Neuronal communication requires the propagation of precisely regulated action potentials. Although a myriad of ion channels and modulatory proteins contribute to the action potential waveform and firing properties of each neuron, voltage-gated sodium (Nav) channels typically generate the crucial depolarizing event. Early Nav channel biochemistry identified two beta subunits (1), thus generating excitement over how accessory proteins might be involved in Nav channel physiology. In recent years, Nav channel interactors have grown to include intracellular fibroblast growth factor homologous factors (iFGFs) (for a recent review of this subject, see ref. 2). Although the majority of FGFs are secreted growth factors, a four-member subfamily now designated FGF11-14 is distinguished by generating nonsecreted proteins that do not interact with FGF receptors. Pioneering efforts by Waxman and colleagues (3, 4) demonstrated that these noncanonical FGFs directly bind the C terminus of Nav channels and influence both current density and gating properties. The current literature includes reports of numerous FGF12-14 interactions with various Nav alpha subunits. The picture is far from complete, however, as expression and functional effects vary not only depending on the FGF and Nav isoform partners but also on the FGF splice variant and cell background (2). Although this diversity has clearly complicated the field, FGFs’ role as Nav channel interacting proteins has been solidified by the discovery of a highly conserved Nav interaction site within the FGF core domain that interacts with specific amino acids within the Nav C termini (2). FGF14 and Nav channels are enriched at the axon initial segment (AIS) of several neuronal types, whereas FGF13 colocalizes with Nav1.6 at nodes of Ranvier of dorsal roots of primary afferents (5). Of note is that nodal localization was seen using a pan FGF13 antibody, but an antibody that is specific to FGF13S (also known as FGF13A or FHF2A) did not immunolabel nodes of Ranvier, showing important isoform and splice variant differences (6). Interestingly, mutations in human FGF14 have been linked to spinocerebellar ataxia (7), and mice lacking FGF14 are ataxic (8). Clearly, FGF−Nav channel interactions are worthy of extensive study, and, in PNAS, Pablo et al. (9) use multiple experimental approaches with cultured hippocampal neurons to support the idea that FGF13 and FGF14 differentially regulate Nav channel cell surface expression within somatodendritic and axonal compartments. Thus, FGFs are likely to be central players in the regulation of Nav channel cell biology.
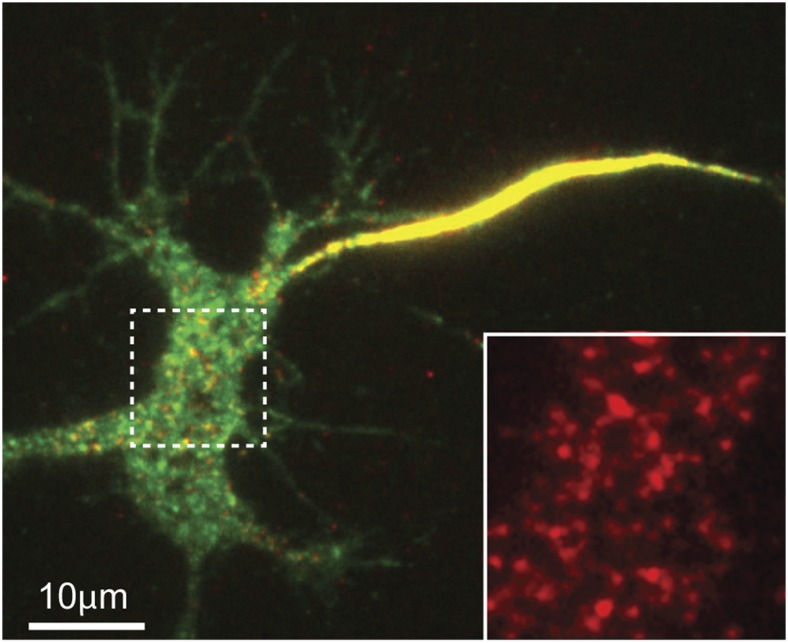
The compartment-specific regulation of FGFs is of great consequence because the polarized distribution of Nav channel isoforms (Nav1.1−Nav1.9) is critical to proper neuronal firing. Fig. 1 illustrates the polarized cell surface distribution of the Nav1.6 isoform in cultured hippocampal neurons. Nav1.6 exhibits a strikingly high plasma membrane density in the AIS compared with the somatodendritic compartment. Nav localization to the AIS has been extensively studied because this domain is vital to action potential initiation (10). In contrast, less is known about Nav channels within the neuronal cell body, even though these somatic channels are likely involved in the transfer of axonal output information to the rest of the neuron (back-propagation) and to synaptic plasticity (11, 12). Studies using chimeric reporter proteins containing the ankyrin-binding motif from the Nav1.2 channel implicate selective endocytosis from the somatic membrane combined with stable tethering to ankyrin within the AIS as a central mechanism in the establishment of Nav channel polarization (13). Experiments using the full-length Nav 1.6 channel and the single-molecule detection sensitivity of total internal reflection fluorescence microscopy demonstrate a direct, ankyrin-dependent vesicular delivery to the AIS where they are immediately immobilized, and ankyrin-independent delivery of mobile channels to the soma (14).
