Significance
Human immunodeficiency viruses (HIV) have developed strategies to interfere with DNA repair in host cells. Some DNA repair pathways represent restriction mechanisms that counteract the virus as soon as it penetrates into the host cell, before the establishment of an interferon response. Here we identify helicase-like transcription factor (HLTF) as a new protein degraded by the viral protein R (Vpr) from HIV-1. HLTF mediates the repair of stalled replication forks to bypass DNA lesions and ensure genome integrity. HLTF is degraded early after Vpr delivery to T lymphocytes or macrophages that represent relevant target cells for HIV. The discovery of HLTF as a DNA repair protein degraded by Vpr in infected cells paves the way for novel unexpected restriction mechanisms.
Keywords: HIV, restriction factor, DNA repair, Vpr target, SILAC
Abstract
Viruses often interfere with the DNA damage response to better replicate in their hosts. The human immunodeficiency virus 1 (HIV-1) viral protein R (Vpr) protein has been reported to modulate the activity of the DNA repair structure-specific endonuclease subunit (SLX4) complex and to promote cell cycle arrest. Vpr also interferes with the base-excision repair pathway by antagonizing the uracil DNA glycosylase (Ung2) enzyme. Using an unbiased quantitative proteomic screen, we report that Vpr down-regulates helicase-like transcription factor (HLTF), a DNA translocase involved in the repair of damaged replication forks. Vpr subverts the DDB1–cullin4-associated-factor 1 (DCAF1) adaptor of the Cul4A ubiquitin ligase to trigger proteasomal degradation of HLTF. This event takes place rapidly after Vpr delivery to cells, before and independently of Vpr-mediated G2 arrest. HLTF is degraded in lymphocytic cells and macrophages infected with Vpr-expressing HIV-1. Our results reveal a previously unidentified strategy for HIV-1 to antagonize DNA repair in host cells.
In addition to their role in maintaining genome integrity, DNA repair proteins participate in other cellular processes including innate immune signaling (1, 2). Immunodeficiency diseases may arise from defects in DNA helicases or translocases involved in the repair of DNA replication forks, as demonstrated, for instance, in Schimke immune-osseous dysplasia (SIOD) (2). Another example is provided by Aicardi–Goutières syndrome (AGS), in which the overproduction of type I interferon (IFN) is associated with mutations of proteins involved in DNA synthesis and repair, namely SAM domain and HD domain-containing protein 1 (SAMHD1), ribonuclease H2 (RNase H2), and three prime repair exonuclease 1 (Trex1) (3).
Cross-talk between the HIV and DNA repair pathways occurs at different steps of the virus life cycle, including reverse transcription, integration, and sensing of viral nucleic acids (4). In this context, the Vpr protein, expressed by both HIV-1 and HIV-2/simian immunodeficiency virus (SIV) sooty mangabey (smm) lineages, has drawn much attention. Vpr is not required for infection of most cell lines or primary CD4+ T cells (5–7). A replication defect for vpr-deleted viruses has been reported in dendritic cells and macrophages, with important donor-to-donor variability (6, 8–11). It was recently suggested that Vpr favors infection of macrophages by counteracting a restriction factor targeting Env expression and viral release (12). Vpr is also necessary for efficient cell-to-cell spread of HIV-1 from macrophages to CD4+ T lymphocytes (13). Vpr plays an important role in vivo. SIVMACΔvpr viruses rapidly revert to a WT version when injected in rhesus macaques (14). A similar reversion has been observed in a laboratory worker accidentally contaminated with a vpr-deficient strain of HIV-1 (15, 16). Several studies also reported mutations in the vpr gene in long-term nonprogressor (LTNP) patients (17–20). Several lines of evidence indicate that Vpr interferes with DNA repair pathways (21). First, the best renowned activity of Vpr, its ability to mediate a G2 arrest of the cell cycle, depends on the activation of the ATR-mediated (ATR: ataxia telangiectasia mutated and Rad3 related) DNA damage response (22). G2 arrest requires Vpr binding to DCAF1, an adaptor of the Cul4A-DDB1 ubiquitin ligase, which is involved in DNA repair in noninfected cells (23–29). More recently, Vpr has been shown to activate the SLX4 complex (SLX4com) with the help of DCAF1 (21, 30). SLX4com associates with several endonucleases, including Mus81, to coordinate the repair of specific replication-born double strand breaks (DSBs) and collapsed replication forks (31–33). It has been proposed that Vpr triggers replication stress and G2 arrest through inappropriate activation of SLX4com (30). This activation would lead to the elimination of viral DNA and, subsequently, virus escape from immune sensing. Vpr also recruits uracil DNA glycosylase (Ung2), an enzyme that prevents mutagenesis by eliminating uracil from DNA molecules, thereby initiating the base-excision repair (BER) pathway (34, 35). Vpr targets Ung2 for degradation through hijacking Cul4A-DDB1 (36). Ubiquitin ligases act on several substrates, which led us to speculate that Vpr may target additional unknown host proteins for proteasomal degradation.
The DNA damage tolerance pathway (DDT) allows stalled replication forks to bypass DNA lesions, such as gaps or DSBs, both in S and G2 phases (37, 38). In response to DNA damage or replicative stress, proliferating cell nuclear antigen (PCNA) is monoubiquitylated by Rad6/Rad18, leading to the recruitment of translesion synthesis polymerases. PCNA can be further polyubiquitinated by the budding yeast Rad5 ubiquitin ligase or its human orthologs helicase-like transcription factor (HLTF) and SNF2 histone linker PHD finger RING finger helicase (SHPRH) (37, 38). By ensuring the completion of S-phase following DNA damage, Rad5/HLTF/SHPRH contribute to genome integrity. HLTF is a DNA-binding protein (39–41) first described as a transcription factor (42, 43), but later authenticated as a protein involved in DNA repair, in tumor suppression, and in the early stages of carcinogenesis (44–47). Although both HLTF and SHPRH are able to polyubiquitinate PCNA, they do not act redundantly (48). HLTF facilitates fork reversal activity but also DNA strand invasion and formation of a d-loop structure in an ATP-independent manner (49–51). Furthermore, HLTF and SHPRH might not be only dedicated to the DDT pathway but more generally involved in DNA repair (48).
Here, we undertook a quantitative proteomic approach, based on a stable-isotope labeling by amino acids in cell culture (SILAC) strategy coupled with LC-MS/MS, to describe global changes in the cellular landscape under Vpr treatment (52). We used the property of Vpr to be incorporated into virions and virus-like particles (VLPs) (53, 54) to deliver the protein to target cells. We report that HIV-1 Vpr induces the early degradation of HLTF in primary cellular targets of HIV and analyzed the underlying molecular mechanisms.
Results
HLTF Is Down-Regulated by HIV-1 Vpr in a Proteasome-Dependent Manner.
We first examined which cellular proteins are modulated on HIV-1 Vpr delivery in HeLa cells. These cells represent a convenient model to perform a SILAC analysis, and Vpr is known to be active in HeLa cells, promoting, for instance, cell cycle arrest (26). To this aim, VLPs containing WT Vpr or a G2 arrest-defective Vpr mutant (Vpr S79A), both tagged with an HA epitope, or Vpr-negative (empty) VLPs were delivered to HeLa cells stably labeled respectively with light, medium, or heavy isotopes (Fig. S1A) (55). Using VLPs enabled us to focus on cellular changes that may occur without de novo Vpr expression. The experiment was performed in pseudoduplicate (S1 and S2) by switching VLPs and isotopes (Fig. S1A). The presence of Vpr in target cells was confirmed by Western blot, with similar levels of WT and Vpr S79A being detected (Fig. S1B). As expected, WT Vpr, but not the Vpr S79A mutant, induced G2 arrest (Fig. S1C) (55). The efficiency of the cell cycle block was modest because cells were harvested 12 h after Vpr delivery, to focus on early events (Fig. S1C). A total of 2,196 and 2,272 proteins were detected by MS, respectively, in S1 and S2 nuclear fractions; among them, 1,572 and 1,601 proteins could be reliably quantified. The levels of most of these proteins (82% in S1; 92% in S2) were not affected by WT Vpr relative to the control (empty VLPs). About 10% of the proteins showed variations in quantity of at least 20% with only 33 proteins reproducibly found in S1 and S2 (Fig. S1D). Most of these proteins were stabilized or enhanced by Vpr (Fig. S1D) and only eight had reduced levels. HLTF, detected with 13 peptides and with a reduction of more than 60% of its relative quantity, appeared as a preferential cellular target of Vpr. Vpr S79A also affected HLTF levels but less than WT Vpr (Fig. S1E). Interestingly, most of the proteins stabilized by WT but not by S79A Vpr are known to be up-regulated in the G2 phase of the cell cycle (Cyclin B/CCNB1, Aurora kinases A/AURKA and B/AURKB, inner centromere protein (INCENP), Annilin/ANLN, or Polo kinase 1/PLK1, for example; Fig. S1E). The presence of this G2 arrest protein signature indirectly validates our strategy. Taken together, these data indicate that SILAC represents a powerful tool to study rapid large-scale proteomic changes in the cellular environment induced by HIV-1 Vpr, as has been recently demonstrated for other HIV proteins (56). Of note, we also validated our SILAC procedure with the observation that SAMHD1 levels were strongly decreased on delivery of HIV-2/SIVsmm Vpx protein as expected (57, 58).
