Abstract
This paper offers a critical review of the properties, methods and potential clinical application of sodium (23Na) magnetic resonance imaging (MRI) in human heart. Because the tissue sodium concentration (TSC) in heart is about ~40µmol/g wet wt, and the 23Na gyromagnetic ratio and sensitivity are respectively about 1/4th and 1/11th that of hydrogen (1H), the signal-to-noise ratio of 23Na MRI in heart is about 1/6000th that of conventional cardiac 1H MRI. In addition, as a quadrupolar nucleus, 23Na exhibits ultra-short and multi-component relaxation behavior (T1~30ms; T2 ~0.5–4ms and 12–20ms), that requires fast, specialized, ultra-short echo-time MRI sequences, especially for quantifying TSC. Cardiac 23Na MRI studies from 1.5–7 T measure a volume-weighted sum of intra- and extra-cellular components present at cytosolic concentrations of 10–15 mM and 135–150 mM in healthy tissue, respectively, at a spatial resolution of about 0.1–1 ml in 10 min or so. Presently, intra- and extra-cellular sodium cannot be unambiguously resolved without the use of potentially toxic shift reagents. Nevertheless, increases in TSC attributable to an influx of intra-cellular sodium and/or increased extra-cellular volume have been demonstrated in human myocardial infarction consistent with prior animal studies, and arguably might also be seen in future studies of ischemia, and cardiomyopathies–especially those involving defects in sodium transport. While technical implementation remains a hurdle, a central question for clinical use is whether cardiac 23Na MRI can deliver useful information unobtainable by other more convenient methods, including 1H MRI.
Keywords: heart, sodium, magnetic resonance imaging (MRI), ultra-short echo-time, T1, T2, quantification, total sodium content, myocardial infarction
Graphical abstract
The properties, methods and clinical potential of sodium MRI in human heart are reviewed. The myocardial sodium concentration is about ~40µmol/g.wet.wt., and its signal-to-noise ratio is about 1/6000th of conventional proton MRI. Sodium’s short multi-component relaxation behavior necessitates fast, ultra-short-echo MRI sequences, especially for quantification. Presently, intra- and extra-cellular sodium cannot be unambiguously resolved, but increased sodium primarily attributable to sodium influx, is demonstrated in human myocardial infarction. The added value of cardiac 23Na MRI vs. existing methods remains key.
INTRODUCTION
Sodium plays a vital role in myocyte function and integrity. The sodium concentration is 8–10 times higher in the extracellular vs. the intracellular space, with the trans-membrane sodium flux and osmotic balance regulated by the sodium-potassium pump. The sodium channel mediates the rapid upbeat of the cardiac action potential and calcium exchange, which are critical to cardiac excitability, conduction and muscular contraction (1–4). Alterations in tissue sodium concentration (TSC) and in trans-membrane sodium flux occur in ischemic heart disease and myocardial infarction (MI)(5,6). Sodium channel dysfunction is also linked to dilated and hypertrophic cardiomyopathies (7–12). While serum sodium levels are routinely measured in patients and may be independent predictors of outcome for certain heart failure etiologies (11), these measures can only detect electrolyte imbalances that affect the entire blood pool. Except for sodium (23Na) magnetic resonance imaging (MRI), direct in vivo sampling of cardiac sodium is only possible via myocardial biopsy during cardiac surgery or image-guided intervention undertaken for other purposes (12). This makes 23Na MRI a uniquely noninvasive tool for accessing myocardial sodium in vivo.
PROPERTIES
Signal-to-noise ratio (SNR)
Fifty years have passed since the first 23Na nuclear magnetic resonance (MR) studies of sodium in muscle (13). 23Na MRI of isolated rodent hearts (14), and the first human cardiac 23Na MRI (15,16) were reported in the 1980s following the innovation of 1.5 T whole-body scanners, which provided the requisite signal-to-noise ratio (SNR) unavailable to early 0.04–0.3 T scanners (17). Indeed, after accounting for the myocardial sodium tissue concentration of ~40 mmol/kg wet weight, the lower 23Na MR sensitivity and gyromagnetic ratio (©) of about 1/4th that of 1H, the SNR of 23Na MRI is some 6000 times lower than that of conventional cardiac proton (1H) MRI under optimum but equivalent detection conditions for both nuclei (Table 1, bottom row). Overcoming this severe, intrinsic SNR handicap to perform 23Na MRI, demands considerable effort.
Table 1.
NMR properties and predicted sensitivity of 23Na MRI in tissue relative to 1H.
| 1H MRI in tissue | 23Na MRI in tissue | |
|---|---|---|
| Magnetic moment (in nuclear magnetons) |
2.7927 | 2.2161 |
| Spin quantum number | 1/2 | 3/2 |
| NMR frequency @ 1 Tesla | 42.577 | 11.262 |
| Sensitivity* @ constant field per nucleus ∝ υ3I(I+1) |
1 | 0.0925 |
| Relative SNR at constant B0/nucleus* | 1 | 0.35 |
| In vivo concentration (mol/kg) | 42.8 | 0.04 |
| In vivo conc. of nucleus (mol/kg) | 85.6 | 0.04 |
| SNR of moiety in tissue | 85.6 | 0.014 |
| SNR relative to 1H of tissue water† | 1 | 1.64 × 10−4 |
υ =NMR frequency,
I =nuclear spin quantum number.
Assumes SNR ∝ υ1.
Optimized detectors, and front-end scanner electronics are certainly essential for 23Na MRI: they are already near-optimal for 1H MRI. Field strength (B0) is important, but the increase in SNR anticipated from higher B0 is at best only linear in B0, assuming that all detectors satisfy the condition that coil noise is negligible compared to sample noise (18). Adjusting the 23Na MRI operating parameters, on the other hand, can have far greater impact. Reducing spatial resolution from 0.5×0.5×3 mm (0.0075 ml)–typical for a 1H MRI study–to 5×5×10 mm or 0.25 ml for 23Na MRI, for example, yields a 333-fold increase in SNR, which can cut the SNR deficit from 6000 to just 18. Being able to image sodium, the prime mover in cellular ion transport, at sub-milliliter resolution by 23Na MRI, is by no means unattractive.
No formal experimental comparison of the B0-dependence of 23Na SNR under equivalent conditions yet exists, but published 23Na images of human heart acquired at 1.5 T (19–22), 3 T (23–25) and 7 T (26,27) clearly benefit from the huge SNR advantage gained by cutting spatial resolution compared to 1H MRI. Of the most recent human cardiac data, 23Na SNRs of 11–24 are reported in the septum at 3T with a resolution of 4×4×10 mm at 3 T in ~9 min acquisitions (25). Sodium images acquired from heart patients in ~12 min at 1.5 T had 6 mm isotropic resolution, permitting myocardial TSC measurements with an accuracy of ~10% in control subjects (22). The 7T results suggest a SNR of ~24 with 5×5×40 mm resolution in 11 min acquisitions (26), and 10-phase cardiac cine 3Na MRI with 6mm isotropic revolution in ~19 min (27).
Thus, much of the remaining SNR deficit for 23Na–that factor of 18 compared to 1H MRI–is dealt with by signal averaging for 10 minutes or so, as compared to the seconds typically required for 1H MRI with comparable SNR per pixel. The ability to accommodate many averages in practical scan times–the acquisition efficiency–depends critically on the tissue 23Na spin-lattice (T1) and spin-spin (T2) relaxation times.
Relaxation times
Initially, it was thought that only 30%–40% of the total tissue sodium was MR-visible (13,28) due to nuclear quadrupolar interactions of this spin-3/2 nucleus (29), and/or interactions with tissue macromolecules. In fact, 23Na commonly exhibits multi-exponential T2 relaxation in intact biological tissue, with fast (T2f) and slow (T2s) spin-spin relaxation times (30). The T2f component comprises about 40% of the total 23Na signal depending on tissue type (30,31), and is attributable on theoretical grounds, to the motion-averaged electrostatic field gradients arising from reorienting water molecules and charged sites on macromolecules in the vicinity of the ions (29, 32). Experimental data showing a T2s component representing 68±15% of the sodium signal in muscle (32), isolated perfused rat hearts, frog hearts (33), and canine hearts studied in vivo (34), is consistent with this theory. In healthy skeletal and cardiac muscle T2f falls in the range 0.5–4 ms, while T2s is 12–32 ms and contributes 68±15% of the total 23Na heart signal (6,24,34–37). Consequently, a major portion of the T2f signal component is lost when spin-echo or gradient-echo times (TE) are longer than about 3 ms. This signal loss and the effect of short T2s on the useful signal bandwidth (BW), reduces SNR and confounds quantification of absolute TSC. Nevertheless, TSC measured by short-TE 23Na MRI, appears to match tissue [Na] measured by assay (34).
