Significance
It is well established that light regulates mammalian biology. And yet, we have been unable to define and thus harness the underlying mechanisms so as to apply them to alter the course of human disease. In this study we determine that the spectrum of light is a critical determinant of its effect on critical illness. We show that an acute and short (24 h) exposure to high-illuminance (1,400 lx) blue spectrum (peak 442 nm) light prior to ischemia/reperfusion (I/R) significantly attenuates the degree of organ injury. Our characterization of the biological mechanisms through which blue light beneficially alters the cellular response to I/R provides an opportunity to develop novel therapeutics for the prevention and treatment of many diseases.
Keywords: blue light, ischemia, reperfusion, organ injury, circadian rhythms
Abstract
Evidence suggests that light and circadian rhythms profoundly influence the physiologic capacity with which an organism responds to stress. However, the ramifications of light spectrum on the course of critical illness remain to be determined. Here, we show that acute exposure to bright blue spectrum light reduces organ injury by comparison with bright red spectrum or ambient white fluorescent light in two murine models of sterile insult: warm liver ischemia/reperfusion (I/R) and unilateral renal I/R. Exposure to bright blue light before I/R reduced hepatocellular injury and necrosis and reduced acute kidney injury and necrosis. In both models, blue light reduced neutrophil influx, as evidenced by reduced myeloperoxidase (MPO) within each organ, and reduced the release of high-mobility group box 1 (HMGB1), a neutrophil chemotactant and key mediator in the pathogenesis of I/R injury. The protective mechanism appeared to involve an optic pathway and was mediated, in part, by a sympathetic (β3 adrenergic) pathway that functioned independent of significant alterations in melatonin or corticosterone concentrations to regulate neutrophil recruitment. These data suggest that modifying the spectrum of light may offer therapeutic utility in sterile forms of cellular injury.
Sunlight, the sole source of energy for all living organisms, has profound effects on life. Prior studies precipitated excitement regarding the therapeutic value of bright light, noting that more light could benefit certain diseases (1–3). But this initial enthusiasm has been dampened by later studies that failed to replicate these findings (4–8). However, light is complex, defined by illuminance (intensity), photoperiod (duration), and wavelength; and yet, few clinical studies have incorporated this complexity into their design. For example, hospitalized patients suffer simultaneously from considerably decreased intensity of daylight, an expanded photoperiod due to continuous or misdirected and thus excessive and obtrusive artificial light called “light pollution,” and a change in spectrum between sunlight and artificial light (4, 5, 9). Each of these factors could influence the biology and outcome of disease, and yet have not been rigorously controlled in prior studies. This complexity of light may contribute, in part, to the inability of prior observational trials to identify an association between light exposure and the outcomes studied.
Mammals perceive visible light (400–700 nm), and experimental animal evidence suggests that each dimension of light can modify animal biology (10–14). In rodents, acute bright light exposure induced the release of corticosterone (15, 16). Fish raised under light of blue/green spectrum exhibited reduced oxidative stress by comparison with environmental red light (13, 14). And a novel paradigm posits that seasonal variation in environmental stress (e.g., thermal, starvation) evolutionarily selected animals expressing photoperiodism: the ability to interpret environmental day length (17, 18). Responding to a progressively shorter photoperiod, energetic resources are concentrated in endogenous mechanisms (i.e., immune function) necessary for seasonally appropriate survival strategies. Although these collective data suggest that light may influence adaptive responses to and during illness, the ramifications of acute alterations in light exposure on health and disease remain to be defined (1–4, 7).
In this study we focus on whether the spectrum of light, holding illuminance and photoperiod constant, can favorably modify the biology of critical illness. Using two independent, murine models of ischemia/reperfusion (I/R), we determine that high- illuminance blue spectrum light functioning through an optic pathway mediates adaptive changes in the response to the critical stress of I/R that protects against organ injury.
Results
Blue Light Before Liver I/R Attenuates Liver Injury and Cellular Necrosis.
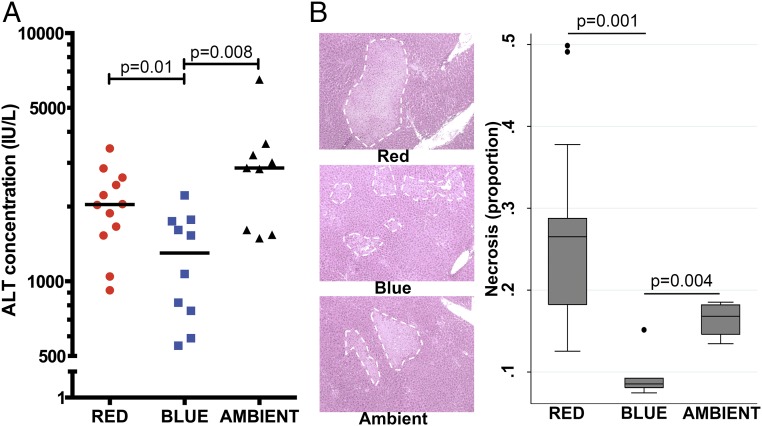
Certain clinical circumstances (e.g., hepatic resection, transplantation) are amenable to pretreatment and characterized by a period of I/R that induces sterile (i.e., absence of infectious etiology) cellular injury. We initially explored whether blue, red, or ambient white fluorescent light altered organ injury when applied before I/R. Pretreatment with blue light protected against liver injury during I/R, as evidenced by reduced serum alanine aminotransferase (ALT) concentration: blue 1,300 IU/L vs. red 2,038 IU/L (P = 0.01) and vs. ambient 2,860 IU/L (P = 0.008; Fig. 1A). Blue light also significantly reduced hepatocellular necrosis (Fig. 1B).
Fig. 1.
Blue light before liver I/R attenuates liver injury and cellular necrosis. Mice were exposed to red, blue, or ambient light for 24 h and then subjected to hepatic I/R (10–12 total mice per group for all four experiments combined). (A) Serum was assayed for ALT concentration (IU/L). Bar, median. (B) Histology (H&E) of liver tissue (200× magnification) was performed to quantify cellular necrosis (representative image of three experiments). White dashed line demarcates regions of necrosis. Corresponding box plots provide summary estimates (bar, median; box, IQR 25–75% range; whiskers, 1.5× IQR) of necrosis. Statistical comparisons were made by nonparametric Mann–Whitney test.
Blue Light Before Kidney I/R Attenuates Acute Kidney Injury and Cellular Necrosis.
