Abstract
Difficulty regulating emotions following stressful events is a hallmark of Major Depressive Disorder (MDD). Although individuals’ ability to regulate their emotions is believed to have direct consequences for both emotional and physical wellbeing, few studies have examined the cardiovascular effects of different emotion regulation strategies in MDD. To the best of our knowledge, the current study is the first to examine the effects of two emotion regulation strategies, cognitive distraction and rumination, on both self-reported sadness and respiratory sinus arrhythmia (RSA) in individuals with MDD and healthy controls (CTLs). Following a forced-failure stressor, participants were randomly assigned to a rumination or cognitive distraction condition. As expected, rumination increased sadness and triggered RSA withdrawal for both MDDs and CTLs. Interestingly, although cognitive distraction reduced sadness, it also triggered RSA withdrawal. Moreover, cognitive distraction was associated with greater RSA withdrawal for MDDs than CTLs. Thus, although depressed individuals are able to use cognitive distraction to emotionally recover from stress, it may be associated with greater cognitive effort. Adding low-cost physiological measures such as RSA into assessments has the potential to offer new and important information about the effects of emotion regulation on mental and physiological health.
Keywords: depression, stress, emotion regulation, respiratory sinus arrhythmia, autonomic nervous system
Exaggerated emotional and cardiovascular responses to stress are central characteristics of Major Depressive Disorder (MDD; e.g., Brown & Harris, 1986; Lazarus & Folkman, 1984; Monroe & Hadjiyannakis, 2002), and can have detrimental effects on mental and physical wellbeing (for a review see Grippo & Johnson, 2009). In fact, an altered response to stress is a primary pathophysiological mechanism linking MDD to cardiovascular morbidity and mortality. Although past research has focused on individual differences in the initial reactivity to stress, studies increasingly emphasize the importance of examining individuals’ ability to regulate their emotions after the stressor ends (Flynn & Rudolph, 2007; Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008). For example, models of stress and health posit that emotion regulation strategies are critical determinants of emotional and cardiovascular health, particularly for those with psychopathology (Brosschot, Gerin, & Thayer, 2006; Kubzansky, Davidson, & Rozanski, 2005). Despite this, little is known about the effects of using different emotion regulation strategies on cardiovascular activity in MDD.
The parasympathetic nervous system plays an important role in regulating cardiovascular activity. More specifically, the parasympathetic nervous system influences oscillations in heart rate via its effect on the vagus nerve, which affects heart rate acceleration and deceleration during respiration. Whereas vagal activity is temporarily suppressed during inhalation, causing heart rate to increase, vagal activity resumes during exhalation, causing heart rate to decrease. Greater parasympathetic input leads to greater heart rate acceleration after inhalation and greater heart rate deceleration after exhalation. Thus, greater parasympathetic activity is associated with greater variability in the interval between heartbeats. Respiratory sinus arrhythmia (RSA) has been identified as an optimal marker of this beat-to-beat variation in heart rate linked to the respiratory cycle. RSA offers advantages over other measures of the parasympathetic nervous system (e.g., low-frequency heart rate variability) given that it is unconfounded by sympathetic activation (Berntson, Cacioppo, & Quigley, 1993). RSA also has advantages over other measures of biological functioning. For one, RSA is relatively inexpensive in that the per subject cost of data collection is minimal. RSA data collection is also far less invasive than other methods for collecting biological data. In fact, with the development of technology to collect ambulatory RSA, it is increasingly feasible to measure RSA data in applied research and clinical settings with minimal or no disruption to the individual (Cullins et al., 2013; Goedhart, de Vries, Kreft, Bakker, & de Geus, 2008; Grossman, Wilhelm, & Spoerle, 2004).
Given the advantages of collecting RSA data, it is not surprising that RSA is used frequently to index parasympathetic activity (see Berntson, Cacioppo, & Grossman, 2007, for an overview). Parasympathetic activity, and thus RSA, is high during times of rest, when energy expenditure is actively reduced (e.g., by inhibiting the sympathetic innervations of the heart; Lovallo & Thomas, 2000; Rottenberg, 2007). However, when environmental demands become more challenging, such as in a time of stress, this “brake” (Rottenberg, 2007) is rapidly withdrawn in order to meet environmental demands. Thus, adaptive parasympathetic responses to stress trigger moderate RSA withdrawal (Blair & Peters, 2003; Kreibig, 2010). RSA withdrawal allows autonomic changes that prime the body for action and facilitate responses to environmental changes, including better executive functioning and social competence (Blair & Peters, 2003; Marcovitch et al., 2010). In contrast to adaptive physiological responses to stress, exaggerated reactivity or delayed recovery (evidenced by greater or longer RSA withdrawal) places the body under excessive strain and increases risk for cardiovascular and other organic diseases (Crowell, Skidmore, Rau & Williams, 2013). To facilitate recovery, the body activates the parasympathetic nervous system, leading to increased RSA. Individual differences in the use of emotion regulation strategies may be associated with different amounts of parasympathetic activation, and these differences may be particularly relevant for individuals with MDD, who are at greater risk for poor health outcomes, including cardiovascular disease (Keicolt-Gaser & Glaser, 2002).
Past research on emotion regulation and recovery from stress in MDD focuses largely on self-reported sadness. Whereas adaptive emotion regulation strategies facilitate recovery from stress, maladaptive emotion regulation strategies prolong depressed mood. Distraction is typically considered an adaptive emotion regulation strategy in the short-term given that it decreases sadness and facilitates engagement in behaviors that are likely to further improve mood (Donaldson & Lam, 2004; Watkins, Teasdale, & Williams, 2000). Both healthy controls and individuals with MDD are able to effectively down-regulate negative emotions when exposed to a distraction induction (for a review see Nolen-Hoeksema et al., 2008). Unlike healthy controls, however, individuals with MDD do not often spontaneously engage in distraction in everyday life. This may be due, in part, to the fact that it is difficult for depressed individuals to disengage from negative thoughts, resulting in an unproductive pattern of ruminative thinking (Joormann, 2010; Nolen-Hoeksema, Morrow, & Fredrickson, 1993). The response styles theory (Nolen-Hoeksema, 1991; Nolen-Hoeksema et al., 2008) defines rumination as repeatedly thinking about negative feelings and the potential antecedents or repercussions of those feelings. In contrast to distraction, ruminative responses to stress increase the duration and severity of depressed moods (Nolen-Hoeksema et al., 1993).