On the plus side, the factors responsible for short 23Na T2s also produce short T1 relaxation times of around 10–40 ms (34,35). This means that MRI experiments performed with pulse sequence repetition times TR~60 ms, are essentially fully-relaxed. Some published 23Na MR T1 and T2 relaxation times for tissues encountered in the chest and heart are summarized in Table 2 (6, 24, 28, 32–45).
Table 2.
Some reported T1 and T2 relaxation times for 23Na in intact muscle, heart and blood.
| Tissue/Sample | T1(ms) a | T2f (ms) a | T2s (ms) a | Bo (T) | Ref |
|---|---|---|---|---|---|
| Blood plasma (human) | 30 ±1 | 17 ±1 | 4.7 | 38 | |
| Blood plasma | 12 | 49.5 | 1.5 | 39 | |
| Blood plasma | 37.3 ±1.0 | 24.5 ±0.8 | 8.5 | 40 | |
| Blood, human in vivo | 20.9 ±6.0 | 1.5 | 41 | ||
| Blood, human | 30.8 ±1.3 | 3.25 ±0.29 | 18.1 ±1.3 | 1.5 | 35 |
| Blood, human LV, in vivo | 31.1 ±7.5 | 19.3 ±3.3 | 1.5 | 36,37 | |
| Blood, human heart | 31.9±4.1 | 20.1 ±1.3 | 3T | 24 | |
| Red blood cells | 30.1 ±3.1 | 4.3 ±1.2 | 19.9 ±1.8 | 1.5 | 35 |
| Red blood cells | 30 ±3 | 14 ±3 | 4.7 | 38 | |
| Red blood cells | 6.3 | 27.2, 17.3b | 1.5 | 42 | |
| Rat muscle | 18.3 ±0.5 | 1.59 ±0.16 | 16.1 ±2.6 | 2.3 | 32 |
| Rat muscle | 12 ±1 | 15 ±8 | 0.89 | 28 | |
| Rat muscle hindlimb | 3–4 | 21–37 | 7.05 | 43 | |
| Human skeletal muscle | 1.2 ±0.2 | 32.5 ±8.2 | 1.91 | 44 | |
| Human skeletal muscle | 0.6–2.5 | 21.2–23.1 | 2 | 45 | |
| Human skeletal muscle | 32.3 ±0.5 | 0.46 ±0.21 | 12.3 ±1.9 | 1.5 | 35 |
| Canine heart-viable tissue | 34.2 ±0.9 | 3.6 ±0.6c | 31.5 ±0.8 | 4.7 | 6 |
| -non-viable | 26.2 ±1.5 | 2.2 ±0.2 c | 21.9 ±1.2 | 4.7 | 6 |
| Canine heart-normal | 2.44 ±0.4 | 15.2 ±1.8d | 1.5 | 34 | |
| -infarcted | 2.04 ±0.2 | 22.7 ±3.4d | 1.5 | 34 | |
| Human heart | 31.6±7.0 | 13.3±4.3 | 1.5 | 36,37 | |
| Human heart | 19.9±6.2 | 12.4±1.8 | 3 | 24 |
Values are mean±SD. Empty cells: values not determined or not reported
Composite T2s relaxation reported.
Significant decrease in non-viable vs. viable tissue
Significant elevation in infarcted vs. normal state (p <0.004)
Because 23Na T1s are about 1/25th of the typical proton (1H) T1s, 23Na experiments can potentially be conducted ~25 times faster than a conventional 1H study for the same MR saturation level. This should result in a gain in SNR efficiency of about √25 ~5. If in addition the BW is cut 4-fold vs. 1H (compromise to the detection of the T2f component, notwithstanding), the combined effect reduces the residual SNR deficit by √(25×4) =10-fold, from 18 to about 2 (46).
Intra- and extra-cellular sodium
The total tissue sodium concentration, [Na]tissue, is a composite of the disparate intra- and extra-cellular sodium concentrations, [Na]intra and [Na]extra (34):
| [1] |
Here [Na]intra is typically 10–15 mM and [Na]extra is 135–150 mM in the cytosol. The V’s are the corresponding tissue volume fractions with Vextra =1-Vintra, and W (in liters/kg or ml/g wet weight) is the tissue water content.
As an example, Vintra occupies about 75% of the water space of cardiac tissue (6,47,48), which in turn is about 77% water (6, 49). Thus, use of Eq. [1] yields a tissue TSC of 0.77×(0.75×15+0.25×145) =37 µmol/g wet weight. This excludes contributions from intravascular blood which occupies 8–10% (50) of the tissue but carries a TSC of ~84 µmol/g (38). If a separate 9% intravascular blood component is accounted for, [Na]tissue increases to (37+0.09×84)/1.09 =41 µmol/g, consistent with a measured value of 43 µmol/g for human heart (21).
Ordinarily, the naturally abundant 23Na resonance is a singlet in biological tissue, and [Na]intra and [Na]extra are not resolvable by 23Na NMR. However, introducing a chemical shift reagent such as dysprosium or thulium into the extra-cellular space can peel off the extra-cellular pool into a separate resonance (51,52), resulting in two resonances–one for each. 23Na NMR spectroscopy can then be used to study ion transport between the two pools, for example, under normal and ischemic conditions (52). Unfortunately, these heavy-metal shift reagents are ultimately toxic and unsuitable for human or non-terminal animal studies in their present form.
Note that the mere existence of dual-component 23Na T2 values has long fueled speculation that each T2 component might be separately assigned to [Na]intra and [Na]extra (53,54). The hypothesis that each or either T2 component can be wholly assigned to intra- or extra-cellular sodium is presently unproven. Moreover, there is some evidence against it. This includes 23Na studies of frog heart that show that when the intracellular sodium signal determined from shift reagent experiments, is subtracted from the total signal, the remaining extra-cellular interstitial sodium remains biexponential with the same T2s (T2f≈1–2ms and T2s≈17ms; 33). Hence the conclusion that the extra-cellular “interstitium contains sodium relaxation times that are similar to the relaxation times found for intracellular sodium” (33). Similarly, studies of fresh rat skeletal muscle up to 24 hrs post-biopsy elicited no significant differences in relaxation components despite equalization of the intra- and extra-cellular sodium concentrations (32). The conclusion was that muscle sodium is “satisfactorily described as distributed in a single compartment” from the NMR standpoint (32). While frog heart and rat muscle are not mammalian heart muscle, observations that neither T2f and T2s nor their relative fractions change in acute reperfused MI vs. healthy canine myocardium measured in vivo despite a doubling of [Na]tissue, are consistent with the same conclusion (34). Thus, from the NMR standpoint, it seems that the view from the Na+ ion is somewhat similar from inside the cell, as it is from the outside.
This is not to say that [Na]intra, [Na]extra, or T2f, and T2s and their relative fractions can never change under various stresses or disease states. Changes in TSC (or [Na]tissue) in response to stress, ischemic disease, infarction, disorders affecting ion transport, therapeutic intervention due to ion pump failure, increased membrane permeability, and/or cell rupture will generally reflect changes in [Na]intra, if [Na]extra, is maintained in homeostasis by the blood pool. Changes in cellular volume Vintra, or vascular volume, due to edema, injury or even exercise would also affect TSC. While this renders 23Na MR sensitive to many cellular level disturbances, it also means that measurements of [Na]tissue alone may not permit resolution of those contributory factors in Eq. [1] that may be causal.
There is hope that newer triple-quantum filtered (TQF) 23Na MRI techniques might help distinguish intra- and extra-cellular cardiac sodium pools, but as in the case of T2f and T2s, support for a hypothesis that it could provide unambiguous resolution of [Na]intra and [Na]extra is presently lacking. Since there are presently no human cardiac TQF 23Na data, we will not address this here.
METHODS
23Na MRI pulse sequence
Table 2 shows that the 23Na tissue MR signal can have multiple contributing T2 components ranging from 0.5 to 33 ms. It is unclear to what extent the scatter in these values reflect real physiological variability, or just the difficulty and error in measuring them by 23Na MR. Certainly, the use of ultra-short 23Na MRI TEs (21,24,25,55–58) would avoid the uncertainty in T2 and minimize the SNR loss from T2 decay. It would also permit quantitative measurements of TSC. Indeed, the presence of the T2f component not only argues against spin-echo MRI (5,59), but also gradient-refocused echo sequences with TE delays of ≥3 ms (6,46,56). Ultra-short TE 23Na MRI pulse sequences and their associated reconstruction algorithms generally require customized programming that can present a hurdle to clinical studies.
The simplest approach to detecting and imaging the T2f component with ultra-short TE is to eliminate both the slice-selection and phase-encoding gradients with their associated delays, altogether. A three-dimensional (3D) projection reconstruction reminiscent of the original MRI experiment (60) fits this bill. Such a sequence comprises a short, hard excitation pulse, followed by a constant radial gradient that is reoriented in subsequent applications, to generate a 3D set of angular projections. To optimize the SNR, the projections can be incremented by an angle (111°) related to the “golden ratio” (61,24,27). By this means, TEs as short as 0.2–0.4ms are possible (57,62)–just short enough to catch a few time points of a T2f ~0.5ms signal (Table 2). The constant gradient radial projections do over-sample the central low-spatial resolution Fourier components, and under-sample the high spatial frequency components. This is not the most efficient trajectory for covering image k-space.
