Abstract
Neuronal populations with unbalanced inhibition can generate interictal spikes (ISs), where each IS starts from a small initiation site and then spreads activation across a larger area. We used in vivo voltage-sensitive dye imaging to map the initiation site of ISs in rat visual cortex disinhibited by epidural application of bicuculline methiodide. Immediately after the application of bicuculline, the IS initiation sites were widely distributed over the entire disinhibited area. After ∼10 min, a small number of sites became “dominant” and initiated the majority of the ISs throughout the course of imaging. Such domination also occurred in cortical slices, which lack long-range connections between the cortex and subcortical structures. This domination of IS initiation sites may allow timing-related plasticity mechanisms to provide a spatial organization where connections projecting outward from the dominant initiation site become strengthened. Understanding the spatiotemporal organization of IS initiation sites may contribute to our understanding of epileptogenesis in its very early stages, because a dominant IS initiation site with strengthened outward connectivity may ultimately develop into a seizure focus.
Keywords: interictal spikes, voltage-sensitive dye imaging, epileptiform focus, seizure initiation, propagating waves
interictal spikes (ISs) are brief, paroxysmal depolarizations of neuronal populations with unbalanced inhibition (de Curtis et al. 1999), and they may play a fundamental role during the early stages of epileptogenesis (Avoli et al. 2006; Sabolek et al. 2012; Staley et al. 2005; Staley and Dudek 2006). At the population level, ISs have a large and stable amplitude, suggesting participation of a large fraction of neurons with nearly synchronized spiking discharges (Traub and Wong 1982). Voltage-sensitive dye (VSD) imaging has revealed that, when rat neocortex is disinhibited by application of bicuculline (a GABAA receptor antagonist), each IS starts from a point initiation site and propagates through the entire disinhibited area (London et al. 1989; Ma et al. 2004; Tsau et al. 1998, 1999). While the entire disinhibited area was activated during each IS, a small initiation site always activated first, followed by outward spreading of population activity.
Our laboratory's previous research suggests that, rather than randomly emerging from the whole disinhibited area, the majority of ISs likely initiate from a few “dominant sites” (Ma et al. 2004; Tsau et al. 1998). However, the development and the spatiotemporal properties of the domination have never been investigated. In this report, we used fast VSD imaging to visualize the formation of the dominant initiation sites, and we found that they form within ∼10 min of IS emergence (induced by epidural application of bicuculline methiodide). Once formed, the vast majority of ISs are initiated by dominant sites. Multiple dominant initiation sites take turns to initiate multiple ISs, with new dominant sites occasionally forming and existing dominant sites occasionally terminating. Further analysis of multiple initiation sites suggests that an IS initiated from a particular dominant site increases the probability that the following IS will initiate from the same site.
Our results suggest that the organization of dominant IS initiation sites takes place during the very early stages of epileptiform activity and acts as a spatiotemporal organizer of the ISs, which may in turn play a role in the development of epilepsy.
EXPERIMENTAL PROCEDURES
All animal protocols (acute in vivo and in vitro cortical slices) were approved by the Institutional Animal Care and Use Committee of Georgetown University following the guidelines of the National Institutes of Health.
In vivo experiments.
Surgical and VSD imaging methods were described in detail in our laboratory's previous papers (Huang et al. 2010; Lippert et al. 2007; Xu et al. 2007). Briefly, Sprague-Dawley rats (250–400 g) were pretreated with dexamethasone sodium phosphate (4 mg/kg) and atropine sulfate (40 μg/kg ip) about 30 min prior to anesthetic induction. Animals were induced with 2–3% isoflurane in air, and tracheostomy was performed. Animals were ventilated with isoflurane in room air. Respiration rate (60–100 breaths/min) and volume (2–4 ml) were adjusted such that inspiratory pressure was ∼5 cmH2O and the end-tidal carbon dioxide was maintained at ∼26–28 mmHg. Isoflurane was reduced to 1.5–2% during surgery. A craniotomy for imaging was performed on one hemisphere over the visual cortex. In some experiments (see Fig. 5), an additional craniotomy window was created over the contralateral hemisphere for applying electrical stimulation. The dura was left intact in the imaging craniotomy window for more stable VSD imaging (Lippert et al. 2007). VSD RH 1691 (Optical Imaging) was dissolved at 2 mg/ml in lactated Ringer solution (100 ml Ringer solution contains sodium chloride 600 mg, sodium lactate 310 mg, potassium chloride 30 mg, calcium chloride dehydrate 20 mg), and staining was done in a temporary staining chamber constructed with a thin layer of silicone valve grease around the edges of the imaging craniotomy. During staining, the dye solution was continuously circulated by a custom-made perfusion pump (Lippert et al. 2007; London et al. 1989). Staining lasted for 90–120 min, followed by washing with dye-free Ringer solution for >15 min.
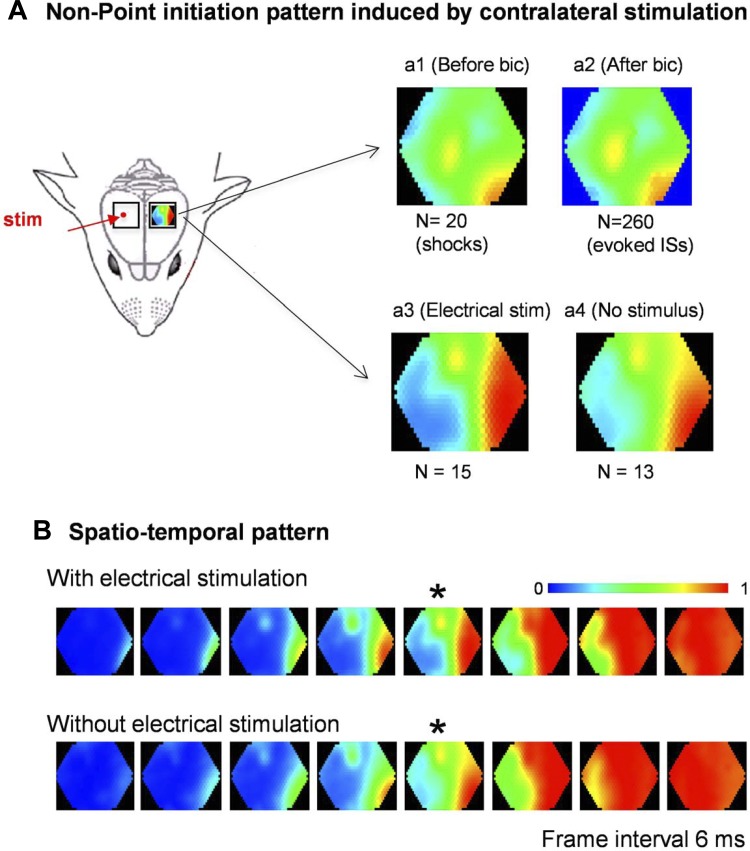
Fig. 5.
Point-stripe pattern induced by electrical stimulation to the contralateral cortex. A: contralateral point stimulation induced neuronal activities in the imaging side with a point-stripe pattern, both before (a1) and after (a2) bicuculline application. In another animal, the stimulation was turned off after bicuculline application to allow ISs to emerge without stimulation. Identical point-stripe initiation patterns are seen with (a3) and without electrical stimulation (a4). B: initiation patterns of the ISs with and without electrical stimulation. The images in a3 and a4 were selected from the frames marked by an asterisk (*).