Although the emotional consequences of rumination and distraction have been well established (see Lyubomirsky & Tkach, 2004, and Nolen-Hoeksema et al., 2008, for reviews), less is known about the effects of these emotion regulation strategies on RSA. Initial evidence indicates that both anger and depressive rumination are associated with parasympathetic withdrawal (Key, Campbell, Bacon, & Gerin, 2008; Ottaviani, Shapiro, Davydov, Goldstein, & Mills, 2009), whereas distraction is associated with parasympathetic activation (Gerin, Davidson, Christenfeld, Goyal, & Schwartz, 2006; Ottaviani et al., 2009; Ottaviani et al., 2011). The majority of studies use passive distraction tasks, which involve passively viewing neutral movies or images (Gerin et al., 2006) or overhearing a fabricated phone conversation (Ottaviani et al., 2009; 2011). By contrast, other studies use cognitive distraction tasks, which require greater cognitive control and demand. Cognitive distraction has been shown to be effective at improving negative mood (Eisenberg & Morris, 2003; Eisenberg, Morris, & Spinrad, 2008). The effect of cognitive distraction on parasympathetic activity, however, is more uncertain and has not been examined in MDD.
The primary goal of the current study was to compare clinically depressed and healthy control participants’ parasympathetic activity during a rumination and cognitive distraction induction. In the process, we hoped to demonstrate the importance and feasibility of adding physiological measures to assessments of emotion regulation. In this study, participants were exposed to a forced-failure stressor, selected for its ability both to induce sad mood (Hammen, 2005) and to trigger biological changes (Kirschbaum, Pirke, & Hellhammer, 1993). Following the forced-failure task, participants were randomly assigned either to ruminate on their poor performance or to cognitively distract themselves from it. We measured RSA reactivity before and during the forced-failure and emotion regulation periods. Whereas we expected rumination to be associated with RSA withdrawal in both the MDD and control groups, we expected cognitive distraction to be associated with RSA activation in both groups.
Method
Participants
Inclusion and exclusion criteria for the current study were determined via an in-person Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 2002), which was conducted by trained graduate students. Strong inter-rater reliability was established, κ = 1.00. Participants were included if they were between the ages of 18 and 60 and were in one of two groups: those who met criteria for current major depressive disorder (MDD; n = 47), and those who did not meet criteria for any past or current Axis I disorder (Control: CTL; n = 51). Participants were excluded due to severe head trauma, learning disabilities, bipolar disorder, psychotic symptoms, alcohol or substance abuse within the past six months, or medical conditions that would affect psychophysiological recordings (e.g., heart murmur). Data from 7 participants with MDD and 4 CTLs could not be included due to excessive movement artefacts or signal error. Thus, the final sample included 40 participants in the MDD group and 47 in the CTL group.
Forced-Failure Stressor
Three forced-failure tasks were used to elicit distress. The first was a facial identification task with false feedback indicating that the participant performed poorly. This 10-minute task was adapted from Tran, Siemer, and Joormann (2011). Participants were asked to identify the emotional expression (happy, sad, angry) depicted in subliminally presented facial expressions. Participants repeatedly received feedback that they were performing poorly relative to others who had already completed the task, and the experimenter urged participants to try harder. The second task was an anagram task, in which approximately 30% of the anagrams were unsolvable. Participants were given 5 minutes to solve as many anagrams as possible but allowed only 30 seconds to solve each anagram. In the third task, participants were given 5 minutes to count backward aloud from 2,083 to zero in 13-step sequences (Kirschbaum et al., 1993).
Rumination and Distraction Induction
Participants were randomly assigned to either a rumination or cognitive distraction condition, which was adapted from the standard rumination and distraction (R/D) task created by Nolen-Hoeksema and Morrow (1993). Regardless of the condition, participants viewed seven prompts one-at-a-time on the computer screen, and they were asked to think and write about each prompt for two minutes. Rumination prompts focused participants’ attention on thoughts that were emotion or self-focused (e.g., ‘why your performance on the tests earlier today made you feel the way it did’ or ‘what people would think of you if they had observed you complete the tests earlier today’). In contrast, cognitive distraction prompts focused participants’ attention on thoughts unrelated to the self (e.g., ‘how to make a peanut-butter-and-jelly sandwich. Describe it in detail, with as many steps as possible.’ or ‘the layout of your local mall. Walk the entire length in your mind and describe the stores, things, or people you would see’). Participants’ written statements were later coded based on Hilt and Pollak (2013) by two independent raters who were blind to group and condition. Rumination score ratings were made on a 5-point Likert scale ranging from 1 (Not at all ruminating) to 5 (Completely ruminating), ICC = .84.
Measures
Sadness ratings
We examined sadness ratings assessed at 4 points: at the end of the baseline period, in the middle of the forced-failure induction, immediately before the R/D induction, and immediately after the R/D induction. Participants rated their sadness on an 11-point Likert-scale ranging from 0 (not at all) to 10 (very much). We focused on sadness as opposed to other metrics of affect (e.g., general negative affect) given that sadness is central to MDD, a common response to forced-failure stressors (Hammen, 2005), and the affective dimension most directly targeted by our rumination induction (Nolen-Hoeksema & Morrow, 1993).