Spiral (25), and “twisted projection imaging” or TPI (56) are alternative k-space trajectories that have been used for human cardiac 23Na MRI. Spirals have been used widely in 1H MRI, while the TPI method is responsible for nearly all quantitative measurements of [Na] in humans (63). In TPI, the constant radial projection gradient is replaced by variable spiral-like k-space trajectories that uniformly sample image k-space in 3D, as illustrated in Fig. 1(a) (56). Combined with a 400µs hard excitation pulse, TEs of ~400µs are possible, measured to the center of the pulse, since 200µs must still be allowed for turning on the TPI gradient (34,35,56,58).
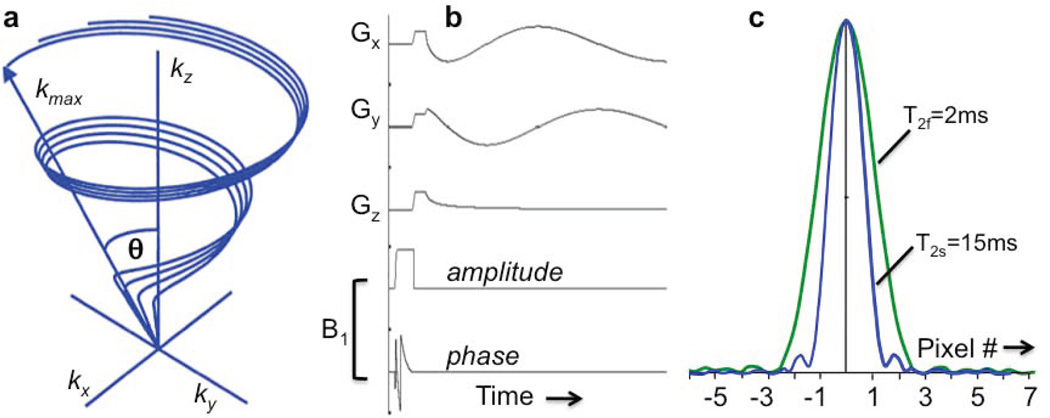
Figure 1.
(a) Partial k-space sampling scheme for twisted projection imaging (TPI). The maximum k forms a sphere of radius kmax, and the projections lie on a surface of a cone whose twist is adjusted to maintain critical sample density (56). (b) The TPI pulse sequence with adiabatic half passage excitation. The hard pulse of the original TPI sequence (56) is replaced by an amplitude (amp.) and phase modulated AHP pulse, but the gradient waveforms, Gx, Gy, and Gz, are the same, and depicted for one of the projections (21). (c) Simulated point spread function for T2f =2ms and T2s =15ms signal components excited by a TPI sequence with TE=0.36ms, a 31.3 kHz bandwidth, 12 ms readout, 22cm FOV, 44×44×44 array size, and Δx =5mm nominal isotropic spatial resolution (58). The T2s component has a full-width at half-maximum (fwhm) of about 1.5Δx (7.5 mm), while the fwhm of the T2f component is 2.4Δx (12.0 mm). (Adapted from Refs. 21,56, 58).
One significant problem with all sequences using hard pulses, is the effect of RF field (B1) inhomogeneity. This may arise, for example, when using surface coils for exciting the cardiac 23Na MR signal. The resultant spatially-varying excitation flip-angle (FA) could be addressed by calibrating the spatial B1 distribution, or by replacing the hard pulse with an adiabatic pulse to provide substantially B1-independent excitation (21). Presently, the most power-efficient adiabatic pulses are adiabatic half-passage (AHP; FA=90°) pulses whose TE period nominally commences at the end of the pulse (vs. the middle of the hard pulse). Use of AHP thus cuts TE by a further half pulse-length (21). A TPI sequence with AHP excitation is depicted in Fig. 1(b).
Of course, while the T2f signal may be detectable at the start of the acquisition window, it is long gone 2 or 3 T2f periods later, so that the spatial information corresponding to the k-space traversed towards the end of the acquisition window–the high spatial frequency components–is still not encoded into the T2f component. Consequently, the image point spread function (PSF) is different for T2f vs. T2s (58), depending on the acquisition parameters (BW, gradient strength, etc). The result can be a blurred 23Na image comprised of a low-resolution T2f image superimposed on a high-resolution T2s image. A comparison of human cardiac images acquired at TEs of 0.4ms and 3.6ms (62) might exemplify such an effect. If spatial resolution is more important than quantifying TSC, setting TE ≥3 ms should eliminate any blurring due to T2f. Fig. 1(c) depicts simulated PSFs for T2f =2 ms and T2s =15ms components excited by a TPI sequence with TE=0.36ms and 12ms readout (58).
For two-dimensional (2D) or restricted 3D 23Na MRI employing slab-selection, the duration of the slice-selection pulse can be cut using truncated selective excitation pulses, achieving TEs as small as 1–1.5ms (24,25). The cost of truncating slice selection is non-uniform slice profiles, which must be accounted for if quantification is the goal.
A final complication for cardiac 23Na MRI pulse sequencing is cardiac motion. In conventional cardiac 1H MRI this is addressed by electro-cardiographic (ECG) gating. If 23Na MRI acquisitions were prospectively gated at a TR equal to the heart-rate, almost the entire SNR advantage of sodium’s short T1 would be lost. Retrospective ECG-gating (24,27) wherein only those frames acquired during active cardiac contraction are omitted, or the acquisition of multiple frames during a timing window limited to a motion-quiet period of say ≤80% of the cardiac cycle (19,20,22,25,55), offer less SNR-costly alternatives. Yet another option is to acquire thousands of projections ungated and bin them into, say, a 10-phase cardiac cine stream (27).
Cardiac 23Na MRI coils
While 23Na MRI can be performed on clinical scanners equipped with broad-band MR transmit/receive capabilities, the limited availability of suitable detection coils and receiver hardware presents another hurdle for patient studies. The usual route after purchasing a 23Na MR channel for the clinical scanner and developing a 23Na MRI pulse sequence capability, is to build (15,21,46) or purchase (20,57) a custom set of 23Na transmit/receive coil systems tailored to the specific application.
The best detectors are those that provide the optimum SNR. From 1.5T to 7T, the 23Na MR frequency ranges from only 16.9 to 79 MHz–a regime ruled by conventional loop detectors. For cardiac 23Na MRI, the choice is invariably surface coils. The diameter of individual surface coils should approximately equal the depth of interest, say 10–15cm for the center of the heart (46). Phased-arrays of surface coils will extend the size of the region of optimum SNR, but require multi-channel 23Na MRI receiver hardware (including preamplifiers, transmit/receive switches, and MR receivers) and phased-array reconstruction, that may or may not be available to the scanner (23,26,46).
