Abstract
Hippocampal theta and gamma oscillations coordinate the timing of multiple inputs to hippocampal neurons and have been linked to information processing and the dynamics of encoding and retrieval. One major influence on hippocampal rhythmicity is from cholinergic afferents. In both humans and rodents, aging is linked to impairments in hippocampus-dependent function along with degradation of cholinergic function. Cholinomimetics can reverse some age-related memory impairments and modulate oscillations in the hippocampus. Therefore, one would expect corresponding changes in these oscillations and possible rescue with the cholinomimetic physostigmine. Hippocampal activity was recorded while animals explored a familiar or a novel maze configuration. Reexposure to a familiar situation resulted in minimal aging effects or changes in theta or gamma oscillations. In contrast, exploration of a novel maze configuration increased theta power; this was greater in adult than old animals, although the deficit was reversed with physostigmine. In contrast to the theta results, the effects of novelty, age, and/or physostigmine on gamma were relatively weak. Unrelated to the behavioral situation were an age-related decrease in the degree of theta-gamma coupling and the fact that physostigmine lowered the frequency of theta in both adult and old animals. The results indicate that age-related changes in gamma and theta modulation of gamma, while reflecting aging changes in hippocampal circuitry, seem less related to aging changes in information processing. In contrast, the data support a role for theta and the cholinergic system in encoding and that hippocampal aging is related to impaired encoding of new information.
Keywords: field potentials, oscillations, learning, encoding, novelty, physostigmine, theta frequency, EEG
theta and gamma oscillations have been linked to information processing in the hippocampus, a structure known to play an important role in episodic and spatial information (Gupta et al. 2012; Hirshhorn et al. 2012; Lega et al. 2012; Manns et al. 2007a; Smith and Mizumori 2006). In particular, theta activity has been correlated with faster learning of conditioned eye blink response (Griffin et al. 2004; Seager et al. 2002). Fluctuations in theta are noted to co-occur with novelty detection and successful encoding (Jeewajee et al. 2008; Jutras and Buffalo 2010) and memory retrieval (Klimesch et al. 2001). Gamma oscillations are also prominent in the hippocampus and increase in amplitude during “theta behaviors” such as movement or sniffing (Bragin et al. 1995; Csicsvari et al. 2003). In humans increases in gamma power correspond to successful encoding and retrieval (Nyhus and Curran 2010).
Most notably, it has been hypothesized that theta and gamma may be important for separating encoding and retrieval states within the hippocampus (Colgin et al. 2009; Cutsuridis et al. 2010; Hasselmo et al. 2002). In addition, there may be a discrete functional role for theta-gamma coupling, such that it is the interplay between these rhythmic oscillations that supports this separation or integration of discrete information (Canolty and Knight 2010; Chrobak and Buzsaki 1998b; Tort et al. 2009).
The cholinergic system has also been associated with spatial memory and novelty (Acquas et al. 1996; Decker et al. 1988; Fadda et al. 2000; Giovannini et al. 2001; McIntyre et al. 2003). Disruptions to cholinergic transmission impair spatial learning (Lamberty and Gower 1991; Opello et al. 1993; Whishaw 1985), while increasing acetylcholine (ACh) can reverse these impairments (Hagan et al. 1989; Janas et al. 2005; Wang and Tang 1998). In particular, ACh levels are highest when learning new information and thus facilitate encoding while hindering retrieval (Hunsaker et al. 2007; Rogers and Kesner 2003).
Lesions to the medial septum (MS), a major source of ACh to the hippocampus (Frotscher and Leranth 1985), impair memory (Gray and McNaughton 1983). These lesions also alter hippocampal unit firing (Ikonen et al. 2002; Leutgeb and Mizumori 1999; Markowska et al. 1995; Sava and Markus 2008) and theta activity (Lawson and Bland 1993; Lee et al. 1994; Stewart and Vanderwolf 1987). Similarly, blockade of muscarinic receptors in the hippocampus alters theta and gamma rhythmicity and theta-gamma coupling (Hentschke et al. 2007). The present study focused on the link between information processing, hippocampal oscillations, and cholinergic activation, particularly within the context of aging.
One hallmark of aging is an impairment in hippocampus-dependent memory (Barnes et al. 1980; Light 1991; Rapp et al. 1987; Zyzak et al. 1995), along with degenerative changes to the septo-hippocampal cholinergic system (see Schliebs and Arendt 2011). Treatments facilitating cholinergic transmission can improve memory in impaired old animals (Brandeis et al. 1990; Hernandez et al. 2006; Quirion et al. 1995). Single-unit studies indicate that aging deficits may stem from impaired encoding of change (Oler and Markus 2000; Sava and Markus 2008). Similar to aging deficits, lesions to the septo-hippocampal system disrupt the development of distinct representations of new environments (Ikonen et al. 2002; Leutgeb and Mizumori 1999). Sava and Markus (2008) demonstrated that an intraseptal cholinomimetic facilitated aged rats in forming a more stable representation of a changed environment, suggesting that MS activation was beneficial under conditions where encoding is required.
While the link between spatial processing, novelty, and theta activity in the hippocampus has been extensively studied (Barry et al. 2012), less is known regarding the role of novelty and ACh on gamma activity or the interactions between theta and gamma and the extent to which manipulations to cholinergic transmission will affect adult and old rats similarly. The aim of the present experiments was to address these open questions. Local field potentials were recorded in both young adult and old rats during exploration of both a familiar (presumably a retrieval situation) and a novel (presumably an encoding situation) maze trajectory after administration of either saline or physostigmine (an acetylcholinesterase inhibitor). Given the fact that old rats show deficits in encoding new information and the possible link to a degraded cholinergic system, we assessed how several measures of oscillatory activity were affected by aging, a change in the environment, and cholinergic activation.
METHODS
Subjects
Data was collected from 7 adult and 10 old Fischer 344 male rats (Harlan and Taconic), aged 12.2 ± 0.15 mo and 23.3 ± 0.32 mo, respectively, at the time of recordings. Rats were individually housed in clear Plexiglas cages and maintained on a 12:12-h light-dark cycle in a temperature- and humidity-controlled room. Animals were allowed ad libitum access to water. All procedures were performed in accordance with protocols reviewed and approved by the University of Connecticut's Institutional Animal Care and Use Committee.
Pretraining Procedure
All animals were food restricted to 85% of their ad libitum weight and acclimated to chocolate sprinkle reinforcement. Rats were initially trained to alternate on a U-shaped maze or a linear runway for 20 min a day until they reached a criterion of 50 trials a day for 3 consecutive days. After reaching the criterion, rats underwent surgery to permanently implant electrode arrays into the hippocampus.
Surgery and Retraining
Animals were anesthetized with isoflurane (1.5–2.5%) and placed in a stereotaxic apparatus (ASI Instruments, Warren, MI). Once anesthetized, the rats were given Metacam (0.06–0.1 mg/kg sc) and penicillin-streptomycin (0.1 ml), the scalp was shaved and aseptically cleaned, and ophthalmic ointment was applied to the eyes. A midline incision was made, and several small anchor screws were fastened to the skull. Electrode arrays (four 50-μm tungsten wires; California Fine Wire, Grover Beach, CA) arranged and spaced with fused silica tubing (Polymicro Tubing, Phoenix, AZ) were implanted into the dorsal hippocampus (AP −3.5, ML 2.5 from bregma, and DV 2.5 from the skull and AP −4.5, ML 3 from bregma, and DV 3.3 from the skull). Each array was cut at an angle such that each of the four wires was a different length and targeted to straddle the CA1 layer. Two old rats had custom-made headstages with seven tetrodes and two electrodes (50-μm tungsten wires, California Fine Wire) (unit data not presented here). Electrodes were targeted ventral to the stratum pyramidale and dorsal to the hippocampal fissure (AP −4.5, ML 3 from bregma, and DV 3.3 from the skull). Two stainless steel screws above the cerebellum served as reference and ground. Dental acrylic then secured the arrays and anchors. After surgery, animals were placed in a clean cage with a heating pad and monitored until ambulatory.
Animals were allowed to recover for 1 wk before maze training commenced. Training took place on an eight-position maze atop a table 60 cm off the ground; two Plexiglas runways (19 cm in height by 61 cm in length) radiated out from a center platform (24 cm in diameter). The configuration of the maze for retraining was fixed across days and defined as the familiar maze trajectory during recording. Recordings began once animals were again well trained and alternating.