At the start of imaging, the solution inside the chamber was replaced by 60–100 μl bicuculline methiodide solution (1 mM in Ringer solution). The chamber was then sealed by a glass coverslip. In some experiments, penicillin solution (6,000–10,000 unit/ml) was used to replace bicuculline to examine IS initiation induced by a different chemical convulsant. The VSD imaging was conducted either as intermittent trials or continuously, beginning right after ISs started to emerge.
The cortex was imaged by a ×5 macroscope (made of a Navitar 25 mm F 0.95 video lens, Lippert et al. 2007) with a field of view of about 4 mm in diameter. A halogen tungsten filament lamp (12 V, 100 W, Zeiss) was used for illumination. The light was filtered with a 630 ± 15 nm interference filter (Chroma Technology) and then reflected down onto the cortex via a 655-nm dichroic mirror (Chroma Technology). The dye fluorescence (∼700 nm) from the stained cortex was collected via the macroscope, filtered through a 695-nm long-pass filter (RG-695, Edmund Scientific) and projected onto the fiber-optic aperture of a 464-channel photodiode array (WuTech Instruments). Each channel (detector) of the array received light from a cortical area ∼160 μm in diameter. The resting florescent light intensity was about 1 V. This VSD signal was filtered at 400 Hz and digitized at 1.6 kHz. A silver ball electrode was placed at the edge of the optical recording field to record the epidural local field potential (LFP). The LFP signal was digitized concurrently with the optical channels, and an electrocardiogram that was used to remove pulsation artifacts offline.
In vitro experiments.
Experiments in brain slices were described in detail in our laboratory's previous papers (Jin et al. 2002; Tsau et al. 1998). Briefly, C57BL/6 mice (n = 8) of both sexes from postnatal days 21–30 were used in the experiments. After deep anesthesia with isoflurane or an intraperitoneal injection of ketamine (100–200 mg/kg), the animals were decapitated. The whole brain was then quickly removed and chilled in cold (0°C) sucrose-based artificial cerebrospinal fluid (ACSF) containing the following (in mM): 252 sucrose, 3 KCl, 2 CaCl2, 2 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 10 dextrose and bubbled with 95% O2/5% CO2. Neocortical slices (400 μm thick) were cut in coronal sections with a vibratome (Leica, VT1000S) between bregma 1 to −3 mm. After sectioning, the slices were transferred into an incubation chamber with ACSF containing the following (in mM): 132 NaCl, 3 KCl, 2 CaCl2, 2 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 10 dextrose, and saturated with 95% O2/5% CO2 at 26°C. The slices were incubated for about 90–120 min before experiments. During experiments, the slices were kept at room temperature (26°C) and perfused (∼30–50 ml/min) on both sides in ACSF with added 10 μM N-methyl-d-aspartate. Bicuculline methiodide was added to the perfusion solution after the slice was stabilized in the recording chamber for >15 min. Two glass microelectrodes (∼100 kΩ tip resistance) were used to record the LFPs of the ISs. The time difference (Δt) for each IS to reach the two electrodes was measured to estimate the location of the IS initiation site.
Data analysis.
Data were processed in Neuroplex (RedShirt Imaging) and using scripts written in MATLAB (Mathworks). Pseudocolor images were generated such that each detector was individually scaled to a linear color scale (Grinvald et al. 1982; Jin et al. 2002) for each IS.
Initiation site, initiation frame, and averaged initiation frame.
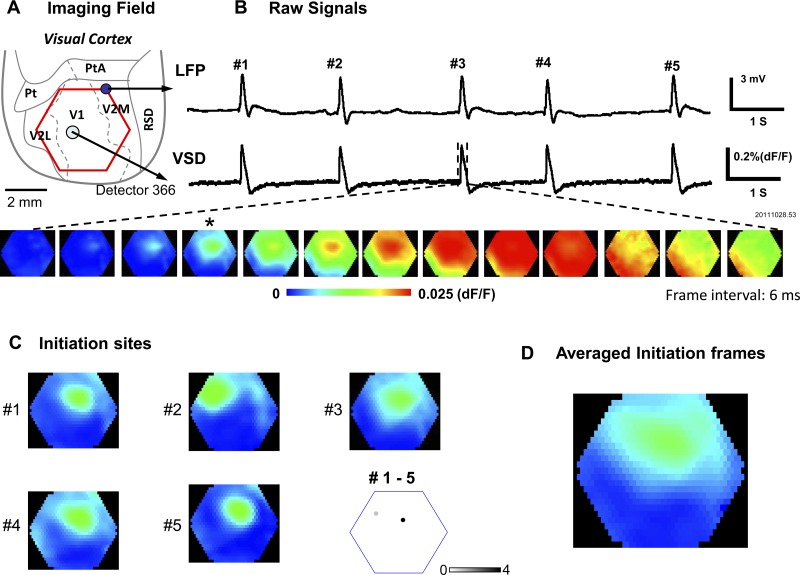
We define the initiation site of an IS as the location with the earliest onset time in the imaging field. Although in other studies the onset time is often calculated as the time when the signal first rises significantly above noise level (such as reaching two times the standard deviation of the background signal), it is difficult to apply this method to our data because the noise and artifact can vary largely on individual detectors. In addition, the signal amplitude varies at different locations due to staining and illumination, and each IS may have a different amplitude. For these reasons, we normalized each IS to its own peak on each detector (peak = 1, baseline = 0) and used 50% of the peak amplitude (Ma et al. 2004, 2012) for calculating the onset time. Fifty percent of the peak is far above noise level for all detectors, allowing automatic marking of the onset time for all of them. The detector (or a few detectors) with earliest 50%-of-peak amplitude time was found for each IS and was defined as the IS “initiation site” (Fig. 1B, bottom images). The frame in which the signal at the initiation site is closest to its 50%-of-peak amplitude is referred to as the “initiation frame” (Fig. 1C). The linear average of initiation frames is referred to as the “averaged initiation frame” (AIF; Fig. 1D). The location of initiation sites and timing of the initiation frames for each IS were automatically generated using scripts written in MATLAB and were verified by the human operator.
Fig. 1.
Interictal spike (IS) initiation sites. A: imaging field (red hexagon) over visual cortex. Cortical area boundaries are approximately sketched following the rat atlas anatomical sections by Paxinos and Watson (2005). Pt, paratenial nucleus; PtA, parietal association cortex; V2M, secondary visual cortex, medial area; V1, primary visual cortex; V2L, secondary visual cortex, lateral area; RSD, retrosplenial dysgranular cortex. B, top traces: local field potential (LFP) signal recorded by a silver ball electrode and voltage-sensitive dye (VSD) signal from one optical detector at the location labeled in A. Both signals are band-pass filtered from 1–200 Hz. Bottom images are composed from all 464 optical detectors. The initiation frame is marked by the asterisk (*). C: images 1–5 are the initiation frames from ISs 1–5 of this recording trial. Bottom right map marks the initiation sites of ISs 1–5. The initiation sites are defined as the detector that first reached 50% of peak amplitude. Note that the initiation sites of ISs 1, 3, 4, and 5 are at the same location, while that of IS 2 has a different location. D: the “averaged initiation frames” of ISs 1–5. It is the linear average of the five frames in C. Note that 4 out of 5 ISs were initiated from the same location, and the averaged initiation frame shows high amplitude (warm color) at this dominant location.