Respiratory sinus arrhythmia (RSA)
Electrocardiograph (ECG) and respiration frequency were recorded with a computer-based data acquisition system (MP150, Biopac Systems). Three standard electrodes were attached bilaterally to participants’ left and right upper rib cage and right collarbone. To measure respiration frequency, a strain-gauge transducer belt was attached around the chest above the ribcage and below the bust. Data was collected using BIOPAC bioamplifiers. The ECG and respiration frequency signals were sampled at a rate of 1,000 Hz, digitized with a 16-bit analog-to-digital converter, and processed using AcqKnowledge and MindWare software. Following recommendations of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996), we used a frequency domain measure of heart rate variability, namely RSA. Thus, vagal activation was indexed by measuring heart rate variability in the frequency band between .14 and .4 Hz (high frequency- heart rate variability). R-wave markers in the ECG signal were evaluated for artifacts by visual inspection and the MAD/MED artifact detection algorithm implemented in MindWare software (Mindware Heart Rate Variability Application, version 2.51; Mindware Technologies Ltd.). Identified artifacts were then manually corrected. Beat-to-beat interval series were obtained from the ECG and converted into time series of instantaneous beat-to-beat intervals with a resolution of 4 Hz. Spectral analysis using the Welch method determined the power spectral density in the frequency band between .14 and .4 Hz. This value is then automatically log-transformed to correct for the non-normal distribution, and minute-by-minute estimates of RSA were determined. This approach accords with best-practice guidelines for frequency domain methods to calculate RSA (Berntson et al., 1993; Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). Data were then analyzed in 5-minute increments, or as close to this interval as tasks would allow. Specifically, RSA was measured during the following four periods: 1. During the 5-minute nature video (baseline), which occurred immediately before the forced-failure stressor; 2. During the first 5 minutes of the forced-failure stressor; 3. During the 5 minutes immediately before the R/D induction, when participants were completing the forced-failure stressor (approximately 15–20 minutes after the forced-failure onset); and 4. During the first 6 minutes of the R/D induction (i.e., during the first three R/D prompts).
Questionnaires
Participants completed the Beck Depression Inventory-II (BDI; Beck, Steer, & Brown, 1996), a 21-item measure assessing depressive symptom severity (α = .97). Additionally, participants completed the Ruminative Responses Scale of the Response Style Questionnaire (RRS; Nolen-Hoeksema & Morrow, 1991), a 22-item self-report questionnaire assessing individual differences in the tendency to ruminate when sad (α = .99).
Procedure
After participants provided informed consent, approximately 5 minutes were taken to attach psychophysiological equipment and ensure the accurate collection of RSA data. Participants then watched a 5-minute nature video followed by the forced-failure stressor. Next, participants were randomly assigned to the rumination or cognitive distraction condition of the R/D induction. Before leaving the laboratory, participants watched a calming nature video in order to provide ample time for mood to return to baseline. Participants completed demographic and clinical questionnaires at the end of the experiment.
Data Analysis
Analyses were conducted with SPSS version 20.0. Baseline and demographic data were analyzed via one-way analysis of variance (ANOVA) and chi square tests. To examine the effect of the forced-failure stressor and R/D induction on self-reported sadness and RSA, we examined the changes in sadness and RSA as a result of the forced-failure stressor and R/D induction. For example, sadness reactivity to the forced-failure stressor was calculated as sadness rated during the forced-failure induction minus sadness at baseline. Sadness reactivity to the R/D induction was calculated as sadness rated after the R/D induction minus sadness immediately before the R/D induction. Positive scores indicate increases in sadness; negative scores indicate decreases in sadness. Similarly, RSA reactivity during the forced-failure stressor was calculated as RSA measured during the first five minutes of the forced-failure stressor minus RSA measured during the five minutes immediately before the forced-failure stressor. RSA reactivity during the R/D induction was calculated as RSA measured during the first six minutes of the R/D induction minus RSA measured during the five minutes immediately before the R/D induction. Positive scores indicate RSA activation; negative scores indicate RSA withdrawal. Univariate ANOVAs were used to examine reactivity and recovery scores by group (MDD, CTL) and condition (rumination, cognitive distraction).
Results
Participant and Baseline Characteristics
Demographic and clinical data are presented in Table 1. There was no significant difference in ethnicity across group or condition, χ2s(1, N=86) < 1, and ethnicity did not significantly differ by group within the rumination, χ2s(1, N=43) < 1, or cognitive distraction conditions, χ2s(1, N=43) < 1, ps > .05. Age also did not significantly differ across groups or conditions, Fs(1, 83) < 1, and age did not significantly differ by group within the rumination, t(42) = 1.66, or cognitive distraction conditions, t(41) = 1.40, ps > .05. Although there was a significant difference in proportion who were female across group, χ2(1, N=87) = 3.86, p = .05, and condition, χ2(1, N=87) = 5.09, p < .05, the proportion female did not differ across diagnostic group within the rumination, χ2(1, N=44) = 2.93, or cognitive distraction condition, χ2(1, N=43) = 1.28, ps > .05. As expected, BDI (depressive symptoms), RRS (trait rumination), and baseline sadness ratings were significantly higher in the MDD versus CTL group, F(1, 82) > 39.19, ps < .001. Scores, however, did not differ by R/D condition, and the group by condition interaction was not significant, Fs(1, 82) < 1, ps > .05. Baseline RSA did not differ by group or condition, and the group by condition interaction was not significant, Fs(1, 83) < 1, ps > .05.
Table 1.
Participant Characteristics
| CTL (N = 47) |
MDD (N = 40) |
|||
|---|---|---|---|---|
| Variable | Rumination | Distraction | Rumination | Distraction |
| Age, M (SD) | 34.63 (12.00) | 40.74 (11.30) | 40.65 (11.96) | 35.70 (12.28) |
| Sex (female:male) | 5:19 | 11:12 | 9:11 | 13:7 |
| Caucasian, % | 39.13 | 39.13 | 35.00 | 30.00 |
| BDI, M (SD) | 3.21 (6.26) | 4.00 (5.29) | 29.84 (13.10) | 31.95 (11.07) |
| RRS, M (SD) | 31.91 (10.33) | 31.13 (8.07) | 66.16 (9.01) | 68.00 (11.12) |
| Rumination Score, M (SD) | 10.58 (3.61) | 7.23 (0.43) | 16.50 (4.88) | 7.80 (2.02) |
R/D Induction Manipulation Check
Participants’ written responses during the R/D induction were coded for the extent to which they were ruminating (see Rumination Score in Table 1). There was a main effect of R/D condition, F(1, 82) = 76.38, p < .001, η2 = .48, and a main effect of diagnostic group, F(1, 82) = 22.13, p < .001, η2 = .21. However, these main effects were qualified by a significant group by condition interaction, F(1, 82) = 15.01, p < .001, η2 = .16. Although MDDs in the rumination condition received higher rumination scores than CTLs in the rumination condition, F(1, 42) = 21.31, p < .001, rumination scores did not differ between MDDs and CTLs in the cognitive distraction condition, F(1, 40) = 1.70, p > .05. Importantly, individuals assigned to the rumination condition received higher rumination scores than participants in the cognitive distraction condition in both the MDD, F(1, 40) = 54.25, p < .001, η2 = .59, and CTL group, F(1, 44) = 18.73, p < .001, η2 = .30.