Recording Procedure
Recordings were performed while rats were sitting in the home cage and during running on the maze both before and after saline or drug injections (Fig. 1).
Fig. 1.
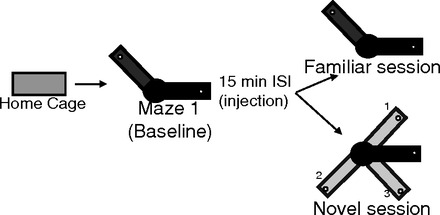
Schematic of maze trajectories and procedures. Recording sessions started as the rats sat in their home cage outside the maze room. The first maze epoch (maze 1) was always the familiar trajectory, followed by an injection of saline or physostigmine (0.1 mg/kg). The second maze epoch (maze 2) was either the same familiar trajectory or 1 of 3 novel trajectories. ISI, intersession interval.
Familiar maze trajectory.
Data were collected while rats sat quietly in the home cage and then while they ran the familiar trajectory. After 5 min on the maze [number of trials (mean ± SE): 19.5 ± 0.95 adult; 10.8 ± 0.34 old], animals were taken off and given an injection of saline or physostigmine (0.1 mg/kg sc) and then allowed to rest for 15 min while the drug took effect. Animals were then placed back on the same maze trajectory and recorded for another 5 min, followed by an additional 2 min in the home cage.
Novel maze trajectory.
The procedure before injection was the same as during the familiar trajectory; data were collected in the home cage and then while rats ran the familiar trajectory. After injections (either saline or physostigmine) and rest, animals were recorded in a novel trajectory. One maze arm remained stationary while the second arm was rotated to another position at least 90° away from the familiar position (Sava and Markus 2008).
Time course of physostigmine.
In addition, after all maze recordings were completed animals underwent four sessions of long-term recordings while they remained in their home cage outside the testing room. A 3-min baseline recording was done before injection of either saline (2 sessions) or physostigmine (0.1 mg/kg sc; 2 sessions). After injections, recordings were conducted for 2 h; 3-min recordings were taken every 10 min (minutes 8–11, 18–21, etc.).
Histology
After testing, rats were euthanized with CO2 and perfused intracardially with saline followed by 10% phosphate-buffered formalin solution. Brains were extracted and further fixed in formalin, cryoprotected in 30% sucrose, and then sliced into 40-μm sections, stained with 0.25% thionin, coverslipped, cleaned, and examined for electrode placements.
Data Analysis
Wide-band electrical activity was recorded (1–2,000 Hz, 3,787 samples/s) with a Neuralynx Data Acquisition System (Bozeman, MT). Light-emitting diodes attached to the headstage were tracked with an overhead camera. Data were selected and analyzed off-line. All data were initially inspected visually (Neuraview, Neuralynx) to remove any segments of bad signal (e.g., due to loose connection, bumping head) then downsampled to 473.4 samples/s (Neuralynx). Data were then segmented with a Neuralynx Video Tracker File Playback and Event Session Splitter to exclude data during food consumption, not running, and turning at the end of each arm. All signal analysis was conducted with custom-written programs in MATLAB (MathWorks, Natick, MA). Statistical analysis was then carried out in SPSS or Excel.
Spectral Indexes
Power spectral density estimates were obtained in MATLAB (MathWorks) with Welch's averaged modified periodogram method (Welch 1967). Each epoch was then blocked, and power estimates were obtained for each running segment (∼1 trial). Running speed for each trial was calculated as the positional difference between successive tracking samples and then low-pass filtered (cutoff = 0.25 Hz) in order to minimize the contribution of head movements and movement artifacts to the overall speed. Theta frequency was obtained for each trial with the Hilbert transform of the band-pass filtered signal (4.5–12 Hz) and calculation of the change in phase divided by the change in time between each sample. For correlation and regression analyses, the running speed and power for each trial were run within a session and pooled across conditions for each rat. For power analyses, each trial of running within each behavioral epoch was concatenated into a single continuous string of data with a cross fading procedure where the first and last 100 ms of each data trial are ramped or faded respectively with a smooth B-spline window with continuous second-order derivates (Roark and Escabi 1999). Adjacent start and end blocks from subsequent trials were then overlapped and morphed by adding the signals overlapping the ramp and fade regions. Power estimates were obtained for separate theta and gamma ranges based on the upper and lower ranges of previous studies (Colgin et al. 2009; Dimpfel 2005; Manns et al. 2007b; Sullivan et al. 2011), theta range (4.5–12 Hz), low gamma range (25–55 Hz), and high gamma range (65–140 Hz), and represented as decibels (dB) relative to 1 μV (also see Fig. 9B).
Fig. 9.
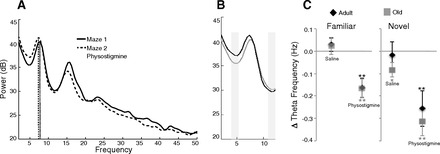
Physostigmine lowers theta frequency. A: representative power spectrum density plot of running on the first maze epoch (solid line) and the second maze epoch after an injection of physostigmine (dashed line). Physostigmine lowered the peak frequency of theta. B: power spectrum of theta range before (gray line) and after physostigmine (black line) emphasizes the shift in theta range to lower frequencies. Shaded rectangles demarcate the lower and upper cutoff values. C: change in peak theta frequency across conditions in adult and old rats. *P < 0.05, **P < 0.01.
Theta-Gamma Coupling
Modulation of the gamma signal by theta phase was measured separately between theta and low gamma and between theta and high gamma. Vector strength (Goldberg and Brown 1969) was used to quantify the degree of modulation of the gamma envelope to the phase of theta, such that higher amplitude of gamma centered at certain phases of theta yields a higher value than if gamma amplitude is uniform across theta phase.
The raw signal was filtered for theta (4.5–12 Hz), low gamma (25–55 Hz), and high gamma (65–140 Hz), and the theta phase and gamma envelope were extracted (Hilbert transform). Theta frequency was also extracted as the rate of change of the phase. Next, theta phase (0–360°) was binned into eighteen 20° intervals [p(ϕ)] and the mean of the gamma (low and high) envelope amplitude over each phase bin was calculated and then normalized by dividing the value of each bin over the sum of all bins [d(ϕ)]. Degree of modulation was calculated with a vector strength index, such that it can be detected if the gamma amplitude envelope is concentrated within certain phase bins (Freund et al. 2010).
RESULTS
Electrode tracts were recreated by evaluating serial histological sections and based on the presence of strong theta in the raw local field potential signal. For consistency, one electrode located within the stratum radiatum layer of CA1 of each rat was used for all analyses (Fig. 2). Theta and gamma oscillations were prominent in both young adult and aged rats, although notable in the traces from old rats are a lower frequency of theta activity and little variation in gamma amplitude across the theta cycle (Fig. 2C). Baseline measures to assess differences in adult and old rats were taken as the animals ran on the first maze epoch (always a familiar trajectory and no treatment). For data examining effects of different environments or drug conditions, results are represented as the within-session (day) change in power from maze 1 (baseline) to maze 2 (see Fig. 1).
Fig. 2.
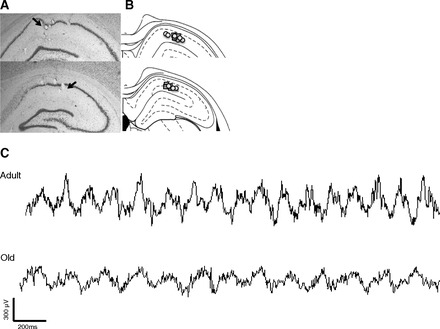
Depiction of electrode placements. A: photomicrographs of electrode locations. For power and theta gamma coupling measures a single electrode was chosen from each rat from the stratum radiatum layer (arrow). B: electrode locations of all rats; placements in young rats are represented by squares and placements in old rats by circles. C: examples of unfiltered traces during maze running show prominent theta activity in both young and old rats. Note the slower frequency in the trace from the old rat and the difference in differences in gamma amplitude across the theta cycle in young adult and old rats.
Power and Running Speed in a Familiar Environment
Measures of absolute power of theta, low gamma, and high gamma were compared in old and adult rats while running in the baseline condition (maze 1) (Fig. 3). Overall, power in the theta, low gamma, or high gamma range was similar in both age groups (all P > 0.1) (Fig. 3A). Table 1 shows the average power for each rat, along with the total number of recordings in each condition.
