = 23; 20 | P < 0.001 | P < 0.001 | P = 0.4 | P = 0.001 | |
| LDL cholesterol (mg/dL)d | –3.19 ± 23.23 | –15.10 ± 27.52 | –20.24 ± 25.93 | –5.20 ± 28.49 | –15.22 ± 27.57 | 13.03 (8.32 to 17.74) | 18.06 (12.37 to 23.75) | 3.07 (–4.64 to 10.78) | 12.78 (3.55 to 22.01) |
| n = 243; 182 | n = 125; 101 | n = 67; 56 | n = 35; 26 | n = 23; 19 | P < 0.001 | P < 0.001 | P = 0.4 | P = 0.007 | |
Note: Conversion factors for units: cholesterol in mg/dL to mmol/L, ×0.02586; creatinine in mg/dL to μmol/L, ×88.4; SUN in mg/dL to mmol/L, ×0.357.
Abbreviations: AC, active control; CA, calcium acetate; FC, ferric citrate; LDL, low-density lipoprotein; SC, sevelamer carbonate; SC + CA, combination of sevelamer carbonate and calcium acetate; SUN, serum urea nitrogen; WBC, white blood cell.
Analyses were performed using last-value-carried-forward imputation for missing measurements. Sample sizes indicate the number of patients with nonmissing baseline and follow-up data after the last-value-carried-forward imputation, followed by the sample size for patients with nonmissing baseline and 12-month follow-up measurements. Values represent mean change from baseline ± standard deviation.
Shown are the estimated mean differences (95% confidence interval) in the change from baseline to 12 months between the indicated treatment groups, after adjustment for the baseline measurement of the outcome variable.
Proportion of WBCs that are lymphocytes.
SC versus CA had P = 0.003 for total cholesterol and P = 0.001 for LDL cholesterol.
Aluminum
For 185 participants in the ferric citrate group with available measurements, median aluminum level was 6.0 (range, 5-24) μg/L at baseline and 7.0 (5-23) μg/L at the end of the 52-week active control period. In the active control group (n = 107), corresponding levels at these points were 6.0 (5-14) and 6.0 (5-15) μg/L, respectively (P = 0.1 between groups at 52 weeks). No clinically meaningful changes in aluminum levels were observed in the study in either group.
Adverse Events
Numbers of deaths were similar in the ferric citrate and active control groups (ferric citrate, 4.5%; active control, 5.4%). Fewer participants in the ferric citrate group had SAEs compared to the active control group (39.1% vs 49.0%, respectively; P = 0.05). The single most common SAE was hospitalization: 34.6% of the ferric citrate group was hospitalized at least once versus 45.6% of the active control group.17 The most common SAEs are shown in Table 5. Fewer participants had at least one gastrointestinal SAE in the ferric citrate group versus the active control group (P = 0.05). There were no musculoskeletal adverse events in the active control group, while 3 occurred in the ferric citrate group (joint swelling and pain in an extremity). No fractures occurred during the study. Importantly, the study did not enroll patients who had previous intolerance to sevelamer or calcium acetate.
Table 5.
Common SAEs
| FC (n = 289) | AC (n = 149) | P | |
|---|---|---|---|
| Any SAE | 39.1 | 49.0 | 0.05 |
| Infections and infestations | 12.5 | 18.1 | 0.1 |
| Surgical and medical procedures | 7.6 | 6.7 | 0.9 |
| Kidney transplantation | 4.2 | 4.0 | 0.9 |
| Vascular | 7.3 | 10.1 | 0.4 |
| General disorders and administration site | 7.3 | 7.4 | 0.9 |
| Cardiac | 7.3 | 12.1 | 0.1 |
| Gastrointestinal | 6.9 | 12.8 | 0.05 |
| Respiratory, thoracic, and mediastinal | 6.6 | 8.7 | 0.4 |
| Injury, poisoning, and procedural | 5.2 | 6.7 | 0.5 |
| Metabolism and nutrition | 5.5 | 6.7 | 0.7 |
Note: Values are given as percentages. Common SAE indicates occurring in >5% of participants. Percentage of patients with treatment-emergent SAEs in the indicated categories, including adverse events occurring after study drug initiation and prior to 30 days after discontinuation of study drug. P values were computed using Fisher exact test. The number of participants analyzed includes all who received at least 1 dose of study drug.