Self-Reported Sadness
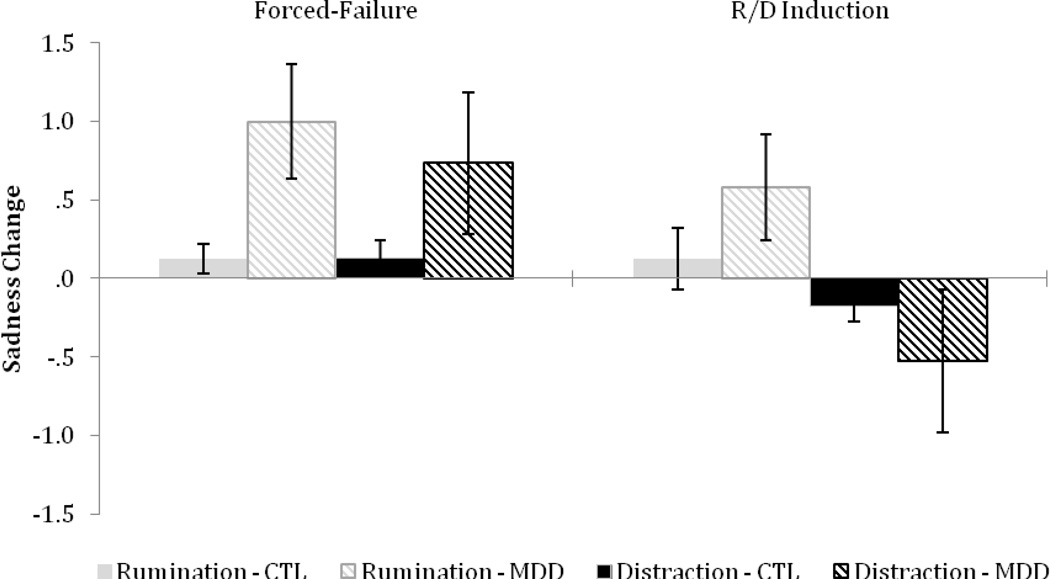
Self-reported sadness was examined to ensure the forced-failure induction and R/D induction had the anticipated effects (see Figure 1). As expected, compared to CTLs, the MDD group reported greater sadness reactivity to the forced-failure stressor, F(1, 81) = 7.36, p < .01, η2 = .08. Sadness reactivity to the failure stressor did not significantly differ by R/D condition, and the group by condition interaction was not significant, F(1, 81) < 1, ps > .05, η2 < .01, suggesting that random assignment was effective. Sadness reactivity to the R/D induction did not differ by group, F(1, 81) < 1, p > .05, η2 < .01. However, individuals in the cognitive distraction and rumination condition differed in their emotional response to the R/D induction, F(1, 81) = 6.20, p < .05, η2 = .07. As was anticipated, participants’ self-reported sadness decreased in the cognitive distraction condition but increased in the rumination condition. The group by condition interaction was not significant, F(1, 81) = 2.04, p > .05, η2 = .03.
Figure 1.
Change in sadness in response to the forced-failure stressor and the emotion regulation induction in CTLs and MDDs in the rumination and distraction condition. Higher scores indicate increased sadness during the forced-failure or Rumination and Distraction (R/D) induction period. Error bars indicate +/− 1 SE.
RSA
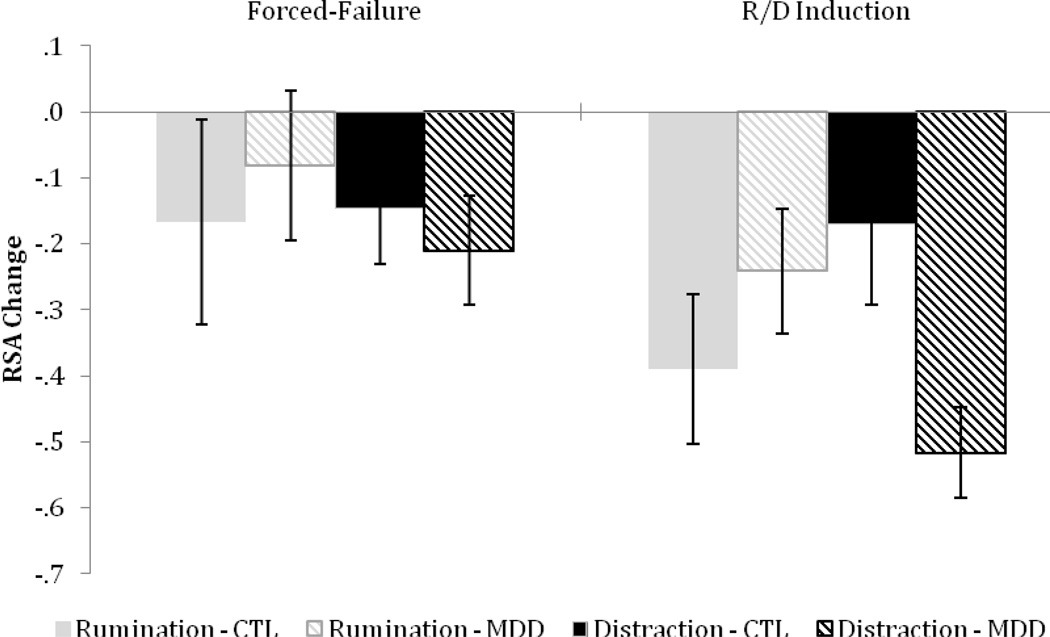
We first conducted a repeated-measures ANOVA with diagnostic-group and R/D condition as the between-subject factors and time as the within-subject factor. This yielded a main effect of time, F(3, 249) = 15.51, p < .001, η2 = .16, which was qualified by the interaction between diagnostic group, R/D condition, and time at the cubic level, F(1, 83) = 3.81, p = .05, η2 = .04. No other main or interaction effects were significant, ps > .05. Follow-up tests conducted on RSA change scores indicated that, as expected, there was a significant RSA withdrawal in response to the forced-failure stressor, t(86) = 2.64, p = .01 (see Figure 2). Importantly, RSA change during forced-failure induction did not significantly differ by group or condition, and the group by condition interaction was not significant, Fs(1, 83) < 1, ps > .05, η2 < .01.
