Significance
Hypoxia-inducible factor 1α (HIF1α) is important for cell growth and survival. It modulates Wnt signaling, regulating cell differentiation and fate. Osteoarthritis (OA) is an increasingly frequent joint disorder characterized by progressive cartilage breakdown in which Wnt/β-catenin signaling triggers matrix metalloproteinase 13 (MMP13) expression and chondrocyte catabolism. Here we demonstrate HIF1α inhibits β-catenin signaling by blocking transcription factor 4 (TCF4)–β-catenin interaction and down-regulates MMP13 expression, thereby alleviating cartilage lesions, whereas the TCF4–β-catenin signaling induces an OA phenotype in mice. In OA joints, PKF118-310, a small molecule that blocked TCF4–β-catenin interaction, significantly reduced the progression of OA cartilage lesions. Thus, blockade of TCF4–β-catenin signaling by HIF1α represents a promising strategy to prevent articular cartilage loss in OA.
Keywords: hypoxia-inducible factor 1α, chondrocyte, osteoarthritis, Wnt signaling, matrix metalloprotease 13
Abstract
Low oxygen tension (hypoxia) regulates chondrocyte differentiation and metabolism. Hypoxia-inducible factor 1α (HIF1α) is a crucial hypoxic factor for chondrocyte growth and survival during development. The major metalloproteinase matrix metalloproteinase 13 (MMP13) is also associated with chondrocyte hypertrophy in adult articular cartilage, the lack of which protects from cartilage degradation and osteoarthritis (OA) in mice. MMP13 is up-regulated by the Wnt/β-catenin signaling, a pathway involved in chondrocyte catabolism and OA. We studied the role of HIF1α in regulating Wnt signaling in cartilage and OA. We used mice with conditional knockout of Hif1α (∆Hif1αchon) with joint instability. Specific loss of HIF1α exacerbated MMP13 expression and cartilage destruction. Analysis of Wnt signaling in hypoxic chondrocytes showed that HIF1α lowered transcription factor 4 (TCF4)–β-catenin transcriptional activity and inhibited MMP13 expression. Indeed, HIF1α interacting with β-catenin displaced TCF4 from MMP13 regulatory sequences. Finally, ΔHif1αchon mice with OA that were injected intraarticularly with PKF118-310, an inhibitor of TCF4–β-catenin interaction, showed less cartilage degradation and reduced MMP13 expression in cartilage. Therefore, HIF1α–β-catenin interaction is a negative regulator of Wnt signaling and MMP13 transcription, thus reducing catabolism in OA. Our study contributes to the understanding of the role of HIF1α in OA and highlights the HIF1α–β-catenin interaction, thus providing new insights into the impact of hypoxia in articular cartilage.
Low oxygen tension (hypoxia) orchestrates several cell functions and is critical in health and disease (1–4). Hypoxia-inducible factor 1α (HIF1α) is an essential factor to maintain chondrocyte homeostasis and allow cell differentiation (5, 6). HIF1 is a heterodimeric DNA-binding complex containing a constitutive HIF1β subunit and HIF1α subunit. In hypoxia, HIF1 binds to the hypoxia response elements of target genes, whereas in normoxia, HIF1α is hydroxylated, thereby leading to its degradation. Indeed, HIF1α hydroxylation is recognized by the von Hippel–Lindau tumor suppressor protein (pVHL), an E3 ubiquitin ligase that targets HIFα for proteolysis in the proteasome (7). The HIF1α pathway interacts with different cell signaling pathways, among them Wnt signaling. Indeed, HIF1α interacts with β-catenin in regulating cell growth and survival. In embryonic stem cells, HIF1α–β-catenin complexes up-regulate lymphoid enhancer-binding factor 1 and transcription factor 1 (TCF1), which activates Wnt signaling (8), whereas in colorectal cancer cells, HIF1α blocks the TCF4–β-catenin interaction and transcriptional activity, thus inhibiting canonical Wnt signaling (9).
Cartilage loss characterizes osteoarthritis (OA), one of the most frequent joint disorders, but available treatments are poorly efficient to prevent joint destruction (10, 11). Therefore, the need for novel drug targets to treat OA is paramount. Matrix metalloproteinase 13 (MMP13) triggers the degradation of articular cartilage. Indeed, chondrocyte-specific deletion of MMP13 alleviated OA in mice; the Wnt family members were candidates for the regulation of MMP13 expression in chondrocytes because its expression was increased in chondrocytes from mice with conditional activation of β-catenin (12). Cumulative data showed that Wnt activity is low under physiological conditions, and activation of Wnt signaling contributes to cartilage breakdown in OA (13, 14). The modulation of Wnt inhibitors had significant effects on chondrocyte catabolism of mice. Indeed, loss of sclerostin enhanced cartilage degradation (15) and the overexpression of Dkk-1 alleviated OA (14). Despite the hypoxic status of cartilage (16), the involvement of hypoxia in regulating Wnt signaling and MMP13 expression in cartilage is still unclear.
We studied the role of HIF1α in regulating Wnt signaling in cartilage of mice with conditional knockout of Hif1α (ΔHif1αchon) and induced OA. Hypoxia maintained low Wnt/β-catenin signaling via HIF1α, which lowered MMP13 expression, then prevented chondrocyte catabolism and cartilage loss. Here we therefore highlight the role of HIF1α in cartilage remodeling and loss during OA and provide a previously unidentified mechanism of microenvironmental regulation of Wnt signaling in cartilage.
Results
HIF1α Deletion in Chondrocytes Enhanced OA Development and MMP13 Expression in Mice.
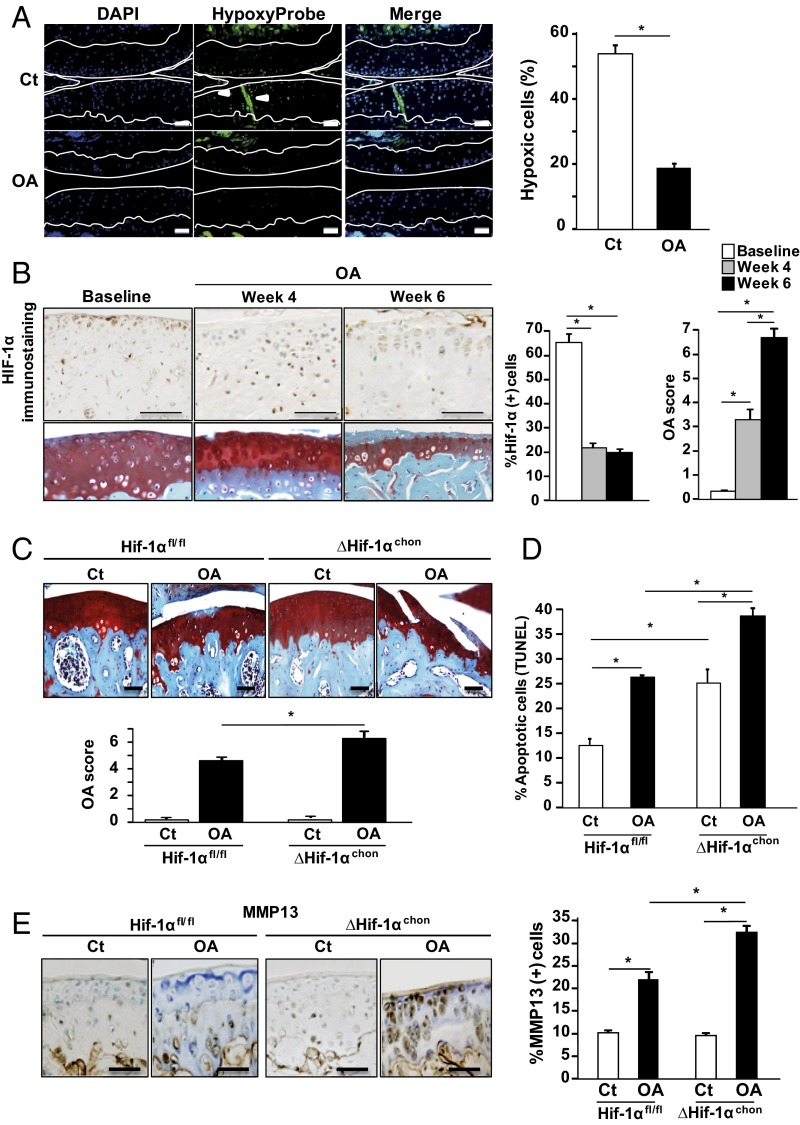
Articular chondrocytes are physiologically in a hypoxic state that might be altered in OA (5). To monitor oxygen tensions in OA chondrocytes, we administered a hypoxyprobe (pimonidazole hydrochloride) to control and OA mice. Hypoxia levels were markedly decreased in all layers of the OA articular cartilage, as shown by immunohistochemistry and by the decreased number of hypoxic cells (Fig. 1A). HIF1α was expressed in undamaged articular cartilage at baseline but its level decreased significantly in OA joints along with the increased cartilage damage (Fig. 1B). Noteworthy, the number of Hif1α-expressing cells was steadily down-regulated after destabilization of the medial meniscus (4 and 6 wk), whereas OA damage increased in a time-dependent manner.
