Abstract
Background
Chagas disease has a long clinically silent period following Trypanosoma cruzi infection and before development of overt clinical pathology; detectable biomarkers of infection and pathogenesis are urgently needed. We tested 22 biomarkers known to be associated with cardiomyopathy to evaluate if a biomarker signature could successfully classify T. cruzi seropositive subjects into clinical Chagas disease stage groups.
Methods
This cross-sectional retrospective case-control study enrolled T. cruzi seropositive blood donors (BD) that were further characterized as having chronic Chagas cardiomyopathy (CC-BD) or not (nonCC-BD) and seronegative (SN) control donors; we also included clinically diagnosed Chagas cardiomyopathy (CC-P). All subjects underwent a health history questionnaire, medical examination, electro- and echocardiograms (ECG and Echo) and phlebotomy. Biomarkers were measured on blinded samples by luminex bead array and Ortho VITROS.
Results
A clear biomarker pattern was observed only in more severe cardiac disease; this pattern included significantly elevated levels of inflammatory cytokines IFN-γ IL-6, IL-10 and TNF-α and soluble cardiovascular disease biomarkers CK-MB, troponin, myoglobin, VCAM and NTproBNP while there were lower levels of MPO, PAI-1, and MCP-1. The markers determined to be most predictive of disease by ROC curve analysis were NTproBNP and T. cruzi PCR status.
Conclusions
Although many biomarkers demonstrated increased or decreased concentrations among the clinical forms of Chagas disease, NTproBNP and T. cruzi PCR were the only tests that would independently be of clinical value for disease staging, in concert with ECG, Echo and clinical assessments.
Keywords: Trypanosoma cruzi (T. cruzi), cytokines, chemokines, inflammation, cardiomyopathy
INTRODUCTION
Chagas disease is caused by Trypanasoma cruzi, a parasite which is naturally transmitted through several species of haematophagous reduviid bugs (e.g., Triatoma infestans). It causes considerable morbidity and mortality in T. cruzi endemic regions of South and Central Americas and is becoming an increasing problem in non-endemic regions due to migration of infected individuals. Of particular concern is the susceptibility of acquisition of the parasite by nontraditional methods. Infections with T. cruzi can also occur via congenital transmission, organ transplantation, and blood transfusion [1]. The great majority of acute T. cruzi infections are unapparent and most symptomatic patients present with minor clinical manifestations. Most untreated acute cases evolve into the indeterminate stage of chronic Chagas disease (seropositive but no sign of the cardiac or digestive forms of the disease as evaluated by ECG and X ray) [1]. However some individuals will develop cardiomyopathy (CC) and/or the mega-syndromes approximately 10 to 20 years after infection in a slow but progressive fashion. Previous studies from our group have suggested an incidence rate of 1.85% for the cardiac form of the disease [2].
Chronic Chagas cardiomyopathy is the most important clinical presentation of Chagas disease. It comprises a wide range of manifestations including heart failure, arrhythmias, heart-blocks, sudden death, thromboembolism and stroke [3]. Clinical presentation typically varies widely according to the degree of myocardial damage. Some patients present a mild form of heart disease, frequently characterized only by the presence of asymptomatic abnormalities on the ECG or in other complimentary exams as was the case of the CC-BD in our study[4]. However some patients will develop heart failure and/or severe arrhythmias, with high mortality rates, typically in adult male patients[3], and also in the elderly [5].
Biomarkers are urgently needed in the case of T. cruzi infection since there is a long clinically silent period from acute infection to development of clinical signs and symptoms of chronic Chagas which occur in ~30% of infected individuals [6]. During the early phase, vector-derived and blood-form parasite trypomastigotes actively infect macrophages and tissue, forming intracytoplasmic cyst-like collections. Although early immunological responses play a role in parasite control, many infected individuals continue onto chronic infection with parasite- and inflammation-related pathology. As the disease progresses to chronic infection, intracellular parasite numbers diminish and the parasites almost disappear from the peripheral blood. It can also be difficult to detect parasites in diseased tissue from individuals who died from clinical Chagas disease manifestations. Low parasite numbers and the lack of a good marker of active infection or incipient disease hinder the investigation of the etiology of parasite-induced pathogenesis and prevent evaluation of new treatments for Chagas disease [7].
The pathogenesis of chronic Chagas cardiomyopathy (CC) is not completely understood. There is now good evidence to indicate that parasitemia is important for the development of the disease [8,9]. However, parasite PCR positivity alone is not a highly informative marker of disease progression; for example, in our previous study we found that approximately 20% of the CC patients were PCR negative whereas 50% of asymptomatic T. cruzi seropositive individuals were PCR positive. For the past five years, our group has searched for sensitive and specific markers of parasite replication and disease progression [8,10–14]. Although some markers have shown promising correlations with disease status, most studies were performed on relatively small sets of samples. Another problem is that many markers have a high degree of overlap between groups. These findings suggest that the use of one single clinical test or laboratory analyte may not be optimal as an effective biomarker, whereas a composite set of biomarkers and an algorithm approach that integrates clinical and biomarker data may be more appropriate to achieve maximal predictive value for both prognostic and therapeutic monitoring applications.
The hallmark of CC histopathology is the presence of a mononuclear infiltrate, composed of macrophages (50%), T cells (40%), B cells (10% or less) with virtually no NK cells. These activated lymphocytes initiate and maintain activity in cardiac tissue, resulting in the local production of inflammatory cytokines such as IFN-γ and TNF-α [15–17]. Our hypothesis is that individuals with more advanced disease would present with higher circulating levels of inflammatory cytokines and cardiac markers underlying or mediating cardiac pathogenesis.
We describe here the results obtained by testing 4 plasma markers of cardiac damage and 18 serum markers of inflammation in samples collected from a well-characterized cohort of T. cruzi seropositive blood donors that were further classified as having cardiomyopathy (CC-BD) or not (nonCC-BD), as well as matched seronegative (SN) control donors. This blood donor cohort is supplemented with patients presenting with more advanced Chagas disease recruited from a large Cardiology Hospital (CC-P). This study is based on analyzing repository samples collected from T. cruzi-infected individuals. It is one of the largest retrospective analyses to date where the primary aim of this study is to characterize biomarkers of inflammation and cardiac disease that correlate with parasitemia and clinical outcomes of cardiomyopathy. Our secondary objective is to identify a composite set of biomarkers associated with the early development of Chagas cardiomyopathy (CC) and severity of cardiac disease.
METHODS
Study design
This retrospective cohort study, developed as part of the National Heart, Lung and Blood Institute (NHLBI) Retrovirus Epidemiological Donor Study-II (REDS-II), enrolled 499 T. cruzi seropositive (SP) blood donors (cases) identified by blood bank screening in 1996–2002 (255 from the city of São Paulo and 244 from the city of Montes Claros in the State of Minas Gerais, Brazil) and 488 seronegative (SN) control donors frequency matched by site, donation date (year), age and gender. This blood donor cohort was supplemented by parallel enrollment and evaluation of 101 previously diagnosed cases of CC from the Heart Institute of University of São Paulo Medical School; inclusion criteria included a physician diagnosis of CC, confirmed T. cruzi seropositivity, no previous treatment with benznidazole (BZN), and no co-morbidities such as diabetes, hypertension or renal failure. Study subjects were recruited by letter and telephone call using the blood center and hospital databases. Recruited patients gave informed consent to participate in this study. From July 2008 to October 2010, recruited individuals (blood donors and CC patients) underwent standardized health questionnaires and medical evaluations including electrocardiogram (ECG), echocardiogram (ECHO), and phlebotomy with processing and cryopreservation of samples for subsequent batched blinded analyses of cardiac markers, T. cruzi PCR and other biomarkers in the United States (US; see below). All blood samples were collected in EDTA and serum tubes, processed for parasite detection (described below) or spun and aliquotted. All specimens were frozen in Brazil at −20°C until shipped to the US REDS-II Central Laboratory (BSRI) on dry ice and maintained at −70°C.
