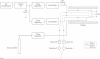
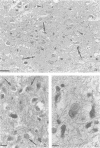
Abstract
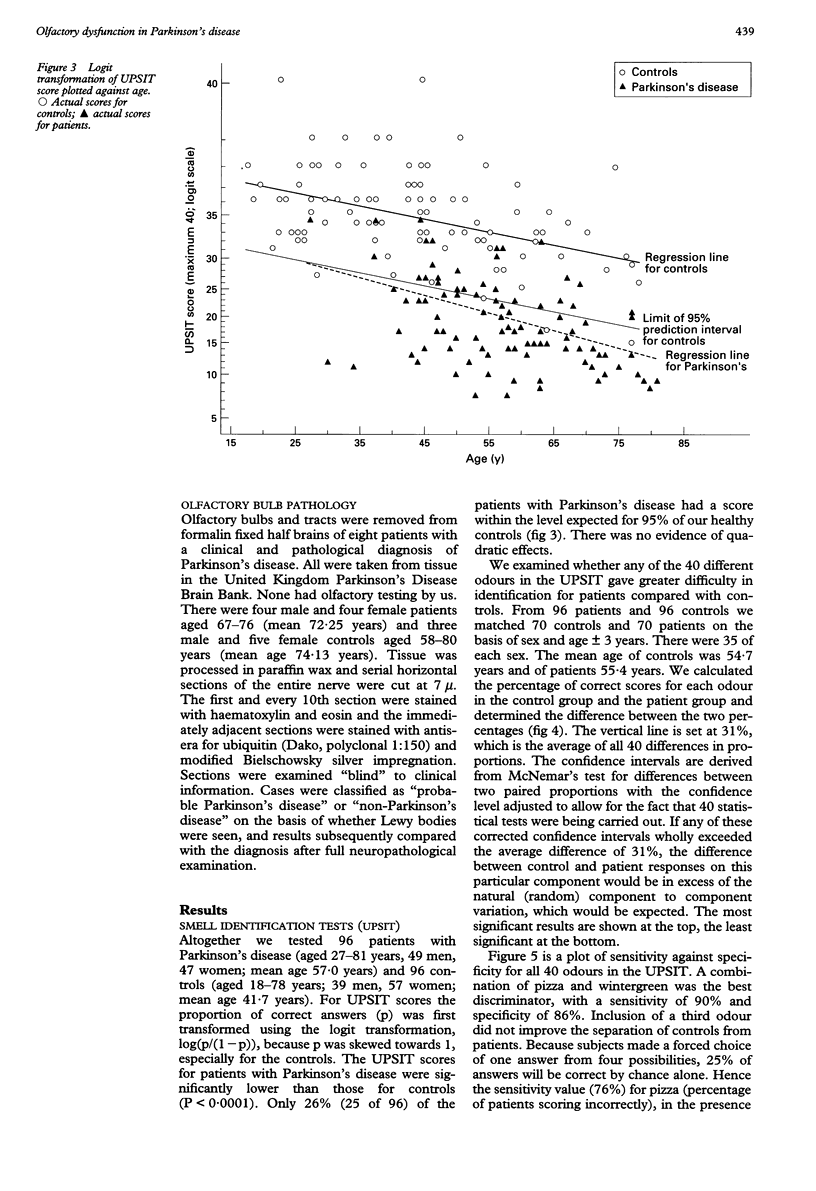
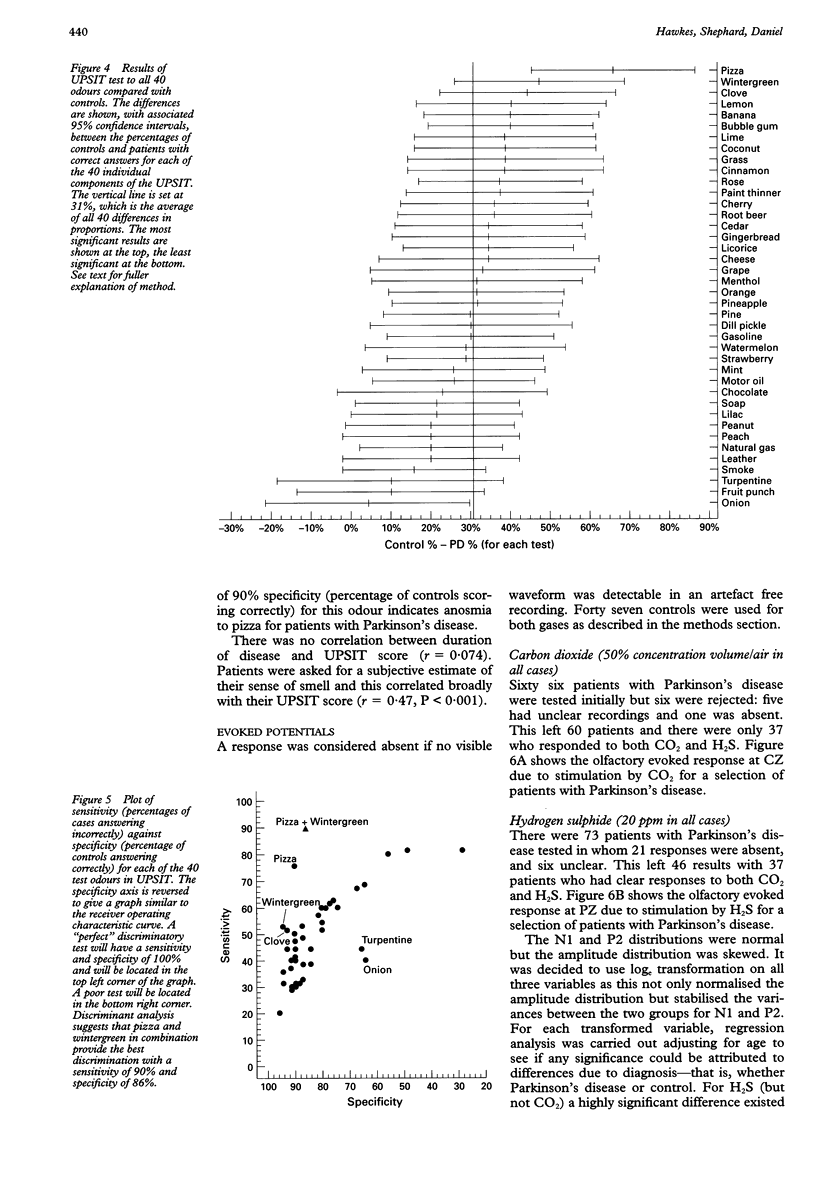
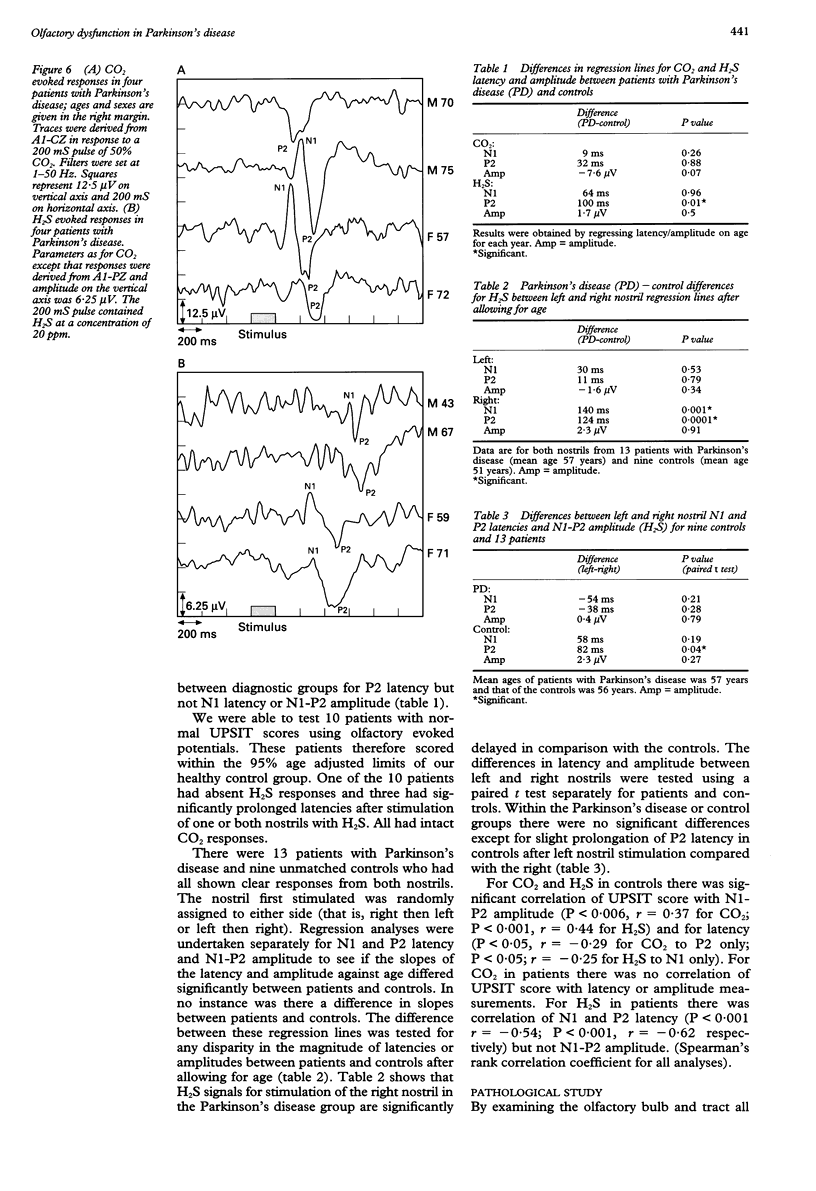
OBJECTIVE: To evaluate olfactory function in Parkinson's disease. METHODS: A standardised odour identification test was used, together with an evoked potential assessment with hydrogen sulphide. In addition, histological analysis was performed on the olfactory bulbs of cadavers who died from Parkinson's disease. RESULTS: Over 70% of patients studied (71 of 96) were outside the 95% limit of normal on the identification test in an age matched sample and there was an unusual pattern of selective loss to certain odours, not hitherto described. The evoked potentials were significantly delayed but of comparable amplitude to a control matched population. Of the 73 patients studied only 37 had a technically satisfactory record containing a clear response to both gases and of these, 12 were delayed. For H2S there was more delay on stimulating the right nostril than the left. Some patients with normal smell identification test scores had delayed evoked potentials. In the pathological examination of olfactory bulbs from eight brains, changes characteristic of Parkinson's disease (Lewy bodies) were seen in every olfactory bulb, particularly in the anterior olfactory nucleus, and were sufficiently distinct to allow a presumptive diagnosis of Parkinson's disease. CONCLUSIONS: Olfactory damage in Parkinson's disease is consistent and severe and may provide an important clue to the aetiology of the disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsterdam J. D., Settle R. G., Doty R. L., Abelman E., Winokur A. Taste and smell perception in depression. Biol Psychiatry. 1987 Dec;22(12):1481–1485. doi: 10.1016/0006-3223(87)90108-9. [DOI] [PubMed] [Google Scholar]
