Abstract
Endothelium lining the interior of cardiovascular system and most visceral organs plays an important role in vascular function. Its dysfunction occurs in some of the most challenging diseases. An important function of the endothelium is to release vasoactive substances that act on the smooth muscle to change vascular tones. Substance secretion from endocrine cells relies on membrane potentials and firing activity, while it is unclear whether the membrane potential regulates substance release from the ECs. Understanding of this requires selective intervention to membrane potentials of the endothelial cells in situ. Here we show a novel intervention to endothelial cells using the optogenetic approach. A strain of transgenic mice was developed with the Cre-loxP recombination system. These transgenic mice expressed channelrhodopsin (ChR) in endothelial cells driven by the vascular endothelial cadherin or cdh5 promoter. Linked in a tandem with YFP, the ChR expression was detected by YFP fluorescence in various endothelium-lining tissues and organs. The YFP fluorescence was observed in the lumen of blood vessels and pericardium, but not in tissues beneath the endothelium lining. Optostimulation of dissociated endothelial cells evoked inward currents and depolarization. In the isolated and perfused heart, surprisingly, optostimulation of endothelial cells produced fast, robust, reproducible and long-lasting vasoconstriction that was not blocked by either ET-1A or TXA2 receptor antagonist. Similar optical vasoconstriction was found in the isolated and perfused kidney. These results indicate that the optogenetics is an effective intervention to vascular endothelium where optostimulation produces vasoconstriction.
Keywords: Endothelium, optogenetics, endothelial cells, channelrhodopsin, transgenic mice, cdh5 promoter
Graphical abstract
Introduction
Endothelium, a single layer of cell lining in the cardiovascular system, lymph vessels and several internal organs, plays a critical role in vascular function including substance exchange, vascular tone regulation, angiogenesis and thrombosis (Lerman and Zeiher, 2005). Endothelial dysfunction contributes to several cardiovascular diseases such as hypertension, shock, stroke and diabetic vascular complications (Endemann and Schiffrin, 2004).
The endothelium is a major tissue with approximately 1013 cells in an adult human (Cines et al., 1998), a number that is 100 times more than all neurons in the brain. The endothelium tissue not only is a mechanical barrier between blood and other tissues, but also resembles the endocrine system (Inagami et al., 1995). Indeed, a prominent function of endothelial cells (ECs) is to release vasoactive substances that act on the smooth muscle (SM) to produce vasoconstriction or vasodilation, maintaining a homeostatic state of vascular tones under different conditions. How the ECs selectively release vasodilators or vasoconstrictors is unclear.
Most endocrine cells are excitable, which secrete hormones and transmitters via depolarization and firing activity (Lowe et al., 1988). In contrast, ECs are nonexcitable. Whether the membrane potential regulates substance release from the ECs remains elusive. It is known that the membrane potential can affect a number of ion channels, transporters, intracellular Ca2+, intracellular pH, etc. Activity of these molecules can in turn change cellular functions. Therefore, it is reasonable to believe that depolarization can affect the endothelium function. If such an assumption is proven correct, then it would be possible to reveal whether the endothelial depolarization results in vasodilation or vasoconstriction. To address these issues, novel methods are needed to selectively intervene to the membrane potentials of ECs when they remain interacting with vascular SM in situ, which will help to understand EC function in vascular tone regulation, and may have impacts on therapeutic designs for several cardiovascular diseases.
The successful development of optogenetics in neuroscience research (Boyden et al., 2005; Nagel et al., 2005) provides a unique way to access the endothelial membrane potentials with light. Generally, the optogenetics is a combination of genetics, electrophysiology and optics to control well-defined events within specific cells of living tissue by expressing an opsin in the cells. This approach allows to control membrane potentials and excitability of the opsin-expressing cells, which has been achieved in various types of neurons (Williams and Deisseroth, 2013), glia (Figueiredo et al., 2011), myocardium (Arrenberg et al., 2010), skeletal muscle cells (Sakar et al., 2012) and the vascular smooth muscle (Wu et al., 2015).
To demonstrate whether the EC function can be manipulated by optogenetics, we developed a new strain of mice that expressed channelrhodopsin (ChR) in ECs. The expression of ChR was detected in the lumen of blood vessels from multiple organs. Characteristics of optoactivation of ECs, including membrane excitability and vasomotor reactivity, were studied in the heart and kidney using combined optostimulation and traditional physiological techniques. Surprisingly, our results indicated that optostimulation of ECs can produce fast, reproducible, long-lasting vasoconstriction that is comparable in strength to the popular vasoconstrictor phenylephrine (PE).
Methods and Materials
Generation of the cdh5-ChR Transgenic Mouse
All experimental procedures in the animal were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the Georgia State University Institutional Animal Care and Use Committee. Transgenic cdh5-ChR mice with fluorescence labeled endothelium were generated by mating the cdh5 promoter-driven Cre mice (B6.FVB-Tg(Cdh5-Cre)7Mlia/J, Stock No. 006137; JaxMice) and cross-bred them with ChR-loxP mice (B6; 129S-Gt(ROSA)26Sortm32(CAG-COP4*H134R/EYFP)Hze/J, Stock No.012569; JaxMice). Animals were genotyped by PCR using punched ear or tail tissue. The presence of the Cre transgene (~100 bp) was detected using the following primers: Cre fw: 5′ GCGGTCTGGCAG TAAAAACTA TC and Cre re: 5′ GTGAAACAGCATTGCTGTCACTT, which would not distinguish hemizygous from homozygous mice. The presence of loxP was detected by using following primers with indication of ChR-loxP (212 bp), heterozygote (212 bp and 297 bp), or WT (297 bp): WT ChR fw: 5′ AAGGGAGCTGCAGTGGAGTA and WT ChR re: 5′ CCGAAAATCTGTGGGAAGTC; ChR fw: 5′ ACATGGTCCTGCTGGAGTTC and ChR re: 5′ GGCATTAAAGCAGCGTATCC.
