Significance
Human activities disrupt ecosystem functioning and jeopardize biodiversity, especially in the tropics. Increasing evidence shows this disturbance is happening even within reserves, but the underlying causative mechanisms are unclear. This paper provides a unique long-term (four decades) empirical, experimental, and modeling study showing how cascading effects of human disturbances affect the structure, dynamics, and functioning of a tropical rainforest reserve. In particular, we provide evidence of how fragmentation and defaunation, operating in synergy, stimulated the population explosion of a long-lived understory palm that, in turn, was followed by reductions in biodiversity and changes in some ecosystem properties.
Keywords: plant demography, fragmentation, defaunation, conservation, cascading effects
Abstract
Anthropogenic disturbances affecting tropical forest reserves have been documented, but their ecological long-term cumulative effects are poorly understood. Habitat fragmentation and defaunation are two major anthropogenic threats to the integrity of tropical reserves. Based on a long-term (four decades) study, we document how these disturbances synergistically disrupt ecological processes and imperil biodiversity conservation and ecosystem functioning at Los Tuxtlas, the northernmost tropical rainforest reserve in the Americas. Deforestation around this reserve has reduced the reserve to a medium-sized fragment (640 ha), leading to an increased frequency of canopy-gap formation. In addition, hunting and habitat loss have caused the decline or local extinction of medium and large herbivores. Combining empirical, experimental, and modeling approaches, we support the hypothesis that such disturbances produced a demographic explosion of the long-lived (≈120 y old, maximum height of 7 m) understory palm Astrocaryum mexicanum, whose population has increased from 1,243–4,058 adult individuals per hectare in only 39 y (annual growth rate of ca. 3%). Faster gap formation increased understory light availability, enhancing seed production and the growth of immature palms, whereas release from mammalian herbivory and trampling increased survival of seedlings and juveniles. In turn, the palm’s demographic explosion was followed by a reduction of tree species diversity, changing forest composition, altering the relative contribution of trees to forest biomass, and disrupting litterfall dynamics. We highlight how indirect anthropogenic disturbances (e.g., palm proliferation) on otherwise protected areas threaten tropical conservation, a phenomenon that is currently eroding the planet’s richest repositories of biodiversity.
Human activities operate as dominant drivers of biodiversity change and disruption of ecosystem processes in tropical forest ecosystems (1). Forest fragmentation modifies the structure, diversity, dynamics, and species composition of arboreal communities through habitat reduction and edge effects on tree mortality and recruitment rates (2, 3), liana proliferation (4), and invasive species (5). In addition, fragmentation reduces area of habitat necessary for many vertebrates (herbivores, seed predators, or seed dispersers) to maintain viable populations (6, 7). Fragmentation also facilitates poaching of game animals (7, 8), which, in turn, disrupts trophic interactions that are critical for the maintenance of species diversity (9, 10). More specifically, fragmentation reduces population sizes of some shade-tolerant tree species while fostering the presence of generalist and pioneer plant species (5, 11). Additionally, defaunation is differential, leading to the disappearance or decline of medium and large herbivores (12), and favors the proliferation of large-seeded, shade-tolerant tree species (13). All these changes reduce tree diversity in forest fragments (14, 15). Studies documenting the effects of forest fragmentation on tree communities have mostly focused on canopy species (16). In contrast, effects on long-lived, understory plants have been studied poorly (17), although such plants may play important roles for maintaining forest structure, functioning, and dynamics (18).
Ecosystem-decay processes are not only happening in unprotected, human-modified landscapes but also within protected areas (19). A recent expert assessment analysis (5) uncovered that direct and indirect human disturbances are seriously affecting tropical forest reserves around the world. In the past three decades, 85% of 60 surveyed reserves suffered reductions in cover of the adjacent forest (fragmentation) and increased levels of hunting pressures. Although such study shows that human disturbances are major drivers of biodiversity loss and ecosystem decay in reserves, the underlying mechanisms and long-term cumulative effects are understood poorly.