Figure 2.
Change in RSA in response to the forced-failure stressor and emotion regulation induction in CTLs and MDDs in the rumination and distraction condition. Higher scores indicate increased RSA during the forced-failure or Rumination and Distraction (R/D) induction period. Error bars indicate +/− 1 SE.
We examined RSA change during the R/D induction to test our main hypotheses. Across conditions, there was a significant RSA withdrawal during the R/D induction, t(86) = 6.10, p < .001. Whereas RSA withdrawal during the R/D induction did not significantly differ by diagnostic group or R/D condition, Fs(1, 83) < 1, ps > .05, η2 < .01, the group by condition interaction was significant, F(1, 83) = 5.53, p < .05, η2 = .06.1 Follow-up tests showed that RSA withdrawal during rumination did not significantly differ between CTL and MDD groups, F(1, 42) < 1, p > .05, η2 = .02. In contrast, RSA withdrawal during cognitive distraction was significantly greater for MDDs than CTLs, F(1, 41) = 5.66, p < .03, η2 = .12. Within-group follow-up tests indicated that RSA withdrawal did not differ between CTLs in the rumination and distraction condition, F(1, 45) = 1.74, p > .05, η2 = .04, but RSA withdrawal was significantly greater for MDDs in the distraction versus rumination condition, F(1, 38) = 5.54, p < .03, η2 = .13.
Discussion
Even though previous studies have examined emotional responses to rumination and distraction in MDD, little is known about the consequences of using these emotion regulation strategies on RSA. To the best of our knowledge, the current study is the first to examine whether clinically depressed and healthy control participants display different affective and parasympathetic responses to a rumination and cognitive distraction induction. As expected, rumination increased sadness and triggered RSA withdrawal for both MDDs and CTLs. Interestingly, although cognitive distraction reduced sadness, it also triggered RSA withdrawal. Moreover, cognitive distraction was associated with greater RSA withdrawal for MDDs than CTLs. For MDDs, RSA withdrawal was also greater during cognitive distraction than rumination.
RSA withdrawal during rumination is in line with numerous studies reporting that both anger and depressive rumination is associated with less parasympathetic activation in analogue samples (e.g., Gerin et al., 2006; Key et al., 2008; Ottaviani et al., 2009). It also parallels the broader literature, which finds cardiovascular activation during perseverative thinking – including worry, rumination, or trauma recall – in analogue samples (Brosschot et al., 2006; Verkuil et al., 2009). Our data extend this literature by comparing clinically depressed and healthy control participants: Results demonstrate that the effect of rumination on RSA withdrawal did not significantly differ between MDDs and CTLs. This finding is relevant to models of cardiovascular disease that include rumination as a mechanism through which MDD heightens risk for cardiovascular morbidity and mortality (Brosschot et al., 2006; Rozanski, Blumenthal, & Kaplan, 1999; Rugulies, 2002). More specifically, results from our study suggest that depressed individuals may be at heightened risk for cardiovascular disease not because rumination has a greater effect on RSA in MDDs than in CTLs, but because MDDs spend more time using an emotion regulation strategy that is associated with parasympathetic withdrawal. Given that rumination produced similar RSA withdrawal in the MDD and CTL groups, models of cardiovascular risk might include not only the cardiovascular effects of rumination but also the frequency with which individuals ruminate in everyday life.
In addition, the current study examined the effects of cognitive distraction on RSA and found RSA withdrawal instead of RSA activation. Our results stand in contrast to observations of cardiovascular recovery during passive distraction (e.g., Gerin et al., 2006; Ottaviani et al., 2008; Ottaviani et al., 2009). For example, cardiovascular recovery from stress – indexed via increased parasympathetic activity or decreased sympathetic activity – has been observed when passively viewing neutral pictures and posters (Gerin et al., 2006) or when distracted by overhearing a fabricated phone conversation (Ottaviani et al., 2008; 2009; 2011). An important difference between our distraction task and passive distraction tasks is that our task required participants to engage in effortful control over their thoughts. In fact, it is this effort that has been posited to drive parasympathetic withdrawal during cognitive tasks (Luft et al., 2009; Reynard et al., 2011). Interestingly, greater effort has long been associated with parasympathetic withdrawal (Lundberg & Frankenhaeuser, 1980). Moreover, evidence from other experimental paradigms shows that tasks requiring cognitive effort lead to parasympathetic withdrawal (Duschek, Muckenthaler, Werner, & Reys del Paso, 2009; Luft, Takase, & Darby, 2009; Reynard, Gevirtz, Berlow, Brown, & Boutelle, 2011; Verkuil, Brosschot, Borkovec, & Thayer, 2009; although see also Butler, Wilhelm, & Gross, 2006, and Segerstrom & Solberg Nes, 2007, for evidence of parasympathetic activation). For example, studies report less parasympathetic activity when participants control the content of thoughts by thinking and writing about an assigned topic compared to baseline parasympathetic activity (Reynard et al., 2011) or when participants think about moral questions (“Is it appropriate for your friend to misrepresent his curriculum vitae in order to get a job?”; Verkuil et al., 2009). Luft et al. (2009) also reported lower parasympathetic activity during tasks requiring high versus low cognitive effort. More specifically, greater parasympathetic withdrawal was observed during a one-back working memory task compared to a simple reaction time task.