Abbreviations: AC, active control; FC, ferric citrate; SAE, serious adverse event.
DISCUSSION
Ferric citrate, an iron-based phosphate binder, effectively decreased serum phosphorus levels, with similar effects on other markers of bone and mineral metabolism as active control in hemodialysis and peritoneal dialysis patients. This occurred with a favorable safety profile and no significant increase in aluminum levels. Ferric citrate pill burden was similar to calcium acetate and slightly less than sevelamer. Ferric citrate use was not accompanied by evidence of increased systemic inflammation or PEW.
Phosphate binders are recommended to manage hyperphosphatemia in dialysis patients who do not achieve adequate phosphate control with dietary restriction alone.2 Selecting the appropriate binder requires consideration of individual binders’ adverse effects, as well as patient comorbid conditions, including markers of bone and mineral metabolism. Aluminum-based binders are infrequently used for long-term therapy due to the increased risk of aluminum toxicity, including potential osteomalacia and encephalopathy.5,18 Calcium-containing binders carry the risk of hypercalcemia and are potentially associated with increased vascular and soft-tissue calcification.7,19 Non—calcium-containing binders such as sevelamer minimize the risks related to hypercalcemia or vascular calcification, but can be poorly tolerated due to gastrointestinal adverse effects, high pill burden, and in some cases elevated cost.8 Lanthanum carbonate, which has a lower pill burden, is also associated with frequent gastrointestinal adverse effects20 and may also be associated with unpredictable absorption and deposition in critical tissues.21 In comparison to these agents, ferric citrate appears to have a comparable or favorable safety profile while also facilitating anemia management.11
Results of this study demonstrate similar control of mineral and bone disease markers, including calcium, phosphorus, and PTH, over a 1-year period. This confirms findings from other studies evaluating ferric citrate, but over a longer period and in more patients.10,22,23 Serum calcium levels increased in all groups, and we attribute this to the lowering of serum phosphorus levels with the re-initiation of phosphate-binder treatment following the washout. Vitamin D analogue use decreased in all groups, making this an unlikely explanation for the increase in serum calcium levels. Cinacalcet use was not considered to be ascertained reliably because this non—study drug oral medication was not subject to strict monitoring. The absolute increase in calcium levels was highest with calcium acetate. Although this was not statistically significant compared with the other groups, the results may be underpowered. PTH levels decreased in all participants, which may have been a function of improved adherence to phosphate binders and associated reduction of serum phosphorus levels in the context of a clinical trial.
Ferric citrate achieved these overall similar results with a mean pill burden similar to that of calcium acetate but significantly less than that of sevelamer. Although mean numeric differences for the groups as a whole were small, there may be some patient subgroups for which the pill burden difference was clinically relevant.
Metabolic acidosis also has adverse effects on bone. An increase in serum bicarbonate levels was seen in both the ferric citrate and active control groups, likely due to the potential bicarbonate absorbed from the citrate moiety in ferric citrate and the carbonate in participants receiving sevelamer. It has not yet been established whether ferric citrate influences other markers of bone metabolism, such as fibroblast growth factor 23, in patients with ESRD; these data were not collected in this study.
In healthy individuals, taking aluminum hydroxide gel concomitantly with calcium citrate caused significantly higher serum aluminum levels compared to taking aluminum hydroxide alone or with calcium acetate.12 The proposed mechanism was formation of a soluble citrate-aluminum compound in the intestine, which enhanced intestinal absorption of aluminum and increased serum aluminum levels.24,25 Historically, use of aluminum-based phosphate binders in dialysis patients was associated witha risk for aluminum toxicity.3-5 Potential risks of aluminum toxicity also exist with exposure to aluminum from cooking implements, dial-ysate water, and other sources. Our study demonstrated similar serum aluminum levels among participants receiving ferric citrate and active control. Of note, we did not allow the use of aluminum binders concurrent with ferric citrate. However, we did not restrict exogenous aluminum exposure from any other sources.