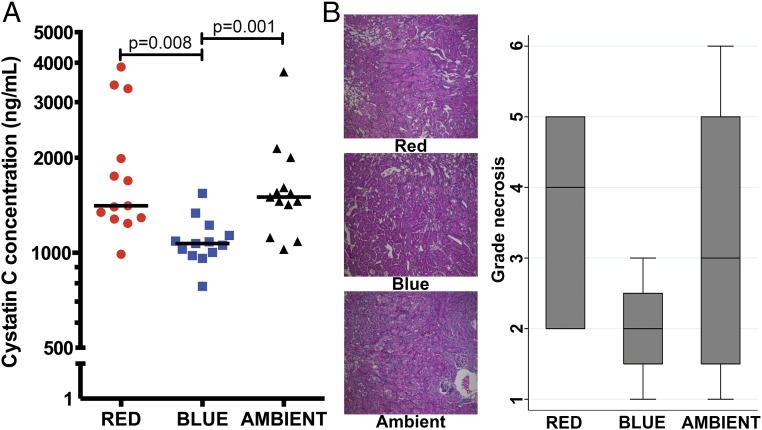
We next explored whether blue light protected against organ injury in an independent model of unilateral renal I/R. Pretreatment with blue light protected against acute kidney injury (AKI), as evidenced by reduced serum cystatin C concentration: blue 1,069 ng/mL vs. red 1,409 ng/mL (P = 0.008) and vs. ambient 1,500 ng/mL (P = 0.001; Fig. 2A). Blue light also reduced renal tubular cellular necrosis (Fig. 2B).
Fig. 2.
Blue light before kidney I/R attenuates acute kidney injury and cellular necrosis. Mice were exposed to red, blue, or ambient light for 24 h and then subjected to unilateral kidney I/R (13 total mice per group for all four experiments combined). (A) Serum was assayed for cystatin C concentration (ng/mL). Bar, median. (B) Histology (H&E) of kidney cortical tissue (200× magnification) was performed to quantify cellular necrosis, blebbing, vacuolization, and cast formation (representative image of four experiments). Corresponding box plots provide summary estimates (bar, median; box, IQR 25–75% range; whiskers, 1.5× IQR) of necrosis. Statistical comparisons were made by nonparametric Mann–Whitney test.
The Effects of Blue Light Are Mediated Through an Optic Pathway.
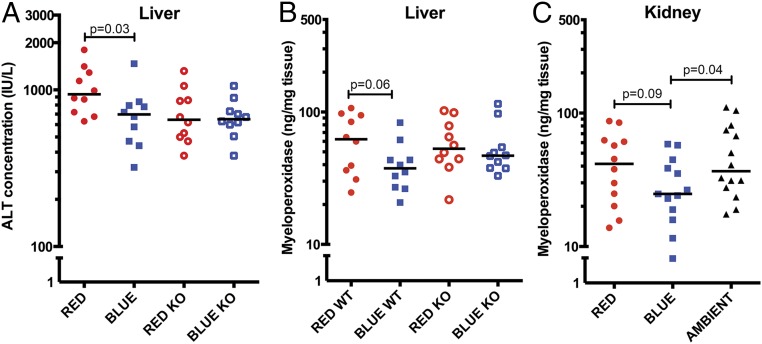
Known pathways through which light may mediate its biological effects are the eyes and skin. We initially interrogated for an optic pathway using Vsx2 KO mice, which undergo optic nerve degeneration (19). Wild-type mice exposed to blue light again exhibited reduced hepatic injury by comparison with red light: ALT 698 IU/L vs. 938 IU/L, P = 0.03 (Fig. 3A). However, this difference was lost in Vsx2 KO mice: 650 IU/L vs. 645 IU/L, P = 0.94.
Fig. 3.
Blue light functions through an optic pathway and reduces liver and kidney MPO activity during I/R. (A) Wild-type 129S1 (closed circles and squares) and Vsx2 Optic KO (open circles and squares) mice were exposed to red or blue light for 24 h and then subjected to hepatic I/R (10 total mice per group for all three experiments combined). Serum was assayed for ALT concentration (IU/L). Bar, median. (B) Liver tissue was assayed for MPO (ng/mg tissue protein). Bar, median. (C) Mice were exposed to red, blue, or ambient light for 24 h and then subjected to unilateral kidney I/R (12–14 total mice per group for all four experiments combined). Kidney tissue was assayed for MPO (ng/mg tissue protein). Bar, median. Statistical comparisons were made by nonparametric Mann–Whitney test.
Blue Light Reduces Liver and Kidney Myeloperoxidase Activity During I/R.
The neutrophil is regarded as the ultimate effector of the cellular injury of I/R (20–22). We observed in both models that blue light reduced neutrophil influx into the ischemic organ, as evidenced by reduced liver and kidney myeloperoxidase (MPO) (Fig. 3 B and C). In addition, the effects of blue light on neutrophil recruitment during liver I/R required an optic pathway (Fig. 3B).
Blue Light Attenuates High-Mobility Group Box 1 Release During I/R.
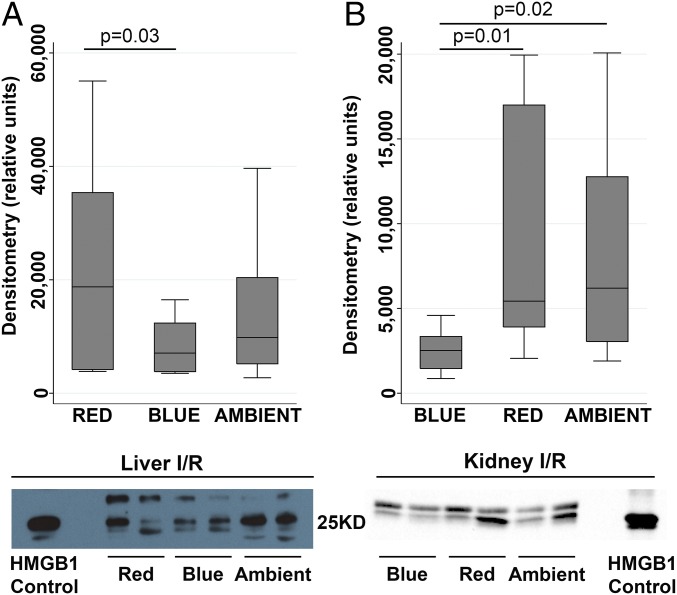
Danger-associated molecular pattern (DAMP) molecules are nuclear or cytosolic proteins that are released outside the cell following tissue injury, such as I/R, and can initiate and perpetuate a noninfectious inflammatory/immune response. High-mobility group box 1 (HMGB1), a nuclear DAMP, is a key mediator in the causal pathway of I/R-mediated neutrophil damage (23, 24). We observed that during I/R, mice exposed to blue light exhibited reduced concentrations of serum HMGB1 (Fig. 4). Two bands migrated to the appropriate molecular weight, which has been observed by us and others, and may be due to posttranslational modification, including phosphorylation and acetylation (23, 25, 26).
Fig. 4.