Histology of ChR labeled Endothelium in Multiple Organs
Heart and kidney from cdh5-ChR and wild type (WT) mice were fixed in 1% paraformaldehyde at room temperature for over 4 hrs and then dehydrated in 30% sucrose in phosphate buffered saline (PBS) at 4°C for 24 hrs. Fixed tissues were embedded in the Tissue-TekTM CRYO-OCT Compound (Andwin Scientific, Torrance, CA, 4583), and cut into 8–10 μm slices by using the Microm HM 550 Cryostats system (Thermo Scientific, PA, 22-050-337). Dura mater was peeled off from inner side of skull and mount on glassed slices directly. YFP fluorescence was detected with 514/527 nm (excitation/emission wavelength) filters under the microscope (Carl Zeiss, Gottingen, Germany, Axiovert 200).
Acute Dissociation of Aortic ECs
ECs were acutely dissociated from aorta obtained from both cdh5-ChR and WT mice by enzymatic dissociation for 10 – 15 min at 37°C using neutral protease (8 U/ml, obtained from Sigma, St. Louis, MO) and elastase (2 U/ml, Worthington, Lakewood, NJ, USA) mg/ml in the following digestion buffer (in mM): 138 NaCl, 5 KCl, 1.5 MgCl2, 0.42 Na2HPO4, 0.44 NaH2PO4, 0.1 CaCl2, 10 HEPES, 4.2 NaHCO3 n 0.3% BSA. This was followed by a 1–2 min 37°C incubation with collagenase type IA (120 U/ml, obtained from Sigma, St. Louis, MO). The tissue segments were then washed with digestion buffer and gently triturated with a fire polished glass pipettes. The trituration solution containing ECs was dropped on a Petri dish coated with poly-L-lysine (Sigma, P8920) and easily identified via fluorescence microscopy.
Electrophysiology
Dissociated ECs were placed in the recording chamber and identified by YFP fluorescence using same microscope set up as described under histology section. Whole-cell currents and membrane potentials of the dissociated ECs were recorded at room temperature in the voltage and current clamp mode, respective. Recorded signals were amplified with an Axopatch 200B amplifier (Molecular Devices, Union City, CA), digitized at 10 kHz, filtered at 2 kHz, and collected with the Clampex 10 data acquisition software (Molecular Devices, Union City, CA). The patch pipettes with resistance of 4–6 MΩ were made with 1.2 mm borosilicate glass capillaries (Sutter Instrument CO., Novato, CA). The optical stimulation was delivered by using a xenon arc lamp with high-speed switcher (Lambda DG-4, Sutter Instruments, Novato, CA). The light source was connected to the incident-light illuminator port of the microscope, and passed through a 470 nm bandpass filter (~20 mW/mm2). Light pulse trains were generated with the Digitimer D4030 pulse generator (Digitimer Ltd, Letchworth Garden City, UK). The solution applied to the bath contained (in mM) 130.0 NaCl, 10.0 KCl, 1.0 MgCl2, 1.5 CaCl2, 10.0 glucose, 10.0 HEPES and 3.0 NaOH (pH 7.4). The internal (pipette) solution contained (in mM) 10.0 KCl, 133.0 K-gluconate, 5.0 EGTA, 5.0 glucose, 1.0 K2-ATP, 0.5 Na-ADP, and 10.0 HEPES (pH 7.4), and the final Mg2+ concentration was adjusted to 1 mM using a [Ca2+] / [Mg2+] calculation software Maxchelator (Chris Patton, Stanford University, Pacific Grove, CA).
Langendorff Heart Perfusion
Coronary circulation resistance was studied in the Langendorff isolated and perfused heart preparation. The heart and lungs were entirely removed from a terminally euthanized mice, and placed in the ice-cold Krebs-Henseleit (KH) solution containing (in mM): 119 NaCl, 4.7 KCl, 2.5 MgSO4, 2.5 CaCl2, 10 glucose, 0.5 disodium EDTA, 25 NaHCO3. All of pulmonary arteries and veins were tightened by cotton thread followed by removal of lungs. Then a cannula was inserted in the ascending aorta connected to the perfusion apparatus. This cannula is attached to the outflow of a reservoir containing an oxygenated KH solution, maintained at > ~35°C, and continuously gassed with 5% CO2 and 95% O2. The perfusion solution was delivered to coronary arteries in the retrograde direction through the aorta. After the perfusion speed was determined in each heart at a hydrostatic pressure (80 cm H2O), perfusion at constant flow (0.2 – 0.4 ml/min) was carried out with a calibrated roller pump (Syringe Pump, Farmingdale, NY, NE-300, NE-4000). The perfusate exited the coronary venous circulation through the coronary sinus in the open vena cava. The viability of the isolated and perfused heart was assessed by its spontaneous beating (> 250 beats/min), coronary vasoconstriction to 10−5 M PE and 60 mM KCl, and coronary vasodilation response to the β receptor agonist isoproterenol (Isop, 10−5 M).
Renal Perfusion
Kidney from both cdh5-ChR and WT mice were isolated, cannulated through renal artery, and perfused at a constant rate (0.2 – 0.4 ml/min at baseline) with KH solution at 37°C as described above. The viability of the isolated and perfused kidney was also assessed by renal vascular responses to 10−5 M PE, Isop, and KCl.
Data Analysis
The electrophysiological data were analyzed with Clampfit 10.3 software. Data are presented as means ± SE. Student’s t-test and one-way ANOVA were used to perform the statistical analysis. Difference was considered significant when P ≤ 0.05.
