Abstract
Acute inhalation of airborne pollutants alters cardiovascular function and evidence suggests that pollutant-induced activation of airway sensory nerves via the gating of ion channels is critical to these systemic responses. Here, we have investigated the effect of capsaicin [transient receptor potential (TRP) vanilloid 1 (TRPV1) agonist], AITC [TRP ankyrin 1 (TRPA1) agonist], and ATP (P2X2/3 agonist) on bronchopulmonary sensory activity and cardiovascular responses of conscious Sprague-Dawley (SD) rats. Single fiber recordings show that allyl isothiocyanate (AITC) and capsaicin selectively activate C fibers, whereas subpopulations of both A and C fibers are activated by stimulation of P2X2/3 receptors. Inhalation of the agonists by conscious rats caused significant bradycardia, atrioventricular (AV) block, and prolonged PR intervals, although ATP-induced responses were lesser than those evoked by AITC or capsaicin. Responses to AITC were inhibited by the TRP channel blocker ruthenium red and the muscarinic antagonist atropine. AITC inhalation also caused a biphasic blood pressure response: a brief hypertensive phase followed by a hypotensive phase. Atropine accentuated the hypertensive phase, while preventing the hypotension. AITC-evoked bradycardia was not abolished by terazosin, the α1-adrenoceptor inhibitor, which prevented the hypertensive response. Anesthetics had profound effects on AITC-evoked bradycardia and AV block, which was abolished by urethane, ketamine, and isoflurane. Nevertheless, AITC inhalation caused bradycardia and AV block in paralyzed and ventilated rats following precollicular decerebration. In conclusion, we provide evidence that activation of ion channels expressed on nociceptive airway sensory nerves causes significant cardiovascular effects in conscious SD rats via reflex modulation of the autonomic nervous system.
Keywords: nociceptive sensory nerves, airways, TRPA1, autonomic nervous system, cardiovascular response
inhalation of noxious irritants and pollutants have been shown to have profound acute cardiovascular effects (8, 42). Common air pollutants derived from industrial processes, combustion engines, and tobacco smoking include particulate matter (PM), ozone, isocyanates, aldehydes, and acrolein. In particular, it has become increasingly evident that a rise in levels of PM positively correlates with rises in cardiovascular morbidity and mortality (8). The deleterious effects of pollutant inhalation have been shown to occur in both chronic and acute exposures. It is believed that effects caused by chronic exposures may occur through inflammatory pathways, while the effects seen after acute exposures are less well understood. Recent studies have identified the importance of two sensory nerve ion channels, transient receptor potential (TRP) ankyrin 1 (TRPA1) and TRP vanilloid 1 (TRPV1), in the cardiovascular effects of PM, suggesting a role for cardiovascular reflexes initiated from the airways (2, 15, 16, 22).
The trachea, bronchi, and lungs are densely innervated with vagal sensory (afferent) nerves. More than 80% of the pulmonary afferent nerves are unmyelinated C fibers termed “nociceptors” due to their sensitivity to noxious stimuli (11, 24, 51, 66). Nociceptive C fibers selectively express TRPV1 channels, whose activation by noxious stimuli (e.g., capsaicin, heat, and pH) (47, 48, 66) initiates defensive mechanisms such as cough, hypersecretion, and bronchospasm (9, 61). The other 20% of pulmonary afferents are comprised of the mechanosensitive myelinated rapidly adapting receptors (RAR) and slowly adapting receptors (SAR) that lack TRPV1 (24, 71), which contribute to the control of eupnic breathing. TRPA1 is also expressed on vagal nociceptive sensory nerves (47) and rat pulmonary C fibers respond robustly to cinnamaldehyde and allyl isothiocyanate (AITC; TRPA1 agonists) (37, 57, 72). However, little is known about the expression of TRPA1 on myelinated mechanosensitive fibers innervating the rat lung. TRPA1 is a polymodal channel that is the target of multiple irritants and pollutants (65). For example, acute treatment of PM has been shown to activate nociceptive sensory nerves in a manner that was substantially reduced by knockout or inhibition of TRPA1 and TRPV1 channels (1-3, 15, 16). Similarly, ozone, aldehydes, and acrolein have been shown to selectively activate nociceptive sensory nerves via TRPA1 (4, 63, 64).
PM inhalation evokes bradycardia and increased heart rate variability in healthy animals (20, 68), suggesting that PM evoked reflex-mediated increases in parasympathetic activity. PM-induced ECG changes were shown to be inhibited by TRPV1 (20) and TRPA1 (22) inhibition, suggesting that these autonomic reflexes occur through activation of TRP channel-expressing afferents in the lung. Nevertheless, PM and other air pollutants have multiple cellular effects (38), which may limit our ability to directly correlate pulmonary sensory nerve activation through TRP channel gating with modulation of cardiac function. Furthermore, the use of anesthetics in ECG studies, which can cause variable block of homeostatic control of cardiovascular and respiratory rhythms (13, 18, 59, 67), may obscure some of the reflex effects evoked by irritant inhalation.
In the case of selective TRPV1 activation in the airways, previous studies have shown that activation of the normally quiescent nociceptive C fibers with capsaicin (TRPV1 agonist) causes an increase in parasympathetic drive to the heart, resulting in bradycardia (14, 27, 52). Here, we provide evidence of the powerful effect airway nerve TRPA1 activation has on cardiovascular function using the selective TRPA1 agonist AITC. Using ex vivo electrophysiological recordings of single fibers innervating the rat airways and ECG and blood pressure recordings of conscious, freely moving rats, we show that AITC, via the activation of airway C fibers, evokes significant atropine-sensitive bradycardia and atrioventricular (AV) block in healthy Sprague-Dawley rats. AITC-induced reflex bradycardia is almost abolished by anesthesia but not decerebration.
METHODS
Ethical Approval
All experiments were approved by the University of South Florida Institutional Animal Care and Use Committee and they comply with regulations and policies stipulated by the Journal of Physiology (21). In total, 101 rats were used in this study.
Bronchopulmonary C-Fiber Extracellular Recordings
Male Sprague-Dawley rats (Harlan) were killed by CO2 asphyxiation followed by exsanguination. The innervated isolated lung preparation was prepared similarly to methods described for the mouse (47, 49). Briefly, the airways and lungs with their intact extrinsic innervation (vagus nerve including vagal ganglia) were taken and placed in a dissecting dish containing Krebs bicarbonate buffer solution composed of the following (in mM): 118 NaCl, 5.4 KCl, 1.0 NaH2PO4, 1.2 MgSO4, 1.9 CaCl2, 25.0 NaHCO3, and 11.1 d-glucose and equilibrated with 95% O2-5% CO2 (pH 7.3–7.4) and containing 3 μM indomethacin. Connective tissue was trimmed away leaving the trachea and lungs with their intact nerves. The airways were then pinned to the larger compartment of a custom-built two-compartment recording chamber that was lined with silicone elastomer (Sylgard). The left vagal ganglia was pulled into the adjacent compartment of the chamber through a small hole and pinned. Both compartments were separately superfused with Krebs bicarbonate buffer (35°C). Furthermore, the trachea was cannulated to allow for Krebs bicarbonate buffer (35°C, 3–5 ml/min) perfusion into the lumen of the airways. The right bronchi was ligated to prevent perfusion of the right lobes. Five to eight punctures using a 20-gauge needle were made in the left lobes to provide an exit path for the perfusate. A sharp glass electrode was pulled by a Flaming Brown micropipette puller (P-87; Sutter Instruments, Novato, CA) and filled with 3 M NaCl solution. The electrode was inserted and placed near the cell bodies of vagal ganglion. Our search paradigm consisted of sweeping longitudinally along the ganglia, alternating from a rostral start point to a caudal start point with each rat. The recorded action potentials were amplified (Microelectrode AC amplifier 1800; A-M Systems, Everett, WA), filtered (0.3 kHz of low cut-off and 1 kHz of high cut-off), and monitored on an oscilloscope (TDS1002B; Tektronix, Beaverton, OR). The scaled output from the amplifier was captured and analyzed by a Macintosh computer using NerveOfIt software (Phocis, Baltimore, MD). Action potential discharge was quantified offline and recorded in 1-s bins.