Blue light attenuates HMGB1 release during hepatic and renal I/R. Mice were exposed to red, blue, or ambient light for 24 h and then subjected to (A) hepatic I/R (10–12 total mice per group for all four experiments combined) or (B) unilateral kidney I/R (13 total mice per group for all four experiments combined). Serum HMGB1 (29KD) expression (representative immunoblot of four experiments). Corresponding box plots provide summary estimates (bar, median; box, IQR 25–75% range; whiskers, 1.5× IQR) of densitometry of HMGB1 concentration. Statistical comparisons were made by nonparametric Mann–Whitney test.
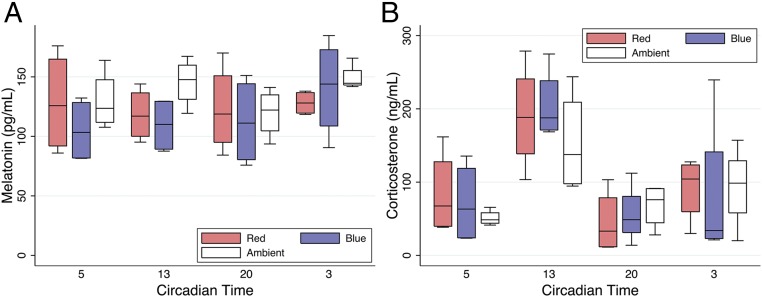
Blue Light Does Not Alter Serum Melatonin and Corticosterone Concentrations.
Many facets of immunity exhibit a circadian pattern and appear under the regulatory influence of melatonin and light (27–29). However, the serum of mice exposed to blue, red, and ambient light demonstrated similar circadian concentrations of melatonin (Fig. 5A). Corticosteroid (i.e., cortisol, corticosterone) hormones also exhibit circadian rhythm and may influence immunity (30). However, we observed little difference in systemic corticosterone concentrations in blue-, red-, and ambient-exposed mice (Fig. 5B). Finally, acute sleep deprivation has been shown to attenuate neuroinflammation and cell death after ischemia and to attenuate systemic inflammation in murine models of endotoxemia (31, 32). However, we observed similar activities of mice exposed to a 24-h photoperiod of blue and red light (Fig. S1A).
Fig. 5.
Blue light does not alter serum melatonin or corticosterone concentration. Mice (C57BL/6) were exposed to red, blue, or ambient light for 24 h (six total mice per group for two experiments combined). Serum was isolated at CT5, CT13, CT20, and CT3 and analyzed for (A) melatonin (pg/mL) and (B) corticosterone (ng/mL) concentrations. Corresponding box plots provide summary estimates (bar, median; box, IQR 25–75% range; whiskers, 1.5× IQR) of melatonin and corticosterone concentrations. Statistical comparisons were made by nonparametric Mann–Whitney test.
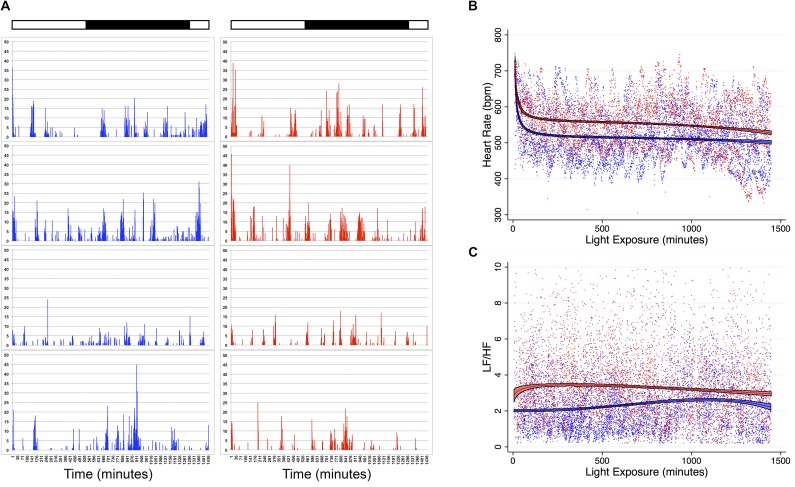
Fig. S1.
Activity, heart rate, and heart rate variability (LF/HF) of mice exposed to blue and red spectrum light. Mice underwent laparotomy and implantation of a DSI HD-X11 telemetry device. After 24 h of recovery, mice were exposed to a 24-h photoperiod of high illuminance blue or red spectrum light and monitored for 24 h. Dark horizontal bar indicates circadian night (CT12 to CT24). White horizontal bar indicates circadian day (CT0 to CT12). (A) Activity of mice during exposure to blue or red light (n = 4 mice per group). (B) Heart rate of mice during exposure to blue or red light. Each point is a single measurement of a single mouse at a single time point. Lines and colored areas represent a fractional polynomial estimate of the mean ± 95% confidence interval of heart rate for each cohort of blue (n = 4) and red (n = 4) mice. (C) LF/HF ratio was calculated as a parameter of sympathetic tone. Each point is a single measurement of a single mouse at a single time point. Lines and colored areas represent a fractional polynomial estimate of the mean ± 95% confidence interval of LF/HF ratio for each cohort of blue (n = 4) and red (n = 4) mice.
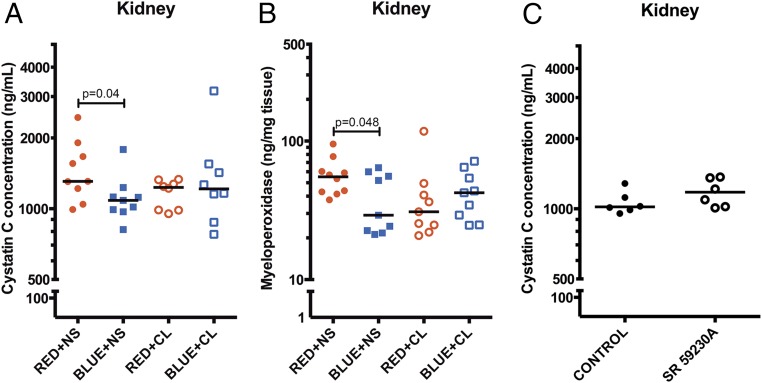
Blue Light Functions Through an Adrenergic Pathway.