Maximum amplitude of the AIFs.
We use the maximum amplitude of AIF as a numerical indicator for the domination of IS initiation sites. In an initiation frame, the amplitude on each detector is a relative value, with a maximum of ∼0.5 (50% of the peak) at the initiation site. In an AIF, if all of the ISs are started from different locations, then the averaged amplitude would be low at all detectors. In contrast, if all ISs are initiated from the same location, then the averaged amplitude would be high near the initiation site, reaching ∼0.5. In Fig. 2B, the AIFs were obtained for each trial, and the maximum amplitude of each AIF was calculated from an average of the five detectors with highest amplitude in the frame.
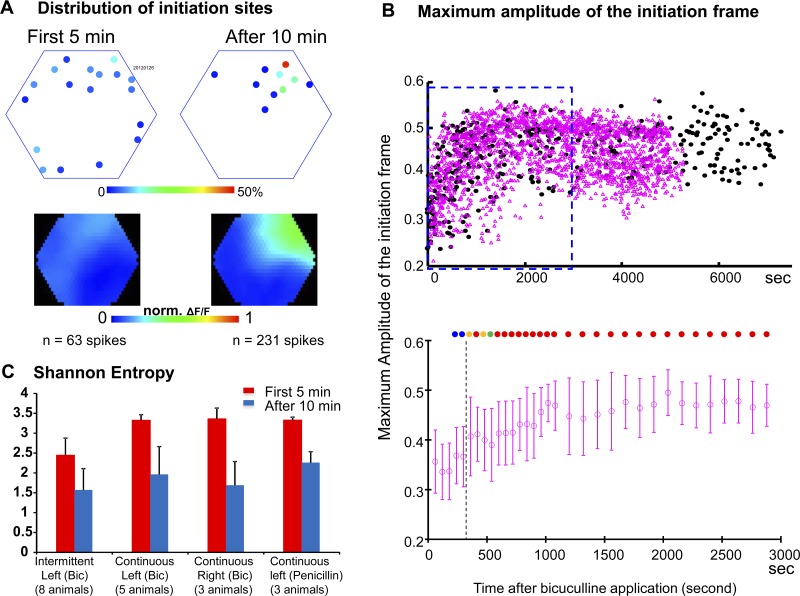
Fig. 2.
Domination of IS initiation sites. A: distribution of initiation sites from a representative animal. Top maps mark the initiation sites during the initial and later periods after bicuculline application. The density of initiation sites on each detector is marked by pseudocolor scale. Bottom images are the averaged initiation frames of the two periods. B: aggregated data from 13 animals. Top graph, maximum amplitude of averaged initiation frames from all recording trials. Bottom graph, statistics of the continuous recording data within the blue square in the top graph. Each point is the average within a certain period (1 or 2 min). The color-filled circles on the top of the plot indicate the P value of the t-test between the current point and the first 3 min: blue: 0–0.05; green: 0.05-0.01; orange: 0.01-0.0001; red: <0.0001. C: Shannon entropy of the initiation sites after drug application. In all four conditions (intermittent recording for bicuculline-induced ISs in the left hemisphere, continuous recording for bicuculline-induced ISs in the left hemisphere, continuous recording for bicuculline-induced ISs in the right hemisphere, continuous recording for penicillin-induced ISs in the left hemisphere), the Shannon entropy of the initiation sites is significantly lower after 10 min (blue bars) than during the first 5 min (red bars), P < 0.001.
Shannon entropy.
From the occurrence rate of IS initiation sites across 464 detectors (X), we determined the probability (p) of sampling a random initiation event for each detector (i), from which we calculated its self-information content (I).
| 1) |
Shannon entropy (S) is the expected value of I and measures the uncertainty in the expected detector (spatial location) of the following initiation sites.
Entropies are low when a consolidated focus generates most of the spikes, while wide distribution of initiation sites increases the entropy. If all ISs initiated from the same site (an entirely predictable outcome), then the entropy value would be zero. Before calculating the Shannon entropy, we regrouped the initiation sites so that any initiation site within a distance of 2 detectors from the previous seed would be included in the same group. In the continuous recording experiments, the entropy was calculated during each 5-min block (30 recording trials, Fig. 2C) or 1-min block (to estimate the time needed for the dominant sites). For the intermittent recording trials, entropy was calculated in 10-trial blocks, since intermittent recordings have fewer trials in a given period.
RESULTS
IS initiation sites.
The ISs emerged at a rate of 0.3–3 spikes/s a few minutes after epidural application of bicuculline methiodide (1 mM) onto the cortex. In the LFP recording, all ISs had a similar amplitude and waveform (Fig. 1B, top trace). The VSD imaging of cortical neuronal populations during each IS showed a rapid depolarization that corresponded to the spikes in the LFP (Fig. 1B, top and bottom traces). We had 464 optical detectors over an imaging field 4 mm in diameter; the earliest of these detectors to reach 50% of peak amplitude for each IS determined the “initiation site” of the IS (Fig. 1B, frame marked with asterisk; see Data analysis). Spatiotemporally, the activity of each IS emerges from its initiation site and expands to the entire imaging field (Fig. 1B, bottom frames). Figure 1C shows the initiation sites of ISs 1–5 from a sample 10-s recording trial. While ISs 1, 3, 4, and 5 had the same initiation site, IS 2 was initiated from a different site, as illustrated in Fig. 1C, bottom right. Figure 1D shows the AIF for ISs 1–5. It was constructed for each IS by taking a single imaging frame at the time when the signal first reached 50% of the peak amplitude at the initiation site (Fig. 1B, bottom image marked with asterisk). The initiation frames for each IS (Fig. 1C) were then all averaged together to generate the averaged optical signal at initiation for these 5 ISs (Fig. 1D).
Domination of IS initiation sites.
During the first few minutes after bicuculline application, the IS initiation sites were distributed widely over the imaging area. Within ∼10 min, the initiation sites began to consolidate into a single or a few initiation sites that initiated the majority of the ISs during a given period. “Domination of initiation sites” refers to the phenomenon where a large fraction of ISs (>30% during consecutive measuring periods with >100 ISs total) emerge from the same initiation site. In a representative animal shown in Fig. 2A, during the first 5 min after bicuculline application, 63 ISs were sampled from 8 imaging trials, and 18 initiation sites were identified. Most of these initiation sites only generated one to three ISs, with the largest density at a single detector generating nine ISs (Fig. 2A, First 5 min). The distribution of initiation sites had changed substantially by 10 min after bicuculline application: of the 231 ISs identified in 24 imaging trials in this time period, 115 ISs (49.7%) were initiated from a single location; the other 116 ISs were from 9 additional sites (Fig. 2A, After 10 min).
Domination of initiation sites was observed in all 13 animals examined and was quantified by two independent methods. The first method was taking a linear average of the “initiation frames” to generate an AIF as shown in Fig. 1D. In Fig. 2A, we generated separate AIFs for the first 5 min after IS emergence and for the rest of the recording time. For the first 5 min, the initiation sites were widely distributed, and the AIF showed an evenly distributed low-amplitude blue color (Fig. 2A, bottom left). In contrast, the AIF for the rest of the recording time showed a high-amplitude “hot spot” over the dominant site (Fig. 2A, bottom right). This large difference in the AIFs was seen in all 13 animals.