Nerve fiber characterization.
Using our ex vivo vagal-lung preparation, we were able to study single sensory fiber responses independent of ventilator mechanics. To measure conduction velocity, an electrical stimulation (S44; Grass Instruments, Quincy, MA) was applied to the center of the receptive field in the lungs or to the pulmonary vagal nerve. The conduction velocity of an individual bronchopulmonary afferent was calculated by dividing the distance along the nerve pathway by the time delay between the shock artifact and the action potential evoked by electrical stimulation. Punctate mechanical stimulation was determined using manual application of calibrated von Frey fibers to the center of the receptive field. Sensitivity to mechanical stretch was determined by increasing the pressure within the tracheal perfusion to 25 cmH20, using a fluid column. Chemical stimuli were intratracheally applied as a 1-ml bolus over 10 s (for C fibers) and over 30 s (for A fibers). We chose to use 30 μM α,β-methylene ATP (αβmATP), 100 μM AITC, and 300 nM capsaicin, as these concentrations had previously been shown to selectively activate P2X2/3, TRPA1, and TRPV1 receptors maximally (29, 47, 49). A response to either mechanical or chemical stimuli was considered positive if the number of action potentials in any 1-s bin was twice the average baseline response. All excitatory chemical treatments were separated by at least 15-min wash. Due to potential heterologous desensitization, capsaicin was only given at the end of the experiment.
ECG Acquisition and Agonist Exposure
Healthy, male, 15-wk-old Sprague-Dawley rats were implanted with a radiotelemetric device (4ET; Data Sciences International) through a midline incision in the abdomen under controlled anesthetic (1–5% isoflurane). A trocar was then used to tunnel through the abdominal and pectoral muscle layers allowing for two sets of ECG leads to be fed rostrally through the trocar and secured by a single polyethylene suture in the lead II and Lewis lead positions. Seven to ten days following surgery, ECGs were recorded from freely moving rats contained in a plexiglass chamber (4.5 × 11 inches) placed on top of the DSI receiver (RPC-1). The receiver was connected to a computer running Ponemah software via an A/D converter. ECG was recorded continuously for 30 min wherein the rat was exposed (in sequential 10-min intervals) to ambient air, nebulized vehicle (4% ethanol in PBS), and either capsaicin (3 mM), AITC (30 mM), or ATP (30 mM) made up in 4% ethanol and PBS. Exposures were performed using a Trek S (PARI Respiratory Equipment) nebulizer (4 l/min), which produces 1- to 5-μm particles. All experiments were performed at the same time of day (0900–1100) to minimize physiological variation due to circadian rhythms. Data were recorded at 1,000 Hz, and the cardiac cycle, including P, QRS, and T waves, was resolved. These data were then analyzed for electrical parameters: e.g., RR interval (RR-I) and PR interval (PR-I), and the ECG was assessed for arrhythmias including sinus arrhythmia, AV block, and ventricle arrhythmia.
Blood Pressure Acquisition
Healthy, male, 15-wk-old Sprague-Dawley rats were implanted with a carotid cannula for invasive blood pressure recording using a method similar to that described by Parasuraman and Raveendran (53). An 18-in. catheter was used to enable acquisition from inside the chamber used for exposing the rats to nebulized agonist. A 1-in. incision was made on the dorsal side of the neck and a pocket was created using blunt dissection. The catheter was then run dorsally under the dermal layers and the extra length was coiled and laid flat in the pocket. The incision was closed using wound clips leaving the end of the catheter exteriorized and secured with a wound clip in a manner that did not occlude the catheter but kept it in place. The rats were given a recovery period of 48 h before being exposed to nebulized agonist as previously described. Data were acquired using the PowerLab 4/35 with a Quad Bridge Amp (AD Instruments), and analysis was performed in LabChart 7 (AD Instruments). Responses to inhalation of nociceptive stimuli were subdivided into “early phase” (30-s period beginning 10 s into the exposure) and “late phase” (30-s period beginning 2 min and 10 s into the exposure).
Urethane and Ketamine Anesthetics
Both urethane (1.2 g/kg) and ketamine (100 mg/kg) were dissolved in saline and separately administered via intraperitoneal injection. Onset of the anesthetic was confirmed through absence of the hind limb withdrawal reflex to interdigital pinch. Two minor incisions (2 cm) were then made exposing the right pectoral and left oblique muscle layers. The negative and positive ECG leads were then fixed onto the pectoral and oblique muscle layers, respectively, with tissue adhesive. The animals were then placed into the chamber and exposed to AITC as mentioned previously.
Isoflurane Study
Healthy, male, 15-wk-old Wistar Kyoto rats were implanted with radiotelemetry modules as previously described. Two studies were performed in which ECG was acquired during two 10-min AITC exposures. In both studies, the first exposure was given before being induced with 5% isoflurane and maintained with 3% isoflurane via a nosecone for a period of 45 min. In the first study, a period of 10–15 min was then given allowing for the rats to come out of anesthesia before the second exposure was administered. For the second study, a period of 45 min was given after isoflurane before the second exposure was administered. Analysis of the ECGs was then performed as before.
Precollicular Decerebration
Decerebration was carried out using a method similar to that described by Sapru and Krieger (58). Anesthesia was induced with 5% isoflurane and maintained with 3% isoflurane through a nosecone for the duration of the procedure. To minimize cerebral hemorrhage, the right common carotid artery was isolated and ligated. A bilateral craniotomy was performed using a set of rongeurs to burr holes into the parietal skull. Subsequently, a suture was placed inferior to the central sagittal sinus and the portion of bone superior to the central sagittal sinus was removed. The dura mater was breached and reflected. The cerebral cortex was then gently aspirated to visualize the superior and inferior colliculi. With the use of a blunt instrument, the brain was sectioned directly rostral to the superior colliculus (and caudal to the hippocampus/thalamus) and the transected forebrain aspirated. At this point, the animal was taken off of isoflurane. Small pieces of oxidized regenerated cellulose (Ethicon; Johnson & Johnson) were placed on the exposed surfaces of the brain and mineral oil was used to fill the remaining cavity to prevent the tissue from desiccating. The trachea was then exposed and a hand-made endotracheal cannula was inserted into the trachea via a transverse tracheotomy incision and gently secured with braided suture. An initial bolus injection of vecuronium (0.3 mg/kg ip) was then administered and maintained (0.15 mg/kg ip every 30 min) to prevent spontaneous skeletal muscle activity. The animal was then put on a dual mode ventilator (Kent Scientific) with respiration rate set to 70–80 beats/min and inspiration percentage of 35, and the ETCO2 was confirmed to be between 30 and 45 mmHg using a capnograph (MDS Matrix). A minimum recovery period of 1 h was employed postdecerebration before data collection began, as we found this time period to be sufficient for the effects of isoflurane anesthesia to be eliminated from the preparation. Nebulized agonist was introduced into the “postventilator” inspiratory cannula using an Aeroneb Pro-X nebulizer (which produces 1- to 5-μm particles) to avoid any increases in inspiration pressure.
Statistics
Data were analyzed using GraphPad software. Where appropriate paired or unpaired Student's t-tests were used. P < 0.05 was taken as significant. All data are expressed as mean ± SE unless otherwise noted.