Fig. 3.
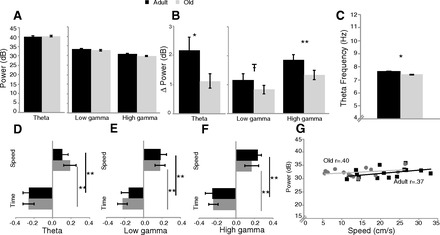
Baseline measures during exploration of a highly familiar environment (maze 1 epoch). A: the amplitude (power) of theta, low gamma, and high gamma did not differ between age groups. B: change in power as animals transitioned from sitting to running; power increased more in the adult rats during running. C: theta frequency was lower in old rats. D–F: regression analysis of the contribution of running speed and time on the maze. β-Values are greater for time for theta power (D), low gamma power (E), and high gamma power (F). G: example of the correlation between running speed and theta power. Old rats had a lower running speed but similar correlations with theta power as adult rats. *P < 0.05, **P < 0.01; т-0.05 < P < 0.1.
Table 1.
Theta and gamma power during baseline running
| Power |
No. of Recordings |
||||||
|---|---|---|---|---|---|---|---|
| Rat ID | Theta | Low gamma | High gamma | Fam Saline | Fam Physo | Nov Saline | Nov Physo |
| 411Y | 39.81 ± 0.45 | 34.68 ± 0.23 | 32.72 ± 0.15 | 3 | 6 | 1 | 2 |
| 412Y | 44.85 ± 1.08 | 34.32 ± 0.67 | 31.25 ± 0.36 | 3 | 5 | 1 | 2 |
| 475Y | 34.09 ± 0.13 | 28.96 ± 0.12 | 27.25 ± 0.17 | 3 | 3 | 1 | 1 |
| 484Y | 35.63 ± 0.12 | 31.22 ± 0.13 | 28.67 ± 0.23 | 4 | 3 | 2 | 1 |
| 494Y | 47.18 ± 0.48 | 37.66 ± 0.61 | 33.92 ± 0.54 | 5 | 3 | 2 | 1 |
| 495Y | 43.43 ± 1.24 | 37.39 ± 0.55 | 32.10 ± 0.39 | 5 | 5 | 2 | 1 |
| 520Y | 30.76 ± 0.51 | 25.32 ± 0.47 | 27.56 ± 0.30 | 4 | 3 | 2 | 1 |
| Mean young | 39.39 ± 2.31 | 32.79 ± 1.72 | 30.50 ± 1.00 | ||||
| 437O | 38.35 ± 0.59 | 30.60 ± 0.22 | 29.06 ± 0.15 | 6 | 6 | 2 | 1 |
| 441O | 37.27 ± 0.69 | 31.21 ± 0.77 | 29.55 ± 0.71 | 2 | 1 | 2 | 0 |
| 443O | 35.79 ± 1.17 | 29.13 ± 1.37 | 26.92 ± 1.58 | 4 | 4 | 1 | 2 |
| 444O | 41.84 ± 1.08 | 35.88 ± 0.60 | 32.27 ± 1.00 | 2 | 2 | 2 | 0 |
| 487O | 39.66 ± 0.33 | 31.87 ± 0.85 | 29.38 ± 0.55 | 5 | 3 | 2 | 1 |
| 488O | 39.78 ± 0.69 | 35.55 ± 0.71 | 30.55 ± 0.31 | 4 | 5 | 2 | 1 |
| 489O | 39.53 ± 1.29 | 33.15 ± 1.21 | 30.43 ± 0.87 | 3 | 3 | 1 | 1 |
| 491O | 45.94 ± 1.39 | 36.99 ± 0.92 | 31.79 ± 0.53 | 3 | 3 | 1 | 2 |
| 492O | 46.33 ± 0.42 | 35.80 ± 0.018 | 31.84 ± 0.13 | 4 | 3 | 1 | 2 |
| 493O | 40.18 ± 0.93 | 28.40 ± 0.20 | 26.17 ± 0.17 | 3 | 4 | 2 | 1 |
| Mean old | 40.47 ± 1.07 | 32.86 ± 0.97 | 29.80 ± 0.64 | ||||
Values are absolute power (means ± SE) in the theta, low gamma, and high gamma frequency ranges and the number of recording sessions in each condition for each rat. There was no difference between young (Y) and old (O) rats in baseline measures of power in any frequency range. Fam, familiar; Nov, novel; Physo, physostigmine.
Comparing the change in power from periods of sitting to running (Fig. 3B), old rats showed less of an increase than younger rats in the theta range (t222 = 2.54, P < 0.05) and the high gamma range (t222 = 3.26, P < 0.001). The same trend was present in the low gamma range (t222 = 1.82, P = 0.07). The baseline (only maze 1 epoch) measure of theta frequency was also compared and was lower in old rats (t172 = 6.61, P < 0.001) (Fig. 3C).
During the baseline maze condition the contributions of both running speed and time (trial number) to theta power (Fig. 3D), low gamma power (Fig. 3E), and high gamma power (Fig. 3F) were assessed with a regression analysis. To compare these contributions, t-tests compared the regression β-values (indicating the slope) for running speed and time. There were no age differences in the β-values for theta, low gamma, or high gamma, indicating that the strength of the influence of both speed and time on power is similar for both age groups. For both adult and old rats there was a stronger relationship (greater slope value) between power and time, with power decreasing over successive trials; this was significant for theta power (adult: t12 = 3.5, P < 0.01; old: t18 = 4.9, P < 0.01), low gamma power (adult: t12 = 2.9, P < 0.05; old: t18 = 5.3, P < 0.01), and high gamma power (adult: t12 = 4.7, P < 0.01; old: t18 = 4.5, P < 0.01) (Fig. 3, D–F).
A correlation was also run between power and running speed. Old rats ran slower than adult rats on the maze 1 epoch (t172 = 10.57, P < 0.001) but had a similar correlation between running speed and power (Fig. 3G).
A correlation between power and speed was also run for the second maze epoch in the different conditions. Table 2 shows the means (±SE) of the resulting r values on the second maze epoch after saline treatment, indicating how power in the theta, low gamma, and high gamma ranges corresponded with the animal's running speed. An ANOVA comparing the r values across conditions revealed that running on the novel trajectory decreased the correlation between running speed and theta power for both adult and old rats (F1,88 = 5.04, P < 0.05), while there were no other significant main effects or interactions (all P > 0.1). Overall, the correlations are very small, explaining very little of the variability in theta power. While old rats ran slower, there was no difference between adult and old rats in how running speed correlated to power, and novelty decreased this correlation for both age groups.
Table 2.
Running speed and power correlations
| 2nd Maze Epoch |
|||
|---|---|---|---|
| 1st Maze Epoch | Familiar | Novel | |
| Speed, cm/s | |||
| Adult | 17.0 ± 0.6 | 14.1 ± 1.3 | 11.3 ± 0.8 |
| Old | 10.0 ± 0.3 | 8.3 ± 0.6 | 6.5 ± 0.4 |
| age P < 0.01 | age P < 0.001; env P < 0.001; age × env n.s. | ||
| Correlations | |||
| Theta | |||
| Adult | 0.24 ± 0.06 | 0.28 ± 0.07 | 0.12 ± 0.06 |
| Old | 0.25 ± 0.03 | 0.18 ± 0.07 | 0.17 ± 0.09 |
| age n.s. | age n.s.; env P < 0.05; age × env n.s. | ||
| Low gamma | |||
| Adult | 0.22 ± 0.04 | 0.15 ± 0.07 | 0.04 ± 0.07 |
| Old | 0.33 ± 0.04 | 0.20 ± 0.08 | 0.11 ± 0.09 |
| age P < 0.05 | age n.s.; env n.s.; age × env n.s. | ||
| High gamma | |||
| Adult | 0.20 ± 0.03 | 0.12 ± 0.07 | 0.16 ± 0.07 |
| Old | 0.31 ± 0.04 | 0.19 ± 0.06 | 0.11 ± 0.10 |
| age P < 0.05 | age n.s.; env n.s.; age × env n.s. | ||
Values are speeds (means ± SE) from each maze epoch during the saline condition and resulting Pearson r values (means ± SE) from correlations of speed with theta, low gamma, and high gamma power. An ANOVA for age group × environment (env) shows no difference in the magnitude of the correlation between adult and old rats, although the novel maze decreased this correlation for both age groups. n.s., Not significant.