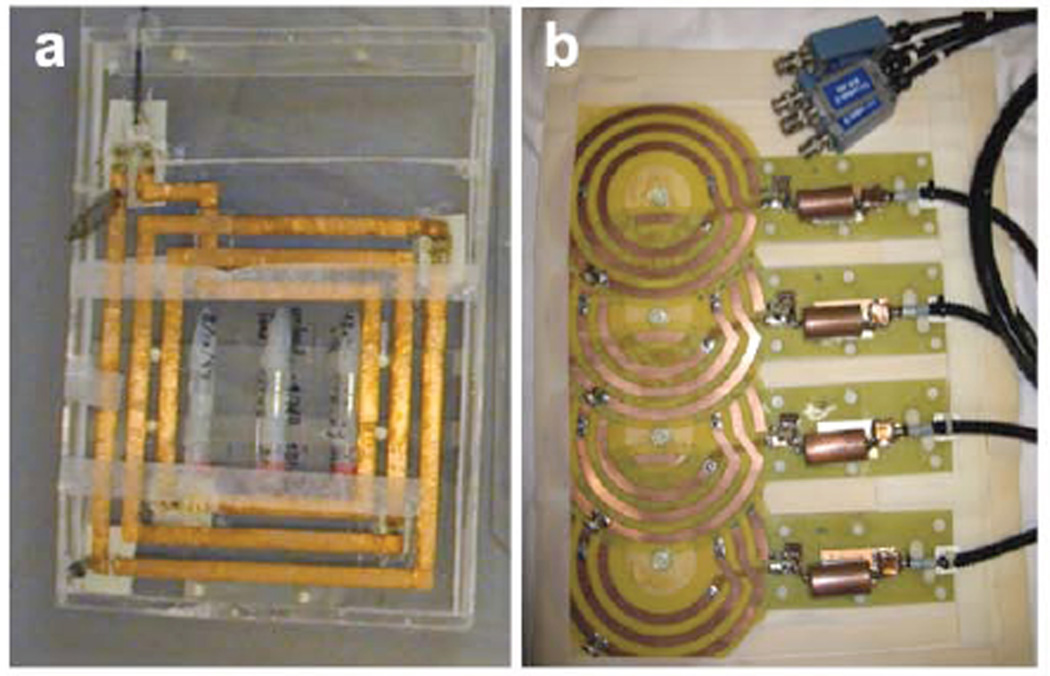
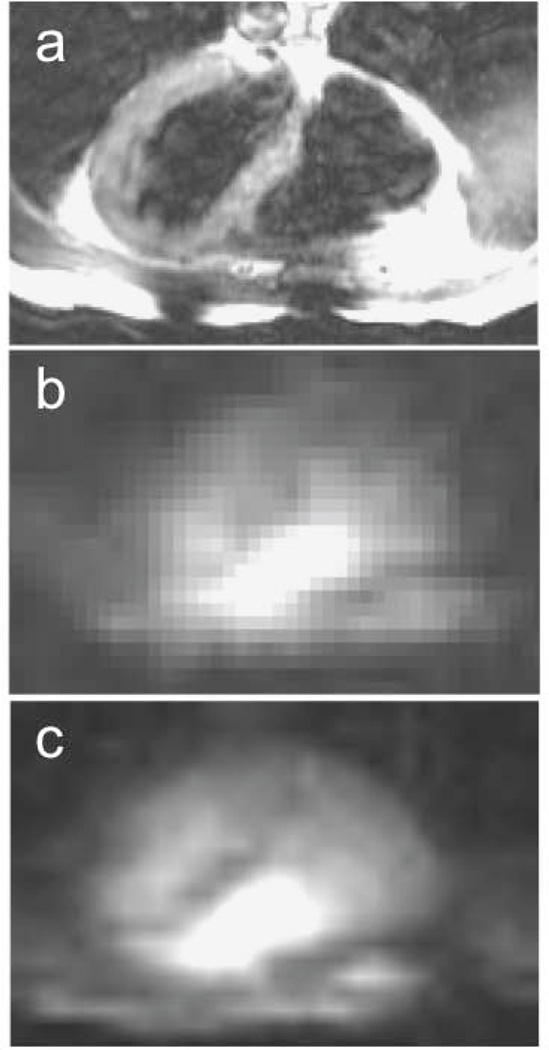
Photographs of custom surface and flexible phased-array 23Na MRI coils for human cardiac use at 1.5 T are shown in Fig. 2 (21,22,46). The low 23Na MR frequency at 1.5T necessitates the use of multiple windings and distributed tuning capacitance to achieve the desirable “sample-dominant noise” condition (18). The receiver coils can also be used for MR excitation. Optimized individual surface coils have intrinsically nonuniform B1s, which must be dealt with for quantitative work (e.g. with the aforementioned AHP pulses; 21). Phased-arrays require either multi-channel excitation (typically unavailable for human cardiac 23Na MRI), or a large separate transmit coil (46). Fig. 3 shows sodium images of the human heart acquired in 4 s and 200 s at 1.5T with the 4-channel cardiac phased-array in Fig. 2, and a separate 40cm surface transmit coil (46).
Figure 2.
Custom-built coils for human cardiac 23Na MRI at 1.5 Tesla (16.9 MHz). (a) Square 25×25 cm three-turn transmit/receive surface coil used for quantitative cardiac TSC studies employing adiabatic excitation (21). The coil has 3 embedded vials of saline gel to serve as sensitivity references, and a mount permitting exchange with a conventional cardiac 1H coil for cardiac MRI (21,22). (b) A 4-channel 23Na phased-array constructed from 15-cm, 3-turns coils for increased inductance at the lower 23Na frequency (46). The detectors were fabricated on 0.25-mm thick flexible printed circuit board, with a separate 40-cm square single-turn transmit coil for excitation.
Figure 3.
(a) Conventional 1H trans-axial image, and (b) corresponding single slice 23Na MRI of the human heart acquired with the phased-array in Fig. 2(b) using a slice-selective gradient refocused echo (GRE) sequence in 4s, and (c) 200s (array size, 32 × 32; resolution, 1 × 1 × 4 cm3; TR/TE= 33/5 ms; from Ref. 46).
RF power deposition
Another down-side of 23Na’s low gyromagnetic ratio (©) is that it requires 4-times the B1 for the same FA and duration, yet short pulses are needed to avoid T2f losses. In addition, the RF power deposited in biological tissue, measured as the specific absorption rate, SAR in W/kg, is proportional to [υB1]2 (64). Consequently, the SAR for a 23Na study done with the B1 required to produce the same FA, pulse length, and TR as a 1H MR study at a 4-fold higher MR frequency in the same magnet, is essentially the same, although RF penetration effects will be less for 23Na due to the lower 23Na RF (64). The benefit of faster scanning due to the short 23Na T1 may thus be limited in practice by potential RF heating concerns in vivo, especially as one moves to higher B0 in search of higher SNR.
Note also that surface coil excitation has a much greater potential for causing local heating than whole-body excitation due to the presence of high SAR gradients close to the surface coil. In addition, the MRI scanner’s inbuilt protections on RF power deposition typically overstate SAR (65). Because the cardiac 23Na coils are likely to be custom-built anyway (Fig. 2), the most prudent course for ensuring safety probably includes testing the coil set for heating using a body phantom of saline gel (23,66).
Quantification
In 23Na MRI signals are quantified as relative changes in signal intensity, or absolute concentration–TSC. Measuring changes in 23Na image intensity is subject to the confounding effects of T1, T2s and T2f relaxation, nonuniform B1, and nonuniform detection sensitivity, on reproducibility and the interpretation of any results. Generally, it is prudent to eliminate as many variables as possible (eg, FA variations, T1 saturation etc.) through experimental design and observing a rigorous protocol. Signal differences can be measured relative to adjacent uninvolved or healthy tissue in a sample region over which sensitivity and/or FA is essentially constant. While such changes cannot be conclusively attributed to changes in TSC, the 23Na MRI signal is often linearly correlated with tissue [Na], for example, as seen in MI (34,46, 67–69).
Absolute TSC at a location (x, y, z) can be measured from the ratio of the 23Na image signal intensity to that in the same location of a reference phantom of known concentration recorded with the same resolution and FA (21). Saline is a typical concentration reference. In general, the tissue and reference signals require corrections for T1 saturation and T2 decay, and for differences in the coil sensitivity (ϕ) that occur when the coil is loaded with the body vs. the reference phantom. The TSC in the tissue at (x,y,z) is given by:
| [2] |
Here, superscripts tissue and ref denote factors associated with sample and reference acquisitions; and F and E are the T1 saturation and T2 decay correction factors, respectively. The coil loading,ϕ, can be determined from the ratio of 23Na signals from a small phantom embedded in the coil, recorded during tissue and reference experiments (21). For a TPI or projection sequence employing TE ≤0.4ms, and TR >60ms, the relaxation corrections E and F approximate unity, so only the signals and loading factors need be measured.
An alternative to measuring TSC that avoids the separate reference acquisition, is to use an external concentration reference in the field-of-view of the 23Na acquisition, at some location, say (x1, y1, z1). Then, although loading is the same, ϕ must be replaced in Eq. (2) by the ratio, Ψ, of the coil sensitivity at (x1, y1, z1) to that at (x, y, z). Note that except for uniform detectors, Ψ =Ψ(x1, y1, z1, x, y, z) is not a scalar. The reference phantom can also be replaced by an internal reference of known [Na] that changes minimally between subjects. While the ratio of myocardial to ventricular blood 23Na signals has been reported as a potentially useful semi-quantitative measure (55), extending this to absolute TSC measurements with corrections for differences in spatial sensitivity and relaxation times has yet to be validated.
In the event that FA differs between the reference and tissue acquisitions, or that FA at the tissue differs from its value at the reference location, the signal and F terms in Eq. [2] will be modulated accordingly. In the simple case of TR>>T1, Eq. [2] is multiplied by the ratio [sin{α(x1, y1, z1)}/sin{α(x, y, z)}], where α is the FA at the two locations. For TR~T1, the correction would need to be modified further to reflect the FA dependence of the steady-state magnetization of a partial saturation experiment. The potential effects of nonuniform B1 and partial saturation effects on the accuracy of TSC measurements, are an argument for performing studies at TR>>T1 with constant FAs provided by separate large excite coils or adiabatic pulses (21,22).
Table 3 summarizes in vivo measurements of TSC in chest and heart likely to be encountered in a 23Na MRI study (21, 34, 35, 58, 70–73). Note that cardiac TSC measurements are prone to partial volume errors from voxels close to high-TSC blood (22,50). The canine 23Na MRI measurement of TSC was confirmed by post-mortem atomic absorption spectrophotometry (34).