We conducted a long-term ecological study (1975–2013) to assess the impacts of human disturbance on a reserve (Los Tuxtlas Tropical Field Station, hereafter referred to as LTS) that protects a fraction of the northernmost tropical rainforest in the Americas (20) (Fig. S1). In particular, we focus on impacts on the dominant (>1,000 mature individuals per hectare), long-lived, understory palm Astrocaryum mexicanum Liebm., and analyze the consequences of such impacts on the ecosystem as a whole. During 1975–1981, we found that the population of this palm was in demographic equilibrium (21). However, over the past three decades, the palm population has been increasing rapidly, evincing a demographic explosion. This explosion is having cascading consequences for the ecosystem, including changes in the understory plant community. For example, tree species richness and tree sapling abundance are negatively related to palm abundance, suggesting that this palm plays a critical role in structuring the tree community (22, 23).
Fig. S1.
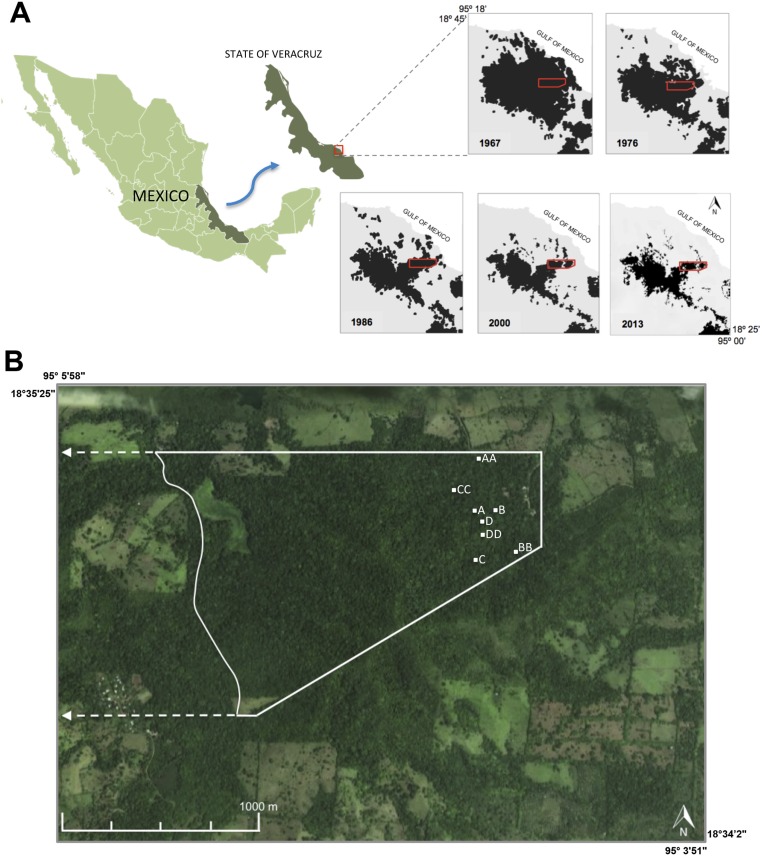
Location of study area, deforestation in the Los Tuxtlas region, southern Mexico, and location of the permanent study plots at the LTS. (A) Location of study area in Mexico and deforestation of tropical rainforest in the Los Tuxtlas region over the past five decades [modified from previous studies (24)]; the red polygon indicates the area of the LTS. (B) Study area within the LTS; the continuous polygon represents the area dedicated to education and research programs. White dots indicate the position of the eight surveyed permanent plots: A and AA, high population density of A. mexicanum; B and BB, medium population density; C and CC, low population density; D and DD, forest gap sites. Satellite image courtesy from © 2013 Google, © 2013 DigitalGlobe, and © 2013 INEGI.
Since the second half of the 20th century, high rates of deforestation have dramatically reduced forest cover in the vicinity of the study site (24). As a consequence, the reserve’s area has been reduced to a medium-sized forest fragment (640 ha), mostly surrounded by pasture (Fig. S1). This disturbance has exposed the reserve to considerable edge effects. Furthermore, synergistic effects of hunting and habitat loss have dramatically reduced populations of several medium and large mammalian herbivores (including tapirs, white-tailed deer, brocket deer, and white-lipped peccaries), causing, their local extirpation in some cases (25, 26). The population explosion of A. mexicanum has resulted from these two convergent disturbances, leading to (i) increased seed production and palm growth as canopy-gap opening rates increased due to edge effects, and (ii) an increase of survival rates of seeds and young palms as mammalian seed predation, herbivory, and trampling declined due to defaunation.
