Significance
The proliferation of vascular smooth muscle cells (VSMCs) in response to excessive cyclic stretch is crucial in vascular remodeling in hypertension. To elucidate the molecular mechanism, we studied the mechanobiological roles of emerin and lamin A/C, two important components of nuclear envelope proteins localized beneath the inner nuclear membrane. We found that emerin and lamin A/C play significant roles in the mechanical modulation of VSMC proliferation. The repressed expression of emerin and lamin A/C mediates the stretch-induced VSMC proliferation, which is important in vascular remodeling during hypertension. Emerin and lamin A/C bind to the respective sequencing-specific motifs of transcription factors to mediate the molecular mechanisms underlying the hyperstretch-induced VSMC dysfunction.
Keywords: mechanobiology, emerin, laminA/C, specific-binding sequence, transcription factors
Abstract
Cyclic stretch is an important inducer of vascular smooth muscle cell (VSMC) proliferation, which is crucial in vascular remodeling during hypertension. However, the molecular mechanism remains unclear. We studied the effects of emerin and lamin A/C, two important nuclear envelope proteins, on VSMC proliferation in hypertension and the underlying mechano-mechanisms. In common carotid artery of hypertensive rats in vivo and in cultured cells subjected to high (15%) cyclic stretch in vitro, VSMC proliferation was increased significantly, and the expression of emerin and lamin A/C was repressed compared with normotensive or normal (5%) cyclic stretch controls. Using targeted siRNA to mimic the repressed expression of emerin or lamin A/C induced by 15% stretch, we found that VSMC proliferation was enhanced under static and 5%-stretch conditions. Overexpression of emerin or lamin A/C reversed VSMC proliferation induced by 15% stretch. Hence, emerin and lamin A/C play critical roles in suppressing VSMC hyperproliferation induced by hyperstretch. ChIP-on-chip and MOTIF analyses showed that the DNAs binding with emerin contain three transcription factor motifs: CCNGGA, CCMGCC, and ABTTCCG; DNAs binding with lamin A/C contain the motifs CVGGAA, GCCGCYGC, and DAAGAAA. Protein/DNA array proved that altered emerin or lamin A/C expression modulated the activation of various transcription factors. Furthermore, accelerating local expression of emerin or lamin A/C reversed cell proliferation in the carotid artery of hypertensive rats in vivo. Our findings establish the pathogenetic role of emerin and lamin A/C repression in stretch-induced VSMC proliferation and suggest mechanobiological mechanism underlying this process that involves the sequence-specific binding of emerin and lamin A/C to specific transcription factor motifs.
The cyclic stretch caused by the rhythmical distention and relaxation of the arterial wall during the cardiac cycle is an important factor in the regulation of vascular modeling and remodeling (1, 2). There is growing evidence that mechanical cyclic stretch modulates the functions (e.g., apoptosis, proliferation, and migration) of vascular smooth muscle cells (VSMCs) in the media of the arterial wall (2) and that chronically elevated cyclic stretch stimulates VSMC functions to mediate vascular remodeling during hypertension (3, 4).
It has been shown that there are various mechano-sensors in the vascular cell membrane, including lipids (5), glycocalyx (6), and proteins such as integrins (7), G proteins and G protein-coupled receptors (8), receptor tyrosine kinase (9), and Ca2+ channel (10) and intercellular junction proteins (2, 11). In recent years, it has been suggested that nuclear envelope (NE) proteins, a hallmark of eukaryotic cells, participate in the mechano-transduction networks. Our previous proteomic analysis revealed that lamin A/C, one kind of NE protein, is mechano-responsive and may contribute to the shear stress-induced proliferation and migration of VSMCs (12). Recently, Swift et al. (13) reported that tissue micromechanics influence the expression of nuclear lamin A, which contributes to matching nuclear mechanics to the stiffness of surrounding tissues and participates in the regulation of stem cell differentiation. These studies revealed that mechano-responsive NE proteins impact on cell behavior, but their roles in the mechano-sensing and regulation of gene expression are still unclear.
NE proteins form an architectural complex on the nucleus membrane through the association of SUN (Sad1 and UNC-84 homology domain) proteins, nesprin proteins, A-type (the most common are lamin A and lamin C) and B-type lamins, and emerin. This complex provides a physical connection between the cytoskeleton and the nucleoplasm and hence is named the “LINC” (linker of the nucleoskeleton and cytoskeleton) complex (14, 15). This physical structure allows mechanical transduction from the cytoskeleton directly into the nucleus. Furthermore, the nucleus is the largest and stiffest organelle in the cell that contains the genome and is the site of transcriptional regulation (16). Using magnetic tweezers, Guilluy et al. (17) introduced mechanical stretch directly to the isolated nuclei and revealed that mechano-transduction, which requires intact nuclear lamin A/C and emerin, can occur within the nucleus.
Nuclear lamin A/C and emerin are important components of the inner nuclear membrane (INM) that link with chromatin and participate in the spatial organization of chromosomes and gene transcription (14, 15). Defects in lamins or emerin cause a number of diseases, such as cardiomyopathy and muscular dystrophy (14, 15, 18). Because our previous work revealed that lamin A/C might contribute to the vascular remodeling in response to shear stress (12), we hypothesized that NE proteins might be mechanical-sensitive molecules that participate in the VSMC functions induced by cyclic stretch.
In the present study, we investigated the effects of emerin and lamin A/C on VSMC proliferation in response to hypertension and studied the mechanobiological mechanisms involved. The results provided insights into the roles of these two important INM proteins in the transcriptional regulation of VSMC proliferation.
Results
Hypertension Represses the Expression of NE Proteins in Vivo.
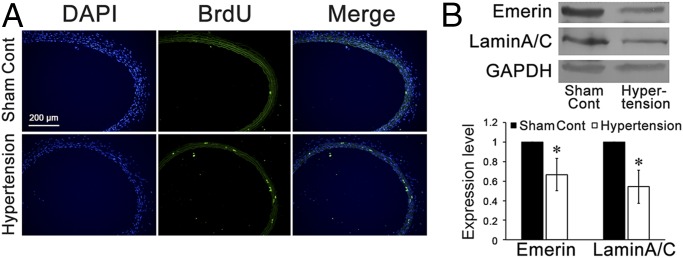
After 1 wk of abdominal aorta coarctation, the operated rats exhibited a marked level of hypertension (mean arterial pressure 161.7 ± 14.9 mmHg in hypertensive rats vs. 96.9 ± 9.9 in sham controls, P < 0.01), a marked increase of cell proliferation in the media of common carotid arteries (Fig. 1A), and a significant suppression of emerin and lamin A/C expression in the common carotid arteries as compared with the sham-operated controls (Fig. 1B and Fig. S1).
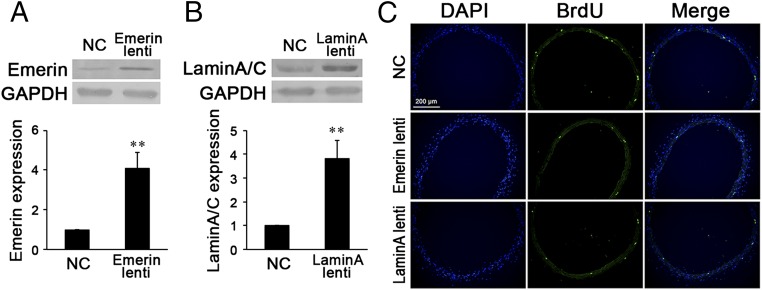
Fig. 1.