One prevalent condition in patients with ESRD is PEW/inflammation, which is also known as malnutrition-inflammation complex syndrome (MICS).26 Ferric citrate increases levels of serum ferritin, a biomarker associated with MICS, compared to active control. We attribute an increase in iron available for utilization into hemoglobin to be the primary mechanism responsible for the reduction in erythropoiesis-stimulating agent use seen with ferric citrate.27 However, because serum ferritin level can also be a marker for inflammation, we examined other markers of inflammation and nutrition collected in this study. Changes in levels of serum albumin, white blood cell count, percentage of white blood cells that are lymphocytes, serum bicarbonate, and SUN were similar in the ferric citrate and active control groups, supporting the hypothesis that changes in ferritin levels seen with ferric citrate were not related to inflammation. Interestingly, SUN levels decreased in both groups, generating the question of whether protein intake decreased. Mean serum albumin level was relatively high at baseline and did not change over the 52 weeks in either the ferric citrate or active control groups, suggesting there was no significant decrease in protein intake. Stable serum creatinine levels similarly do not that SUN level changes were due to worse health, because creatinine level in dialysis is largely a function of muscle. Participants with PEW also can have low cholesterol levels as a manifestation of inflammation. There was a greater reduction in both LDL and total cholesterol levels in the active control group compared to the ferric citrate group, apparently related to sevelamer use. This most likely re-flects the known cholestyramine-like effect of sevelamer.28,29 This finding establishes one potential pleiotropic benefit of sevelamer that cannot be obtained with ferric citrate. Overall, these findings suggest that oral iron absorption with ferric citrate does not promote PEW/inflammation.
Ferric citrate overall had a favorable safety profile, with fewer patients having SAEs or hospitalization in the ferric citrate group versus active control. Gastrointestinal adverse effects have been described with the use of all phosphate binders in naive patients.30 It is important to note that participants were excluded from this trial if they had known intolerance to sevelamer and calcium acetate, possibly accounting for differences in mild to moderate gastrointestinal adverse effects associated with ferric citrate compared to active control.11 Darkening of the stool (likely related to the iron compound) and diarrhea have been reported previously with ferric citrate,10,23 although gastrointestinal SAEs occurred in fewer patients receiving ferric citrate versus active control. Because the tolerability of these adverse effects may vary between individuals, these factors ultimately need to be taken into consideration when individualizing binder choice.
One limitation of the study was its open-label design, which was implemented due to the known effects of ferric citrate on stool discoloration, an effect that would make it difficult to conduct a double-blinded study. Second, the 1-year duration cannot allow assessment of effects on longer-term outcomes, including theorized effects of longer-term iron loading. Notably, the median achieved ferritin level of 858 (interquartile range, 568-1,105) ng/mL at the completion of the 52-week active control period11 is similar to the median level of 794 ng/mL reported by the DOPPS (Dialysis Outcomes and Practice Pattern Study) Practice Monitor for US dialysis facilities, consistent with current US anemia management trends.31 While the study included both peritoneal dialysis and hemodialysis patients, most included participants were receiving hemodialysis. The study had several major strengths as well. First, ferric citrate was compared to the 2 most commonly used phosphate binders in clinical practice, allowing for extrapolation to current care environments. Second, detailed and frequent central laboratory results were obtained, showing sustained similar bone and mineral disease control over the course of an entire year. Also, all SAEs were collected and adjudicated by independent study monitors.