To test the hypothesis that the demographic explosion of A. mexicanum is at least partly explained by fragmentation and defaunation, we measured population growth rate during the past four decades and assessed to what extent changes in density-dependent regulation affect population growth. We also examined whether fragmentation accelerates gap formation rates, and if so, what were the demographic consequences for the palm. In addition, we examined whether defaunation affected the demographic rates of A. mexicanum, exploring the palm’s life cycle stages more affected by fragmentation and defaunation, and the contribution of these stages to population growth. Finally, we assessed relationships between population increase of the palm and tree species diversity, forest composition, tree biomass, and litterfall dynamics. Our results strongly suggest that fragmentation and defaunation significantly correlate with the growth of an understory plant and this increase, in turn, affects understory plant diversity and ecosystem properties. Given the global importance of tropical rainforest ecosystems, and the widespread anthropogenic impacts on them, our findings are of broad significance.
Results and Discussion
Long-Term Population Growth of A. mexicanum.
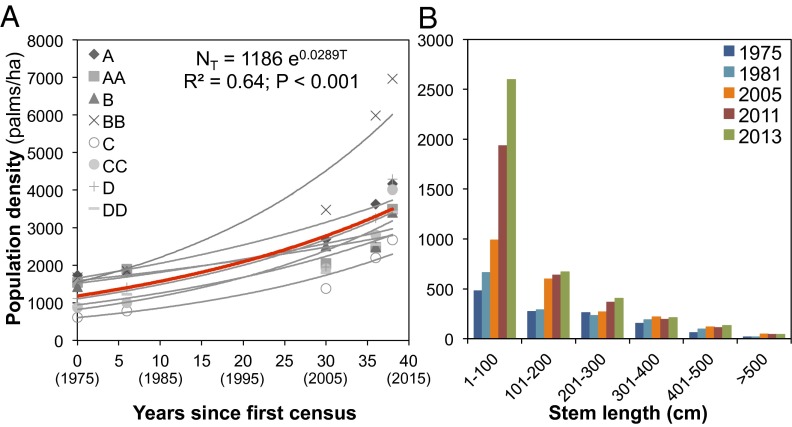
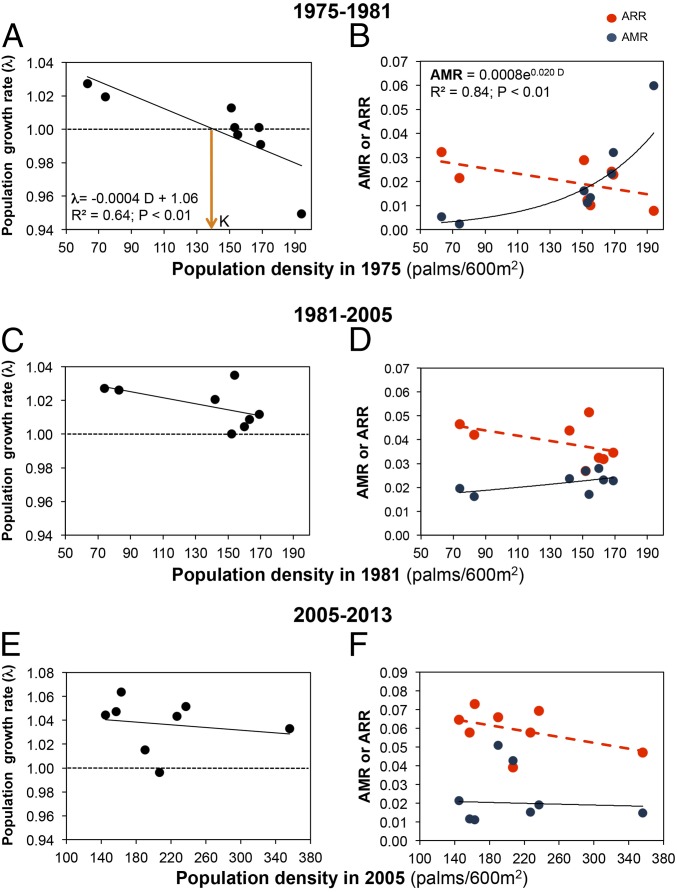
During the past few decades (1975–2013), the palm population grew exponentially in all studied plots (Methods), with an average increase of 326% (λ = 1.029 or 2.9% per year; Fig. 1A). This increase corresponds to a change in population density from 1,243–4,058 adult palms per hectare, with the age structure increasingly dominated by juveniles (Fig. 1B). This change strongly contrasts with the annual population growth rate estimated through matrix modeling using data from 1975 to 1981 (λ = 1.004 or 0.4% per year), which predicted a population increase of only 17% from 1975 to 2013. In the past, λ-values decreased significantly with population size, indicating negative density-dependent regulation of the population and a carrying capacity of ca. 135 palms per 600 m2 (2,250 palms per hectare; Fig. 2A). Such negative density dependence operated on mortality (mostly in younger stages) but not on recruitment rates (27) (Fig. 2B). However, this regulation weakened over time (Fig. 2 C–F), and is no longer evident in recent years (2005–2013), resulting in an increase in population size. The diminution of the density-dependent regulation may occur, as has been found in other systems, due to higher availability of resources (light from canopy gaps in the forest edges in this case) or to the elimination of its natural enemies (28).