All data were centralized by the REDS-II Data Coordinating Center (Westat). A pre-defined set of abnormalities in the Echo or ECG measurements triggered an expert panel composed of three Brazilian cardiologists to review cardiac findings blinded to the subject’s serostatus. The expert panel was asked to reach a consensus regarding the following question “If this patient were seropositive for T. cruzi how would you classify them: definite CC, probably CC, possible CC, or no CC”. Further details of the cohort procedures, rates and clinical correlates of CC have been previously reported [2]. The local physician also received all test results and counseled the participants when necessary. For the purpose of this study we excluded all seropositive blood donors with history of treatment with BZN, because we had no data concerning their drug regimen or treatment duration and timing.
Ethics Statement
This study is approved by the UCSF CHR, Comissão de Ética para Análise de Projetos de Pesquisa (CAPPesq), Comitê de Ética em Pesquisa da Fundação Hemominas (CEP Hemominas) and National IRB - Brasília: A Comissão Nacional de Ética em Pesquisa (CONEP). Informed written consent was given by the patients for their information to be stored in the hospital database and used for research.
PCR procedures
At the time of interviews and medical examinations 20 mL of EDTA-anti-coagulated blood was collected from each enrolled subject and was immediately mixed with an equal volume of 6M guanidine HCl-0.2 M EDTA solution. The guanidine-EDTA blood mixture was maintained at room temperature until boiled for 15 min, followed by vortexing and aliquoting (1.0 mL). Aliquots were frozen in Brazil at −20°C until shipped to the US REDS-II Central Laboratory (BSRI) on dry ice, followed by maintenance at −70°C. The target-capture (TC) T. cruzi real-time (RT) PCR assay used in this study [18] was developed based on the PCR method described by Virreira M et al.[19] targeting kinetoplast minicircle T. cruzi DNA.
Clinical cardiac measurements
Resting 12-lead ECG’s were recorded using the same model of machine at both sites (General Electric MAC 1200 electrocardiograph; GE Healthcare, Waukesha, WI) using standardized procedures. All ECGs were processed blindly by the central ECG laboratory (Epidemiological Cardiology Research Center, Wake Forest University, Winston-Salem, NC). ECGs were analyzed electronically, with manual over-reading by trained cardiologists to ensure quality control. ECGs were classified by Minnesota code criteria using variables that were derived from the median complex of the Marquette measurement matrix [20,21].
Echo studies were performed using a Sequoia 512 ultrasound instrument (Acuson, Mountain View, CA, USA) at Sao Paulo site and GE Vivid3 (GE Healthcare, Waukesha, WI) at Montes Claros site. Cardiac measurements were performed according to the guidelines of the American Society of Echocardiography [22,23]. Studies were recorded in digital format and all measurements were performed on digital loops using a Digisonics offline analysis station (version 3.2 software, Digisonics, Houston, Tex) at the Cardiovascular Branch, Echocardiography Laboratory, National Heart, Lung, and Blood Institute, Bethesda, Maryland, US. Left ventricle (LV) ejection fraction was calculated based on modified form of Simpson’s biplane method [22].
Plasma and Serum Biomarkers
Biomarkers associated with T. cruzi infection, Chagas and cardiovascular disease were identified and selected for assessment in this study. Blinded serum samples were tested using cardiac marker Milliplex kits (Millipore) with antibody coated beads for detection of matrix metalloproteinase (MMP)-9, myeloperoxidase (MPO), plasminogen activation inhibitor (PAI)-1, sE-Selectin, soluble vascular cell adhesion molecule (VCAM), soluble intracellular adhesion molecule (ICAM)-1, adiponectin, (CVD Panel I; 1:100 dilution); C-reactive protein (CRP), serum amyloid A (SAA), serum amyloid P (SAP) (Millipore CVD Panel II; 1:2000 dilution) and monocyte chemotactic protein (MCP)-1, interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, IL-8, IL-10, Interferon (IFN)-γ and vascular endothelium growth factor (VEGF) (Millipore CVD Panel III; no dilution). The standard curve detection ranged from 2 – 25000 ng/mL for CVD I, 40 ng/mL – 500μg/mL for CVD II and 0.64 – 50,000pg/mL for CVD III. Testing was performed following the manufacturer’s protocols. Standard curves and samples were tested in duplicate. Results were acquired on a Labscan 200 analyzer (Luminex) using Bio-Plex manager software v6.1 (Bio-Rad). The biomarker that was predominantly above the threshold of detection was adiponectin (58% of samples) and below the threshold of detection were IFN-γ (57%) and IL-1β (82% of samples). When samples were above the threshold of detection, we assigned the value to twice the highest value in the set; when samples were below the threshold of detection, we assigned the result to one-half the lowest data point in the set. Blinded EDTA plasma specimens were used to measure CK-MB, troponin, myoglobin and NTproBNP (N-terminal of the prohormone brain natriuretic peptide) with a US FDA cleared assays on the VITROS System (Ortho Clinical Diagnostics, Raritan, NJ, US). Results for the groups measured are summarized in Table 2.
Table 2. Summary of cytokine results for biomarkers.
The median and interquartile range is reported for all biomarkers tested.
| Chagas Cardiomyopathy | Cardiomyopathy Chagas Ab+ | Non- Cardiomyopathy Chagas Ab+ | Seronegative | |
|---|---|---|---|---|
|
T. cruzi Parasitemia |
1.8 (0.16,5) | 0.31 (0,2.3) | 0.06 (0,1.8) | 0 (0,0) |
| Clinical Cardiac Measurements | ||||
| EF | 30 (20,40) | 58 (48,65) | 63 (60,65) | 62 (60,65) |
| ECG QTCCalculation |
457 (438,502) | 447 (423,462) | 424 (408,439) | 427 (411,442) |
| ECG QRSDuration |
140 (110,158) | 134 (112,144) | 88 (82,92) | 88 (82,95) |
| Cardiac Biomarkers | ||||
| Troponin | 0.012 (0.012,0.021) | 0.022 (0.013,0.04) | 0.012 (0.012,0.012) | 0.012 (0.012,0.012) |
| Myoglobin | 44 (35,55) | 40 (32,50) | 37 (30,46) | 37 (30,47) |
| NTproBNP | 747 (358,2224) | 76 (40,187) | 44 (26,72) | 39 (24,66) |
| CK-MB | 0.85(0.5407,1.269) | 1.06(0.6437,1.791) | 0.79(0.4853,1.297) | 0.68(0.417,1.17) |
| Adiponectin | 647800(647800,647800) | 647800(10050,647800) | 647800(8815,647800) | 647800(8536,647800) |