In conclusion, ferric citrate maintains similar effects on bone and mineral disease and other important clinical parameters in dialysis patients compared with other commonly used calcium-containing and non—calcium-containing phosphate binders. Calcium, PTH, and aluminum levels were not different in participants receiving either ferric citrate or active control after 1 year of use. There was no evidence that the increase in ferritin levels promoted or reflected PEW/inflammation. Based on its positive effects on these important clinical outcomes and its overall comparable or favorable adverse-effect profile, ferric citrate could be considered as a first-line phosphate binder for many hyperphosphatemic dialysis patients.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge the additional members of the Collaborative Study Group, including the following: United States: I. Cohen, N. Lizzul (Phoenix, AZ); R. Cohen, E. Camp (Tempe, AZ); A. Felsenfeld, S. Graham, E. Daza, K. Knibloe (Los Angeles, CA); C. Sun, L. Estrada (Riverside, CA); W. Chiang, R. Darwish, S. Amini (Whittier, CA); D. Jalal, D. Spiegel, B. Farmer (Denver, CO); I. Chang, H. Beeson (Westminster, CO); K. Kapatkin, T. Laneve (Brandon, FL); P. Fitzpatrick, J. Wright (Jacksonville, FL); A. Rabiei, S. Asghari (Jupiter, FL); M. Seek, D. Usrey (Ocala, FL); S. Zeig, V. Gervais (Pembroke Pines, FL); M. Smith, M. Collins (August, GA); Z. Sharon, D. Darwin (Decatur, GA); M. Sinsakul, D. Jones-O'Brien, K. Lockwood (Chicago, IL); S. Arfeen, S. Martin (Michigan City, IN); E. Reisin, S. Barry (Kenner, LA); D. Weiner, L. Chan, A. Well (Boston, MA); B. Athreya, A. Burkhart (Holyoke, MA); B. Greco, J. Whitbeck (Springfield, MA); K. Nossuli, V. Sharma (Bethesda, MD); K. Umanath, M. Zidan (Detroit, MI); F. Al-Saghir, J. Powell (Pontiac, MI); A. Hiremath, D. Udell (Southgate, MI); A. Pfleuger, D. Hamiel (Rochester, MN); A. Goel, A. Hurst, C. Pope (Kansas City, MO); J. Manley, T. Mueller (Asheville, NC); P. Chuang, D. Griswell (Charlotte, NC); J. Middleton, D. Schumm (Durham, NC); R. Moore, F. Abbot (Wilmington, NC); I. Bowline, V. Mauck (Winston-Salem, NC); W. Shapiro, R. Liang (Brooklyn, NY); F. Whittier, D. Dziegelesk (Canton, OH); S. Kant, H. Duncan (Cincinnati, OH); R. Heyka, R. Naude (Cleveland, OH); U. Bhatt, C. Stratton (Columbus, OH); C. Sholer, D. Dion (Oklahoma City, OK); S. Goral, R. Neubauer (Philadelphia, PA); R. Burgos-Calderon, P.F. Fontanez (Rio Piedras, PR); C. Galphin, C. Yancy-Spurgeon (Chattanooga, TN); D. Linfert, G. Schulman, A. Fortner, J. Giese, S. Meier, J. Zirchenbach (Nashville, TN); P. Van Buren, J. Inrig, T. Tyler, T. Lightfoot (Dallas, TX); A. Basford, S. Fadem, A. Frome, J. Olivero, B. Armentrout, N. Dickson, F. Ricks (Houston, TX); J. Abraham, K. Raphael, J. Zitterkoph (Salt Lake City, UT); K. Bolton, N. Mchedlishviii (Charlottesville, VA); A. Assefi, R. Cheriyan, R. Dadmarz, M. Obeid (Fairfax, VA); O. Ayodeji, L. Jones-Brandon (Hampton, VA); G. Feldman, M. Nicholas (Richmond, VA); D. Negoi, D. de Waal (Burlington, VT); S. Blumenthal, C. Veenendaal (Milwaukee, WI). Israel: Y. Yagil, D. Pinhas (Ashkelon); D. Schwartz, N. Platner (Tel Aviv).
Support: All authors report receiving research support and some received travel support from Keryx Biopharmaceuticals. This study was supported in part by a grant from the Patient Protection and Affordable Care Act of 2010. The Internal Revenue Service issued the funding under the Qualifying Therapeutic Discovery Project administered under section 48D of the Internal Revenue Code. Dr Van Buren receives institutional support as the Dedman Family Scholar in Clinical Care at University of Texas Southwestern Medical Center. He also receives financial support from NIH grant 1K23DK096007-01A1.
Footnotes
Because an author of this article is an editor for AJKD, the peer-review and decision-making processes were handled entirely by an Associate Editor (Rajnish Mehrotra, MD) who served as Acting Editor-in-Chief. Details of the journal's procedures for potential editor conflicts are given in the Information for Authors & Editorial Policies.
This work was presented in part as posters at the American Society of Nephrology's Kidney Week in Atlanta, GA, November 5-10, 2013.
Financial Disclosure: Dr Van Buren has received compensation from Keryx Biopharmaceuticals for participation in an advisory board meeting. The other authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: JBL, JPD, TG, JM, MS; data acquisition: PNVB, JBL, JPD, TG, JM, MS, KU, JDA, SSA, IGB, GC, SZF, SG, MK, MVS, DEW; data analysis/interpretation: PNVB, JBL, JPD, TG, JM, MS, KU, JDA, SSA, IGB, GC, SZF, SG, MK, MVS, DEW; statistical analysis: TG, MS; supervision or mentorship: JBL, JPD, TG, JM, MS, KU, DEW. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. PNVB takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and registered) have been explained.