Fig. 1.
Hypoxia and hypoxia-inducible factor 1α (HIF1α) are reduced in cartilage of mice with OA. (A) Immunohistofluorescence staining of Hypoxyprobe adducts in healthy and OA wild-type mouse cartilage. Hypoxyprobe adducts were revealed in hypoxic cells (PO2 < 10 mmHg) by a fluorescein-conjugated antibody (HP-FITC-MAb). Graph shows proportion of hypoxic positive cells in cartilage of the tibial plateau and internal femoral condyle. Data are mean ± SEM. *P < 0.05 compared with control (n = 7 animals per group). (Scale bar, 100 μm.) (B, Upper) HIF1α immunostaining and OA score (Safranin-O staining) in control mice at 0, 4, and 6 wk post-OA induction. Graphs show percentage of HIF1α(+) cells and OA score in articular cartilage of the tibial plateau and internal femoral condyle. (Scale bar, 100 μm.) *P < 0.05 compared with control (n = 7 animals per group) (C) Safranin-O staining and (D) TUNEL assay of Hif1αfl/fl and ΔHif1αchon mouse joints with OA or sham operation (ct) at week 6. (Scale bar, 100 μm.) OA score in OA and sham-operated knees of Hif1αfl/fl and ΔHif1αchon mice (week 6). *P < 0.05 compared with control. #P < 0.05 (n = 8–11 animals per group). (E) Immunostaining for MMP13 in Hif1αfl/fl and ΔHif1αchon mouse joints (week 6) and quantification. (Scale bar, 100 μm.) *P < 0.05 compared with control. #P < 0.05 (n = 8–11 animals per group).
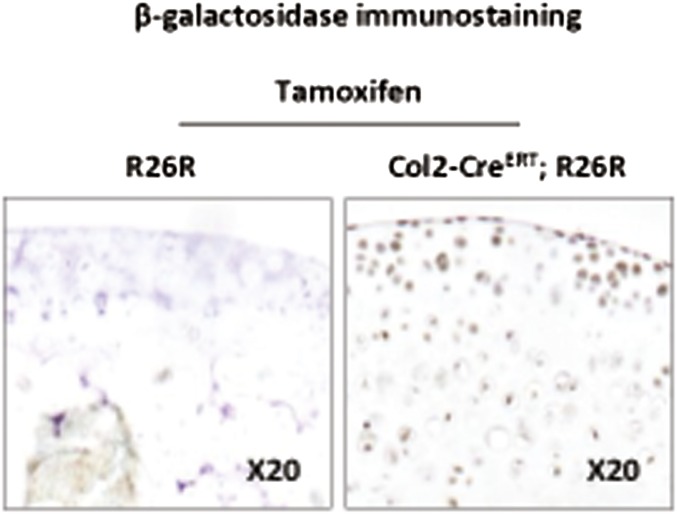
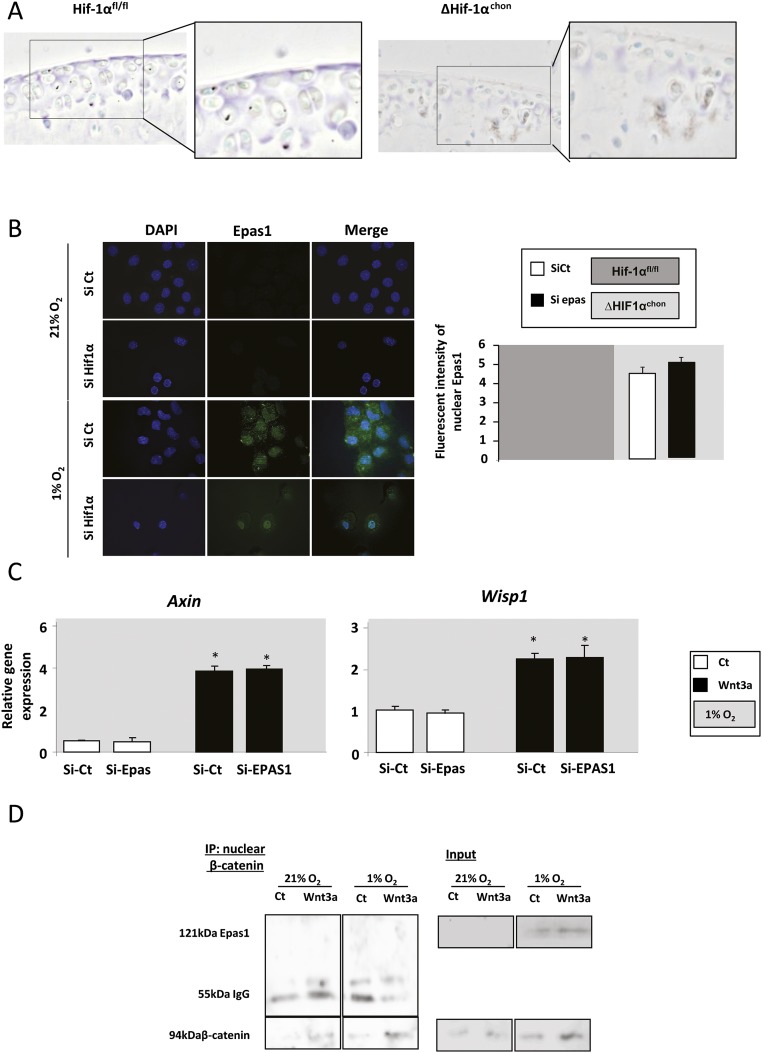
To determine the role of HIF1α in OA development, we generated mice with inducible conditional knockout of Hif1α by mating Col2-CreERT mice with Hif1αfl/fl mice in which the recombination was induced by tamoxifen. We first verified that the Cre-lox recombination occurred correctly in cartilage in Col2-CreERT; R26R-LacZ mice and R26R-LacZ mice were used as controls. β-Galactosidase was expressed in the articular cartilage of Col2-CreERT; R26R-LacZ mice, thus tamoxifen induced Cre-lox recombination in chondrocytes (Fig. S1). OA was induced in ΔHif1αchon mice 1 wk after tamoxifen injections. OA cartilage lesions were increased in ΔHif1αchon mice as shown by the osteoarthritis score (Fig. 1C). As HIF1α is a survival factor, we assessed chondrocyte apoptosis in OA in the absence of HIF1α. The number of apoptotic cells was increased in ΔHif1αchon mice although the OA score remained unchanged (Fig. 1D). TUNEL positive cells were further increased in ΔHif1αchon mice with destabilization of the medial meniscus (DMM). As previously described, the expression of MMP13 was induced in OA mice. This increase is enhanced in ΔHif1αchon mice along with the exacerbated cartilage loss (Fig. 1E). Thus, OA is associated with loss of hypoxia in articular cartilage, decreased HIF1α protein levels, and increased MMP13 expression and cartilage loss in mice. To rule out the hypothesis that ΔHif1αchon could induce endothelial PAS domain-containing protein 1 (EPAS1 or Hif2α) expression and therefore contribute to the phenotype, we found that EPAS1 was expressed at the same level in Hif1αfl/fl mice and ΔHif1αchon mice, suggesting the absence of compensatory increase of EPAS1 (Fig. S2A).