| MMP-9 | 174 (130,212) | 163 (118,225) | 158 (120,239) | 178 (127,243) |
| MPO | 35 (26,48) | 43 (26,79) | 40 (28,69) | 41 (28,96) |
| PAI-1 | 78 (54,106) | 83 (54,131) | 101 (65,132) | 106 (70,154) |
| Soluble Cell Adhesion Molecules | ||||
| sEselectin | 59 (46,71) | 64 (49,77) | 61 (43,77) | 58 (42,76) |
| ICAM | 339 (281,479) | 339 (270,406) | 338 (289,438) | 333 (270,400) |
| VCAM | 2229 (1922,2503) | 2185 (1806,2559) | 2042 (1680,2466) | 1963 (1657,2364) |
| Soluble Inflammatory Mediators | ||||
| CRP | 8387 (2971,14570) | 6055 (2314,13210) | 7362 (2411,14770) | 8095 (3055,15330) |
| SAA | 12390 (6428,27830) | 9487 (4648,18390) | 9301 (4785,19970) | 9407 (5114,18220) |
| SAP | 44670 (33060,58630) | 60660 (38990,87730) | 63780 (43540,88290) | 60770 (45370,88870) |
| IFNg | 0.21 (0.015,0.95) | 0.015 (0.015,0.67) | 0.015 (0.015,0.59) | 0.015 (0.015,0.55) |
| IL-10 | 4.3 (1.6,8) | 2.3 (0.12,4.7) | 1.5 (0.008,3.8) | 1.5 (0.008,4.3) |
| IL-1b | 0.024 (0.024,0.7) | 0.024 (0.024,0.069) | 0.024 (0.024,0.024) | 0.024 (0.024,0.024) |
| IL-6 | 1.6 (0.64,3.1) | 0.77 (0.091,1.7) | 0.57 (0.09,1.5) | 0.71 (0.09,1.7) |
| IL-8 | 2.2 (1.4,3.2) | 1.9 (1.2,3.4) | 1.8 (0.99,3.1) | 2 (1.2,3.2) |
| MCP-1 | 111 (91,143) | 118 (78,154) | 116 (82,158) | 131 (96,174) |
| TNF-a | 3.6 (2.5,5.5) | 3.4 (1.8,4.7) | 3 (1.7,4.8) | 3 (1.8,4.4) |
| VEGF | 41 (24,53) | 32 (10,53) | 32 (14,47) | 32 (7.5,49) |
Statistical Methods
All results from testing were compiled and analyzed for differences between clinical groups and for correlations between parasite load or specific clinical parameters and cytokine, chemokine, growth factor or cardiac biomarker. Analyses were performed by log-transforming biomarker measurements and compared using a non-parametric ANOVA (Wilcoxon Rank or Kruskal Wallis with Dunn’s multiple comparison tests). Results were adjusted for age and gender; significance was determined if the p value was <0.05. Biomarker measurements were compared to parasite load to determine if there were associations between the two. Parasite level and biomarkers were log-transformed and correlations were measured using a Pearson correlation. In the heatmap analysis, we plotted the medians of each biomarker across each subgroup. For each row, the medians were scaled to mean 0 and standard deviation 1. The dendrogram is generated based on hierarchical clustering with complete linkage and 1-Pearson correlation as distance. We performed receiver operating characteristic (ROC) analysis to identify a potential biomarker panel that was indicative of cardiomyopathy status. First we performed univariate logistic regression using individual biomarkers as predictors and binary cardiomyopathy status as dependent variable [24,25]. Then we used the univariately significant (P<0.05) biomarkers and constructed a multivariate logistic regression model. Next we constructed a final biomarker model by combining the biomarkers that were both univariately and multivariately correlated to cardiomyopathy. To measure how this multivariate model could be used to predict cardiomyopathy at the level of the individual patient, we compared predicted with reported cardiomyopathy and computed false positive and true positive rates. We plotted ROC (curves and computed AUCs (Area under curve) as prediction performance measures. Analyses were performed using Graphpad Prism and R.
RESULTS
Clinical cardiac pathology and inflammation in T. cruzi positive subjects
Of the original 1088 subjects that completed the clinical parameter measurements, 1077 had samples available for biomarker testing. Forty-nine enrolled patients were excluded from the analysis due to prior treatment for T. cruzi infection. The demographic and clinical characteristics of these subjects are described in Table 1. The gender and age distributions were similar between the blood donor groups and CC-P group. As expected, the CC-P had more severe cardiac disease: 42% had a left ventricular ejection fraction (LVEF) <30%, whereas only 7% of CC-BD group had LVEF <30%. Results for the groups measured are summarized in Table 2.
Table 1.
Summary of study participant demographics.
| N(%) | Patient | Seropositive Blood donors (BD) |
Seronegative (BD) | |
|---|---|---|---|---|
| CC-P | CC-BD | nonCC-BD | Controls | |
| 101 | 109 | 341 | 488 | |
| Gender | ||||
| Male | 60(59) | 67(61) | 169(50) | 241(49) |
| Female | 41(41) | 42(39) | 172(50) | 247(51) |
| Age | ||||
| 0–29 | 1(1) | 0 | 3(1) | 4(1) |
| 30–39 | 8(7) | 23(21) | 73(21) | 90(18) |
| 40–49 | 46(46) | 34(31) | 110(32) | 151(31) |
| 50–59 | 46(46) | 29(27) | 101(30) | 152(31) |
| 60+ | 0 | 23(21) | 54(16) | 91(19) |
Markers associated with inflammation were elevated in the CC-P group compared to nonCC-BD. These included IL-10 (p<0.005) and IL-6 (p<0.005). Also markers associated with cardiac dysfunction such as myoglobin (p<0.005), CK-MB (p=0.03), troponin (p<0.005), NTproBNP (p<0.005) and adiponectin (p<0.005) were elevated in the CC-P group compared to nonCC-BD (Figure 1 and 2). Biomarker concentrations that were decreased in CC-P compared to nonCC-BD included PAI-1 (p=0.001) and SAP (p<0.005) (Figure 2 and Table 3). While inflammatory markers were not significantly elevated among CC-BD compared to nonCC-BD, two cardiac markers, troponin and NTproBNP, were significantly higher (both p<0.005) in blood donors with cardiac pathology. In CC-BD and nonCC-BDs there was an inflammatory profile associated with T. cruzi infection, with elevations in IL-1β (p<0.005) compared to the seronegative donors (Figure 1).
Figure 1. Elevations in inflammatory cytokines in Chagas disease.
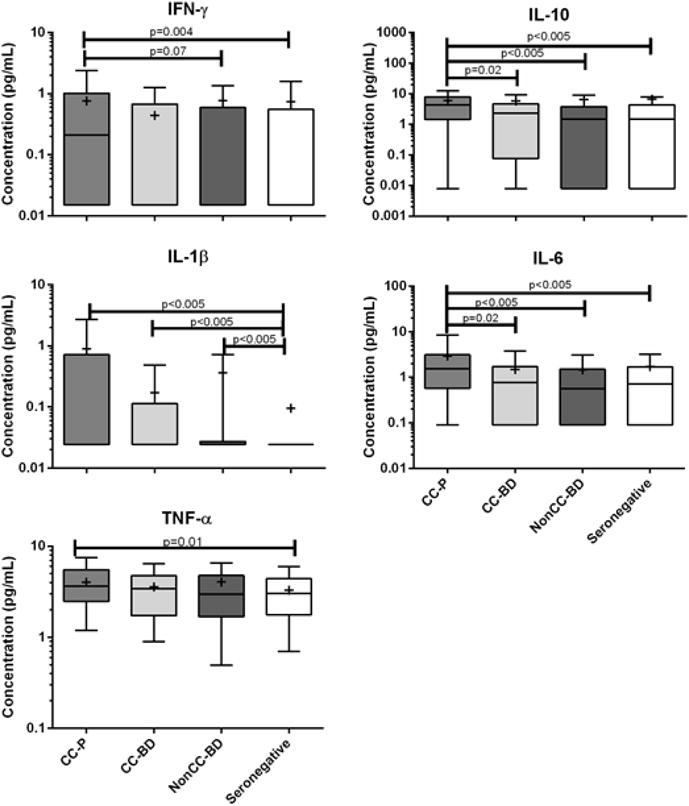
There are higher inflammatory cytokines in chronic Chagas cardiomyopathy compared to the other groups of T. cruzi infected and seronegative individuals. IL-6 and IL-10 were significantly elevated compared to all groups (p<0.05). IFN-γ were significantly elevated compared to the seronegative donor group (p=0.004). TNF-α was elevated compared to T. cruzi seronegative donors (p=0.01). IL-1β concentrations were significantly elevated in T. cruzi infected groups compared to seronegative control donors (p<0.005).
Figure 2. Soluble cardiac biomarkers are differentially expressed in chronic Chagas cardiomyopathy disease stages.