Recently it has been shown that sympathetic signals govern a circadian oscillation in leukocyte emigration into tissues that alters the susceptibility to and outcome from inflammatory diseases (27, 33). Thus, we explored whether blue light regulates sympathetic tone and thereby reduces neutrophil influx and organ injury during I/R. We initially quantified the heart rate variability of mice exposed to blue and red light, focusing on the low frequency/high frequency (LF/HF) ratio as a physiologic parameter of sympathetic activity (34–38). We observed that mice exposed to blue light exhibited lower sympathetic tone, as evidenced by reduced heart rates and LF/HF, in contrast to mice exposed to red light (Fig. S1 B and C). We next explored whether augmenting adrenergic tone reversed the protective effects of blue light. As shown in Fig. 6A, control (saline) mice exposed to blue light exhibited reduced AKI by comparison with control mice exposed to red light: cystatin C concentration: blue + normal saline (NS) 1,068 ng/mL vs. red + NS 1,307 ng/mL (P = 0.04). However, when mice were administered the β3 agonist CL-316,243, blue light no longer was protective: cystatin C concentration, blue + CL 1,215 ng/mL vs. red + NS 1,307 ng/mL (P = 0.25). Similarly, β3 agonism partly reversed the reduction in MPO in mice exposed to blue light (Fig. 6B). Alternatively, administering the β3 antagonist SR 59230A (39) to mice before I/R did not reduce AKI: SR 1,154 ng/mL vs. NS 1,019 ng/mL (P = 0.20) (Fig. 6C).
Fig. 6.
Blue light functions through an adrenergic pathway. Mice were administered either equivolume saline (0.9%, i.p.) or the β3 agonist CL316,243 (1 mg/kg, i.p.), and exposed to red, blue, or ambient light for 24 h. Mice were then subjected to unilateral kidney I/R (nine total mice per group for all four experiments combined). (A) Serum was analyzed for cystatin C concentration (ng/mL). Bar, median. (B) Kidney tissue was analyzed for MPO concentration (ng/mg tissue protein). Bar, median. (C) Mice were administered either equivolume saline (0.9%, i.p.) or the β3 antagonist SR 59230A (5 mg/kg, i.p.) and then subjected to unilateral kidney I/R (six total mice per group for two experiments combined). Serum was analyzed for cystatin C concentration (ng/mL). Bar, median. Statistical comparisons were made by nonparametric Mann–Whitney test.
Discussion
Prior studies precipitated excitement regarding the therapeutic value of bright light or windows in beneficially altering human disease, yet more contemporary studies have failed to replicate these observations (1, 3–8). Thus, whether light can acutely modify the biology of critical illness has never been described. Here we report that a 24-h photoperiod of high-intensity blue spectrum light attenuates organ injury in two clinically relevant, animal models of hepatic and renal I/R. Importantly, the benefits of blue light are translationally relevant to circumstances permitting pretreatment (e.g., transplantation). The mechanism involves an optic pathway that functions, in part, by a permissive withdrawal of β3 adrenergic tone to reduce neutrophil recruitment. Additionally, blue light inhibits the release of HMGB1, a key mediator of I/R-induced organ injury. Collectively, our data support the potential of blue light as a therapy.
Little data are published on the effects of light spectra on mammalian biology. Fish exposed to green or blue LEDs during starvation exhibited less oxidative stress by comparison with red LED exposure (13, 14). Whether blue and green light were protective or red light was injurious could not be definitively determined. We observed that during I/R, blue light reduced organ injury and cellular necrosis. Our experimental conditions included an ambient fluorescent light exposure to replicate the typical artificial indoor lighting environment (i.e., the hospital). For nearly every outcome parameter assessed, mice exposed to this ambient light exhibited organ injury similar to mice exposed to red light. Thus, our data strongly support that it is blue light that is protective.
One mechanism by which light may alter the response to stress is through changes in immunity: photoimmunomodulation (4, 5, 10, 11, 17, 18, 40). The neutrophil is a fundamental effector of organ injury during I/R, and inhibiting neutrophil influx or function during I/R markedly reduces organ injury (20–22). We observed that the kidneys and livers of mice exposed to blue light had reduced infiltration of active neutrophils (i.e., MPO). Damage-associated molecular patterns (DAMP), such as HMGB1, are released during I/R, and others and we have shown that HMGB1 is a key mediator of inflammation and cellular damage during liver I/R and ischemic AKI (23, 41). Recently it has been shown that HMGB1 is released by necrotic cells and leads to neutrophil recruitment and injury amplification (24). We observed that blue light also reduced the systemic release of HMGB1. Thus, a reduction in the release of HMGB1 and an inhibition of neutrophil recruitment are two biological mechanisms by which blue light may protect against ischemia.
The physiologic mechanism through which blue light regulated these biological changes required an intact optic pathway. The prototypical mediator of central circadian rhythms and photoimmunomodulation is melatonin (29). Nearly every facet of immunity exhibits a circadian pattern and appears under the regulatory influence of melatonin and light (27, 28, 42, 43). Similarly, corticosteroid stress hormones also exhibit circadian rhythm, are modulated by acute light exposure and may influence immunity (15, 16). However, the effects of blue light occurred without notable differences in systemic melatonin or corticosterone concentrations in comparison with red light. Furthermore, the experimental conditions of blue and red light were characterized by identical photoperiods, illuminances, and temperatures. These observations highlight the importance of the spectrum of light in modifying the biological response to I/R, and that mechanisms distinct from central circadian and adrenocortical axes are operant. Notably, degeneration of the optic nerve as occurs in VsX2 KO mice correlated with reduced AKI during I/R and a phenotype more closely approximating that of mice exposed to blue light. These data may provide sufficient impetus for a clinical investigation of the ramifications of optical blindness on ischemic diseases.
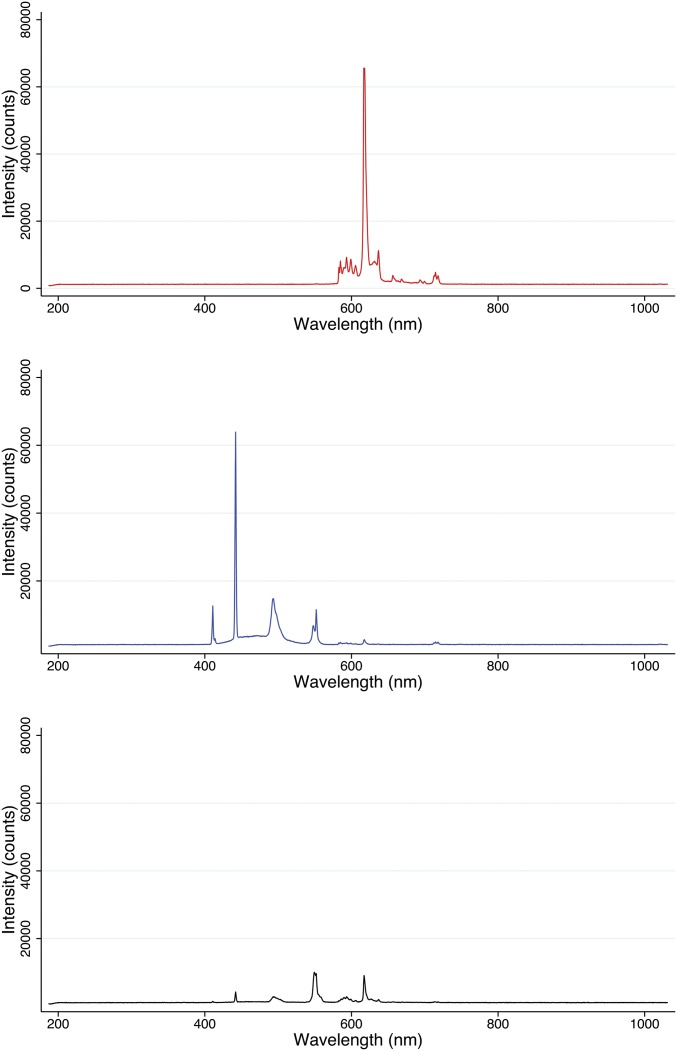
Our analyses of melatonin and corticosteroids were attempts to study the central circadian clock (i.e., SCN, melatonin) and determine whether blue light altered these rhythms relative to red light; our data suggest that it does not. These results do not address whether circadian rhythms were altered, in general, by any or all of the lighting conditions. Although corticosterone concentrations exhibited a circadian pattern of release, melatonin was suppressed by each lighting condition. And, although the capacity for light to suppress melatonin is dependent upon the spectrum, contemporary studies demonstrate that red light (600, 630, 700 nm) of sufficient intensity can also suppress melatonin (44, 45). We speculate that the high illuminance of our intervention (Fig. S2) rendered similar suppression of melatonin for both the red and blue light groups.