In situ proliferation and expression of emerin and lamin A/C in the common carotid artery of hypertensive rats and sham-treated controls. (A) Immunofluorescence staining against BrdU revealed that after 1 wk of abdominal aorta coarctation, VSMC proliferation in the media of common carotid arteries increased markedly. (B) The expression of emerin and of lamin A/C was repressed significantly in the common carotid arteries of the aorta-coarctation–induced hypertensive rats. GAPDH was used for normalization. Values are expressed as mean ± SD. *P < 0.05 vs. the sham-treated control (n = 5).
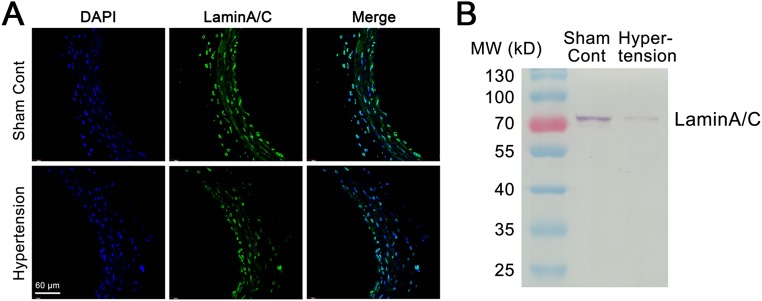
Fig. S1.
The expression of lamin A/C in the common carotid arteries of hypertensive rats was repressed in comparison with the expression in sham controls. (A) Immunofluorescence staining of lamin A/C in the common carotid artery is higher in a normal control rat than in a hypertensive rat. (B) Entire immunoblots of lamin A/C in the common carotid arteries of aorta-coarctation hypertensive rats and sham-operated controls.
The increased cyclic mechanical stretch in hypertension has been shown to play an important role in VSMC proliferation (19). We hypothesized that cyclic stretch may modulate VSMC proliferation by inducing the expression of emerin and lamin A/C.
Cyclic Stretch Modulates the Expression of NE Proteins and the Proliferation of VSMCs in Vitro.
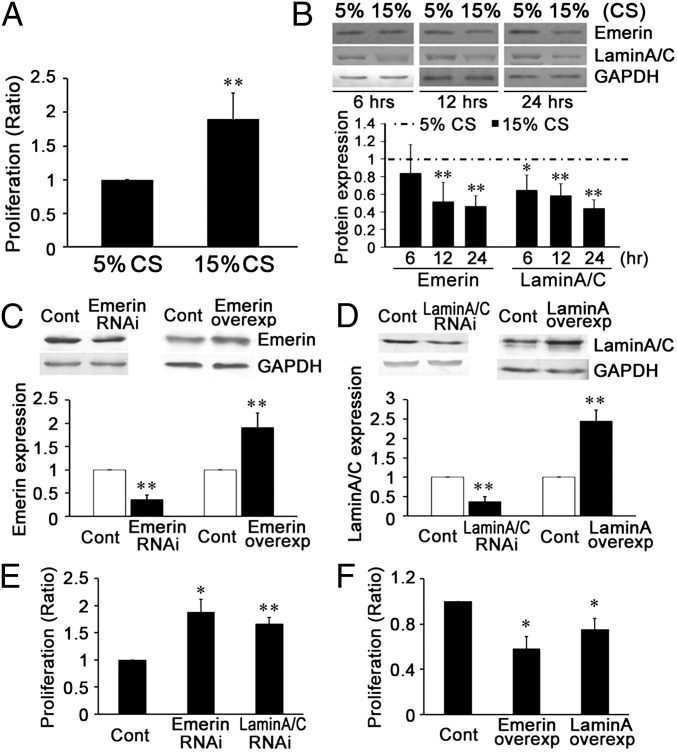
Compared with the group subjected to the normal level (5%) of cyclic stretch (20) for 24 h, a high level (15%) of cyclic stretch (21) significantly increased the proliferation of VSMCs, as shown by BrdU ELISA (Fig. 2A).
Fig. 2.
Cyclic stretch (CS) modulated the expression of NE proteins emerin and lamin A/C, which participate in the stretch-induced proliferation of VSMCs in vitro. (A) A 15% cyclic stretch increased VSMC proliferation in comparison with a 5% cyclic stretch. (B) A 15% cyclic stretch decreased the expression of emerin and lamin A/C in comparison with a 5% cyclic stretch. (C) Under static conditions, target siRNA transfection significantly decreased and plasmid transfection significantly increased the expression of emerin. (D) Under static conditions, target siRNA transfection significantly decreased and plasmid transfections significantly increased the expression of lamin A/C. (E) Under static conditions, specific RNAi of both emerin and lamin A/C increased VSMC proliferation. (F) Up-regulated expression of emerin and lamin A/C decreased VSMC proliferation. For Western blots, GAPDH was used for normalization. Values are expressed as mean ± SD. *P < 0.05, **P < 0.01 vs. the respective control (Cont) (n = 5).
The effects of different levels of cyclic stretch on the expression of emerin and lamin A/C proteins were studied at 6, 12, and 24 h. Compared with the normal 5% cyclic stretch, the 15% cyclic stretch decreased the expression of emerin (at 12 and 24 h but not at 6 h) and lamin A/C (at all time points: 6, 12, and 24 h) (Fig. 2B).
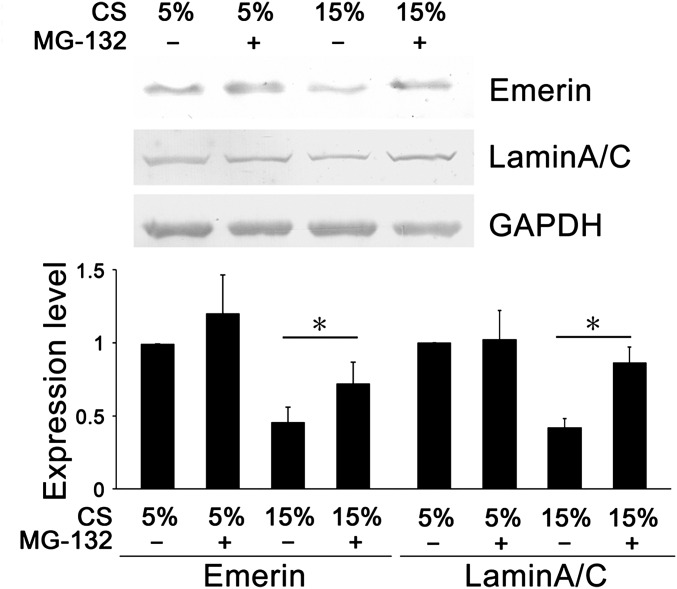
Compared with 5% cyclic stretch, 15% cyclic stretch decreased the mRNA level of emerin at the 24-h time point but not at 6 and 12 h. The 15% cyclic stretch showed no significant effect on mRNA level of lamin A/C at any of the time points (6, 12, and 24 h) (Table S1). The proteasome inhibitor MG-132(R) (C26H41N3O5) (10 μM) was used to detect possible lamin A/C and emerin degradation during the application of cyclic stretch. We found that MG-132(R) reversed, at least partially, the repression of emerin and lamin A/C protein expression induced by 15% cyclic stretch (Fig. S2). These results suggest that pathological (15%) cyclic stretch may increase the degradation of lamin A/C and emerin and that the repressed levels of these two NE proteins may be important in the VSMC hyperproliferation induced by hyperstretch.