Fig. S1.
β-Galactosidase immunostaining in Col2-CreERT; R26R-LacZ and R26R-LacZ mice with injection of tamoxifen (n = 3).
Fig. S2.
(A) EPAS1 expression in Hif1αfl/fl and ΔHif1αchon mice at week 6 (immunohistochemistry) in control knees. (B) Immunocytofluorescence staining of EPAS1 in normoxic and hypoxic chondrocytes (n = 4). Quantification of EPAS1 translocation in chondrocytes: intensity of Epas1 signal into the nucleus of chondrocytes cultured in hypoxia and normoxia after Hif1α siRNA silencing (pixels) (n = 198–277). (C) PCR analysis of relative gene expression in primary chondrocytes with 21% and 1% O2 for Wnt targets (Axin and Wisp1) with EPAS1 siRNA silencing (n = 6). (D) Coimmunoprecipitation of β-catenin in nuclear protein extracts. Western blot (WB) analysis of protein levels of EPAS1 and β-catenin.
HIF1α Inhibits MMP13 Expression and the Transcription of Wnt Targets.
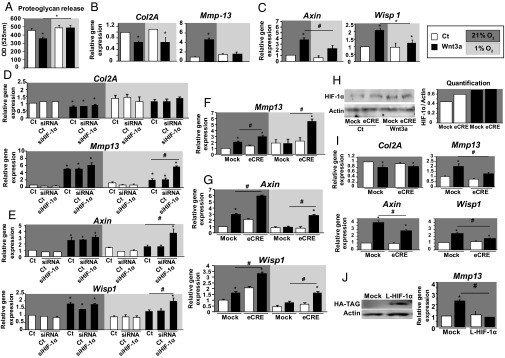
HIF1α is a major HIF that regulates chondrocyte metabolism (17). We first assessed the role of hypoxia in the metabolic effects of Wnt signaling in primary chondrocytes. As expected in normoxia, Wnt3a reduced proteoglycan release and Col2A expression while increasing Mmp13 expression (Fig. 2 A and B). In contrast, under hypoxic conditions, Wnt3a failed to modulate the proteoglycan release as well as the expression of catabolic markers. Furthermore target genes such as Axin and Wisp1 were not regulated by Wnt3a in hypoxia (Fig. 2C). To investigate whether HIF1α modulates MMP13 expression induced by Wnt, we first deleted HIF1α chondrocytes and analyzed the expression of MMP13 and Wnt target genes under Wnt stimulation. Using siRNA silencing, there was no effect of HIF1α knockdown in COL2A and Mmp13 in normoxia (Fig. 2D). Furthermore, HIF1α knockdown has no effect on the nuclear translocation of EPAS1 (Fig. S2B).
Fig. 2.
HIF1α inhibits the transcription of Wnt targets in Wnt3a-induced chondrocytes. (A) Proteoglycan release in chondrocyte culture media (n = 9). qPCR analysis of relative gene expression in primary chondrocytes with 21% and 1% O2 for: (B) anabolic marker (collagen 2A, COL2A) and catabolic marker (Mmp13) (n = 7); and (C) direct transcriptional targets of Wnt3a (Axin and Wisp1) (n = 14). (D) Expression of anabolic and catabolic genes (COL2A and Mmp13) with HIF1α siRNA silencing (n = 6). (E) Direct transcriptional targets of Wnt3a (Axin and Wisp1) with HIF1α siRNA silencing (n = 6). (F) Catabolic marker Mmp13 in HIF1α-lacking chondrocytes (Cre-lox recombination in vitro) (n = 5). (G) Direct transcriptional targets of Wnt3a (Axin and Wisp1) in HIF1α-lacking chondrocytes (Cre-lox recombination in vitro) (n = 5). Data are mean ± SEM. *P < 0.05 compared with control, #P < 0.05. (H) Western blot analysis of HIF1α expression in von Hippel–Lindau tumor suppressor protein (VHL)-lacking chondrocytes (with 21% O2), and quantification (n = 3). qPCR analysis of relative gene expression in VHL-lacking chondrocytes (Cre-lox recombination in vitro) for: (I) anabolic marker COL2A; catabolic marker MMP13; and direct transcriptional targets of Wnt (Axin and Wisp1) (with 21% O2). (J) Western blot analysis of stabilized HIF1α expression using a tag antibody (HA) in primary chondrocytes and qPCR analysis of relative gene expression of Mmp13. Data are mean ± SEM (n = 5 experiments); *P < 0.05 compared with control; #P < 0.05.
Loss of HIF1α promoted the Wnt-induced Mmp13 expression and the transcriptional Wnt targets (Fig. 2E) and was confirmed by Cre-lox recombination (Fig. 2 F and G). This was not observed when EPAS1 was knocked down (Fig. S2C).
To confirm that HIF1α inhibits Mmp13 expression by blocking transcription of Wnt targets, we stabilized HIF1α in normoxic chondrocytes upon von Hippel–Lindau tumor suppressor (Vhl) deletion using Cre-lox recombination. HIF1α increase with VHL deletion was confirmed by Western blot analysis (Fig. 2H) and led to increased Mmp13, Axin, and WNT1--inducible-signaling pathway protein 1 (Wisp1) expression in normoxia (Fig. 2I). Moreover, stabilization of HIF1α did not affect the transcription of the anabolic marker COL2A1 (Fig. 2I). Thus, the regulation of anabolic markers is independent of the HIF1α pathway. Because loss of VHL stabilizes both HIF1 and EPAS1, we used an overexpression of constitutive stabilized HIF1 in chondrocytes (18). We found that HIF1α abolished Mmp13 expression induced by Wnt (Fig. 2J). Thus, HIF1α alone is able to inhibit Wnt-induced Mmp13 expression.
HIF1α–β-Catenin Interaction Reduced TCF4 Binding to the Mmp13 Regulatory Region.