Fig. S2.
Intensity and spectrum of red, blue, and ambient lights. Spectroradiometric measurements of light intensity counts and wavelengths of each lighting condition were analyzed with an ISP-80-8-I integrating sphere (Ocean Optics). The sphere was placed into each cage to replicate the distance between the filtered light source and the animal population of each experimental condition. To avoid the intensity fluctuation of the light source, the measurement was integrated within 100 ms.
Nearly all visceral organs receive direct innervation from the autonomic nervous system (ANS). The two branches of the ANS are key regulators of immune responses (33, 46–48). Acute exposure to bright light has been shown to rapidly induce sympathoexcitation of adrenal, renal, and hepatic tissue (49–51). These data may appear at odds with our overarching paradigm: blue light leads to a withdrawal of sympathetic tone in reducing immune-mediated injury. However, the exposure used in these earlier studies was typically to white fluorescent (49) or incandescent (16, 50, 51) light of brief (10–30 min) duration, the subsequent assessment of sympathetic activity was performed within minutes, and the duration of sympathoexcitation transient (20–180 min). In contrast, our intervention of a prolonged 24-h photoperiod of rich, blue spectrum that is instituted in mice already exposed to ambient “day” illumination is distinct. In the study of Ishida et al. (16), light exposure did not significantly heighten adrenal sympathetic nerve activity when instituted at daytime. And although Mutoh et al. (49) did observe increased renal sympathetic nerve activity when mice were exposed to light during the day, this sympathoexcitation was minimal and transient (∼10 min) with an illuminance less than 2,000 lx; our red and blue lights were 1,400 lx. When we exposed mice to a 24-h photoperiod of blue spectrum light, they exhibited reduced sympathetic tone (i.e., heart rate, LF/HF). Thus, we perceive that our experimental conditions are distinct from the prior literature, our data unique and complementary to those previously published, and that a unifying theme can explain these seemingly disparate results. Although acutely white or fluorescent light may heighten sympathetic activity, during more prolonged exposure, particularly to blue spectrum light, a transition to a withdrawal of adrenergic tone may occur.
Chronic exposure to dim light at night (dLAN) has been shown in rodents to suppress both humoral and cellular immunity (9). Thus, the absence of a true night (<5 lx) could also account for our observations. However, in these prior reports, mice were chronically exposed (3–4 wk) to dLAN before immune challenge. Furthermore, if dLAN were operant in our experiments, its effects would have to have been spectrum specific, because red and ambient light possessed a similar exposure to light at night (LAN) as blue light, yet afforded no protection. Alternatively, a continuous photoperiod may also disrupt sleep, which too has been shown to be immunosuppressive (31, 32). However, mice exhibited similar activity levels with each light condition, and yet blue light was distinctly protective. Thus, we perceive that mechanisms distinct from light pollution or sleep disturbances are at play.
Recently, it has been described that long-range sympathetic signals, modulated by light, govern a circadian oscillation in leukocyte emigration into certain tissues (27, 33). In rodents, sympathetic tone and leukocyte recruitment peak at night, a time of maximal animal activity (27, 33). These circadian rhythms in leukocyte migration alter the outcome from inflammatory diseases; injury is maximal at night when adrenergic tone and neutrophil emigration are highest. Our data suggest that blue light reduces adrenergic tone and thereby, attenuates neutrophil influx. We observed that the administration of a β3 agonist inhibited the protective effects of blue light and rendered the outcomes with blue light similar to those of red light. By contrast, β3 antagonism did not afford protection. However, SR 59230A also possesses α1 antagonism, which potentiates arterial vasodilatation, and thus may induce systemic hypotension and worsen organ ischemia (52). Thus, the lack of protection with SR 59230A may be the consequence of the offsetting effects of β3 and α1 antagonism. Alternatively, these results may be interpreted to indicate that a withdrawal of β3 adrenergic tone is permissive, although not sufficient for attenuating organ injury. This interpretation would also be in agreement with our data demonstrating that β3 agonism reverses the protection of blue light. Thus, we propose that blue light, functioning through an optic pathway, leads to a withdrawal in β3 sympathetic tone that permissively reduces neutrophil influx and organ injury. The reduction in cellular necrosis may further lead to a reduction in the release of HMGB1, and thereby “break” an injury amplification loop, characterized by additional neutrophil recruitment and cellular injury.
In conclusion, our data support that light can be harnessed to acutely modify the biology, physiology and outcome of critical illness. Pretreatment with blue light is protective during I/R, and thus represents a possible therapeutic to target and control systemic inflammation and mitigate organ injury in clinical circumstances permitting pretreatment. However, there are distinct differences between the visual, circadian, and immune biology of nocturnal mammals, as studied herein, and that of diurnal Homo sapiens. Thus, if light does impart therapeutic value, further investigation to determine the precise characteristics (illuminance, photoperiod, and wavelength) will be critically important to optimize its potential clinical translation.
Materials and Methods
Study Design.
Our study was a randomized, controlled laboratory experiment to study the biologic effects of three light spectrums on the organ injury induced by I/R using murine models of kidney and liver I/R. The primary a priori hypothesis is that high-illuminance blue spectrum light reduces kidney and liver organ injury from I/R, by comparison with high-illuminance red and standard illuminance, ambient, white fluorescent light. The primary endpoints are serum cystatin C and ALT concentrations, as validated markers of renal and hepatic cellular injury.
Reagents.
Antibodies for HMGB1 (ab79823) and tubulin (ab4074) were obtained from Abcam. The β3 agonist CL316,243 and β3 antagonist SR 59230A were obtained from Sigma-Aldrich.