Effects of Novelty on Power
To assess how running on the novel trajectory affected the power in each frequency range, a within-animal comparison was used. The change in running speed and power from the baseline maze (1st epoch) was calculated for each session (2nd maze − 1st maze epoch) (see Fig. 1) and averaged for either the familiar or the novel condition after saline injections (Fig. 4A). Note that the values in Table 2 include all sessions regardless of whether the second epoch was the familiar maze again or the novel trajectory, resulting in minor differences. Both adult and old rats decreased running speed on the novel trajectory (F1,88 = 12.35, P < 0.001), and the effect was stronger in the adult rats (main effect of age: F1,88 = 8.621, P < 0.01; age × environment interaction: F1,88 = 7.14, P < 0.01). On the novel trajectory, a direct comparison of the change in running speed between young adult and old rats found that running speed decreased more in adult than old rats (t29 = 3.46, P < 0.001).
Fig. 4.
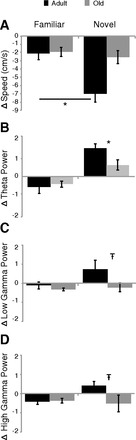
Changes in running speed and power as animals encountered a familiar maze again or a novel trajectory. A: adult rats showed a greater decrease in running speed on encountering a novel trajectory. B: changes in theta power; both adult and old rats showed increased theta power on the novel trajectory, although the extent of the increase was greater in adult rats. C and D: there was a trend for adult, but not old, rats to show increased low gamma (C) and high gamma (D) power on the novel trajectory. *P < 0.05; т-0.05 < P < 0.1.
Despite the decreased running speed on the novel trajectory, theta power increased (Fig. 4B) (ANOVA: main effect of environment, F1,91 = 30.82, P < 0.001) for both adult and old rats (no main effect of age, P > 0.10), and there was a trend for an interaction (F1,91 = 3.57, P = 0.06). A direct comparison on the novel trajectory shows that the extent of the increase was greater in the adult rats (t30 = 2.53, P < 0.01).
The change in low gamma power across environments revealed a main effect of both age (F1,91 = 7.02, P < 0.01) and environment (F1,91 = 4.77, P < 0.05) and again a trend for an interaction (F1,91 = 2.86, P = 0.09). Within the novel trajectory, a direct comparison shows there was a trend for the adult rats to have a greater increase in power than the old rats (t30 = 1.88, P = 0.07).
Examination of the change in high gamma power across environments revealed a main effect of age (F1,91 = 4.07, P < 0.05) but not environment (P > 0.1) and an age × environment interaction (F1,91 = 5.06, P < 0.05). Similar to results for low gamma, a direct comparison on the novel trajectory shows a trend for increased high gamma power in adult but not old rats (t30 = 1.87, P = 0.07).
Thus we see that theta power increased during exploration of a novel maze configuration in adult but not old rats. This increase occurred despite a reduction in running speed, therefore decoupling the relationship between speed and power that was observed in the familiar situation.
Theta-Gamma Coupling
Modulation of the gamma amplitude across theta phase was calculated for both the low and high gamma ranges. Baseline (maze 1 epoch only) modulation was calculated from the first maze epoch. While in the younger animals low gamma amplitude changed with theta phase, this was largely absent in the old rats. Figure 5, A and B, depicts the strongest, median, and weakest modulation found in the adult (Fig. 5A) and old (Fig. 5B) animals. Note how even the weakest modulation found in the adult animals is still greater than the strongest modulation found in the aged animals. A similar result is seen for high gamma (Fig. 5, C and D). This age difference is also apparent in the example of raw waveforms shown in Fig. 2.
Fig. 5.
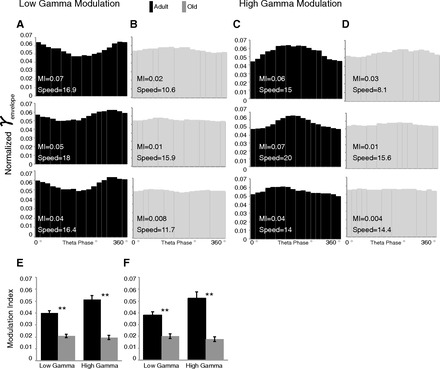
Theta phase modulation of gamma envelope. A and B: distributions of theta phase modulation of the low gamma amplitude envelope in young (A) and old (B) rats representing the highest, median, and lowest modulation values (modulation index) and corresponding running speed (cm/s) for each age group. C and D: distributions of theta phase modulation of high gamma amplitude envelope in young (C) and old (D) rats representing the highest, median, and lowest modulation values for each age group. E: mean (±SE) modulation values of low and high gamma during maze 1; young rats had higher modulation of both low and high gamma. F: low and high gamma during maze 1 segments matched for speed for adult and aged rats. **P < 0.001.
A modulation index was calculated for each recording, and then the mean was taken of each maze condition. A statistical comparison of the modulation index shows that it was higher in the young adult versus old rats for both low (t140 = 7.89, P < 0.001) and high (t140 = 8.63, P < 0.001) gamma (Fig. 5E).
To rule out that the reduced modulation in the aged rats was related to their lower running speed, this issue was examined in greater depth. The modulation value was also compared between adult and old rat data matched for speed (running speed mean ± SE for adult 12.71 ± 0.81 cm/s and old 12.29 ± 0.56 cm/s; t65 = 0.43, P > 0.1) (Fig. 5F). Adult rats still had higher modulation values for both low gamma (t65 = 5.44, P < 0.001) and high gamma (t65 = 6.06, P < 0.001).
To assess effects of environment, a within-animal comparison determined the change in modulation as animals encountered the familiar maze again or a novel trajectory (Fig. 5, E and F). The low gamma modulation values did not change upon encountering either maze environment in adult or old animals (all P > 0.1). High gamma modulation values showed more change; there was an effect of age (F1,78 = 4.54, P < 0.05) and no main effect of novelty (P > 0.1) and age × environment interaction (F1,78 = 4.86, P < 0.05), such that high gamma modulation decreased on the novel trajectory only in adult rats.
Thus we have observed that old rats have a lower degree of theta-gamma modulation, but this degree of modulation was not affected by different maze environments in either adult or old rats.
Effects of Physostigmine on Power and Coupling
Previous studies have reported that doses of 0.1 mg/kg of the anticholinesterase physostigmine (Beninger et al. 1995; Stemmelin et al. 1999) improve spatial performance in rats. In addition, there are reports of two distinct types of theta activity: theta sensitive to cholinergic modulation is lower in frequency than theta related to movement (Kramis et al. 1975; Lawson and Bland 1993; Olpe et al. 1987; Podol'skii et al. 2001). Therefore, the effects of physostigmine treatment on theta power were examined within the lower range of theta (low theta = 4.5–8 Hz) and the higher range of theta (high theta = 8–12 Hz) separately.
To verify the time course of physostigmine effects, power was examined while the rats sat in their home cage before and after either physostigmine (0.1 mg/kg sc) or saline control. Three-minute recordings were taken every 10 min, and the change in power from baseline at each 10-min interval was calculated (Fig. 6). Repeated-measures ANOVA between physostigmine and saline was carried out for each group at each frequency range. Saline treatment did not differ from baseline in any range (all P > 0.1). In the low theta range, old rats had increased power in the physostigmine condition (F1,154 = 9.0, P < 0.01), while adult rats showed no change (P > 0.1) (Fig. 6A). In the high theta range, physostigmine-treated adult rats showed decreased power from baseline (F1,66 = 42.18, P < 0.001). The same trend was present in old rats (F1,154 = 3.8, P = 0.07) (Fig. 6B).
Fig. 6.
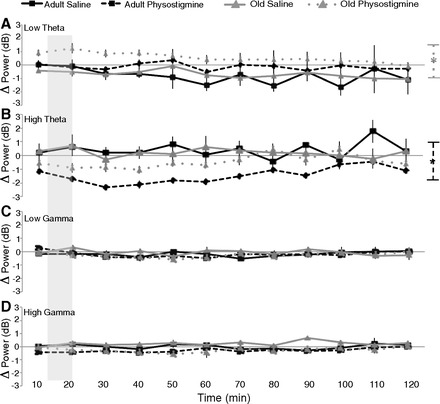
Effects of physostigmine while sitting in the home cage. To verify the time during which running the second maze epoch corresponded to changes in power after subcutaneous injections, recordings were carried out over an extended time period following injections of both saline and physostigmine. The shaded portion of each panel represents the period of time during which rats underwent the second maze exposure. The change in power from baseline (preinjection) at each 10-min interval spanning 2 h after saline (solid line) or physostigmine (dashed line) for both young (black) and old (gray) rats is shown. A: in the low theta range, there was no difference for young rats between saline and physostigmine. Old rats had increased low theta power after physostigmine. B: in the high theta range there was a decrease in power from physostigmine in young rats. C and D: low gamma (C) and high gamma (D) power did not differ between physostigmine and saline treatment in either young or old rats. *P < 0.01.