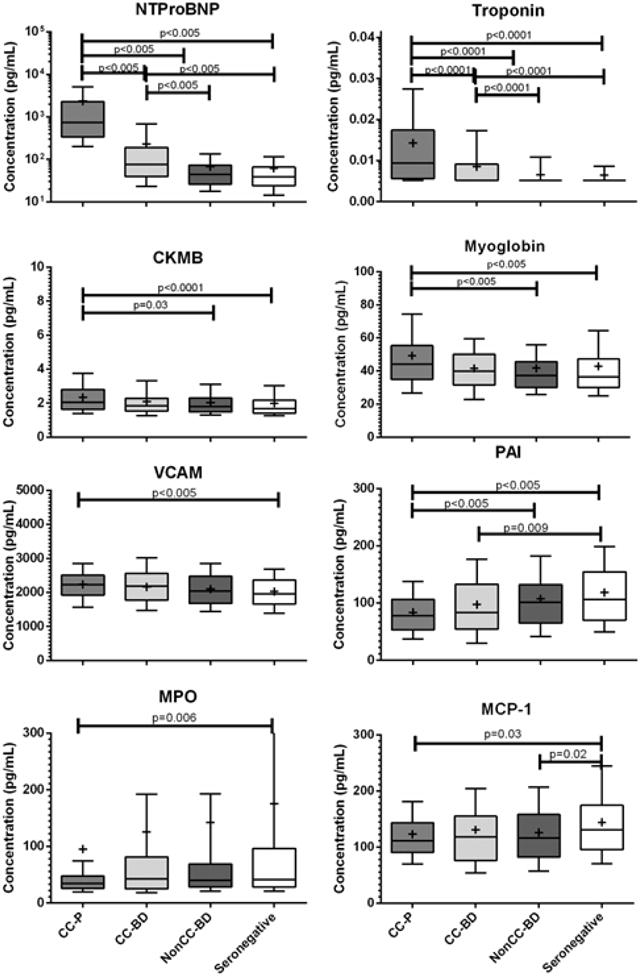
NTproBNP and Troponin were significantly elevated in CC-P and CC-BD compared to seronegative and/or Non-CC BD groups (p<0.005). CKMB was elevated in CC compared to other T. cruzi Ab+ and seronegative donor groups (p<0.05). Myoglobin and VCAM were elevated in CC-P compared to non-CC-BD and seronegative groups (p<0.005). There were reductions in other circulating biomarkers with disease severity; these markers included plasminogen activated inhibitor (PAI-1), myeloperoxidase (MPO) and monocyte chemotactic protein (MCP-1) (p<0.05).
Table 3. Differential expression of biomarkers with disease stage.
Overall Kruskal-Wallis test compares the medians of four groups to determine if the samples have come from different populations. This table summarizes the test median differences between selected group pairs; p-values are adjusted for age and gender.
| CC-P vs nonCC-BD | CC-BD vs nonCC-BD | nonCC-BD vs Seronegative | |
|---|---|---|---|
| Parasitemia | < 0.005 | 0.05 | < 0.005 |
| Troponin | < 0.005 | < 0.005 | . |
| NTproBNP | < 0.005 | < 0.005 | . |
| EF | < 0.005 | < 0.005 | . |
| ECG QTCalculation | < 0.005 | < 0.005 | . |
| ECG QRSDuration | < 0.005 | < 0.005 | . |
| Myoglobin | < 0.005 | . | . |
| Adiponectin | < 0.005 | . | . |
| SAP | < 0.005 | . | . |
| IL-10 | < 0.005 | . | . |
| IL-6 | < 0.005 | . | . |
| IL-1β | 0.1 | . | < 0.005 |
| PAI-1 | 0.001 | . | . |
| CK-MB | 0.03 | . | . |
| MCP-1 | . | . | 0.02 |
| SAA | 0.07 | . | . |
| TNF-α | 0.07 | . | . |
| IFNγ | 0.07 | . | . |
| VEGF | 0.1 | . | . |
| MPO | 0.1 | . | . |
| VCAM | 0.1 | . | . |
| IL-8 | . | . | . |
| ICAM | . | . | . |
| MMP-9 | . | . | . |
| CRP | . | . | . |
| sE-selectin | . | . | . |
Biomarker pattern across the spectrum of Chagas disease
A heat map was developed to visualize all data together and determine if we could detect a marker pattern that would differentiate CC vs nonCC groups (Figure 3). The heatmap demonstrated that the Chagas cardiopmyopathy group show a pattern that is distinct from the other groups. It also demonstrated the CC-BD pattern was similar to nonCC-BD and controls. However, there were higher levels in cardiac markers (ECG QTc and QRS), TNF-α and cell adhesion molecules (sE-selectin, ICAM, VCAM) in the CC-BD compared to nonCC-BD although differences were not statistically significant.
Figure 3. Heatmap of group medians of all biomarkers and cardiac parameters.
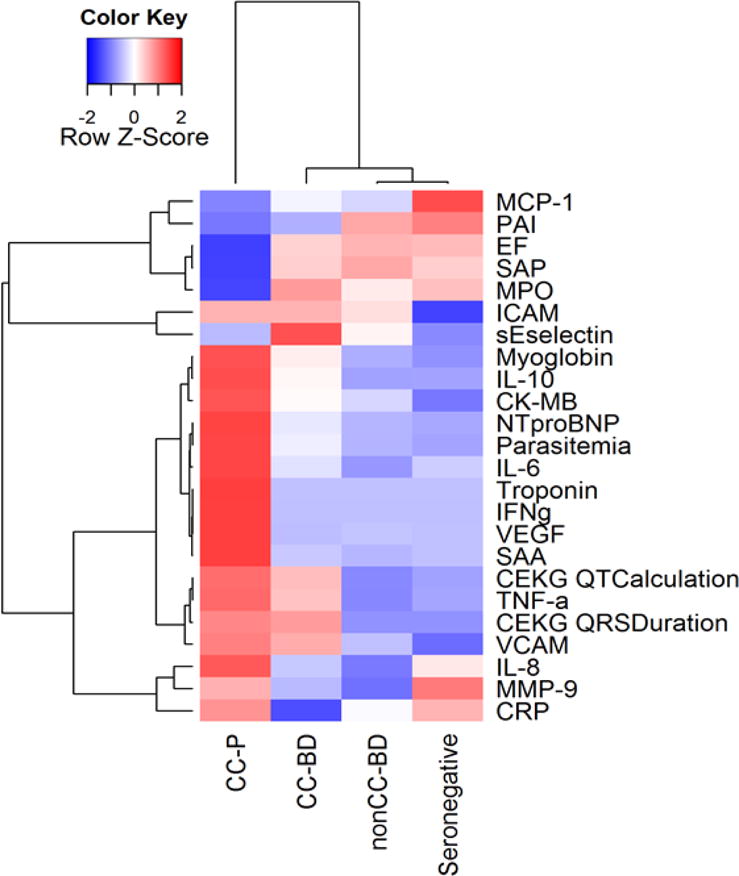
Each cell represents the median of the biomarker for the corresponding group. For each row, the medians are then scaled to mean 0 and standard deviation 1. The dendrogram is generated based on hierarchical clustering with complete linkage and 1-Pearson correlation as distance.
Parasitemia correlates with biomarkers
We measured biomarker correlations with parasitemia and found that there were a number of positive correlations with log parasite load including ICAM (p=0.02), IFN-γ (p=0.005), NTproBNP (p=0.01) and troponin (p=0.005); MPO inversely correlated with parasitemia (p=0.01). We next analyzed the biomarkers in relation to cardiac measurements known to be associated with Chagas disease. We found that there were negative correlations between EF and log-transformed biomarkers including adiponectin (p=0.006), IL-10 (p<0.005), IL-6 (p=0.009), NTproBNP and troponin (both p<0.005). There were also biomarkers that had positive correlations with EF including PAI-1 (p<0.005) and SAP (p=0.02). There were positive correlations between ECG QTc and biomarkers including adiponectin (p=0.02), CRP (p=0.01), myoglobin (p=0.005), NTproBNP and troponin (both p<0.005). We also found positive correlations between ECG QRS duration and IL-10 (p=0.003), myoglobin (p=0.003), NTproBNP and Troponin (both p<0.005) with negative correlations between ECG QRS duration and biomarkers PAI-1 (p<0.005) and SAP (p=0.005).