The second method for quantifying the initiation frames from different animals was to calculate the maximum amplitude of AIFs in each 10-s trial (see Data analysis). In Fig. 2B, top, the black dots are the maximum amplitudes of the AIF in each trial from 13 animals. The plot shows a clear trend of elevation after bicuculline application, suggesting that, over time, more ISs were initiated from a few dominant initiation sites. The statistics of the continuous recording (pink points in Fig. 2B, top) were further plotted in the bottom panel, with each point representing the average amplitude of AIFs within a certain period (1 min for the first 18 points and 2 min for the rest). The color-filled dots indicate the P values generated from two-sample t-tests comparing all amplitudes from the current time period to those from the first 3 min (blue: 0–0.05; green: 0.05-0.01; orange: 0.01-0.0001; red: <0.0001). After about 5 min, all further time periods showed a significant difference compared with those during the first 3 min, suggesting that initiation sites were becoming dominant.
Eight of the 13 animals were imaged with intermittent imaging trials to avoid possible phototoxicity from the VSD (Lippert et al. 2007; London et al. 1989). The other five animals were studied with continuous optical recording. The organization of the dominant sites showed no apparent difference between intermittent and continuous recordings (black and pink points in Fig. 2B, top), suggesting that light exposure during continuous optical recordings did not affect the organization of dominant initiation sites.
We used spatial Shannon entropy as an additional independent method for quantifying the domination of IS initiation sites (see experimental procedures, Data analysis). Within all animals examined, there was a large decrease in the entropy of IS initiation sites after the initial period, in both left and right hemispheres, for ISs induced by bicuculline (Fig. 2C), which indicates that the IS initiation sites became more predictable. We used Shannon entropy to estimate the time needed for the dominant sites to form: in eight animals with continuous recordings, the imaging trials were divided into 1-min blocks, and the Shannon entropy was calculated for each block. The first three blocks were used as a control pool for evaluating the rest of the blocks, and the following blocks were each compared with the control with a two-sample t-test. The entropy became significantly different from the control samples after 10.6 ± 5.6 min (mean ± SD), averaged across all eight animals (with five imaged on the left hemisphere and three on the right). The ISs initiated in the penicillin model showed the same decrease in Shannon entropy as those in the bicuculline model (Fig. 2C, rightmost columns).
Multiple dominant initiation sites.
Our analyses show that, by the end of the initial period, there were still multiple IS initiation sites. We questioned whether there was a single dominant site with a few nondominant sites or multiple dominant sites taking turns to initiate the ISs. We addressed this below using continuous VSD imaging experiments to examine large numbers of ISs after the initial domination period.
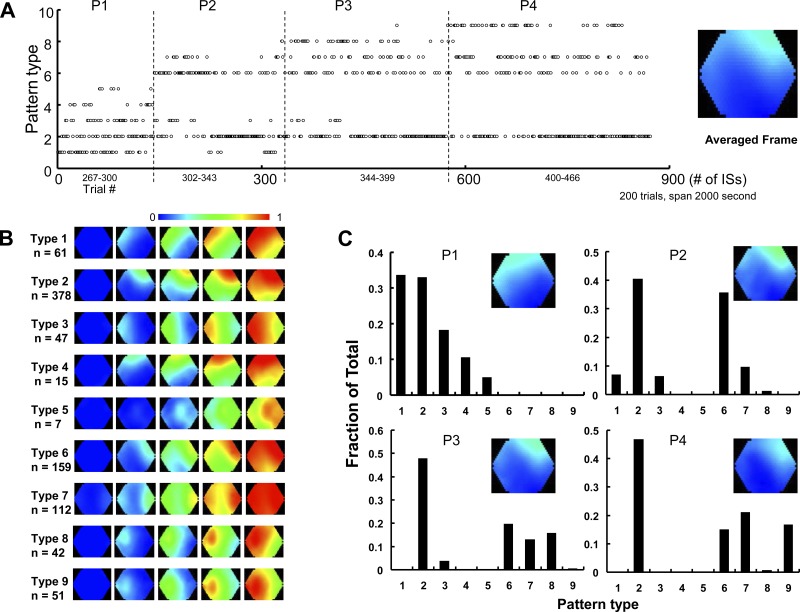
In five animals, we reduced the intensity of illumination light to reduce the light-bleaching of the dye and extend the imaging time (Lippert et al. 2007). We were able to continuously image the cortex for up to 2 h. Figure 3 displays data collected from one representative animal, for which we divided the continuous recording into more than 500 trials (10 s each). Among them, the 200 consecutive trials that started 46 min after bicuculline application are shown in Fig. 3. Nine types of dominant IS initiation were found, which together constituted 873 ISs (Fig. 3A). For this experiment, the nine dominant types were defined not only by their initiation sites (Fig. 3B, frame 2 in each row), but also by their spatiotemporal patterns of spread (Fig. 3B, frames 3–5 in each row). The spatiotemporal pattern of spread further distinguished IS initiations with similar initiation locations. For example, types 2 and 6 were both initiated in the top right of the imaging field, but type 6 can be distinguished by the rapid right rotation of its wave front. All 873 observed ISs were of these nine initiation types, and none initiated at other “random” locations or organized differently in space. Among the nine types of IS initiation, type 2 was the most commonly seen (378/873 ISs) and was dominant through all 200 trials. To better describe the distribution of the initiation types, we arbitrarily divided the total recording time into four periods (Fig. 3A, P1-4) and plotted the occurrence rate of each type in each time period (Fig. 3C). In each time period, the probability was normalized to one for all initiation patterns so that changes in pattern frequency could be compared across time periods. We found that type 2 was most prominent throughout the entire recording, while types 1 and 3 had high occurrence rates during period 1 but later disappeared. Type 9 only occurred in period 4, and types 6–8 occurred only in periods 2–4. These results suggest that ISs are initiated by multiple dominant sites, that new dominant sites can form, and that existing dominant sites can terminate. Of the nine pattern types, types 4 and 5 were seen least commonly (15 and 7 out of the 873 ISs) and are the only ones to not meet our definition of “dominant” (30 of 100 consecutive recorded ISs); this suggests that some initiation patterns may occur repeatedly but fail to become dominant. However, the overwhelming majority (>97%) of ISs were initiated by dominant sites.
Fig. 3.
Multiple dominant sites and initiation patterns. A: pattern type of each IS during 200 continuous trials. Nine types of propagation patterns were found among all the ISs, as defined in B. According to the trend of the pattern, the recording time was arbitrarily divided into four periods: P1, P2, P3, and P4. The pseudocolor image at the right side is an average of the initiation frame of all the spikes. B: the nine types of propagation patterns. C: histograms showing the occurrences of each pattern type within different periods. The pseudocolor image insets are the average of all the initiation frames during that period.
After domination, the preceding IS may greatly increase the probability for a successive IS to initiate from the same site. In the animal shown in Fig. 3A, the overall probability for type 2 to occur was 0.42; If the immediately preceding IS was type 2, then the probability for the next IS to be type 2 increased to 0.55. A similar tendency was seen in the other initiation types with lower overall probability (type 1: 0.068/0.33; type 3: 0.052/0.17; type 4: 0.017/0.29; type 5: 0.08/0.16; type 6: 0.18/0.3; type 7: 0.12/0.32; type 8: 0.047/0.29; type 9: 0.013/0.24). This tendency suggests that all nine types possess a feature of “domination”: an increased likelihood for the following ISs to be initiated from the same site/type. The switching of IS initiation sites is organized dynamically on a time scale of minutes.