In the home cage, low and high gamma ranges were unaffected by physostigmine (all P > 0.1) (Fig. 6, C and D).
Before running on the second maze epoch, animals were either given saline (data presented above) or the cholinomimetic physostigmine (see Fig. 1). Again, the correlation between power and running speed were examined; Table 3 shows the correlation r values for the familiar and novel conditions after injection of physostigmine. Similar to the saline data, after physostigmine injections the correlation between theta power and running speed decreased on the novel trajectory.
Table 3.
Running speed and power correlations after physostigmine
| 2nd Maze Epoch Familiar |
2nd Maze Epoch Novel |
|||
|---|---|---|---|---|
| Saline | Physo | Saline | Physo | |
| Speed, cm/s | ||||
| Adult | 14.1 ± 1.3 | 9.79 ± 0.9 | 11.3 ± 0.8 | 6.75 ± 0.97 |
| Old | 8.3 ± 0.6 | 5.18 ± 0.33 | 6.5 ± 0.4 | 4.12 ± 0.56 |
| age P < 0.001; physo P < 0.001; age × physo n.s. | age P < 0.001; physo P < 0.001; age × physo n.s. | |||
| Correlations | ||||
| Theta | ||||
| Adult | 0.28 ± 0.07 | 0.26 ± 0.06 | 0.12 ± 0.06 | 0.13 ± 0.12 |
| Old | 0.18 ± 0.07 | 0.17 ± 0.08 | 0.17 ± 0.09 | 0.09 ± 0.16 |
| age n.s.; physo n.s.; age × physo n.s. | age n.s.; physo n.s.; age × physo n.s. | |||
| Low gamma | ||||
| Adult | 0.15 ± 0.07 | 0.28 ± 0.06 | 0.04 ± 0.07 | 0.26 ± 0.17 |
| Old | 0.20 ± 0.08 | 0.17 ± 0.07 | 0.11 ± 0.09 | 0.15 ± 0.16 |
| age n.s.; physo n.s.; age × physo n.s. | age n.s.; physo n.s.; age × physo n.s. | |||
| High gamma | ||||
| Adult | 0.12 ± 0.07 | 0.30 ± 0.07 | 0.16 ± 0.07 | 0.30 ± 0.19 |
| Old | 0.19 ± 0.06 | 0.30 ± 0.06 | 0.11 ± 0.10 | 0.14 ± 0.17 |
| age n.s.; physo P < 0.05; age × physo n.s. | age n.s.; physo n.s.; age × physo n.s. | |||
Values are speeds (means ± SE) from each maze epoch during the physostigmine conditions and resulting Pearson r values (means ± SE) from correlations of speed with theta, low gamma, and high gamma power. ANOVA for age group × physostigmine shows no difference in the magnitude of the correlation after drug injections.
Similar to the examination of the saline data above, a two-way ANOVA (age × environment) compared the within-session change in power when the environment was again familiar or was a novel configuration (see Fig. 1) after physostigmine injections.
Running speed decreased after injections of physostigmine; this effect was greater for the adult rats in both environments (main effect of age: F1,82 =7.43, P < 0.01), although again the novel environment decreased running speed more than the familiar environment in both age groups (main effect of environment: F1,82 = 4.38, P < 0.05) (Fig. 7A).
Fig. 7.
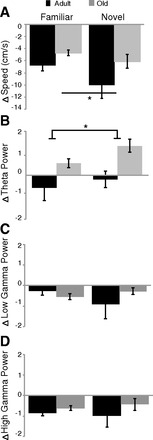
Effect of physostigmine on encountering a familiar maze again or a novel trajectory. A: change in speed; physostigmine decreased running speed in adult rats more than old rats, although both age groups still slowed down more on the novel trajectory. B: change in theta power; physostigmine increased theta power only in old rats. C and D: there was no effect of either age or environment on low (C) or high (D) gamma power. *P < 0.05.
Physostigmine also affected theta power in adult and old rats differently (main effect of age: F1,79 = 6.56, P < 0.05), while there was no effect of environment and no interaction. Figure 7B shows that old rats had increased theta power on the second maze when it was both familiar and novel, whereas adult rats showed either a decrease or no change in theta power after physostigmine that could be explained by the decrease in running speed. This is in contrast to the effects of saline, in which old rats showed little change in theta power.
There was no effect of age or environment on either low or high gamma power after physostigmine injections (all P > 0.1) (Fig. 7, C and D).
When comparing the change in theta-gamma modulation from the first to second epochs on the maze, there was no effect of physostigmine (Fig. 8). There was no effect of physostigmine on low or high gamma, in either the familiar (Fig. 8A) or novel (Fig. 8B) environment (all P > 0.1).
Fig. 8.
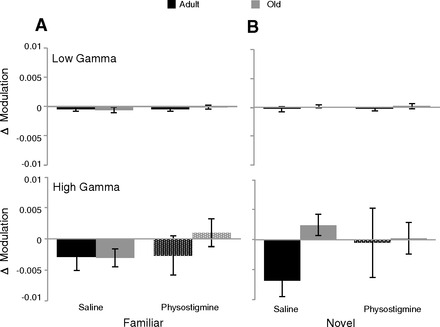
Change in theta-gamma coupling. Neither encountering a familiar environment again (A) nor encountering a novel trajectory (B) changed theta modulation of either low (top) or high (bottom) gamma. There was also no change in the degree of modulation from physostigmine.
Physostigmine treatment also lowered the frequency of theta. An example power spectrum density plot shows the power distribution across all frequencies (Fig. 9A). There is a prominent peak in the theta range (“theta bump”), while notably after physostigmine injections there was increased power in the lower theta range (shift in “theta bump”) (Fig. 9B). A within-session change in frequency from maze 1 to maze 2 was calculated and compared to 0 (1-sample t-test) (Fig. 9B). Old rats had lower theta frequency (Fig. 3) but similar decrease in frequency as adult rats after physostigmine. In the familiar situation, saline did not change theta frequency for either adult or old rats (all P > 0.1), although after physostigmine there was significant decrease in frequency for both adult (t26 = −3.7, P < 0.01) and old (t28 = −3.9, P < 0.01) rats. In the novel situation, the frequency decreased in old (t13 = −2.4, P < 0.05) but not adult (P > 0.1) rats after saline, although again physostigmine decreased the frequency in both groups (adult: t9 = −3.2, P < 0.01; old: t8 = −5.0, P < 0.01). Jeewajee et al. (2008) similarly showed a decrease in theta frequency with exposure to a completely novel environment in rats, possibly reflecting encoding.
Physostigmine selectively affected theta power, specifically only increasing theta power in old rats under encoding conditions.
DISCUSSION
Theta and gamma oscillations have been linked to hippocampal information processing (Colgin and Moser 2010; Hasselmo 2005). Given the aging deficits in hippocampus-based learning, one would expect corresponding changes in oscillations. The present experiment examined the power of theta and gamma and the degree of theta-gamma coupling while animals explored a familiar (presumably a retrieval situation) or a novel (presumably an encoding situation) maze configuration, known to alter CA1 place fields of young rats (Frank et al. 2004; Sava and Markus 2008).
One aspect of aging is a degradation of cholinergic function (Bartus et al. 1982), with activation of the cholinergic system restoring behavior (Markowska et al. 1995) and single-unit activity (Sava and Markus 2008). Thus the effects of aging and cholinergic activation were also examined.
Theta Power
Theta power was similar in the adult and old rats when running an overtrained/familiar trajectory. Others have found, under familiar conditions, differences in theta power between young (4 mo) and adult (9 mo) animals (Huxter et al. 2012) and between young (2 mo) and middle-aged (15 mo) animals (Kuo et al. 2010). Given that our adult animals were 12 mo old, it is possible that this age effect plateaus later in the life span. Alternatively, differences in the precise electrode placements could result in large between-animal variability masking a difference between age groups in this study. We therefore used within-animal analyses to compare changes in power across different situations. Our findings that older rats show less of an increase in theta power from sitting to running a familiar maze are in agreement with the results of Kuo et al. (2010) from placing the animals on a familiar treadmill.