Fig. 1.
Long-term population growth trajectory of A. mexicanum at LTS. (A) Population growth trajectories of adult palms over 39 y; the red line corresponds to the best-fit exponential model (generalized linear model with Poisson error; NT is population size at year T, 1,186 is the mean population density in 1975 (year 0), and 0.0289 is the intrinsic annual rate of increase). The red line corresponds to the best-fit relationship considering data from all plots and dates; gray lines (and letters) correspond to plots in closed sites with high (A, AA), medium (B, BB), and low (C, CC) population densities in 1975, and in tree-fall gap sites (D, DD). (B) Change in population size structure from 1975 to 2013.
Fig. 2.
Population dynamics of A. mexicanum at the LTS. Population growth rate (λ) vs. initial population density (D) during three periods: 1975–1981 (A), 1981–2005 (B), and 2005–2013 (C). Annual mortality rate (AMR; ind/ind per year; continuous line) and annual recruitment rate (ARR; ind/ind per year; dashed line) vs. initial population density for 1975–1981 (D), 1981–2005 (E), and 2005–2013 (F). In all cases, λ was calculated as (Nt/N0)(1/t), where N0 and Nt are population density (juvenile and adult palms) at year 0 and t years after, respectively. In A and D, best-fit significant parameter regressions are shown; in all other cases, the trends were nonsignificant.
Causes of the Population Explosion.
Fragmentation effects.
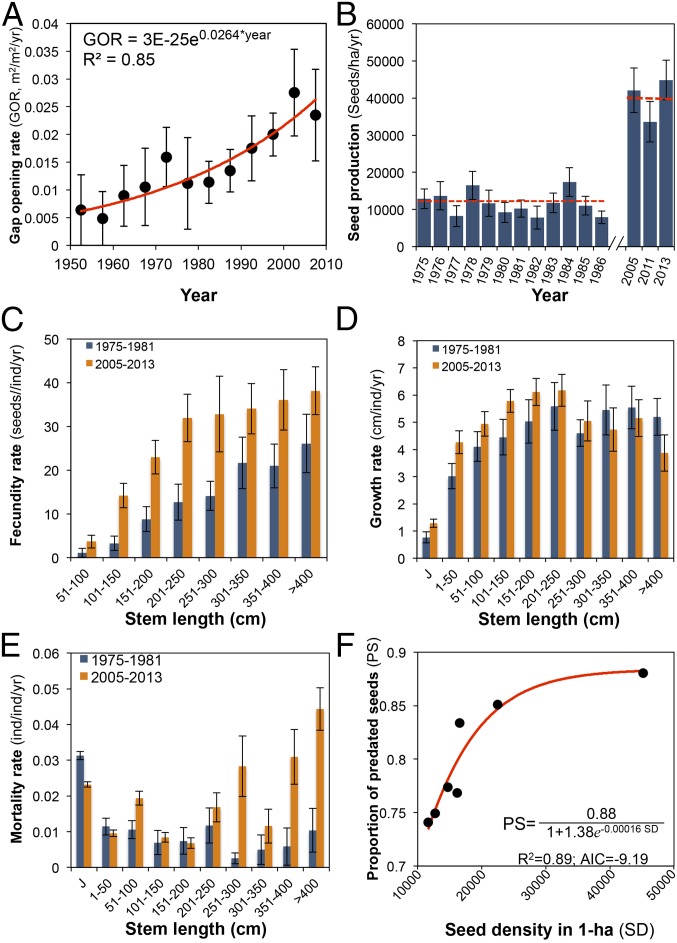
As a consequence of fragmentation, the LTS has been subject to edge effects. Edges of forest fragments experience stronger wind exposure and root desiccation, increasing the risk for fall of trees and large limbs (3, 4). This dynamic results in a more open canopy, augmenting light availability in the understory. Using a gap formation dating protocol (29) (Methods), we estimated that in our permanent plots, gap formation rate (percentage of forest area opened per year) increased 2.7-fold during the study period, from 1.3% in 1975 to 3.6% in 2013 (Fig. 3A). In a previous study, we showed that seed production and growth rates of A. mexicanum increase within tree-fall gaps until forest regeneration shades the palms (27). Also, smaller gaps that result from the fall of tree limbs produce pulses of high seed production (30). Paralleling the increased rates of gap opening, seed production of A. mexicanum increased significantly over time. On average, at the plot and individual levels, seed production rates were more than 200% higher in the later years of the study (2005–2013) than during the previous 30–40 y (Fig. 3 B and C). Positive responses in seed production to increased light availability occur frequently in shade-tolerant tropical rainforest palms, as has been documented elsewhere (e.g., 31, 32).
Fig. 3.
Temporal changes in gap formation dynamics and demographic rates of A. mexicanum. (A) Mean (±SE) annual rates of gap formation between 1953 and 2013 at 5-y intervals; the red line represents the best-fitted regression line (parameters are shown inside the panel). (B) Mean (±1.96 SE) variation in annual seed production in plots initially established at closed-canopy forest sites (n = 6); dashed lines represent averages in the 1975–1986 and 2005–2013 periods. (C–E) Changes in size-specific fecundity, growth, and mortality rates (±1.96 SE), respectively, in past and recent periods; in all cases, ANOVA detected significant size × time effects (P < 0.05). (F) Proportion of seeds predated as a function of available seeds; the best-fit nonlinear model (logistic of three parameters) is shown. GOR, gap opening rate; ind, individual.
