Significance
The obstetrical dilemma hypothesis states that the human female pelvis represents a compromise between designs most suitable for childbirth and bipedal locomotion, respectively. This hypothesis has been challenged recently on biomechanical, metabolic, and biocultural grounds. Here we provide evidence for the pelvis’ developmental adaptation to the problem of birthing large-headed/large-bodied babies. We show that the female pelvis reaches its obstetrically most adequate morphology around the time of maximum fertility but later reverts to a mode of development similar to that of males, which significantly reduces the dimensions of the birth canal. These developmental changes are likely mediated by hormonal changes during puberty and menopause, indicating “on-demand” adjustment of pelvic shape to the needs of childbirth.
Keywords: pelvis, development, evolution, obstetrical dilemma, sex steroids
Abstract
The bony pelvis of adult humans exhibits marked sexual dimorphism, which is traditionally interpreted in the framework of the “obstetrical dilemma” hypothesis: Giving birth to large-brained/large-bodied babies requires a wide pelvis, whereas efficient bipedal locomotion requires a narrow pelvis. This hypothesis has been challenged recently on biomechanical, metabolic, and biocultural grounds, so that it remains unclear which factors are responsible for sex-specific differences in adult pelvic morphology. Here we address this issue from a developmental perspective. We use methods of biomedical imaging and geometric morphometrics to analyze changes in pelvic morphology from late fetal stages to adulthood in a known-age/known-sex forensic/clinical sample. Results show that, until puberty, female and male pelves exhibit only moderate sexual dimorphism and follow largely similar developmental trajectories. With the onset of puberty, however, the female trajectory diverges substantially from the common course, resulting in rapid expansion of obstetrically relevant pelvic dimensions up to the age of 25–30 y. From 40 y onward females resume a mode of pelvic development similar to males, resulting in significant reduction of obstetric dimensions. This complex developmental trajectory is likely linked to the pubertal rise and premenopausal fall of estradiol levels and results in the obstetrically most adequate pelvic morphology during the time of maximum female fertility. The evidence that hormones mediate female pelvic development and morphology supports the view that solutions of the obstetrical dilemma depend not only on selection and adaptation but also on developmental plasticity as a response to ecological/nutritional factors during a female’s lifetime.
Females and males of most mammalian species differ in various morphological characteristics, such as the size and shape of the body as a whole and of soft and hard tissue structures (1). Sex-specific differences are also well documented in humans and nonhuman primates, particularly in the pelvis, and various hypotheses have been proposed to explain how pelvic sexual dimorphism evolves and develops (2–11). There is general agreement that the female pelvis is under obstetric selection to be adequately capacious for childbirth. However, the exact nature of selective pressures and developmental mechanisms yielding female and male pelvic phenotypes is still largely unknown, and whether obstetric adaptations involve trade-offs with other aspects of pelvic function, such as locomotor efficiency and abdominal stabilization, continues to be debated (12, 13).
One key hypothesis discussed in this context is Washburn's obstetrical dilemma (OD) (14). In its original form (14), the OD hypothesis posits a conflict between the evolution of bipedal locomotion (selection for biomechanically efficient, narrow pelves) and of large brains (selection for large-brained neonates, and obstetrically efficient, wide pelves). According to Washburn, the dilemma is “solved by delivery of the fetus at a much earlier stage of development” (ref. 14, p. 74) than in our closest living relatives, the great apes. Although the OD hypothesis thus primarily seeks to explain the early timing of birth and human altriciality (15), it also provides an explanation for pelvic sexual dimorphism: Selection favored wider female pelves to reduce the risks involved in birthing large-brained/large-bodied babies, but did so at the expense of locomotor efficiency (2, 5, 7). According to this hypothesis, the tight fit between the neonate head and maternal pelvis (obstetric constraints) and the high prevalence of obstructed labor in humans (16–18) reflect a trade-off between obstetric and locomotor selection pressures on the female pelvis.
Over the past years, the OD hypothesis has been reexamined extensively and has been challenged on various grounds (10–13, 19–22). The energetics of gestation and growth (EGG) hypothesis (12, 20, 23) provides a new perspective, proposing that the timing of birth is constrained by the limited metabolic output of the mother rather than by spatial limitations of her pelvis. Furthermore, inverse-dynamics models and experimental data indicate that a wide pelvis does not reduce bipedal locomotor efficiency (12, 13). Because these studies effectively falsify a major tenet of the OD, the tight fit between neonate head and maternal pelvis and the high prevalence of obstructed labor require alternative explanations. It has been proposed that solutions to the OD can be renegotiated (11) through ecologically mediated phenotypic plasticity of pelvic and fetal dimensions but that rapid changes in environmental conditions may result in fetopelvic mismatch (10, 11, 23). Obstructed labor thus would be a consequence of a mismatch between maternal and neonate developmental plasticity (23, 24) or of biocultural factors (22) rather than an evolutionary trade-off between obstetrics and locomotion.
On the other hand, indirect evidence for gene-mediated constraints on fetopelvic proportions comes from a recent study demonstrating that mothers with large heads (who, because of the high heritability of cranial dimensions, are likely to have large-headed babies) tend to have obstetrically more favorable pelvic dimensions than mothers with small heads (25). However, correlation between head size and these pelvic dimensions is also present in males (25), although the correlation is less pronounced than in females. Thus the extent to which the observed patterns represent female-specific obstetric selection, sex-neutral genetic–developmental integration, and/or developmental plasticity remains to be clarified.
Somatic sexual dimorphism such as that of the pelvis is largely the result of hormonally regulated sex-biased gene expression (26, 27). Previous research on the development of pelvic sexual dimorphism in mammals reveals a wide variety of modes of divergence. Several studies in rodents (28–30) suggest that the pubertal developmental trajectory of the male pelvis deviates from the prepubertal mode shared by both sexes, presumably under testosterone influence. This hypothesis also was proposed for humans and for other primates (6, 31). Other studies suggest that estrogen effects are crucial for female pelvic development during puberty (4, 32).
Here we reevaluate the evidence for the OD and alternative hypotheses from a developmental perspective. We propose the developmental obstetric dilemma (DOD) hypothesis, which posits that pelvic morphology reflects changing obstetric needs (versus other, possibly locomotor, needs) during a female’s lifetime. Given that female fertility (measured as birth rate per year) reaches its peak around the age of 25–30 y (33, 34) and declines toward 40–45 y, the DOD hypothesis predicts that (i) sex-specific differences in human pelvic morphology become pronounced after puberty; (ii) the female pelvis reaches its obstetrically most adequate morphology around the age of highest fertility; (iii) during postmenopausal life, the female pelvis reverts to an obstetrically less adequate morphology, which is probably most adequate for locomotion and other functions; (iv) the male pelvis does not show these developmental changes.
To test the DOD hypothesis, we track pelvic development from late fetal stages to late adulthood in an anonymized known-age and known-sex forensic/clinical sample (n = 275) (Materials and Methods). The bony elements constituting the pelvis fuse relatively late during development, so that the 3D morphology of the pelvis critically depends on the presence of ligaments and other soft tissue structures. Thus computed tomography (CT) was used to analyze pelvic morphology in the context of surrounding tissues. Pelvic size and shape were quantified with a total number of k = 377 3D anatomical landmarks. Sex-specific patterns of shape variation during development were analyzed and visualized with methods of geometric morphometrics (GM) (Materials and Methods).