Results
1. Generation of transgenic mice with ChR expression in ECs
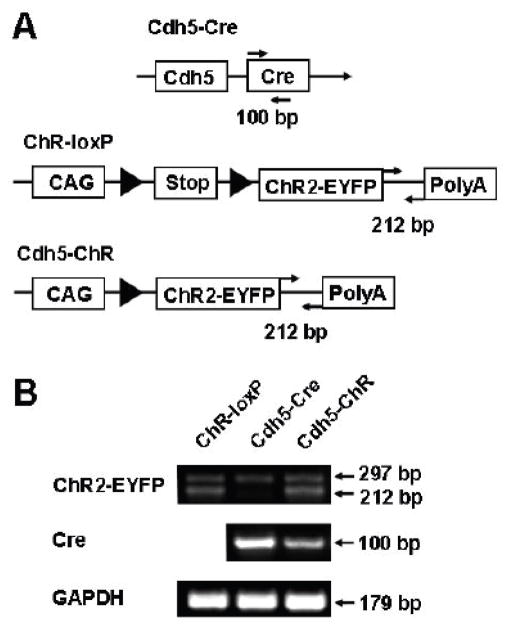
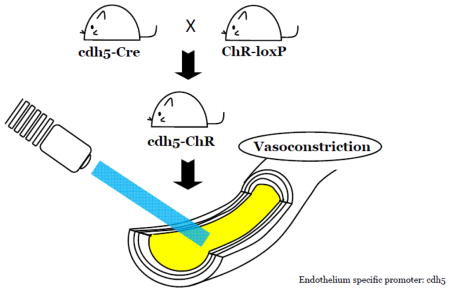
To achieve endothelial expression of ChR, we took the advantage of two strains of commercially available mice, cdh5 promoter-driven Cre mice (B6.FVB-Tg(Cdh5-Cre)7Mlia/J, Stock No. 006137; JaxMice) (Alva et al., 2006) and ChR-loxP mice (B6; 129S-Gt(ROSA)26Sortm32(CAG-COP4*H134R/EYFP)Hze/J, Stock No.012569; JaxMice) (Madisen et al., 2012), to generate a new strain of transgenic mice driven by the cdh5 promoter. The cdh5 is gene encoding VE-cadherin, also known as cadherin 5 or CD144, expressed mainly in endothelium. The cdh5 promoter has been previously demonstrated in adult/quiescent endothelium (Gory et al., 1999; Hisatsune et al., 2005), and also successfully used to direct EC-specific expression of several genes (Liu et al., 2013). Therefore, we employed the homozygous cdh5-Cre mice to cross-bred with heterozygotous ChR-loxP mice. Fifty percentage of their offspring mice were tested positive for both Cre and ChR-loxP expression (Fig. 1B), suggesting that these cdh5-ChR mice have gained the expression of ChR as a result of the Cre-loxP mediated crossover.
Figure 1.
Generation of cdh5-ChR transgenic mice. A. Construction pattern of parental cdh5-Cre and ChR-loxP mice as well as their expected offspring that express both Cre and ChR2-eYFP. In the presence of Cre recombinase, the stop codon flanked by loxP (solid triangle) in the ChR-loxP mouse will be removed, resulting in expression of the ChR2-eYFP in a tandem driven by the CAG promoter in cdh5-ChR mice. Note that arrows indicate primers for genotyping with expected PCR fragment shown below. B. Primers targeted at the 3′ UTR of ChR2-eYFP produced a 212 bp PCR product, and primers targeted at the Cre open-reading frame yielded a 100 bp. The presence of both 212 bp and 100 bp bands indicated positive Cre-loxP recombination producing the cdh5-ChR strain, while cdh5-Cre and ChR-loxP mice have only the 100 bp and 212 bp bands, respectively. Note that a ~300 bp non-specific band was found in all mice. Abbreviations: CAG, cytomegalovirus-immediate-early (CMV-IE) enhancer/chicken β-actin/rabbit β-globin hybrid promoter; PolyA, bovine growth hormone polyadenylation signal and flippase recognition target flanked phosphoglycerate kinase-Neo-polyA cassette; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
2. Endothelium expression of ChR-YFP
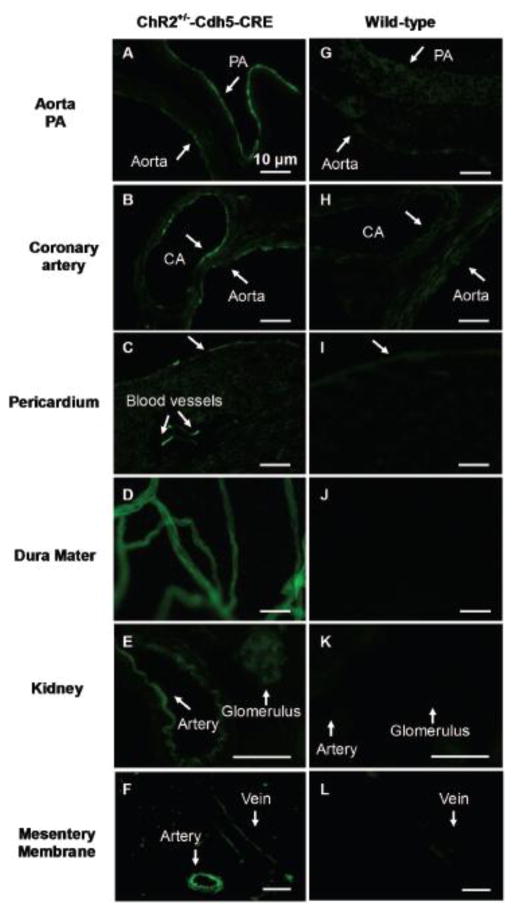
The expression of ChR was indicated by YFP fluorescence as it was fused to a tandem protein (Fig. 1A). Indeed, the Cre and ChR-loxP positive mice showed strong YFP fluorescence on the luminal side of the aorta, pulmonary artery and coronary artery (Fig. 2A, B). In the transverse section of the heart, YFP fluorescence was detected in the surface pericardium and interior blood vessels, while the myocardia did not show YFP fluorescence (Fig. 2C). A large number of blood vessels in the dura matter of the brain were YFP-positive (Fig. 2D). In the transverse section of the kidney, glomeruli, arcuate artery, and interlobular artery were also fluorescent in cdh5-ChR mice (Fig 2E). In mesentery membrane from cdh5-ChR, both of vein and artery were clearly seen fluorescent (Fig. 2F). In contrast, such YFP fluorescence was not seen in any of these tissues obtained from the WT mice (Fig 2G–L).