Table 3.
Some in vivo 23Na MRI measurements of total sodium content in normal tissue.
| Tissue | n | [Na] mmol/kg wet wt | Ref. | MRI method |
|---|---|---|---|---|
| Human skeletal muscle | 10 | 28.4 ±3.6 | 35 | TPI |
| 5 | 26.2 ±3.3 | 58 | TPI | |
| 5 | 26 ±4 | 70 | 3DGRE | |
| Canine heart | 6 | 34.4 ±2.8 | 34 | TPI |
| Human heart | 10 | 43 ±4 | 21 | TPI |
| Human adipose tissue | 10 | 17 ±4 | 21 | TPI |
| 20 ±2 | 71 | TPI | ||
| Human ventricular blood | 10 | 79 ±8 | 21 | TPI |
| Human breast, fibrous | 9 | 28 ro | 71 | TPI |
| 19 | 33 roua | 72 | TPI | |
| 2 | 57 ±14 | 73 | 3DDR | |
Values are mean ±SD; TPI=twisted projection imaging; 3DGRE=three-dimensional (3D) gradient refocused echo; 3DDR=3D density-adapted radial acquisition.
Converted from mmoles/liter of wet tissue assuming a specific gravity of 1.04 (46).
CLINICAL AND PRE-CLINICAL RESEARCH
Cardiac 23Na MRI is presently limited to patients with chronic MI (19,20,22). The findings are that 23Na MRI shows areas of elevated myocardial signal that correlate with wall-motion abnormalities identified by 1H MRI in 30 subjects studied at 8 days and 6 months post-MI (19). An additional 10 patients studied on days 9, 14, and 90 post-MI revealed a decline in the level of sodium signal elevation from 39%, to 31% to 28% at the three times, respectively, in infarcted vs non-infarcted tissue (20). Another study measuring absolute TSC found 30% elevations in 20 patients with chronic MI measured ≥90 days post-onset (59 ±10 vs 45 ±5 µmol/g in non-MI and 43 ±4µmol/g in controls; 22). This study confirmed that changes in 23Na MR relaxation times were not a confounding factor in the two earlier studies (19,20) that reported changes in sodium signal levels in MI.
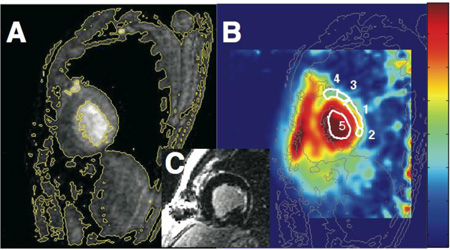
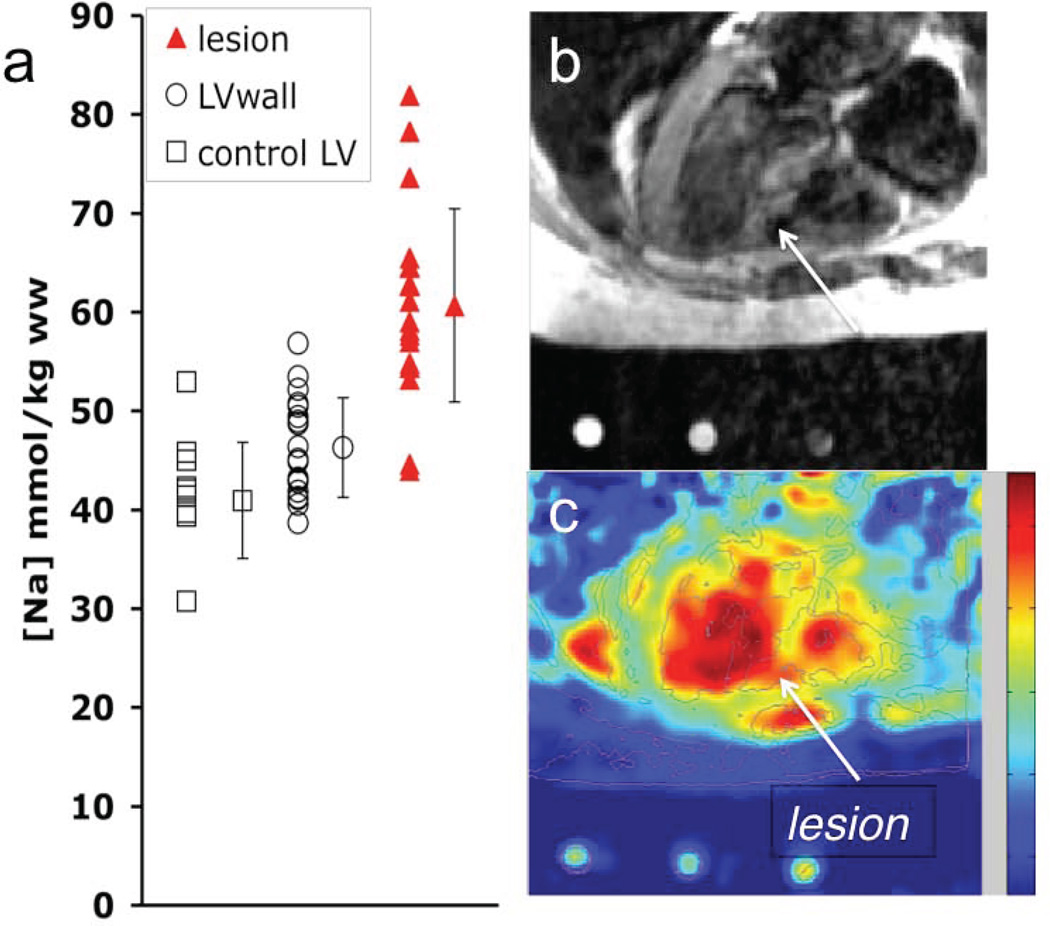
Fig. 4 shows TSC levels in normal, MI, and adjacent tissues in the cohort of 20 MI patients, and a 23Na image showing elevated TSC in a patient with a septal MI (22). This study also showed that the magnitude of the TSC increases in MI identified by contrast-enhanced 1H MRI, was unrelated to infarct age or size after 90 days post-onset, or to global ventricular function. An intermediate elevation in TSC was also reported in viable tissue immediately adjacent to the infarction (22). Although intracellular sodium is important for myocellular excitability, TSC measured at rest in these chronic MI patients was not closely associated with an increased risk of arrhythmia, as assessed by electro-physiologic testing for inducibility of monomorphic ventricular tachycardia (22).
Figure 4.
(a) TSC values in infarcted and remote myocardium in 20 MI patients, and in adjacent tissue in 11 of the patients. Vertical bars denote means ±SD. TSC is significantly increased in MI vs remote tissue (P <0.001). (b) Trans-axial gated 1H fast spin-echo image, and (c) corresponding 23Na image from a 3D TPI data set (TR/TE=85/0.4 ms; isotropic spatial resolution =6 mm) from a 61-year-old man with a remote septal MI (arrow). Color scale is proportional to TSC and includes B1 correction; LV=left ventricle. (Adapted from Ref. 22).
The result that sodium 23Na MR levels are elevated in MI is consistent with prior animal work (5,6,67–69). For example, canine studies of acute MI show TSC levels increasing by up to two-fold or more in the first 8–9 hrs following reperfusion (69). To the extent that the local extracellular sodium pool is buffered at or near 140mM by residual perfusion or diffusion routes, the elevated TSC observed in acute MI as it relates to Eq. [1] is attributable to either: (i) increased [Na]intra due to energetically compromised cellular Na+/K+ pump activity; or (ii) an increase in Vextra associated with edema, myocyte necrosis or intra-myocardial hemorrhage; or (iii) a combination of both.
Of these, insight on the role of the intracellular component is provided by 23Na MR spectroscopy studies of rodent hearts using shift reagents, which demonstrate huge increases of up to 5-fold in [Na]intra following 30 min of ischemia and dysfunction (74,75). Histochemical measurements suggest that the increase in the Vintra factor is, on the other hand, much smaller: up to about 13% in the first hour post-occlusion in isolated canine heart (3,4). On the extracellular side of Eq. [1], microscopic analysis using diffusible markers suggests that about 12–19% of the total myocardial tissue volume in rodents is extracellular (1,67,75), with microscopy evidence that Vextra is little-changed in acute reperfused MI (67). Increases in total tissue water content, W, are even more modest: 2–6% increases in reperfused canine MI up to 26 hours post-onset (2,4), or no significant difference from non-MI in acute (57) or chronic MI (49). Thus, these studies in aggregate, point to the bulk of the change in TSC following MI being borne by an increased [Na]intra, at least in the acute phase (69).