Results
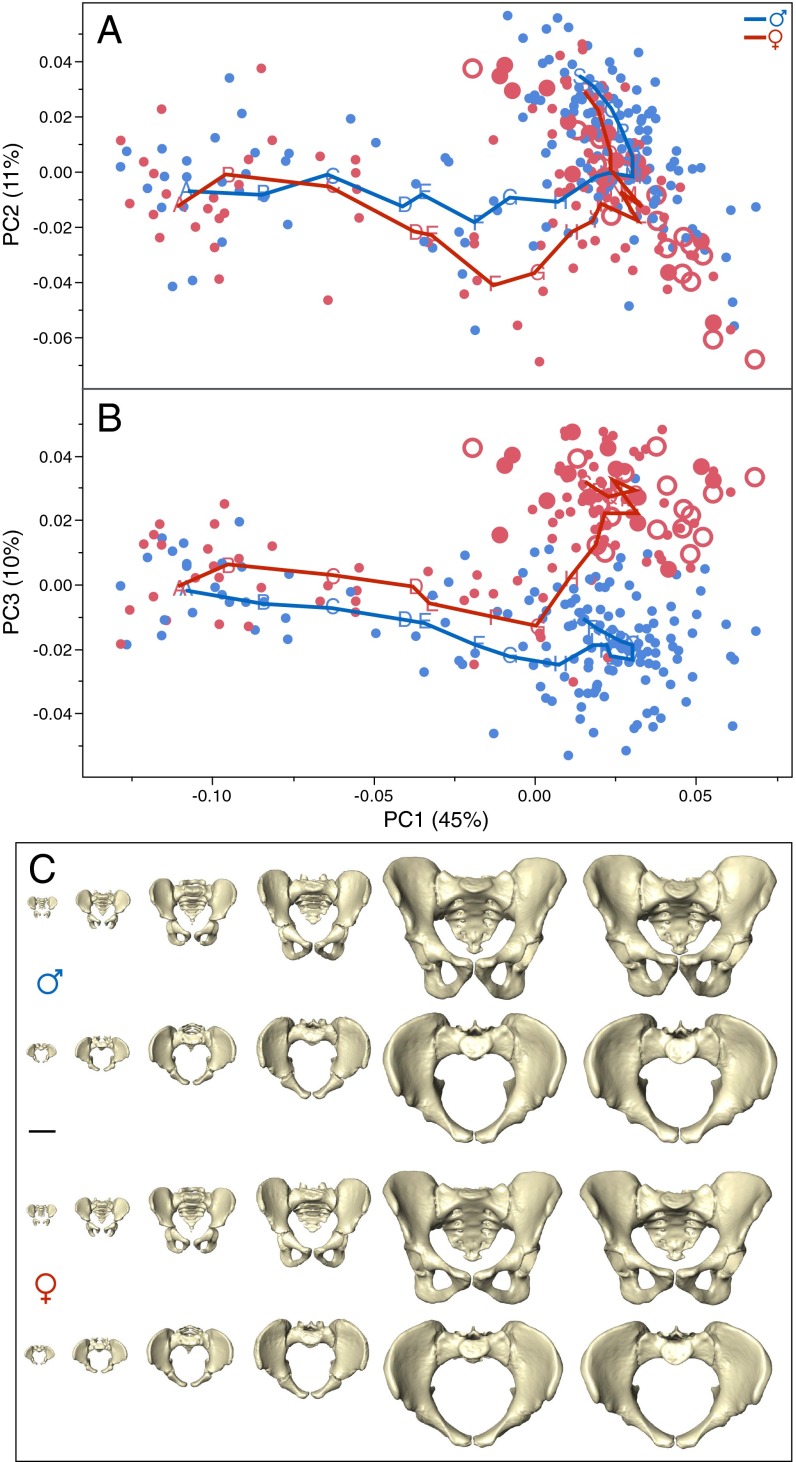
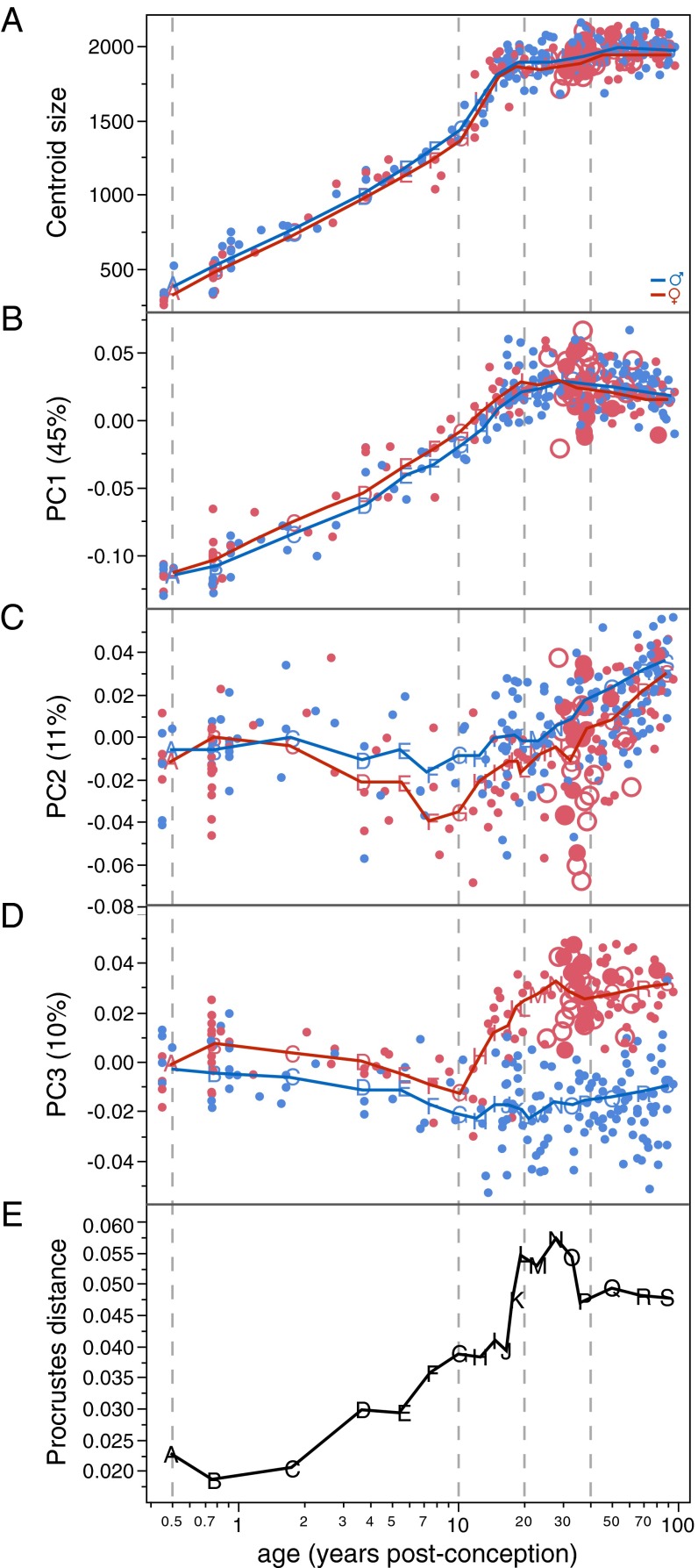
Fig. 1 graphs sex-specific trajectories of pelvic shape change along the first three principal components (PCs) of shape space and visualizes actual pelvic morphologies at six developmental stages from birth to late adulthood. Fig. 2 graphs the temporal course of pelvic size and shape change. Pelvic growth trajectories (i.e., age-related increase in size) of females and males are largely similar (Fig. 2A). PC1, which accounts for 45% of the total shape variation in the sample, captures a shared male/female mode of shape change (Fig. 2B). It is closely correlated with increase in size (females: r2 = 0.91; males: r2 = 0.92) and thus represents ontogenetic allometry (i.e., growth-related change in shape). PC2 (accounting for 11% of the total shape variation) and PC3 (accounting for 10%) track the development of sex-specific differences in pelvic shape. Female and male trajectories diverge early during infancy (see PC3 in Figs. 1B and 2D) and exhibit further separation during late childhood (see PC2 in Figs. 1A and 2C), resulting in moderate but significant sexual dimorphism at the onset of puberty (age 10–12 y) (Fig. 2E and Table S1). These findings confirm previous studies on the early development of sexual dimorphism in pelvic substructures (7, 35–37).
Fig. 1.