We also observed greater RSA withdrawal during cognitive distraction in the MDD versus CTL group. Given evidence connecting effort and parasympathetic withdrawal (Lundberg & Frankenhaeuser, 1980), greater RSA withdrawal in the MDD group could reflect greater effort required for depressed individuals to engage in cognitive distraction following sadness-inducing failure. This interpretation is in line with behavioral data showing that depressed participants are slower to disengage from negative material (see reviews by Gotlib & Joormann, 2010; Joormann, 2010; Koster, De Lissnyder, Derakshan, & De Raedt, 2011). It is further supported by evidence that depressed individuals show greater activation in brain regions subserving cognitive control (dorsal anterior cingulate cortex and the insula) when removing negative material from working memory (Foland-Ross, Hamilton, Joormann, Berman, Jonidees, & Gotlib, 2013). Furthermore, during cognitive distraction, individuals with remitted depression exhibited increased neural activity compared to healthy controls in the regulating control-network, including the anterior cingulate (Kanske, Heissler, Schönfelder, & Wessa, 2012). The idea of cognitive effort leading to parasympathetic withdrawal is also in line with research showing lower parasympathetic activity in other clinical groups when using adaptive, yet effortful, emotion regulation strategies (Aldao & Mennin, 2012). Specifically, Aldao and Mennin found that individuals with generalized anxiety disorder exhibited lower parasympathetic activity when using acceptance or reappraisal to regulate their emotions compared to when passively viewing a film. Importantly, in the current study, despite group differences in parasympathetic withdrawal during cognitive distraction, both healthy controls and clinically depressed participants were able to use distraction to repair the sad mood that was induced during the forced-failure period. Thus, although cognitive distraction appears to facilitate emotional recovery from stress, it may be associated with greater cognitive effort for individuals with MDD. In fact, for individuals with MDD, RSA withdrawal was even greater during cognitive distraction than rumination, further emphasizing the effort required for this population to engage in cognitive distraction. The fact that we observed a different pattern of findings for RSA and self-reported affect in the MDD versus CTL group underscores the importance of adding physiological measures to clinical assessments.
It is also noteworthy that, in this study, baseline RSA did not differ between MDD and CTL participants. Interestingly, the literature on baseline RSA in depression is mixed. Whereas some studies report lower RSA levels in depressed compared to control participants (Dalack & Roose, 1990; Lehofer et al., 1999; Rottenberg, Clift, Bolden, & Salomon, 2007), others report no differences in RSA levels (e.g., Lehofer et al., 1997; Moser et al., 1998). Although a meta-analysis conducted by Rottenberg (2007) found that depression was associated with lower levels of resting RSA (d = .33), only 6 of the 39 comparisons indicated a significant difference between depressed and control participants, and depression accounted for only about 2% of the variance in RSA. Moreover, Rottenberg (2007) concludes that the overall effect of depression on RSA likely reflects an upper-bound estimate that may be very modest after accounting for confounds and unpublished studies, thereby casting doubt on the effects of depression on baseline RSA. When incorporating our findings on baseline RSA into the broader literature, it is important to consider the fact that we measured baseline RSA while participants were watching a calming nature video, which may have served as a passive distraction task, which has been shown to increase parasympathetic activity (Gerin et al., 2006; Ottaviani et al., 2009; 2011). We, therefore, caution against direct comparison between baseline RSA measured in the current study with that measured in studies where participants are not engaging in any activity.
The current study has several limitations. For one, comorbid health conditions were determined based on participants’ self report, and it is possible that participants may have been unaware of prior cardiovascular conditions that would have affected RSA. However, using reactivity and recovery, as opposed to raw scores, minimized external sources of variability. Nonetheless, future work might consider including a physical checkup. Future studies might also include additional measures of cardiovascular health, including blood pressure or pre-ejection period (PEP). A second limitation is that the current study did not directly assess self-reported effort, which prevents us from drawing a direct connection between effort and parasympathetic withdrawal during the cognitive distraction induction. Lastly, despite random assignment to the cognitive distraction and rumination conditions, there was an unequal proportion of female participants across cells. Importantly, however, the group by condition interaction on RSA recovery remained significant when sex was included in the model.
Despite these limitations, the current study offers a unique examination of parasympathetic activity during a rumination and cognitive distraction induction in clinically depressed versus healthy control participants. Findings demonstrate that the effect of rumination on RSA did not significantly differ between the MDD and CTL groups. In addition, the current study provides the first evidence of the effects of cognitive distraction on RSA in MDD. Our results demonstrate that distraction leads to greater parasympathetic withdrawal, indexed via RSA withdrawal, for MDDs than CTLs, which may reflect that depressed individuals require additional effort to disengage from negative material. We hope that this study also demonstrates the importance and feasibility of incorporating physiological measures into assessments of emotion regulation. Assessing RSA activity can provide information about parasympathetic activation that cannot be obtained from self-report measures. Thus, information on RSA could help understand the effects of different emotion regulation strategies on patients’ physical health, which is especially relevant for patients with comorbid health conditions. In addition, to the extent that RSA withdrawal indexes cognitive effort, examining changes in RSA during cognitive distraction may prove a useful tool to assess skill integration as treatment progresses. With practice, cognitive distraction should become less effortful for depressed individuals, leading to less RSA withdrawal. These assessment options are possible given that RSA provides a low-cost physiological measure that can be assessed with minimal disruption in applied research and clinical settings. With the development of devices to collect ambulatory RSA, barriers to data collection have decreased even further. Future work might examine parasympathetic activation during other emotion regulation strategies, including the use of emotion regulation strategies in naturalistic settings. Additionally, investigators conducting research in applied or clinical settings might test whether parasympathetic withdrawal during cognitive distraction decreases with treatment or practice.
Acknowledgements
This work was supported by grant F31-MH086246.
Footnotes
Including sex as a covariate did not affect the significant diagnostic group by R/D condition interaction, F(1, 79) = 6.76, p = .01, η2 = .08. There were no significant main or interactive effects with sex, Fs < 1.