CONCLUSIONS AND FUTURE
Despite the 6000-fold SNR hit compared to 1H MRI, 23Na is the second most viable nucleus for performing routine human MRI in its natural endogenous form. Yet, cardiac applications remain quite limited. Undoubtedly a declining appetite for supporting non-1H nuclei by the scanner manufacturers has taken a toll: the specialized technical requirements of pulse sequences, detectors and image reconstruction can present insurmountable obstacles for many would-be clinical 23Na MRI researchers. But there are other issues as well. To be useful in a clinical setting, cardiac 23Na MRI needs to add value by providing functional information relating to diagnosis and/or prognosis that is not obtainable by regular 1H MRI or other more convenient means.
Because changes in the tissue 23Na MRI signal primarily reflect changes in intracellular sodium concentration and/or the extracellular volume fraction when the extra-cellular sodium is in homeostasis with the blood pool, 23Na MRI may be particularly sensitive to disorders affecting sodium-potassium pump function, channelopathies (54), as well as injuries or interventions that disrupt the extra-cellular space. Thus, while cardiac applications are presently limited to MI patients, future 23Na MRI studies could reveal altered myocardial TSC associated with sodium pump dysfunction in some cardiomyopthies (7–12). If serum sodium can independently predict outcomes in certain forms of heart failure (11), then TSC measured directly in the heart, might be even more outcome sensitive. Also, just as arm and leg exercise in healthy subjects causes TSC to increase in skeletal muscle (31,70,76)–due to hyperaemia and a net Na+ influx into the myocytes (1)–it is conceivable that exercise stress could elicit changes in cardiac TSC as well, which might find value in patients with symptoms of myocardial ischemia.
Of course, the potential for false positive detections of altered TSC in the myocardial wall is also high. The adjacent blood pool has twice the sodium; the myocardial wall is relatively thin compared to the spatial resolution of 23Na MRI, and is thinner still in dilated cardiomyopathy and chronic MI; while exercise tends to introduce motion artefacts. All too often, the main competition to 23Na MRI is 1H MRI which, in its many forms–including contrast enhancement, cine, functional tagging, etc.–is already a sensitive, high resolution diagnostic tool for probing cardiac function.
Acknowledgments
The 23Na MRI work in our laboratory was supported by US NIH grants R01 HL61695, the DW Reynolds foundation, and many fruitful collaborations with Ronald Ouwerkerk, Robert G Weiss, and Ray F Li. PAB is supported by the Russell H Morgan Professorship of the Johns Hopkins University Dept. of Radiology and grant 13GRNT17050100 from the American Heart Association.
Abbreviations
- TSC
tissue sodium concentration
- MI
myocardial infarction
- SNR
signal-to-noise ratio
- TE
echo-time
- BW
bandwidth
- FA
flip-angle
- TPI
twisted projection imaging
- PSF
point spread function
- AHP
adiabatic half passage
- ECG
electro-cardiographic
- T1
spin, lattice relaxation time
- T2
spin-spin relaxation time
- TR
pulse sequence repetition time
References
- 1.Barclay JA, Harley EJ, Houghton H. Electrolyte content of rat heart atria and ventricles. Circ Res. 1960;8:1264–1267. doi: 10.1161/01.res.8.6.1264. [DOI] [PubMed] [Google Scholar]
- 2.Jennings RB, Sommers HM, Kaltenbach JP, West JJ. Electrolyte alterations in acute myocardial ischemic injury. Circ Res. 1964;14:260–264. doi: 10.1161/01.res.14.3.260. [DOI] [PubMed] [Google Scholar]
- 3.Willerson JT, Scoles F, Mukherjee A, Platt M, Templeton GH, Fink GS, Buja LM. Abnormal myocardial fluid retention as an early manifestation of ischemic injury. Am J Pathol. 1972;87:159–188. [PMC free article] [PubMed] [Google Scholar]
- 4.Whalen DA, Hamilton DG, Ganote CE, Jennings RB. Effect of a transient period of ischemia on myocardial cells I. Effect in cell volume regulation. Am J Pathology. 1974;74(3):381–397. [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon PJ, Maudsley AA, Hilal SK, Simon HE, Cassidy F. Sodium nuclear magnetic resonance imaging of myocardial tissue of dogs after coronary artery occlusion and reperfusion. J Am Coll Cardiol. 1986;7:573–579. doi: 10.1016/s0735-1097(86)80467-3. [DOI] [PubMed] [Google Scholar]
- 6.Kim RJ, Lima JOAC, Chen EL, Reeder SB, Klocke FJ, Zerhouni EA, Judd RM. Fast 23Na magnetic resonance imaging of acute reperfused myocardial infarction: potential to assess myocardial viability. Circulation. 1997;95:1877–1885. doi: 10.1161/01.cir.95.7.1877. [DOI] [PubMed] [Google Scholar]
- 7.Steimle AE, Stevenson LW, Fonarow GC, Hamilton MA, Moriguchi JD. Prediction of improvement in recent onset cardiomyopathy after referral for heart transplantation. J Am Coll Cardiol. 1994;23(3):553–559. doi: 10.1016/0735-1097(94)90735-8. [DOI] [PubMed] [Google Scholar]
- 8.Hesse M, Kondo CS, Clark RB, Su L, Allen FL, Geary-Joo CTM, Kunnathu S, Severson DL, Nygren A, Giles WR, Cross JC. Dilated cardiomyopathy is associated with reduced expression of the cardiac sodium channel SCN5aA. Cardiovasc. Res. 2007;75:498–509. doi: 10.1016/j.cardiores.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Ge J, Sun S, Paajanen V, Wang S, Su C, Yang Z, Li Y, Wang S, Jia J, Wang K, Zou Y, Gao L, Wang K, Fan Z. Molecular and Clinical Characterization of a Novel SCN5A Mutation Associated With Atrioventricular Block and Dilated Cardiomyopathy. Circulation: Arrhythmia and Electrophysiology. 2008;1:83–92. doi: 10.1161/CIRCEP.107.750752. [DOI] [PubMed] [Google Scholar]
- 10.Bezzina CR, Remme CA. Dilated Cardiomyopathy due to Sodium Channel Dysfunction What Is the Connection. Circulation: Arrhythmia and Electrophysiology. 2008;1:80–82. doi: 10.1161/CIRCEP.108.791434. [DOI] [PubMed] [Google Scholar]
- 11.Vilas Boas LGC, Bestetti RB, Otaviano AP, Cardinalli-Neto A, Nogueira PR. Outcome of Chagas cardiomyopathy in comparison to ischemic cardiomyopathy. Internat J Cardiol. 2013;167:486–490. doi: 10.1016/j.ijcard.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 12.Coppini R, Ferrantini C, Yao L, Fan P, Del Lungo M, Stillitano F, Sartiani L, Tosi B, Suffredini S, Tesi C, Yacoub M, Olivotto I, Belardinelli L, Poggesi C, Cerbai E, Mugelli A. Late Sodium Current Inhibition Reverses Electromechanical Dysfunction in Human Hypertrophic Cardiomyopathy. Circulation. 2013;127:575–584. doi: 10.1161/CIRCULATIONAHA.112.134932. [DOI] [PubMed] [Google Scholar]
- 13.Cope FW. Nuclear magnetic resonance evidence for complexing of sodium ions in muscle. Proc Natl Acad Sci U S A. 1965;54:225–227. doi: 10.1073/pnas.54.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delayre JL, Ingwall JS, Malloy C, Fossel ET. Gated sodium-23 nuclear magnetic resonance images of an isolated perfused working rat heart. Science. 1981;212:935–936. doi: 10.1126/science.7233188. [DOI] [PubMed] [Google Scholar]
- 15.Ra JB, Hilal SK, Oh CH, Mun IK. In vivo magnetic resonance imaging of sodium in the human body. Magn Reson Med. 1988;7:11–22. doi: 10.1002/mrm.1910070103. [DOI] [PubMed] [Google Scholar]
- 16.Granot J. Sodium imaging of human body organs and extremities in vivo. Radiology. 1988;167:547–550. doi: 10.1148/radiology.167.2.3357970. [DOI] [PubMed] [Google Scholar]
- 17.Bottomley PA, Hart HR, Edelstein WA, Schenck JF, Smith LS, Leue WM, Mueller OM, Redington RW. Anatomy and metabolism of the normal human brain studied by magnetic resonance at 1.5 Tesla. Radiol. 1984;150:441–446. doi: 10.1148/radiology.150.2.6691099. [DOI] [PubMed] [Google Scholar]