Figure 2.
YFP fluorescence in various tissues and organs in cdh5-ChR (left column) and WT mice (right column). In cdh5-ChR mice, strong fluorescence was seen on lumen side of aorta (A, B), pulmonary artery (PA) (A) and coronary artery (CA) (B). YFP fluorescence was also seen in pericardium (C), blood vessels in dura mater (D), renal arcuate artery (E), glomerulus (E), mesenteric artery (F) and mesenteric vein (F). In contrast, YFP fluorescence was not observed in these tissues and organs from WT mice (G–L). Scale bar is 10 μm.
3. Effects of ChR activation on membrane potentials and ionic currents in acutely dissociated ECs
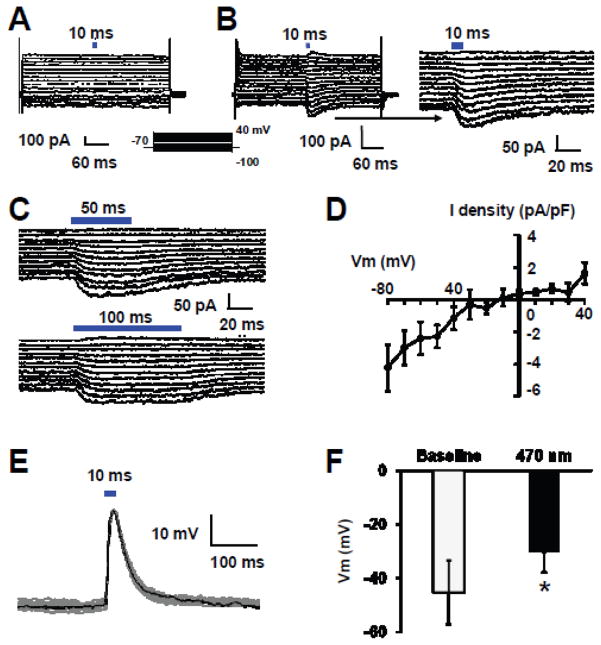
The presence of YFP fluorescence in a variety of blood vessels suggests that ChR is expressed in ECs. To demonstrate that is indeed the case, we performed functional studies of ionic currents in acutely dissociated ECs. In voltage clamp, ECs from WT mice showed whole-cell currents that did not respond to optostimulation (Fig. 3A). In ECs obtained from cdh5-ChR mice, optostimulation at 10, 50 and 100 ms of blue light (470 nm) produced an inward current (Fig. 3B). The amplitude of this current was large at hyperpolarizing membrane potential, decreased with depolarization, and reversed its polarity when the membrane potentials were more positive than 0mV. The current showed almost no adaptation with long-lasting optostimulation (Fig. 3C). In I/V plot, the current showed a weak inward rectification with a reversal potential at −10mV (Fig. 3D). Activation of this current led to strong depolarization of the ECs, which averaged by −15.2 mV from baseline at −45.3 ± 11.6 mV (n=4) (Fig. 3E, F). The optical depolarization was also seen in longer time optostimulation up to 50 and 100 ms (Data are not shown).
Figure 3.
Optical excitation of acutely dissociated ECs from aorta. A. Whole-cell currents were recorded from an EC from a WT mouse in voltage clamp. Light stimulation (10 ms) did not produce any change of membrane currents. Steps of voltage commands (from −100 to 40 with a 10 mV increment) were applied to the cell at a holding potential of −70 mV. B. Optostimulation with 10 ms pulses of blue light (470 nm) to an EC from a cdh5-ChR mouse evoked inward currents. The amplitude of this current decreased with depolarization, and reversed its polarity when the membrane potentials were more positive than 0mV. The photo currents decayed slowly after turning off the light, which are better seen in the expanded display on the right panel. C. The inward photo currents responding to longer durations (50 and 100 ms) of blue light stimulations showed almost no reduction in current amplitudes. D. Current-voltage (I–V) relationship of the photo currents showed a reversal potential at −10 mV with moderate inward rectification. E. In current clamp, blue light stimulation produced strong depolarization in an EC from cdh-ChR mouse. F. Optostimulation produced significant depolarization. Data are presented as means ± SE (*, P<0.05, student’s t-test; n=4 cells).