Animal experimentation.
We used 8- to 12-wk-old male C57BL/6J, 129S1/Sv-Vsx2or-J/J (Vsx2, visual system homeobox 2), and their control 129S1/SvImJ mice (The Jackson Laboratory). Homozygous 129S1/Sv-Vsx2or-J/J mice exhibit abnormal eye development: microphthalmia, small lens, and absence of the optic nerve; otherwise, these mice are fertile and exhibit no other phenotypic abnormalities (19). All experimentation was initiated at CT2 (CT, circadian time set 0 as previous dawn and 12 previous dusk). All animals had ad libitum access to water and LabDiet Prolab IsoPro RMH 3000 diet pellets (LabDiet).
Liver I/R.
A segmental (70%) hepatic warm I/R with 60 min of ischemia was used as previously performed (23).
Renal I/R.
A unilateral renal I/R model was used (53). A laparotomy was performed, the left kidney was exposed, and the renal pedicle was occluded for 30 min. Reperfusion was then initiated, the contralateral kidney was removed, and the abdomen closed.
The temperature during ischemia was maintained at 31 °C using a warming incubator chamber. At the end of 6 h (liver I/R) or 24 h (renal I/R) of reperfusion, the mice were anesthetized with isoflurane and killed by exsanguination.
For all studies, one investigator allocated each mouse to a single light exposure and a second investigator, blinded to the lighting conditions, performed the surgical experimentation and collected the samples. An investigator blinded to the specific treatment analyzed the data.
Exposure to light.
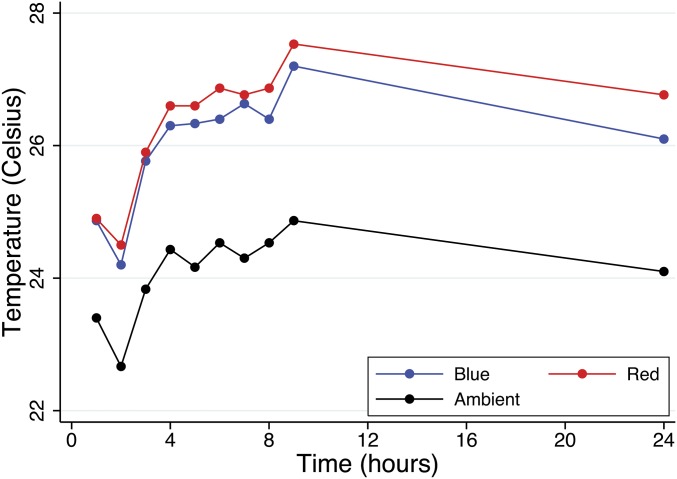
All experiments were conducted in a climatic room maintained on a day–night 12:12 h light:dark cycle (lights on from 0800 to 2000 hours) at an ambient temperature of 23 ± 2 °C and a relative humidity of 60%. Mice were randomly assigned to one of three different spectral illuminances: red (617 nm, 1,400 lx), blue (442 nm, 1,400 lx), and ambient light (fluorescent white light, 400 lx) (SI Materials and Methods). For hepatic and renal I/R these lighting environments were administered continuously for the 24-h period preceding experimentation and the respective 6- and 24-h periods of reperfusion. We continuously measured cage temperature and demonstrate that the heat emission modestly affected the cage temperatures (25 ± 2 °C; Fig. S3). Illuminance was measured with a hand-held digital lux meter (Digital Light Level Meter LX1330B; Mastech). Spectroradiometric measurements were made with an ISP-80-8-I integrating sphere (Ocean Optics).
Fig. S3.
Temperature of red, blue, and ambient light experimental conditions. A thermometer was placed in the bottom of each cage, and temporal measurements of cage temperature were recorded over a 24-h period at the times indicated. Thermal measurements began at the same time that all experiments were initiated: CT2 (CT, circadian time set 0 as previous dawn and 12 previous dusk). Mean temperature of n = 3 independent experiments.
Heart rate variability.
Mice underwent laparotomy and implantation of a DSI HD-X11 wireless telemetry monitor (DSI) within the peritoneal cavity as previously published (SI Materials and Methods) (54). The HD-X11 is a reusable 2.2-g, 1.4-cc wireless telemetry device capable of continuous measurement of one biopotential (e.g., electrocardiogram) and animal activity. After a 24-h period of recovery, mice were exposed to blue and red spectrum light and monitored for 24 h. Data collection and analysis were performed using Ponemah version 5.20 (DSI). Frequency domain parameters of heart rate variability, specifically the LF/HF ratio, were calculated as published (34–38).
Organ physiology.
Serum ALT concentration was determined using a DRI-CHEM 4000 Chemistry Analyzer System (Heska) (23). Renal function was determined by assaying serum for cystatin C [interassay coefficient of variability (CV): 2.8%] using an enzyme immunoassay kit (R&D) (55).
Histology.
The extent of parenchymal necrosis was graded using H&E-stained histological sections at 200× magnification with evaluation of 10 fields per sample (SI Materials and Methods).
Immunoblot and HMGB1 concentration.
Serum was electrophoresed in a 15% SDS/PAGE gel and developed as previously described (23). Densitometry was performed by the NIH Image program (NIH) (56).
Melatonin and corticosterone concentrations.
Serum melatonin concentration was quantified using an enzyme-linked immunosorbent assay (interassay CV: 6.5%; IBL). Serum corticosterone concentration was determined by an enzyme immunoassay kit (interassay CV: 2.5%; Enzo Life Science).
Myeloperoxidase.
MPO concentration was determined using an ELISA assay kit (interassay CV: 7.6%; Hycult Biotech).
Statistics.
Statistical analyses were performed using Stata 12SE software. Values are expressed as medians. Groups are compared by Mann–Whitney rank sum. A P < 0.05 was considered statistically significant.
Study approval.
We performed all animal experiments in accordance with the NIH Guide for the Care and Use of Laboratory Animals under protocols approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
SI Materials and Methods
Animal Experimentation.
Liver ischemia/reperfusion.
A segmental (70%) hepatic warm I/R with 60 min of ischemia was used as previously performed (23). Briefly, a midline laparotomy was performed, and all structures in the portal triad to the left and median liver lobes were occluded with an atraumatic microvascular clamp (Fine Science Tools) for 60 min. Ischemia was confirmed by visualizing the pale blanching of the ischemic lobes. After the clamp was removed, gross evidence of reperfusion, which was based on immediate color change, was ensured before closing the abdomen.
Heart rate variability.