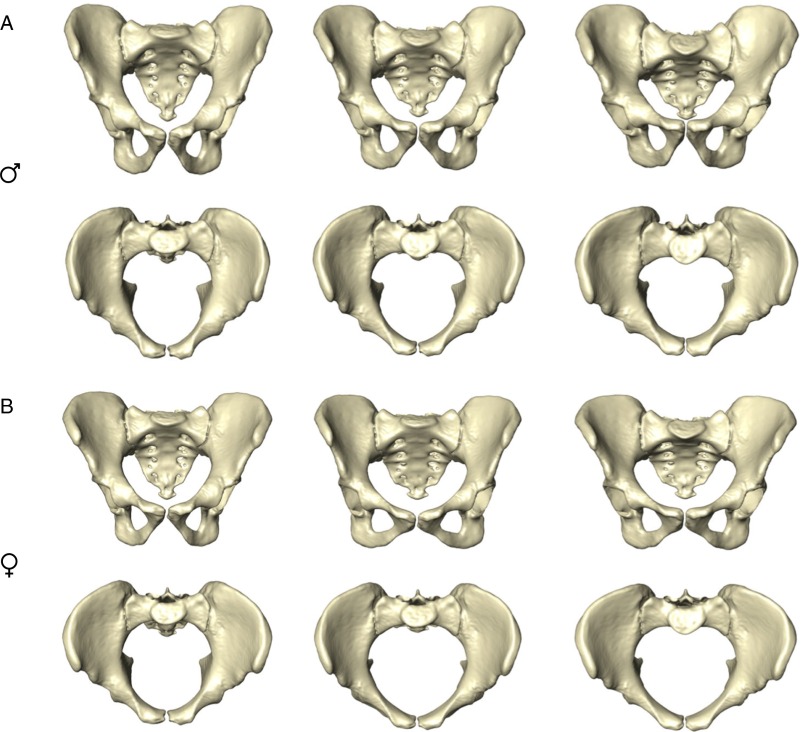
Developmental changes in human pelvic morphology from late fetal stages to late adulthood. (A and B) Bivariate plots of shape variation along PC1 (45% of total sample variation) and PC2 (11%) (A), and along PC1 and PC3 (10%) (B). Red symbols represent females; dots indicate immature or unknown parity status; filled and open circles indicate parous and nonparous status, respectively. Blue symbols represent males. Points A–S denote moving-average positions calculated at the ages indicated in Fig. 2. (C) Anterior and superior views of sex-specific pelvic mean shapes at birth and around 2, 6, 13, 25, and 80 y. (Scale bar, 5 cm.)
Fig. 2.
Age-related change in human pelvic size (A), shape (B–D), and shape dimorphism (E). Colors and symbols are as in Fig. 1; note that the age axis is scaled logarithmically in postconception years.
Table S1.
Differences between male and female pelvic shapes in immature, prime reproductive age, and old adult samples (Procrustes ANOVA)
| Age range, y | df | SS | MS | Rsq | F | P |
| >0 to <13 | 1 | 0.00705 | 0.00705 | 0.05781 | 3.98873 | 0.00276 |
| Residuals | 65 | 0.11491 | 0.00176 | |||
| Total | 66 | 0.12196 | ||||
| ≥19 to <40 | 1 | 0.04584 | 0.04584 | 0.19299 | 15.78333 | 0.00033 |
| Residuals | 66 | 0.19168 | 0.00290 | |||
| Total | 67 | 0.23752 | ||||
| ≥40 to ≤95 | 1 | 0.05741 | 0.05741 | 0.19990 | 24.48527 | 0.00033 |
| Residuals | 98 | 0.22979 | 0.00234 | |||
| Total | 99 | 0.28720 |
Df, degrees of freedom; F, F-value; MS, mean square; P = P value; Rsq, R2; SS, sum of squares.
From the age of ∼10 y onward the female trajectory changes its direction substantially, whereas the male trajectory continues its earlier course (Figs. 1B and 2D and Table S2). Around the age of 40–45 y, the female trajectory changes again, assuming a direction that is largely parallel to that of the male trajectory (Figs. 1B and 2D and Table S2). Overall, the mean difference between male and female pelvic shapes (i.e., pelvic sexual dimorphism) reaches a peak during early adulthood and is reduced during later adult life (Fig. 2E), as has been observed earlier (38).
Table S2.
Comparison of trajectory directions among sexes and age groups
| Comparison | Angle, degrees | P |
| f1–f2 | 59.6 | 0.003* |
| f2–f3 | 76.5 | 0.020* |
| m1–m2 | 17.2 | 0.496 |
| m2–m3 | 83.5 | 0.074 |
| f1–m1 | 6.7 | 0.280 |
| f2–m2 | 57.5 | 0.002* |
| f3–m3 | 9.2 | 0.719 |
Trajectory directions were evaluated by multivariate regression of PC1-3 on log age (years postconception) for six groups (f1: females, age ≤8 y, n = 31; f2: females, age >8 y ≤35 y, n = 39; f3: females, age >35 y, n = 55; m1: males, age ≤8y, n = 31; m2: males, age >8 y ≤ 35 y, n = 52; m3: males, age >35 y, n = 68).
P values ≤0.02 indicate significant divergence between trajectory segments. Note divergence between males and females from puberty to prime reproductive age (f2–m2), but no divergence between sexes during childhood (f1–m1) and late adulthood (f3–m3).
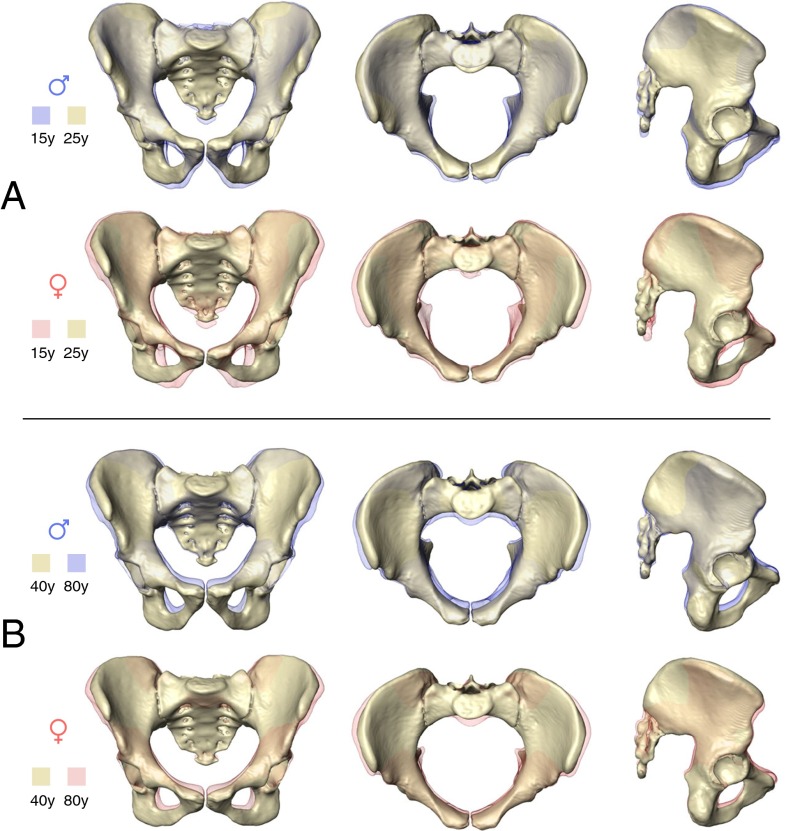
Fig. 3 visualizes the corresponding modes of sex-specific change in pelvic shape (for additional visualizations and animations, see Fig. S1 and Movies S1–S6). In males, pelvic development from ∼15 y to young adulthood (∼25 y) is characterized by a relative reduction of anteroposterior and superoinferior dimensions (Fig. 3A and Movies S1–S3). During this process the superior portion of the sacrum is tilted ventrally, and the greater sciatic notch becomes narrower.
Fig. 3.
Anterior, superior, and lateral views showing male and female patterns of pelvic shape change from ∼15 y (transparent) to ∼25 y (solid) (A), and from ∼40 y (solid) to ∼80 y (transparent) (B). For additional visualizations of the same patterns of shape change, see Movies S1–S6.