Chemicals
AITC, ATP disodium salt hydrate, atropine (free base), capsaicin, and ruthenium red were purchased from Sigma with the exception of αβmATP trisodium salt and terazosin hydrochloride (Tocris).
RESULTS
Extracellular Recording from Bronchopulmonary Afferents
Electrical stimulation of the pulmonary vagus nerve invariably yielded compound action potentials comprised of mostly slow C fibers (between 1.0 and 0.3 m/s; Fig. 1A). The short distance between the vagal ganglion and the rat lung made conduction velocity measurements of fast fibers difficult, although on occasion fibers with conduction velocities up to 10 m/s could be detected in distal portions of the lung (Fig. 1B).
Fig. 1.
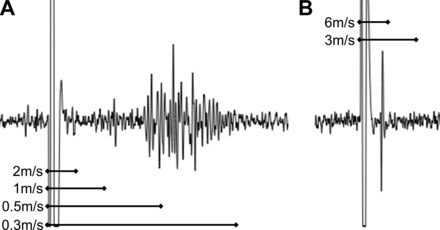
Electrically evoked compound potentials in vagal ganglia. Vagus nerve stimulated by concentric stimulating electrode, action potentials recorded extracellularly within the vagal sensory ganglia. Conduction velocities calculated from physical distance traveled divided by time separating shock artifact from electrical potentials. A: representative image showing the majority of sensory fibers are slowly conducting C fibers. B: rare example of more rapid A fiber (likely myelinated).
We studied 19 C fibers (mean conduction velocity of 0.49 m/s, range of 0.36 to 0.70), which all responded to punctate mechanical stimulation of a receptive field within the lung. C fibers were generally quiescent with a peak baseline activity of 0.68 ± 0.2 Hz. The majority of C fibers responded to more than one chemical stimulus (Fig. 2). Fourteen of nineteen C fibers responded to the P2X2/3 receptor agonist αβmATP (30 μM), evoking 55.1 ± 12.4 action potentials with a peak discharge of 11.5 ± 2.1 Hz. Fifteen of seventeen C fibers responded to the TRPA1 receptor agonist AITC (100 μM), evoking 127.1 ± 32.0 action potentials with a peak discharge of 12.1 ± 1.3 Hz. Consistent with previous studies, five of five C fibers responded to the TRPV1 receptor agonist capsaicin (300 nM), evoking 144.0 ± 43.6 action potentials with a peak discharge of 26.2 ± 6.1 Hz. In this sample of C fibers, there was no correlation of insensitivity to αβmATP and insensitivity to AITC. There was no difference in the conduction velocities between αβmATP-sensitive and αβmATP-insensitive C fibers (P = 0.29).
Fig. 2.
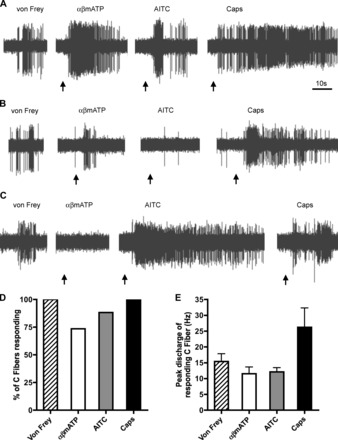
Electrophysiological characterization of C fibers innervating rat bronchopulmonary airways. A: representative example of action potentials evoked from an individual bronchopulmonary C fiber to punctate mechanical stimulus (von Frey), α,β-methylene ATP (αβmATP; 30 μM), allyl isothiocyanate (AITC; 100 μM), and capsaicin (Caps; 300 nM). B and C: 2 further representative C fibers showing selective sensitivity to αβmATP and AITC. D: group data showing the %C fibers responding to each stimulus. E: mean ± SE peak discharge (Hz) in response to each stimulus (only responsive C fibers included).
We studied 19 fast fibers (Fig. 3), all of which responded to at least one type of mechanical stimulation of the lung (von Frey punctate or 25 cmH2O pressure). The majority of these fibers had conduction velocities faster than our limit of detection (∼10 m/s), although 5 of the 19 were between 5 and 10 m/s. As such the fast fibers were designated as A fibers, which had a peak baseline activity of 2.6 ± 1.0 Hz. Fourteen of sixteen A fibers responded to distention of the lung with 25 cmH20 pressure with a peak discharge of 31.5 ± 6.2 Hz. These studies were performed ex vivo with lungs perfused with buffer (and exiting through small punctures made in the lungs); thus it is impossible to determine/interpret any apparent adaptation index of these mechanically sensitive fibers. Twelve of nineteen A fibers responded to 30 μM αβmATP, evoking 778 ± 125 action potentials with a peak discharge of 44.7 ± 5.2 Hz. In marked contrast to bronchopulmonary C fibers, no bronchopulmonary A fibers responded to either 100 μM AITC (17 fibers tested) or 300 nM capsaicin (13 fibers tested). We also tested the response of some A fibers, particularly those that failed to respond to αβmATP, to the nicotinic acetylcholine receptor agonist nicotine (100 μM). Seven of nine A fibers responded to nicotine, evoking 481 ± 200 action potentials with a peak discharge of 19.6 ± 4.8 Hz.
Fig. 3.
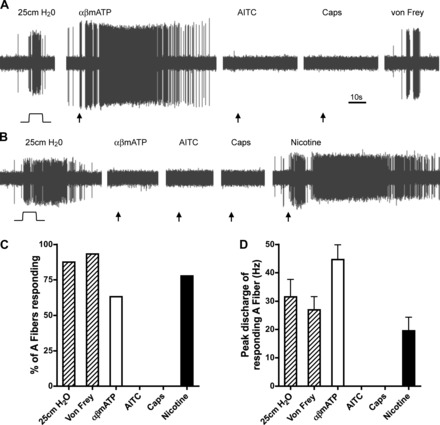
Electrophysiological characterization of A-fiber innervating rat bronchopulmonary airways. A and B: representative examples of action potentials evoked from an individual bronchopulmonary A fiber to mechanical distention (25 cmH2O), αβmATP (30 μM), AITC (100 μM), capsaicin (300 nM), nicotine (100 μM), and punctate mechanical stimulus (von Frey). C: group data showing the %A fibers responding to each stimulus. D: mean ± SE peak discharge (Hz) in response to each stimulus (only responsive A fibers included).
From these single fiber recordings, it is clear that the rat bronchopulmonary system is innervated by multiple afferent fiber subtypes. Whereas both A and C fibers are often sensitive to αβmATP, only C fibers are sensitive to AITC and capsaicin. Furthermore, the activation of C fibers via P2X2/3-, TRPA1-, and TRPV1-mediated activation is approximately equivalent.
Bradycardia Evoked by Irritant Inhalation
Given that pulmonary C fiber activation by capsaicin has been shown to cause bradycardia (27, 52) and that both TRPA1 and P2X2/3 channels are coexpressed with TRPV1 on some airway sensory nerves, we hypothesized that activation of vagal nociceptors through inhalation of specific TRPA1 and P2X2/3 channel agonists would evoke bradycardia. As expected, exposure of conscious rats to capsaicin (3 mM), AITC (30 mM), or ATP (30 mM) caused substantial bradycardia with prolonged RR-Is (Fig. 4). Analysis of the ECGs showed that a significant RR-I increase occurred upon exposure to capsaicin (234 ± 25 ms compared with 169 ± 12 ms, P < 0.05), AITC (270 ± 15 ms compared with 153 ± 3 ms, P < 0.05), and ATP (165 ± 3 ms compared with 150 ± 4 ms, P < 0.05; Fig. 4). Furthermore, by plotting the relationships of consecutive RR-Is (Fig. 5), it was clear that the bradycardia was unstable, with slow beats following faster beats in no particular rhythm.