Monitors were implanted within the peritoneal cavity according to manufacturer instructions and as previously published (54). At the time of laparotomy, skin flaps were raised lateral to the incision on both sides of the abdomen for placement of biopotential leads. The telemetry device was then implanted in the peritoneal cavity. Biopotential leads were brought through the rectus bilaterally, and the fascia was closed with interrupted 4-0 Vicryl suture (Ethicon). Biopotential leads were then tunneled s.c. to achieve positioning analogous to Lead II in human electrocardiography. The skin was closed with simple interrupted 4-0 silk sutures (Ethicon).
Lighting Apparatus.
Two-Day Light Classic DL930 light (3 × 36 W compact fluorescent: at 12 inches, 4,000 K, 10,000 lx at 30 cm) were used (Uplift Technologies). These lamps were fitted with blue (peak 442 nm) and red (peak 617 nm) filters (Lee Filters) and positioned over each cage to ensure identical irradiances and ∼1,400 lx when measured at the middle of the cage (Fig. S2).
Histology.
Liver.
The necrotic area was quantitatively assessed using ImageJ (NIH), as previously performed (57). Results were presented as the mean percentage of necrotic area (mm2) with respect to the entire area of one capture (mm2).
Kidney.
The percentage of tubules in the corticomedullary junction showing injury (i.e., cell necrosis, blebbing, vacuolization, and cast formation) was quantified using the criteria of Peng et al. (58) and a 7-point scale: 0, no injury; 1, 0–15%; 2, 15–30%; 3, 30–45%; 4, 45–60%; 5, 60–75%; and 6, >75% (59).
Acknowledgments
This work was supported by NIH Grant R01 GM082852 (to M.R.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1515296113/-/DCSupplemental.
References
- 1.Beauchemin KM, Hays P. Dying in the dark: Sunshine, gender and outcomes in myocardial infarction. J R Soc Med. 1998;91(7):352–354. doi: 10.1177/014107689809100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walch JM, et al. The effect of sunlight on postoperative analgesic medication use: A prospective study of patients undergoing spinal surgery. Psychosom Med. 2005;67(1):156–163. doi: 10.1097/01.psy.0000149258.42508.70. [DOI] [PubMed] [Google Scholar]
- 3.Ulrich RS. View through a window may influence recovery from surgery. Science. 1984;224(4647):420–421. doi: 10.1126/science.6143402. [DOI] [PubMed] [Google Scholar]
- 4.Castro R, Angus DC, Rosengart MR. The effect of light on critical illness. Crit Care. 2011;15(2):218. doi: 10.1186/cc10000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castro RA, et al. Light and the outcome of the critically ill: An observational cohort study. Crit Care. 2012;16(4):R132. doi: 10.1186/cc11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohn R, Harhay MO, Cooney E, Small DS, Halpern SD. Do windows or natural views affect outcomes or costs among patients in ICUs? Crit Care Med. 2013;41(7):1645–1655. doi: 10.1097/CCM.0b013e318287f6cb. [DOI] [PubMed] [Google Scholar]
- 7.Wunsch H, Gershengorn H, Mayer SA, Claassen J. The effect of window rooms on critically ill patients with subarachnoid hemorrhage admitted to intensive care. Crit Care. 2011;15(2):R81. doi: 10.1186/cc10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simons KS, et al. Effect of preadmission sunlight exposure on intensive care unit-acquired delirium: A multicenter study. J Crit Care. 2014;29(2):283–286. doi: 10.1016/j.jcrc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Bedrosian TA, Fonken LK, Walton JC, Nelson RJ. Chronic exposure to dim light at night suppresses immune responses in Siberian hamsters. Biol Lett. 2011;7(3):468–471. doi: 10.1098/rsbl.2010.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson RJ. Seasonal immune function and sickness responses. Trends Immunol. 2004;25(4):187–192. doi: 10.1016/j.it.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Prendergast BJ, Hotchkiss AK, Bilbo SD, Kinsey SG, Nelson RJ. Photoperiodic adjustments in immune function protect Siberian hamsters from lethal endotoxemia. J Biol Rhythms. 2003;18(1):51–62. doi: 10.1177/0748730402239676. [DOI] [PubMed] [Google Scholar]
- 12.Haldar C, Ahmad R. Photoimmunomodulation and melatonin. J Photochem Photobiol B. 2010;98(2):107–117. doi: 10.1016/j.jphotobiol.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Choi CY, et al. Effect of LED light spectra on starvation-induced oxidative stress in the cinnamon clownfish Amphiprion melanopus. Comp Biochem Physiol A Mol Integr Physiol. 2012;163(3-4):357–363. doi: 10.1016/j.cbpa.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Shin HS, Lee J, Choi CY. Effects of LED light spectra on oxidative stress and the protective role of melatonin in relation to the daily rhythm of the yellowtail clownfish, Amphiprion clarkii. Comp Biochem Physiol A Mol Integr Physiol. 2011;160(2):221–228. doi: 10.1016/j.cbpa.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Jung CM, et al. Acute effects of bright light exposure on cortisol levels. J Biol Rhythms. 2010;25(3):208–216. doi: 10.1177/0748730410368413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishida A, et al. Light activates the adrenal gland: Timing of gene expression and glucocorticoid release. Cell Metab. 2005;2(5):297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Walton JC, Weil ZM, Nelson RJ. Influence of photoperiod on hormones, behavior, and immune function. Front Neuroendocrinol. 2011;32(3):303–319. doi: 10.1016/j.yfrne.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson RJ, Demas GE. Seasonal changes in immune function. Q Rev Biol. 1996;71(4):511–548. doi: 10.1086/419555. [DOI] [PubMed] [Google Scholar]
- 19.Zou C, Levine EM. Vsx2 controls eye organogenesis and retinal progenitor identity via homeodomain and non-homeodomain residues required for high affinity DNA binding. PLoS Genet. 2012;8(9):e1002924. doi: 10.1371/journal.pgen.1002924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61(6):647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- 21.Uchida Y, Freitas MC, Zhao D, Busuttil RW, Kupiec-Weglinski JW. The inhibition of neutrophil elastase ameliorates mouse liver damage due to ischemia and reperfusion. Liver Transpl. 2009;15(8):939–947. doi: 10.1002/lt.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirayama S, Shiraishi T, Shirakusa T, Higuchi T, Miller EJ. Prevention of neutrophil migration ameliorates rat lung allograft rejection. Mol Med. 2006;12(9-10):208–213. doi: 10.2119/2006-00036.Hirayama. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsung A, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204(12):2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huebener P, et al. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J Clin Invest. 2015;125(2):539–550. doi: 10.1172/JCI76887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evankovich J, et al. Calcium/calmodulin-dependent protein kinase IV limits organ damage in hepatic ischemia-reperfusion injury through induction of autophagy. Am J Physiol Gastrointest Liver Physiol. 2012;303(2):G189–G198. doi: 10.1152/ajpgi.00051.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lippai D, et al. Alcohol-induced IL-1β in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J Leukoc Biol. 2013;94(1):171–182. doi: 10.1189/jlb.1212659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol. 2013;13(3):190–198. doi: 10.1038/nri3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arjona A, Silver AC, Walker WE, Fikrig E. Immunity’s fourth dimension: Approaching the circadian-immune connection. Trends Immunol. 2012;33(12):607–612. doi: 10.1016/j.it.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson RJ, Drazen DL. Melatonin mediates seasonal changes in immune function. Ann N Y Acad Sci. 2000;917:404–415. doi: 10.1111/j.1749-6632.2000.tb05405.x. [DOI] [PubMed] [Google Scholar]