Fig. S1.
Setup and results of the SILAC experiment. (A) Experimental procedure for SILAC-based strategy. HeLa cells were cultured in medium containing light, medium, or heavy isotopes for 30 d. Cells were then transduced for 2 h with empty VLP, VLP containing Vpr S79A or Wt Vpr. Two independent biological replicates were performed named Silac (S1) and Switch (S2), in which isotopic conditions were inverted. At 12 h, treated cells were mixed in 1:1:1 ratio, separated into cytoplasmic and nuclear fractions, and subjected to proteomics experiments. (B) Vpr expression in HeLa cells treated with the VLP used for SILAC. HA-Vpr expression level was checked by Western blot. (C) WT Vpr-containing VLP used for SILAC trigger a G2 arrest. An aliquot of cells used for SILAC was fixed and then stained with propidium iodide, and DNA content was monitored by flow cytometry. The histogram displays the ratio between cells in G2/M and cells in G1 phases. (D) Identification of a new Vpr target by SILAC. The values were normalized taking as 100% the mock condition treated with empty VLPs. Two experiments were performed: Silac (S1) and Switch (S2), the values displayed for down- and up-regulated proteins by HIV-1 Vpr are the means of S1 and S2 ± SD. Only proteins with at least 20% variation are shown. *Group of peptides shared by several proteins. (E) Representation of significantly down- and up-regulated proteins by HIV-1 VprS79A compared with the mock condition (empty VLP). The values displayed for down- and up-regulated proteins by HIV-1 Vpr are the means of S1 and S2 ± SD. Only proteins with at least 20% variation are shown.
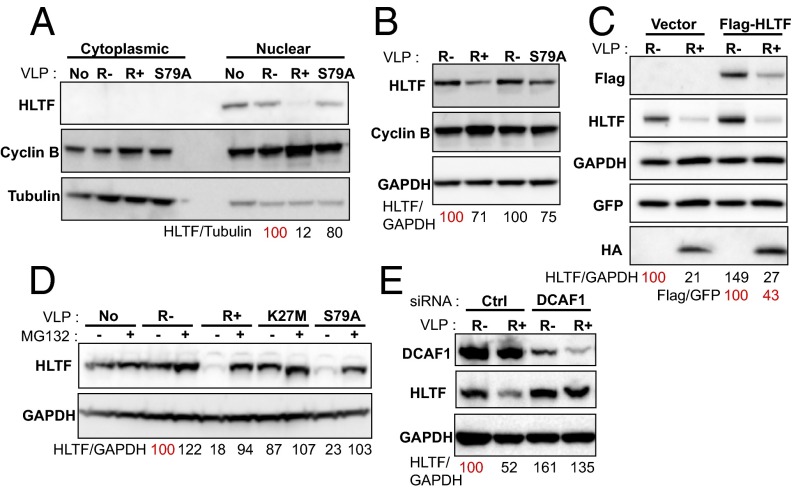
Western blot analysis of the nuclear fraction of HeLa cells that had been incubated with VLPs containing WT Vpr confirmed the HLTF steady-state amount reduction together with cyclin B/CCNB1 stabilization (Fig. 1A). HLTF was not detected in the cytoplasmic fraction (Fig. 1A). HLTF down-regulation was also observed in Jurkat T cells similarly treated with VLPs (Fig. 1B). The G2 arrest-defective Vpr S79A mutant also decreased HLTF levels, but to a lesser extent than WT Vpr (Fig. 1 A, B, and D), supporting our SILAC results. A Flag-tagged HLTF expressed under the control of a CMV promoter was also down-regulated by Vpr, suggesting that Vpr does not reduce HLTF expression through transcriptional modulation of the HLTF promoter (Fig. 1C, Flag panel for exogenous HLTF and HLTF panel for total HLTF).
Fig. 1.
HIV-1 Vpr down-regulates HLTF in a DCAF1-dependent manner. (A) HLTF expression in HeLa cells is down-regulated by VLP-encapsidated WT HIV-1 Vpr. Cells were transduced with the VLP used for SILAC. After 24 h, cells were harvested and fractioned. Protein expression was analyzed by Western blotting. (B) HLTF expression in Jurkat T cells is down-regulated by VLP-encapsidated WT HIV-1 Vpr. Cells were treated 48 h with VLP and lysed, and whole cell extracts were analyzed by Western blot. (C) Endogenous and exogenous HLTF expression levels are reduced by VLP-encapsidated WT HIV-1 Vpr. HeLa cells were cotransfected with a vector expressing Flag-HLTF or an empty vector together with a transfection control vector encoding the GFP (ratio10:1). Twenty-four hours after transfection, cells were transduced with the VLP and were harvested 24 h later. Protein expression was analyzed by Western blotting. (D) HLTF depletion induced by HIV-1 Vpr is proteasome-dependent. HeLa cells were treated with or without MG132 during 6 h after VLP incubation. Cells were transduced with the different VLP: empty VLP (R−), VLP containing wt Vpr (R+), or VLP containing Vpr K27M or Vpr S79A. (E) Vpr hijacks DCAF1 to mediate HLTF degradation. HeLa cells were treated with siRNA Control or siRNA against DCAF1 for 24 h. Cells were then transduced with the VLP and harvested 24 h later. Protein expression was analyzed by Western blotting. In each panel, quantification was performed: ratios between signals were calculated relative to a 100% reference indicated in red. All Western blots are representative of three independent experiments except B, which corresponds to a direct analysis of cells used for the SILAC.
We then asked whether HLTF down-regulation was proteasome dependent. To this aim, we incubated cells with MG132, a proteasome inhibitor. HLTF down-regulation in the presence of WT or S79A Vpr was inhibited by MG132 (Fig. 1D). Of note, a second G2 arrest-defective Vpr mutant, Vpr K27M, was unable to decrease HLTF levels (Fig. 1D). Furthermore, knockdown by siRNA of the DCAF1 ubiquitin ligase adaptor, previously shown to be hijacked by Vpr, inhibited Vpr-mediated HLTF down-regulation (Fig. 1E). Altogether, these results support a model in which HIV-1 Vpr uses the Cul4A-DDB1 ubiquitin ligase through DCAF1 binding to induce the proteasomal degradation of HLTF.
HLTF Degradation Occurs Independently from Vpr-Mediated G2 Arrest in HeLa Cells.
HLTF is an enzyme that stabilizes and repairs stalled replication forks and controls G2/M transition (49, 59, 60). Considering HLTF activities led us to investigate its role in Vpr-mediated G2 arrest. In an experiment in which HLTF levels were monitored over time, we observed its down-regulation 6 h after VLP treatment (Fig. 2A). The effect of Vpr on G2 cell cycle arrest was modest at 6 h and increased over time (Fig. 2B). Rad18, a protein that interacts with HLTF at the replication fork, was not affected by Vpr (Fig. 2A) (48). We then examined the effect of Vpr at earlier time points. Strikingly, down-regulation of HLTF was detected as soon as 30 min after VLP treatment (Fig. 2C, Upper) and intensified over time from 0 to 4 h (Fig. 2C). An increase in G2 arrested cell number also started to be detected at 4 h after VLP treatment (Fig. 2D). Vpr S79A induced HLTF degradation, but not as efficiently as Vpr WT as previously mentioned (Figs. 1 and 2C), with 23% and 31% HLTF remaining with the WT and mutant protein, respectively, 4 h after VLP addition. HLTF levels did not significantly changed from 0 to 4 h in the presence of Vpr K27M, as already observed in Fig. 1E. Thus, Vpr-mediated HLTF degradation is unlikely a consequence of Vpr-mediated G2 arrest but rather precedes this arrest. Furthermore, the partial ability of the G2 arrest-defective Vpr S79A mutant to induce HLTF degradation suggests that a block in the cell cycle is not a prerequisite for the modulation of HLTF expression.
Fig. 2.