Fig. 1.
GFP and extracellular biotin acceptor domain tagged Nav1.6 expressed in a cultured hippocampal neuron. Surface channels were detected with streptavidin-conjugated CF640R and are indicated by the red color. Inset shows an enhancement of the surface labeling within the dashed line box in the soma.
Pablo et al. (9) focused on FGF13 and FGF14 regulation of Nav channel surface expression in cultured DIV 9–12 hippocampal neurons. Immunolabeling of hippocampal neurons showed a concentration of FGF14 within the AIS in agreement with previous work (15). In contrast, FGF13 showed both an axonal and somatodendritic localization pattern. High-resolution images acquired using structured illumination microscopy showed that even the AIS localization of these proteins differed from each other, suggesting different functional roles. The shRNA-mediated knockdown of these FGFs had differing effects on Nav channel localization. FGF14 knockdown decreased the localization of Nav channels to the AIS consistent with the work of Laezza et al. (16) where a mutation in FGF14 that disrupts its interaction with Nav channels also showed loss of AIS localization, decreased current density, and reduced excitability of hippocampal neurons. Conversely, shRNA-based knockdown of FGF13 had no effect on Nav protein expression within the AIS. Whole-cell voltage clamp was used to show that shRNA-based knockdown of FGF13 increased current density, whereas knockdown of FGF14 decreased current density.
Based on these results, Pablo et al. (9) hypothesized that FGF14 is involved in trafficking Nav channels to the AIS, whereas FGF13 mediates endocytosis of Nav channels within the somadendritic domain. The authors next used a biotinylation assay to measure surface expression of Nav channels in cultured hippocampal neurons after either FGF13 or FGF14 knockdown. FGF14 knockdown decreased Nav channel surface expression whereas FGF13 knockdown increased it. The decreased surface expression after FGF14 knockdown is consistent with the decreased channel expression within the AIS. The authors indirectly addressed the role that FGF13 may play in Nav channel endocytosis by observing the steady-state Nav channel levels after Dynasore treatment. When Dynasore was used to inhibit dynamin-mediated endocytosis, Nav current levels increased, consistent with the idea that Nav channels are constantly internalized from the soma surface at steady state. Importantly, FGF13 knockdown failed to increase current levels in the presence of Dynasore, suggesting that without FGF13 Nav endocytosis was already at a minimum. Together, these data indicate FGF13 and FGF14 differentially regulate Nav channel localization in a compartment-specific manner.
As the pieces of the FGF−Nav channel puzzle are put together, it will be important to investigate isoform- and splice variant-specific effects. Pablo et al. (9) show that expression of shRNA-resistant FGF13VY, but not FGF13S, restored the current density after FGF13 knockdown. Interestingly, both FGF13S and FGF13U (also known as FGF13B or FHF2B) expression increase Nav1.6 current density in DRG-derived ND7/23 cells (5, 6), in direct contrast to the role of FGF13VY in decreasing current density as discussed above. Whether these opposing effects are specific to each FGF isoform, Nav isoform, or cell type will be important to determine. FGF13S and FGF13U both increase current density in ND7/23 cells, but it is not clear whether they alter surface expression of the channels in addition to modifying channel properties. In fact, modification of biophysical properties has been a recurring theme in the study of FGF and Nav channel interactions. FGF14 has been shown to inhibit neuronal excitability due to a hyperpolarizing shift in the voltage dependence of steady-state
Pablo et al. use multiple experimental approaches with cultured hippocampal neurons to support the idea that FGF13 and FGF14 differentially regulate Nav channel cell surface expression within somatodendritic and axonal compartments.
inactivation in both cerebellar granule neurons (17) and Purkinje neurons (18) without altering Nav channel axonal localization. Together, these data suggest that FGFs can alter both Nav channel localization and biophysical properties in an isoform- and cell-type-specific manner.