Fig. 4.
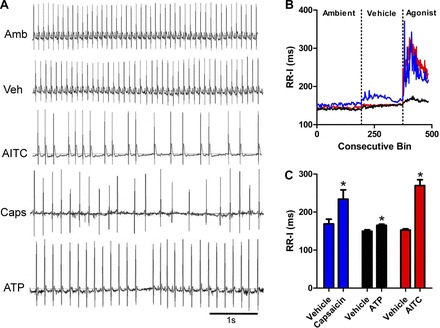
Inhalation of nociceptive stimuli evoke bradycardia. A: representative ECG recorded during ambient (Amb), vehicle (Veh), AITC (30 mM), capsaicin (3 mM), and ATP (30 mM) exposures. B: mean consecutive bin averages of RR interval (RR-I) during ambient, vehicle, and agonist exposures [capsaicin (blue, n = 3), ATP (black, n = 4), and AITC (red, n = 11)]. C: mean ± SE RR-I. *Significant difference to vehicle response (P < 0.05).
Fig. 5.
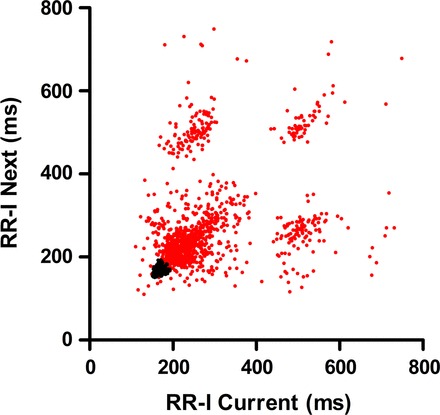
AITC-evoked bradycardia is unstable. Single example of individual RR-I plotted against subsequent RR-I during vehicle (black) and AITC (30 mM; red) exposures.
AV Block Evoked by Irritant Inhalation
The severity of bradycardia seen upon exposure to these nociceptive receptor agonists was found to be capable of evoking second degree AV block along with PR-I prolongation. No AV block was identified upon vehicle exposure. AITC and capsaicin evoked substantial increases in AV block events (112 ± 37 and 25 ± 10 ms, respectively), while ATP produced minimal AV block events (1 ± 1; Fig. 6). In addition PR-I increased upon exposure to AITC (93 ± 13 ms compared with 44 ± 1 ms, P < 0.05) and capsaicin (57 ± 1 ms compared with 46 ± 0.4 ms, P < 0.05), although the effect of ATP (49 ± 0.3 ms compared with 48 ± 1 ms) failed to reach significance (Fig. 6).
Fig. 6.
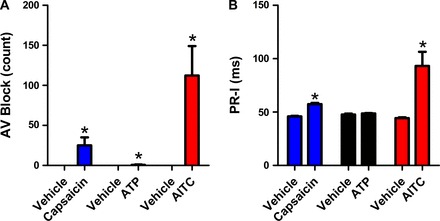
Inhalation of nociceptive stimuli evoke second degree atrioventricular (AV) block. Derived from ECG data recorded during exposure to vehicle, capsaicin (3 mM, blue, n = 3), ATP (30 mM, black, n = 3), and AITC (30 mM, red, n = 11 and 8, respectively). A: mean ± SE AV Block. B: mean ± SE PR interval (PR-I). *Significant difference to vehicle response (P < 0.05).
Cardiac Responses to AITC Inhalation Are Blunted by TRP Channel Inhibition
We further investigated the mechanism underlying the potent pulmonary-cardiac reflexes evoked by AITC. The TRP channel blocker ruthenium red (4 mg/kg ip) decreased responses to AITC by 45% in the case of RR-I, 86% in PR-I, and 83% of AV block compared with control (P < 0.05; Table 1).
Table 1.
AITC evokes bradyarrythymia via TRPA1-mediated parasympathetic reflex in conscious rats
| RR Interval, ms | AV Block, count | PR Interval, ms | |
|---|---|---|---|
| Control | |||
| Ambient | 145 ± 3 | 0 ± 0 | 44.4 ± 0.7 |
| Vehicle | 153 ± 3 | 0 ± 0 | 44.2 ± 0.8 |
| AITC | 270 ± 15* | 112 ± 37* | 93.1 ± 13* |
| n | 11 | 11 | 8 |
| Ruthenium red (4 mg/kg ip) | |||
| Ambient | 156 ± 9 | 0 ± 0 | 48.6 ± 1.9† |
| Vehicle | 159 ± 9 | 0 ± 0 | 48.6 ± 1.8† |
| AITC | 224 ± 9*† | 19 ± 9*† | 56.2 ± 2.2*† |
| n | 9 | 9 | 5 |
| Atropine (1 mg/kg ip) | |||
| Ambient | 149 ± 6 | 0 ± 0 | 46.8 ± 0.6 |
| Vehicle | 140 ± 5† | 0 ± 0 | 44.8 ± 0.5 |
| AITC | 156 ± 3*† | 0 ± 0† | 46 ± 0.7† |
| n | 5 | 5 | 4 |
| Ketamine (100 mg/kg ip) | |||
| Ambient | 304 ± 33† | N.D. | N.D. |
| Vehicle | 298 ± 35† | N.D. | N.D. |
| AITC | 310 ± 29 | N.D. | N.D. |
| n | 4 | ||
| Urethane (1.2 g/kg ip) | |||
| Ambient | 228 ± 5† | N.D. | N.D. |
| Vehicle | 228 ± 8† | N.D. | N.D. |
| AITC | 229 ± 10 | N.D. | N.D. |
| n | 5 | ||
| Decerebration | |||
| Ambient | 164 ± 10† | 0 ± 0 | 50.8 ± 2.5† |
| Vehicle | 170 ± 10† | 0.3 ± 0.3 | 50.5 ± 2.3† |
| AITC | 235 ± 26* | 10 ± 10*† | 54 ± 2.7† |
| n | 5 | 4 | 5 |
Values are mean ± SE. RR interval, atrioventricular (AV) block, and PR interval upon exposure to ambient, vehicle, and AITC (30 mM).
AITC, allyl isothiocyanate; transient receptor potential ankyrin 1 (TRPA1); N.D., data not determined.
P < 0.05, significant difference between AITC and vehicle responses.
P < 0.05, significant difference between control and pretreatments.
Muscarinic Inhibition Prevents Cardiac Responses to Inhaled AITC
We hypothesized that the parasympathetic branch of the autonomic nervous system was required for AITC-induced reflex responses (14, 27, 52). Atropine, a selective muscarinic inhibitor (1 mg/kg ip), caused a mild tachycardia (140.2 ± 4.6 ms compared with 152.7 ± 3.2 ms, P < 0.05), consistent with the limited tonic parasympathetic control of the heart in rodents. Atropine reduced RR-I and PR-I prolongation to AITC exposure by 86 and 98%, respectively, compared with control (P < 0.05; Table 1). Atropine was also found to completely abolish the ability of AITC to evoke AV block (P < 0.05; Table 1).