Fig. S2.
MG-132(R), a proteasome inhibitor, increased expression of emerin and lamin A/C in VSMCs subjected to 15% cyclic stretch for 24 h. GAPDH was used for normalization. Values are expressed as mean ± SD. *P < 0.05 vs. the respective control (n = 4). CS, cyclic stretch.
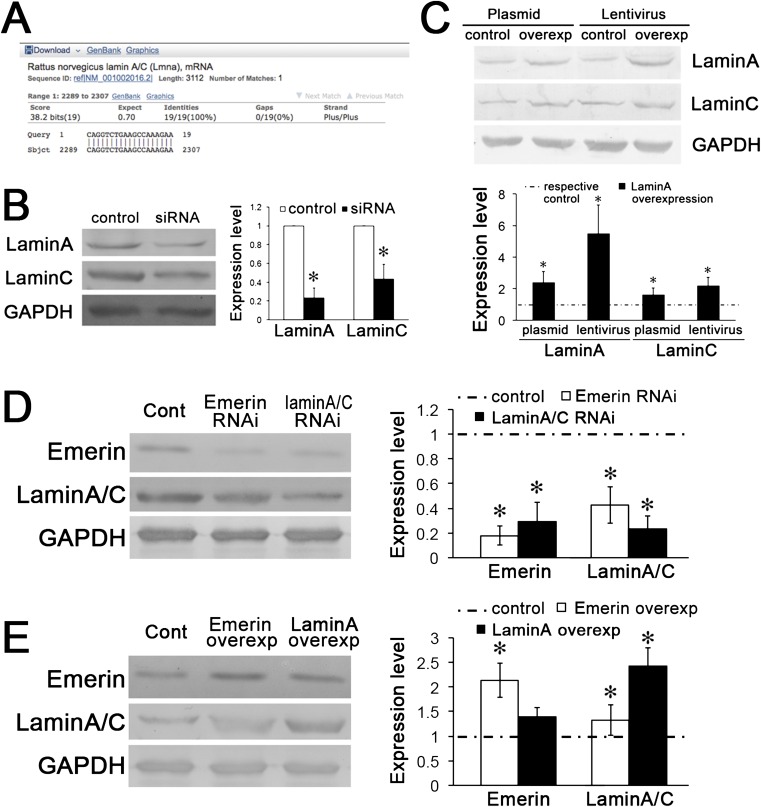
To assess the roles of these NE proteins in modulating VSMC proliferation, the expression of emerin or lamin A/C under static conditions was repressed by specific siRNA transfection (Fig. 2 C and D and Fig. S3 A and B). The results indicate that the proliferation of VSMCs under static conditions is increased significantly by the repression of either emerin or lamin A/C (Fig. 2E). Transfection of a plasmid overexpressing lamin A increased the expression of both lamin A and lamin C (Fig. S3C), and plasmids overexpressing emerin and lamin A significantly decreased VSMC proliferation (Fig. 2F).
Fig. S3.
The expressions of emerin, lamin A, and lamin C are correlated with one other. (A) The siRNA (CAGG UCUG AAGC CAAA GAAT T) targets on LMNA, which is the template of both lamin A and lamin C. (B) Transfection of target siRNA to lamin A/C modulated the expression of both lamin A and lamin C. GAPDH was used for normalization. Values are expressed as mean ± SD. *P < 0.05 vs. the respective control (n = 4). (C) Overexpression of lamin A with either plasmid or lentivirus markedly increased the expression of both lamin A and lamin C. GAPDH was used for normalization. Values are expressed as mean ± SD. *P < 0.05 vs. the respective control (n = 4). (D) After transfection of emerin siRNA for 48 h, the expression of lamin A/C was decreased; conversely, lamin A/C siRNA also decreased the expression of emerin. (E) Emerin overexpression increased the expression of lamin A/C; lamin A overexpression increased the expression of lamin A/C but had no significant effect on the expression of emerin. GAPDH was used for normalization. Values are expressed as mean ± SD. *P < 0.05 vs. the respective control (n = 6).
These results indicate that a pathological (15%) level of cyclic stretch (21) decreases the expression of emerin and lamin A/C and increases the proliferation of VSMCs.
Cyclic Stretch Modulates the Proliferation of VSMCs via NE Proteins in Vitro.
To confirm the effects of emerin and lamin A/C on cyclic stretch-induced VSMC proliferation, VSMCs were transfected with emerin or lamin A/C target siRNA (Fig. 3A and Fig. S4A) and then were subjected to normal (5%) cyclic stretch. The results show that, compared with negative control, transfection of either emerin- or lamin A/C-target siRNA significantly increased VSMC proliferation under 5% cyclic stretch (Fig. 3B). In contrast, transfection with a plasmid overexpressing emerin or lamin A (Fig. 3C and Fig. S4B) reversed the effect of high (15%) cyclic stretch on VSMC proliferation (Fig. 3D).
Fig. 3.
Cyclic stretch modulates the proliferation of VSMCs via NE proteins in vitro. (A) The expression of emerin or lamin A/C was repressed by target siRNA transfection during the application of 5% cyclic stretch. (B) Emerin- or lamin A/C-target siRNA transfection increased the proliferation of VSMCs. (C) The expression of emerin and of lamin A/C was increased by plasmid transfection. (D) Transfection with plasmid overexpressing emerin and lamin A decreased VSMC proliferation under 15% cyclic stretch. For Western blots, GAPDH was used for normalization. Values are expressed as mean ± SD. *P < 0.05, **P < 0.01 vs. the respective control (n = 6).
Fig. S4.
Entire immunoblots of lamin A/C in cultured VSMCs and common carotid arteries. (A) Entire immunoblots of lamin A/C in VSMCs transfected with siRNA during the application of 5% cyclic stretch. (B) Entire immunoblots of lamin A/C in VSMCs transfected with overexpression plasmid of lamin A during the application of 15% cyclic stretch. (C) Entire immunoblots of lamin A/C in the common carotid arteries of hypertensive rats locally injected with lentiviruses of lamin A or the respective control. NC, negative control.
These results suggest that the NE proteins emerin and lamin A/C are crucial mechano-responsive molecules participating in the modulation of VSMC proliferation. The increase in VSMC proliferation induced by the decreased expression of emerin and lamin A/C in response to pathologically increased cyclic stretch may be involved in the pathogenesis of vascular remodeling during hypertension.
ChIP with Microarray Analysis Revealed the Binding Between NE Proteins and Chromatin.
To demonstrate the possible mechanism by which NE proteins regulate VSMC proliferation, ChIP with microarray (ChIP-on-chip) and bioinformatics analyses were used to detect the DNA-binding ability of emerin and lamin A/C and to demonstrate whether this binding affects transcription factors related to proliferation. DNA extractions from VSMCs were incubated with emerin or lamin A/C antibody, and the pulled-down DNA was analyzed by CpG Island Plus Refseq Promoter arrays. The results reveal that 1,030 DNA segments were immunoprecipitated with emerin (Table S2), and 1,046 DNA segments were immunoprecipitated with lamin A/C (Table S2).