The increase in gap formation rates also affected growth and survival of palms. Stem growth increased notably in juvenile and young adult palms (<200-cm stem length), showing 20–70% higher growth rates in recent years than in past years (Fig. 3D). Such increases have important demographic consequences, because faster growth rates in gaps reduce age to first reproduction (27); shortening the prereproductive period increases population growth rates in expanding populations (33, 34). Other studies have shown that growth rates of small understory palms increase with light availability (32). However, taller A. mexicanum showed similar growth rates and suffered increased mortality over time (Fig. 3E). Such behavior can be attributed to the increase in physical damage caused by the frequent fall of trees and branches (22, 27, 29) and to physiological damage on A. mexicanum palms directly exposed to sunlight for extended periods, as has been documented for other understory, shade-tolerant palms (31).
Defaunation effects.
A. mexicanum produces single-seeded nuts (3–5 cm in width × 4–6 cm in length) that are rich in lipids and nutrients, and are an important food for several mammals (35, 36). Seed predation by rodents (e.g., mice, squirrels, pacas, agouties) and peccaries represents the most important cause of mortality during the A. mexicanum life cycle (21). From 1975 to 1981, we found that vertebrates removed 93% of seeds from the ground (21) in 90 d. Similar figures were recorded in 1989 [93% (37)], 2000 and 2005 (94% and 94%, respectively), and 2010 (92%), which indicates that pressures from seed removal have remained virtually unchanged over time. This constancy can result from an increase in seed removal by small mammals (mice and squirrels). Small mammals have increased in the LTS due to reduced competition with other granivorous vertebrates (decimated or extinct) or due to the disappearance of their major predators (10, 38). Indeed, mouse density was fivefold higher in the LTS than in other tropical rainforest reserves (39). However, seed removal by rodents does not compensate for the seed consumption by large mammals (white-tailed deer, brocket deer, peccaries, and tapirs) affected by defaunation (12). The proportion of consumed seeds increased monotonically with seed availability (Methods) until a threshold was reached, indicating a satiation effect on predators (Fig. 3F). Furthermore, seed removal by rodents does not necessarily result in seed death. Indeed, small rodents and squirrels disperse the seeds of A. mexicanum (40). In Belize, up to 30% of A. mexicanum seeds removed by mice were buried in the ground but subsequently germinated (41). Similar behavior has been observed in agouties that remove seeds of Astrocaryum standleyanum in Barro Colorado Island (42). Although seed removal has remained high and relatively constant over time, the partial seed damage (nonlethal) by rodents, as well as their caching and scatter-hoarding behavior, and the satiation effect result in more seeds transitioning to the seedling stage.