The specific mechanisms and extent to which FGFs influence surface expression are areas for further study, e.g., does FGF13 mediate Nav channel capture into clathrin-coated pits? FGF knockdown rescue experiments strongly suggest the action is direct, for FGF13 mutations that prevent Nav channel binding fail to rescue or prevent the current increase seen upon knockdown. Thus far, only indirect evidence suggests that FGF13 is involved in steady-state clathrin-mediated endocytosis. Direct evidence would include measures of Nav channel membrane surface stability using single-molecule imaging techniques of tagged channels (14) and observed recruitment of Nav channels to clathrin-coated pits. Such experiments have been done for other ion channels, such as Kv2.1 (19). Pablo et al. (9) suggest FGF14 may enhance trafficking of Nav channels to the axonal compartment. The authors also performed rescue experiments with FGF14 that indicate direct binding is required for the regulation of Nav expression within the AIS. Given the highly specific FGF localization within the AIS, it will be interesting to determine, via either FRET or superresolution methods, when and where Nav channel isoforms assemble with FGF13 and FGF14. It is unclear, in light of the intracellular AIS trafficking barrier, how some trafficking vesicles enter the axon (20). Perhaps FGF14 plays a role in getting Nav channel-containing vesicles past this barrier. It will also be important to determine whether the loss of axonal Nav channels is due to altered stability within the axonal membrane postdelivery. Indeed, a recent paper demonstrated a link between FGF14 and casein kinase 2 (21), a protein shown to enhance Nav localization to the AIS by regulating Nav interactions with ankyrinG (22).
Pablo et al. (9) provide a valuable contribution to our understanding of FGFs’ complex influence on neuronal excitability. Although previous studies have tackled FGF isoforms separately, these investigators explore the differing effects of two FGF isoforms in the same neuronal type. Furthermore, they show that these effects are compartment-specific, with FGF13VY knockdown increasing current density in the somatodendritic region and FGF14B knockdown decreasing current density in the axonal compartment, both of which are likely due to changes in Nav channel surface expression. It is exciting that, even though the Nav channel field has come a long way since the discovery of beta subunits, so many interesting questions remain.
Footnotes
The authors declare no conflict of interest.
See companion article on page E2665.
References
- 1.Hartshorne RP, Messner DJ, Coppersmith JC, Catterall WA. The saxitoxin receptor of the sodium channel from rat brain. Evidence for two nonidentical beta subunits. J Biol Chem. 1982;257(23):13888–13891. [PubMed] [Google Scholar]
- 2.Pablo JL, Pitt GS. Fibroblast growth factor homologous factors: New roles in neuronal health and disease. Neuroscientist. 2016;22(1):19–25. doi: 10.1177/1073858414562217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu CJ, Dib-Hajj SD, Renganathan M, Cummins TR, Waxman SG. Modulation of the cardiac sodium channel Nav1.5 by fibroblast growth factor homologous factor 1B. J Biol Chem. 2003;278(2):1029–1036. doi: 10.1074/jbc.M207074200. [DOI] [PubMed] [Google Scholar]
- 4.Liu Cj, Dib-Hajj SD, Waxman SG. Fibroblast growth factor homologous factor 1B binds to the C terminus of the tetrodotoxin-resistant sodium channel rNav1.9a (NaN) J Biol Chem. 2001;276(22):18925–18933. doi: 10.1074/jbc.M101606200. [DOI] [PubMed] [Google Scholar]
- 5.Wittmack EK, et al. Fibroblast growth factor homologous factor 2B: Association with Nav1.6 and selective colocalization at nodes of Ranvier of dorsal root axons. J Neurosci. 2004;24(30):6765–6775. doi: 10.1523/JNEUROSCI.1628-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rush AM, et al. Differential modulation of sodium channel Na(v)1.6 by two members of the fibroblast growth factor homologous factor 2 subfamily. Eur J Neurosci. 2006;23(10):2551–2562. doi: 10.1111/j.1460-9568.2006.04789.x. [DOI] [PubMed] [Google Scholar]