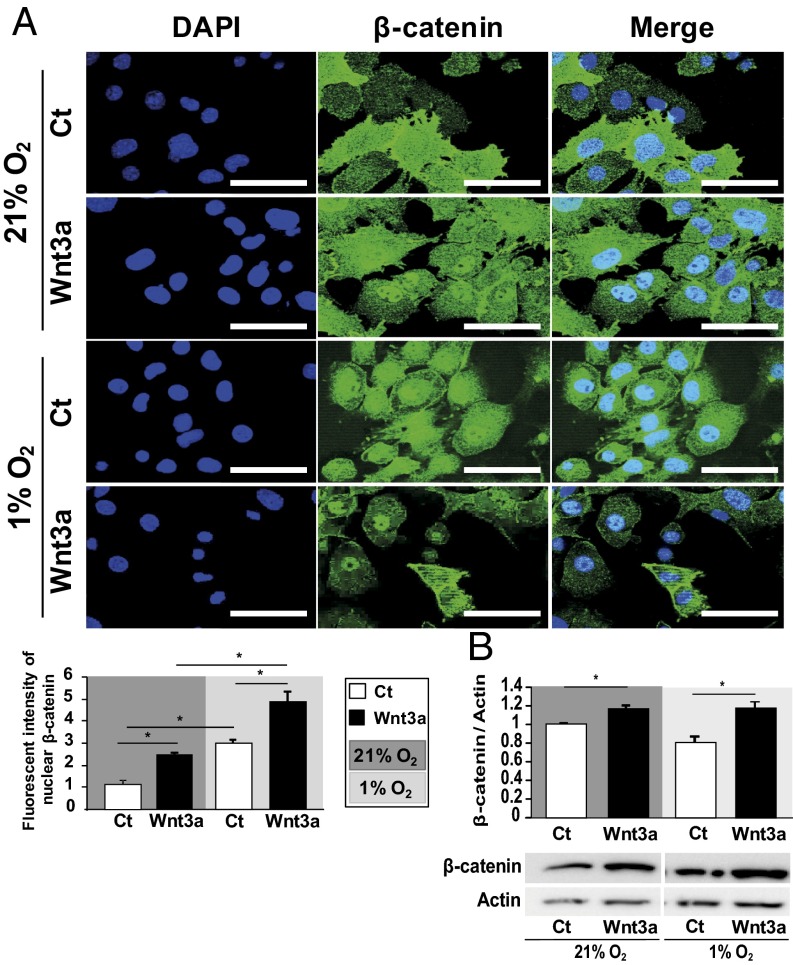
Upon Wnt pathway activation, β-catenin accumulates in the cytoplasm, translocates into the nucleus, binds to TCF transcription factor, and activates the transcription of target genes (19). Wnt activation increases the levels of catabolic markers under normoxia (16). We then investigated whether the translocation of β-catenin is reduced in hypoxia. Wnt3a promoted β-catenin translocation into the nucleus and its protein expression regardless of O2 level (Fig. 3 A and B). Thus, hypoxia reduced transcriptional activity of β-catenin independently of nuclear β-catenin translocation.
Fig. 3.
Wnt3a promotes β-catenin translocation into the nucleus in hypoxia and normoxia. (A) Immunocytofluorescence staining of β-catenin in normoxic and hypoxic chondrocytes. Bar 100 μm (n = 4). Quantification of β-catenin translocation in chondrocytes: intensity of β-catenin signal into the nucleus of chondrocytes cultured in hypoxia and normoxia after Wnt3a stimulation (pixels) (n = 198–277). (B) Western blot analysis of β-catenin protein level in normoxic and hypoxic chondrocytes and quantification. Data are mean ± SEM. *P < 0.05 compared with control (Ct).
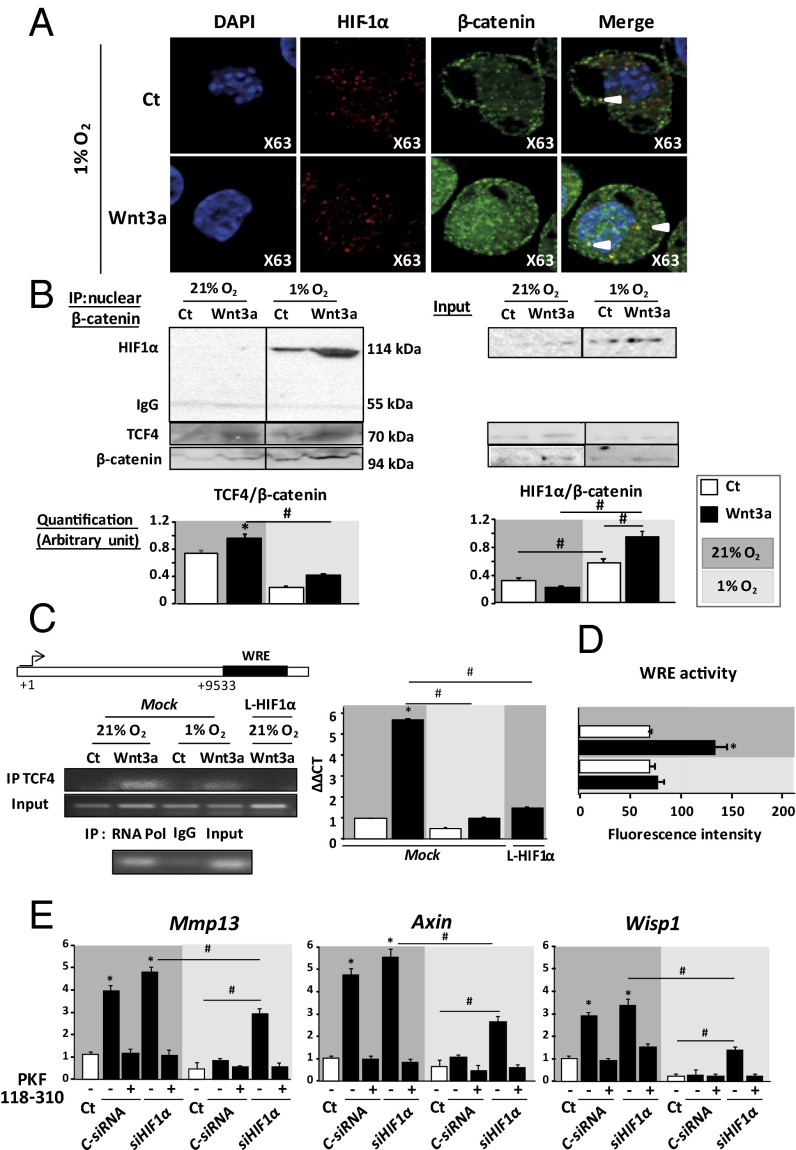
We investigated whether HIF1α inhibits Wnt activity by direct interaction between HIF1α and β-catenin. In hypoxia, HIF1α and β-catenin colocalized in the nucleus of Wnt3a-induced chondrocytes (Fig. 4A), suggesting a possible interaction within nuclear complexes. Indeed, coimmunoprecipitation in nuclear extracts assay revealed HIF1α–β-catenin interaction complexes in hypoxia (Fig. 4B). However, EPAS1 failed to coimmunoprecipitate with β-catenin in normoxia and hypoxia (Fig. S2D). We next assessed the impact of HIF1α–β-catenin interaction on the transcriptional activity of TCF4–β-catenin complexes. In chondrocyte stimulated by Wnt3a, the formation of TCF4–β-catenin nuclear complexes was decreased in hypoxia compared with normoxia (Fig. 4B). These data suggest that under hypoxia, β-catenin may bind preferentially to HIF1α rather than TCF4, thus reducing Wnt/β-catenin signaling.
Fig. 4.