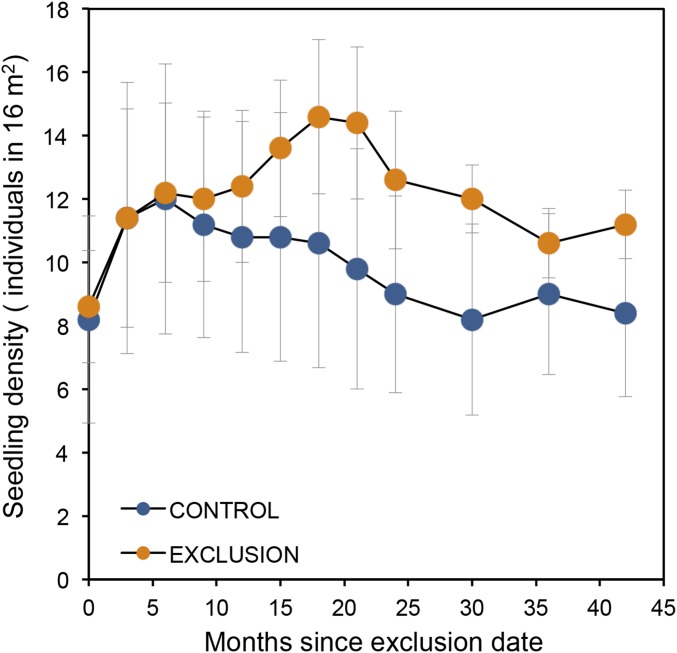
In historical times, seedling and juvenile A. mexicanum was undoubtedly browsed and trampled by deer, tapirs, and peccaries. This idea is supported by observations that large domestic mammals (cows and horses) consume palm leaves when they occasionally intrude into the forest. Experimental evidence suggests that a single total defoliation event increases mortality of juvenile palms by 50% (43), but such damage is absent in the LTS (26). A vertebrate exclusion experiment between 1985 and 1991 (Methods) did not find significant differences in density of seedlings between enclosure and control (open to vertebrate activity) treatments (Fig. S2), a fact that supports the idea that folivorous mammals presently do not represent a mortality factor for A. mexicanum seedlings or saplings.
Fig. S2.
Seedling density of A. mexicanum as a function of two treatments of animal manipulation at Los Tuxtlas, Mexico. The control treatment corresponds to quadrats (1 × 1 m) open to vertebrate animals; exclusion corresponds to quadrats surrounded by metallic mesh (1.2 m width × 1.2 m length × 1 m height, 0.5-inch aperture) to exclude vertebrates. Each treatment had 16 quadrats randomly established within each of five (30 × 30 m) closed-canopy sites. Censuses of seedlings were conducted over 42 mo (1986–1990). Circles are average values (n = 5), and vertical lines are 1 SE. A repeated-measures ANOVA did not detect temporal differences between treatments (treatment × time interaction: F4,28 = 0.30, P = 0.87).
During 2005–2013, the annual mortality rate of juvenile palms decreased 26% in comparison to rates from 1975 to 1981 [from 0.031 to 0.023 individual per individual (ind/ind) per year; Fig. 3]. The lack of herbivory and trampling by large vertebrates (26), as well as increased light conditions in the understory, likely contributes to this decrease.
Exploring Causal Factors.