Fig. S1.
Anterior and superior views showing male (A) and female (B) pelvic mean shapes at age ∼15 y (Left), ∼25 y (Center), and ∼80 y (Right). For additional visualizations of the same patterns of shape change, see Fig. 3 and Movies S1–S6.
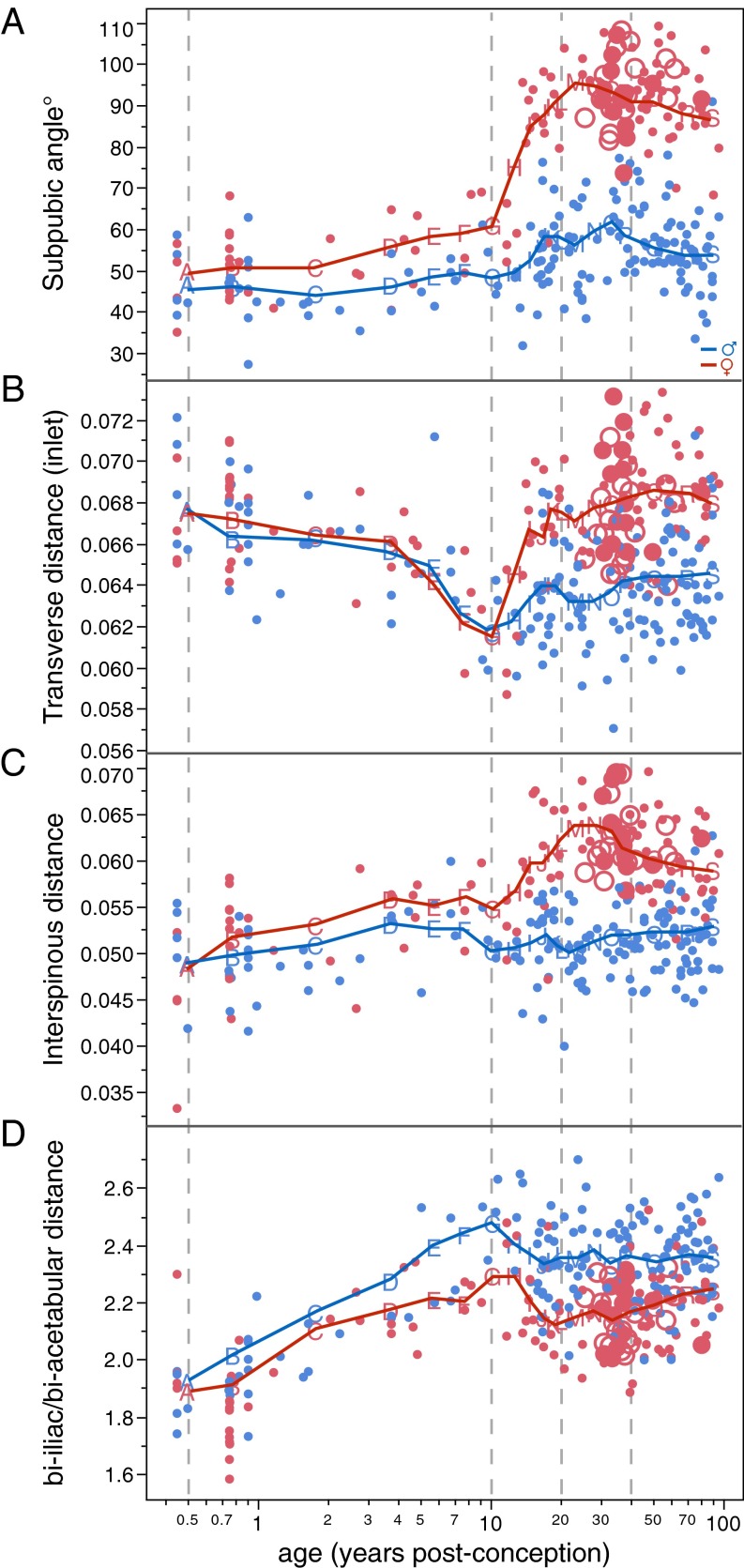
Development of the female pelvis during the same period (∼15 to ∼25 y) (Fig. 3A and Movies S1–S3) differs substantially from the male mode (Table S2). The sacrum and the ischiopubic region undergo substantial eversion, and the iliac blades undergo inversion. As a result, the anteroposterior dimensions of the pelvic midplane and outlet and the transverse dimensions of the pelvic inlet and outlet become larger (Figs. 3A and 4). Also, the subpubic angle (Fig. 4A) and the angle formed by the greater sciatic notch become wider. As an additional effect, the biacetabular distance becomes relatively wider, and bi-iliac width is relatively reduced (Fig. 4D). Overall, these developmental changes result in a wide, obstetrically favorable birth canal.
Fig. 4.
Sex-specific changes in angular (A) and size-normalized linear (B and C) pelvic dimensions and in pelvic proportions (D). Colors and symbols are as in Figs. 1 and 2.
It should be noted that the contrasting patterns of male and female pelvic development from puberty to young adulthood visualized here (Fig. 3) were described in part by Coleman (39), who used anteroposterior radiographs and a precursor of GM methods to track pelvic development in a longitudinal sample of the Fels Longitudinal Study begun in 1929. Using the same sample, a multivariate analysis of linear pelvic dimensions yielded similar results (7).
Around the age of 40–45 y, the female pelvis resumes a mode of shape change which is similar to that of males (Figs. 2 and 3B, Table S2, and Movies S4–S6). This pattern largely corresponds to that present in ∼15- to 25-y-old males (Table S2). Anteroposterior and superoinferior pelvic dimensions become relatively shorter. Interspinous distance is reduced, and the iliac blades become more everted. At the same time, the subpubic angle and the greater sciatic notch become narrower, as observed earlier (38, 40). In females, this mode of shape change results in a significant reduction of obstetrically relevant birth canal dimensions (Fig. 4).
Although our data show that female and male trajectories diverge substantially before the attainment of sexual maturity, we further assessed whether maternity (pregnancy and lactation) has an influence on the development of pelvic shape, as reported, for example, in mice (41). To this end, we analyzed pelvic shape variation in a subsample of females with known maternity status. Results show that pelvic morphologies of parous and nonparous females (both groups with an average age of 34 y) are statistically indistinguishable (Figs. 1, 2, and 4 and Table S3).
Table S3.
Comparison of pelvic shape of parous and nonparous women
| Age range, y | df | SS | MS | Rsq | F | P |
| 28–41 | 1 | 0.00578 | 0.00578 | 0.07291 | 1.88757 | 0.10330 |
| Residuals | 24 | 0.07344 | 0.00306 | |||
| Total | 25 | 0.07922 |
Sample structure: nonparous: n = 13, mean age = 34.1 y; parous: n = 12 (9 multiparae), mean age = 34.4 y. Df, degrees of freedom; F, F-value; MS, mean square; P = P value; Rsq, R2; SS, sum of squares.
Discussion
The findings presented here provide support for the DOD hypothesis along several lines of evidence: With the onset of puberty, the female developmental trajectory diverges substantially from the childhood trajectory, whereas the male trajectory essentially continues its earlier course (Table S2). As a result, the female pelvis attains its obstetrically most favorable morphology around the age of 25–30 y, i.e., at the age of highest fertility (33, 34). Furthermore, pelves in postmenopausal women assume a developmental mode that is largely similar to that of males (Table S2), with the effect that the birth canal becomes constricted.
Sexual dimorphism is largely the outcome of the sex-biased expression of autosomal genes, which in turn are regulated by sex-specific hormone levels and/or differential hormone receptor sensitivity (26, 27, 42). In mice, for example, testicular-feminized males (i.e., males lacking androgen receptors) and gonadectomized males develop female-like pelvic morphologies, whereas experimental administration of androgens to females induces male-like morphologies (28–30). In humans, direct evidence for hormone-mediated sex-specific bone remodeling patterns of the pelvis is not yet available. Nevertheless, studies on long bone morphology indicate that sexual skeletal dimorphism develops via complex interactions between sex-specific steroid hormone levels, sex-biased gene expression, and gender differences in sensitivity to bone-loading conditions and to hormones such as the growth hormone/insulin-like growth factor 1 (IGF1) axis (42–45).