HIF1α binds β-catenin and inhibits TCF4 binding to the MMP13 regulatory region. (A) Immunocytofluorescence staining of β-catenin and HIF1α in hypoxic chondrocytes (63×). (n = 3). (B) Coimmunoprecipitation of β-catenin in nuclear protein extracts. Western blot (WB) analysis of protein levels of HIF1α, TCF4, and β-catenin and quantification of HIF1α–β-catenin and TCF4–β-catenin complexes. (C) ChIP analysis of TCF4 binding to the Mmp13 regulatory region. Sequence contains Wnt responsive elements. RNA Pol, RNA polymerase (positive control); IgG, mouse IgG (negative control). qPCR analysis of TCF4 binding to Mmp13 regulatory regions (n = 3). *P < 0.05 compared with control; #P < 0.05. (D) Luciferase reporter assay in C3H10 cells. Data are ratio of firefly luciferase to control (Renilla) luciferase activity (n = 3). (E) qPCR analysis of relative gene expression (with HIF1α siRNA silencing) with 21% and 1% O2 for Mmp13 (n = 8) and direct transcriptional targets of Wnt (Axin and Wisp1) (n = 8). Data are mean ± SEM. *P < 0.05 compared with control; #P < 0.05.
MMP13 is regulated by both HIF and Wnt signaling (20–24). To better characterize the role of HIF1α–β-catenin interaction in inhibiting Wnt/β-catenin signaling and Mmp13 expression under hypoxia, we assessed TCF4 binding to the Mmp13 regulatory region by ChIP assay (Fig. 4C). We used a Mmp13 regulatory 3′ region downstream of coding area that includes Wnt responsive elements (WREs) (24) (Fig. 4C). TCF4 binding to WREs was decreased in hypoxia compared with normoxia (Fig. 4C). To confirm the role of HIF1α in inhibiting TCF4 binding to WREs, we overexpressed the stabilized form of HIF1α in normoxic chondrocytes and found that TCF4 binding to WREs was abolished (Fig. 4C). Therefore, TCF4 binding to the Mmp13 regulatory region was reduced by HIF1α. We further investigated the transcriptional activity of Mmp13 under hypoxia. The regulatory region was cloned downstream of a luciferase reporter gene and the plasmid was transfected in C3H10 cells. This assay revealed down-regulation of Wnt3a-induced luciferase activity when cultured under hypoxia (Fig. 4D) and confirmed the functional regulation of this sequence by hypoxia and Wnt3a.
PKF118-310 Reduced OA Progression in ΔHif1αchon Mice.
Given the sequestration of β-catenin by HIF1α, we hypothesized that HIF1α deletion may increase the TCF4–β-catenin complex level, thereby leading to chondrocyte catabolism. To verify this hypothesis, we deleted HIF1α in chondrocytes by siRNA silencing and blocked TCF4–β-catenin complexes by using PKF118-310, which blocks the interaction between TCF4 and β-catenin. In normoxia, PKF118-310 totally suppressed Wnt-induced Mmp13 and target gene expression (Fig. 4E). Loss of HIF1α increased the expression of Mmp13 and that of the bona fide Wnt target genes Axin and Wisp1. Hence, the PKF118-310 addition inhibited the increased expression of the canonical Wnt targets Mmp13, Axin, and Wisp1 induced by loss of HIF1α. Thus, HIF1α prevented Wnt from inducing Mmp13 expression by blocking TCF4–β-catenin complexes.
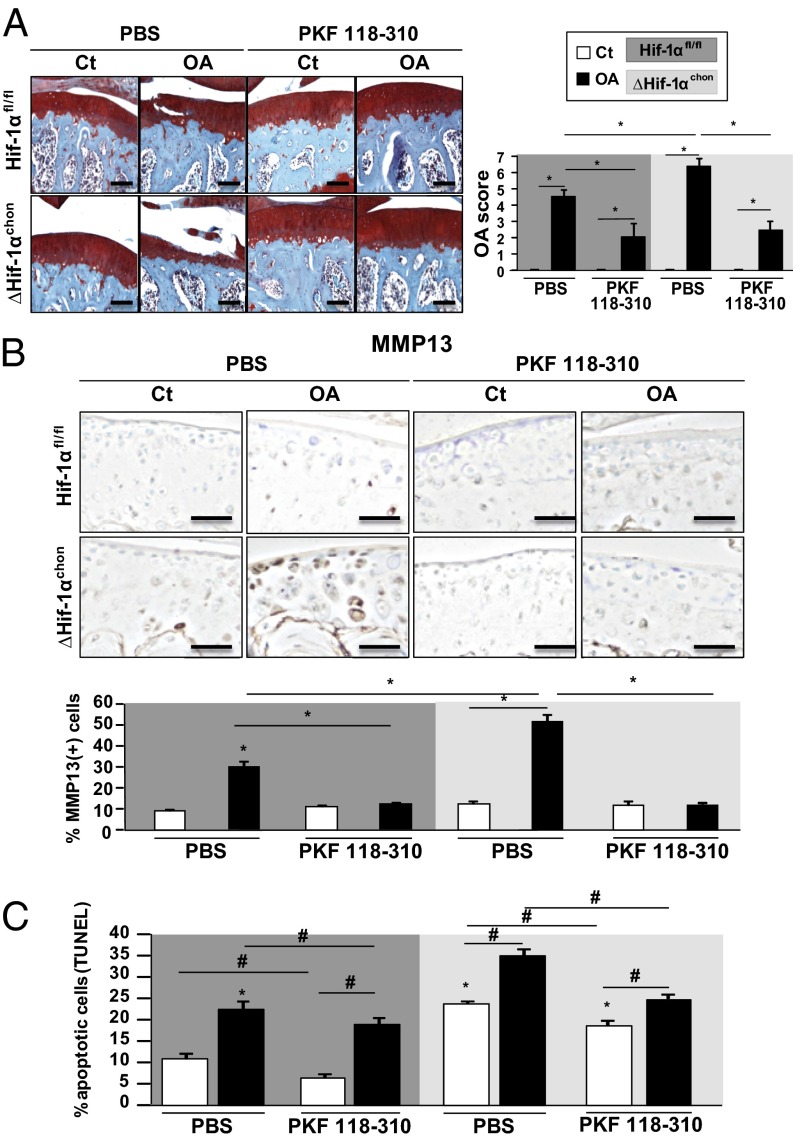
In OA mice, conditional loss of HIF1α increased MMP13 expression and cartilage lesions (Fig. 5A). Increased TCF4–β-catenin complex level may promote the phenotype observed in ΔHif1αchon mice. Articular injection of the β-catenin inhibitor PKF118-310 in ΔHif1αchon mice prevented cartilage lesions and reduced MMP13 expression compared with PBS treatment. Thus, PKF118-310 administration in ΔHif1αchon mice blocked the increased MMP13 expression and cartilage damage (Fig. 5 A and B). Moreover, PKF118-310 resulted in a reduced number of apoptotic cells in OA mice (Fig. 5C). Loss of HIF1α enhanced cartilage damage by increasing TCF4–β-catenin complexes, which activated MMP13 expression.
Fig. 5.