Biphasic Blood Pressure Response Evoked by AITC Inhalation
Given that inhalation of AITC caused bradycardia, we predicted that it would also cause hypotension, perhaps secondary to the bradycardic effects. AITC (30 mM) caused an increase in pulse interval derived from the blood pressure recordings shown in Fig. 7. Surprisingly, AITC inhalation evoked a biphasic response with an initial hypertension followed by a prolonged hypotension (Fig. 7). Atropine (1 mg/kg ip), in addition to preventing AITC-induced bradycardia, potentiated the early hypertensive phase and prevented the late hypotensive phase (Fig. 7). Increases in blood pressure can cause a decrease in heart rate via a baroreceptor-mediated reflex. To determine whether the AITC-induced bradycardia was secondary to the hypertensive effect, we pretreated the rats with terazosin (0.3 mg/kg), a selective α1-inhibitor that has limited central nervous system penetration. As expected, terazosin decreased baseline blood pressure (100.62 ± 4 mmHg compared with 128.36 ± 2 mmHg, P < 0.05), due to its inhibition of sympathetic vascular tone, but did not have a significant effect on baseline pulse interval (148.9 ± 6.8 ms compared with 164.9 ± 5.4 ms, P = 0.07). Furthermore, terazosin prevented AITC-induced hypertension in the early phase of the exposure (Fig. 7). Nevertheless, AITC produced robust bradycardia in the presence of terazosin (254.4 ± 49.3 ms compared with vehicle, 145.04 ± 6.8 ms, P < 0.05), suggesting that the decrease in heart rate is not secondary to hypertension (Fig. 7).
Fig. 7.
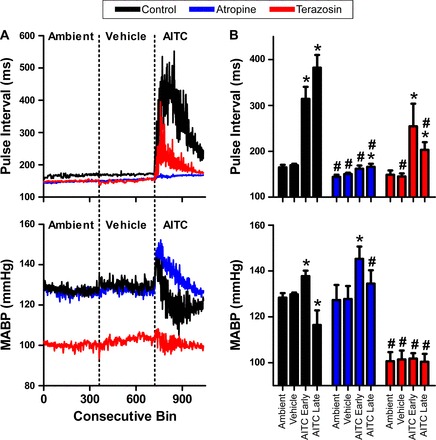
AITC evokes a biphasic blood pressure response. Control data shown in black (n = 6), atropine (1 mg/kg ip, blue, n = 7), and terazosin (0.3 mg/kg ip, red, n = 4). A: mean consecutive bin averages of pulse interval (top) and mean arterial blood pressure (MABP; bottom) during ambient, vehicle, and AITC (30 mM) exposures. B: mean ± SE pulse interval (top) and MABP (bottom) upon exposure to ambient, vehicle, and both early and late phases of AITC (30 mM) response. *Significant difference to vehicle response (P < 0.05). #Significant difference to control response with either atropine or terazosin (P < 0.05).
Effects of Anesthesia and Decerebration on Cardiac Responses to Inhaled AITC
Inhalation of AITC had virtually no effect on heart rate following administration of both urethane and ketamine, although both anesthetics produced bradycardia independent of the nociceptive stimulus (Table 1). Furthermore, no changes in RR-I were seen in rats upon AITC inhalation 15 min after cessation of isoflurane anesthesia (Fig. 8), despite the observation that these rats were able to walk and groom with relative ease. Such data suggest that residual anesthesia has a profound effect on airway-cardiac reflexes evoked by AITC. Nevertheless, in rats allowed to recover for 45 min after cessation of isoflurane anesthesia, AITC caused significant bradycardia (Fig. 8).
Fig. 8.
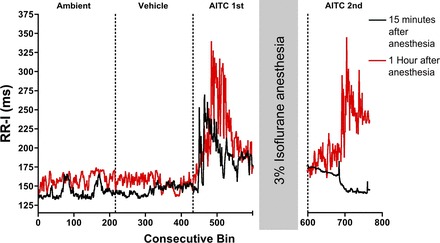
Isoflurane reversibly inhibits AITC response. Mean consecutive bin averages of RR-I during ambient, vehicle, and AITC (30 mM) exposures before 45 min 3% isoflurane (AITC 1st) and after (AITC 2nd). Black data: AITC 2nd exposure 15 min after anesthesia (n = 2); red data: AITC 2nd exposure 60 min after anesthesia (n = 2).
Lastly, we investigated AITC-evoked cardiac reflexes in decerebrate and paralyzed rats. Nebulized AITC was administered directly into the lower airways via a tracheal cannula; thus responses were likely due to the stimulation of pulmonary afferents rather than afferents from the upper airways. AITC caused a significant increase in RR-I (235 ± 26 ms compared with 170 ± 10 ms, P < 0.05; Fig. 9 and Table 1) and AV block (13 ± 13 events compared with 0.3 ± 0.3 ms, P < 0.05; Table 1). An increase in PR-I (53 ± 3 ms compared with 51 ± 2 ms; Table 1) was also seen; however, these results failed to reach significance. Sectioning of the brain stem caudal of the medulla prevented any AITC-induced changes in ECG (data not shown).
Fig. 9.
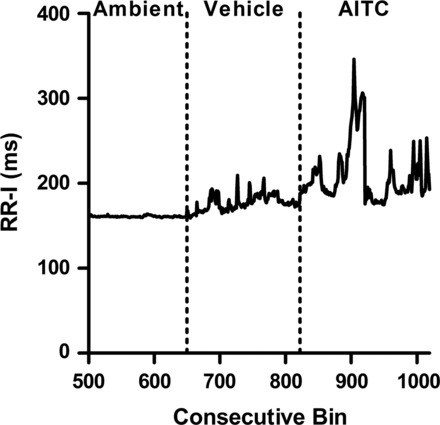
AITC response is maintained after precollicular decerebration. Mean consecutive bin averages of RR-I during ambient, vehicle, and AITC (30 mM) exposures after decerebration (n = 4).
DISCUSSION
We found that the majority of slowly conducting C fibers innervating the rat lung were stimulated by AITC, consistent with previous reports (37, 72). These fibers were also sensitive to capsaicin, the canonical nociceptive (TRPV1) stimulus. Although we did not directly determine the role of TRPA1 in AITC-evoked action potential discharge in this study, we have previously shown in mouse nociceptors that responses to AITC at these concentrations are entirely mediated by TRPA1 (49, 63). Mechanically sensitive A fibers did not respond to AITC, suggesting that TRPA1 expression in vagal sensory nerves is limited to unmyelinated subsets.
On the other hand αβmATP, the selective P2X2/3 receptor, activated subsets of both C fibers and A fibers. Previous studies in guinea pig and mouse have identified P2X2/3 receptors as exclusively expressed in airway sensory nerves innervated by the vagal nodose ganglion but not by the vagal jugular ganglion (which are unresponsive to ATP) (31, 48). Although in the rat the nodose and jugular ganglia are fused into a single complex, it is likely that the fibers activated in this study by αβmATP are also specifically innervated from “nodose” neurons. The action potential discharge from C fibers evoked by αβmATP and AITC (both at approximate maximal concentrations) was qualitatively similar.
Both capsaicin and AITC evoked immediate and substantial bradycardia in conscious rats, accompanied by substantial increases in PR-I and dropped QRS waves indicative of AV block. Such responses suggested a strong involvement of increased parasympathetic drive to the heart (14, 36, 55), which was confirmed by the abolition of AITC-induced responses (i.e., RR-I, PR-I, and AV block) by the muscarinic antagonist atropine. Previous studies have also shown that both capsaicin and AITC given intravenously evoke an apneic reflex (34, 37), likely due to activation of airway vagal C fibers. Thus the similarities between both respiratory and cardiac reflexes evoked by TRPA1- and TRPV1-stimulated pathways suggest a large degree of overlap between the TRPA1- and TRPV1-modulated central neural circuits.
We have previously shown that 30 mM AITC evoked substantial bradypnea when instilled into the nasal cavity of wild-type mice but had no effect on TRPA1−/− mice (62), thus demonstrating the selectivity of AITC for TRPA1. Here ruthenium red, an inhibitor of both TRPA1 and TRPV1, reduced AITC-induced bradycardia, suggesting the direct involvement of TRP channels in these responses. In this study ruthenium red was more effective in reducing the effects of AITC on AV block compared with RR-I, possibly due to the inhibitor curbing high intensity action potential discharge from the airway C fibers but not abolishing all C fiber activity (47).