Identification of predictive biomarkers for cardiomyopathy status
Parasitemia, adiponectin, MPO, VCAM, IFNγ, IL-10, IL-1β, IL-6, MCP-1, NTproBNP, CK-MB, troponin, and myoglobin were univariately correlated to cardiomyopathy status. By including all above biomarkers as independent variables in a multivariate logistic regression model, we found NTproBNP (P<10−5), myoglobin, troponin, CK-MB, and quantitative parasitemia (P<0.05) remained correlated with cardiomyopathy. We then constructed a final regression model by combining these 5 biomarkers. The 5-marker combined model showed the highest AUC of 0.86 (Fig 4). However, NTproBNP, as the strongest predictor, achieved an AUC of 0.83. The second best independent predictor was parasitemia, with an AUC of 0.78. The combination AUC was significantly higher than the strongest independent predictor (p=0.02). When we included only the seropositive individuals in the ROC analysis, we found similar results. Independently, troponin, CK-MB, and myoglobin showed lower predictability. The predictive modeling performed was exploratory in nature; a model validation from independent data sets would be required to obtain true prediction performance.
Figure 4. Receiver operator curve identifies potentially predictive biomarkers of Chagas cardiac pathology.
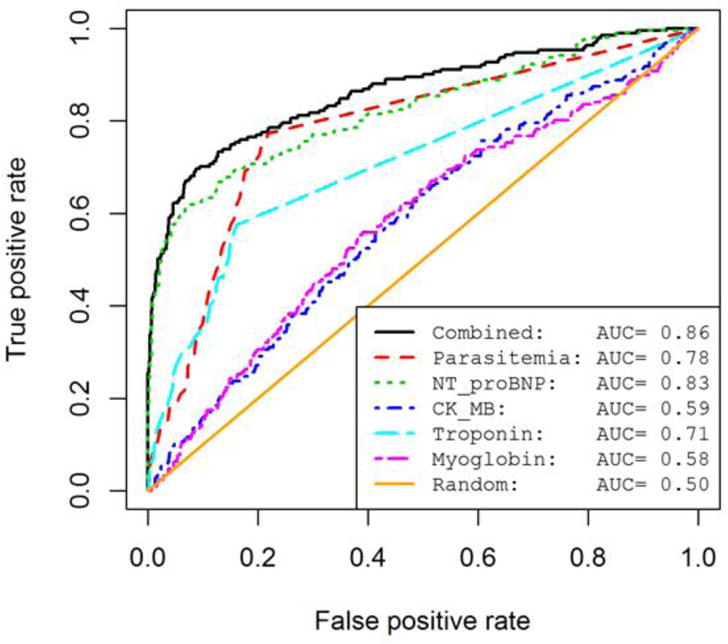
The ROC curves show the prediction performance across all false positive rates (False Positives/False Positives + True Negatives) and true positive rates (True Positives/True Positives + False Negatives). AUCs (Area under curve) were calculated as prediction performance measures for each predictive model. We show 5 univariate biomarker models and the combined model which includes all 5 predictors. A random predictive model is shown for comparison.
DISCUSSION
This study is one of the largest and most extensive analyses of biomarkers in Chagas cardiomyopathy. We tested 22 biomarkers in over 1000 individuals with 449 who were T. cruzi seropositive blood donors and 101 individuals who had clinical Chagas cardiomyopathy. The markers selected were known to be associated with cardiomyopathy or inflammation and were chosen to evaluate if multi-marker signatures were able to successfully classify T. cruzi seropositive subjects into clinical Chagas disease stage groups. These markers, encompassing indications of both systemic and cardiac inflammation, were selected to identify parasite-driven immune activation and Chagas cardiomyopathy prior to the onset of clinical symptoms.
Indeed a clear pattern was found in CC-P group, with high levels of inflammatory markers including cytokines IL-10 and IL-6 and markers of cardiac dysfunction including myoglobin, CK-MB, troponin, NTproBNP and adiponectin, as shown previously in other studies[26–29]. Disappointingly, we did not see much difference between the CC and non-CC blood donors; the CC-BD did not present with higher levels of inflammatory markers. There could be two explanations for this: one reason is that the CC-BD may represent a group with a mild form of CC with low grade inflammation that is not sufficient to develop heart fibrosis and consequently important LV dysfunction. In favor of this hypothesis is the fact that CC-BD was slightly (but not significantly) younger than the CC-P, and so far had not yet developed the more severe form of the disease as manifested by the moderate magnitude of ECG and ECHO abnormalities. In this case a pattern of near normal inflammatory markers may predict favorable outcomes of disease. Alternatively the levels of inflammatory markers may increase in the bloodstream only in the later stage of disease and the assays are not sufficiently sensitive to identify individuals who are at risk of progressing to the more severe form of the CC. In favor of this hypothesis is the fact that early markers of cardiac damage, such as troponin and NTproBNP, were significantly increased among the CC-BD, suggesting that cardiac damage is occurring in those patients and they are earlier markers of disease progression than the inflammatory biomarkers that were the focus of the present study.
In the ROC curve analysis we identified the best parameters that would indicate CC (independent of being severe or not severe). In this analysis CC-BD and CC-P were combined and the best markers were parasitemia (T. cruzi quantitative PCR) and NTproBNP. We also found that IL-1β significantly elevated in all T. cruzi-seropositive groups (donors with and without CC, and patients with overt Chagas cardiomyopathy) compared to seronegative donors, suggesting an effect of chronic infection on immune activation; however, the values of these parameters overlapped, precluding their use as independent prognostic tools.
Our results show that NTproBNP was the strongest predictor of the presence of Chagas cardiomyopathy patients, confirming previous studies that described that natriuretic peptides are useful markers of left ventricular dysfunction in Chagas disease [30–33]. BNP and NTproBNP levels in Chagas disease are related to NYHA functional class [34], to the presence of ventricular arrhythmias [34], and to left ventricular diastolic dysfunction [35]; these markers are recognized as powerful prognostic markers in prospective studies of patients with diagnosed CC [36]. However, the role of natriuretic peptides in detecting early cardiac involvement is controversial [30]. Garcia-Alvarez et al. showed similar levels of BNP among Chagas disease patients in the indeterminate form and in non-infected subjects, but described that BNP levels are significantly higher in those patients in the indeterminate form with diastolic dysfunction[37]. In the large number of individuals evaluated in this study, NTproBNP concentrations did not differentiate nonCC-BD when compared to non-infected BD, further suggesting that natriuretic peptides are not useful biomarkers of early cardiac damage in Chagas disease.
Inflammatory markers (IFNγ, IL-10, IL-1β, IL-6, and MCP-1) were distinctly elevated in patients with Chagas cardiomyopathy compared to the other clinical groups; however, these markers were not effective in identifying clinical Chagas disease in the ROC analysis. Similarly, these cytokines have also been shown to be elevated in different stages of T. cruzi infection and Chagas disease progression in other studies [27]. Contrary to other studies [38,39], in this study the MPO concentration was lower in CC-P, CC-BD and NonCC-BD and significantly lower than seronegative individuals. While other studies show a significant increase in MPO activity related to cardiovascular disease [38,39], this study found decreases in MPO concentration; these two measurements may not be same. Another study of heart failure found MPO and NTproBNP concentrations to correlate [40]; similarly, when focusing on the CC groups, we found that there was a significant correlation between the two markers (p=0.02). Although we had selected to measure MPO concentration in this study, this may not be the most comprehensive marker; future studies of MPO would include investigating both concentration and activity.