HLTF degradation induced by HIV-1 Vpr precedes G2/M arrest. (A) Kinetic of HLTF disappearance. HeLa cells were transduced with empty VLP or WT Vpr VLP for 2 h. Cells were harvested 6, 12, 24, and 48 h after VLP treatment, and whole cell lysates were analyzed by Western blot. Quantification was performed: ratios between the HLTF and the GAPDH signals were calculated relative to a 100% reference indicated in red. The Western blot is representative of two independent experiments. (B) Kinetic of Vpr-mediated G2 arrest. Cell cycle analysis after VLP treatment, same time points as in A. (C) Short time kinetic of HLTF disappearance. HeLa cells were transduced with empty VLP (R−), VLP containing WT Vpr (R+), or Vpr mutants K27M or S79A. Cells were harvested at time 0 min, 30 min, 2 h, and 4 h after the 2-h VLP treatment, and whole cell lysates were analyzed by Western blot as in A. Quantification was performed as in A. (D) HLTF degradation precedes Vpr-mediated cell cycle arrest. Cell cycle analysis performed after VLP treatment at the indicated time points (same as in C) for the 6-h time point: 6(−) indicates with no MG132 treatment and 6(+) with MG132 all along the kinetic.
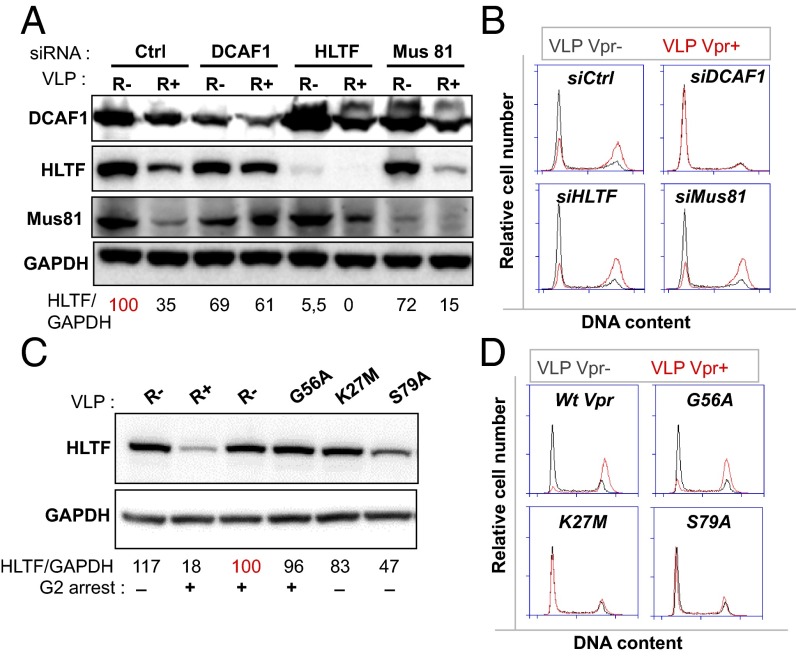
Vpr triggers the degradation of the SLX4-associated Mus81 endonuclease, although conflicting results have been reported regarding the link between Vpr-mediated Mus81 degradation and G2 arrest (30, 61). We confirmed that Vpr degrades both Mus81 and HLTF and that this degradation required DCAF1 (Fig. 3A). In addition, silencing of HLTF did not inhibit Mus81 degradation and, inversely, silencing of Mus81 did not prevent HLTF degradation, suggesting that the two events occur independently of each other (Fig. 3A). Although DCAF1 siRNA inhibited Vpr-mediated G2 arrest as expected, four distinct HLTF siRNA did not affect Vpr-mediated cell cycle arrest (Fig. 3B and Fig. S2 A and B). Furthermore, HLTF siRNA alone did not perturb the cell cycle (Fig. 3B and Fig. S2 A and B).
Fig. 3.
HLTF degradation and G2 arrest are two independent activities of HIV-1 Vpr. (A) Mus81 depletion does not trigger HLTF degradation and, conversely, HLTF depletion does not trigger Mus81 degradation. HeLa cells were treated with siRNA control or siRNA against DCAF1, HLTF, or Mus81 for 24 h. Cells were then transduced with the VLP and harvested 24 h later. Protein expression was analyzed by Western blotting. Quantification was performed: ratios between the HLTF and the GAPDH signals were calculated relative to a 100% reference indicated in red. The Western blot is representative of three independent experiments. (B) Depletion of HLTF by siRNA does not perturb cell cycle and does not inhibit Vpr-mediated G2 arrest. HeLa cells were treated as in A, and cell cycle was analyzed 24 h after VLP treatment. (C) The Vpr G56A mutant, defective for HLTF degradation, is still able to arrest the cell cycle. HeLa cell were treated with VLP [empty VLP (R−), VLP containing WT Vpr (R+), or mutants G56A, K27M, or S79A] for 24 h. Protein expression was then assessed by Western blot and quantification performed as in A and the cell cycle analyzed (D).
Fig. S2.
(A) Efficient silencing of DCAF1 and HLTF by siRNA in cells used for cell cycle analysis. (B) Depletion of HLTF by several siRNA does not perturb cell cycle and does not inhibit Vpr-mediated G2 arrest. Western blot was performed using antibodies directed against the indicated proteins. (C) Screening of Vpr mutants for their ability to induce HLTF degradation. HeLa cells were transfected with plasmids encoding HA-Vpr or indicated mutants. Cells were lysed 2 d after transfection, and an immunoblot against relevant proteins was done. *Interesting Vpr mutants that are still able to arrest cells in G2/M but not to induce the degradation of HLTF.
We further studied the links that may exist between HLTF degradation and G2 arrest. To this aim, we analyzed the ability of a panel of Vpr mutants to block the cell cycle and to degrade HLTF (Fig. S2C). The proteins were expressed in HeLa cells by transfection. We identified mutants that displayed various abilities to degrade HLTF (Fig. S2C). We then selected three mutants (G56A, K27M, and S79A) that we delivered in HeLa cells through VLPs (Fig. 3 C and D). The K27M mutant was inactive in both assays, whereas S79A partly degraded HLTF (47% HLTF remaining, compared with 18% with the WT protein) without altering the cell cycle (Fig. 3 C and D). In contrast, the Vpr G56A mutant induced G2 arrest but did not degrade HLTF (Fig. 3 C and D). Altogether our results strongly suggest that HLTF degradation and G2 arrest can be genetically uncoupled.
Vpr Down-Regulates HLTF in Infected T Cells and Primary Macrophages.
We then investigated whether HLTF is degraded in HIV-1–infected cells. MT4 and Jurkat T cells, as well as HeLa cells, were infected with WT or ∆Vpr HIV-1, and HLTF levels were measured after 3 d. The levels of HLTF were strongly decreased in the presence of Vpr in the three cell types (Fig. 4A; 1%, 16%, and 38% HLTF remaining after infection with WT HIV-1 in comparison with the ∆Vpr virus in MT4, Jurkat, and HeLa cells, respectively). Nonetheless, as expected from the literature, Vpr had no effect on viral replication in these cells. WT or ∆Vpr HIV similarly infected MT4 cells, as assessed by measuring Gag expression by flow cytometry at different time points (Fig. S3). Moreover, HLTF was silenced in MT4 cells by using a doxycycline-inducible shRNA (Fig. 4A, Left). HLTF silencing did not alter infection levels of WT or ∆Vpr HIV (Fig. S3).
Fig. 4.
HLTF degradation is induced by HIV-1 Vpr in infected T cells and HeLa cells. (A) HLTF is degraded in MT4 cells by HIV-1 viruses expressing WT Vpr (Left), in Jurkat T cells (Center), and in HeLa cells (Right). MT4 cells were transduced with lentiviruses expressing shRNA against HLTF and cultured in the presence of doxycycline for 3 d. Cells were then either not infected or infected with HIV-1 NL4.3 WT or Vpr-deleted viruses (∆Vpr) for 48 h. Jurkat and HeLa cells were either not infected or infected with NL4.3 WT or Vpr- deleted viruses (∆Vpr). Cells were lysed 48 h after infection, and cell lysates were analyzed by Western blot. (B) Vpr from HIV-1 VLP triggers HLTF degradation in macrophages. Monocyte-derived macrophages were differentiated for 4 or 7 d and then exposed to HIV-1 VLP containing or not Vpr for 24 h [empty VLP (R−), VLP containing WT Vpr (R+)]. Cells were then lysed, and HLTF expression was analyzed by Western blot. In each panel, quantification was performed: ratios between signals were calculated relative to a 100% reference indicated in red. All Western blots are representative of three independent experiments. (C) HLTF levels are reduced in macrophages following infection with the YU2 WT virus, in comparison with the ∆Vpr virus. Monocyte-derived macrophages (MDMs) were treated with siRNA control or siRNA against HLTF for 24 h. Cells were then infected with either YU2 WT virus or YU2 ∆Vpr virus (∆Vpr). Analysis of HLTF and GADPH expression was done in extracts collected at day 16 after infection. The kinetic of replication is shown Fig. S4.
Fig. S3.