Loss of HIF1α increases TCF4–β-catenin complexes and cartilage lesions in OA mice with control or PKF118-310. (A) Safranin-O staining and OA score of Hif1αfl/fl and ΔHif1αchon mouse knees after OA induction or control (Ct) at week 6 after treatment or not with PKF118-310. (Scale bar, 100 μm.) (B) MMP13 expression in Hif1αfl/fl and ΔHif1αchon mice at week 6 and quantification. (Scale bar, 100 μm.) (C) TUNEL assay, quantification in Hif1αfl/fl and ΔHif1αchon mouse joints with OA or control at week 6. Data are mean ± SEM. *P < 0.05, #P < 0.05 compared with control Hif-1αfl/fl (n = 8–11 animals per group).
Discussion
Hypoxia is a characteristic of physiological articular cartilage (5, 25). We addressed the specific function of HIF1α in chondrocytes and in OA in mice. With inducible conditional knockout of HIF1α in mice, we showed that HIF1α alleviated OA development by down-regulating MMP13 through inhibition of β-catenin transcriptional activity in chondrocytes. The hypoxic avascular nature of the articular cartilage maintains the chondrocyte phenotype and homeostasis. Indeed, we observed that hypoxia and HIF1α were down-regulated in OA cartilage. Our findings are consistent with lower chondrocyte hypertrophy during hypoxia, which may contribute to the maintenance of cartilage homeostasis (26–28). Moreover, we show that HIF1α is necessary to maintain a physiologic chondrocyte microenvironment and function. Consistent with our data, the inhibition of HIF1α by 2-methoxyestradiol induced an OA phenotype in mice (5), which suggests that HIF1α modulation is an important event that triggers chondrocyte differentiation in OA. With our conditional HIF1α-knockout mouse model, we show that chondrogenic HIF1α directly maintains cartilage metabolism. Moreover, we show that HIF1α is also a physiological antiapoptotic factor in articular cartilage as its loss enhanced chondrocyte apoptosis. Six weeks after HIF1α deletion, the increase of apoptotic chondrocyte did not induce cartilage lesion as chondrocyte apoptosis alone is not sufficient to induce cartilage lesion (15). Here, the increase in procatabolic enzymes in addition to increased apoptosis can exacerbate the cartilage lesion during OA. Finally the cartilage erosion observed in OA ΔHif1αchon mice results from the double function of HIF1α in articular cartilage as a Wnt inhibitor and an antiapoptotic protein. However, we cannot discard the possibility that Epas1 could be involved in cartilage erosion, because it was described as an inhibitor of survival and catabolic activator (22, 29).
Wnt/β-catenin signaling is one of the key pathways involved in OA (30). Its activation triggers the osteoarthritic differentiation of chondrocytes and OA in mice (16, 31). Thus, understanding the molecular regulators of Wnt signaling is of great therapeutic interest. Oxygen level is an important regulator of Wnt activity (9, 32). Indeed, high oxygen levels promote Wnt signaling and the differentiation of stem cells (33). Hypoxia inhibited the destruction of human cartilage explants by reducing MMP13 production in a HIF1α-dependent manner (25). Because MMP13 is an important target of both HIF signaling and canonical Wnt pathways (20–24), we assessed whether HIF1α regulates MMP13 and Wnt signaling to prevent OA. Here we demonstrated that hypoxia down-regulated canonical Wnt signaling, thereby preventing chondrocyte catabolism. HIF1α deficiency exacerbated cartilage catabolism, thus HIF1α is necessary to prevent OA development. We show that Wnt-induced MMP13 expression was promoted in ΔHifα chondrocytes but was blunted by stabilized HIF1α in chondrocytes. We demonstrate that HIF1α down-regulates the transcription of MMP13 driven by canonical Wnt signaling.
Given the critical role of β-catenin to drive Wnt signaling, understanding the role of HIF1α–β-catenin interaction may reveal novel mechanisms in regulating Wnt signaling. Because HIF1α inhibits TCF4–β-catenin interaction and transcriptional activity (9), we investigated whether HIF1α modulates MMP13 through a Wnt/β-catenin pathway. Hypoxia promoted the translocation of β-catenin and binding to HIF1α but not MMP13 expression. These results are consistent with transcriptional blockade of β-catenin activity despite the stabilization of β-catenin in the nucleus. Our study brings an unidentified mechanism into the protein regulation of HIF1α and β-catenin interaction (9) and its impact on Mmp13 transcription in chondrocytes. Under hypoxia, HIF1α–β-catenin complexes are preferentially formed, which results in a lower TCF4–β-catenin complex level and therefore markedly reduced TCF4 binding to the Mmp13 regulatory region. Interestingly, when stabilized in normoxic chondrocytes, HIF1α blocked TCF4 binding to the Mmp13 regulatory region. Furthermore, the inhibition of TCF4–β-catenin complexes by PKF118-310 abolished Wnt3a-induced MMP13 expression in ΔHIF1α chondrocytes. These results confirm the role of TCF4–β-catenin complexes triggering MMP13 expression in ΔHIF1α chondrocytes. Thus, HIF1α is a nuclear negative regulator of TCF4–β-catenin complexes that inhibits the shift toward a catabolic phenotype in chondrocytes.
We further demonstrated that HIF1α signaling protected against cartilage damage by blocking TCF4–β-catenin. Indeed, PKF118-310 administration reduced cartilage breakdown and the expression of MMP13 observed in ΔHif1αchon mice. Our findings support a procatabolic role of Wnt/β-catenin signaling in OA and bring new insights into the modulation of Wnt/β-catenin signaling by hypoxia. Furthermore, we provide evidence that HIF1α is a potent inhibitor of β-catenin and Wnt signaling and is required to block cartilage degradation. The use of HIF1α agonists might be a useful strategy for treating cartilage lesions in OA.
Methods
Harvesting, Expansion, and Transfection of Primary Chondrocytes.
Hif1αfl/fl and Vhlfl/fl mice (The Jackson Laboratory) were used for chondrocyte cultures. Chondrocytes were harvested from 6-d-old mice and cultured with 10% (vol/vol) FBS. Recombination, transient overexpression, and plasmid (18) information are detailed in SI Methods.
Real-Time PCR.
Real-time PCR involved use of SYBR green (Applied Biosystems) in five to eight independent experiments. Averaged Ct values were normalized to the averaged Ct value of Rpl13a. Adjusted average Ct values were used to calculate relative expression versus control. Primer sequences are detailed in SI Methods.
Immunocytochemistry.
Cells were cultured on cover glasses, fixed with 4% (wt/vol) formaldehyde. Cultures were saturated with 3% (wt/vol) BSA for 60 min, then incubated with the antibodies rabbit primary anti-HIF1α, mouse primary anti–β-catenin, and anti-EPAS1 (all Santa Cruz Biotechnology) for 1 h. Cultures were incubated for 60 min with Alexa dye 488-conjugated rabbit secondary antibody or Cy3 dye-conjugated mouse secondary antibody and observed with Axio Observer Z1 (Zeiss).
Quantification of Proteoglycan Release, Western Blot Analysis, and Immunoprecipitation.
Proteoglycan release was measured in the supernatant by the colorimetric method. Proteins were extracted from whole cell lysates and nuclear extracts (n = 3 independent experiments). Immunoprecipitation, cloning, and reporter gene assay are described in SI Methods.
Mice.