Our data on pelvic development thus may be tentatively linked to hormonal change in the following way: The early differentiation of sex-specific pelvic shape might be related to the transient hormonal “minipuberty” during the first year of life (46). Directional changes in developmental trajectories during the prepubertal stage (see PC2 in Figs. 1 and 2) may be linked to the increase in IGF1 (47). The substantial divergence of the female developmental trajectory during puberty (see PC3 in Figs. 1 and 2) is most likely caused by the sex-specific rise in estradiol levels, triggering a change in pelvic bone-remodeling patterns (46, 47). We further hypothesize that the obstetrically favorable shape of the female pelvis is maintained by the high estradiol levels during the time of maximum fertility and that the significant reduction of obstetric dimensions from age 40 y onwards is related to the premenopausal decline in estradiol levels (46). Further testing of these hypotheses will require combined hormonal, morphometric, and life-history data.
Short-term hormonal effects of pregnancy and birthing on sacroiliac and pubic joint motility, as well as the effects of body position on pelvic obstetric dimensions, are well documented (48–50). However, the age-matched sample of parous and nonparous individuals studied here does not provide evidence for major effects of pregnancy and lactation on the development of female-specific pelvic morphology. The weak or absent influence of the growing fetus on its mother's pelvic development thus might be one of the reasons for fetopelvic disproportion and obstructed labor.
What are the possible evolutionary implications of hormone-mediated development of pelvic obstetric dimensions? Our data suggest that estrogens have a strong influence on the development of the female pelvic morphology during puberty. At the same time, they imply a weak-to-absent influence of androgens on human male-specific pelvic development during puberty, although such influences are well documented in other developmental modules such as the face (51–53). As proposed earlier (4), testosterone may be involved in the maintenance of the human male pelvic morphology throughout development but does not lead to developmental divergence during puberty.
Referring to recent hypotheses on ecological and nutritional factors influencing the OD (23), we postulate that the female pelvis is highly sensitive to in vivo modification via environmental modulation of hormone levels. As proposed in the framework of human reproductive ecology (23), and specifically by the predictive adaptive response hypothesis (54), an individual’s developmental trajectory may be modified according to the environmental conditions “expected” (i.e., likely) during its reproductive phase. The relationship between the term fetus and its mother’s pelvic morphology thus might be mediated via estrogen levels, which in turn are sensitive to the current state of ecological parameters relevant for prenatal and postnatal development.
Based on the evidence presented here, the DOD hypothesis predicts that higher levels of estrogen in females during puberty/young adulthood result in development/maintenance of an obstetrically more favorable pelvic morphology, which facilitates the delivery of larger babies. The relationships between sex hormone levels, maternal pelvic morphology, fetal size, and pre/postnatal development are complex and are topics of intense research (10, 11, 23, 55, 56). For example, it has been shown that females who are large at birth have comparatively high estradiol levels during adulthood (57). Estradiol levels also are influenced by diet and nutritional status (58–60) and are good predictors of fertility (61), and, likely, of adult pelvic shape (this study). Given this network of cause and effect, there is ample opportunity for in vivo feedback between ecological/nutritional conditions, sex hormone levels, neonate size, and maternal body and pelvic dimensions. For instance, the observed within-subject correlation of pelvic obstetric dimensions with body size and head size (25) could partly be an effect of higher estradiol levels in larger females (57), resulting in obstetrically more favorable morphologies of their pelves.
Evidence for estradiol-mediated female-specific patterns of pelvic development in humans (this study), nonhuman primates (4), and rodents (32) may indicate either evolutionarily conserved or convergent developmental mechanisms of sexual dimorphism in mammalian species exhibiting obstetric constraints. Because pelvic width does not correlate with locomotor efficiency (13), the question remains why the female pelvis did not evolve and/or does not develop wider obstetric dimensions, which would significantly reduce the existing perinatal risks for the mother and the infant. Pelvic size might be limited by nutritional conditions, which impose global constraints on body growth (11). The high prevalence of obstructed labor thus might largely represent a modern phenomenon resulting from a mismatch between secular increases in neonate size and maternal size. However, additional factors must be advanced to explain both the limited expansion of female pelvic dimensions during pubertal development and the reversal to more constricted dimensions during postmenopausal development. One conspicuous feature of the female expansion/reversal pattern is the widening/shortening of the distance between the ischial spines (Figs. 3 and 4C). The ischial spines are larger in humans than in nonhuman primates, because they constitute important attachment sites for the ligaments and fasciae forming the pelvic floor (62). The spines and associated ligamentous structures substantially constrain the birth canal dimensions, but they provide support for the abdominal and pelvic organs and contribute to sagittal stabilization of the sacrum (62–64). Intraabdominal hydrostatic pressure reaches high peak values during walking and running (65), and although that pressure positively influences the stability of the lumbar spine, it results in high strains in the pelvic floor (66). Pelvic floor strains thus might represent a limiting factor of birth canal dimensions, and this hypothesis receives support from the observation that wider dimensions correlate with a higher prevalence of pelvic floor disorders (67).
Based on these considerations, we hypothesize that the evolutionary and developmental dilemma of the female pelvis reflects a trade-off between obstetrics and abdominopelvic stability. During a female’s lifetime, the dilemma is alleviated first in one direction, by widening the birth canal during the time of highest fertility, and then in the other, by restricting its dimensions during postmenopausal life. Although our data provide support for the obstetric side of the dilemma, testing its locomotor side will require a shift of focus from bipedal locomotor economy toward locomotion-related abdominopelvic stability. It remains to be clarified whether the female postmenopausal reversal to more constricted birth canal dimensions evolved under selective pressures acting on postreproductive life (68) or whether it represents a proximate effect of reduced estrogen levels and developmental plasticity. Also, when during human evolution the developmental mode of the female pelvis started to diverge from the male mode remains to be investigated.
Materials and Methods
The study is based on an anonymized known-age and known-sex forensic/clinical sample of nonsymptomatic humans (n = 275) ranging from late fetal stages to late adulthood (Table S4). Data sources are the Collections of the Anthropological Institute, the Virtopsy database of the Institute of Forensic Medicine of the University of Zurich, Children's Hospital of Zurich, the Institute of Diagnostic and Interventional Radiology of the University of Zurich, the digital autopsy database of the Catholic University of Leuven, Belgium (KU Leuven), and clinical datasets freely available from the OsiriX web-page (www.osirix-viewer.com).
Table S4.
Sample structure
| Developmental stage | Age range, y | Males | Females | Total |
| Perinatal | −0.3 to 0.08 | 10 | 16 | 26 |
| Infancy | 0.16–2 | 13 | 5 | 18 |
| Early childhood | 3–8 | 9 | 10 | 19 |
| Middle childhood to adolescence | 9–18 | 27 | 16 | 43 |
| Prime reproductive age | 19–40 | 33 | 36 | 69 |
| Old adults | >40–95 | 59 | 41 | 100 |
Volumetric data were acquired with medical CT (beam collimation 128 × 0.6 mm; in-plane pixel size 0.2 × 0.2–0.7 × 0.7 mm2, slice increment 0.2–1.0 mm). 3D surface models of the bony pelvis were generated with Avizo 6.3.1 (FEI Visualization Sciences Group), and subsequent mesh cleaning was performed with Geomagic XOS (3D Systems). Only well-preserved pelves were used. Several specimens (n = 9 with ages <8 y, n = 5 with ages 12–15 y, and n = 14 with ages 50–80 y) required minor virtual reconstruction (69, 70).