- 18.Edelstein WA, Glover GH, Hardy CJ, Redington RW. The Intrinsic Signal-to-Noise Ratio in NMR Imaging. Magn Reson Med. 1986;3:604–618. doi: 10.1002/mrm.1910030413. [DOI] [PubMed] [Google Scholar]
- 19.Sandstede JJW, Pabst T, Beer M, Lipke C, Baurle K, Butter F, Harre K, Kenn W, Voelker W, Neubauer S, Hahn D. Assessment of Myocardial Infarction in Humans with 23Na MR Imaging: Comparison with Cine MR Imaging and Delayed Contrast Enhancement. Radiology. 2001;221:222–228. doi: 10.1148/radiol.2211001563. [DOI] [PubMed] [Google Scholar]
- 20.Sandstede JJW, Hillenbrand H, Beer M, Pabst T, Butter F, Machann W, Bauer W, Hahn D, Neubauer S. Time Course of 23Na Signal Intensity After Myocardial Infarction in Humans. Magn Reson Med. 2004;52:545–551. doi: 10.1002/mrm.20165. [DOI] [PubMed] [Google Scholar]
- 21.Ouwerkerk R, Weiss RG, Bottomley PA. Measuring Human Cardiac Tissue Sodium Concentrations Measured Using Surface Coils, Adiabatic Excitation and Twisted Projection Imaging With Minimal T2 Losses. J Magn Reson Imag. 2005;21:546–555. doi: 10.1002/jmri.20322. [DOI] [PubMed] [Google Scholar]
- 22.Ouwerkerk R, Bottomley PA, Solaiyappan M, Spooner A, Tomaselli G, Wu KC, Weiss RG. Tissue sodium concentration in myocardial infarction in humans: A quantitative 23Na MR imaging study. Radiology. 2008;248:88–96. doi: 10.1148/radiol.2481071027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.AJames JR, Lin C, Stark H, Dale BM, Bansal N. In: Teck Chwee Lim, Goh James CH., editors. Optimization and Characterization of Sodium MRI Using 8-channel 23Na and 2-channel 1H RX/TX Coil; 13th International Conference on Biomedical Engineering IFMBE Proceedings; 2009. pp. 138–141. http://link.springer.com/chapter/10.1007%2F978-3-540-92841-6_34) [Google Scholar]
- 24.Konstandin S, Schad LR. Two-Dimensional Radial Sodium Heart MRI Using Variable-Rate Selective Excitation and Retrospective Electrocardiogram Gating with Golden Angle Increments. Magnetic Resonance in Medicine 70. 2013:791–799. doi: 10.1002/mrm.24523. [DOI] [PubMed] [Google Scholar]
- 25.Gai ND, Rochitte C, Nacif MS, Bluemke DA. Optimized Three-Dimensional Sodium Imaging of the Human Heart on a Clinical 3T Scanner. Magnetic Resonance in Medicine. 2014 doi: 10.1002/mrm.25175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graess A, Ruehle A, Renz Winter L, Pfeiffer H, Ruff2 J, Rieger J, Niendorf T. Sodium imaging of the heart at 7T: design, evaluation and application of a four-channel transmit/receive surface coil array. J Cardiovasc Magn Reson. 2013;15(Suppl 1):W14. [Google Scholar]
- 27.Resetar A, Hoffmann SH, Graessel A, Waiczies H, Niendorf T, Nagel AM. Retrospectively gated CINE 23Na imaging of the heart at 7.0 T using density-adapted 3D projection reconstruction. Proc. Intl. Soc. Mag. Reson. Med. 2014;22:436. doi: 10.1016/j.mri.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Cope FW. Spin-echo nuclear magnetic resonance evidence for complexing of sodium ions in muscle, brain, and kidney. Biophys J. 1970;10:843–858. doi: 10.1016/S0006-3495(70)86339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hubbard PS. Nonexponential nuclear magnetic resonance by quadrupole interactions. J Chem Phys. 1970;53:985–987. [Google Scholar]
- 30.Berendsen HJC, Edzes HT. The observation and general interpretation of sodium magnetic resonance in biological material. Ann N Y Acad Sci. 1973;204:459–485. doi: 10.1111/j.1749-6632.1973.tb30799.x. [DOI] [PubMed] [Google Scholar]
- 31.Rooney W, Springer C. A comprehensive approach to the analysis and interpretation of the resonances of spins 3/2 from living systems. NMR Biomed. 1991;4:209–226. doi: 10.1002/nbm.1940040502. [DOI] [PubMed] [Google Scholar]
- 32.Chang DC, Woessner DE. Spin-echo study of 23 Na relaxation in skeletal muscle: evidence of sodium ion binding inside a biological cell. J Magn Reson. 1978;30:185–191. [Google Scholar]
- 33.Foy BD, Burnstein D. Interstitial sodium nuclear magnetic resonance relaxation times in perfused hearts. Biophys J. 1990;58:127–134. doi: 10.1016/S0006-3495(90)82358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Constantinides CD, Kraitchman DL, O’Brien K, Boada FE, Gillen J, Bottomley PA. Noninvasive quantification of total sodium concentrations in acute reperfused myocardial infarction using 23Na MRI. Magn Reson Med. 2001;46:1144–1151. doi: 10.1002/mrm.1311. [DOI] [PubMed] [Google Scholar]
- 35.Constantinides C, Gillen JS, Boada FE, Pomper MG, Bottomley PA. Human skeletal muscle: 23Na MR imaging and quantification-potential applications in exercise and disease. Radiology. 2000;216:559–568. doi: 10.1148/radiology.216.2.r00jl46559. [DOI] [PubMed] [Google Scholar]
- 36.Pabst T, Sandstede J, Beer M, Kenn W, Neubauer S, Hahn D. Sodium T2* relaxation times in human heart muscle. J Magn Reson Imaging. 2002;15:215–218. doi: 10.1002/jmri.10046. [DOI] [PubMed] [Google Scholar]
- 37.Pabst T, Sandstede J, Beer M, Kenn W, Neubauer S, Hahn D. Evaluation of sodium T1 relaxation times in human heart. J Magn Reson Imaging. 2003;17:726–729. doi: 10.1002/jmri.10310. [DOI] [PubMed] [Google Scholar]
- 38.Pettegrew JW, Woessner DE, Minshew NJ, Glonek T. Sodium-23 NMR analysis of human whole blood, erythrocytes, and plasma: chemical shift, spin relaxation and intracellular sodium concentration studies. J Magn Reson. 1984;57:185–196. [Google Scholar]
- 39.Perman WH, Turski PA, Houston LW, Glover GH, Hayes CE. Methodology of in vivo human sodium MR imaging at 1.5 T. Radiology. 1986;160:811–820. doi: 10.1148/radiology.160.3.3737922. [DOI] [PubMed] [Google Scholar]
- 40.Shinar H, Navon G. Sodium-23 NMR relaxation times in body fluids. Magn Reson Med. 1986;3:927–934. doi: 10.1002/mrm.1910030613. [DOI] [PubMed] [Google Scholar]
- 41.Winkler SS, Thomasson DM, Sherwood K, Perman WH. Regional T2 and sodium concentration estimates in the normal brain by sodium-23 MR imaging at 1.5T. J Comput Assist Tomogr. 1989;13:561–566. doi: 10.1097/00004728-198907000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Perman WH, Turski PA. Biomedical magnetic resonance imaging: principles, methodology and applications. New York, NY: VCH; 1984. pp. 421–468. [Google Scholar]
- 43.Buist JR, Deslauriers R, Saunders JK, Mainwood GW. 23Na and flame photometric studies of the NMR visibility of sodium in rat muscle. Can J Physiol Pharmacol. 1991;69:1663–1669. doi: 10.1139/y91-247. [DOI] [PubMed] [Google Scholar]
- 44.Kushnir T, Knubovet T, Itzchak Y, Eliav U, Sadeh M, Rapoport L, Kott E, Navon G. In vivo 23Na NMR studies of myotonic dystrophy. Magn Reson Med. 1997;37:192–196. doi: 10.1002/mrm.1910370209. [DOI] [PubMed] [Google Scholar]
- 45.Kemp-Harper R, Styles P, Wimperis S. Measurement of 23Na transverse relaxation in vivo: the flip-angle-independent experiment. J Magn Reson Ser B. 1995;109:223–228. [Google Scholar]
- 46.Lee RF, Giaquinto R, Constantinides, Souza S, Weiss RG, Bottomley PA. A broadband phased-array system for direct phosphorus and sodium metabolic MRI on a clinical scanner. Magn Reson Med. 2000;43:269–277. doi: 10.1002/(sici)1522-2594(200002)43:2<269::aid-mrm14>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 47.Snyder WS, Cook MJ, Nasset ES, Karhausen LR, Howells GP, Tipton IH. Re- port of the task group on reference man: International Commission on Radiological Protection No. 23. Oxford, England: Pergamon Press; 1984. pp. 26–32. 214–215, 280–285, 196. [Google Scholar]