Running speed can alter theta and gamma power (Chen et al. 2011; Hinman et al. 2011); therefore we examined the correlation between power and running speed to address this potential influence. Both age groups showed a similar positive correlation between theta power and running speed in the familiar maze, but these correlations were low, and more of the variation in power was explained by the number of trials run on the maze.
When the second epoch involved running a novel trajectory, both groups slowed. Despite this reduction in running speed, theta power increased for both adult and old rats, thus uncoupling any relationship between speed and theta power. Furthermore, the younger animals had a greater reduction in speed but showed a greater increase in theta power than old rats.
Theta power increases in young rats during exploration of a novel environment (Kocsis et al. 2007). Given the reduced cholinergic activity in the aged (Bartus et al. 1982) and the correlation between ACh release and low theta power (Crouzier et al. 2006), the data here support the prediction that one would expect: a reduced theta response to novelty in the aged.
Similar to the present findings of increased theta in response to novelty despite the reduction in running speed, others have shown that cognitive factors can account for a similar or greater amount of variance in theta power than running speed (Hinman et al. 2011; Montgomery et al. 2009; Schmidt et al. 2013; Wyble et al. 2004). Previously, little was known regarding age-related changes in theta in response to novelty. Taken together, the present data indicate a weaker theta response to exploration/novelty in the older animals.
Physostigmine treatment had different effects in old and adult rats; at rest in the home cage physostigmine selectively increased theta power in the lower frequency range in old rats while it decreased theta power in the higher frequency range in adult rats. Overall, old rats had a lower theta frequency than adult rats; this age-related shift in theta frequency has been reported previously (Abe and Toyosawa 1999; Markowska et al. 1995). The present data support findings of two distinct types of theta and that lower-frequency theta was sensitive to cholinergic modulation (Bland 1986; Lawson and Bland 1993). It is likely that because of the degraded cholinergic system of old rats (Bartus et al. 1982; Sugaya et al. 1998) treatment with physostigmine only increased low theta power in the old rats. Similarly, previous reports demonstrate that administration of physostigmine in young rats elicits only low theta activity in both anesthetized (5–6 Hz; Olpe et al. 1987) and freely moving (4.3–5.7 Hz; Podol'skii et al. 2001) rats.
Importantly, in addition to the fact that physostigmine shifted the frequency of theta, it also increased the magnitude of theta power response to novelty, to levels similar to those found in younger animals (under saline). This is similar to previous reports of cholinergic activation (with carbachol) causing hippocampal place fields in aged animals to encode a trajectory change like their younger counterparts (Sava and Markus 2008). No such effects were found during a familiar trajectory. Taken together, the data indicate that the cholinergic system plays an important role in encoding new information, and this is affected by aging.
Gamma Power
“Theta behaviors,” such as movement or sniffing, are also linked to increased gamma power (Bragin et al. 1995; Csicsvari et al. 2003). Gamma oscillations align periods of inhibition and may allow precise coordination of neuronal input from behaviorally relevant stimuli, providing temporal precision of pyramidal cell firing (Chrobak and Buzsaki 1998a; Jutras and Buffalo 2010; Womelsdorf et al. 2007). Low gamma has been linked to CA3 and possibly pattern retrieval, while high gamma has been linked to the MEC and encoding (Bragin et al. 1995; Colgin et al. 2009; Montgomery and Buzsaki 2007). Little is known regarding the effects of novelty on gamma; however, in humans gamma was related to successful encoding (Sederberg et al. 2007a, 2007b). To our knowledge, there are no data regarding gamma and aging.
In the present study there were no differences in basal levels of gamma power between adult and old rats. However, younger rats showed a greater increase, especially in high gamma power, as they transitioned from sitting to running on the maze. Notably, there was little effect of the novel trajectory on either low or high gamma. Presumably the present manipulation was not sufficient to engage a strong gamma response (Penley et al., unpublished observation). Similarly, gamma power was not a good predictor of successful performance in a match-to-place task requiring rats to remember a new goal location within the same testing environment across days (Shirvalkar et al. 2010).
Unlike its effects on theta, physostigmine did not change low or high gamma power while animals were sitting in their home cage. Similarly, there were no systematic effects of the drug when the animals ran the second epoch. These data suggest that activation of the cholinergic system has little impact on hippocampal gamma oscillations.
Theta-Gamma Coupling
The interplay between theta and gamma oscillations may integrate brain activity at both a local and a distributed level (Canolty and Knight 2010; Chrobak and Buzsaki 1998a; Tort et al. 2010). Theta phase modulates gamma, with a correlation found between theta phase and gamma power (Jensen and Colgin 2007). This modulation may support different cognitive processes (Tort et al. 2009) and a neural code (Lisman and Idiart 1995). Currently, the literature regarding phase modulation is focused on young adult animals (Tort et al. 2009) or humans under different task conditions (Canolty and Knight 2010; Holz et al. 2010; Schack et al. 2002), with no data on how modulation changes with age.
The present experiment found an age-related decrease in the degree of theta-gamma coupling. This was true for both low and high gamma and tended to be unaffected by the novel trajectory. Furthermore, unlike the effects of physostigmine on the theta power response to novelty, there was no effect of physostigmine on theta-gamma coupling. These data suggest that coupling does not simply reflect changes in theta power and may reflect the underlying circuitry of the hippocampus.
General Conclusions
The interplay between theta and gamma oscillations is thought to play a role in hippocampal information processing. In fact, we found an age-related reduction in theta modulation of gamma. This could be the result of changes in CA1 interneuron circuitry (Stanley et al. 2012). However, why manipulations of task demands and/or cholinergic activation had no impact on theta-gamma modulation is puzzling. Clearly, further in vivo and in vitro investigation of this issue is needed.
Power in the low and high gamma ranges were also less affected by novelty, age, or physostigmine. There are situations that show a greater role for gamma (Montgomery and Buzsaki 2007; Sederberg et al. 2007a, 2007b). In the present situation, presumably changing trajectory while keeping the room and task constant was not sufficient to engage a strong gamma response (Shirvalkar et al. 2010; Penley et al., unpublished observation). It is possible that gamma activity is related to the degree to which the task involves competition between previous and new information. In tasks where encoding and retrieval are contrasted, age-related differences in the low and high gamma response may be found.
There was, however, an increased theta response to novelty in the younger animals compared with older animals. This age-related decline was reversed with physostigmine. The aging and physostigmine effects were predominantly found under conditions of novelty rather than under familiar conditions. These data support a role for theta and the cholinergic system in encoding new information. The findings underscore that hippocampal aging is related to impaired encoding of new information.
GRANTS
This work was supported by the University of Connecticut Research Foundation (UCRF).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.K.J. and E.J.M. conception and design of research; T.K.J. and M.D.H. performed experiments; T.K.J., M.D.H., B.S., J.R.H., and M.A.E. analyzed data; T.K.J. and E.J.M. interpreted results of experiments; T.K.J. prepared figures; T.K.J. drafted manuscript; T.K.J., B.S., J.R.H., and E.J.M. edited and revised manuscript; T.K.J., M.D.H., B.S., J.R.H., M.A.E., and E.J.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Stephanie Bohannon and Kevin Mastro for assistance with training and data analysis.