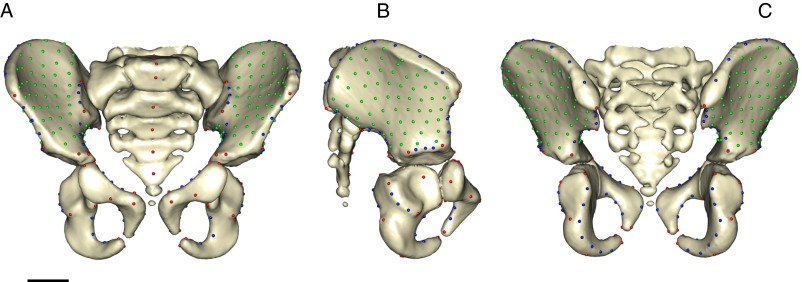
The shape of the pelvis was quantified with a total number of k = 377 3D anatomical landmarks, which denote locations of biological and/or geometric homology among specimens of the sample. These comprise fixed landmarks (LMs) (kf = 63), curve semi-landmarks (SLMs) (kc = 90), and surface SLMs (ks = 224) (Fig. S2 and Tables S5 and S6). The fixed-LM set comprises 14 LM pairs, which eventually fuse during pelvic development. For geometric morphometric analyses, the mean position was calculated for each pair, resulting in kf = 49 fixed LMs and a total of k = 363 LMs. Surface SLMs were generated from an arbitrary specimen’s point cloud, and iterative SLM sliding procedures were applied as described in ref. 71. SLM sliding was performed relative to the symmetrized mean configuration, using the minimum bending energy criterion. These data were submitted to generalized Procrustes analysis. All procedures were performed with the R package Morpho, version 2.3.1.1 (72).
Fig. S2.
Anterior (A), lateral (B), and posterior (C) views of an immature pelvis showing pelvic LMs and SLMs. Fixed LMs are shown in red, curve SLMs in blue, and surface SLMs in green. (Scale bar, 2 cm.)
Table S5.
Definition of fixed LMs
| LM number | Description |
| 1–2 | Pubic symphysis: superior-most anterior point |
| 3–4 | Pubic symphysis: superior-most posterior point |
| 5–6 | Ischiopubic juncture: pubis posterior fusion point |
| 7–8 | Ischiopubic juncture: ischium posterior fusion point |
| 9–10 | Ischiopubic juncture: pubis–obturator foramen inferior fusion point |
| 11–12 | Ischiopubic juncture: ischium–obturator foramen inferior fusion point |
| 13–14 | Ischiopubic juncture: pubis–obturator foramen superior fusion point |
| 15–16 | Ischiopubic juncture: ischium–obturator foramen superior fusion point |
| 17–18 | Ischium: posterior-most point (superior from ischial tuberosity) |
| 19–20 | Ischium: inferior-most midpoint |
| 21–22 | Ilioischial juncture: ischium fusion point |
| 23–24 | Ilioischial juncture: ilium fusion point |
| 25–26 | Ilioischial juncture: ischium–acetabulum lateral fusion point |
| 27–28 | Ilioischial juncture: ilium–acetabulum lateral fusion point |
| 29–30 | Acetabulum: superior-most lateral point |
| 31–32 | Acetabulum: point on pubic part of lunate surface |
| 33–34 | Iliopubic juncture: pubis superior fusion point |
| 35–36 | Iliopubic juncture: ilium superior fusion point |
| 37–38 | Iliopubic juncture: pubis fusion point on pelvic brim |
| 39–40 | Iliopubic juncture: ilium fusion point on pelvic brim |
| 41–42 | Pubis: anterior midpoint |
| 43–44 | Acetabulum: inferior-most lateral point on lunate surface |
| 45–46 | Acetabulum: deepest point on acetabular center (acetabular fossa) |
| 47–48 | Pelvic brim and sacroiliac joint: intersection point |
| 49–50 | Sacroiliac joint: superior-most point |
| 51 | S1: superior-most anterior point |
| 52 | S1: superior-most posterior point |
| 53 | S1: anterior midpoint |
| 54 | S2: anterior midpoint |
| 55 | S3: anterior midpoint |
| 56 | S4: anterior midpoint |
| 57–58 | Anterior superior iliac spine |
| 59–60 | Iliac crest: posterior most point (end point) |
| 61–62 | Posterior inferior iliac spine |
| 63 | S5: anterior midpoint |
Table S6.
Definition of SLMs
| Name | Description |
| Iliac crest | Right: start LM 57, end LM 59; Left: start LM 58, end LM 60 with eight subdivisions |
| Posterior iliac spines | Right: start LM 59, end LM 61; Left: start LM 60, end LM 62 with four subdivisions |
| Greater sciatic notch | Right: start LM 61, end LM 23; Left: start LM 62, end LM 24 with four subdivisions |
| Acetabulum: on ilium | Right: start LM 27, end LM 29; Left: start LM 28, end LM 30 with four subdivisions |
| Anterior iliac spines | Right: start LM 57, end LM 29; Left: start LM 58, end LM 30 with four subdivisions |
| Acetabulum: on ischium | Right: start LM 25, end LM 43; Left: start LM 26, end LM 44 with four subdivisions |
| Obturator foramen posterior | Right: start LM 15, end LM 11; Left: start LM 16, end LM 12 with four subdivisions |
| Obturator foramen anterior | Right: start LM 13, end LM 9; Left: start LM 14, end LM 10 with four subdivisions |
| Sacroiliac joint | Right: start LM 49, end LM 47; Left: start LM 50, end LM 48 with four subdivisions |
| Pelvic brim1 | Right: start LM 47, end LM 39; Left: start LM 48, end LM 40 with four subdivisions |
| Pelvic brim2 | Right: start LM 37, end LM 3; Left: start LM 38, end LM 4 with four subdivisions |
| Pubis posterior | Right: start LM 3, end LM 5; Left: start LM 4, end LM 6 with two subdivisions |
| Ischium posterior | Right: start LM 7, end LM 21; Left: start LM 8, end LM 22 with eight subdivisions |
Principal component analysis was used to reduce the dimensionality of shape space and visualize major patterns of shape variation in the sample. Sex-specific moving averages of PC scores, centroid size, and angular and linear pelvic dimensions were calculated to explore patterns of morphological change along developmental trajectories. To test for differences between group-specific pelvic shapes (Tables S1 and S3), Procrustes ANOVA was performed using the R package geomorph, version 3.0.0-1 (73). Directions of developmental trajectories through shape space were compared using the methods proposed in ref. 74 (Table S2).
Supplementary Material
Acknowledgments
We thank Michael Thali, Lars Ebert, Steffen Ross, and Wim Develter for preparing the anonymized forensic data and Stefan Schlager and Emma Sherratt for their suggestions regarding R packages Morpho and geomorph, respectively. We also thank the three anonymous reviewers for their constructive comments and suggestions. This study was supported by Swiss National Science Foundation Grant 31003A_135470/1 (to C.P.E.Z.), and the A. H. Schultz Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1517085113/-/DCSupplemental.