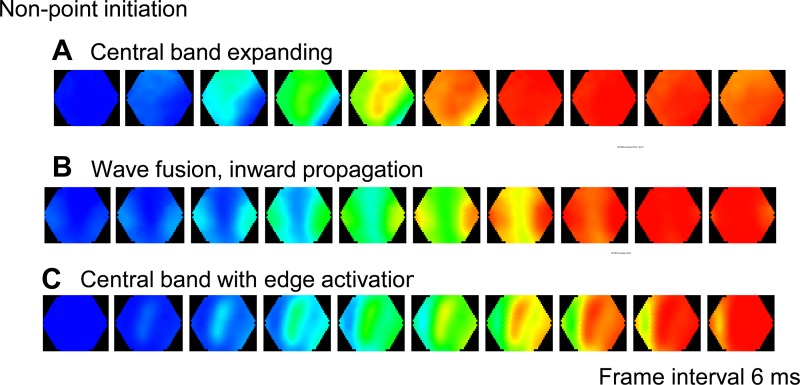
Nonpoint initiation patterns.
We noticed that some ISs are not initiated from a single point. The most obvious example is type 7 (Fig. 3B), where two waves from both left and right edges of the imaging field move toward each other and fuse at the center of the field. Examples from other animals (Fig. 4) also show that ISs may initiate as an expanding central band (Fig. 4A), as two waves starting from different edges of the imaging field and propagating inward (Fig. 4B), or as a combination of both (Fig. 4C). The most likely explanation for the nonpoint patterns is that the real initiation site may be located outside the imaging field, but projects simultaneously to multiple areas in the imaging field where we observe the IS. We explored the possibility that a nonpoint IS initiation pattern could be created by activity outside the disinhibited area, e.g., in the contralateral cortex.
Fig. 4.
Nonpoint initiation patterns. Examples of ISs initiated from nonpoint initiation sites. A: an IS started from a band of activation in the center of the imaging field. B: two waves propagating inward from the edge of the imaging field. C: an IS started from the center and the side bands.
In the experiment shown in Fig. 5, we created two craniotomy windows, one over each hemisphere (Fig. 5A), so that point electrical shocks could be applied to the contralateral cortex. We applied low-intensity stimulation (10 μA, 0.2 ms, delivered by a 2 MΩ monopolar tungsten electrode) to the contralateral hemisphere (stimulation side) prior to the bicuculline application and found that the stimulus could create a “point-stripe” pattern in the imaging side (Fig. 5A, image a1) as an evoked response. This finding demonstrates that the evoked activity in the contralateral cortex is able to simultaneously activate two locations in the imaging cortex even without the disinhibition from bicuculline. In the same animal, after bicuculline was applied to the imaging side, the contralateral stimulus-evoked ISs initiated with a similar point-stripe pattern (Fig. 5A, image a2). The movie frames of the “point-stripe” pattern (Fig. 5B) reveals that the point and stripe appeared with indistinguishable timing, suggesting that the two locations are activated by the same source. In another animal, we turned off electrical stimulation after bicuculline application to allow ISs to emerge without the stimulation. We found that the stimulation-free and electrically-evoked point-stripe initiation patterns were indistinguishable (Fig. 5A, images a3 and a4). In the 64 ISs recorded during the period without electrical stimulation, 24 (37.5%) were initiated with the same pattern as those induced by contralateral stimulations, and the others were initiated from three dominant single-point patterns. This contralateral stimulation experiment suggests that nonpoint initiation patterns may be related to the activity outside the imaging area, such as in the contralateral cortex.
Domination of IS initiation sites in cortical slices.
One concern regarding the in vivo experiments was the nonuniform diffusion of epidurally applied bicuculline. Furthermore, the brain is vigorously perfused by blood circulation, and, therefore, topically applied bicuculline would most likely have uneven concentration in different areas. Uneven distribution of bicuculline in space may affect the probability of initiating ISs in different regions. To minimize this potential effect, we investigated domination of IS initiation sites in cortical slices.
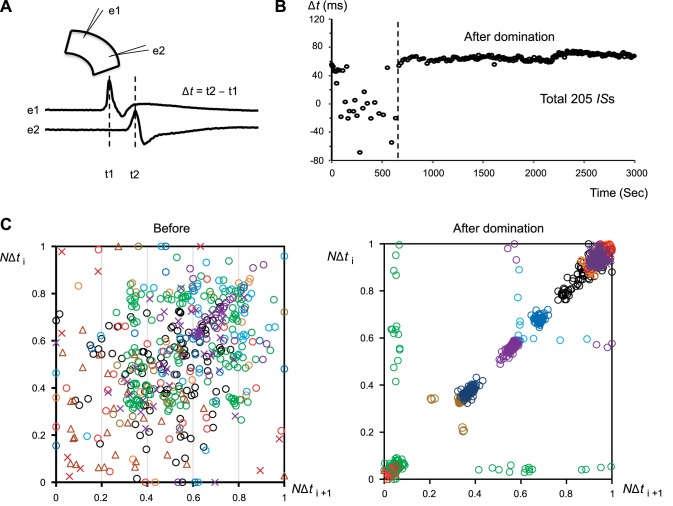
In the experiments shown in Fig. 6, about 1 min after bicuculline methiodide (2 μM) was added to the perfusion solution, interictal-like spikes emerged, which were recorded by two glass electrodes (∼100 KΩ tip resistance) placed in the superficial layers of the somatosensory cortex. The location of the IS initiation sites can be estimated by the time it takes for each IS to reach the two electrodes. When the Δt to reach the two electrodes is the same for two ISs, it is highly likely that the ISs originate from the same location (Tsau et al. 1998). The plotted Δt shows the initial wide distribution change to a clear clustering after about 700 s, suggesting that dominant initiation sites develop over time. Aggregated data from multiple slices are presented in Fig. 6C, where each Δt from each slice is normalized to the longest Δt in that slice (normalized Δt, NΔt). We plot the NΔt for each IS against the NΔt of its successor IS (NΔti+1) in Fig. 6C. After domination (Fig. 6C, right), the NΔt plots show a vast majority of dots along the diagonal, suggesting a high likelihood that each IS is initiated from the same location as its predecessor. In contrast, before domination (Fig. 6C, left), the dots are widely scattered, suggesting a broad distribution of initiation sites. While the time for domination of IS initiation varied from slice to slice, a transition to the domination was observed in all slices (16 slices from 8 animals).
Fig. 6.
Formation of domination of IS initiation sites in a cortical slice. A: ISs induced by 2 μM bicuculline were recorded by two glass electrodes placed in the superficial layers of a coronal slice. The time difference (Δt) between an IS peak reaching the two electrodes (e1 and e2) is used as a measurement to assess domination. B: plotting of Δt against time. A sudden transition in Δt distribution (vertical broken line) demonstrates the formation of a new dominant site, with relatively fixed Δt afterwards. C: scatter plots of normalized Δt (NΔti) for each IS against that of its successor IS (NΔti+1) before (left) and after domination (right). Colors and symbols represent the data from different slices. In each slice, every Δt is normalized between the longest negative Δt (as 0) and the longest positive Δt (as 1) in each slice.