References
- Aldao A, Mennin DS. Paradoxical cardiovascular effects of implementing adaptive emotion regulation strategies in generalized anxiety disorder. Behaviour Research and Therapy. 2012;50(2):122–130. doi: 10.1016/j.brat.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the beck depression inventory. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Berntson GG, Cacioppo JT, Grossman P. Whither vagal tone. Biological Psychology. 2007;74(2):295–300. doi: 10.1016/j.biopsycho.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30(2):183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Blair C, Peters R. Physiological and neurocognitive correlates of adaptive behavior in preschool among children in Head Start. Developmental Neuropsychology. 2003;24(1):479–497. doi: 10.1207/S15326942DN2401_04. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research. 2006;60(2):113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris T. Stressor, vulnerability and depression: a question of replication. Psychological Medicine. 1986;16(04):739–744. doi: 10.1017/s0033291700011740. [DOI] [PubMed] [Google Scholar]
- Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43:612–622. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Crowell SE, Skidmore CR, Rau HK, Williams PG. Psychosocial Stress, Emotion Regulation, and Resilience in Adolescence. In: O’Donohue WT, Benuto LT, Tolle LW, editors. Handbook of Adolescent Health Psychology. New York: Springer; 2013. pp. 129–141. [Google Scholar]
- Cullins SW, Gevirtz RN, Poeltler DM, Cousins LM, Harpin RE, Muench F. An exploratory analysis of the utility of adding cardiorespiratory biofeedback in the standard care of pregnancy-induced hypertension. Applied Psychophysiology and Biofeedback. 2013;38(3):161–170. doi: 10.1007/s10484-013-9219-4. [DOI] [PubMed] [Google Scholar]
- Dalack GW, Roose SP. Perspectives on the relationship between cardiovascular disease and affective disorder. Journal of Clinical Psychiatry. 1990;51:4–11. [PubMed] [Google Scholar]
- Donaldson C, Lam D. Rumination, mood and social problem-solving in major depression. Psychological Medicine. 2004;34(7):1309–1318. doi: 10.1017/s0033291704001904. [DOI] [PubMed] [Google Scholar]
- Duschek S, Muckenthaler M, Werner N, Reyes de Paso GA. Relationships between features of autonomic cardiovascular control and cognitive performance. Biological Psychology. 2009;81(2):110–117. doi: 10.1016/j.biopsycho.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Morris AS. Children’s emotion-related regulation. Advances in Child Development and Behavior. 2003;30:189–229. [PubMed] [Google Scholar]
- Eisenberg N, Morris AS, Spinrad TL. Emotion-related regulation: The construct and its measurement. In: Teti DM, editor. Handbook of Research Methods in Developmental Science. Vol. 6. 2008. pp. 423–442. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/ PSY SCREEN) New York: Biometrics Research, New York State Psychiatric Institute; 2002. Nov, [Google Scholar]
- Flynn M, Rudolph KD. Perceptual asymmetry and youths’ responses to stress: Understanding vulnerability to depression. Cognition and Emotion. 2007;21(4):773–788. doi: 10.1080/02699930600824635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross LC, Hamilton JP, Joormann J, Berman MG, Jonides J, Gotlib IH. The neural basis of difficulties disengaging from negative irrelevant material in major depression. Psychological Science. 2013;24(3):334–344. doi: 10.1177/0956797612457380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerin W, Davidson KW, Christenfeld NJ, Goyal T, Schwartz JE. The role of angry rumination and distraction in blood pressure recovery from emotional arousal. Psychosomatic Medicine. 2006;68(1):64–72. doi: 10.1097/01.psy.0000195747.12404.aa. [DOI] [PubMed] [Google Scholar]
- Goedhart AD, de Vries M, Kreft J, Bakker FC, de Geus EJ. No effect of training state on ambulatory measures of cardiac autonomic control. Journal of Psychophysiology. 2008;22(3):130–140. [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annual review of clinical psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: A review of neurobiological mechanisms and the integration of research from preclinical disease models: Review. Stress: The International Journal on the Biology of Stress. 2009;12(1):1–21. doi: 10.1080/10253890802046281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Wilhelm FH, Spoerle M. Respiratory sinus arrhythmia, cardiac vagal control, and daily activity. American Journal of Physiology-Heart and Circulatory Physiology. 2004;56(2):H728–H734. doi: 10.1152/ajpheart.00825.2003. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annual Review in Clinical Psychology. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hilt LM, Pollak SD. Characterizing the ruminative process in young adolescents. Journal of Clinical Child & Adolescent Psychology. 2013;42(4):519–530. doi: 10.1080/15374416.2013.764825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J. Cognitive inhibition and emotion regulation in depression. Current Directions in Psychological Science. 2010;19(3):161–166. [Google Scholar]
- Joormann J, Gotlib IH. Updating the contents of working memory in depression: interference from irrelevant negative material. Journal of Abnormal Psychology. 2008;117(1):182–192. doi: 10.1037/0021-843X.117.1.182. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Emotion regulation in depression: Relation to cognitive inhibition. Cognition and Emotion. 2010;24(2):281–298. doi: 10.1080/02699930903407948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schönfelder S, Wessa M. Neural correlates of emotion regulation deficits in remitted depression: The influence of regulation strategy, habitual regulation use, and emotional valence. Neuroimage. 2012;61(3):686–693. doi: 10.1016/j.neuroimage.2012.03.089. [DOI] [PubMed] [Google Scholar]