- 30.Weibel L, Follenius M, Spiegel K, Ehrhart J, Brandenberger G. Comparative effect of night and daytime sleep on the 24-hour cortisol secretory profile. Sleep. 1995;18(7):549–556. [PubMed] [Google Scholar]
- 31.Ashley NT, et al. Sleep deprivation attenuates endotoxin-induced cytokine gene expression independent of day length and circulating cortisol in male Siberian hamsters (Phodopus sungorus) J Exp Biol. 2013;216(Pt 14):2581–2586. doi: 10.1242/jeb.083832. [DOI] [PubMed] [Google Scholar]
- 32.Weil ZM, et al. Sleep deprivation attenuates inflammatory responses and ischemic cell death. Exp Neurol. 2009;218(1):129–136. doi: 10.1016/j.expneurol.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheiermann C, et al. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 2012;37(2):290–301. doi: 10.1016/j.immuni.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howden R, et al. The genetic contribution to heart rate and heart rate variability in quiescent mice. Am J Physiol Heart Circ Physiol. 2008;295(1):H59–H68. doi: 10.1152/ajpheart.00941.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84(2):482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- 36.Pagani M, et al. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation. 1997;95(6):1441–1448. doi: 10.1161/01.cir.95.6.1441. [DOI] [PubMed] [Google Scholar]
- 37.Presciuttini B, Duprez D, De Buyzere M, Clement DL. How to study sympatho-vagal balance in arterial hypertension and the effect of antihypertensive drugs? Acta Cardiol. 1998;53(3):143–152. [PubMed] [Google Scholar]
- 38.Thireau J, Zhang BL, Poisson D, Babuty D. Heart rate variability in mice: A theoretical and practical guide. Exp Physiol. 2008;93(1):83–94. doi: 10.1113/expphysiol.2007.040733. [DOI] [PubMed] [Google Scholar]
- 39.Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452(7186):442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 40.Nelson RJ, Demas GE, Klein SL, Kriegsfeld LJ. Seasonal Patterns of Stress, Immune Function and Disease. Cambridge Univ Press; New York: 2002. [Google Scholar]
- 41.Wu H, et al. HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol. 2010;21(11):1878–1890. doi: 10.1681/ASN.2009101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young MR, et al. Circadian rhythmometry of serum interleukin-2, interleukin-10, tumor necrosis factor-alpha, and granulocyte-macrophage colony-stimulating factor in men. Chronobiol Int. 1995;12(1):19–27. doi: 10.3109/07420529509064496. [DOI] [PubMed] [Google Scholar]
- 43.Castanon-Cervantes O, et al. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185(10):5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanifin JP, et al. High-intensity red light suppresses melatonin. Chronobiol Int. 2006;23(1-2):251–268. doi: 10.1080/07420520500521988. [DOI] [PubMed] [Google Scholar]
- 45.Ho Mien I, et al. Effects of exposure to intermittent versus continuous red light on human circadian rhythms, melatonin suppression, and pupillary constriction. PLoS One. 2014;9(5):e96532. doi: 10.1371/journal.pone.0096532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moynihan J, Kruszewska B, Madden K, Callahan T. Sympathetic nervous system regulation of immunity. J Neuroimmunol. 2004;147(1-2):87–90. doi: 10.1016/j.jneuroim.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 47.Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93(7):773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. J Exp Med. 2012;209(6):1057–1068. doi: 10.1084/jem.20120571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mutoh T, Shibata S, Korf HW, Okamura H. Melatonin modulates the light-induced sympathoexcitation and vagal suppression with participation of the suprachiasmatic nucleus in mice. J Physiol. 2003;547(Pt 1):317–332. doi: 10.1113/jphysiol.2002.028001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niijima A, Nagai K, Nagai N, Akagawa H. Effects of light stimulation on the activity of the autonomic nerves in anesthetized rats. Physiol Behav. 1993;54(3):555–561. doi: 10.1016/0031-9384(93)90249-f. [DOI] [PubMed] [Google Scholar]
- 51.Niijima A, Nagai K, Nagai N, Nakagawa H. Light enhances sympathetic and suppresses vagal outflows and lesions including the suprachiasmatic nucleus eliminate these changes in rats. J Auton Nerv Syst. 1992;40(2):155–160. doi: 10.1016/0165-1838(92)90026-d. [DOI] [PubMed] [Google Scholar]
- 52.Bexis S, Docherty JR. Role of alpha 1- and beta 3-adrenoceptors in the modulation by SR59230A of the effects of MDMA on body temperature in the mouse. Br J Pharmacol. 2009;158(1):259–266. doi: 10.1111/j.1476-5381.2009.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colombaro V, et al. Lack of hyaluronidases exacerbates renal post-ischemic injury, inflammation, and fibrosis. Kidney Int. 2015;88(1):61–71. doi: 10.1038/ki.2015.53. [DOI] [PubMed] [Google Scholar]
- 54.Lewis AJ, et al. Use of biotelemetry to define physiology-based deterioration thresholds in a murine cecal ligation and puncture model of sepsis. Crit Care Med. February 9, 2016 doi: 10.1097/CCM.0000000000001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song S, et al. Serum cystatin C in mouse models: A reliable and precise marker for renal function and superior to serum creatinine. Nephrol Dial Transplant. 2009;24(4):1157–1161. doi: 10.1093/ndt/gfn626. [DOI] [PubMed] [Google Scholar]
- 56.Zhang X, et al. CaMKIV-dependent preservation of mTOR expression is required for autophagy during LPS-induced inflammation and acute kidney injury. J Immunol. 2014;193(5):2405–2415. doi: 10.4049/jimmunol.1302798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang H, et al. Hepatic arterial perfusion is essential for the spontaneous recovery from focal hepatic venous outflow obstruction in rats. Am J Transpl. 2011;11(11):2342–2352. doi: 10.1111/j.1600-6143.2011.03682.x. [DOI] [PubMed] [Google Scholar]
- 58.Peng Q, et al. C3a and C5a promote renal ischemia-reperfusion injury. J Am Soc Nephrol. 2012;23(9):1474–1485. doi: 10.1681/ASN.2011111072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou W, et al. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest. 2000;105(10):1363–1371. doi: 10.1172/JCI8621. [DOI] [PMC free article] [PubMed] [Google Scholar]