One of the cytokines, IL-10, has been shown to have anti-inflammatory effects in previous Chagas studies [41]. In one ten-patient study, IL-10 production from PBMCs from Chagasic patients was found to positively correlate with EF [41]. In other studies, IL-10 has been attributed to eliciting an increased susceptibility to infection, regulation of chemokine production (eg. MCP-1) recruiting monocytes/macrophages, endothelial activation with IL-6 production, and increased nitric oxide production [29,42,43]. This study found that IL-10 levels were higher in CC and there was an inverse correlation with EF. The difference in our findings could be due to the larger sample size of our study or that plasma or serum rather than in vitro PBMC stimulation cultures was used for the measurement. It is also possible that cellular production of IL-10 is increased in Chagas patients with low EF as an attempt to trigger the Th2 immune response to control the effects of pro-inflammatory cytokines [44].
Markers of cardiac necrosis were also independently related to cardiomyopathy status in our study. Troponin represents a highly sensitive marker for heart muscle damage that can be detected in patients with heart failure. Its presence may help to identify a high-risk sub-group with a very poor short-term prognosis [45]. A few previous studies have shown that patients with Chagas cardiomyopathy have higher levels of troponin when compared to controls and patients in the indeterminate form [26,46]. Cardiac troponin levels correlate with the severity of the cardiomyopathy and may be useful for diagnosis and monitoring of heart injury in chronic Chagas disease [46]. Few experimental and clinical studies have evaluated increased CK-MB levels in Chagas disease [46,47] and we could not find data on myoglobin measurement in this disease; the mechanism related to the increased levels of these markers observed in cardiomyopathy patients in our study may be related to heart injury typical of advanced cardiomyopathies.
Since adipose tissue and adipocytes are primary sites of T. cruzi replication during acute infection and act as reservoirs during chronic disease [11], adipokines may be an informative marker in T. cruzi infection and Chagas disease progression. Studies show that there is a high parasite number within the first months of infection that declines over time [48], but adipose tissue may have a role as a chronic reservoir from which infection can recrudesce during periods of immunosuppression and/or lipoatrophic states [49]. Pro-inflammatory cytokines (TNF-α, IL-1β, and IFN-γ), chemokines (MCP-1) and fibrinogen (PAI-1) are expressed by adipocytes during acute infection. Adiponectin isoforms, also secreted by adipocytes, are known to play many roles in metabolic homeostasis and have some evidence of beneficial effects of reducing cardiopathology [50]. Its relation to cardiovascular disease progression in the context of chronic Chagas cardiomyopathy is not well known, but since adiponectin null mice have a cardiomyopathic phenotype, it is possible that the reduction in adiponectin contributes to the pathogenesis of Chagasic cardiomyopathy[51]. In this study we found that all infected groups had adiponectin concentrations in the upper range of the assay. Adiponectin levels in the CC group were above the threshold of detection suggesting elevated levels in chronic disease. This increased adiponectin expression may have been driven by inflammation caused by parasite sequestered in the adipose tissue reservoir. More work is needed to elucidate the role of adipokines and adipose tissue in harboring parasites to determine if this is a useful measurement of Chagas disease progression.
One interesting finding was the inverse correlation with PAI-1 and parasitemia. Although PAI-1 is known to be overexpressed in inflammatory diseases such as sepsis [52], in this study there was lower PAI-1 detected in CC. PAI-1, also known as Serpin E1, is a serine protease inhibitor and its main activity is fibrinolysis after clot formation. It is known that the T. cruzi parasite relies on serine protease for cell invasion, supporting endocytosis and degrading the cellular matrix of the host cell [53,54]. This has been an active target for drug intervention in treating Chagas disease [55]. In this study we find that there is higher expression of PAI-1 with lower parasite numbers. It is possible that the serine protease inhibitor may be beneficial to T. cruzi infected individuals by acting on the parasite and preventing cell invasion and parasite growth.
With any clinical study, the results need to be balanced with the limitations of the study design and analyses. As clinical Chagas disease course evolves over decades, it was necessary to design a retrospective cross-sectional study, using stored serum and plasma specimens, to capture disease outcomes in initially asymptomatic seropositive blood donors. Since we were not yet able to follow individuals prospectively for disease progression, there may be bias introduced by the retrospective study design. We attempted to overcome this with a large sample size; this study was considerably larger than many Chagas studies in the literature. We found that some of the analytes measured were above or below the threshold of detection; although this is a limitation when doing statistical analyses, we can use the significance of group analyses to direct further investigation into specific analytes using more sensitive or focused methods. Additional studies measuring the same markers in follow-up time points will be important in determining whether biomarkers predict disease progression. Ultimately, we are interested in using quantitative parasite measurements, immune status and clinical cardiac information to develop a composite prognostic score for accurate prognosis of CC disease onset and progression as indicators for therapeutic interventions and monitoring treatment outcomes.
In this study we focused on markers of inflammation and cardiac damage. We found that there are elevations in inflammatory markers during Chagas cardiomyopathy, in addition to elevations in NTproBNP and parasitemia. Although these were not effective at determining disease status, they do give some insight into the persistent inflammation that may play a role in pathogenesis during chronic disease. NTproBNP and parasite concentrations were the two most effective predictors of disease. Altogether, the markers identified here, in addition to other proteomic markers and lipoproteins, will be important in future studies to measure improved diagnosis, to monitor patient prognosis, to identify higher risk individuals who need more intensive and earlier treatment, and to predict and monitor efficacy after treatment. This work will be reliant on the development of a repository of well-characterized specimens, like the ones described here, and will be vital in the identification and validation of markers for understanding Chagas disease progression and determination of post-treatment T. cruzi eradication.
Acknowledgments
The REDS II Chagas study group from the NHLBI Retrovirus Epidemiology Donor Study-II (REDS-II), International Component consists of the following investigators.
Brazil:
Fundação Pró-Sangue/Hemocentro São Paulo (São Paulo):C Almeida-Neto, GM Patavino, NA Salles, M Otani.
Hemominas (Belo Horizonte, Minas Gerais): ABF Carneiro-Proietti,
HeartInstitute/Universityof São Paulo: V Salemi, B Ianni, LNastari, F Fernandes, J C A Ferreira-Filho, C Mady
Centro de Ciências Biológicas e da Saúde, Prontosocor de Montes: Claros, Minas Gerais:Antunes AP, Menezes M
Federal Universityof Sao Joao del Rey, Divinópolis (Minas Gerais): CL Oliveira
Hospital das Clínicas/Faculdade de Medicina, Universidade Federal de Minas Gerais: AL Ribeiro
Department of Infectious Disease/Institute of Tropical Medicine/University of São Paulo: EC Sabino, L Capuani.
US:
Blood Systems Research Institute andUniversity of California San Francisco: M.P. Busch, E.L. Murphy, B. Custer, M Seielstad, X Deng, TH Lee, SM Keating and T. Gonçalez
Coordinating center: Westat, Inc.: J. Schulman, M. King, and K. Kavounis
National Heart, Lung, and Blood Institute, NIH: V Sachdeva, S.A. Glynn, S. Kleinman
Funding: NHLBI HHSN268200417175C; NIAID P50AI098461
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
These authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
See Footnote Authorship for list of individuals responsible for the NHLBI REDS-II Brazil Study.