(A) MT4 cells expressing doxycycline-inducible shRNA against HLTF together with RFP were obtained under puromycine selection. Percentage of RFP-positive cells expressing shRNA was determined by flow cytometry (NTd, nontransduced cells; shCtrl, cells transduced with a control shRNA; and shHLTF, cells transduced with an shRNA against HLTF). Data show a mean of three experiments ± SD. (B) The presence of Vpr or the absence of HLTF did not alter infection by HIV-1. Cells were infected with VSV-G pseudotyped NL4-3 viruses expressing or not Vpr [NI, noninfected; NL WT, cells infected with NL4-3 (VSV) expressing Vpr; and NL ∆Vpr, cells infected with NL4-3 (VSV) lacking Vpr]. Infection levels were assessed by measuring Gag expression by flow cytometry at 24 and 48 h postinfection (pi). Data show a mean of three experiments ± SD.
We then examined the activity of Vpr in primary human macrophages, in which Vpr has been reported to give an advantage to the virus in multicycle infection assays (6, 8–11). The role of HLTF has not yet been examined in such nondividing cells. We found that HLTF is expressed at day 4 or day 7 following differentiation of freshly isolated monocytes into macrophages (MDMs) (Fig. 4B; two donors). HLTF levels were decreased by two- to sixfold following macrophage incubation for 24 h with Vpr-containing HIV-1 VLPs compared with Vpr-negative VLP (Fig. 4B). This decrease was similarly observed with macrophages at day 4 or 7 after differentiation (Fig. 4B). This experiment demonstrated that incoming Vpr is sufficient to decrease endogenous levels of HLTF on viral entry. HLTF levels were also reduced following productive infection with the YU2 macrophage-tropic strain expressing Vpr compared with the isogenic ∆Vpr virus (Fig. 4C; two donors, Western blot at day 16 after infection). As expected, viral replication was delayed and less efficient in the absence of Vpr (Fig. S4) (6, 8–11). However, depletion of HLTF by siRNA did not impact viral replication (Fig. S4).
Fig. S4.
HLTF silencing does not affect HIV-1 replication in macrophages. MDMs were treated with siRNA Ctrl or siRNA against HLTF for 24 h. The cells were then not infected (NI) or infected with either Yu-2 wt virus (WT) or Yu-2 ∆Vpr virus (∆Vpr). A second siRNA treatment was done 48 h after infection. Cell supernatants were collected at the indicated time points, and p24 levels were measured by ELISA. Error bars represent SEM of triplicates from one donor. Same results were obtained with a second donor.
Altogether, our results indicate that Vpr degrades HLTF in natural cell targets of HIV-1.
Discussion
HLTF, a Bona Fide Cellular Substrate of HIV-1 Vpr.
SILAC provides an unbiased view of global changes in protein levels under different parallel conditions. Surprisingly, HLTF was the sole cellular target that was clearly down-regulated in the presence of HIV-1 Vpr, among more than 2,000 proteins quantified. Several other host proteins have been proposed to be targeted by Vpr, but previous results were mostly based on candidate-based approaches and with often modest down-regulation (62–64). Importantly, HLTF is down-regulated by Vpr in infected T cells. The effect of Vpr on HLTF is reminiscent of Vpx-induced SAMHD1 degradation (57, 58). Both Vpr and Vpx act rapidly, when incoming virions enter the cell, to trigger proteasomal degradation of their target proteins by hijacking ubiquitin ligases. Vpr is closely related to Vpx. These two proteins have a common evolutionary origin (65, 66), share similar amino acid sequences, and are incorporated into virions (67, 68). Despite these similarities, the two proteins display different activities (69). HIV-1 Vpr does not degrade SAMHD1. It will be worth examining the effect of a large panel of Vpr proteins from HIV-1, HIV-2, and SIV strains on HLTF, to understand the evolutionary pressures associated with this novel activity. It will also be of interest to determine whether this degradation involves a direct interaction between HLTF and HIV-1 Vpr.
Why Does Vpr Induce HLTF Degradation?
How could HLTF degradation impact HIV-1 replication and pathogenicity? As part of the repair of damaged replication forks, one obvious possibility was that HLTF was involved in Vpr-mediated G2 arrest. This hypothesis was attractive because both Vpr and depletion of some DNA translocases having apparent redundant function with HLTF interfere with SLX4com. For example, SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily A-like protein 1 (SMARCAL1) was reported to interfere with MUS81 structure-specific endonuclease subunit (Mus81) or the SLX4 endonuclease complex and the repair of damaged replication forks (30, 70, 71). However, several lines of evidence suggest that HLTF is not involved in the cytostatic activity of Vpr. Silencing of HLTF by siRNA did not perturb the cell cycle, nor did it affect Vpr-mediated G2 arrest. In addition, some Vpr mutants that do not measurably degrade HLTF were still able to arrest the cell cycle. Thus, HLTF degradation represents a new activity of Vpr apparently distinct from G2 arrest, at least in HeLa cells. Further work will help determine how the removal of HLTF by Vpr may impact DNA repair on HIV-1 infection of macrophages, dendritic cells, and T cells.
HLTF is a DNA translocase involved in postreplication DNA repair occurring in the S phase of the cycle (49, 72–74). As such, it has been mostly studied in dividing cells, in which DNA repair processes are important to avoid aberrant DNA synthesis that would be a source of mutations or DSBs. It is tempting to speculate that HLTF could exert additional functions, because we report here that the protein is expressed in nondividing macrophages. Cell cycle-related proteins, for instance, the cyclin/Cdk inhibitor p21, which was first thought to be only dedicated to the control of cell cycle progression, were later on identified in macrophages as a repressor of HIV-1 replication (75, 76). Our experiments did not demonstrate a rescue of Vpr-deleted HIV-1 replication in HLTF-silenced macrophages. It will be interesting to further explore a potential antiviral role of HLTF in other nondividing cells such as dendritic cells and in primary lymphocytes. One alternative and non-mutually exclusive possibility is that HLTF degradation is related to the ability of Vpr to interfere with the immune response and the cooperation between immune cells (77–81). For example, whether HLTF degradation is related to the ability of Vpr to escape or promote immune detection in primary cells should be investigated. A role of HLTF in DNA sensing could be a consequence of its capacity to bind ssDNA ends (82). It is also possible that HLTF is not a direct target of Vpr and that its degradation results from the inactivation of another cellular protein that was not detected in our SILAC experiments. Looking for interacting partners of HLTF and their potential antiviral role could help addressing this issue.
HLTF belongs to a small family of DNA translocases, including SMARCAL1 and zinc finger Ran-binding domain-containing protein 3 (ZRANB3)/AH2, that catalyze fork regression activity in vitro (83). SMARCAL1 deficiency causes the SIOD human disease, which is associated with immune deficiency (84). This observation, together with our finding that HLTF is antagonized by a viral protein, may suggest that these DNA translocases could play a role in the immune defense against pathogens.
HIV-1 and HIV-2/SIV Interfere with Distinct DNA Repair Pathways.
HIV-1 and HIV-2/SIV have likely developed distinct strategies to interfere with DNA repair pathways. Vpx from HIV-2/SIV targets SAMHD1, an enzyme that hydrolyses nucleotides potentially required for DNA repair and that prevents viral reverse transcription (85–87), whereas we show here that HIV-1 Vpr triggers HLTF degradation and thus manipulates the DDT pathway. Vpr also likely interferes with the BER pathway through Ung2 recruitment/degradation and prematurely activates the SLX4 complex. The use of distinct strategies to impact the DNA repair pathway may underline the different pathological outcomes associated with HIV-1 and HIV-2/SIV. Future studies should aim to analyze the ability of divergent lentiviruses to induce the degradation of human and simian HLTF, to evaluate its importance in pathology and in cross-species transmission (88). It will also be worthwhile to search for signatures of positive selection in HLTF sequences originating from various species. Such analysis will complement functional studies and will help evaluate the impact of HLTF at the host–virus interface.
Experimental Procedures
SILAC.
Full details for the SILAC procedure are provided in the SI Experimental Procedures.
Viruses and VLP Production.
VLPs and viruses were produced in 293 T cells cotransfected by the calcium-phosphate method. The Δ-Env HIV-1 viruses (DHIV NL4.3 viruses) were produced by using pNL4.3 deltaEnv HIV-1 constructs lacking the gene encoding Vpr (DHIVΔVpr) or encoding WT Vpr (DHIV WT) along with a plasmid encoding the vesicular stomatitis virus glycoprotein G (VSV-G). The proviral plasmids were a kind gift from Vincente Planelles, University of Utah, Salt Lake City (89). HIV-1 VLPs were produced by using psPAX2 lentiviral packaging plasmid along with the plasmid encoding VSV-G and a plasmid encoding either HA-tagged WT or mutant Vpr. Briefly, 48 h after transfection, the culture supernatants were collected and filtered through 0.45-μm pore filters. The viral particles were then concentrated in 10% (wt/vol) polyethylene glycol 6000 (Sigma) containing 300 mM NaCl. Viral and VLP productions were measured by quantification of p24 (HIV) levels by using an ELISA (Innotest; Fujirebio, or ZeptoMetrix Corporation). Viruses were titrated by using the reporter cells TZM-bl obtained through the National Institutes of Health (NIH) AIDS Reagent Program, Division of AIDS, NIH (TZM-bl from John C. Kappes, Xiaoyun Wu, and Tranzyme Inc., catalog number 8129).