DISCUSSION
The principal findings of this report are as follows. 1) The initiation sites of the ISs undergo a spatial consolidation within the first few minutes. During this period, a few dominant sites quickly develop (Figs. 1 and 2). 2) There may be multiple dominant sites, taking turns to initiate the ISs (Fig. 3). 3) Nonpoint initiation patterns were observed, e.g., two spatially distinct locations activated simultaneously (Figs. 3 and 4), and they could be mimicked by electrical stimulus to the contralateral cortex. The pattern evoked by electrical stimulation later became a dominant pattern for ISs emerging under inhibitory blockade (Fig. 5). Our findings suggest that the initiation of ISs undergoes dynamic organization at a very early stage and, consequently, most of the ISs start from a few dominant sites. Domination of IS initiation sites may establish a spatiotemporal framework that could be associated with the early stages of epileptogenesis.
Is the phenomenon real?
We demonstrated a process happening in the first few minutes of ISs initiation, where widely distributed IS initiation sites consolidate into a few dominant sites that initiate the majority of the ISs thereafter. There are two potential factors which may argue against our claim of dominant initiation sites. One factor is the diffusion pattern of the applied drug: topical application of bicuculline to the cortex will likely create a diffusion gradient from the surface down into the cortex. The gradient may become larger as blood circulation constantly washes out drug from the cortical tissue. Presumably, locations with higher bicuculline concentration may be more active and likely to initiate ISs. To deal with this diffusion problem, we used a very high concentration of bicuculline (1 mM) on the dura, about 100 times the concentration that completely blocks GABAA receptors (Chagnac-Amitai and Connors 1989). At this high concentration, a complete blockade would be quickly created across the superficial cortical layers of exposed cortex, despite the concentration gradient (e.g., some locations would have 20 μM and other places 200 μM, both saturating the receptors). The wide distribution of initiation sites observed within the first 5 min strongly suggests a nearly homogenous blockade of inhibition in the cortex exposed to bicuculline (Fig. 2A). The domination of initiation sites happened much later, usually 5–10 min after bicuculline application. It is thus unlikely to be caused by uneven distribution of bicuculline. This issue is further addressed by in vitro brain slice experiments where inhomogeneous diffusion of the bicuculline was minimized. In these experiments, IS initiation showed a clear trend from a widely distributed time latency to a consistent latency within a few minutes (Fig. 6), suggesting that inhomogeneous diffusion is unlikely the cause for domination of initiation sites.
The second factor is related to the technical issues of VSD imaging: our VSD signals mainly originate from layers 1–3 (Lippert et al. 2007). It is possible that the original initiation sites of ISs are located down in the deeper layers (Connors 1984) or outside the imaging field, and thus the activity in our imaging may be a secondary or tertiary event. However, a domination seen in the secondary initiation sites would still suggest a domination process in the primary initiation sites.
It is also possible that phototoxicity, if it is unevenly distributed in the field of view, might become a concern. We argue that the dominant initiation sites are not artifacts of phototoxicity because in our brain slice experiments there is no optical recording and no phototoxicity, yet we still see domination of the initiation sites. In our imaging experiments, with 10-s second recording trials used during intermittent recording, there is no detectable phototoxicity up to 100 trials, based on the fact that the amplitude of the LFPs does not decrease (Huang et al. 2010; Lippert et al. 2007; Xu et al. 2007). For the continuous recording, we greatly reduced the illuminating light, and as a consequence the optical recording time could be extended to 2,000 s. The formation of dominant sites happens within the first 300–600 s following bicuculline application, which is very unlikely to be caused by phototoxicity. Additionally, the whole imaging field is more or less evenly exposed under illuminating light. The reduction of VSD signal (due to bleaching of the VSD) is proportional among individual detectors over the field. The initiation site is the location where the interictal spike appears the earliest, and the amplitude of the signal at the initiation site is similar at other locations. Thus any phototoxicity should be evenly distributed across the field of view and cannot adequately explain why the ISs consistently emerge from the same location.
From all of the data in this report, we believe the domination of IS initiation sites is a real phenomenon and not related to uneven drug diffusion or phototoxicity.
Possible mechanisms underlying the domination.
The rodent visual cortex has local connectivity that is highly convergent and divergent. For example, both excitatory and inhibitory visual cortical neurons receive highly convergent input from neurons with a broad range of preferred orientations (Bock et al. 2011). How can such highly specialized networks develop dominant IS initiation sites? We believe that the emergence of dominant sites is a dynamic process: once GABAergic inhibition is blocked by bicuculline, excitatory activity will become regenerative, i.e., the activity will expand through the highly divergent local excitatory networks, resulting in a large fraction of neurons activated within a short time frame. This will be seen in the LFP as an IS (de Curtis et al. 1999; Jefferys 1990). Spatiotemporally, the excitation starts from one location and expands as a propagating wave in space, moving outward in all directions (Fig. 1B).
Our experiments do not provide direct evidence of a mechanism for the emergence of dominant sites. However, the center-first/surrounding-later pattern of wave propagation might play a role in the development of a dominant site due to short-term plasticity, such as spike timing-dependent potentiation (STDP; Bi and Poo 1998; Markram et al. 1997). During the outward spreading of the IS, neurons closer to the initiation site (proximal neurons) would be more likely to fire before neurons farther away from the initiation site (distal neurons). The increased likelihood of presynaptic/proximal neurons firing before postsynaptic/distal may facilitate STDP potentiation of the synapses that mediate outward spread. In contrast, there would be an increased likelihood of postsynaptic/proximal neurons firing before presynaptic/distal, which may facilitate depression of synapses mediating inward spread of activation toward the initiation site. Therefore, if ISs repeatedly start from one location, the outward connections from the initiation site would be potentiated while inward connections would be depressed. Thus STDP could establish a stable pattern of wave propagation if consecutive trains of ISs are initiated from the same location.
We found that an IS had a higher probability of being initiated from the same site of its predecessor; this tendency supports the idea that an outwardly propagating IS could “imprint” the local circuit with a spatial pattern of synaptic strengthening/depression via STDP or other timing-dependent mechanisms. During the initial state, immediately following the blockade of inhibition, all locations would have equal potential to become an IS initiation site, as observed in Fig. 2, where early IS initiation sites were widely distributed in the disinhibited area. These IS initiation sites may disrupt each other via the outward synaptic strengthening and compete to become dominant. The competition for domination may be similar to the formation of Turing patterns in reaction-diffusion models where multiple sites with center potentiation and outward suppression compete (Fiete et al. 2010; Kondo and Miura 2010). As more and more ISs occur, the effects of STDP would become stronger around a few initiation sites, with subsequent ISs being initiated from those sites. Similarly, the chance for an IS to be initiated outside the dominant sites would be gradually reduced. These would eventually lead to a “winner-takes-all” scenario, where all ISs are initiated from a few dominant sites. A surprising finding of the current report is that the dynamic site domination process takes place within only a few minutes.
Multiple dominant sites and nonpoint initiation patterns.