References
- 1.Ribeiro AL, Nunes MP, Teixeira MM, Rocha MO. Diagnosis and management of Chagas disease and cardiomyopathy. Nat Rev Cardiol. 2012;9:576–589. doi: 10.1038/nrcardio.2012.109. [DOI] [PubMed] [Google Scholar]
- 2.Sabino EC, Ribeiro AL, Salemi VM, Di Lorenzo Oliveira C, Antunes AP, et al. Ten-year incidence of Chagas cardiomyopathy among asymptomatic Trypanosoma cruzi-seropositive former blood donors. Circulation. 2013;127:1105–1115. doi: 10.1161/CIRCULATIONAHA.112.123612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rocha MO, Ribeiro AL, Teixeira MM. Clinical management of chronic Chagas cardiomyopathy. Front Biosci. 2003;8:e44–54. doi: 10.2741/926. [DOI] [PubMed] [Google Scholar]
- 4.Rocha MO, Teixeira MM, Ribeiro AL. An update on the management of Chagas cardiomyopathy. Expert Rev Anti Infect Ther. 2007;5:727–743. doi: 10.1586/14787210.5.4.727. [DOI] [PubMed] [Google Scholar]
- 5.Lima-Costa MF, Matos DL, Ribeiro AL. Chagas disease predicts 10-year stroke mortality in community-dwelling elderly: the Bambui cohort study of aging. Stroke. 2010;41:2477–2482. doi: 10.1161/STROKEAHA.110.588061. [DOI] [PubMed] [Google Scholar]
- 6.Requena-Mendez A, Lopez MC, Angheben A, Izquierdo L, Ribeiro I, et al. Evaluating Chagas disease progression and cure through blood-derived biomarkers: a systematic review. Expert Rev Anti Infect Ther. 2013;11:957–976. doi: 10.1586/14787210.2013.824718. [DOI] [PubMed] [Google Scholar]
- 7.Neva FA, Brown HW. Basic Clinical Parasitology. East Norwalk, Connecticut: Appleton & Lange; 1994. pp. 65–71. [Google Scholar]
- 8.Cunha-Neto E, Nogueira LG, Teixeira PC, Ramasawmy R, Drigo SA, et al. Immunological and non-immunological effects of cytokines and chemokines in the pathogenesis of chronic Chagas disease cardiomyopathy. Mem Inst Oswaldo Cruz. 2009;104(Suppl 1):252–258. doi: 10.1590/s0074-02762009000900032. [DOI] [PubMed] [Google Scholar]
- 9.Sabino EC, Ribeiro AL, Lee TH, Oliveira CL, Carneiro-Proietti AB, et al. Detection of Trypanosoma cruzi DNA in blood by PCR is associated with Chagas cardiomyopathy and disease severity. Eur J Heart Fail. 2015 doi: 10.1002/ejhf.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lula JF, Rocha MO, Nunes Mdo C, Ribeiro AL, Teixeira MM, et al. Plasma concentrations of tumour necrosis factor-alpha, tumour necrosis factor-related apoptosis-inducing ligand, and FasLigand/CD95L in patients with Chagas cardiomyopathy correlate with left ventricular dysfunction. Eur J Heart Fail. 2009;11:825–831. doi: 10.1093/eurjhf/hfp105. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira RC, Ianni BM, Abel LC, Buck P, Mady C, et al. Increased plasma levels of tumor necrosis factor-alpha in asymptomatic/“indeterminate” and Chagas disease cardiomyopathy patients. Mem Inst Oswaldo Cruz. 2003;98:407–411. doi: 10.1590/s0074-02762003000300021. [DOI] [PubMed] [Google Scholar]
- 12.Fonseca SG, Moins-Teisserenc H, Clave E, Ianni B, Nunes VL, et al. Identification of multiple HLA-A*0201-restricted cruzipain and FL-160 CD8+ epitopes recognized by T cells from chronically Trypanosoma cruzi-infected patients. Microbes Infect. 2005;7:688–697. doi: 10.1016/j.micinf.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Marin-Neto JA, Cunha-Neto E, Maciel BC, Simoes MV. Pathogenesis of chronic Chagas heart disease. Circulation. 2007;115:1109–1123. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- 14.Talvani A, Rocha MO, Barcelos LS, Gomes YM, Ribeiro AL, et al. Elevated concentrations of CCL2 and tumor necrosis factor-alpha in chagasic cardiomyopathy. Clin Infect Dis. 2004;38:943–950. doi: 10.1086/381892. [DOI] [PubMed] [Google Scholar]
- 15.Reis DD, Jones EM, Tostes S, Jr, Lopes ER, Gazzinelli G, et al. Characterization of inflammatory infiltrates in chronic chagasic myocardial lesions: presence of tumor necrosis factor-alpha+ cells and dominance of granzyme A+, CD8+ lymphocytes. Am J Trop Med Hyg. 1993;48:637–644. doi: 10.4269/ajtmh.1993.48.637. [DOI] [PubMed] [Google Scholar]
- 16.Reis MM, Higuchi Mde L, Benvenuti LA, Aiello VD, Gutierrez PS, et al. An in situ quantitative immunohistochemical study of cytokines and IL-2R+ in chronic human chagasic myocarditis: correlation with the presence of myocardial Trypanosoma cruzi antigens. Clin Immunol Immunopathol. 1997;83:165–172. doi: 10.1006/clin.1997.4335. [DOI] [PubMed] [Google Scholar]
- 17.Abel LC, Rizzo LV, Ianni B, Albuquerque F, Bacal F, et al. Chronic Chagas’ disease cardiomyopathy patients display an increased IFN-gamma response to Trypanosoma cruzi infection. J Autoimmun. 2001;17:99–107. doi: 10.1006/jaut.2001.0523. [DOI] [PubMed] [Google Scholar]
- 18.Sabino EC, Lee TH, Montalvo L, Nguyen ML, Leiby DA, et al. Antibody levels correlate with detection of Trypanosoma cruzi DNA by sensitive polymerase chain reaction assays in seropositive blood donors and possible resolution of infection over time. Transfusion. 2013;53:1257–1265. doi: 10.1111/j.1537-2995.2012.03902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Virreira M, Torrico F, Truyens C, Alonso-Vega C, Solano M, et al. Comparison of polymerase chain reaction methods for reliable and easy detection of congenital Trypanosoma cruzi infection. Am J Trop Med Hyg. 2003;68:574–582. doi: 10.4269/ajtmh.2003.68.574. [DOI] [PubMed] [Google Scholar]
- 20.Prineas RJ, Crow RS, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings. Littleton, MA: John Wright-PSG; 1982. [Google Scholar]
- 21.Ribeiro AL, Sabino EC, Marcolino MS, Salemi VM, Ianni BM, et al. Electrocardiographic abnormalities in Trypanosoma cruzi seropositive and seronegative former blood donors. PLoS Negl Trop Dis. 2013;7:e2078. doi: 10.1371/journal.pntd.0002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 23.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 25.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 26.Arias R, Bastos C, Mota G, Sodre F, Moreira A, et al. Troponin in chagas disease. Braz J Infect Dis. 2003;7:358–359. doi: 10.1590/s1413-86702003000600001. [DOI] [PubMed] [Google Scholar]
- 27.Sousa GR, Gomes JA, Fares RC, Damasio MP, Chaves AT, et al. Plasma cytokine expression is associated with cardiac morbidity in chagas disease. PLoS One. 2014;9:e87082. doi: 10.1371/journal.pone.0087082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbosa M, Nunes M, Ribeiro A, Barral M, Rocha M. N-terminal proBNP levels in patients with Chagas disease: a marker of systolic and diastolic dysfunction of the left ventricle. Eur J Echocardiogr. 2007;8:204–212. doi: 10.1016/j.euje.2006.03.011. Epub 2006 May 2002. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto E, Sherbuk J, Clark E, Marks M, Gandarilla O, et al. Biomarkers in Trypanosoma cruzi-Infected and Uninfected Individuals with Varying Severity of Cardiomyopathy in Santa Cruz, Bolivia. PLoS Negl Trop Dis. 2014;8:e3227. doi: 10.1371/journal.pntd.0003227. doi: 3210.1371/journal.pntd.0003227. eCollection 0002014 Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribeiro AL, dos Reis AM, Barros MV, de Sousa MR, Rocha AL, et al. Brain natriuretic peptide and left ventricular dysfunction in Chagas’ disease. Lancet. 2002;360:461–462. doi: 10.1016/S0140-6736(02)09638-1. [DOI] [PubMed] [Google Scholar]