- Brittebo E. B., Eriksson C., Feil V., Bakke J., Brandt I. Toxicity of 2,6-dichlorothiobenzamide (chlorthiamid) and 2,6-dichlorobenzamide in the olfactory nasal mucosa of mice. Fundam Appl Toxicol. 1991 Jul;17(1):92–102. doi: 10.1016/0272-0590(91)90242-v. [DOI] [PubMed] [Google Scholar]
- Chui H. C., Mortimer J. A., Slager U., Zarow C., Bondareff W., Webster D. D. Pathologic correlates of dementia in Parkinson's disease. Arch Neurol. 1986 Oct;43(10):991–995. doi: 10.1001/archneur.1986.00520100013007. [DOI] [PubMed] [Google Scholar]
- Daniel S. E., Hawkes C. H. Preliminary diagnosis of Parkinson's disease by olfactory bulb pathology. Lancet. 1992 Jul 18;340(8812):186–186. doi: 10.1016/0140-6736(92)93275-r. [DOI] [PubMed] [Google Scholar]
- Ding X. X., Coon M. J. Immunochemical characterization of multiple forms of cytochrome P-450 in rabbit nasal microsomes and evidence for tissue-specific expression of P-450s NMa and NMb. Mol Pharmacol. 1990 Apr;37(4):489–496. [PubMed] [Google Scholar]
- Doty R. L., Bromley S. M., Stern M. B. Olfactory testing as an aid in the diagnosis of Parkinson's disease: development of optimal discrimination criteria. Neurodegeneration. 1995 Mar;4(1):93–97. doi: 10.1006/neur.1995.0011. [DOI] [PubMed] [Google Scholar]
- Doty R. L., Deems D. A., Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988 Aug;38(8):1237–1244. doi: 10.1212/wnl.38.8.1237. [DOI] [PubMed] [Google Scholar]
- Doty R. L., Shaman P., Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984 Mar;32(3):489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- Doty R. L., Stern M. B., Pfeiffer C., Gollomp S. M., Hurtig H. I. Bilateral olfactory dysfunction in early stage treated and untreated idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1992 Feb;55(2):138–142. doi: 10.1136/jnnp.55.