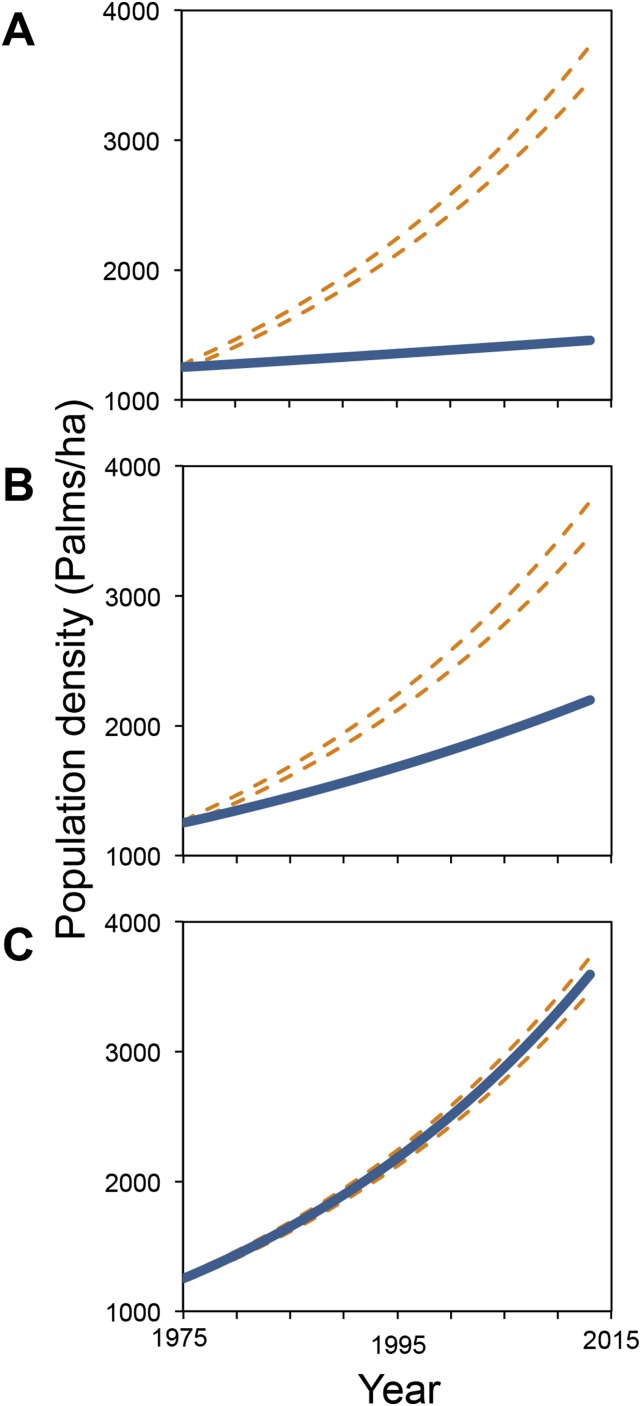
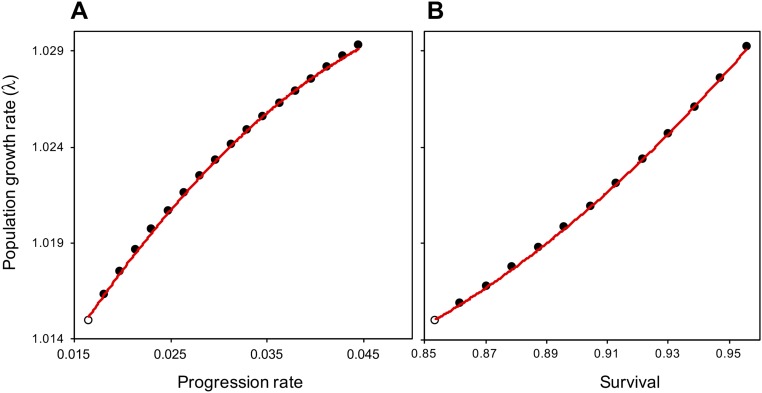
To assess whether the observed demographic changes account for the exponential growth of A. mexicanum during the past four decades (Fig. 1A), we constructed a matrix model using demographic data from 2005 to 2013 (Methods and Fig. S3B). Given that we lack data to assess disturbance effects on seedlings, we conservatively assumed that survival and growth rates at this stage remained unaltered over time. Our model projected a population growth rate of λ = 1.015 (i.e., an annual increase of 1.5%), which was lower than the empirical annual population growth rate (λ = 1.029 or 2.9% per year; Fig. S4B). To explore to what extent the potential effects of anthropogenic disturbances on seedling survival and growth could contribute to the palm population explosion, we conducted a sensitivity analysis with a manual perturbation approach (Methods). By increasing the annual progression probability of seedlings to the juvenile stage from 0.017 to 0.045 (Fig. S5A), simulating the fact that seedling growth was affected positively by increased light conditions in the understory as shown elsewhere (44), λ reached the empirical value [95% confidence interval of this λ value was 1.012–1.046 by Monte Carlo procedure calculation (45)]. The same result is obtained by increasing the annual seedling survivorship rate from 0.85 to 0.96 (Fig. S5B). Such change is in line with the empirical increase in the survival rate of juvenile palms (discussed above). Indeed, the population growth trajectory based on the new λ tightly matches the observed one (Fig. S4C), indicating that positive effects of disturbance on survival or growth of seedlings likely played a role in the population explosion. Also, a retrospective sensitivity analysis (46) (details are provided in Methods) showed that the increases in survival and growth rates at early life stages had the greatest contribution to the change in λ (Fig. S6).