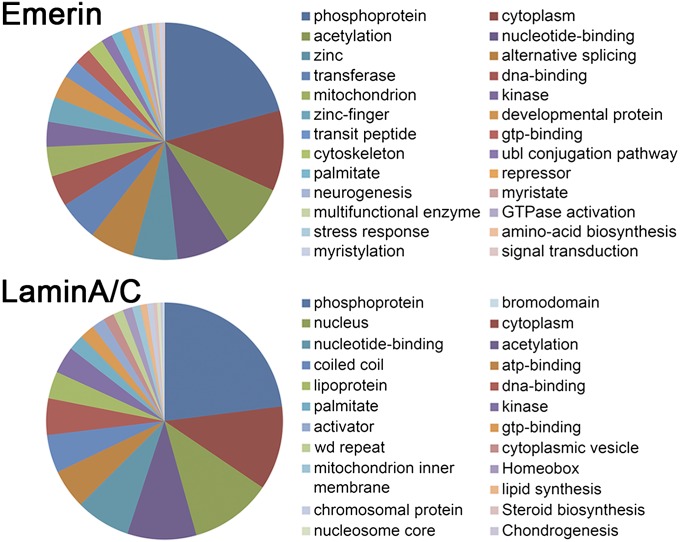
The primary functions of the immunoprecipitated DNA segments were classified by Gene Ontology (GO) annotations using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) v. 6.7 (https://david.ncifcrf.gov/summary.jsp). Fig. 4 shows that the DNA segments immunoprecipitated with emerin or lamin A/C are involved in different functional categories. The top five prominent functional categories for DNA segments immunoprecipitated with emerin were phosphoprotein (232 DNA segments), cytoplasm (122 DNA segments), acetylation (103 DNA segments), nucleotide-binding (81 DNA segments), and zinc (68 DNA segments) (Table S3); the top five prominent functional categories for DNA segments immunoprecipitated with lamin A/C were phosphoprotein (249 DNA segments), cytoplasm (124 DNA segments), nucleus (121 DNA segments), acetylation (102 DNA segments), and nucleotide-binding (81 DNA segments) (Table S3).
Fig. 4.
GO annotations show the primary functional categories of the DNA segments immunoprecipitated with emerin or lamin A/C (Table S2).
These results suggest that both emerin and lamin A/C can bind with DNA segments, but the cellular functions involved differ somewhat.
Motifs of Transcription Factors Enriched in DNA Segments Binding with NE Proteins.
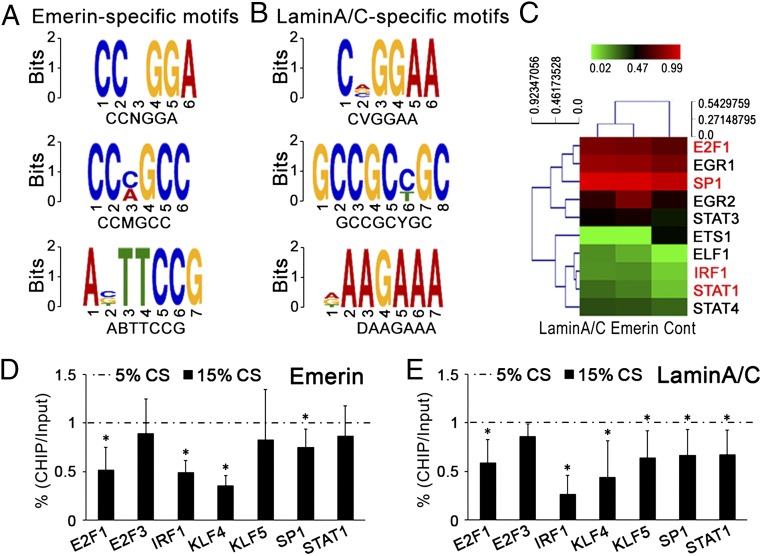
To assess whether the binding of DNA segments with emerin or lamin A/C is sequence specific, we used Multiple Em for Motif Elicitation (MEME) (22) and Discriminative Regular Expression Motif Elicitation (DREME) (23) web service to visualize the locations of matches to the promoters of transcription factor (24). The specific motifs in the DNA segments binding with emerin were CCNGGA, CCMGCC, and ABTTCCG (Fig. 5A), and those binding with lamin A/C were CVGGAA, GCCGCYGC, and DAAGAAA (Fig. 5B). Table S4 shows the transcription factors revealed by the ChIP-on-chip assay and includes the sequence-specific motifs in their promoter regions.
Fig. 5.
Motifs of transcription factors enriched in DNA segments binding with emerin or lamin A/C. (A) The specific motifs in the DNA segments immunoprecipitated with emerin. (B) The specific motifs in the DNA segments immunoprecipitated with lamin A/C. (C) Emerin or lamin A/C-target siRNA modulated the activations of transcription factors in Table S4. The red font indicates the factors involved in the proliferation of smooth muscle cells. (D and E) After immunoprecipitation with emerin (D) or lamin A/C (E), qPCR detected the ChIP levels of promoter regions of transcription factors related with “proliferation of (vascular) smooth muscle cells” in Table S4 after cyclic stretch was applied for 24 h. Values are expressed as mean ± SD (n = 4).
These results suggest that emerin and lamin A/C bind to specific DNA segments, including the promoter regions of transcription factors. To detect whether this sequence-specific binding is involved in VSMC proliferation, Ingenuity Pathway Analysis (IPA), ChIP-quantitative PCR (qPCR), and Protein/DNA array were used to detect the binding of emerin or lamin A/C to the promoter regions of specific transcription factors.
Binding of Emerin and Lamin A/C with the Promoter Regions of Transcription Factors Participates in VSMC Proliferation.
Using IPA software (content version: 14197757) (www.ingenuity.com/products/ipa), we investigated the functions of the transcription factors whose promoter regions were immunoprecipitated with emerin or lamin A/C (24). Six transcription factors related to emerin and four related to lamin A/C were involved in VSMC proliferation (Table S4). We then detected the role of altered emerin and lamin A/C expression in the activation of these transcription factors.
Using the Protein/DNA array, we detected the activation of 10 transcription factors revealed by ChIP-on-chip and motif analyses (Fig. 5C). The activation of four transcription factors related to VSMC proliferation showed a tendency to increase when emerin and lamin A/C were repressed by siRNA transfection.
Cyclic Stretch Alters the Ability of Emerin and Lamin A/C to Bind to the Promoter Region of Transcription Factors.
After different level of cyclic stretch were applied for 24 h, DNA extracts from the VSMCs were incubated with emerin or lamin A/C antibody, and the immunoprecipitated DNA segments were analyzed by qPCR to quantify the promoter regions of transcription factors related to VSMC proliferation (Table S4).
The results showed that, as compared with 5% cyclic-stretch, 15% stretch decreased the binding of emerin to the promoter regions of E2F1, IRF1, KLF4, and SP1 and decreased the binding of lamin A/C the to the promoter regions of E2F1, IRF1, KLF4, KLF5, SP1, and STAT1 (Fig. 5 D and E).
Increased Expression of NE Proteins Represses VSMC Proliferation in Vivo.
To demonstrate further the possible protective effects of emerin and lamin A/C on VSMC proliferation during hypertension, their overexpression was induced by the transcutaneous injection of lentiviruses around the common carotid artery of hypertensive rats in vivo (Fig. 6 A and B). The lentivirus of lamin A significantly increased the expression of both lamin A and lamin C (Fig. 6B and Figs. S3C and S4C). In comparison with the negative control, emerin and lamin A/C overexpression significantly decreased cell proliferation in the media of the common carotid arteries of hypertensive rats (Fig. 6C).