SUPPLEMENTARY MATERIAL
Table S1: Baseline and follow-up values for phosphorus, calcium, and PTH for ferric citrate, active control, and subgroups.
Table S2: Baseline and follow-up values for PEW/inflammation variables for ferric citrate, active control, and subgroups.
Figure S1: Participant flow through study.
Note: The supplementary material accompanying this article (http://dx.doi.org/10.1053/j.ajkd.2015.03.013) is available at www.ajkd.org
REFERENCES
- 1.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 2.National Kidney Foundation K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4)(suppl 3):S1–S201. [PubMed] [Google Scholar]
- 3.Boyce B, Fell G, Elder H, et al. Hypercalcaemic osteomalacia due to aluminum toxicity. Lancet. 1982;2:1009–1013. doi: 10.1016/s0140-6736(82)90049-6. [DOI] [PubMed] [Google Scholar]
- 4.Swartz R, Dombrouski J, Burnotowska M, Mayor G. Microcytic anemia in dialysis patients: reversible marker of aluminum toxicity. Am J Kidney Dis. 1987;9:217–223. doi: 10.1016/s0272-6386(87)80058-6. [DOI] [PubMed] [Google Scholar]
- 5.Alfrey A, LeGendre G, Kaehny W. The dialysis encephalopathy syndrome. Possible aluminum intoxication. N Engl J Med. 1976;294:184–188. doi: 10.1056/NEJM197601222940402. [DOI] [PubMed] [Google Scholar]
- 6.Qunibi W, Hootkins R, McDowell L, et al. Treatment of hyperphosphatemia in hemodialysis patients: the Calcium Acetate Renagel Evaluation (CARE Study). Kidney Int. 2004;65:1914–1926. doi: 10.1111/j.1523-1755.2004.00590.x. [DOI] [PubMed] [Google Scholar]
- 7.Chertow G, Burke S, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 8.Navaneethan S, Palmer S, Craig J, Elder G, Strippoli G. Benefits and harms of phosphate binders in CKD: a systematic review of randomized controlled trials. Am J Kidney Dis. 2009;54:619–637. doi: 10.1053/j.ajkd.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Yang W, Yang C, Hou C, Wu T, Young E, Hsu C. Anopen-label, crossover study of a new phosphate-binding agent in haemodialysis patients: ferric citrate. Nephrol Dial Transplant. 2002;17:265–270. doi: 10.1093/ndt/17.2.265. [DOI] [PubMed] [Google Scholar]
- 10.Dwyer JP, Sika M, Schulman G, et al. Dose-response and efficacy of ferric citrate to treat hyperphosphatemia in hemodialysis patients: a short-term randomized trial. Am J Kidney Dis. 2015;61(5):759–766. doi: 10.1053/j.ajkd.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 11.Lewis J, Sika M, Koury M, et al. Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol. 2015;26:493–503. doi: 10.1681/ASN.2014020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nolan C, Califano J, Butzin C. Influence of calcium acetate or calcium citrate on intestinal aluminum absorption. Kidney Int. 1990;38:937–941. doi: 10.1038/ki.1990.294. [DOI] [PubMed] [Google Scholar]
- 13.Umanath K, Sika M, Niecestro R, et al. Rationale and study design of a three-period, 58-week trial of ferric citrate as a phosphate binder in patients with ESRD on dialysis. Hemodial Int. 2013;17(1):67–74. doi: 10.1111/j.1542-4758.2012.00711.x. [DOI] [PubMed] [Google Scholar]
- 14.Sinsakul M, Sika M, Koury M, et al. The safety and tolerability of ferric citrate as a phosphate binder in dialysis patients. Nephron Clin Pract. 2012;121(1-2):c25–c29. doi: 10.1159/000341922. [DOI] [PubMed] [Google Scholar]
- 15.PhosLo [package insert] Fresenius Medical Care North America; Waltham, MA: 2007. [Google Scholar]
- 16.Renvela [package insert] Genzyme Corp; Cambridge, MA: 2010. [Google Scholar]