REFERENCES
- Abe Y, Toyosawa K. Age-related changes in rat hippocampal theta rhythms: a difference between type 1 and type 2 theta. J Vet Med Sci 61: 543–548, 1999. [DOI] [PubMed] [Google Scholar]
- Acquas E, Wilson C, Fibiger HC. Conditioned and unconditioned stimuli increase frontal cortical and hippocampal acetylcholine release: effects of novelty, habituation, and fear. J Neurosci 16: 3089–3096, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, Nadel L, Honig WK. Spatial memory deficit in senescent rats. Can J Psychol 34: 29–39, 1980. [DOI] [PubMed] [Google Scholar]
- Barry C, Heys JG, Hasselmo ME. Possible role of acetylcholine in regulating spatial novelty effects on theta rhythm and grid cells. Front Neural Circuits 6: 5, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science 217: 408–414, 1982. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Wirsching BA, Mallet PE, Jhamandas K, Boegman RJ. Physostigmine, but not 3,4-diaminopyridine, improves radial maze performance in memory-impaired rats. Pharmacol Biochem Behav 51: 739–746, 1995. [DOI] [PubMed] [Google Scholar]
- Bland BH. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol 26: 1–54, 1986. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsaki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci 15: 47–60, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandeis R, Dachir S, Sapir M, Levy A, Fisher A. Reversal of age-related cognitive impairments by an M1 cholinergic agonist, AF102B. Pharmacol Biochem Behav 36: 89–95, 1990. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci 14: 506–515, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Resnik E, McFarland JM, Sakmann B, Mehta MR. Speed controls the amplitude and timing of the hippocampal gamma rhythm. PLoS One 6: e21408, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsaki G. Gamma oscillations in the entorhinal cortex of the freely behaving rat. J Neurosci 18: 388–398, 1998a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsaki G. Operational dynamics in the hippocampal-entorhinal axis. Neurosci Biobehav Rev 22: 303–310, 1998b. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser EI. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 462: 353–357, 2009. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Moser EI. Gamma oscillations in the hippocampus. Physiology (Bethesda) 25: 319–329, 2010. [DOI] [PubMed] [Google Scholar]
- Crouzier D, Baubichon D, Bourbon F, Testylier G. Acetylcholine release, EEG spectral analysis, sleep staging and body temperature studies: a multiparametric approach on freely moving rats. J Neurosci Methods 151: 159–167, 2006. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron 37: 311–322, 2003. [DOI] [PubMed] [Google Scholar]
- Cutsuridis V, Cobb S, Graham BP. Encoding and retrieval in a model of the hippocampal CA1 microcircuit. Hippocampus 20: 423–446, 2010. [DOI] [PubMed] [Google Scholar]
- Decker MW, Pelleymounter MA, Gallagher M. Effects of training on a spatial memory task on high affinity choline uptake in hippocampus and cortex in young adult and aged rats. J Neurosci 8: 90–99, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimpfel W. Pharmacological modulation of cholinergic brain activity and its reflection in special EEG frequency ranges from various brain areas in the freely moving rat (Tele-Stereo-EEG). Eur Neuropsychopharmacol 15: 673–682, 2005. [DOI] [PubMed] [Google Scholar]
- Fadda F, Cocco S, Stancampiano R. Hippocampal acetylcholine release correlates with spatial learning performance in freely moving rats. Neuroreport 11: 2265–2269, 2000. [DOI] [PubMed] [Google Scholar]
- Frank LM, Stanley GB, Brown EN. Hippocampal plasticity across multiple days of exposure to novel environments. J Neurosci 24: 7681–7689, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund JA, Nikitin A, Stocks NG. Phase locking below rate threshold in noisy model neurons. Neural Comput 22: 599–620, 2010. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Leranth C. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol 239: 237–246, 1985. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Rakovska A, Benton RS, Pazzagli M, Bianchi L, Pepeu G. Effects of novelty and habituation on acetylcholine, GABA, and glutamate release from the frontal cortex and hippocampus of freely moving rats. Neuroscience 106: 43–53, 2001. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Brown PB. Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sound localization. J Neurophysiol 32: 613–636, 1969. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. Comparison between the behavioural effects of septal and hippocampal lesions: a review. Neurosci Biobehav Rev 7: 119–188, 1983. [DOI] [PubMed] [Google Scholar]
- Griffin AL, Asaka Y, Darling RD, Berry SD. Theta-contingent trial presentation accelerates learning rate and enhances hippocampal plasticity during trace eyeblink conditioning. Behav Neurosci 118: 403–411, 2004. [DOI] [PubMed] [Google Scholar]
- Gupta AS, van der Meer MA, Touretzky DS, Redish AD. Segmentation of spatial experience by hippocampal theta sequences. Nat Neurosci 15: 1032–1039, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JJ, Jansen JH, Broekkamp CL. Hemicholinium-3 impairs spatial learning and the deficit is reversed by cholinomimetics. Psychopharmacology (Berl) 98: 347–356, 1989. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. What is the function of hippocampal theta rhythm?—linking behavioral data to phasic properties of field potential and unit recording data. Hippocampus 15: 936–949, 2005. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Bodelon C, Wyble BP. A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput 14: 793–817, 2002. [DOI] [PubMed] [Google Scholar]
- Hentschke H, Perkins MG, Pearce RA, Banks MI. Muscarinic blockade weakens interaction of gamma with theta rhythms in mouse hippocampus. Eur J Neurosci 26: 1642–1656, 2007. [DOI] [PubMed] [Google Scholar]
- Hernandez CM, Gearhart DA, Parikh V, Hohnadel EJ, Davis LW, Middlemore ML, Warsi SP, Waller JL, Terry AV., Jr Comparison of galantamine and donepezil for effects on nerve growth factor, cholinergic markers, and memory performance in aged rats. J Pharmacol Exp Ther 316: 679–694, 2006. [DOI] [PubMed] [Google Scholar]
- Hinman JR, Penley SC, Long LL, Escabi MA, Chrobak JJ. Septotemporal variation in dynamics of theta: speed and habituation. J Neurophysiol 105: 2675–2686, 2011. [DOI] [PubMed] [Google Scholar]
- Hirshhorn M, Grady C, Rosenbaum RS, Winocur G, Moscovitch M. Brain regions involved in the retrieval of spatial and episodic details associated with a familiar environment: an fMRI study. Neuropsychologia 50: 3094–3106, 2012. [DOI] [PubMed] [Google Scholar]
- Holz EM, Glennon M, Prendergast K, Sauseng P. Theta-gamma phase synchronization during memory matching in visual working memory. Neuroimage 52: 326–335, 2010. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Rogers JL, Kesner RP. Behavioral characterization of a transection of dorsal CA3 subcortical efferents: comparison with scopolamine and physostigmine infusions into dorsal CA3. Neurobiol Learn Mem 88: 127–136, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxter JR, Miranda JA, Dias R. The hippocampal physiology of approaching middle-age: early indicators of change. Hippocampus 22: 1923–1940, 2012. [DOI] [PubMed] [Google Scholar]
- Ikonen S, McMahan R, Gallagher M, Eichenbaum H, Tanila H. Cholinergic system regulation of spatial representation by the hippocampus. Hippocampus 12: 386–397, 2002. [DOI] [PubMed] [Google Scholar]
- Janas AM, Cunningham SC, Duffy KB, Devan BD, Greig NH, Holloway HW, Yu QS, Markowska AL, Ingram DK, Spangler EL. The cholinesterase inhibitor, phenserine, improves Morris water maze performance of scopolamine-treated rats. Life Sci 76: 1073–1081, 2005. [DOI] [PubMed] [Google Scholar]
- Jeewajee A, Lever C, Burton S, O'Keefe J, Burgess N. Environmental novelty is signaled by reduction of the hippocampal theta frequency. Hippocampus 18: 340–348, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Colgin LL. Cross-frequency coupling between neuronal oscillations. Trends Cogn Sci 11: 267–269, 2007. [DOI] [PubMed] [Google Scholar]
- Jutras MJ, Buffalo EA. Synchronous neural activity and memory formation. Curr Opin Neurobiol 20: 150–155, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Yonelinas A, Kroll NE, Lazzara M, Rohm D, Gruber W. Theta synchronization during episodic retrieval: neural correlates of conscious awareness. Brain Res Cogn Brain Res 12: 33–38, 2001. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Li S, Hajos M. Behavior-dependent modulation of hippocampal EEG activity by the selective norepinephrine reuptake inhibitor reboxetine in rats. Hippocampus 17: 627–633, 2007. [DOI] [PubMed] [Google Scholar]
- Kramis R, Vanderwolf CH, Bland BH. Two types of hippocampal rhythmical slow activity in both the rabbit and the rat: relations to behavior and effects of atropine, diethyl ether, urethane, and pentobarbital. Exp Neurol 49: 58–85, 1975. [DOI] [PubMed] [Google Scholar]