To evaluate the expression of HIF1α during OA, we induced joint instability in 10-wk-old male C57BL6 mice (Janvier Labs) by DMM of the right knee and sham surgery at the left knee as described (34). Mice were killed at weeks 0 (n = 3), 4 (n = 5), and 6 (n = 5) after OA induction. fl/fl HIF1α, R26R-LacZ, and Col2-CreERT mice were supplied by The Jackson Laboratory. At 9 wk of age, Col2-CreERT; fl/fl HIF1α, Col2-CreERT, and R26R-LacZ; and fl/fl HIF1α and R26R-LacZ mice were injected with tamoxifen (1 mg/10 g) (Sigma) daily for 5 d. Joint instability was induced in 10-wk-old male ΔHif1αchon mice (n = 8–10 per group) and fl/fl HIF1α littermates (n = 9–12 per group) in the right knee, with sham operation performed in the left knee. ΔHif1αchon and fl/fl HIF1α were injected intraarticularly once a week with PKF118-310 or PBS (n = 8 per group). Mice were killed 6 wk after surgery. This time point is suitable to quantify the expression of MMP13 in the remaining cartilage. To monitor hypoxia level in healthy and OA cartilage, we injected the hypoxia marker pimonidazole hydrochloride (Hypoxyprobe) in mice. Male FVB mice (The Jackson Laboratory) at 16 wk old were injected intraperitoneally with 0.6 mg pimonidazole/10 g weight and killed 17 h later. Mice were treated in accordance with the Guidelines for Animal Experimentation issued by the local committee (Lariboisière-Villemin no. CEEALV/2012–02-01, Paris).
Histology.
Knees were fixed in 4% (wt/vol) PFA for 24 h at 4 °C and decalcified in Osteosoft; tissue was embedded in paraffin. Serial 5-µm-thick sagittal sections of medial femorotibial joints were collected at three depths at 70-µm intervals. Sections of tibias and femurs were stained with Safranin-O. The scoring method was used for tibias and femurs, with a total severity score ranging from 0 to 12 (35).
Statistical Analysis.
Data are reported as mean ± SEM. Statistical analyses involved ANOVA and the Mann–Whitney test (Statview, SAS Institute). P < 0.05 was the threshold of statistical significance.
SI Methods
Transfection of Primary Chondrocytes.
Recombination was induced with adenovirus expressing Cre recombinase (200 multiplicity of infection) (Vector Biolabs) or HIF1α and EPAS1 siRNA (0.125 µg/mL) (Santa Cruz Biotechnology). Transient overexpression of HIF1α involved use of a retrovirus expressing a mutant construct (P402A/P564A) with both Pro402 and Pro564 replaced by alanines, thereby rendering HIF1α resistant to prolylhydroxylase-dependent degradation (l-HIF1α cells) The plasmid HA-HIF1α P402A/P564A-pBabe-puro was from Addgene (18). Retroviral plasmids were transfected into the 293T cell line by using FuGene (Promega). Culture medium was collected after 48 h and added to cells with 8 μg/mL polybrene. A second transduction was performed with 72-h culture medium. Then, Wnt3a-conditioned medium at 30% (vol/vol) was added for 48 h. Culture media of l-cells was added to the control conditions.
PCR Methods.
The following primer sequences were used: Mmp13, 5′-TGATGGCACTGCTGACATCAT-3′ and 5′-TGTAGCCTTTGGAACTGCTT-3′; collagen 2A1 (Col2A1), 5′-CCG TCATCGAGTACCGATCA-3′ and 5′-CAGGTCAGGTCAGCCATTCA-3′; ribosomal protein L13 (Rpl13a), 5′-GGATCCCTCCACCCTATGACA-3′ and 5′-AGCCGAACAACCTTGAGAGC-3′; Axin, 5′-CCATGACGGACAGTAGCGTA-3′ and 5′-GCCATTGGCCTTCACACT-3′; and Wisp1, 5′-GTGGACATCCAACTACACATCAA-3′ and 5′-AAGTTCGTGGCCTCCTCTG-3′.
Western Blot Analysis and Immunoprecipitation.
Whole cell lysates were prepared and proteins were extracted (n = 3 independent experiments). To verify the stabilization of the HIF1α mutant in chondrocytes, we used rabbit primary HA-TAG antibody for protein analysis (Sigma). For nuclear extracts, chondrocytes were scraped in cold PBS, spun down (12,000 × g for 30 s), resuspended in 400 μL of buffer A (10 mM Hepes, pH 7.9; 1.5 mM MgCl2; 10 mM KCl) with 25 μL of 10% (vol/vol) Nonidet P-40, and then spun down. Nuclei in the pellet were resuspended in buffer B [20 mM Hepes, pH 7.9; 25% (vol/vol) glycerol; 1.5 mM MgCl2; 0.2 mM EDTA; 0.46 M NaCl], and protein concentration was determined. Immunoprecipitation was performed with Dynabeads (Invitrogen). Immunoprecipitation and detection involved use of the antibodies rabbit primary anti-HIF1α and mouse primary anti–β-catenin and anti-EPAS1 (all Santa Cruz Biotechnology).
Chromatin Immunoprecipitation.
Primary chondrocytes were cultured until confluence in p150 dishes (106 cells per dish). Fixation of cultured cells and their processing for chromatin immunoprecipitation (ChIP) followed the manufacturer’s instructions (Millipore). Immunoprecipitation with Dynabeads followed the manufacturer’s instructions (Invitrogen). Immunoprecipitation and detection involved use of mouse primary anti-HIF1α (Santa Cruz Biotechnology) and mouse primary anti-TCF4, mouse anti-RNA polymerase, and mouse IgG (all Upstate). The probes used for PCR to amplify the fragment that contains Wnt responsive elements (WREs) were 5′-CATGCCAACAAATTCCATATTG-3′ and 5′-CCACGCATAGTCATATAG-3′. Results are average Ct values reported to input Ct values.
Cloning and Reporter Gene Assay.
The pGL3-positive luciferase reporter vector (Promega) was used as a positive vector. The 3′-untranslated region (UTR) of MMP13 (Chr 9: 7,271,980–7,283,333) containing the TCF/lymphoid enhancer-binding factor binding sites (WRE) (Seq1) was cloned downstream of firefly luciferase at the BamH1 site in the pGL3-positive luciferase reporter vector. This construct was named vector + Seq1. In brief, with use of exgen 500 (Thermo Scientific), positive vector or vector + Seq1 constructs were transiently cotransfected with TK Renilla vector in C3H10 cells. Cells were then cultured with Wnt3a conditioned medium in hypoxia (1% O2) and normoxia (21% O2) for 24 h. Luciferase activity was measured in cellular lysates by the Dual-Glo substrate system (Promega). Measurements involved use of the SAFAS luminometer (Monaco). Data are represented as the ratio of experimental (Firefly) luciferase to control (Renilla) luciferase.
Immunohistochemistry.
These experiments were performed in serial 5-µm-thick sagittal sections. Antigen retrieval involved use of citrate at 10 mM, pH 6, for 4 h at 70 °C. Hyaluronidase was then added for 15 min at 37° (1 mg/mL) (HIF1α, MMP13, and Hypoxyprobe adducts). Sections were incubated with the antibodies rabbit primary anti-HIF1α (Santa Cruz Biotechnology) and rabbit primary anti–β-galactosidase and anti-MMP13 (both Abcam) at 4 °C overnight. Hypoxyprobe adducts were revealed in hypoxic cells (PO2 < 10 mmHg) with a fluoroscein-conjugated monoclonal antibody (HP-FITC-MAb), then rabbit horseradish peroxidase-conjugated anti-FITC antibody. Negative controls were rabbit nonspecific IgG antibodies. Positive cells were counted on the tibial cartilage surface and expressed as a percentage of total cells. The TUNEL assay was carried out with a Millipore kit.