4. Contraction of coronary arteries by optostimulation in isolated and perfused hearts
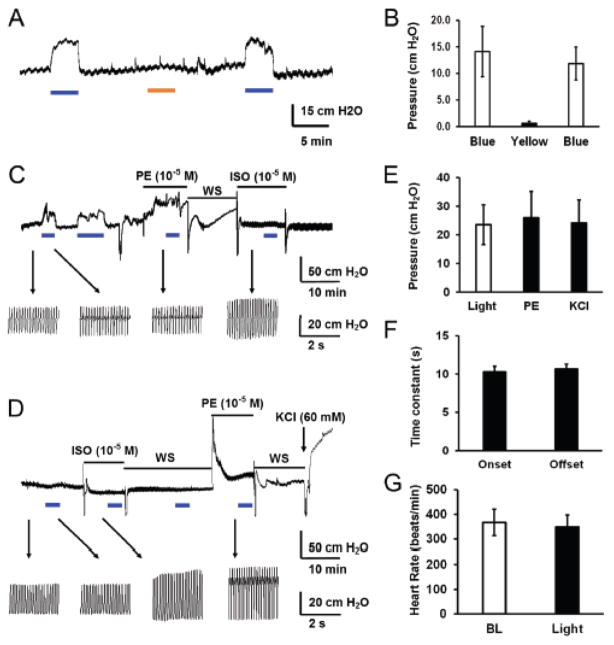
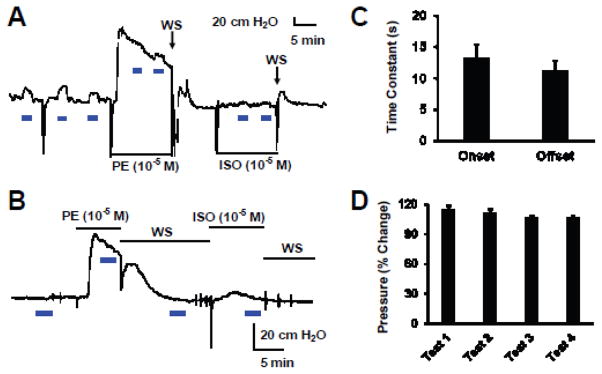
The large inward current and depolarization produced by optostimulation should have impacts on endothelial function and vascular tones. To reveal the functional consequences of the optostimulation, we studied the coronary circulation in the Langendorff heart preparation. The specificity of optostimulation was tested with both blue (470 ± 40 nm) and yellow light (602 ± 70 nm). Only blue light triggered significant vasoconstriction, but not yellow light (Fig. 4A, B). When the heart was exposed to blue light covering either left or right coronary arteries on ventricle, the resistance of coronary circulating increased markedly with perfusion pressure changes by 23.5 ± 6.9 cm H2O (n=5), indicating coronary vasoconstriction (Fig. 4C, E). The light beam covered main left coronary artery, left circumflex branch, left anterior descending branch, left marginal artery and great cardiac vein on the left side of ventricle. On the right ventricle surface, the light beams covered main coronary artery and major branches, including conus arteriosus branch, right anterior ventricular and right marginal arteries, and anterior and small cardiac veins. No obvious different responses were seen between the left and right optical approaches to the heart ventricle. The heart rate and pulse pressure were not affected by optostimulation (Fig. 4C, G). The coronary vasoconstriction by optostimulation was nicely reproducible with repetitive light exposures, and showed no adaption with a long optostimulation up to 6 min (Fig. 4C). Interestingly, a prior exposure to PE (10−5 M) or Isop (10−5 M) largely diminished the coronary vasoconstriction by optostimulation, suggesting that they may share the same ionic mechanisms in the SM. In comparison, the optostimulation produced coronary vasoconstriction to the degree that was comparable to 10−5 M PE (26.0 ± 9.1 cm H2O, n=4) and 60 mM KCl (24.2 ± 7.9 cm H2O, n=4) with no significant difference (P > 0.05, Fig. 4C, E). The coronary vasoconstriction by optostimulation had fast time response with the onset and offset time to be ~ 10 sec (Fig 4F). Such coronary vasoconstriction was not found in WT heart when it was exposed to the same light stimulation (Fig. 4D).
Figure 4.
Optical vasoconstriction of coronary arteries by optostimulation. A, B. In isolated and perfused hearts from cdh5-ChR mice, significant coronary constriction was produced by blue light stimulation (470 nm), but not by yellow light (602 nm). C. Coronary constriction was produced by optostimulation followed by PE and isoproterenol (Isop) treatments. The optical coronary vasoconstriction was strong, long-lasting, reproducible with repetitive optostimulation, and did not cause evident changes in heart rate and pulse pressure. The latter both were augmented by Isop. Heart rate and pulse pressure were indicated by arrows toward the enlarged pattern on the bottom of the figure. D. Optostimulation did not produce any vasoconstriction in a heart from WT mouse. E. In the perfused heart from cdh5-ChR mouse, the optical vasoconstriction with ~20 mW/mm2 blue light was competitive comparing to that produced by 10−5 M PE and 60 mM KCl. F. The optostimulation triggered fast response in heart perfusion with onset and offset time less than 11s. G. Optical vasoconstriction had no significant effect on heart rate.
5. Optical vasoconstriction in isolated and perfused kidney
Optostimulation also produced vasoconstriction in the renal circulation. When blue light was applied to the surface of the isolated and perfused kidney. In the kidney, repetitive optostimulations gave rise to repetitive increases in the perfusion pressure (13.9 ± 3.3 cm H2O, n=9) with no clear adaptions (Fig. 5A). The optostimulation-induced renal vasoconstriction was significantly reduced when vasoconstriction was induced by PE (10−5 M) (Fig 5A). It was partially recovered when the effect of PE largely declined (Fig. 5A). The onset and offset times of the optical vasoconstriction was similar to those in the heart (11–14 sec) (Fig. 5C). The optical vasoconstriction was reproducible without marked adaptation (Fig. 5A, D). Compared to the vasoconstriction produced by 10−5 M PE (110.0 ± 35.2 cm H2O) and 60 mM KCl (84.9 ± 20.9cm H2O), the optical vasoconstriction of the renal circulation was significantly smaller (P < 0.001, freedom=11 and 14, separately). In contrast, optostimulation did not induce any response in the circulation resistance of WT kidney (Fig. 5C).
Figure 5.