Inhalation of AITC in conscious rats yielded a biphasic blood pressure response, with an early hypertension followed by hypotension. When pretreated with atropine, AITC simply evoked a sustained hypertension, suggesting the involvement of a parasympathetic depressor effect (55). It is presently unclear if such a depressor effect is exclusively due to a decrease in cardiac output due to bradycardia or if a reduction in peripheral resistance also contributes. Acute increases in blood pressure can cause reflex bradycardia secondarily to baroreceptor firing. Previous studies have shown that a 10-mmHg increase in pressure caused a pulse interval increase of 15 ms (17, 19, 23, 41, 50). In this study the 10-mmHg increase coincided with a 145-ms increase in pulse interval. With the use of terazosin, which blocked the AITC-evoked hypertension through inhibition of peripheral α1-receptors, AITC evoked a 109-ms increase in pulse interval. This suggests that much of the bradycardia is a direct effect of AITC on reflex parasympathetic signaling to the heart and not secondary to baroreceptor activity (70).
Anesthesia drastically inhibited the bradycardia evoked by inhalation of AITC in the freely-breathing rat. Similarly, Nakamura and Hayashida (45) demonstrated that bradycardia evoked by smoke inhalation was completely abolished by either pentobarbital or chloralose, whereas White and McRitchie (69) showed that pentobarbital attenuated this reflex substantially. There are, to our knowledge, no studies comparing the effects of anesthetics on capsaicin- or AITC-evoked cardiovascular reflexes. Nevertheless, there are reports of relatively mild bradycardia evoked by capsaicin (TRPV1) and acrolein (TRPA1) nebulized directly into the lung under anesthesia with chloralose and urethane (32), chloralose alone (7), and pentobarbital (5), although there are other reports of strong bradycardic responses under urethane anesthesia (52). Indeed, there are many reports of the bradycardic response to capsaicin injection either intravenously or into the right atria under anesthesia (10, 14, 30, 32, 34, 35, 52), and such responses are thought to be mediated by vagal nociceptive fibers innervating the airways. However, without comparisons with unanesthetized animals it is difficult to put these data into context. In some cases we were able to derive breathing rates from our intramuscular electrodes implanted to measure ECG data in combination with video recording of rat thoracic inflation. Despite anesthesia abolishing AITC-evoked bradycardia, anesthesia did not abolish the AITC-induced modulation of respiratory rhythm (conscious: vehicle 70 ± 2, AITC 22 ± 1, n = 3, P < 0.005; ketamine: vehicle 50 ± 6, AITC 29 ± 2, n = 3, P < 0.05, data not shown). This suggests significant differences between the central neural circuits involved with reflexes initiated from the airways.
Despite robust activation of bronchopulmonary C fibers by αβmATP, inhalation of ATP only produced minor bradycardia and limited AV block. The reason for this discrepancy is as yet unclear. ATP activates vagal sensory nerves via P2X2/3 receptors with similar potency to αβmATP (29, 66). ATP does activate other purinergic receptors, and it can also be metabolized in vivo, although this is unlikely responsible for the lack of significant cardiovascular responses as, with an n of 2, we found that inhalation of 30 mM αβmATP also had little effect (159.4 ± 1 ms compared with vehicle 152.7 ± 8 ma, data not shown). It is possible that simultaneous activation of A fibers expressing P2X2/3 receptors may increase heart rate (60), thus functionally antagonizing the C-fiber-mediated bradycardia. Alternatively, there is some evidence to suggest that vagal nodose C fibers and vagal jugular C fibers differentially modulate the cough reflex (61). αβmATP selectively activates nodose fibers, whereas AITC and capsaicin activate both jugular and nodose C fibers. Thus it is possible that jugular C-fiber activation evokes greater bradycardic reflexes compared with nodose C fibers, although definitive proof requires further study. It is important to note that only one concentration of ATP was studied, and there remains the possibility that robust bradycardia may be evoked with higher concentrations.
We cannot definitely ascribe the cardiovascular reflexes evoked by AITC inhalation to a specific group of nociceptors. Given the size of the nebulized irritant particles, it is likely that nociceptors throughout the airways (nasal, larynx, trachea, and bronchopulmonary) are activated in the conscious rat. The initial hypertensive effect may be due to the activation of TRPA1-expressing upper airways nociceptive fibers (44, 62) innervating the nasal airways or the larynx (28, 39, 43), whereas the later hypotension suggests specific activation of lower airways nociceptive reflexes (10, 14, 33, 34, 52). AITC nebulized directly to the lungs evoked bradyarrhythmia in decerebrate rats, indicating that the afferents characterized in our single fiber recordings are capable of eliciting these responses. It is possible that the nebulized stimuli may have entered the pulmonary circulation and caused effects distal to the airways. Brain stem removal abolished the responses, arguing against a direct effect on the heart. Stimulation of dorsal root ganglion/“sympathetic afferents” nociceptors by circulating irritant may contribute, although activation of these nerves by intra-arterial and left atrial injections of capsaicin evoke reflex tachycardia (10, 12). Carotid bodies express P2X receptors, whose activation causes reflex bradycardia (40). However, direct injection of ATP into the left common carotid artery caused minor reflex bradycardia compared with right atrial ATP injections (26), suggesting that receptors in the airways are likely to mediate most of the response to inhaled P2X agonists.
AITC and capsaicin evoked significant bradycardia in conscious animals. Surprisingly, given the constant exposure, there was little consistency or rhythmicity of RR-I on a beat-to-beat basis, thus precluding analysis by conventional heart rate variability methods (56). Lung volume changes (as observed by video recording) appeared not to be a controlling factor (data not shown). The rat heart can respond with rhythmic bradycardia to high intensity electrical stimulation of vagal efferents (6, 25); thus it is likely that the lack of rhythmicity seen is due to either sporadic afferent activity or sporadic efferent activity.
TRPA1 (and to a certain extent TRPV1) is the target of multiple inhaled pollutants including PM, cigarette smoke, ozone, diisocyanates, formadehyde, crotonaldehyde, and acrolein. Inhibition or knockout of TRPA1 substantially limits neuronal responses to these pollutants. Inhalation of these pollutants is associated with acute exacerbation of cardiovascular and respiratory disease. Here, we have shown that AITC causes robust bradycardia and biphasic blood pressure responses via reflex modulation of the autonomic nervous system. These findings are in agreement with previous studies that found inhalation of PM caused an increase in heart rate variability (suggestive of increased parasympathetic drive) and second degree AV block in healthy animals (20, 46, 54, 68). It is likely that similar acute reflexes occur with exposure to TRPA1-activating pollutants.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.S.H., K.F.M., J.W.B., J.B.D., and T.E.T.-C. conception and design of research; J.S.H. and S.H. performed experiments; J.S.H. and S.H. analyzed data; J.S.H. and T.E.T.-C. interpreted results of experiments; J.S.H. prepared figures; J.S.H. drafted manuscript; J.S.H. and T.E.T.-C. edited and revised manuscript; J.S.H. and T.E.T.-C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Carol Landon and Haley Legato for assistance with the animal surgeries. We also thank Dr. Jerome Breslin and his laboratory colleagues Dr. Travis Doggett and Natascha Alves for assistance with the blood pressure recordings.