Transduction and Infection.
Cells transduced by VLPs were incubated for 2 h in DMEM or RPMI supplemented with 5 µg/mL Dextran (Sigma) and an equivalent of 50–100 ng p24 or p27 of HIV-1 or SIV VLP, respectively, for 2 × 105 cells, and then complete medium was added. The proteasome inhibitor Mg132 (Sigma) was used at a final concentration of 20 µM for 6 h from the beginning of VLP incubation or with DMSO as a control. Jurkat cells were infected for 2 h in complete RPMI medium supplemented with 5 mM Hepes and 5 µg/mL Dextran.
Peripheral blood mononuclear cells (PBMCs) from the blood of anonymous donors (obtained in accordance with the ethical guidelines of the Institut Cochin) were isolated by Ficoll (Sigma) density-gradient separation. Monocytes were isolated by positive selection with CD14 magnetic MicroBeads (Miltenyi Biotec). MDMs were obtained by culturing the monocytes for 7 d in RPMI containing 10 ng/mL granulocyte-macrophage colony stimulating factor and 20 ng/mL macrophage colony-stimulating factor. YU2 WT and YU2 ΔVpr viruses were kind gifts from Serge Benichou, Institut Cochin, Paris.
MDM cells were infected (at a multiplicity of infection of 0.1) for 3 h in complete RPMI medium without serum at 37 °C. Cells were washed twice with PBS to remove inoculum and cultured in RPMI complete medium. Viral production was measured by quantification of p24 levels in the cellular supernatants collected at times 0, 1, 2, 5, 9, 12, and 16 d after infection by using an ELISA (Innotest; Fujirebio).
Cells, plasmid constructs, siRNA, shRNA, and the procedures for cell cycle analysis and Western blots are described in the SI Experimental Procedures.
SI Experimental Procedures
Cells.
Cells were cultured in DMEM (Sigma) or RPMI (Sigma) containing 10% heat-inactivated FBS (Biowest), 1,000 U/mL penicillin, 1,000 µg/mL streptomycin, and 2 mM glutamine (Life Technologies). PBMCs from the blood of anonymous donors (obtained in accordance with the ethical guidelines of the Institut Cochin) were isolated by Ficoll (GE Healthcare) density-gradient separation. Monocytes were isolated by positive selection with CD14 magnetic MicroBeads (Miltenyi Biotec). MDMs were obtained by culturing the monocytes for 7–9 d as described in ref. 86.
Plasmid Constructions and Transfections.
p3XFlag HLTF plasmid was obtained by amplification of HLTF sequence from pcDNA3 Flag HLTF, a kind gift of K. A. Cimprich, Stanford University School of Medicine, Stanford, CA. The HLTF gene was inserted between HindIII and KpnI restriction sites into the p3XFlag-CMV vector (Sigma). Plasmid transfections were performed with Fugene6 (Promega).
siRNA and shRNA Silencing.
siRNA transfections were performed with DharmaFECT1 (Dharmacon-Gelifesciences) in HeLa cells or INTERFERin in MDM cells (Polyplus-transfection). The following siRNAs were used. HLTF: N9, GGAUUUGUGUUUACUCGUU; N8, GCAGGAUCUUCUAAGGUUA; N7, GAUAGAGAAUGGUGGCAUA; or N6, CCAGAUGACUUUCUAACUA (Gelifesciences). MUS81: siRNA pool of CUCAGGAGCCCGAGUGAUA, UGACCCACACGGUGCGCAA, CAUUAAGUGUGGGCGUCUA, and CAGCCCUGGUGGAUCGAUA (Gelifesciences). DCAF1: GGAAUGACACUGUGCGCUU or CGGAGUUGGAGGAGGACGA (Dharmacon). The final concentration of all siRNAs was 100 nM. Cell lines expressing the pTRIPZ-inducible shRNA against HLTF (V2THS_153143, V2THS_ 153146, and V3THS_393629; Gelifesciences) were obtained under puromycine selection as described in the manual of the manufacturer.
Cell Cycle Analysis.
After VLP treatment, cells were detached with PBS supplemented with 0.5 mM EDTA or by trypsinization, washed twice with PBS, and fixed in 70% (vol/vol) ethanol. Following treatment for 30 min at 37 °C with 0.2 mg/mL RNase A and 50 µg/mL propidium iodide in buffer H (20 mM Hepes, 160 mM NaCl, 1 mM EGTA), cells were analyzed for their DNA content using FC500 (Beckman Coulter) or C6 Accuri (BD Biosciences) flow cytometers. At least 10,000 cells were analyzed for their distribution in the different phases of the cell cycle.
Western Blot and Antibodies.
Cells were lysed with SDS-DTT sample buffer (Laemmli) and then homogenized through a 25-gauge syringe needle with benzonase (Sigma). Total protein extracts were separated by 4–12% (wt/vol) gel in Mops buffer (ThermoFisher Scientific-Life Technologies), transferred onto PVDF membranes, and revealed by a chemiluminescence procedure (CDPStarW; Applied Biosystems). Signals were acquired by a LAS 3000 apparatus (Fujifilm) and quantified using the Multigauge (Fujifilm) or ImageJ software (National Institutes of Health). The following antibodies were used: anti-HLTF (ab17984; Abcam), anti-MUS81 (ab14387; Abcam), anti-GAPDH (SC-32233; Santa Crus), anti-flag (M2 clone-F1804; Sigma), anti-tubulin (T9026; Sigma), anti-hemagglutinin (HA tag, clone 16B12, HA.11; Covance), anti-DCAF1 (bs-9393R; Gentaur), anti-SAMHD1 (ab67820; Abcam), anti-RAD18 (ab18959; Abcam), and anti-GFP (ab6556; Abcam).
SILAC.
HeLa cells were cultured for 4 wk in SILAC DMEM minus l-arginine and l-lysine (Gibco-Life Technologies) supplemented with antibiotics and glutamine and with heat-inactivated and dialyzed (Sigma) FBS and 80 µg/mL proline. The medium was supplemented with 100 µg/mL of various stable carbon or nitrogen isotopes (all from EURISO-TOP),13C6 15N4 l-arginine and 13C6 15N2-l-lysine, or 13C6 l-arginine and D4 l-lysine, or natural l-arginine and l-lysine for heavy, medium, or light labels, respectively. After complete labeling monitored by MS analysis, 2 × 106 cells of each condition were incubated with VLP. In the SILAC1, heavy-labeled cells were incubated with VLP containing WT Vpr of HIV-1, whereas medium-labeled cells were incubated with VLP containing VprS79A and light-labeled cells with empty VLP. In the SILAC2, conditions were switched: heavy labeling was associated with VLP-containing VprS79A, medium labeling with empty VLP, and light labeling with VLP containing WT Vpr. Cells were harvested by trypsinization 12 h later and washed several times with PBS, and cell pellets were resuspended in 1 mL subcellular fractionation buffer [10 mM Hepes, pH 7, 1.5 mM MgCl2, 10 mM KCl, 50 mM sucrose, 5% (wt/vol) glycerol, and 0.1% Triton X-100] and incubated at 4 °C for 15 min. The mixture was centrifuged at 1,300 × g for 7 min at 4 °C. The supernatant containing predominantly cytoplasmic proteins, and the pellet corresponding to the nuclear fraction was collected and frozen until peptide preparation.
Cytosolic Peptide Preparation.
Cytoplasmic fractions of the same SILAC were pooled (2.4 mL in total, 0.6 mL left for cell cycle and Western blot analyses) and then precipitated by 1-h incubation at −20 °C with 15 mL ice-cold acetone (Carlo Erba). Pellets were obtained after 30-min centrifugation at 1,800 × g at 4 °C and resuspended in 1 mL of 50 mM ammonium bicarbonate (ABC; Sigma). Cysteins were reduced by 30-min incubation at 56 °C in 30 mM DTT (Sigma) and then alkylated by 30-min incubation at room temperature in 55 mM chloroacetamid (Fluka). Protein homogenates were digested overnight at 30 °C with 15 µg Sequencing Grade Modified Trypsin (Promega); pH 8 was checked before incubation.
Nuclear Peptide Preparation.