References
- 1.McPherson FJ, Chenoweth PJ. Mammalian sexual dimorphism. Anim Reprod Sci. 2012;131(3-4):109–122. doi: 10.1016/j.anireprosci.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Schultz AH. Sex differences in the pelves of primates. Am J Phys Anthropol. 1949;7(3):401–423. doi: 10.1002/ajpa.1330070307. [DOI] [PubMed] [Google Scholar]
- 3.Leutenegger W. [Relationships between the size of the new-born and sexual dimorphism in the pelvis of the simian primates] Folia Primatol (Basel) 1970;12(3):224–235. doi: 10.1159/000155292. [DOI] [PubMed] [Google Scholar]
- 4.Gingerich PD. The development of sexual dimorphism in the bony pelvis of the squirrel monkey. Anat Rec. 1972;172(3):589–595. doi: 10.1002/ar.1091720312. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg KR. The evolution of modern human childbirth. Am J Phys Anthropol. 1992;35(S15):89–124. [Google Scholar]
- 6.Tague RG. Variation in pelvic size between males and females in nonhuman anthropoids. Am J Phys Anthropol. 1995;97(3):213–233. doi: 10.1002/ajpa.1330970302. [DOI] [PubMed] [Google Scholar]
- 7.LaVelle M. Natural selection and developmental sexual variation in the human pelvis. Am J Phys Anthropol. 1995;98(1):59–72. doi: 10.1002/ajpa.1330980106. [DOI] [PubMed] [Google Scholar]
- 8.Lovejoy CO. The natural history of human gait and posture. Part 1. Spine and pelvis. Gait Posture. 2005;21(1):95–112. doi: 10.1016/j.gaitpost.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Wittman AB, Wall LL. The evolutionary origins of obstructed labor: Bipedalism, encephalization, and the human obstetric dilemma. Obstet Gynecol Surv. 2007;62(11):739–748. doi: 10.1097/01.ogx.0000286584.04310.5c. [DOI] [PubMed] [Google Scholar]
- 10.Gruss LT, Schmitt D. The evolution of the human pelvis: Changing adaptations to bipedalism, obstetrics and thermoregulation. Philos Trans R Soc Lond B Biol Sci. 2015;370(1663):20140063. doi: 10.1098/rstb.2014.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells JCK. Between Scylla and Charybdis: Renegotiating resolution of the ‘obstetric dilemma’ in response to ecological change. Philos Trans R Soc Lond B Biol Sci. 2015;370(1663):20140067. doi: 10.1098/rstb.2014.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunsworth HM, Warrener AG, Deacon T, Ellison PT, Pontzer H. Metabolic hypothesis for human altriciality. Proc Natl Acad Sci USA. 2012;109(38):15212–15216. doi: 10.1073/pnas.1205282109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warrener AG, Lewton KL, Pontzer H, Lieberman DE. A wider pelvis does not increase locomotor cost in humans, with implications for the evolution of childbirth. PLoS One. 2015;10(3):e0118903. doi: 10.1371/journal.pone.0118903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Washburn SL. Tools and human evolution. Sci Am. 1960;203(3):63–75. [PubMed] [Google Scholar]
- 15.Portmann A. Die Tragzeiten der Primaten und die Dauer der Schwangerschaft beim Menschen: Ein Problem der vergleichenden Biologie. Rev Suisse Zool. 1941;48:511–518. [Google Scholar]
- 16.Hofmeyr GJ. Obstructed labor: Using better technologies to reduce mortality. Int J Gynaecol Obstet. 2004;85(Suppl 1):S62–S72. doi: 10.1016/j.ijgo.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 17.McClure EM, Goldenberg RL, Bann CM. Maternal mortality, stillbirth and measures of obstetric care in developing and developed countries. Int J Gynaecol Obstet. 2007;96(2):139–146. doi: 10.1016/j.ijgo.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Harrison MS, et al. A prospective population-based study of maternal, fetal, and neonatal outcomes in the setting of prolonged labor, obstructed labor and failure to progress in low- and middle-income countries. Reprod Health. 2015;12(Suppl 2):S9. doi: 10.1186/1742-4755-12-S2-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurki HK. Pelvic dimorphism in relation to body size and body size dimorphism in humans. J Hum Evol. 2011;61(6):631–643. doi: 10.1016/j.jhevol.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Dunsworth H, Eccleston L. The evolution of difficult childbirth and helpless hominin infants. Annu Rev Anthropol. 2015;44(1):55–69. [Google Scholar]
- 21.Brown KM. Selective pressures in the human bony pelvis: Decoupling sexual dimorphism in the anterior and posterior spaces. Am J Phys Anthropol. 2015;157(3):428–440. doi: 10.1002/ajpa.22734. [DOI] [PubMed] [Google Scholar]
- 22.Stone PK. Biocultural perspectives on maternal mortality and obstetrical death from the past to the present. Am J Phys Anthropol. 2016;159(Suppl 61):S150–S171. doi: 10.1002/ajpa.22906. [DOI] [PubMed] [Google Scholar]
- 23.Ellison P. Energetics, reproductive ecology, and human evolution. PaleoAnthropology. 2008;2008:172–200. [Google Scholar]
- 24.Jasienska G, Thune I, Ellison PT. Fatness at birth predicts adult susceptibility to ovarian suppression: An empirical test of the Predictive Adaptive Response hypothesis. Proc Natl Acad Sci USA. 2006;103(34):12759–12762. doi: 10.1073/pnas.0605488103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer B, Mitteroecker P. Covariation between human pelvis shape, stature, and head size alleviates the obstetric dilemma. Proc Natl Acad Sci USA. 2015;112(18):5655–5660. doi: 10.1073/pnas.1420325112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams TM, Carroll SB. Genetic and molecular insights into the development and evolution of sexual dimorphism. Nat Rev Genet. 2009;10(11):797–804. doi: 10.1038/nrg2687. [DOI] [PubMed] [Google Scholar]
- 27.Parsch J, Ellegren H. The evolutionary causes and consequences of sex-biased gene expression. Nat Rev Genet. 2013;14(2):83–87. doi: 10.1038/nrg3376. [DOI] [PubMed] [Google Scholar]
- 28.Bernstein P, Crelin ES. Bony pelvic sexual dimorphism in the rat. Anat Rec. 1967;157(3):517–525. [Google Scholar]
- 29.Iguchi T, Irisawa S, Fukazawa Y, Uesugi Y, Takasugi N. Morphometric analysis of the development of sexual dimorphism of the mouse pelvis. Anat Rec. 1989;224(4):490–494. doi: 10.1002/ar.1092240406. [DOI] [PubMed] [Google Scholar]
- 30.Uesugi Y, Taguchi O, Noumura T, Iguchi T. Effects of sex steroids on the development of sexual dimorphism in mouse innominate bone. Anat Rec. 1992;234(4):541–548. doi: 10.1002/ar.1092340409. [DOI] [PubMed] [Google Scholar]
- 31.Tague RG. Big-bodied males help us recognize that females have big pelves. Am J Phys Anthropol. 2005;127(4):392–405. doi: 10.1002/ajpa.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berdnikovs S, Bernstein M, Metzler A, German RZ. Pelvic growth: Ontogeny of size and shape sexual dimorphism in rat pelves. J Morphol. 2007;268(1):12–22. doi: 10.1002/jmor.10476. [DOI] [PubMed] [Google Scholar]
- 33.Migliano AB, Vinicius L, Lahr MM. Life history trade-offs explain the evolution of human pygmies. Proc Natl Acad Sci USA. 2007;104(51):20216–20219. doi: 10.1073/pnas.0708024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: Final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65. [PubMed] [Google Scholar]
- 35.Boucher BJ. Sex differences in the foetal pelvis. Am J Phys Anthropol. 1957;15(4):581–600. doi: 10.1002/ajpa.1330150409. [DOI] [PubMed] [Google Scholar]
- 36.Wilson LAB, Ives R, Cardoso HFV, Humphrey LT. Shape, size, and maturity trajectories of the human ilium. Am J Phys Anthropol. 2015;156(1):19–34. doi: 10.1002/ajpa.22625. [DOI] [PubMed] [Google Scholar]
- 37.Bilfeld MF, et al. Ontogeny of size and shape sexual dimorphism in the pubis: A multislice Computed Tomography study by geometric morphometry. J Forensic Sci. 2015;60(5):1121–1128. doi: 10.1111/1556-4029.12761. [DOI] [PubMed] [Google Scholar]
- 38.Walker PL. Greater sciatic notch morphology: Sex, age, and population differences. Am J Phys Anthropol. 2005;127(4):385–391. doi: 10.1002/ajpa.10422. [DOI] [PubMed] [Google Scholar]