Continuous VSD imaging in vivo revealed multiple dominant sites taking turns to lead IS generation (Fig. 3). It also indicated that some of the ISs may be initiated with nonpoint patterns, where wave propagation appears to originate from multiple locations. This is different from the evidence obtained during in vitro experiments (Fig. 6; Tsau et al. 1998, 1999), where in most cases the ISs are initiated with a point pattern and fewer dominant sites. It is possible that the complicated long-range connections in vivo, including thalamocortical and interhemispheric connections, may create more dominant sites and the nonpoint patterns.
Indeed, our contralateral cortical stimulation experiment (Fig. 5) strongly suggests that long-range connectivity, such as callosal projections, may be associated with the nonpoint initiations patterns of ISs and multiple initiation sites. In our experiments, stimulus to the contralateral cortex evoked ISs with a distinct “point-stripe pattern” (Fig. 5), similar to the pattern of stimulation-evoked response prior to bicuculline application. This suggests that such initiation patterns originate in the contralateral cortex and are conducted via two groups of callosal fibers, which simultaneously activate the point and the stripe. Callosal fibers at the V1/V2 border may influence circuits in the contralateral hemisphere and help establish an IS pacemaker outside the disinhibited area. This possibility should be further explored, in particular to determine whether the V2/V2 border is associated with the stripe pattern.
Repetitive stimulation of the contralateral cortex prior to applying bicuculline was able to create similar initiation patterns in subsequent ISs, suggesting that incoming connections from the contralateral cortex can establish dominant IS initiation sites. Repetitive ISs consume a large amount of energy (Schwartz and Bonhoeffer 2001), and the metabolic burden in the disinhibited cortex may become a limiting factor for the rate of IS occurrence (Milton 2010). The higher-frequency activity coming from the contralateral cortex via callosal fibers may thus be able to facilitate the ISs in the disinhibited region and potentially even act as a pacemaker of IS activity. Together with the mechanisms related to STDP, it is reasonable to speculate that connectivity with the contralateral cortex may be potentiated after repetitive ISs, and eventually form dominant sites that pace ISs. Similarly, other structures outside the disinhibited areas may also act as pacemakers of ISs. On the other hand, we cannot completely rule out the possible effect of drug diffusion, especially for trials carried out at later time periods. The high rate of nonpoint patterns in later periods and their larger initiation sites could suggest a contribution from drug diffusion.
Glial cells are important contributors to both ISs and epileptogenesis (Devinsky et al. 2013). While glia may contribute to the formation of IS initiation sites, the glial signals are relatively slow (Chemla and Chavane 2009) and unlikely to play a role in the short time frames where STDP occurs. In our experiments, glial membranes are also stained and are likely to contribute to the VSD signals. However, identification of the IS initiation sites is mostly related to the initial rise in VSD signal occurring over ∼30 ms (Fig. 1); glial signals are too slow to directly contribute to the rising phase of the IS spike.
Role of ISs and clinical implications.
The role of ISs in epileptogenesis remains controversial (Staley et al. 2011). It may include suppressing seizure activity (Barbarosie and Avoli 1997), preventing seizures from spreading (de Curtis and Avanzini 2001), facilitating seizures over time (Bains et al. 1999; Staley and Dudek 2006), or directly triggering seizures (Rogawski 2006).
Clinically, brain regions with frequent ISs are often located within the ictal onset zone and are included in surgical resection (Gilliam et al. 1997; Radhakrishnan et al. 1998). However, they can also be discordant in terms of initiation and propagation, suggesting that IS and ictal activation might be two different processes (Usui et al. 2008). Frequent ISs may contribute to the long-term development of a seizure focus but through an accumulating effect rather than directly triggering a seizure. This possibility was suggested by recent research where ISs in a rat model become self-reinforced in frequency and amplitude and produced accumulated changes in gene expressions similar to those observed in human seizure foci (Barkmeier et al. 2012).
Such an “accumulating” or “facilitating” effect could arise through the process of IS initiation site domination. We speculate that if ISs were generated by a dominant site, then repetitive spatiotemporal patterns of activity would accumulate, facilitating a spatial pattern of timing-dependent plasticity in the cortex. Consequently, such patterns may promote long-term reorganization of some hyperexcitable foci into seizure onset foci, possibly involving activity-dependent signaling and gene expression (Barkmeier et al. 2012). In contrast, if ISs are randomly initiated, there should be no spatial patterns of long-term changes. In fact, random ISs may even wash out any tendency to form spatial plasticity patterns.
Our finding that the IS initiation sites can undergo spatial consolidation over the course of minutes may support the case for early anti-epileptogenic interventions (i.e., rapid application of treatment modalities such as benzodiazepines or electrical stimulation) following potentially epileptogenic brain insults, such as penetrating head trauma. Recently, it has been shown in a rat model of blunt head trauma with very high risk of epileptogenesis that early application of hypothermia can effectively block this process (D'Ambrosio et al. 2013). Transcranial or direct cortical electrical stimulation can reduce ISs in animal models and in human epilepsy (Berenyi et al. 2012; Labar et al. 2013; Morrell 2011), and cortical stimulation via an implanted device may change the frequency of ISs even when applied to a remote region (Labar et al. 2013). It is plausible that such stimulation may disrupt the formation of dominant IS initiation sites and by doing so avert the spatial STDP patterns fueled by dominant sites. Such early interventions may have the potential to diminish the epileptogenic process.
In conclusion, our findings suggest that, in the very early stages of IS generation, a spatiotemporal organization occurs such that a few dominant initiation sites generate the majority of ISs. Manipulating the spatial organization of dominant sites may become a new disease-modifying strategy in epilepsy.
GRANTS
This study was supported by National Institute of Neurological Disorders and Stroke (NINDS) Grant NS-059034 (J. Y. Wu); fellowships from the Epilepsy Foundation of America (to D. Vitantonio); Natural Science Foundation of China Grant 61302035 and Joint Research Fund for the Doctoral Program of Higher Education of China Grant 20111107120018 (to X. Geng); National Science Foundation Partnerships for International Research and Education OISE-0730255 (to B. S. Wolff); NINDS Training Grants T32-NS41218 and T32-NS041231 (to B. S. Wolf); and the Humboldt Foundation/Stiftung (to K. Takagaki).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.V., W.X., and J.-y.W. conception and design of research; D.V. and W.X. performed experiments; D.V., W.X., X.G., and B.S.W. analyzed data; D.V., W.X., G.K.M., and J.-y.W. interpreted results of experiments; D.V., W.X., and J.-y.W. prepared figures; D.V., W.X., K.T., G.K.M., and J.-y.W. drafted manuscript; D.V., W.X., B.S.W., G.K.M., and J.-y.W. edited and revised manuscript; D.V., W.X., X.G., B.S.W., K.T., G.K.M., and J.-y.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. L. B. Cohen and P. A. Forcelli for helpful discussion and comments on the manuscript.
Present address of D. Vitantonio: Neurology Residency Program, University of California, Los Angeles Medical Center, 710 Westwood Plaza, Los Angeles, CA 90095.