Nuclear fractions were lysed in 20 µL 25 mM ABC, pH 8, and 1% Rapigest acid-labile surfactant (Waters); 180 µL 25 mM ABC was added, and lysates were incubated at 95 °C for 5 min. Cysteins were reduced and alkylated as above, and nuclear proteins were digested with 2 µg Trypsin. Rapigest was cleaved by a 2-h incubation at 37 °C in 1% trifluoroacetic acid (TFA; Fluka). After 15 min of 18,000 × g centrifugation, pellets of cleaved Rapigest were eliminated.
IEF Off-Gel Peptide Fractionation.
Peptides were desalted using a Sep-Pak C18 column (Waters). Briefly, the C18 column was activated with 3 mL 90% (vol/vol) acetonitrile (ACN; VWR) and 0.1% TFA and equilibrated with 3 mL 0.1% TFA. Peptides were percolated on the column, and the retained fraction was washed with 2 mL 0.1% TFA, eluted with 2 mL 70% ACN and 0.1% TFA, and then dried in a vacuum concentrator (Eppendorf).
Peptides were prepared for an Off-Gel isoelectrofocalization on a 13-cm strip, pH 3–10, (GE Healthcare) as indicated in the Agilent 3100 Off-Gel fractionator kit-quick start guide. After focusing, each of the 12 fractions was collected. Trapped peptides in the gel strip were extracted. Briefly, 200 µL 50% (vol/vol) methanol (Carlo-Erba) and 1% formic acid (FA; Sigma) were added to each tank of the frame and incubated for 30 min. Methanol-extracted peptides were pooled with their respective fraction and then dried in a vacuum concentrator and resuspended in 100 µL 10% ACN and 0.1% TFA.
LC-MS/MS Analysis.
One microliter of each fraction was analyzed using an Ultimate 3000 Rapid Separation liquid chromatographic system coupled to a Linear Trap Quadrupole (LTQ) Orbitrap Velos mass spectrometer (Thermo Fisher Scientific). Briefly, peptides were loaded and washed on a C18 reverse phase precolumn from Dionex (3-µm particle size, 100-Ǻ pore size, 150-µm internal diameter, 1-cm length). The loading buffer contained 98% H2O, 2% ACN, and 0.1% TFA. Peptides were then separated on a C18 reverse phase column from Dionex (2-µm particle size, 100-Ǻ pore size, 75-µm ID, 15-cm length) with a 2-h gradient from 99% A (0.1% FA in H2O) to 40% B (80% ACN, 0.085% FA, and 20% H2O).
The hybrid LTQ-Orbitrap mass spectrometer acquired data throughout the elution process and operated in a data-dependent scheme with full MS high-resolution spectra acquired with the Orbitrap, followed by up to 20 LTQ MS/MS collision induced dissociation (CID) spectra on the most abundant ions detected in the MS spectra. Mass spectrometer settings were as follows: full MS (AGC: 1.106, resolution: 6.104, m/z range 400–2,000, maximum ion injection time: 500 ms); MS/MS (AGC: 5.103, maximum injection time: 200 ms, minimum signal threshold: 500, isolation width: 2 Da, dynamic exclusion time setting: 30 s). Fragmentation was permitted for precursors with charge states of two or more.
Spectra Processing, Peptide Identification, and Quantification.
The software used to generate .mgf files was Proteome discoverer 1.3. The threshold of signal to noise for extraction values was 3. MS/MS spectra were submitted to Maxquant version 1.3. The database used is a concatenation of Human sequence of Swissprot (www.uniprot.org) database and a list of common contaminant sequences. The oxidation of methionines was permitted partially, whereas the carbamidomethylations of cysteins were considered complete. Peptide matches were filtered to maintain probability of a false positive less than 1%.
Bioinformatics Analysis.
A MySQL database was built from raw results using EasyPHP software, and candidates were selected based on three criteria met on both the SILAC1 and the SILAC2 condition: number of detected peptides ≥ 2 (ensuring the peptide presence in the sample), number of peptides used for protein quantification ≥ 3 (for accurate quantification) and a ratio variation ≥ 20% (up- or down-regulation compared with the control condition).
Acknowledgments
We thank Baek Kim and all members of our “Retrovirus, Quiescence and Proliferation” laboratory for fruitful discussions. We thank Karlene Cimprich and Dr. Myung for the gift of mammalian vectors expressing Flag-HLTF, Vicente Planelles for the gift of NL4-3 proviral constructs (wt and ΔVpr), Serge Benichou for the gift of proviral pYU2 and pYU2-ΔVpr plasmids and for some Vpr mutants, and Nicolas Manel for the gift of the psPAX2 vector. We thank the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program for kindly providing reagents and Daniel Aaron Donahue for critical reading of the manuscript. We acknowledge the Cytometry and Immunobiology Facility of the Cochin Institute and the 3P5 Proteomic Facility of Paris Descartes University. This work was supported by grants from the “Agence Nationale de la Recherche sur le SIDA et les hépatites virales” (ANRS), SIDACTION, Fondation de France, and Fondation pour la Recherche Médicale (FRM; Grant DEQ20140329528 to F.M.-G.). H.L. received a fellowship from ANRS, SIDACTION, and Fondation de France, M.-L.B. from Fondation pour la Recherche Médicale and ANRS, and G.C. received a fellowship from the French government. Work in the O.S. laboratory was supported by grants from the ANRS, SIDACTION, AREVA Foundation, the Vaccine Research Institute, the Labex Integrative Biology of Emerging Infectious Diseases (IBEID) program, the FP7 program HIT Hidden HIV (Health-F3-2012-305762), and Institut Pasteur.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600485113/-/DCSupplemental.
References
- 1.Chatzinikolaou G, Karakasilioti I, Garinis GA. DNA damage and innate immunity: Links and trade-offs. Trends Immunol. 2014;35(9):429–435. doi: 10.1016/j.it.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Zeman MK, Cimprich KA. Causes and consequences of replication stress. Nat Cell Biol. 2014;16(1):2–9. doi: 10.1038/ncb2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crow YJ, Manel N. Aicardi-Goutières syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15(7):429–440. doi: 10.1038/nri3850. [DOI] [PubMed] [Google Scholar]
- 4.Brégnard C, Benkirane M, Laguette N. DNA damage repair machinery and HIV escape from innate immune sensing. Front Microbiol. 2014;5:176. doi: 10.3389/fmicb.2014.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dedera D, Hu W, Vander Heyden N, Ratner L. Viral protein R of human immunodeficiency virus types 1 and 2 is dispensable for replication and cytopathogenicity in lymphoid cells. J Virol. 1989;63(7):3205–3208. doi: 10.1128/jvi.63.7.3205-3208.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balliet JW, et al. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: Mutational analysis of a primary HIV-1 isolate. Virology. 1994;200(2):623–631. doi: 10.1006/viro.1994.1225. [DOI] [PubMed] [Google Scholar]
- 7.Eckstein DA, et al. HIV-1 Vpr enhances viral burden by facilitating infection of tissue macrophages but not nondividing CD4+ T cells. J Exp Med. 2001;194(10):1407–1419. doi: 10.1084/jem.194.10.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206(2):935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 9.Jacquot G, et al. Localization of HIV-1 Vpr to the nuclear envelope: Impact on Vpr functions and virus replication in macrophages. Retrovirology. 2007;4:84. doi: 10.1186/1742-4690-4-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zander K, et al. Cyclophilin A interacts with HIV-1 Vpr and is required for its functional expression. J Biol Chem. 2003;278(44):43202–43213. doi: 10.1074/jbc.M305414200. [DOI] [PubMed] [Google Scholar]
- 11.de Silva S, Planelles V, Wu L. Differential effects of Vpr on single-cycle and spreading HIV-1 infections in CD4+ T-cells and dendritic cells. PLoS One. 2012;7(5):e35385. doi: 10.1371/journal.pone.0035385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mashiba M, Collins DR, Terry VH, Collins KL. Vpr overcomes macrophage-specific restriction of HIV-1 Env expression and virion production. Cell Host Microbe. 2014;16(6):722–735. doi: 10.1016/j.chom.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins DR, Lubow J, Lukic Z, Mashiba M, Collins KL. Vpr promotes macrophage-dependent HIV-1 infection of CD4+ T lymphocytes. PLoS Pathog. 2015;11(7):e1005054. doi: 10.1371/journal.ppat.1005054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang SM, et al. Importance of vpr for infection of rhesus monkeys with simian immunodeficiency virus. J Virol. 1993;67(2):902–912. doi: 10.1128/jvi.67.2.902-912.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaumont T, et al. Reversal of human immunodeficiency virus type 1 IIIB to a neutralization-resistant phenotype in an accidentally infected laboratory worker with a progressive clinical course. J Virol. 2001;75(5):2246–2252. doi: 10.1128/JVI.75.5.2246-2252.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goh WC, et al. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: A mechanism for selection of Vpr in vivo. Nat Med. 1998;4(1):65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- 17.Caly L, Saksena NK, Piller SC, Jans DA. Impaired nuclear import and viral incorporation of Vpr derived from a HIV long-term non-progressor. Retrovirology. 2008;5:67. doi: 10.1186/1742-4690-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadi K, et al. Human immunodeficiency virus type 1 Vpr polymorphisms associated with progressor and nonprogressor individuals alter Vpr-associated functions. J Gen Virol. 2014;95(Pt 3):700–711. doi: 10.1099/vir.0.059576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacquot G, et al. Characterization of the molecular determinants of primary HIV-1 Vpr proteins: Impact of the Q65R and R77Q substitutions on Vpr functions. PLoS One. 2009;4(10):e7514. doi: 10.1371/journal.pone.0007514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tzitzivacos DB, Tiemessen CT, Stevens WS, Papathanasopoulos MA. Viral genetic determinants of nonprogressive HIV type 1 subtype C infection in antiretroviral drug-naive children. AIDS Res Hum Retroviruses. 2009;25(11):1141–1148. doi: 10.1089/aid.2009.0080. [DOI] [PubMed] [Google Scholar]