Fig. 6.
(A and B) Local injection of emerin (A) or lamin A (B) lentiviruses (lenti) significantly increased the expression of emerin or lamin A/C in common carotid arteries the hypertensive rats. (C) Immunofluorescence staining against BrdU revealed that locally injected lentiviruses of emerin or lamin A remarkably decreased VSMC proliferation in the media of common carotid arteries of hypertensive rats. For Western blot, GAPDH was used for normalization. Values are expressed as mean ± SD. **P < 0.01 vs. the negative control (NC) (n = 6).
Discussion
The nuclear lamins form a mesh-like network of intermediate filaments localized mainly at the INM and play a major role in maintaining the mechanical stability and shape of the nucleus (13, 25). There are four lamin isoforms in mammalian cells: A-type lamins, including lamin A and lamin C, which are splice variants encoded by LMNA, and B-type lamins, including lamin B1 and B2 encoded by LMNB1 and LMNB2, respectively (25, 26). Lamin B1 and B2 are essential proteins that are expressed in all cells throughout development (4, 8, 26), whereas lamin A and C are expressed only in differentiated cells. The differential expression patterns of lamins suggest that lamin A/C may be important for inducing or maintaining the differentiated state. Recently, the differential expression of lamin A/C has been detected in cells subjected to different kinds of mechanical stimuli and has been shown to be involved in differential cellular functions. Harada et al. (27) reported that differentially expressed lamin A/C can modulate cell migration and survival in human mesenchymal stem cells and two cancer cell lines. Swift et al. (13) found that lamin A, but not lamin B, is changed by matrices of different stiffness and participates in the differentiation of mesenchymal stem cells induced by the stiffness of the culture matrix. Consistent with the results reported here indicating that cyclic stretch may posttranslationally regulate lamin A/C, a 40-kDa lower band of lamin A/C (Fig. S4) indicates cleavage of lamin A/C that is regulated by adhesion and cell mechanics, as shown by Buxboim et al. (28). Furthermore, our current study demonstrates that, in addition to lamin A/C, the expression of emerin in VSMCs also is modulated by mechanical cyclic stretch and subsequently induces VSMC proliferation.
Emerin acts as an anchor of NE to INM and binds to lamin A/C at the nucleoplasm (26). The mutations in EMD (encoded emerin, located on chromosome X in humans and mice) and LMNA (encoded lamin A and lamin C, located on chromosome 1 in humans and chromosome 3 in mice) are related to similar diseases characterized by progressive skeletal muscle weakening, abnormal fat deposition, premature aging, and complex cardiac syndrome, including dilated cardiomyopathy and life-threatening irregular heart rhythms (29, 30). Embryo fibroblasts cultured from lamin A-deficient (Lmna−/−) mice (30, 31) and from emerin-deficient (Emd-/y for males or Emd−/− for females) mice (30) demonstrate defective nuclear mechanics and impaired transcription of mechanically activated genes. In the aortic media, the single-nucleotide mutation of LMNA at G608G decreases the expression of mechanotransduction proteins such as vinculin, transgelin, and vimentin in VSMCs under high (but not under normal) shear stress (32). The results of this study using the hypertensive animal model and cultured VSMCs suggest that emerin and lamin A/C have protective effects and that the repressed expression of emerin and lamin A/C by pathologically elevated (15%) cyclic stretch (21) may be important in the abnormal VSMC proliferation in hypertension. All these results suggest that both emerin and lamin A/C are important mechano-responsive molecules that play important roles in cardiovascular functions.
Using ChIP-on-chip analysis to analyze the molecular mechanisms by which emerin and lamin A/C modulate VSMC functions, we found 1,030 DNA segments binding with emerin and 1,046 binding with lamin A/C. Using IPA software, we further analyzed the DNA segments immunoprecipitated with emerin or lamin A/C (Table S3) that participate in the modulation of cell proliferation. Interestingly, in addition to their role in the proliferation of smooth muscle cells (Table S4), these DNA segments are involved in the proliferation of many other types of cells (e.g., neuronal cells, fibroblasts, and others), suggesting that emerin and lamin A/C may have universal effects on cellular proliferation. Our present research revealed that the decreased expression of emerin and lamin A/C attenuates their binding to the promoter region of transcription factors and that these changes negatively regulate VSMC proliferation under different conditions (static, 5% cyclic stretch, and 15% cyclic stretch) (Fig. S5). Our previous study also revealed that 15% cyclic stretch accelerated apoptosis of VSMCs (33); that finding, combined with our present results, suggests that a pathological level of cyclic stretch may induce rapid VSMC turnover. It will be very informative to demonstrate the potential roles of emerin and lamin A/C in VSMC apoptosis induced by mechanical stimuli. However, the molecular mechanisms involved in cell turnover probably are quite complex, and this subject deserves further investigation beyond our present work, which focuses on the mechanobiological mechanisms of VSMC proliferation.
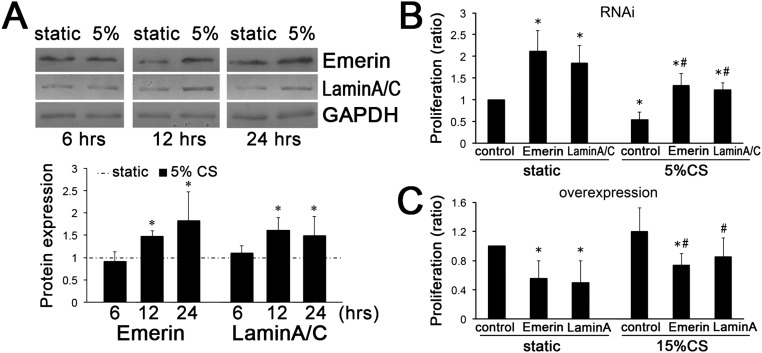
Fig. S5.
Emerin and lamin A/C negatively regulate VSMC proliferation under different conditions (static control, 5% cyclic stretch, and 15% cyclic stretch). (A) Emerin and lamin A/C expression was increased after 5% cyclic stretch (CS) applied for 12 h and 24 h, compared with static control. GAPDH was used for normalization. Values are expressed as mean ± SD; *P < 0.05 vs. the static control (n = 5). (B and C) Emerin and lamin A/C were repressed by RNAi (B) or overexpressed by plasmid transfection (C). Then VSMCs were subjected to 5% and 15% cyclic stretch. The results indicate that both emerin and lamin A/C exert an inhibitory effect on cell proliferation under stretch conditions. *P < 0.05 vs. the static control, #P < 0.05 vs. the respective control under cyclic stretch application (n = 4).
Although it had been reported that nuclear lamin A/C and emerin link with chromatin (14, 15), the mechanism and character of their binding are still unclear. It has been demonstrated that some kinds of chromatin-binding proteins link the INM to the chromatin and are involved in the organized localization of chromatin within the nucleus, DNA replication, and mRNA synthesis (34). For example, BAF binds chromatin with both emerin (35) and lamin A (36) in vivo and in vitro. In addition to its binding via other proteins, there is evidence that lamin A may bind to DNA with some specific sequences. Zullo et al. (37) reported that discrete DNA regions characterized as (GA)n repeats are associated with the binding of chromatin and lamin A and are not affected by BAF knockdown. Artificially generated tandem arrays containing (GA)n repeats revealed their colocalization with lamin A (37). Ottaviani et al. (38) demonstrated the ability of cis-acting elements containing (GA)n and CCCTC-binding factor (CTCF) to bind with lamin A/C. Our results showed that the DNA sequences binding with lamin A/C do not have the (GA)n repeats but do have two motifs with a high G/A ratio, CVGGA and DAAGAAA, and one motif with a high G/C ratio, GCCGCYGC. Interestingly, emerin also has three sequencing-specific binding motifs, two of which, CCNGGA and CCMGCC, have a high G/C ratio. The DNA fragments that bind with emerin are not same as those that bind with lamin A/C, suggesting that emerin and lamin A/C regulate cellular functions via different mechanisms.