REFERENCES
- 1.Agopyan N, Bhatti T, Yu S, Simon SA. Vanilloid receptor activation by 2- and 10-microm particles induces responses leading to apoptosis in human airway epithelial cells. Toxicol Appl Pharmacol 192: 21–35, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Agopyan N, Head J, Yu S, Simon SA. TRPV1 receptors mediate particulate matter-induced apoptosis. Am J Physiol Lung Cell Mol Physiol 286: L563–L572, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Agopyan N, Li L, Yu S, Simon SA. Negatively charged 2- and 10-microm particles activate vanilloid receptors, increase cAMP, and induce cytokine release. Toxicol Appl Pharmacol 186: 63–76, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124: 1269–1282, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bergren DR, Ustinova EE, Schultz HD. Pulmonary C-fiber activation before and after peptidase inhibition in rats. Respir Physiol 107: 99–109, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Berntson GG, Quigley KS, Fabro VJ, Cacioppo JT. Vagal stimulation and cardiac chronotropy in rats. J Auton Nerv Syst 41: 221–226, 1992. [DOI] [PubMed] [Google Scholar]
- 7.Bootle DJ, Adcock JJ, Ramage AG. Involvement of central 5-HT1A receptors in the reflex activation of pulmonary vagal motoneurones by inhaled capsaicin in anaesthetized cats. Br J Pharmacol 117: 724–728, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr, Whitsel L, Kaufman JD; American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121: 2331–2378, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Carr MJ, Undem BJ. Pharmacology of vagal afferent nerve activity in guinea pig airways. Pulm Pharmacol Ther 16: 45–52, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Coleridge HM, Coleridge JC, Kidd C. Role of the pulmonary arterial baroreceptors in the effects produced by capsaicin in the dog. J Physiol 170: 272–285, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleridge HM, Coleridge JC, Luck JC. Pulmonary afferent fibres of small diameter stimulated by capsaicin and by hyperinflation of the lungs. J Physiol 179: 248–262, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleridge JC, Coleridge HM, Roberts AM, Kaufman MP, Baker DG. Tracheal contraction and relaxation initiated by lung and somatic afferents in dogs. J Appl Physiol 52: 984–990, 1982. [DOI] [PubMed] [Google Scholar]
- 13.Cullen PM, Turtle M, Prys-Roberts C, Way WL, Dye J. Effect of propofol anesthesia on baroreflex activity in humans. Anesth Analg 66: 1115–1120, 1987. [PubMed] [Google Scholar]
- 14.Davis B, Roberts AM, Coleridge HM, Coleridge JC. Reflex tracheal gland secretion evoked by stimulation of bronchial C-fibers in dogs. J Appl Physiol 53: 985–991, 1982. [DOI] [PubMed] [Google Scholar]
- 15.Deering-Rice CE, Johansen ME, Roberts JK, Thomas KC, Romero EG, Lee J, Yost GS, Veranth JM, Reilly CA. Transient receptor potential vanilloid-1 (TRPV1) is a mediator of lung toxicity for coal fly ash particulate material. Mol Pharmacol 81: 411–419, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deering-Rice CE, Romero EG, Shapiro D, Hughen RW, Light AR, Yost GS, Veranth JM, Reilly CA. Electrophilic components of diesel exhaust particles (DEP) activate transient receptor potential ankyrin-1 (TRPA1): a probable mechanism of acute pulmonary toxicity for DEP. Chem Res Toxicol 24: 950–959, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.el-Mas MM, Abdel-Rahman AA. Estrogen enhances baroreflex control of heart rate in conscious ovariectomized rats. Can J Physiol Pharmacol 76: 381–386, 1998. [PubMed] [Google Scholar]
- 18.Elsner R, Franklin DL, Van Citters RL, Kenney DW. Cardiovascular defense against asphyxia. Science (New York, NY) 153: 941–949, 1966. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari AU, Daffonchio A, Franzelli C, Mancia G. Potentiation of the baroreceptor-heart rate reflex by sympathectomy in conscious rats. Hypertension 18: 230–235, 1991. [DOI] [PubMed] [Google Scholar]
- 20.Ghelfi E, Rhoden CR, Wellenius GA, Lawrence J, Gonzalez-Flecha B. Cardiac oxidative stress and electrophysiological changes in rats exposed to concentrated ambient particles are mediated by TRP-dependent pulmonary reflexes. Toxicol Sci 102: 328–336, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Grundy D. Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. Exp Physiol 100: 755–758, 2015. [DOI] [PubMed] [Google Scholar]
- 22.Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, Farraj AK. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ Health Perspect 119: 951–957, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Head GA, McCarty R. Vagal and sympathetic components of the heart rate range and gain of the baroreceptor-heart rate reflex in conscious rats. J Auton Nerv Syst 21: 203–213, 1987. [DOI] [PubMed] [Google Scholar]
- 24.Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol 127: 113–124, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Hotta H, Lazar J, Orman R, Koizumi K, Shiba K, Kamran H, Stewart M. Vagus nerve stimulation-induced bradyarrhythmias in rats. Auton Neurosci 151: 98–105, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Katchanov G, Xu J, Hurt CM, Pelleg A. Electrophysiological-anatomic correlates of ATP-triggered vagal reflex in the dog. III. Role of cardiac afferents. Am J Physiol Heart Circ Physiol 270: H1785–H1790, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Kaufman MP, Iwamoto GA, Ashton JH, Cassidy SS. Responses to inflation of vagal afferents with endings in the lung of dogs. Circ Res 51: 525–531, 1982. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi M, Cheng ZB, Nosaka S. Inhibition of baroreflex vagal bradycardia by nasal stimulation in rats. Am J Physiol Heart Circ Physiol 276: H176–H184, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Kollarik M, Dinh QT, Fischer A, Undem BJ. Capsaicin-sensitive and -insensitive vagal bronchopulmonary C-fibres in the mouse. J Physiol 551: 869–879, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwong K, Hong JL, Morton RF, Lee LY. Role of pulmonary C fibers in adenosine-induced respiratory inhibition in anesthetized rats. J Appl Physiol 84: 417–424, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Kwong K, Kollarik M, Nassenstein C, Ru F, Undem BJ. P2X2 receptors differentiate placodal vs. neural crest C-fiber phenotypes innervating guinea pig lungs and esophagus. Am J Physiol Lung Cell Mol Physiol 295: L858–L865, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee BP, Morton RF, Lee LY. Acute effects of acrolein on breathing: role of vagal bronchopulmonary afferents. J Appl Physiol 72: 1050–1056, 1992. [DOI] [PubMed] [Google Scholar]
- 33.Lee LY, Beck ER, Morton RF, Kou YR, Frazier DT. Role of bronchopulmonary C-fiber afferents in the apneic response to cigarette smoke. J Appl Physiol 63: 1366–1373, 1987. [DOI] [PubMed] [Google Scholar]
- 34.Lee LY, Lundberg JM. Capsazepine abolishes pulmonary chemoreflexes induced by capsaicin in anesthetized rats. J Appl Physiol 76: 1848–1855, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Lee LY, Morton RF. Pulmonary chemoreflex sensitivity is enhanced by prostaglandin E2 in anesthetized rats. J Appl Physiol 79: 1679–1686, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Levy MN, Zieske H. Autonomic control of cardiac pacemaker activity and atrioventricular transmission. J Appl Physiol 27: 465–470, 1969. [DOI] [PubMed] [Google Scholar]