Optical vasoconstriction in isolated kidney. A. Blue light produced vasoconstriction in the kidney obtained from a cdh5-ChR mouse but not that from a WT mouse (B). C. The optostimulation has fast onset and offset (10–14s). D. Repetitive blue light stimulation produced up to 114.7% vasoconstriction in perfused kidney from cdh5-ChR mouse with slight decline in the amplitude.. Blue light intensity is ~20 mW/mm2 and stimulation duration is 2 min with 5 min interval.
6. Mechanism underlying optostimulation induced vasoconstriction
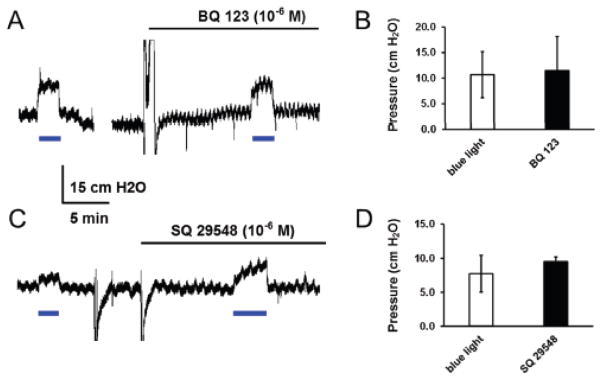
To address the underlying mechanism, two preeminent endothelium-derived vasoconstrictors, endothelin 1 (ET-1) and thromboxane A2 (TXA2) were tested in perfused hearts of cdh5-ChR mice. In the presence of the ET-1A receptor blocker BQ-123 (10−6 M), blue light produced the same levels of vasoconstriction (Fig. 6A, B). The vasoconstriction by optostimulation was not attenuated with the TXA2 receptor antagonist SQ-29548 (10−6 M) either (Fig. 6C, D). Therefore, the optostimulation-induced vasoconstriction does not seem to be mediated by these two vasoconstrictors released from endothelium.
Figure 6.
Effects of ET-1A and TXA2 receptor antagonists on optical vasoconstriction. A, B. In isolated and perfused hearts from cdh5-ChR mice, coronary constriction was produced by blue light stimulation. The optical vasoconstriction remained in the presence of the ET-1A receptor antagonist BQ-123 (10−6 M). C, D. The optical vasoconstriction was not suppressed by the TXA2 receptor antagonist SQ-29548 (10−6 M) either. Data are presented as means ± SE (n = 3 hearts, P > 0.05). Note that there is a gap of 10 min in A.
Discussion
To our knowledge this is the first report demonstrating the application of optogenetics to the endothelium tissue. By taking the advantage of Cre-LoxP recombination system, we created a new strain of mice that express ChR in ECs directed by the cdh5 promoter. In the cdh5-ChR mice, we observed YFP expression in ECs from various tissues and organs. Our functional assays indicated that photo currents and depolarization can be produced in ECs, and optostimulation evokes vasoconstriction in the heart and the kidney.
Several tissue-specific promoters have previously been used to direct gene expression in ECs, such as Tie-1 and Tie-2-, PECAM, Flk-1, and SCL (Gothert et al., 2005; Gustafsson et al., 2001; Kisanuki et al., 2001; Licht et al., 2004; Terry et al., 1997). Although EC expression of desired genes are reported, unexpected expression has been found in these transgenic lines including hematopoietic lineages and other cell types (Alva et al., 2006). Such non-specific expression limits their transgenic applications, although they were widely used for gene deletions. Thus, more selective promoters are needed to generate transgenic models with specific expression in differentiated endothelium.
Cadherin-5 (Cdh5 or CD-144), a transmembrane glycoprotein, is an essential player in the morphogenesis of the vascular system, modulation of blood flow, vascular endothelial growth factor (VEGF) signaling, and vascular permeability in a variety of tissues (Carmeliet et al., 1999; Corada et al., 2001; Venkiteswaran et al., 2002). The cdh5 promoter has been characterized in adult/quiescent endothelium, and used to introduce specific gene expression in ECs (Alva et al., 2006). The success of EC-specific expression thus has led to the generation of mice with Cre recombinase expression in endothelium (Alva et al., 2006), which is now commercially available (Jackson lab). These mice have also been successfully used to modify gene expressions previously (Fioret et al., 2014; Guo et al., 2008). By taking the advantage of the cdh5-Cre mouse strain and another commercially available ChR-loxP strain, we have generated a new strain of mice. This strain of cdh5-ChR mice expresses ChR in a tandem with YFP. Fifty percent offspring (cdh5-ChR) of the mating pair of cdh5-Cre and ChR-loxP mice showed both Cre (100 bp) and ChR-EYFP (212 bp) bands without any detectable physical and behavioral abnormalities.
Our morphological studies have shown that YFP fluorescence is detected in various tissues and organs. The YFP fluorescence is observed in the luminal lining of arteries including the aortic, pulmonary, coronary, renal, mesenteric and basilar arteries. It is also seen in renal, dura, skeletal muscle veins as well as pericardium and mesentery. Therefore, the cdh5-ChR mice appear to express ChR as expected.
These YFP positive ECs indeed express ChRs. Direct activation of these cationic channels with 470 nm blue light in acute dissociated ECs evokes large and long-lasting inward currents in voltage clamp with reversal potential at −10mV consistent to the cationic permeability of the ChR. The photo currents show moderate inward rectification in the I–V plot with no clear adaptation/inactivation in response to prolonged light exposure up to 100 ms. In current clamp, optostimulation produces depolarization of the ECs. These electrophysiological studies indicate that functional ChR is expressed in ECs obtained from the cdh5-ChR mice.