- 21.Blondot ML, Dragin L, Lahouassa H, Margottin-Goguet F. How SLX4 cuts through the mystery of HIV-1 Vpr-mediated cell cycle arrest. Retrovirology. 2014;11(1):117. doi: 10.1186/s12977-014-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roshal M, Kim B, Zhu Y, Nghiem P, Planelles V. Activation of the ATR-mediated DNA damage response by the HIV-1 viral protein R. J Biol Chem. 2003;278(28):25879–25886. doi: 10.1074/jbc.M303948200. [DOI] [PubMed] [Google Scholar]
- 23.Belzile JP, et al. HIV-1 Vpr-mediated G2 arrest involves the DDB1-CUL4AVPRBP E3 ubiquitin ligase. PLoS Pathog. 2007;3(7):e85. doi: 10.1371/journal.ppat.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeHart JL, et al. HIV-1 Vpr activates the G2 checkpoint through manipulation of the ubiquitin proteasome system. Virol J. 2007;4:57. doi: 10.1186/1743-422X-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hrecka K, et al. Lentiviral Vpr usurps Cul4-DDB1[VprBP] E3 ubiquitin ligase to modulate cell cycle. Proc Natl Acad Sci USA. 2007;104(28):11778–11783. doi: 10.1073/pnas.0702102104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Rouzic E, et al. HIV1 Vpr arrests the cell cycle by recruiting DCAF1/VprBP, a receptor of the Cul4-DDB1 ubiquitin ligase. Cell Cycle. 2007;6(2):182–188. doi: 10.4161/cc.6.2.3732. [DOI] [PubMed] [Google Scholar]
- 27.Schröfelbauer B, Hakata Y, Landau NR. HIV-1 Vpr function is mediated by interaction with the damage-specific DNA-binding protein DDB1. Proc Natl Acad Sci USA. 2007;104(10):4130–4135. doi: 10.1073/pnas.0610167104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan L, Ehrlich E, Yu XF. DDB1 and Cul4A are required for human immunodeficiency virus type 1 Vpr-induced G2 arrest. J Virol. 2007;81(19):10822–10830. doi: 10.1128/JVI.01380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen X, Duus KM, Friedrich TD, de Noronha CM. The HIV1 protein Vpr acts to promote G2 cell cycle arrest by engaging a DDB1 and Cullin4A-containing ubiquitin ligase complex using VprBP/DCAF1 as an adaptor. J Biol Chem. 2007;282(37):27046–27057. doi: 10.1074/jbc.M703955200. [DOI] [PubMed] [Google Scholar]
- 30.Laguette N, et al. Premature activation of the SLX4 complex by Vpr promotes G2/M arrest and escape from innate immune sensing. Cell. 2014;156(1-2):134–145. doi: 10.1016/j.cell.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Fekairi S, et al. Human SLX4 is a Holliday junction resolvase subunit that binds multiple DNA repair/recombination endonucleases. Cell. 2009;138(1):78–89. doi: 10.1016/j.cell.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muñoz IM, et al. Coordination of structure-specific nucleases by human SLX4/BTBD12 is required for DNA repair. Mol Cell. 2009;35(1):116–127. doi: 10.1016/j.molcel.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 33.Svendsen JM, et al. Mammalian BTBD12/SLX4 assembles a Holliday junction resolvase and is required for DNA repair. Cell. 2009;138(1):63–77. doi: 10.1016/j.cell.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouhamdan M, et al. Human immunodeficiency virus type 1 Vpr protein binds to the uracil DNA glycosylase DNA repair enzyme. J Virol. 1996;70(2):697–704. doi: 10.1128/jvi.70.2.697-704.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selig L, et al. Uracil DNA glycosylase specifically interacts with Vpr of both human immunodeficiency virus type 1 and simian immunodeficiency virus of sooty mangabeys, but binding does not correlate with cell cycle arrest. J Virol. 1997;71(6):4842–4846. doi: 10.1128/jvi.71.6.4842-4846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schröfelbauer B, Yu Q, Zeitlin SG, Landau NR. Human immunodeficiency virus type 1 Vpr induces the degradation of the UNG and SMUG uracil-DNA glycosylases. J Virol. 2005;79(17):10978–10987. doi: 10.1128/JVI.79.17.10978-10987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saugar I, Ortiz-Bazán MA, Tercero JA. Tolerating DNA damage during eukaryotic chromosome replication. Exp Cell Res. 2014;329(1):170–177. doi: 10.1016/j.yexcr.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Ghosal G, Chen J. DNA damage tolerance: A double-edged sword guarding the genome. Transl Cancer Res. 2013;2(3):107–129. doi: 10.3978/j.issn.2218-676X.2013.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheridan PL, Schorpp M, Voz ML, Jones KA. Cloning of an SNF2/SWI2-related protein that binds specifically to the SPH motifs of the SV40 enhancer and to the HIV-1 promoter. J Biol Chem. 1995;270(9):4575–4587. doi: 10.1074/jbc.270.9.4575. [DOI] [PubMed] [Google Scholar]
- 40.Hayward-Lester A, et al. Cloning, characterization, and steroid-dependent posttranscriptional processing of RUSH-1 alpha and beta, two uteroglobin promoter-binding proteins. Mol Endocrinol. 1996;10(11):1335–1349. doi: 10.1210/mend.10.11.8923460. [DOI] [PubMed] [Google Scholar]
- 41.Gong X, et al. Developmental regulation of Zbu1, a DNA-binding member of the SWI2/SNF2 family. Dev Biol. 1997;183(2):166–182. doi: 10.1006/dbio.1996.8486. [DOI] [PubMed] [Google Scholar]
- 42.Ding H, et al. Characterization of a helicase-like transcription factor involved in the expression of the human plasminogen activator inhibitor-1 gene. DNA Cell Biol. 1996;15(6):429–442. doi: 10.1089/dna.1996.15.429. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Q, Ekhterae D, Kim KH. Molecular cloning and characterization of P113, a mouse SNF2/SWI2-related transcription factor. Gene. 1997;202(1-2):31–37. doi: 10.1016/s0378-1119(97)00446-0. [DOI] [PubMed] [Google Scholar]
- 44.Debauve G, Capouillez A, Belayew A, Saussez S. The helicase-like transcription factor and its implication in cancer progression. Cell Mol Life Sci. 2008;65(4):591–604. doi: 10.1007/s00018-007-7392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandhu S, et al. Loss of HLTF function promotes intestinal carcinogenesis. Mol Cancer. 2012;11:18. doi: 10.1186/1476-4598-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim JJ, et al. Promoter methylation of helicase-like transcription factor is associated with the early stages of gastric cancer with family history. Ann Oncol. 2006;17(4):657–662. doi: 10.1093/annonc/mdl018. [DOI] [PubMed] [Google Scholar]