Our present results demonstrate the sequencing-specific binding of emerin or lamin A/C to promoter regions of various transcription factors, but many other questions remain to be answered. Thus, it is still unclear whether the DNA binding of emerin and lamin A/C modulated by cyclic stretch is direct or involves a complex. Our present work demonstrates that the expressions of emerin, lamin A, and lamin C are correlated with each other (Fig. S3), suggesting complex interactions among different kinds of NE proteins. The mechano-transduction network in the nucleus and the roles of NE protein interactions need further studies.
In summary, our current results indicate that pathologically elevated cyclic stretch represses the expression of two NE proteins, emerin and lamin A/C, which may play important roles in vascular remodeling during hypertension. The repressed expression of emerin and lamin A/C is a pathogenetic factor in the stretch-induced VSMC proliferation. The sequencing-specific DNA binding of both emerin and lamin A/C to the promoter regions of specific transcription factors may play a significant role in the molecular mechanisms underlying stretch-induced VSMC functions.
Materials and Methods
The animal investigation conforms to the guidelines in the Guide for the Care and Use of Laboratory Animals (39) published by the US National Institutes of Health, and the protocol was approved by the Animal Research Committee of Shanghai Jiao Tong University.
Hypertensive Animal Models.
Renal hypertensive rats were generated by abdominal aortic coarctation (40). Male Sprague–Dawley rats, weighing 150 ± 20 g, were randomly assigned to sham-operated and abdominal aorta-constriction groups.
Application of Mechanical Stretch.
For the application of mechanical cyclic stretch, VSMCs were plated on type I collagen-coated flexible silicone-bottomed plates (Flexercell International) and then were subjected to cyclic stretch produced by a computer-controlled vacuum (FX-4000T Strain Unit; Flexcell International) as previously described (4). Cells cultured under the same conditions but not subjected to mechanical strain were used as the static control.
ChIP.
Cells were fixed in 1% formaldehyde for 10 min at room temperature, followed by quenching with glycine and rinsing with cold PBS. Afterward, cells were collected and lysed. DNA were released and sonicated into 200- to 700-bp fragments. A chromatin aliquot was incubated overnight with 5 µg anti-emerin antibody (Abcam), anti-lamin A/C antibody (Abcam), or control rabbit IgG (rIgG) (Abcam). Antibody-coupled magnetic beads (Invitrogen) were added, washed, and eluted. DNA fragments associated with emerin, lamin A/C, or control antibodies were eluted and purified using the One-Day Chromatin Immunoprecipitation Kit (Millipore). Input genomic DNA was obtained through similar elution and purification procedures.
RefSeq Promoter Array and MOTIF Assay.
For the ChIP-on-chip assay, samples were hybridized with the CpG Refseq Prom MeDIP Array (NimbleGen), which covers 15,287 promoters (the design region is about −3,880 bp to +970 bp of the transcription start site). For each ChIP-on-chip dataset, the sequences for the summit region (201 bp) spanning 100 bp up- and downstream from the summit of each peak were retrieved. The top 500 sequences with the highest peak score were selected for MOTIF analysis based on MEME (22) and DREME (23).
Statistical Analysis.
Each experiment was performed at least in triplicate, and all values are expressed as mean ± SD. The Student’s t test was used to compare two groups, and multiple comparisons among the groups were performed using one-way ANOVA and the Student–Newman–Keuls test for post hoc comparisons. A value of P < 0.05 was regarded as statistically significant.
SI Materials and Methods
Hypertensive Animal Models.
Renal hypertensive rats were generated by abdominal aortic coarctation (40). Male Sprague–Dawley rats weighing 150 ± 20 g were randomly assigned to sham-operated and abdominal aorta-constriction groups. Animals were anesthetized via isoflurane inhalation. In the group treated with abdominal aorta constriction, the abdominal aorta was dissected free at 5 mm above the branch of left kidney artery, and then a 21-gauge needle (o.d. 0.82 mm) was placed beside the aorta. A 3-0 gauge (0.2-mm diameter) suture was tied tightly around the aorta and the needle. The needle was removed to cause severe aortic constriction. Animals in the sham-operated group were treated as the animals in the abdominal aorta-constriction group except for the application of aortic constriction.
For in vivo overexpression of emerin or lamin A/C in hypertensive rats, the left common carotid artery was dissected free after the abdominal aorta was constricted, and 150 μL lentivirus (Shanghai GenePharma) was added dropwise along the artery. Rats were maintained under specific pathogen-free conditions in the vivarium facility of Shanghai Jiao Tong University with a 12-h light/dark cycle (light on at 6:00 AM).
Blood pressure of rats in the aorta-constriction and sham-operated groups was measured by carotid cannula (40). The rats were killed with 120 mg/kg sodium pentobarbital; then the full length of the common carotid artery was dissected and isolated. A portion of artery specimen (about 2 mm thick) was analyzed by in situ proliferation, and the remaining portion of the artery was ground into powder with liquid nitrogen for Western blot.
Cell Culture.
Primary VSMCs were cultured from the medial portions of thoracic aortas after the removal of adventitia and endothelium. Male Sprague–Dawley rats weighing 150 ± 20 g were killed with 120 mg/kg sodium pentobarbital, and the thoracic aorta was surgically removed. The media of the aorta was isolated surgically and minced into small pieces, which were plated onto 25-cm2 culture flasks for culture in DMEM (Gibco) containing 10% heat-inactivated FBS (Gibco), 100 U/mL penicillin, and 100 μg/mL streptomycin and were incubated at 37 °C in a humidified incubator (95% air and 5% CO2). VSMCs were characterized by immunohistochemical staining for smooth muscle-specific α-actin monoclonal antibody (Sigma-Aldrich). VSMC monolayers were passaged every 3–4 d after trypsinization, and passages 6–9 were used for experiments.
Cell Proliferation in Vitro Assay.
Cell proliferation in vitro was analyzed using a colorimetric BrdU kit (Roche Diagnostics). Six hours before the end of cyclic stretch application, BrdU labeling reagent was added to the culture medium (1:1,000). After the application of cyclic stretch, VSMCs were seeded at a density of 1.0 × 104 per well in 96-well plates (about 80% confluent) and were labeled according to the manufacturer’s instructions. Briefly, the cells were fixed with FixDenat solution (Roche Diagnostics) for 30 min at room temperature and then were incubated with anti-BrdU peroxidase working solution (freshly diluted 1:100) for 90 min. After three rinses with washing buffer, substrate solution (100 μL per well) was added to the cells and incubated for 20 min at room temperature. Thereafter, 25 μL of 1 M H2SO4 was added to each well, and the 96-well plates were shaken briefly at 300 rounds/min. The absorbance at 450 nm was measured in an ELISA plate reader (Bio-Rad 680).
In Situ Cell Proliferation Analysis of Artery Specimens.