Consistent with the expression of ChR in ECs, we have found that the optostimulation increases perfusion resistance in coronary and renal circulation in isolated organs from the cdh5-ChR mice. This optostimulation is specific for blue light that activates ChR, and optical vasoconstriction is produced in organs obtained from cdh5-ChR mice but not from the WT. This suggests that the optical vasoconstriction is not produced by endogenous photo-sensitive mechanisms. It is known that in addition to endothelium, cdh5 is expressed in other cells including certain blood cells (Alva et al., 2006). The possibility of blood cell involvement may largely be ruled out in our in-vitro organ perfusion, because the blood cells are mostly removed in the preparations, and because the blood cells tend to release vasodilators rather vasoconstrictors. This light-produced vasoconstriction in heart and kidney is fast, reproducible and long-lasting with no obvious decline in perfusion resistance in 6 min. This optical vasoconstriction in coronary circulation is as strong as the popular clinical vasoconstrictor PE at high concentration (10−5 M) and 60mM KCl. In the isolated kidney, the optical vasoconstriction is less potent than both of that produced by 10−5 M PE and 60 mM KCl, which is likely to be a result from the high expression level of α1 adrenoceptor and poor transparency of the organ.
In the heart, only can a part of coronary circulation (~ 30 % of surface area of the whole heart) be illuminated by the light beam during experiments. Despite that, optostimulation still produces potent vasoconstriction, which is comparable to that produced by 10−5 M PE or 60mM KCl in the heart. In addition to the robust response, this optogenetic method also provides a high temporal precision. The onset and offset time constant are short, within 11s in heart and 14s in kidney. This spatial and temporal performance of optostimulation on ECs in isolated and perfused organs seems to allow a selective access and manipulation of ECs with the optogenetic approach.
We have realized some limitations of the optostimulation in the perfused organs: 1) The tissue transparency is limited, especially in the isolated kidney. 2) Not all sections of blood vessels can be covered by the light beam with ~ 3 mm in diameter and 20 mW/mm2 in strength. With the limited light penetration, therefore, the optostimulation seems to be effective only in the vessels on or near to the surface of the organ.
What produces the optical vasoconstriction is still unclear, which may involve several potentials mechanisms. Optostimulation may increase the release of endothelium-derived vasoconstrictors from ECs, such as ET-1, which is a peptide, while most peptide hormones and transmitters are packed in vesicles inside the cell. The vesicle release is a Ca2+-dependent process that usually occurs with depolarization. Other vasoconstrictors including TXA2 and hydroxyeicosatetraenoic acids are lipid metabolites. Their productions are more important in their biological effects than the releasing mechanism. It is possible that some of their biosynthetic and degradative enzymes are affected by certain events associated with depolarization. Our results suggest that neither ET-1 nor TXA2 seems to play a major role in optical vasoconstriction, as the optical vasoconstriction remains when their receptor antagonists are present in the perfusion solution.
A decreased release of endothelium-derived vasodilators from ECs may be attributable as well, including NO, prostacyclin, and 11,12-epoxyeicosatrienoic acids. The release and production of these endothelium-derived vasodilators are regulated by several endothelium-targeting substances such as acetylcholine, bradykinin and histamine. How these molecules are affected by membrane potentials, and whether a decreased release of the endothelium-derived vasodilators occurs with optostimulation are still not clear. K+ is a potential player too, which can be released from ECs by optical depolarization, and subsequently produces depolarization of the SM. Another possibility is the gap junction between ECs and SM cells, with which the light induced EC depolarization may be coupled to the SM leading depolarization and vasoconstriction. Testing these possibilities requires systematic studies including screening potential contributors, pinpointing the critical players and elaborating their action mechanisms, Clearly this is a major undertaking that cannot be accomplished in the present study. Nevertheless, the demonstration of the feasibility of optoactivation of ECs in the present study appears to affirm a powerful approach for further investigation of the vasoconstriction produced by depolarization.
The application of optogenetics to endothelium may have several implications on future research and therapeutical agent tests. 1) This method may allow researchers to identify endothelium-derived substances in situ without introducing any receptor agonists/antagonists that tend to have widespread effects on vasculatures and nearby tissues. 2) The high temporal and spatial precision may provide a new way to approach local ECs lining the vasculature without systematical administration of drugs. 3) The local optostimulation-induced vasoconstriction may be helpful to manipulate the interaction among ECs, SM cells, and immune cells, as well as certain substances, including endothelium derived factors/mediators and cytokines. 4) The optical manipulation of ECs may be useful to control the EC dysfunction, vascular growth and inflammation that are known to participate in cardiovascular pathogenesis. 5) Beyond cardiovascular field, the accuracy in specific location of optical stimulation could be used to reveal the mechanism of neuron-pericyte-vasculature coupling in certain brain area, as neurons regulating homeostatic function may require constant blood supplies. 6) The activation of ECs may affect the permeability of the capillary endothelium (Frohlich, 2002), helping drugs to pass through certain tissue barriers such as the blood brain barrier and placenta barrier, which may have impact on drug delivery. We expect that all of these potential applications of the optical activation of ECs to develop and expand rapidly when further investigations take place.
In conclusion, the present study demonstrates a novel intervention to vascular ECs. This method is based on newly developed optogenetics and the availability to transgenic mice, and allows us to express selectively ChR in ECs. Positive ChR expression is identified with YFP fluorescence in the endothelial lining in various tissues. Optostimulation of the ECs that express ChR triggers large inward currents, leading to depolarization. In isolated and perfused organs, optostimulation produces vasoconstriction that is vigorous, reproducible and sustained. These results indicate that the optogenetics in the endothelium seems to be a powerful intervention to the cardiovascular system.