- 37.Lin YJ, Lin RL, Ruan T, Khosravi M, Lee LY. A synergistic effect of simultaneous TRPA1 and TRPV1 activations on vagal pulmonary C-fiber afferents. J Appl Physiol 118: 273–281, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lippmann M, Chen LC. Health effects of concentrated ambient air particulate matter (CAPs) and its components. Crit Rev Toxicol 39: 865–913, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Liu BY, Tsai TL, Ho CY, Lu SH, Lai CJ, Kou YR. Role of TRPA1 and TRPV1 in the ROS-dependent sensory irritation of superior laryngeal capsaicin-sensitive afferents by cigarette smoke in anesthetized rats. Pulm Pharmacol Ther 26: 364–372, 2013. [DOI] [PubMed] [Google Scholar]
- 40.McQueen DS, Bond SM, Moores C, Chessell I, Humphrey PP, Dowd E. Activation of P2X receptors for adenosine triphosphate evokes cardiorespiratory reflexes in anaesthetized rats. J Physiol 507: 843–855, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller AW, Sims JJ, Canavan A, Hsu T, Ujhelyi MR. Impaired vagal reflex activity in insulin-resistant rats. J Cardiovasc Pharmacol 33: 698–702, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Mills NL, Donaldson K, Hadoke PW, Boon NA, MacNee W, Cassee FR, Sandstrom T, Blomberg A, Newby DE. Adverse cardiovascular effects of air pollution. Nat Clin Pract Cardiovasc Med 6: 36–44, 2009. [DOI] [PubMed] [Google Scholar]
- 43.Mutoh T, Kanamaru A, Kojima K, Nishimura R, Sasaki N, Tsubone H. Effects of perineural capsaicin treatment on cardiopulmonary reflexes elicited by laryngeal instillations of capsaicin and distilled water in sevoflurane-anesthetized dogs. J Vet Med Sci 62: 665–668, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Mutoh T, Taki Y, Tsubone H. Desflurane but not sevoflurane augments laryngeal C-fiber inputs to nucleus tractus solitarii neurons by activating transient receptor potential-A1. Life Sci 92: 821–828, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura T, Hayashida Y. Autonomic cardiovascular responses to smoke exposure in conscious rats. Am J Physiol Regul Integr Comp Physiol 262: R738–R745, 1992. [DOI] [PubMed] [Google Scholar]
- 46.Nalivaiko E, De Pasquale CG, Blessing WW. Electrocardiographic changes associated with the nasopharyngeal reflex in conscious rabbits: vago-sympathetic co-activation. Auton Neurosci 105: 101–104, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, Undem BJ. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol 586: 1595–1604, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nassenstein C, Taylor-Clark TE, Myers AC, Ru F, Nandigama R, Bettner W, Undem BJ. Phenotypic distinctions between neural crest and placodal derived vagal C-fibres in mouse lungs. J Physiol 588: 4769–4783, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nesuashvili L, Hadley SH, Bahia PK, Taylor-Clark TE. Sensory nerve terminal mitochondrial dysfunction activates airway sensory nerves via transient receptor potential (TRP) channels. Mol Pharmacol 83: 1007–1019, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oliveira DR, Santos RA, Santos GF, Khosla M, Campagnole-Santos MJ. Changes in the baroreflex control of heart rate produced by central infusion of selective angiotensin antagonists in hypertensive rats. Hypertension 27: 1284–1290, 1996. [DOI] [PubMed] [Google Scholar]
- 51.Paintal AS. Mechanism of stimulation of type J pulmonary receptors. J Physiol 203: 511–532, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palecek F, Sant'Ambrogio G, Sant'Ambrogio FB, Mathew OP. Reflex responses to capsaicin: intravenous, aerosol, and intratracheal administration. J Appl Physiol 67: 1428–1437, 1989. [DOI] [PubMed] [Google Scholar]
- 53.Parasuraman S, Raveendran R. Measurement of invasive blood pressure in rats. J Pharm Pharmacol 3: 172–177, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pope CA 3rd, Verrier RL, Lovett EG, Larson AC, Raizenne ME, Kanner RE, Schwartz J, Villegas GM, Gold DR, Dockery DW. Heart rate variability associated with particulate air pollution. Am Heart J 138: 890–899, 1999. [DOI] [PubMed] [Google Scholar]
- 55.Rinkema LE, Thomas JX Jr, Randall WC. Effects of individual cardiac nerve stimulation on atrioventricular conduction. J Auton Nerv Syst 5: 357–371, 1982. [DOI] [PubMed] [Google Scholar]
- 56.Rowan WH 3rd, Campen MJ, Wichers LB, Watkinson WP. Heart rate variability in rodents: uses and caveats in toxicological studies. Cardiovasc Toxicol 7: 28–51, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Ruan T, Lin YS, Lin KS, Kou YR. Sensory transduction of pulmonary reactive oxygen species by capsaicin-sensitive vagal lung afferent fibres in rats. J Physiol 565: 563–578, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sapru HN, Krieger AJ. Procedure for the decerebration of the rat. Brain Res Bull 3: 675–679, 1978. [DOI] [PubMed] [Google Scholar]
- 59.Sellgren J, Ejnell H, Elam M, Ponten J, Wallin BG. Sympathetic muscle nerve activity, peripheral blood flows, and baroreceptor reflexes in humans during propofol anesthesia and surgery. Anesthesiology 80: 534–544, 1994. [DOI] [PubMed] [Google Scholar]
- 60.Shepherd JT. The lungs as receptor sites for cardiovascular regulation. Circulation 63: 1–10, 1981. [DOI] [PubMed] [Google Scholar]
- 61.Taylor-Clark TE. Peripheral neural circuitry in cough. Curr Opin Pharmacol 22: 9–17, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taylor-Clark TE, Kiros F, Carr MJ, McAlexander MA. Transient receptor potential ankyrin 1 mediates toluene diisocyanate-evoked respiratory irritation. Am J Respir Cell Mol Biol 40: 756–762, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taylor-Clark TE, McAlexander MA, Nassenstein C, Sheardown SA, Wilson S, Thornton J, Carr MJ, Undem BJ. Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. J Physiol 586: 3447–3459, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taylor-Clark TE, Undem BJ. Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J Physiol 588: 423–433, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taylor-Clark TE, Undem BJ. Sensing pulmonary oxidative stress by lung vagal afferents. Respir Physiol Neurobiol 178: 406–413, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol 556: 905–917, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watkins L, Maixner W. The effect of pentobarbital anesthesia on the autonomic nervous system control of heart rate during baroreceptor activation. J Auton Nerv Syst 36: 107–114, 1991. [DOI] [PubMed] [Google Scholar]
- 68.Watkinson WP, Campen MJ, Costa DL. Cardiac arrhythmia induction after exposure to residual oil fly ash particles in a rodent model of pulmonary hypertension. Toxicol Sci 41: 209–216, 1998. [DOI] [PubMed] [Google Scholar]
- 69.White SW, McRitchie RJ. Nasopharyngeal reflexes: integrative analysis of evoked respiratory and cardiovascular effects. Aust J Exp Biol Med Sci 51: 17–31, 1973. [DOI] [PubMed] [Google Scholar]
- 70.Yavari P, McCulloch PF, Panneton WM. Trigeminally-mediated alteration of cardiorespiratory rhythms during nasal application of carbon dioxide in the rat. J Auton Nerv Syst 61: 195–200, 1996. [DOI] [PubMed] [Google Scholar]
- 71.Zhang G, Lin RL, Wiggers M, Snow DM, Lee LY. Altered expression of TRPV1 and sensitivity to capsaicin in pulmonary myelinated afferents following chronic airway inflammation in the rat. J Physiol 586: 5771–5786, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou Y, Sun B, Li Q, Luo P, Dong L, Rong W. Sensitivity of bronchopulmonary receptors to cold and heat mediated by transient receptor potential cation channel subtypes in an ex vivo rat lung preparation. Respir Physiol Neurobiol 177: 327–332, 2011. [DOI] [PubMed] [Google Scholar]