BrdU (Sigma-Aldrich), 100 mg/kg, was i.p. injected, and 6 h later the common carotid artery was removed surgically. The artery was plunged into freezing isopentane and frozen-sectioned into 6-μm sections. The sections were fixed with 70% ethanol in 50 mmol/L glycine (pH 2.0), digested with 0.05% trypsin and 0.05% CaCl2 in PBS, and denaturized with 4 mol/L HCl. Then the sections were immune-detected with anti–BrdU-FLUOS antibody (Roche Diagnostics) and were observed under a fluorescence microscope (Olympus IX71). The results of six consecutive sections from a vessel were averaged to represent the value for the vessel.
Protein/DNA Arrays.
Nuclear proteins were prepared from VSMCs by using a nuclear extraction kit (Panomics). TranSignal Arrays (Panomics), which identify the sequence-specific DNA-binding properties (activity) of 345 transcription factors, were used according to the manufacturer’s directions. For data quantification, the fluorescence intensity of each spot on the protein/DNA array was normalized to the positive control on the same array after subtracting the fluorescence intensity of the negative control (background).
qPCR Assay.
qPCR assays were performed to confirm the results of ChIP-on-chip with primers amplifying the promoter regions of transcription factors revealed by MOTIF assay. The cycle threshold (Ct) values of ChIP DNA fractions were normalized to the Ct value of the input DNA fraction for the same qPCR assay (ΔCt) to account for differences in chromatin sample preparation. Relative expression of the ChIP fraction was measured by the 2-ΔΔCT method. DNA fractions chromatin immunoprecipitated with IgG were used as the negative control.
Western Blot.
Total protein was extracted from the artery specimen by grinding the specimen into powder in liquid nitrogen, adding lysis buffer [0.15 M Tris (pH 6.8), 1.2% SDS, 15% mercaptoethanol], and vortexing for 10 min on ice. Cells were gently washed twice with cold PBS, scraped into lysis buffer [0.15 M Tris (pH 6.8), 1.2% SDS, 15% mercaptoethanol], and incubated on ice for 10 min. The lysates were centrifuged at 13,000 × g for 3 min, and the supernatants were boiled for 8 min. Cell lysates were quantified using the Bradford analysis kit (Bio-Rad). Thirty micrograms of total protein per line were subjected to electrophoretic separation by 10% SDS/PAGE and were transferred to nitrocellulose membranes (Hybond; Amersham). The membranes were blocked for 1 h at room temperature in Tris-buffered saline Tween-20 buffer containing 5% nonfat milk. The blots were incubated overnight at 4 °C with the following antibodies: lamin A/C (1:500) (Santa Cruz Technologies), emerin (1:500) (Abcam), lamin A (1:500) (Abcam), lamin C (1:500) (Abcam), and GAPDH (1:500) (Santa Cruz Technologies). After three washings, the blots were incubated for 1 h at room temperature with relevant secondary antibodies. The stains were deposited by the 5-bromo-4-chloro-3-indolyl-phosphate (BCIP)/nitro blue tetrazolium (NBT) phosphatase substrate system according to the manufacturer’s instructions (KPL). The results were analyzed using Quantity One imaging software (Bio-Rad). The specific protein expression levels were normalized to GAPDH expression.
RNAi.
VSMCs were seeded at a density of 2 × 105 cells per well in six-well plates or Flexercell plates (Flexcell International) and were grown in 10% FBS/DMEM. After 24 h, the cells were about 60% confluent and were transfected with specific siRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Briefly, 100 nmol siRNA and 5 µL Lipofectamine 2000 (Invitrogen) were diluted in Opti-MEM (Invitrogen) without serum or antibiotics. After mixing for 20 min at room temperature, the mixture was added dropwise to a final volume of 800 µL, and the cells were incubated at 37 °C in a humidified incubator. After 24 h of incubation, the interference mixture was removed, and complete culture medium was added. Nonsilencing siRNA was used as a mock control.
The sequences of the double-stranded siRNA targeting lamin A/C were 5′-CAGG UCUG AAGC CAAA GAAT T-3′ and 5′-UUCU UUGG CUUC AGAC CUGT T-3′; the sequences for siRNA targeting emerin were 5′-CUAC CUUU CUGC UCUU UGUT T-3′ and 5′-ACAA AGAG CAGA AAGG UAGT T-3′; the sequences for the mock control were 5′-UUCU CCGA ACGU GUCA CGUT T-3′ and 5′-ACGU GACA CGUU CGGA GAAT T-3′.
Plasmid Transfection.
The complete coding sequences of emerin mRNA (sequence ID: ref|NM_012948.1|) and lamin A mRNA (sequence ID: emb|X66870.1|) were obtained by gene synthesis with a series of primers (Shanghai GenePharma). Then the fragments were subcloned into the pEX-3 vector (Invitrogen) with restriction sites XhoI and EcoRI (New England Biolabs). Both plasmid constructs were verified by DNA sequencing.
Emerin- or lamin A-expressing plasmid was transfected into VSMCs using X-tremeGENE HP DNA Transfection Reagent (Roche) according to the manufacturer’s protocol. Briefly, 2 × 105 cells per well in six-well or Flexercell plates (about 60% confluent) were transfected with a total amount of 6 μL X-tremeGENE HP DNA transfection reagent and 2 μg pEX-3-Emerin plasmid or pEX-3-Lamin A. Transfection efficiency was confirmed by Western blot. The control group used empty pEX-3.
Proteasome Inhibitor.
VSMCs were seeded at a density of 2 × 105 cells per well in six-well or Flexercell plates (about 60% confluent). Thirty minutes before the application of cyclic stretch, a proteasome inhibitor, MG-132(R) (Sigma-Aldrich), was added to the medium with a final concentration of 10 μM. The same volume of DMSO was used as a mock control.
IPA.
The DNA segments immunoprecipitated with emerin or lamin A/C (Table S2) were uploaded to IPA software (content version: 14197757) (www.ingenuity.com/products/ipa), and the possible biological processes were analyzed. By using the “Grow” tool of IPA, the potential transcription factors correlated with emerin and lamin A/C were revealed as networks. IPA integrates the knowledge about genes, drugs, chemicals, protein families, processes, and pathways based on their interactions and functions derived from the Ingenuity Pathways Knowledge Database literature (IPKDL). The significance values for analyses of network and pathway generation were calculated by comparing the number of proteins that participate in a given function or pathway relative to the total number of occurrences of these proteins in all functional/pathway annotations stored in the IPKDL (24).
Supplementary Material
Acknowledgments
This research was supported by National Natural Science Foundation of China Grants 11232010 (to Z.-L.J.), 11572199 (to Y.-X.Q.), 11222223 (to Y.-X.Q.), and 11428207 (to Y.W.) and by National Institutes of Health Grant HL108735 (to S.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1604569113/-/DCSupplemental.