Acknowledgments
This work was supported by NIH grant R01-NS-073875. SZ is an MBD fellow of Georgia State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Developmental dynamics : an official publication of the American Association of Anatomists. 2006;235:759–767. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- Arrenberg AB, Stainier DY, Baier H, Huisken J. Optogenetic control of cardiac function. Science. 2010;330:971–974. doi: 10.1126/science.1195929. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature neuroscience. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- Corada M, Liao F, Lindgren M, Lampugnani MG, Breviario F, Frank R, Muller WA, Hicklin DJ, Bohlen P, Dejana E. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood. 2001;97:1679–1684. doi: 10.1182/blood.v97.6.1679. [DOI] [PubMed] [Google Scholar]
- Endemann DH, Schiffrin EL. Endothelial dysfunction. Journal of the American Society of Nephrology : JASN. 2004;15:1983–1992. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- Figueiredo M, Lane S, Tang F, Liu BH, Hewinson J, Marina N, Kasymov V, Souslova EA, Chudakov DM, Gourine AV, Teschemacher AG, Kasparov S. Optogenetic experimentation on astrocytes. Experimental physiology. 2011;96:40–50. doi: 10.1113/expphysiol.2010.052597. [DOI] [PubMed] [Google Scholar]
- Fioret BA, Heimfeld JD, Paik DT, Hatzopoulos AK. Endothelial cells contribute to generation of adult ventricular myocytes during cardiac homeostasis. Cell reports. 2014;8:229–241. doi: 10.1016/j.celrep.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich E. Structure and function of blood-tissue barriers. Deutsche medizinische Wochenschrift. 2002;127:2629–2634. doi: 10.1055/s-2002-35932. [DOI] [PubMed] [Google Scholar]
- Gory S, Vernet M, Laurent M, Dejana E, Dalmon J, Huber P. The vascular endothelial-cadherin promoter directs endothelial-specific expression in transgenic mice. Blood. 1999;93:184–192. [PubMed] [Google Scholar]
- Gothert JR, Gustin SE, Hall MA, Green AR, Gottgens B, Izon DJ, Begley CG. In vivo fate-tracing studies using the Scl stem cell enhancer: embryonic hematopoietic stem cells significantly contribute to adult hematopoiesis. Blood. 2005;105:2724–2732. doi: 10.1182/blood-2004-08-3037. [DOI] [PubMed] [Google Scholar]
- Guo W, Lasky JL, Chang CJ, Mosessian S, Lewis X, Xiao Y, Yeh JE, Chen JY, Iruela-Arispe ML, Varella-Garcia M, Wu H. Multi-genetic events collaboratively contribute to Pten-null leukaemia stem-cell formation. Nature. 2008;453:529–533. doi: 10.1038/nature06933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson E, Brakebusch C, Hietanen K, Fassler R. Tie-1-directed expression of Cre recombinase in endothelial cells of embryoid bodies and transgenic mice. Journal of cell science. 2001;114:671–676. doi: 10.1242/jcs.114.4.671. [DOI] [PubMed] [Google Scholar]
- Hisatsune H, Matsumura K, Ogawa M, Uemura A, Kondo N, Yamashita JK, Katsuta H, Nishikawa S, Chiba T, Nishikawa S. High level of endothelial cell-specific gene expression by a combination of the 5′ flanking region and the 5′ half of the first intron of the VE-cadherin gene. Blood. 2005;105:4657–4663. doi: 10.1182/blood-2004-09-3554. [DOI] [PubMed] [Google Scholar]
- Inagami T, Naruse M, Hoover R. Endothelium as an endocrine organ. Annual review of physiology. 1995;57:171–189. doi: 10.1146/annurev.ph.57.030195.001131. [DOI] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Developmental biology. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- Licht AH, Raab S, Hofmann U, Breier G. Endothelium-specific Cre recombinase activity in flk-1-Cre transgenic mice. Developmental dynamics : an official publication of the American Association of Anatomists. 2004;229:312–318. doi: 10.1002/dvdy.10416. [DOI] [PubMed] [Google Scholar]
- Liu S, Zhang Y, Moayeri M, Liu J, Crown D, Fattah RJ, Wein AN, Yu ZX, Finkel T, Leppla SH. Key tissue targets responsible for anthrax-toxin-induced lethality. Nature. 2013;501:63–68. doi: 10.1038/nature12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe AW, Madeddu L, Kelly RB. Endocrine secretory granules and neuronal synaptic vesicles have three integral membrane proteins in common. The Journal of cell biology. 1988;106:51–59. doi: 10.1083/jcb.106.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ, 3rd, Gu X, Zanella S, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsaki G, Ramirez JM, Jones AR, Svoboda K, Han X, Turner EE, Zeng H. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nature neuroscience. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Current biology : CB. 2005;15:2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Sakar MS, Neal D, Boudou T, Borochin MA, Li Y, Weiss R, Kamm RD, Chen CS, Asada HH. Formation and optogenetic control of engineered 3D skeletal muscle bioactuators. Lab on a chip. 2012;12:4976–4985. doi: 10.1039/c2lc40338b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RW, Kwee L, Baldwin HS, Labow MA. Cre-mediated generation of a VCAM-1 null allele in transgenic mice. Transgenic research. 1997;6:349–356. doi: 10.1023/a:1018475031852. [DOI] [PubMed] [Google Scholar]
- Venkiteswaran K, Xiao K, Summers S, Calkins CC, Vincent PA, Pumiglia K, Kowalczyk AP. Regulation of endothelial barrier function and growth by VE-cadherin, plakoglobin, and beta-catenin. American journal of physiology. Cell physiology. 2002;283:C811–821. doi: 10.1152/ajpcell.00417.2001. [DOI] [PubMed] [Google Scholar]
- Williams SC, Deisseroth K. Optogenetics. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:16287. doi: 10.1073/pnas.1317033110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Li SS, Jin X, Cui N, Zhang S, Jiang C. Optogenetic approach for functional assays of the cardiovascular system by light activation of the vascular smooth muscle. Vascular pharmacology. 2015 doi: 10.1016/j.vph.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]