Acknowledgments
We thank Caroline Marty for her contribution for the histology and Dr. W. G. Kaelin Jr. for providing the HA-HIF1α P402A/P564A-pBabe-puro plasmid. This work was supported by the European Program SFP7 Sybil and the Fondation de l’Avenir.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514854113/-/DCSupplemental.
References
- 1.Maes C, Carmeliet G, Schipani E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat Rev Rheumatol. 2012;8(6):358–366. doi: 10.1038/nrrheum.2012.36. [DOI] [PubMed] [Google Scholar]
- 2.Maes C, et al. VEGF-independent cell-autonomous functions of HIF-1α regulating oxygen consumption in fetal cartilage are critical for chondrocyte survival. J Bone Miner Res. 2012;27(3):596–609. doi: 10.1002/jbmr.1487. [DOI] [PubMed] [Google Scholar]
- 3.Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. 2013;123(9):3664–3671. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gezer D, Vukovic M, Soga T, Pollard PJ, Kranc KR. Concise review: Genetic dissection of hypoxia signaling pathways in normal and leukemic stem cells. Stem Cells. 2014;32(6):1390–1397. doi: 10.1002/stem.1657. [DOI] [PubMed] [Google Scholar]
- 5.Gelse K, et al. Role of hypoxia-inducible factor 1 alpha in the integrity of articular cartilage in murine knee joints. Arthritis Res Ther. 2008;10(5):R111. doi: 10.1186/ar2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunwoodie SL. The role of hypoxia in development of the Mammalian embryo. Dev Cell. 2009;17(6):755–773. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008;15(4):621–627. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- 8.Mazumdar J, et al. O2 regulates stem cells through Wnt/β-catenin signalling. Nat Cell Biol. 2010;12(10):1007–1013. doi: 10.1038/ncb2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol. 2007;9(2):210–217. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- 10.Arden N, Nevitt MC. Osteoarthritis: Epidemiology. Best Pract Res Clin Rheumatol. 2006;20(1):3–25. doi: 10.1016/j.berh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Murray CJ, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, et al. Recent progress in understanding molecular mechanisms of cartilage degeneration during osteoarthritis. Ann N Y Acad Sci. 2011;1240:61–69. doi: 10.1111/j.1749-6632.2011.06258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu M, et al. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009;24(1):12–21. doi: 10.1359/JBMR.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funck-Brentano T, et al. Dkk-1-mediated inhibition of Wnt signaling in bone ameliorates osteoarthritis in mice. Arthritis Rheumatol. 2014;66(11):3028–3039. doi: 10.1002/art.38799. [DOI] [PubMed] [Google Scholar]
- 15.Bouaziz W, et al. Loss of sclerostin promotes osteoarthritis in mice via β-catenin-dependent and -independent Wnt pathways. Arthritis Res Ther. 2015;17:24. doi: 10.1186/s13075-015-0540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuasa T, Otani T, Koike T, Iwamoto M, Enomoto-Iwamoto M. Wnt/beta-catenin signaling stimulates matrix catabolic genes and activity in articular chondrocytes: Its possible role in joint degeneration. Lab Invest. 2008;88(3):264–274. doi: 10.1038/labinvest.3700747. [DOI] [PubMed] [Google Scholar]
- 17.Wu L, et al. Insights on biology and pathology of HIF-1α/-2α, TGFβ/BMP, Wnt/β-catenin, and NF-κB pathways in osteoarthritis. Curr Pharm Des. 2012;18(22):3293–3312. doi: 10.2174/1381612811209023293. [DOI] [PubMed] [Google Scholar]
- 18.Yan Q, Bartz S, Mao M, Li L, Kaelin WG., Jr The hypoxia-inducible factor 2alpha N-terminal and C-terminal transactivation domains cooperate to promote renal tumorigenesis in vivo. Mol Cell Biol. 2007;27(6):2092–2102. doi: 10.1128/MCB.01514-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Leeman MF, Curran S, Murray GI. The structure, regulation, and function of human matrix metalloproteinase-13. Crit Rev Biochem Mol Biol. 2002;37(3):149–166. doi: 10.1080/10409230290771483. [DOI] [PubMed] [Google Scholar]
- 21.Leeman MF, McKay JA, Murray GI. Matrix metalloproteinase 13 activity is associated with poor prognosis in colorectal cancer. J Clin Pathol. 2002;55(10):758–762. doi: 10.1136/jcp.55.10.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito T, et al. Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development. Nat Med. 2010;16(6):678–686. doi: 10.1038/nm.2146. [DOI] [PubMed] [Google Scholar]
- 23.Saito T, Kawaguchi H. HIF-2α as a possible therapeutic target of osteoarthritis. Osteoarthritis Cartilage. 2010;18(12):1552–1556. doi: 10.1016/j.joca.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Yun K, Im SH. Transcriptional regulation of MMP13 by Lef1 in chondrocytes. Biochem Biophys Res Commun. 2007;364(4):1009–1014. doi: 10.1016/j.bbrc.2007.10.121. [DOI] [PubMed] [Google Scholar]
- 25.Thoms BL, Dudek KA, Lafont JE, Murphy CL. Hypoxia promotes the production and inhibits the destruction of human articular cartilage. Arthritis Rheum. 2013;65(5):1302–1312. doi: 10.1002/art.37867. [DOI] [PubMed] [Google Scholar]
- 26.Leijten JC, Moreira Teixeira LS, Landman EB, van Blitterswijk CA, Karperien M. Hypoxia inhibits hypertrophic differentiation and endochondral ossification in explanted tibiae. PLoS One. 2012;7(11):e49896. doi: 10.1371/journal.pone.0049896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Provot S, et al. Hif-1alpha regulates differentiation of limb bud mesenchyme and joint development. J Cell Biol. 2007;177(3):451–464. doi: 10.1083/jcb.200612023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Portron S, et al. Inverse regulation of early and late chondrogenic differentiation by oxygen tension provides cues for stem cell-based cartilage tissue engineering. Cell Physiol Biochem. 2015;35(3):841–857. doi: 10.1159/000369742. [DOI] [PubMed] [Google Scholar]
- 29.Ryu JH, et al. Hypoxia-inducible factor-2α regulates Fas-mediated chondrocyte apoptosis during osteoarthritic cartilage destruction. Cell Death Differ. 2012;19(3):440–450. doi: 10.1038/cdd.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loeser RF. Osteoarthritis year in review 2013: Biology. Osteoarthritis Cartilage. 2013;21(10):1436–1442. doi: 10.1016/j.joca.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nalesso G, et al. WNT-3A modulates articular chondrocyte phenotype by activating both canonical and noncanonical pathways. J Cell Biol. 2011;193(3):551–564. doi: 10.1083/jcb.201011051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitani T, Harada N, Nakano Y, Inui H, Yamaji R. Coordinated action of hypoxia-inducible factor-1α and β-catenin in androgen receptor signaling. J Biol Chem. 2012;287(40):33594–33606. doi: 10.1074/jbc.M112.388298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hakim F, et al. High oxygen condition facilitates the differentiation of mouse and human pluripotent stem cells into pancreatic progenitors and insulin-producing cells. J Biol Chem. 2014;289(14):9623–9638. doi: 10.1074/jbc.M113.524363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadri A, et al. Osteoprotegerin inhibits cartilage degradation through an effect on trabecular bone in murine experimental osteoarthritis. Arthritis Rheum. 2008;58(8):2379–2386. doi: 10.1002/art.23638. [DOI] [PubMed] [Google Scholar]
- 35.Glasson SSCM, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative: Recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(3) Suppl 3:S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]