- 39.Coleman WH. Sex differences in the growth of the human bony pelvis. Am J Phys Anthropol. 1969;31(2):125–151. doi: 10.1002/ajpa.1330310202. [DOI] [PubMed] [Google Scholar]
- 40.Tague RG. Maternal mortality or prolonged growth: Age at death and pelvic size in three prehistoric Amerindian populations. Am J Phys Anthropol. 1994;95(1):27–40. doi: 10.1002/ajpa.1330950103. [DOI] [PubMed] [Google Scholar]
- 41.Schutz H, Donovan ER, Hayes JP. Effects of parity on pelvic size and shape dimorphism in Mus. J Morphol. 2009;270(7):834–842. doi: 10.1002/jmor.10723. [DOI] [PubMed] [Google Scholar]
- 42.Callewaert F, Sinnesael M, Gielen E, Boonen S, Vanderschueren D. Skeletal sexual dimorphism: Relative contribution of sex steroids, GH-IGF1, and mechanical loading. J Endocrinol. 2010;207(2):127–134. doi: 10.1677/JOE-10-0209. [DOI] [PubMed] [Google Scholar]
- 43.Devlin MJ. Estrogen, exercise, and the skeleton. Evol Anthropol. 2011;20(2):54–61. doi: 10.1002/evan.20299. [DOI] [PubMed] [Google Scholar]
- 44.Oury F. A crosstalk between bone and gonads. Ann N Y Acad Sci. 2012;1260(1):1–7. doi: 10.1111/j.1749-6632.2011.06360.x. [DOI] [PubMed] [Google Scholar]
- 45.Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends Endocrinol Metab. 2012;23(11):576–581. doi: 10.1016/j.tem.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet. 2008;9(12):911–922. doi: 10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cole TJ, Ahmed ML, Preece MA, Hindmarsh P, Dunger DB. The relationship between Insulin-like Growth Factor 1, sex steroids and timing of the pubertal growth spurt. Clin Endocrinol (Oxf) 2015;82(6):862–869. doi: 10.1111/cen.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elden H, Hagberg H, Olsén MF, Ladfors L, Ostgaard HC. Regression of pelvic girdle pain after delivery: Follow-up of a randomised single blind controlled trial with different treatment modalities. Acta Obstet Gynecol Scand. 2008;87(2):201–208. doi: 10.1080/00016340701823959. [DOI] [PubMed] [Google Scholar]
- 49.Dehghan F, et al. The effect of relaxin on the musculoskeletal system. Scand J Med Sci Sports. 2014;24(4):e220–e229. doi: 10.1111/sms.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reitter A, et al. Does pregnancy and/or shifting positions create more room in a woman’s pelvis? Am J Obstet Gynecol. 2014;211(6):662.e1–662.e9. doi: 10.1016/j.ajog.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 51.Verdonck A, Gaethofs M, Carels C, de Zegher F. Effect of low-dose testosterone treatment on craniofacial growth in boys with delayed puberty. Eur J Orthod. 1999;21(2):137–143. doi: 10.1093/ejo/21.2.137. [DOI] [PubMed] [Google Scholar]
- 52.Emery Thompson M, Zhou A, Knott CD. Low testosterone correlates with delayed development in male orangutans. PLoS One. 2012;7(10):e47282. doi: 10.1371/journal.pone.0047282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lefevre CE, Lewis GJ, Perrett DI, Penke L. Telling facial metrics: Facial width is associated with testosterone levels in men. Evol Hum Behav. 2013;34(4):273–279. [Google Scholar]
- 54.Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends Ecol Evol. 2005;20(10):527–533. doi: 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Trevathan W. Primate pelvic anatomy and implications for birth. Philos Trans R Soc Lond B Biol Sci. 2015;370(1663):20140065. doi: 10.1098/rstb.2014.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moffett A, Hiby SE, Sharkey AM. The role of the maternal immune system in the regulation of human birthweight. Philos Trans R Soc Lond B Biol Sci. 2015;370(1663):20140071. doi: 10.1098/rstb.2014.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jasienska G, Ziomkiewicz A, Lipson SF, Thune I, Ellison PT. High ponderal index at birth predicts high estradiol levels in adult women. Am J Hum Biol. 2006;18(1):133–140. doi: 10.1002/ajhb.20462. [DOI] [PubMed] [Google Scholar]
- 58.Bentley GR, Harrigan AM, Ellison PT. Dietary composition and ovarian function among Lese horticulturalist women of the Ituri Forest, Democratic Republic of Congo. Eur J Clin Nutr. 1998;52(4):261–270. doi: 10.1038/sj.ejcn.1600547. [DOI] [PubMed] [Google Scholar]
- 59.Woods MN, et al. Hormone levels during dietary changes in premenopausal African-American women. J Natl Cancer Inst. 1996;88(19):1369–1374. doi: 10.1093/jnci/88.19.1369. [DOI] [PubMed] [Google Scholar]
- 60.Aubertin-Leheudre M, et al. Fat/fiber intakes and sex hormones in healthy premenopausal women in USA. J Steroid Biochem Mol Biol. 2008;112(1-3):32–39. doi: 10.1016/j.jsbmb.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lipson SF, Ellison PT. Comparison of salivary steroid profiles in naturally occurring conception and non-conception cycles. Hum Reprod. 1996;11(10):2090–2096. doi: 10.1093/oxfordjournals.humrep.a019055. [DOI] [PubMed] [Google Scholar]
- 62.Abitbol MM. Evolution of the ischial spine and of the pelvic floor in the Hominoidea. Am J Phys Anthropol. 1988;75(1):53–67. doi: 10.1002/ajpa.1330750107. [DOI] [PubMed] [Google Scholar]
- 63.Ashton-Miller JA, DeLancey JOL. Functional anatomy of the female pelvic floor. Ann N Y Acad Sci. 2007;1101(1):266–296. doi: 10.1196/annals.1389.034. [DOI] [PubMed] [Google Scholar]
- 64.Tardieu C, et al. How is sagittal balance acquired during bipedal gait acquisition? Comparison of neonatal and adult pelves in three dimensions. Evolutionary implications. J Hum Evol. 2013;65(2):209–222. doi: 10.1016/j.jhevol.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 65.Grillner S, Nilsson J, Thorstensson A. Intra-abdominal pressure changes during natural movements in man. Acta Physiol Scand. 1978;103(3):275–283. doi: 10.1111/j.1748-1716.1978.tb06215.x. [DOI] [PubMed] [Google Scholar]
- 66.Hodges PW, Eriksson AEM, Shirley D, Gandevia SC. Intra-abdominal pressure increases stiffness of the lumbar spine. J Biomech. 2005;38(9):1873–1880. doi: 10.1016/j.jbiomech.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 67.Handa VL, et al. Architectural differences in the bony pelvis of women with and without pelvic floor disorders. Obstet Gynecol. 2003;102(6):1283–1290. doi: 10.1016/j.obstetgynecol.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 68.Hawkes K, O’Connell JF, Jones NG, Alvarez H, Charnov EL. Grandmothering, menopause, and the evolution of human life histories. Proc Natl Acad Sci USA. 1998;95(3):1336–1339. doi: 10.1073/pnas.95.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zollikofer CPE, Ponce de León MS. Virtual Reconstruction: A Primer in Computer-Assisted Paleontology and Biomedicine. Wiley-Liss; Hoboken, NJ: 2005. [Google Scholar]
- 70.Ponce de León MS, et al. Neanderthal brain size at birth provides insights into the evolution of human life history. Proc Natl Acad Sci USA. 2008;105(37):13764–13768. doi: 10.1073/pnas.0803917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gunz P, Mitteroecker P, Bookstein FL. In: Semilandmarks in Three Dimensions. Modern Morphometrics in Physical Anthropology, Developments in Primatology: Progress and Prospects. Slice DE, editor. Kluwer Academic Publishers; New York: 2005. pp. 73–98. [Google Scholar]
- 72.Schlager S. 2016 Morpho: Calculations and visualisations related to geometric morphometrics. R package version 2.3.1.1. Available at cran.r-project.org/web/packages/Morpho/index.html. Accessed February 28, 2016.
- 73.Adams DC, Collyer ML, Sherratt E. 2015 geomorph: Software for geometric morphometric analyses. R package version 2.1.7. Available at cran.r-project.org/web/packages/geomorph/index.html. Accessed February 28, 2016.
- 74.Collyer ML, Sekora DJ, Adams DC. A method for analysis of phenotypic change for phenotypes described by high-dimensional data. Heredity (Edinb) 2015;115(4):357–365. doi: 10.1038/hdy.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.