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esiri M. M., Wilcock G. K. The olfactory bulbs in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1984 Jan;47(1):56–60. doi: 10.1136/jnnp.47.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillner M., Brittebo E. B., Brandt I., Söderkvist P., Appelgren L. E., Gustafsson J. A. Uptake and specific binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin in the olfactory mucosa of mice and rats. Cancer Res. 1987 Aug 1;47(15):4150–4159. [PubMed] [Google Scholar]
- Hext P. M., Lock E. A. The accumulation and metabolism of 3-trifluoromethylpyridine by rat olfactory and hepatic tissues. Toxicology. 1992;72(1):61–75. doi: 10.1016/0300-483x(92)90086-t. [DOI] [PubMed] [Google Scholar]
- Hoehn M. M., Yahr M. D. Parkinsonism: onset, progression and mortality. Neurology. 1967 May;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hughes A. J., Daniel S. E., Kilford L., Lees A. J. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992 Mar;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobal G., Hummel C. Cerebral chemosensory evoked potentials elicited by chemical stimulation of the human olfactory and respiratory nasal mucosa. Electroencephalogr Clin Neurophysiol. 1988 Jul-Aug;71(4):241–250. doi: 10.1016/0168-5597(88)90023-8. [DOI] [PubMed] [Google Scholar]
- Kobal G., Plattig K. H. Methodische Anmerkungen zur Gewinnung olfaktorischer EEG-Antworten des wachen Menschen (objektive Olfaktometrie). EEG EMG Z Elektroenzephalogr Elektromyogr Verwandte Geb. 1978 Sep;9(3):135–145. [PubMed] [Google Scholar]
- Levine H. L. The office diagnosis of nasal and sinus disorders using rigid nasal endoscopy. Otolaryngol Head Neck Surg. 1990 Apr;102(4):370–373. doi: 10.1177/019459989010200411. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Tucker C. M., Yates P. O. Alzheimer's disease: an olfactory connection? Mech Ageing Dev. 1988 Jan;42(1):1–15. doi: 10.1016/0047-6374(88)90058-9. [DOI] [PubMed] [Google Scholar]