- 47.Moinova HR, et al. HLTF gene silencing in human colon cancer. Proc Natl Acad Sci USA. 2002;99(7):4562–4567. doi: 10.1073/pnas.062459899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin JR, Zeman MK, Chen JY, Yee MC, Cimprich KA. SHPRH and HLTF act in a damage-specific manner to coordinate different forms of postreplication repair and prevent mutagenesis. Mol Cell. 2011;42(2):237–249. doi: 10.1016/j.molcel.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blastyák A, Hajdú I, Unk I, Haracska L. Role of double-stranded DNA translocase activity of human HLTF in replication of damaged DNA. Mol Cell Biol. 2010;30(3):684–693. doi: 10.1128/MCB.00863-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torres-Ramos CA, Prakash S, Prakash L. Requirement of RAD5 and MMS2 for postreplication repair of UV-damaged DNA in Saccharomyces cerevisiae. Mol Cell Biol. 2002;22(7):2419–2426. doi: 10.1128/MCB.22.7.2419-2426.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blastyák A, et al. Yeast Rad5 protein required for postreplication repair has a DNA helicase activity specific for replication fork regression. Mol Cell. 2007;28(1):167–175. doi: 10.1016/j.molcel.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ong SE, et al. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics. 2002;1(5):376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 53.Cohen EA, Dehni G, Sodroski JG, Haseltine WA. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J Virol. 1990;64(6):3097–3099. doi: 10.1128/jvi.64.6.3097-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paxton W, Connor RI, Landau NR. Incorporation of Vpr into human immunodeficiency virus type 1 virions: Requirement for the p6 region of gag and mutational analysis. J Virol. 1993;67(12):7229–7237. doi: 10.1128/jvi.67.12.7229-7237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maudet C, et al. Molecular insight into how HIV-1 Vpr protein impairs cell growth through two genetically distinct pathways. J Biol Chem. 2011;286(27):23742–23752. doi: 10.1074/jbc.M111.220780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matheson NJ, et al. Cell surface proteomic map of HIV infection reveals antagonism of amino acid metabolism by Vpu and Nef. Cell Host Microbe. 2015;18(4):409–423. doi: 10.1016/j.chom.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hrecka K, et al. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011;474(7353):658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laguette N, et al. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474(7353):654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Unk I, et al. Human HLTF functions as a ubiquitin ligase for proliferating cell nuclear antigen polyubiquitination. Proc Natl Acad Sci USA. 2008;105(10):3768–3773. doi: 10.1073/pnas.0800563105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helmer RA, et al. Helicase-like transcription factor (Hltf) regulates G2/M transition, Wt1/Gata4/Hif-1a cardiac transcription networks, and collagen biogenesis. PLoS One. 2013;8(11):e80461. doi: 10.1371/journal.pone.0080461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DePaula-Silva AB, et al. Determinants for degradation of SAMHD1, Mus81 and induction of G2 arrest in HIV-1 Vpr and SIVagm Vpr. Virology. 2015;477:10–17. doi: 10.1016/j.virol.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Casey Klockow L, et al. The HIV-1 protein Vpr targets the endoribonuclease Dicer for proteasomal degradation to boost macrophage infection. Virology. 2013;444(1-2):191–202. doi: 10.1016/j.virol.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Romani B, Shaykh Baygloo N, Hamidi-Fard M, Aghasadeghi MR, Allahbakhshi E. HIV-1 Vpr protein induces proteasomal degradation of chromatin-associated class I HDACs to overcome latent infection of macrophages. J Biol Chem. 2015;290:17380–17389. doi: 10.1074/jbc.M115.689018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X, et al. HIV-1 Vpr protein inhibits telomerase activity via the EDD-DDB1-VPRBP E3 ligase complex. J Biol Chem. 2013;288(22):15474–15480. doi: 10.1074/jbc.M112.416735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharp PM, Bailes E, Stevenson M, Emerman M, Hahn BH. Gene acquisition in HIV and SIV. Nature. 1996;383(6601):586–587. doi: 10.1038/383586a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tristem M, Marshall C, Karpas A, Hill F. Evolution of the primate lentiviruses: Evidence from vpx and vpr. EMBO J. 1992;11(9):3405–3412. doi: 10.1002/j.1460-2075.1992.tb05419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Checroune F, Yao XJ, Gottlinger HG, Bergeron D, Cohen EA. Incorporation of Vpr into human immunodeficiency virus type 1: Role of conserved regions within the P6 domain of Pr55gag. J AIDS Human Retrovirol. 1995;10(1):1–7. [PubMed] [Google Scholar]
- 68.Selig L, et al. Interaction with the p6 domain of the gag precursor mediates incorporation into virions of Vpr and Vpx proteins from primate lentiviruses. J Virol. 1999;73(1):592–600. doi: 10.1128/jvi.73.1.592-600.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ayinde D, Maudet C, Transy C, Margottin-Goguet F. Limelight on two HIV/SIV accessory proteins in macrophage infection: Is Vpx overshadowing Vpr? Retrovirology. 2010;7:35. doi: 10.1186/1742-4690-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bétous R, et al. SMARCAL1 catalyzes fork regression and Holliday junction migration to maintain genome stability during DNA replication. Genes Dev. 2012;26(2):151–162. doi: 10.1101/gad.178459.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Couch FB, et al. ATR phosphorylates SMARCAL1 to prevent replication fork collapse. Genes Dev. 2013;27(14):1610–1623. doi: 10.1101/gad.214080.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Achar YJ, Balogh D, Haracska L. Coordinated protein and DNA remodeling by human HLTF on stalled replication fork. Proc Natl Acad Sci USA. 2011;108(34):14073–14078. doi: 10.1073/pnas.1101951108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burkovics P, Sebesta M, Balogh D, Haracska L, Krejci L. Strand invasion by HLTF as a mechanism for template switch in fork rescue. Nucleic Acids Res. 2014;42(3):1711–1720. doi: 10.1093/nar/gkt1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hishiki A, et al. Structure of a novel DNA-binding domain of helicase-like transcription factor (HLTF) and its functional implication in DNA damage tolerance. J Biol Chem. 2015;290(21):13215–13223. doi: 10.1074/jbc.M115.643643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Allouch A, et al. p21-mediated RNR2 repression restricts HIV-1 replication in macrophages by inhibiting dNTP biosynthesis pathway. Proc Natl Acad Sci USA. 2013;110(42):E3997–E4006. doi: 10.1073/pnas.1306719110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bergamaschi A, et al. The CDK inhibitor p21Cip1/WAF1 is induced by FcgammaR activation and restricts the replication of human immunodeficiency virus type 1 and related primate lentiviruses in human macrophages. J Virol. 2009;83(23):12253–12265. doi: 10.1128/JVI.01395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doehle BP, Hladik F, McNevin JP, McElrath MJ, Gale M., Jr Human immunodeficiency virus type 1 mediates global disruption of innate antiviral signaling and immune defenses within infected cells. J Virol. 2009;83(20):10395–10405. doi: 10.1128/JVI.00849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Majumder B, Venkatachari NJ, Srinivasan A, Ayyavoo V. HIV-1 mediated immune pathogenesis: Spotlight on the role of viral protein R (Vpr) Curr HIV Res. 2009;7(2):169–177. doi: 10.2174/157016209787581445. [DOI] [PubMed] [Google Scholar]
- 79.Okumura A, et al. HIV-1 accessory proteins VPR and Vif modulate antiviral response by targeting IRF-3 for degradation. Virology. 2008;373(1):85–97. doi: 10.1016/j.virol.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roesch F, et al. Vpr enhances tumor necrosis factor production by HIV-1-infected T cells. J Virol. 2015;89(23):12118–12130. doi: 10.1128/JVI.02098-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sato K, et al. HIV-1 Vpr accelerates viral replication during acute infection by exploitation of proliferating CD4+ T cells in vivo. PLoS Pathog. 2013;9(12):e1003812. doi: 10.1371/journal.ppat.1003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kile AC, et al. HLTF’s ancient HIRAN domain binds 3′ DNA ends to drive replication fork reversal. Mol Cell. 2015;58(6):1090–1100. doi: 10.1016/j.molcel.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu Y. Unwinding and rewinding: Double faces of helicase? J Nucleic Acids. 2012;2012:140601. doi: 10.1155/2012/140601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baradaran-Heravi A, et al. SMARCAL1 deficiency predisposes to non-Hodgkin lymphoma and hypersensitivity to genotoxic agents in vivo. Am J Med Genet A. 2012;158A(9):2204–2213. doi: 10.1002/ajmg.a.35532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goldstone DC, et al. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature. 2011;480(7377):379–382. doi: 10.1038/nature10623. [DOI] [PubMed] [Google Scholar]
- 86.Lahouassa H, et al. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol. 2012;13(3):223–228. doi: 10.1038/ni.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Powell RD, Holland PJ, Hollis T, Perrino FW. Aicardi-Goutieres syndrome gene and HIV-1 restriction factor SAMHD1 is a dGTP-regulated deoxynucleotide triphosphohydrolase. J Biol Chem. 2011;286(51):43596–43600. doi: 10.1074/jbc.C111.317628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Duggal NK, Emerman M. Evolutionary conflicts between viruses and restriction factors shape immunity. Nat Rev Immunol. 2012;12(10):687–695. doi: 10.1038/nri3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ward J, et al. HIV-1 Vpr triggers natural killer cell-mediated lysis of infected cells through activation of the ATR-mediated DNA damage response. PLoS Pathog. 2009;5(10):e1000613. doi: 10.1371/journal.ppat.1000613. [DOI] [PMC free article] [PubMed] [Google Scholar]