References
- 1.Halka AT, et al. The effects of stretch on vascular smooth muscle cell phenotype in vitro. Cardiovasc Pathol. 2008;17(2):98–102. doi: 10.1016/j.carpath.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Haga JH, Li YS, Chien S. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomech. 2007;40(5):947–960. doi: 10.1016/j.jbiomech.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Morrow D, et al. Cyclic strain inhibits Notch receptor signaling in vascular smooth muscle cells in vitro. Circ Res. 2005;96(5):567–575. doi: 10.1161/01.RES.0000159182.98874.43. [DOI] [PubMed] [Google Scholar]
- 4.Qi YX, et al. Cyclic strain modulates migration and proliferation of vascular smooth muscle cells via Rho-GDIalpha, Rac1, and p38 pathway. J Cell Biochem. 2010;109(5):906–914. doi: 10.1002/jcb.22465. [DOI] [PubMed] [Google Scholar]
- 5.Butler PJ, Tsou TC, Li JY, Usami S, Chien S. Rate sensitivity of shear-induced changes in the lateral diffusion of endothelial cell membrane lipids: A role for membrane perturbation in shear-induced MAPK activation. FASEB J. 2002;16(2):216–218. doi: 10.1096/fj.01-0434fje. [DOI] [PubMed] [Google Scholar]
- 6.Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci USA. 2003;100(13):7988–7995. doi: 10.1073/pnas.1332808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, et al. Shear stress activation of SREBP1 in endothelial cells is mediated by integrins. Arterioscler Thromb Vasc Biol. 2002;22(1):76–81. doi: 10.1161/hq0102.101822. [DOI] [PubMed] [Google Scholar]
- 8.Tzima E, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437(7057):426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 9.Chen KD, et al. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem. 1999;274(26):18393–18400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- 10.Traub O, Ishida T, Ishida M, Tupper JC, Berk BC. Shear stress-mediated extracellular signal-regulated kinase activation is regulated by sodium in endothelial cells. Potential role for a voltage-dependent sodium channel. J Biol Chem. 1999;274(29):20144–20150. doi: 10.1074/jbc.274.29.20144. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto K, et al. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med. 2006;12(1):133–137. doi: 10.1038/nm1338. [DOI] [PubMed] [Google Scholar]
- 12.Qi YX, et al. PDGF-BB and TGF-β1 on cross-talk between endothelial and smooth muscle cells in vascular remodeling induced by low shear stress. Proc Natl Acad Sci USA. 2011;108(5):1908–1913. doi: 10.1073/pnas.1019219108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swift J, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341(6149):1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahl KN, Ribeiro AJ, Lammerding J. Nuclear shape, mechanics, and mechanotransduction. Circ Res. 2008;102(11):1307–1318. doi: 10.1161/CIRCRESAHA.108.173989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature. 2013;497(7450):507–511. doi: 10.1038/nature12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caille N, Thoumine O, Tardy Y, Meister JJ. Contribution of the nucleus to the mechanical properties of endothelial cells. J Biomech. 2002;35(2):177–187. doi: 10.1016/s0021-9290(01)00201-9. [DOI] [PubMed] [Google Scholar]
- 17.Guilluy C, et al. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat Cell Biol. 2014;16(4):376–381. doi: 10.1038/ncb2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crisp M, et al. Coupling of the nucleus and cytoplasm: Role of the LINC complex. J Cell Biol. 2006;172(1):41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shyu KG, Chao YM, Wang BW, Kuan P. Regulation of discoidin domain receptor 2 by cyclic mechanical stretch in cultured rat vascular smooth muscle cells. Hypertension. 2005;46(3):614–621. doi: 10.1161/01.HYP.0000175811.79863.e2. [DOI] [PubMed] [Google Scholar]
- 20.Chen LJ, Wei SY, Chiu JJ. Mechanical regulation of epigenetics in vascular biology and pathobiology. J Cell Mol Med. 2013;17(4):437–448. doi: 10.1111/jcmm.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balachandran K, Sucosky P, Jo H, Yoganathan AP. Elevated cyclic stretch alters matrix remodeling in aortic valve cusps: Implications for degenerative aortic valve disease. Am J Physiol Heart Circ Physiol. 2009;296(3):H756–H764. doi: 10.1152/ajpheart.00900.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machanick P, Bailey TL. MEME-ChIP: Motif analysis of large DNA datasets. Bioinformatics. 2011;27(12):1696–1697. doi: 10.1093/bioinformatics/btr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailey TL. DREME: Motif discovery in transcription factor ChIP-seq data. Bioinformatics. 2011;27(12):1653–1659. doi: 10.1093/bioinformatics/btr261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai L, Li C, Shedden KA, Misek DE, Lubman DM. Comparative proteomic study of two closely related ovarian endometrioid adenocarcinoma cell lines using cIEF fractionation and pathway analysis. Electrophoresis. 2009;30(7):1119–1131. doi: 10.1002/elps.200800505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, Spann TP. Nuclear lamins: Building blocks of nuclear architecture. Genes Dev. 2002;16(5):533–547. doi: 10.1101/gad.960502. [DOI] [PubMed] [Google Scholar]
- 26.Holaska JM. Emerin and the nuclear lamina in muscle and cardiac disease. Circ Res. 2008;103(1):16–23. doi: 10.1161/CIRCRESAHA.108.172197. [DOI] [PubMed] [Google Scholar]
- 27.Harada T, et al. Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. J Cell Biol. 2014;204(5):669–682. doi: 10.1083/jcb.201308029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buxboim A, et al. Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin. Curr Biol. 2014;24(16):1909–1917. doi: 10.1016/j.cub.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muchir A, Worman HJ. Emery-Dreifuss muscular dystrophy. Curr Neurol Neurosci Rep. 2007;7(1):78–83. doi: 10.1007/s11910-007-0025-3. [DOI] [PubMed] [Google Scholar]
- 30.Lammerding J, et al. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J Cell Biol. 2005;170(5):781–791. doi: 10.1083/jcb.200502148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lammerding J, et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113(3):370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song M, San H, Anderson SA, Cannon RO, 3rd, Orlic D. Shear stress-induced mechanotransduction protein deregulation and vasculopathy in a mouse model of progeria. Stem Cell Res Ther. 2014;5(2):41. doi: 10.1186/scrt429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi YX, Yao QP, Zhang P, Jiang ZL. RACK1 regulates Src activity on apoptosis of vascular smooth muscle cells induced by cyclic strain. Cell Mole Bioeng. 2011;4(3):358–367. [Google Scholar]
- 34.Amendola M, van Steensel B. Mechanisms and dynamics of nuclear lamina-genome interactions. Curr Opin Cell Biol. 2014;28:61–68. doi: 10.1016/j.ceb.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Berk JM, et al. O-Linked β-N-acetylglucosamine (O-GlcNAc) regulates emerin binding to barrier to autointegration factor (BAF) in a chromatin- and lamin B-enriched “niche”. J Biol Chem. 2013;288(42):30192–30209. doi: 10.1074/jbc.M113.503060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capanni C, et al. Lamin A precursor induces barrier-to-autointegration factor nuclear localization. Cell Cycle. 2010;9(13):2600–2610. doi: 10.4161/cc.9.13.12080. [DOI] [PubMed] [Google Scholar]
- 37.Zullo JM, et al. DNA sequence-dependent compartmentalization and silencing of chromatin at the nuclear lamina. Cell. 2012;149(7):1474–1487. doi: 10.1016/j.cell.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 38.Ottaviani A, et al. Identification of a perinuclear positioning element in human subtelomeres that requires A-type lamins and CTCF. EMBO J. 2009;28(16):2428–2436. doi: 10.1038/emboj.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Committee on Care and Use of Laboratory Animals (1996) Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85-23.
- 40.Barton CH, Ni Z, Vaziri ND. Enhanced nitric oxide inactivation in aortic coarctation-induced hypertension. Kidney Int. 2001;60(3):1083–1087. doi: 10.1046/j.1523-1755.2001.0600031083.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.