- Kuo TB, Li JY, Hsieh SS, Chen JJ, Tsai CY, Yang CC. Effect of aging on treadmill exercise induced theta power in the rat. Age (Dordr) 32: 297–308, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberty Y, Gower AJ. Cholinergic modulation of spatial learning in mice in a Morris-type water maze. Arch Int Pharmacodyn Ther 309: 5–19, 1991. [PubMed] [Google Scholar]
- Lawson VH, Bland BH. The role of the septohippocampal pathway in the regulation of hippocampal field activity and behavior: analysis by the intraseptal microinfusion of carbachol, atropine, and procaine. Exp Neurol 120: 132–144, 1993. [DOI] [PubMed] [Google Scholar]
- Lee MG, Chrobak JJ, Sik A, Wiley RG, Buzsaki G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience 62: 1033–1047, 1994. [DOI] [PubMed] [Google Scholar]
- Lega BC, Jacobs J, Kahana M. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus 22: 748–761, 2012. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Mizumori SJ. Excitotoxic septal lesions result in spatial memory deficits and altered flexibility of hippocampal single-unit representations. J Neurosci 19: 6661–6672, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light LL. Memory and aging: four hypotheses in search of data. Annu Rev Psychol 42: 333–376, 1991. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Idiart MA. Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science 267: 1512–1515, 1995. [DOI] [PubMed] [Google Scholar]
- Manns JR, Howard MW, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron 56: 530–540, 2007a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Zilli EA, Ong KC, Hasselmo ME, Eichenbaum H. Hippocampal CA1 spiking during encoding and retrieval: relation to theta phase. Neurobiol Learn Mem 87: 9–20, 2007b. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Olton DS, Givens B. Cholinergic manipulations in the medial septal area: age-related effects on working memory and hippocampal electrophysiology. J Neurosci 15: 2063–2073, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CK, Marriott LK, Gold PE. Patterns of brain acetylcholine release predict individual differences in preferred learning strategies in rats. Neurobiol Learn Mem 79: 177–183, 2003. [DOI] [PubMed] [Google Scholar]
- Montgomery SM, Betancur MI, Buzsaki G. Behavior-dependent coordination of multiple theta dipoles in the hippocampus. J Neurosci 29: 1381–1394, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SM, Buzsaki G. Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc Natl Acad Sci USA 104: 14495–14500, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhus E, Curran T. Functional role of gamma and theta oscillations in episodic memory. Neurosci Biobehav Rev 34: 1023–1035, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oler JA, Markus EJ. Age-related deficits in the ability to encode contextual change: a place cell analysis. Hippocampus 10: 338–350, 2000. [DOI] [PubMed] [Google Scholar]
- Olpe HR, Klebs K, Kung E, Campiche P, Glatt A, Ortmann R, D'Amato F, Pozza MF, Mondadori C. Cholinomimetics induce theta rhythm and reduce hippocampal pyramidal cell excitability. Eur J Pharmacol 142: 275–283, 1987. [DOI] [PubMed] [Google Scholar]
- Opello KD, Stackman RW, Ackerman S, Walsh TJ. AF64A (ethylcholine mustard aziridinium) impairs acquisition and performance of a spatial, but not a cued water maze task: relation to cholinergic hypofunction. Physiol Behav 54: 1227–1233, 1993. [DOI] [PubMed] [Google Scholar]
- Podol'skii IY, Vorob'ev VV, Belova NA. Long-term changes in hippocampus and neocortex EEG spectra in response to pharmacological treatments affecting the cholinergic system. Neurosci Behav Physiol 31: 589–595, 2001. [DOI] [PubMed] [Google Scholar]
- Quirion R, Wilson A, Rowe W, Aubert I, Richard J, Doods H, Parent A, White N, Meaney MJ. Facilitation of acetylcholine release and cognitive performance by an M2-muscarinic receptor antagonist in aged memory-impaired. J Neurosci 15: 1455–1462, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Rosenberg RA, Gallagher M. An evaluation of spatial information processing in aged rats. Behav Neurosci 101: 3–12, 1987. [DOI] [PubMed] [Google Scholar]
- Roark RM, Escabi MA. B-spline design of maximally flat and prolate spheroidal-type FIR filters. IEEE Trans Signal Processing 47: 701–716, 1999. [Google Scholar]
- Rogers JL, Kesner RP. Cholinergic modulation of the hippocampus during encoding and retrieval. Neurobiol Learn Mem 80: 332–342, 2003. [DOI] [PubMed] [Google Scholar]
- Sava S, Markus EJ. Activation of the medial septum reverses age-related hippocampal encoding deficits: a place field analysis. J Neurosci 28: 1841–1853, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schack B, Vath N, Petsche H, Geissler HG, Moller E. Phase-coupling of theta-gamma EEG rhythms during short-term memory processing. Int J Psychophysiol 44: 143–163, 2002. [DOI] [PubMed] [Google Scholar]
- Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res 221: 555–563, 2011. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Hinman JR, Jacobson TK, Szkudlarek E, Argraves M, Escabi M, Markus EJ. Dissociation between dorsal and ventral hippocampal theta oscillations during a place and response task. J Neurosci. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seager MA, Johnson LD, Chabot ES, Asaka Y, Berry SD. Oscillatory brain states and learning: impact of hippocampal theta-contingent training. Proc Natl Acad Sci USA 99: 1616–1620, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, Litt B, Brandt A, Kahana MJ. Gamma oscillations distinguish true from false memories. Psychol Sci 18: 927–932, 2007a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A, Tully MS, Kahana MJ. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cereb Cortex 17: 1190–1196, 2007b. [DOI] [PubMed] [Google Scholar]
- Shirvalkar PR, Rapp PR, Shapiro ML. Bidirectional changes to hippocampal theta-gamma comodulation predict memory for recent spatial episodes. Proc Natl Acad Sci USA 107: 7054–7059, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJ. Hippocampal place cells, context, and episodic memory. Hippocampus 16: 716–729, 2006. [DOI] [PubMed] [Google Scholar]
- Stanley EM, Fadel JR, Mott DD. Interneuron loss reduces dendritic inhibition and GABA release in hippocampus of aged rats. Neurobiol Aging 33: 431.e1–431.e13, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmelin J, Cassel JC, Will B, Kelche C. Sensitivity to cholinergic drug treatments of aged rats with variable degrees of spatial memory impairment. Behav Brain Res 98: 53–66, 1999. [DOI] [PubMed] [Google Scholar]
- Stewart DJ, Vanderwolf CH. Hippocampal rhythmical slow activity following ibotenic acid lesions of the septal region. I. Relations to behavior and effects of atropine and urethane. Brain Res 423: 88–100, 1987. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Greene R, Personett D, Robbins M, Kent C, Bryan D, Skiba E, Gallagher M, McKinney M. Septo-hippocampal cholinergic and neurotrophin markers in age-induced cognitive decline. Neurobiol Aging 19: 351–361, 1998. [DOI] [PubMed] [Google Scholar]
- Sullivan D, Csicsvari J, Mizuseki K, Montgomery S, Diba K, Buzsaki G. Relationships between hippocampal sharp waves, ripples, and fast gamma oscillation: influence of dentate and entorhinal cortical activity. J Neurosci 31: 8605–8616, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort AB, Komorowski R, Eichenbaum H, Kopell N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol 104: 1195–1210, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort AB, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H. Theta-gamma coupling increases during the learning of item-context associations. Proc Natl Acad Sci USA 106: 20942–20947, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Tang XC. Reversal of scopolamine-induced deficits in radial maze performance by (-)-huperzine A: comparison with E2020 and tacrine. Eur J Pharmacol 349: 137–142, 1998. [DOI] [PubMed] [Google Scholar]
- Welch PD. Use of fast Fourier transform for estimation of power spectra—a method based on time averaging over short modified periodograms. IEEE Trans Audio Electroacoust 15: 70–73, 1967. [Google Scholar]
- Whishaw IQ. Cholinergic receptor blockade in the rat impairs locale but not taxon strategies for place navigation in a swimming pool. Behav Neurosci 99: 979–1005, 1985. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Schoffelen JM, Oostenveld R, Singer W, Desimone R, Engel AK, Fries P. Modulation of neuronal interactions through neuronal synchronization. Science 316: 1609–1612, 2007. [DOI] [PubMed] [Google Scholar]
- Wyble BP, Hyman JM, Rossi CA, Hasselmo ME. Analysis of theta power in hippocampal EEG during bar pressing and running behavior in rats during distinct behavioral contexts. Hippocampus 14: 662–674, 2004. [DOI] [PubMed] [Google Scholar]
- Zyzak DR, Otto T, Eichenbaum H, Gallagher M. Cognitive decline associated with normal aging in rats: a neuropsychological approach. Learn Mem 2: 1–16, 1995. [DOI] [PubMed] [Google Scholar]