- 31.Puyo AM, Scaglione J, Auger S, Cavallero S, Postan M, et al. Natriuretic peptides as prognostic and diagnostic markers in Chagas’ disease. Regul Pept. 2005;128:203–210. doi: 10.1016/j.regpep.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro AL, Teixeira MM, Reis AM, Talvani A, Perez AA, et al. Brain natriuretic peptide based strategy to detect left ventricular dysfunction in Chagas disease: a comparison with the conventional approach. Int J Cardiol. 2006;109:34–40. doi: 10.1016/j.ijcard.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 33.Mocelin AO, Issa VS, Bacal F, Guimaraes GV, Cunha E, et al. The influence of aetiology on inflammatory and neurohumoral activation in patients with severe heart failure: a prospective study comparing Chagas’ heart disease and idiopathic dilated cardiomyopathy. Eur J Heart Fail. 2005;7:869–873. doi: 10.1016/j.ejheart.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Talvani A, Rocha MO, Cogan J, Maewal P, de Lemos J, et al. Brain natriuretic peptide and left ventricular dysfunction in chagasic cardiomyopathy. Mem Inst Oswaldo Cruz. 2004;99:645–649. doi: 10.1590/s0074-02762004000600020. [DOI] [PubMed] [Google Scholar]
- 35.Oliveira BM, Botoni FA, Ribeiro AL, Pinto AS, Reis AM, et al. Correlation between BNP levels and Doppler echocardiographic parameters of left ventricle filling pressure in patients with Chagasic cardiomyopathy. Echocardiography. 2009;26:521–527. doi: 10.1111/j.1540-8175.2008.00842.x. [DOI] [PubMed] [Google Scholar]
- 36.Lima-Costa MF, Cesar CC, Peixoto SV, Ribeiro AL. Plasma B-type natriuretic peptide as a predictor of mortality in community-dwelling older adults with Chagas disease: 10-year follow-up of the Bambui Cohort Study of Aging. Am J Epidemiol. 2010;172:190–196. doi: 10.1093/aje/kwq106. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Alvarez A, Sitges M, Pinazo MJ, Regueiro-Cueva A, Posada E, et al. Chagas cardiomyopathy: the potential of diastolic dysfunction and brain natriuretic peptide in the early identification of cardiac damage. PLoS Negl Trop Dis. 2010;4 doi: 10.1371/journal.pntd.0000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhiman M, Coronado YA, Vallejo CK, Petersen JR, Ejilemele A, et al. Innate immune responses and antioxidant/oxidant imbalance are major determinants of human Chagas disease. PLoS Negl Trop Dis. 2013;7:e2364. doi: 10.1371/journal.pntd.0002364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhiman M, Estrada-Franco JG, Pando JM, Ramirez-Aguilar FJ, Spratt H, et al. Increased myeloperoxidase activity and protein nitration are indicators of inflammation in patients with Chagas’ disease. Clin Vaccine Immunol. 2009;16:660–666. doi: 10.1128/CVI.00019-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang WH, Brennan ML, Philip K, Tong W, Mann S, et al. Plasma myeloperoxidase levels in patients with chronic heart failure. Am J Cardiol. 2006;98:796–799. doi: 10.1016/j.amjcard.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 41.Costa GC, da Costa Rocha MO, Moreira PR, Menezes CA, Silva MR, et al. Functional IL-10 gene polymorphism is associated with Chagas disease cardiomyopathy. J Infect Dis. 2009;199:451–454. doi: 10.1086/596061. [DOI] [PubMed] [Google Scholar]
- 42.Machado FS, Martins GA, Aliberti JC, Mestriner FL, Cunha FQ, et al. Trypanosoma cruzi-infected cardiomyocytes produce chemokines and cytokines that trigger potent nitric oxide-dependent trypanocidal activity. Circulation. 2000;102:3003–3008. doi: 10.1161/01.cir.102.24.3003. [DOI] [PubMed] [Google Scholar]
- 43.Llaguno M, Pertili LA, da Silva MV, Bunazar P, Reges AM, et al. The relationship between heart rate variability and serum cytokines in chronic chagasic patients with persistent parasitemia. Pacing Clin Electrophysiol. 2011;34:724–735. doi: 10.1111/j.1540-8159.2010.03025.x. [DOI] [PubMed] [Google Scholar]
- 44.Amir O, Rogowski O, David M, Lahat N, Wolff R, et al. Circulating interleukin-10: association with higher mortality in systolic heart failure patients with elevated tumor necrosis factor-alpha. Isr Med Assoc J. 2010;12:158–162. [PubMed] [Google Scholar]
- 45.La Vecchia L, Mezzena G, Zanolla L, Paccanaro M, Varotto L, et al. Cardiac troponin I as diagnostic and prognostic marker in severe heart failure. J Heart Lung Transplant. 2000;19:644–652. doi: 10.1016/s1053-2498(00)00120-0. [DOI] [PubMed] [Google Scholar]
- 46.Saravia SG, Haberland A, Bartel S, Araujo R, Valda G, et al. Cardiac troponin T measured with a highly sensitive assay for diagnosis and monitoring of heart injury in chronic Chagas disease. Arch Pathol Lab Med. 2011;135:243–248. doi: 10.5858/135.2.243. [DOI] [PubMed] [Google Scholar]
- 47.Mercado TI, Garbus J. Creatine phosphokinase isoenzymes and Trypanosoma cruzi infections. Comp Biochem Physiol B. 1979;64:11–15. doi: 10.1016/0305-0491(79)90177-9. [DOI] [PubMed] [Google Scholar]
- 48.Combs TP, Nagajyothi, Mukherjee S, de Almeida CJ, Jelicks LA, et al. The adipocyte as an important target cell for Trypanosoma cruzi infection. J Biol Chem. 2005;280:24085–24094. doi: 10.1074/jbc.M412802200. [DOI] [PubMed] [Google Scholar]
- 49.Tanowitz HB, Jelicks LA, Machado FS, Esper L, Qi X, et al. Adipose tissue, diabetes and Chagas disease. Adv Parasitol. 2011;76:235–250. doi: 10.1016/B978-0-12-385895-5.00010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shibata R, Ouchi N, Murohara T. Adiponectin and cardiovascular disease. Circ J. 2009;73:608–614. doi: 10.1253/circj.cj-09-0057. [DOI] [PubMed] [Google Scholar]
- 51.Nagajyothi F, Desruisseaux MS, Weiss LM, Chua S, Albanese C, et al. Chagas disease, adipose tissue and the metabolic syndrome. Mem Inst Oswaldo Cruz. 2009;104(Suppl 1):219–225. doi: 10.1590/s0074-02762009000900028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skibsted S, Jones AE, Puskarich MA, Arnold R, Sherwin R, et al. Biomarkers of endothelial cell activation in early sepsis. Shock. 2013;39:427–432. doi: 10.1097/SHK.0b013e3182903f0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Landfear SM, Ignatushchenko M. The flagellum and flagellar pocket of trypanosomatids. Mol Biochem Parasitol. 2001;115:1–17. doi: 10.1016/s0166-6851(01)00262-6. [DOI] [PubMed] [Google Scholar]
- 54.Grellier P, Vendeville S, Joyeau R, Bastos IM, Drobecq H, et al. Trypanosoma cruzi prolyl oligopeptidase Tc80 is involved in nonphagocytic mammalian cell invasion by trypomastigotes. J Biol Chem. 2001;276:47078–47086. doi: 10.1074/jbc.M106017200. [DOI] [PubMed] [Google Scholar]
- 55.Silva-López R. Immunocytochemistry of proteases in the study of Leishmania physiology and host-parasite interaction. Intech, Rijeka: H. Dehghani; 2012. [Google Scholar]