- 17.Rodby R, Umanath K, Niecestro R, et al. Phosphorus binding with ferric citrate is associated wtih fewer hospitalizations and reduced hospitalization costs. Exp Rev Pharmacoecon Outcomes Res. 2014;13:1–6. doi: 10.1586/14737167.2015.995169. [DOI] [PubMed] [Google Scholar]
- 18.Walker G, Aaron J, Peacock M, Robinson P, Davison A. Dialysate aluminum concentration in renal bone disease. Kidney Int. 1982;21:411–415. doi: 10.1038/ki.1982.37. [DOI] [PubMed] [Google Scholar]
- 19.Teng M, Wolf M, Ofsthun M, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 20.Hutchison A, Barnett M, Krause R, Kwan J, Siami G. Lanthanum Study Group. Long-term efficacy and safey profile of lanthanum carbonate: results for up to 6 years of treatment. Nephron Clin Pract. 2008;110:15–23. doi: 10.1159/000149239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slatopolsky E, Liapis H, Finch J. Progressive accumulation of lanthanum in the liver or normal and uremic rats. Kidney Int. 2006;68:2809–2813. doi: 10.1111/j.1523-1755.2005.00753.x. [DOI] [PubMed] [Google Scholar]
- 22.Yang WC, Yang CS, Hou CC, Wu TH, Young EW, Hsu CH. An open-label, crossover study of a new phosphate-binding agent in haemodialysis patients: ferric citrate. Nephrol Dial Transplant. 2002;17(2):265–270. doi: 10.1093/ndt/17.2.265. [DOI] [PubMed] [Google Scholar]
- 23.Yokoyama K, Akiba T, Fukagawa M, et al. A randomized trial of JTT-751 versus sevelamer hydrochloride in patients on hemodialysis. Nephrol Dial Transplant. 2014;29:1053–1060. doi: 10.1093/ndt/gft483. [DOI] [PubMed] [Google Scholar]
- 24.Partridge N, Regnier F, White J, Hem S. Influence of dietary constituents on intestinal absorption of aluminum. Kidney Int. 1989;35:1413–1417. doi: 10.1038/ki.1989.142. [DOI] [PubMed] [Google Scholar]
- 25.Molitoris B, Froment D, Mackenzie T, Huffer W, Alfrey A. Citrate: a major factor in the toxicity of orally administered aluminum compounds. Kidney Int. 1989;36:949–953. doi: 10.1038/ki.1989.286. [DOI] [PubMed] [Google Scholar]
- 26.Rattanasompattikul M, Molnar M, Zaritsky J, et al. Association of malnutrition-inflammation complex and responsiveness to erthyropoeisis-stimulating agents in long-term hemodialysis patients. Nephrol Dial Transplant. 2013;28:1936–1945. doi: 10.1093/ndt/gfs368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Umanath K, Jalal DI, Greco BA, et al. Ferric citrate reduces intravenous iron and erythropoeisis-stimulating agent use in ESRD. J Am Soc Nephrol. doi: 10.1681/ASN.2014080842. published online ahead of print March 3, 2015 http://dx.doi.org/10.1681/ASN. 2014080842. [DOI] [PMC free article] [PubMed]
- 28.Chertow G, Burke S, Dillon M, Slatopolsky E. Rena-gel Study Group. Long-term effects of sevelamer hydro-chloride on the calcium x phosphate product and lipid profile of haemodialysis patients. Nephrol Dial Transplant. 1999;14:2907–2914. doi: 10.1093/ndt/14.12.2907. [DOI] [PubMed] [Google Scholar]
- 29.Braunlin W, Zhorov E, Guo A, et al. Bile acid binding to sevelamer HCl. Kidney Int. 2002;62:611–619. doi: 10.1046/j.1523-1755.2002.00459.x. [DOI] [PubMed] [Google Scholar]
- 30.Bleyer A, Burke S, Dillon M, et al. A comparison of the calcium-free phosphate binder sevelamer hydrochloride with calcium acetate in the treatment of hyperphosphatemia in hemodialysis patients. Am J Kidney Dis. 1999;33:694–701. doi: 10.1016/s0272-6386(99)70221-0. [DOI] [PubMed] [Google Scholar]
- 31.Fuller D, Pisoni R, Bieber B, Port F, Robinson B. The DOPPS practice monitor for US dialysis care: update on trends in anemia management 2 years into the bundle. Am J Kidney Dis. 2013;62:1213–1216. doi: 10.1053/j.ajkd.2013.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.