- Pinching A. J., Doving K. B. Selective degeneration in the rat olfactory bulb following exposure to different odours. Brain Res. 1974 Dec 27;82(2):195–204. doi: 10.1016/0006-8993(74)90598-8. [DOI] [PubMed] [Google Scholar]
- Quinn N. P., Rossor M. N., Marsden C. D. Olfactory threshold in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1987 Jan;50(1):88–89. doi: 10.1136/jnnp.50.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger R. B. Comparative deposition of inhaled aerosols in experimental animals and humans: a review. J Toxicol Environ Health. 1985;15(2):197–214. doi: 10.1080/15287398509530647. [DOI] [PubMed] [Google Scholar]
- Shipley M. T. Transport of molecules from nose to brain: transneuronal anterograde and retrograde labeling in the rat olfactory system by wheat germ agglutinin-horseradish peroxidase applied to the nasal epithelium. Brain Res Bull. 1985 Aug;15(2):129–142. doi: 10.1016/0361-9230(85)90129-7. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Gough A. C., Leigh P. N., Summers B. A., Harding A. E., Maraganore D. M., Sturman S. G., Schapira A. H., Williams A. C., Maranganore D. M. Debrisoquine hydroxylase gene polymorphism and susceptibility to Parkinson's disease. Lancet. 1992 Jun 6;339(8806):1375–1377. doi: 10.1016/0140-6736(92)91196-f. [DOI] [PubMed] [Google Scholar]
- Tomlinson A. H., Esiri M. M. Herpes simplex encephalitis. Immunohistological demonstration of spread of virus via olfactory pathways in mice. J Neurol Sci. 1983 Aug-Sep;60(3):473–484. doi: 10.1016/0022-510x(83)90158-2. [DOI] [PubMed] [Google Scholar]
- Yoshimura N. Olfactory bulb involvement in Pick's disease. Acta Neuropathol. 1988;77(2):202–205. doi: 10.1007/BF00687432. [DOI] [PubMed] [Google Scholar]