- 48.Polimeni PI. Extracellular space and ionic distribution in the rat ventricle. Am J Physiol. 1974;227:676–683. doi: 10.1152/ajplegacy.1974.227.3.676. [DOI] [PubMed] [Google Scholar]
- 49.Bottomley PA, Weiss RG. Noninvasive magnetic resonance detection of creatine depletion in non-viable infarcted myocardium. Lancet. 1998;351:714–718. doi: 10.1016/S0140-6736(97)06402-7. [DOI] [PubMed] [Google Scholar]
- 50.Judd RM, Resar JR, Yin FC. Rapid measurements of diastolic intra-myocardial vascular volume. Am J Physiol. 1993;265:H1038–H1047. doi: 10.1152/ajpheart.1993.265.4.H1038. [DOI] [PubMed] [Google Scholar]
- 51.Constantinides CD, Rogers J, Herzka D, Bolar D, Boada FE, Kraitchman DL, Bottomley PA. Superparamagnetic iron oxide MION as a potential contrast agent for 23Na MRI in myocardial infarction. Magn Reson Med. 2001;46:1164–1168. doi: 10.1002/mrm.1313. [DOI] [PubMed] [Google Scholar]
- 52.van Emous JF, van Echteld CJA. Changes of intracellular sodium T2 relaxation times during ischemia and reperfusion in isolated rat hearts. Magn Reson Med. 1998;40:679–683. doi: 10.1002/mrm.1910400505. [DOI] [PubMed] [Google Scholar]
- 53.Narayana PA, Kulkarni MV, Mehta SD. NMR of 23Na in biological systems. In: Partain CL, Price RR, Patton JA, Kulkarni MV, Everette JA Jr, editors. Magnetic resonance imaging. 2nd. Vol. 2. Philadelphia, Pa: Saunders: physical principles and instrumentation; 1998. pp. 1553–1563. [Google Scholar]
- 54.Weber MA, Nielles-Vallespin S, Huttner HB, Wohrle JC, Jurkat-Rott K, Lehmann-Horn F, Schad LR, Kauczor HU, Essig M, Meinck HM. Evaluation of Patients with Paramyotonia at 23Na MR Imaging during Cold-induced Weakness. Radiol. 2006;240:489–500. doi: 10.1148/radiol.2401050737. [DOI] [PubMed] [Google Scholar]
- 55.Pabst T, Sandstede J, Beer M, Kenn W, Greiser A, von Kienlin M, Neubauer S, Hahn D. Optimization of ECG-triggered 3D 23Na MRI of the human heart. Magn Reson Med. 2001;45:164–166. doi: 10.1002/1522-2594(200101)45:1<164::aid-mrm1022>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 56.Boada FE, Gillen JS, Shen GX, Chang SY, Thulborn KR. Fast three dimensional sodium imaging. Magn Reson Med. 1997;37:706–715. doi: 10.1002/mrm.1910370512. [DOI] [PubMed] [Google Scholar]
- 57.Nielles-Vallespin S, Weber MA, Bock M, Bongers A, Speier P, Combs SE, Wohrle J, Lehmann-Horn F, Essig M, Schad LR. 3D radial projection technique with ultrashort echo times for sodium MRI: Clinical applications in human brain and skeletal muscle. Magn Reson Med. 2007;57:74–81. doi: 10.1002/mrm.21104. [DOI] [PubMed] [Google Scholar]
- 58.Lu A, Atkinson IC, Claiborne TC, Damen FC, Thulborn KR. Quantitative Sodium Imaging With a Flexible Twisted Projection Pulse Sequence. Magn Reson Med. 63:1583–1593. 2010. doi: 10.1002/mrm.22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ra JR, Hilal SK, Cho ZH. A method for in vivo MR imaging of the short T2 component of sodium-23. Magn Reson Med. 1986;3:296–302. doi: 10.1002/mrm.1910030213. [DOI] [PubMed] [Google Scholar]
- 60.Lauterbur PC. Image Formation by Induced Local Interactions: Examples Employing Nuclear Magnetic Resonance. Nature. 1973;242:190–191. [PubMed] [Google Scholar]
- 61.Winkelmann S, Schaeffter T, Koehler T, Eggers H, Doessel O. An Optimal Radial Profile Order Based on the Golden Ratio for Time-Resolved MRI. IEEE Trans Med Im. 2007;26:68–76. doi: 10.1109/TMI.2006.885337. [DOI] [PubMed] [Google Scholar]
- 62.Jerecic R, Bock M, Wacker C, Bauer W, Schad LR. 23Na-MRI of the human heart using a 3D radial projection technique. Biomed Tech. 2002;47(Suppl 1/1):458–459. doi: 10.1515/bmte.2002.47.s1a.458. [DOI] [PubMed] [Google Scholar]
- 63.Bottomley PA. Sodium MRI in Man: Technique Findings. In: Harris RK, Wasylishen RE, editors. Encyclopedia of Magnetic Resonance. John Wiley: Chichester; 2012. eMagRes, Vol 1: 353–366. DOI 10.1002/9780470034590.emrstm1252. [Google Scholar]
- 64.Bottomley PA, Andrew ER. RF magnetic field penetration, phase-shift and power dissipation in biological tissue: Implications for NMR imaging. Phys Med Biol. 1978;23:630–643. doi: 10.1088/0031-9155/23/4/006. [DOI] [PubMed] [Google Scholar]
- 65.El-Sharkawy AEM, Qian D, Bottomley PA, Edelstein WA. A Multichannel, Real-Time MRI RF Power Monitor for Independent SAR Determination. Med Phys. 2012;39:2334–2341. doi: 10.1118/1.3700169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.El-Sharkawy AEM, Gabr RE, Schär M, Weiss RG, Bottomley PA. Quantification of human high-energy phosphate metabolite concentrations at 3T with partial volume and sensitivity corrections. NMR Biomed. 2013;26:1363–1371. doi: 10.1002/nbm.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim RJ, Judd RM, Chen EL, Fieno DS, Parish TB, Lima JAC. Relationship of elevated 23Na magnetic resonance image intensity to infarct size after acute reperfused myocardial infarction. Circulation. 1999;100:185–192. doi: 10.1161/01.cir.100.2.185. [DOI] [PubMed] [Google Scholar]
- 68.Constantinides C, Weiss RG, Lee R, Bolar D, Bottomley PA. Restoration of low resolution metabolic images with a priori anatomic information: 23Na MRI in myocardial infarction. Magn Reson Imaging. 2000;18:461–471. doi: 10.1016/s0730-725x(99)00145-9. [DOI] [PubMed] [Google Scholar]
- 69.Rochitte CE, Kim RJ, Hillenbrand HB, Chen EL, Lima JAC. Microvascular integrity and the time course of myocardial sodium accumulation after acute infarction. Circ Res. 2000;87:648–655. doi: 10.1161/01.res.87.8.648. [DOI] [PubMed] [Google Scholar]
- 70.Bansal N, Szczepaniak L, Ternullo D, Fleckenstein JL, Malloy CR. Effect of exercise on 23Na MRI and relaxation characteristics of the human muscle. J Magn Reson Imag. 2000;11:532–538. doi: 10.1002/(sici)1522-2586(200005)11:5<532::aid-jmri9>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 71.Jacobs MA, Ouwerkerk R, Wolff AC, Stearns V, Bottomley PA, Barker PB, Argani P, Khouri N, Davidson NE, Bhujwalla Z, Bluemke DA. Multiparametric and multinuclear magnetic resonance imaging of human breast cancer: current applications. Technology in Cancer Research & Treatment. 2004;3:543–550. doi: 10.1177/153303460400300603. [DOI] [PubMed] [Google Scholar]
- 72.Ouwerkerk R, Jacobs MA, Macura KJ, Wolff AC, Stearns V, Mezban SD, Khouri NF, Bluemke DA, Bottomley PA. Elevated Tissue Sodium Concentration in Malignant Breast Lesions Detected With Noninvasive 23Na MRI. Breast Cancer Research and Treatment. 2007;106:151–160. doi: 10.1007/s10549-006-9485-4. [DOI] [PubMed] [Google Scholar]
- 73.Meike Danisch M, Kalayciyan R, Wetterling F, Schad LR. Bilaterale 23Na-MR-Bildgebung der Mamma und Quantifizierung der Natriumkonzentration. Z. Med. Phys. 2014;14:65–72. doi: 10.1016/j.zemedi.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 74.Malloy CR, Buster DC, Castro MMCA, Geraldes CFGC, Jeffrey FMH, Sherry AD. Influence of global ischemia on intracellular sodium in the perfused rat heart. Magn Reson Med. 1990;15:33–44. doi: 10.1002/mrm.1910150105. [DOI] [PubMed] [Google Scholar]
- 75.van Echteld JA, Kirkels JH, Eijgelshoven MHJ, van der Meer P, Ruigrok TJC. Intracellular sodium during ischemia and calcium-free perfusion: a 23Na NMR study. J Mol Cell Cardiol. 1991;23:297–307. doi: 10.1016/0022-2828(91)90066-u. [DOI] [PubMed] [Google Scholar]
- 76.Ouwerkerk R, Lee RF, Bottomley PA. Dynamic changes in sodium levels in human exercising muscle measured with 23Na MRI. Proc Intl Soc Mag Reson Med. 1999;3:1530. [Google Scholar]