- 7.van Swieten JC, et al. A mutation in the fibroblast growth factor 14 gene is associated with autosomal dominant cerebellar ataxia [corrected] Am J Hum Genet. 2003;72(1):191–199. doi: 10.1086/345488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Q, et al. Ataxia and paroxysmal dyskinesia in mice lacking axonally transported FGF14. Neuron. 2002;35(1):25–38. doi: 10.1016/s0896-6273(02)00744-4. [DOI] [PubMed] [Google Scholar]
- 9.Pablo JL, Wang C, Presby MM, Pitt GS. Polarized localization of voltage-gated Na+ channels is regulated by concerted FGF13 and FGF14 action. Proc Natl Acad Sci USA. 2016;113:E2665–E2674. doi: 10.1073/pnas.1521194113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshimura T, Rasband MN. Axon initial segments: Diverse and dynamic neuronal compartments. Curr Opin Neurobiol. 2014;27:96–102. doi: 10.1016/j.conb.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myoga MH, Beierlein M, Regehr WG. Somatic spikes regulate dendritic signaling in small neurons in the absence of backpropagating action potentials. J Neurosci. 2009;29(24):7803–7814. doi: 10.1523/JNEUROSCI.0030-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams SR, Stuart GJ. Action potential backpropagation and somato-dendritic distribution of ion channels in thalamocortical neurons. J Neurosci. 2000;20(4):1307–1317. doi: 10.1523/JNEUROSCI.20-04-01307.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fache M-P, et al. Endocytotic elimination and domain-selective tethering constitute a potential mechanism of protein segregation at the axonal initial segment. J Cell Biol. 2004;166(4):571–578. doi: 10.1083/jcb.200312155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akin EJ, Solé L, Dib-Hajj SD, Waxman SG, Tamkun MM. Preferential targeting of Nav1.6 voltage-gated Na+ Channels to the axon initial segment during development. PLoS One. 2015;10(4):e0124397. doi: 10.1371/journal.pone.0124397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao M, Bosch MK, Nerbonne JM, Ornitz DM. FGF14 localization and organization of the axon initial segment. Mol Cell Neurosci. 2013;56:393–403. doi: 10.1016/j.mcn.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laezza F, et al. The FGF14(F145S) mutation disrupts the interaction of FGF14 with voltage-gated Na+ channels and impairs neuronal excitability. J Neurosci. 2007;27(44):12033–12044. doi: 10.1523/JNEUROSCI.2282-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldfarb M, et al. Fibroblast growth factor homologous factors control neuronal excitability through modulation of voltage-gated sodium channels. Neuron. 2007;55(3):449–463. doi: 10.1016/j.neuron.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosch MK, et al. Intracellular FGF14 (iFGF14) is required for spontaneous and evoked firing in cerebellar purkinje neurons and for motor coordination and balance. J Neurosci. 2015;35(17):6752–6769. doi: 10.1523/JNEUROSCI.2663-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weigel AV, Tamkun MM, Krapf D. Quantifying the dynamic interactions between a clathrin-coated pit and cargo molecules. Proc Natl Acad Sci USA. 2013;110(48):E4591–E4600. doi: 10.1073/pnas.1315202110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe K, et al. Networks of polarized actin filaments in the axon initial segment provide a mechanism for sorting axonal and dendritic proteins. Cell Reports. 2012;2(6):1546–1553. doi: 10.1016/j.celrep.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu WJ, et al. CK2 activity is required for the interaction of FGF14 with voltage-gated sodium channels and neuronal excitability. FASEB J. 2016;25:201500161. doi: 10.1096/fj.201500161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bréchet A, et al. Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin G. J Cell Biol. 2008;183(6):1101–1114. doi: 10.1083/jcb.200805169. [DOI] [PMC free article] [PubMed] [Google Scholar]