REFERENCES
- Avoli M, Biagini G, de Curtis M. Do interictal spikes sustain seizures and epileptogenesis? Epilepsy Curr 6: 203–207, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bains JS, Longacher JM, Staley KJ. Reciprocal interactions between CA3 network activity and strength of recurrent collateral synapses. Nat Neurosci 2: 720–726, 1999. [DOI] [PubMed] [Google Scholar]
- Barbarosie M, Avoli M. CA3-driven hippocampal-entorhinal loop controls rather than sustains in vitro limbic seizures. J Neurosci 17: 9308–9314, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkmeier DT, Senador D, Leclercq K, Pai D, Hua J, Boutros NN, Kaminski RM, Loeb JA. Electrical, molecular and behavioral effects of interictal spiking in the rat. Neurobiol Dis 47: 92–101, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenyi A, Belluscio M, Mao D, Buzsaki G. Closed-loop control of epilepsy by transcranial electrical stimulation. Science 337: 735–737, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci 18: 10464–10472, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock DD, Lee WC, Kerlin AM, Andermann ML, Hood G, Wetzel AW, Yurgenson S, Soucy ER, Kim HS, Reid RC. Network anatomy and in vivo physiology of visual cortical neurons. Nature 471: 177–182, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnac-Amitai Y, Connors BW. Horizontal spread of synchronized activity in neocortex and its control by GABA-mediated inhibition. J Neurophysiol 61: 747–758, 1989. [DOI] [PubMed] [Google Scholar]
- Chemla S, Chavane F. Voltage-sensitive dye imaging: technique review and models. J Physiol (Paris) 104: 40–50, 2009. [DOI] [PubMed] [Google Scholar]
- Connors BW. Initiation of synchronized neuronal bursting in neocortex. Nature 310: 685–687, 1984. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio R, Eastman CL, Fattore C, Perucca E. Novel frontiers in epilepsy treatments: preventing epileptogenesis by targeting inflammation. Expert Rev Neurother 13: 615–625, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Prog Neurobiol 63: 541–567, 2001. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Radici C, Forti M. Cellular mechanisms underlying spontaneous interictal spikes in an acute model of focal cortical epileptogenesis. Neuroscience 88: 107–117, 1999. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA. Glia and epilepsy: excitability and inflammation. Trends Neurosci 36: 174–184, 2013. [DOI] [PubMed] [Google Scholar]
- Fiete IR, Senn W, Wang CZ, Hahnloser RH. Spike-time-dependent plasticity and heterosynaptic competition organize networks to produce long scale-free sequences of neural activity. Neuron 65: 563–576, 2010. [DOI] [PubMed] [Google Scholar]
- Gilliam F, Wyllie E, Kashden J, Faught E, Kotagal P, Bebin M, Wise M, Comair Y, Morawetz R, Kuzniecky R. Epilepsy surgery outcome: comprehensive assessment in children. Neurology 48: 1368–1374, 1997. [DOI] [PubMed] [Google Scholar]
- Grinvald A, Manker A, Segal M. Visualization of the spread of electrical activity in rat hippocampal slices by voltage-sensitive optical probes. J Physiol 333: 269–291, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Xu W, Liang J, Takagaki K, Gao X, Wu JY. Spiral wave dynamics in neocortex. Neuron 68: 978–990, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JG. Basic mechanisms of focal epilepsies. Exp Physiol 75: 127–162, 1990. [DOI] [PubMed] [Google Scholar]
- Jin W, Zhang RJ, Wu JY. Voltage-sensitive dye imaging of population neuronal activity in cortical tissue. J Neurosci Methods 115: 13–27, 2002. [DOI] [PubMed] [Google Scholar]
- Kondo S, Miura T. Reaction-diffusion model as a framework for understanding biological pattern formation. Science 329: 1616–1620, 2010. [DOI] [PubMed] [Google Scholar]
- Labar D, Dakov P, Kobylarz E, Nikolov B, Schwartz TH, Fisher S. Effects of responsive electrical brain stimulation on intracranial electroencephalogram spikes. Neuromodulation 16: 355–361; discussion 362, 2013. [DOI] [PubMed] [Google Scholar]
- Lippert MT, Takagaki K, Xu W, Huang X, Wu JY. Methods for voltage-sensitive dye imaging of rat cortical activity with high signal-to-noise ratio. J Neurophysiol 98: 502–512, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London JA, Cohen LB, Wu JY. Optical recordings of the cortical response to whisker stimulation before and after the addition of an epileptogenic agent. J Neurosci 9: 2182–2190, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Zhao M, Schwartz TH. Dynamic neurovascular coupling and uncoupling during ictal onset, propagation, and termination revealed by simultaneous in vivo optical imaging of neural activity and local blood volume. Cereb Cortex 23: 885–899, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HT, Wu CH, Wu JY. Initiation of spontaneous epileptiform events in the rat neocortex in vivo. J Neurophysiol 91: 934–945, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275: 213–215, 1997. [DOI] [PubMed] [Google Scholar]
- Milton JG. Epilepsy as a dynamic disease: a tutorial of the past with an eye to the future. Epilepsy Behav 18: 33–44, 2010. [DOI] [PubMed] [Google Scholar]
- Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 77: 1295–1304, 2011. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Elsevier Academic, 2005. [Google Scholar]
- Radhakrishnan K, So EL, Silbert PL, Jack CR Jr, Cascino GD, Sharbrough FW, O'Brien PC. Predictors of outcome of anterior temporal lobectomy for intractable epilepsy: a multivariate study. Neurology 51: 465–471, 1998. [DOI] [PubMed] [Google Scholar]
- Rogawski MA. Point-counterpoint: do interictal spikes trigger seizures or protect against them? Epilepsy Curr 6: 197–198, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabolek HR, Swiercz WB, Lillis KP, Cash SS, Huberfeld G, Zhao G, Ste Marie L, Clemenceau S, Barsh G, Miles R, Staley KJ. A candidate mechanism underlying the variance of interictal spike propagation. J Neurosci 32: 3009–3021, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz TH, Bonhoeffer T. In vivo optical mapping of epileptic foci and surround inhibition in ferret cerebral cortex. Nat Med 7: 1063–1067, 2001. [DOI] [PubMed] [Google Scholar]
- Staley K, Hellier JL, Dudek FE. Do interictal spikes drive epileptogenesis? Neuroscientist 11: 272–276, 2005. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Dudek FE. Interictal spikes and epileptogenesis. Epilepsy Curr 6: 199–202, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley KJ, White A, Dudek FE. Interictal spikes: harbingers or causes of epilepsy? Neurosci Lett 497: 247–250, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Wong RK. Cellular mechanism of neuronal synchronization in epilepsy. Science 216: 745–747, 1982. [DOI] [PubMed] [Google Scholar]
- Tsau Y, Guan L, Wu JY. Epileptiform activity can be initiated in various neocortical layers: an optical imaging study. J Neurophysiol 82: 1965–1973, 1999. [DOI] [PubMed] [Google Scholar]
- Tsau Y, Guan L, Wu JY. Initiation of spontaneous epileptiform activity in the neocortical slice. J Neurophysiol 80: 978–982, 1998. [DOI] [PubMed] [Google Scholar]
- Usui N, Mihara T, Baba K, Matsuda K, Tottori T, Umeoka S, Nakamura F, Terada K, Usui K, Inoue Y. Early seizure propagation from the occipital lobe to medial temporal structures and its surgical implication. Epileptic Disord 10: 260–265, 2008. [DOI] [PubMed] [Google Scholar]
- Xu W, Huang X, Takagaki K, Wu JY. Compression and reflection of visually evoked cortical waves. Neuron 55: 119–129, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]