- Key BL, Campbell TS, Bacon SL, Gerin W. The influence of trait and state rumination on cardiovascular recovery from a negative emotional stressor. Journal of Behavioral Medicine. 2008;31(3):237–248. doi: 10.1007/s10865-008-9152-9. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R. Depression and immune function: central pathways to morbidity and mortality. Journal of Psychosomatic Research. 2002;53(4):873–876. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Koster EH, De Lissnyder E, Derakshan N, De Raedt R. Understanding depressive rumination from a cognitive science perspective: The impaired disengagement hypothesis. Clinical Psychology Review. 2011;31(1):138–145. doi: 10.1016/j.cpr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Kreibig SD. Autonomic nervous system activity in emotion: A review. Biological Psychology. 2010;84(3):394–421. doi: 10.1016/j.biopsycho.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Davidson KW, Rozanski A. The clinical impact of negative psychological states: expanding the spectrum of risk for coronary artery disease. Psychosomatic Medicine. 2005;67(Supplement 1):S10–S14. doi: 10.1097/01.psy.0000164012.88829.41. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Appraisal and Coping. New York: 1984. Stress. [Google Scholar]
- Lehofer M, Moser M, Hoehn-Saric R, McLeod D, Liebmann P, Drnovsek B, Zapotoczky HG. Major depression and cardiac autonomic control. Biological Psychiatry. 1997;42(10):914–919. doi: 10.1016/S0006-3223(96)00494-5. [DOI] [PubMed] [Google Scholar]
- Lehofer M, Moser M, Hoehn-Saric R, McLeod D, Hildebrandt G, Egner S, Zapotoczky HG. Influence of age on the parasympatholytic property of tricyclic antidepressants. Psychiatry Research. 1999;85(2):199–207. doi: 10.1016/s0165-1781(99)00005-0. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Thomas TL. Stress hormones in psychophysiological research: Emotional, behavioral, and cognitive implications. In: Cacioppo JT, Tassinary LG, Bernston GG, editors. Handbook of Psychophysiology. New York: Cambridge University Press; 2000. pp. 342–367. [Google Scholar]
- Luft CDB, Takase E, Darby D. Heart rate variability and cognitive function: Effects of physical effort. Biological Psychology. 2009;82(2):186–191. doi: 10.1016/j.biopsycho.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Lundberg U, Frankenhaeuser M. Pituitary-adrenal and sympathetic-adrenal correlates of distress and effort. Journal of Psychosomatic Research. 1980;24(3):125–130. doi: 10.1016/0022-3999(80)90033-1. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, Tkach C. The consequences of dysphoric rumination. In: Papageorgiou C, Wells A, editors. Rumination: Nature, theory, and treatment of negative thinking in depression. Chichester, England: John Wiley & Sons; 2004. pp. 21–41. [Google Scholar]
- Marcovitch S, Leigh J, Calkins SD, Leerks EM, O’Brien M, Blankson AN. Moderate vagal withdrawal in 3.5- year- old children is associated with optimal performance on executive function tasks. Developmental Psychobiology. 2010;52(6):603–608. doi: 10.1002/dev.20462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Hadjiyannakis K. The social environment and depression: Focusing on severe life stress. In: Gotlib H, Hammen CL, editors. Handbook of Depression. New York, NY: Guilford Press; 2002. pp. 314–340. [Google Scholar]
- Moser M, Lehofer M, Hoehn-Saric R, McLeod DR, Hildebrandt G, Steinbrenner B, Zapotoczky HG. Increased heart rate in depressed subjects in spite of unchanged autonomic balance? Journal of Affective Disorders. 1998;48(2):115–124. doi: 10.1016/s0165-0327(97)00164-x. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100(4):569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta Earthquake. Journal of Personality and Social Psychology. 1991;61(1):115–121. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. Effects of rumination and distraction on naturally occurring depressed mood. Cognition & Emotion. 1993;7(6):561–570. [Google Scholar]
- Nolen-Hoeksema S, Morrow J, Fredrickson BL. Response styles and the duration of episodes of depressed mood. Journal of Abnormal Psychology. 1993;102(1):20–28. doi: 10.1037//0021-843x.102.1.20. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3(5):400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Shapiro D. Do we need a stressor to be stressed? Insights from cardiac regulation. Japanese Psychological Research. 2011;53(2):155–162. [Google Scholar]
- Ottaviani C, Shapiro D, Davydov DM, Goldstein IB. Autonomic stress response modes and ambulatory heart rate level and variability. Journal of Psychophysiology. 2008;22(1):28–40. [Google Scholar]
- Ottaviani C, Shapiro D, Davydov DM, Goldstein IB, Mills PJ. The autonomic phenotype of rumination. International Journal of Psychophysiology. 2009;72(3):267–275. doi: 10.1016/j.ijpsycho.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Reynard A, Gevirtz R, Berlow R, Brown M, Boutelle K. Heart rate variability as a marker of self-regulation. Applied Psychophysiology and Biofeedback. 2011;36(3):209–215. doi: 10.1007/s10484-011-9162-1. [DOI] [PubMed] [Google Scholar]
- Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biological Psychology. 2007;74(2):200–211. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Clift A, Bolden S, Salomon K. RSA fluctuation in major depressive disorder. Psychophysiology. 2007;44(3):450–458. doi: 10.1111/j.1469-8986.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99(16):2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- Rugulies R. Depression as a predictor for coronary heart disease: a review and meta-analysis. American Journal of Preventive Medicine. 2002;23(1):51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Nes LS. Heart rate variability reflects self-regulatory strength, effort, and fatigue. Psychological Science. 2007;18(3):275–281. doi: 10.1111/j.1467-9280.2007.01888.x. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. European Heart Journal. 1996;17:354–381. [PubMed] [Google Scholar]
- Tran TB, Siemer M, Joormann J. Implicit interpretation biases affect emotional vulnerability: A training study. Cognition and Emotion. 2011;25(3):546–558. doi: 10.1080/02699931.2010.532393. [DOI] [PubMed] [Google Scholar]
- Verkuil B, Brosschot JF, Borkovec TD, Thayer JF. Acute autonomic effects of experimental worry and cognitive problem solving: Why worry about worry? International Journal of Clinical Health & Psychology. 2009;9(3):439–453. [Google Scholar]
- Vickers KS, Vogeltanz-Holm ND. The effects of rumination and distraction tasks on psychophysiological responses and mood in dysphoric and nondysphoric individuals. Cognitive Therapy and Research. 2003;27(3):331–348. [Google Scholar]
- Watkins E, Teasdale JD, Williams RM. Decentring and distraction reduce overgeneral autobiographical memory in depression. Psychological Medicine. 2000;30(4):911–920. doi: 10.1017/s0033291799002263. [DOI] [PubMed] [Google Scholar]