Fig. S3.
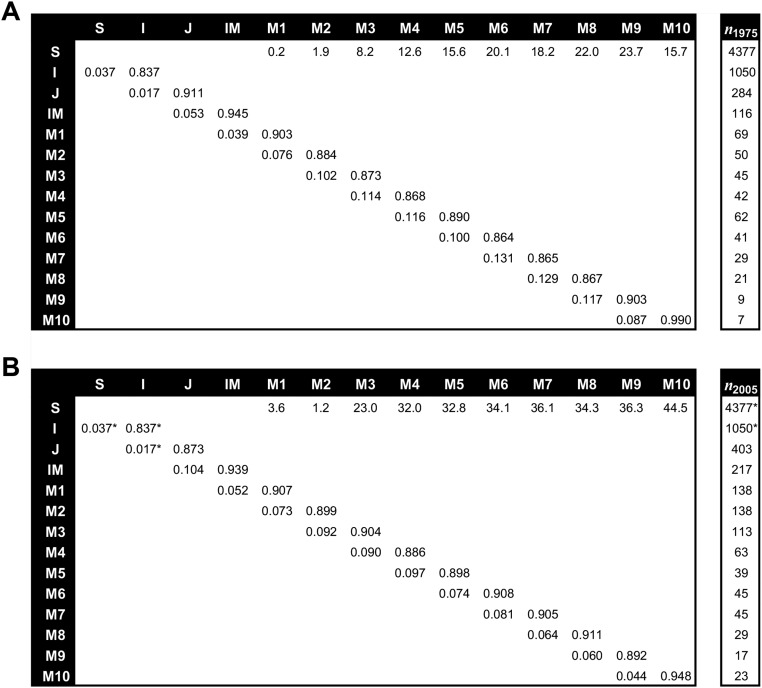
Population projection models for A. mexicanum constructed with data from six closed-canopy forest sites established at Los Tuxtlas. (A) Original matrix population model containing mean annual transition probabilities for 1975–1981. Life stages are as follows: seeds (S), infant palms or seedlings (I; 1- to 7-y-old palms with bifid leaves), juveniles (J; 8- to 17-y-old palms with pinnate leaves and stemless), immature individuals (IM; 1–50 cm in stem length), and mature stages (M1–M10; each one with a 50-cm stem length interval). (B) NPM containing mean annual transition probabilities for 2005–2013; transition probabilities for seeds and infant palms (indicated by an asterisk) were the same as recorded in 1975–1981. In both matrices, the first row contains fecundity rates (annual number of seeds produced per mature palm of a given stage), the main diagonal depicts stage-specific annual permanence probabilities, and the subdiagonal shows stage-specific annual progression probabilities. Adult palms are all individuals in the IM to M10 categories. Adult palms with a stem length longer than 50 cm are mature, reproductive individuals. To the right of each matrix, the vector (n) shows the number of individuals per life cycle stage in year 1975 or 2005.
Fig. S4.
Observed and predicted population growth trajectories for A. mexicanum over a 39-y period. In all panels, dotted lines represent 95% confidence intervals of the observed trajectory generated by fitting an exponential generalized linear model to the data of population density for 1975, 1981, 2005, 2011, and 2013 (as shown in the Fig. 1) and the solid lines represent the predicted trajectories. (A) Population trajectory predicted by the matrix model constructed with data from 1975 to 1981. (B) Trajectory projected by the matrix model constructed with data from 2005 to 2013, with transition probabilities for seeds and infant palms as recorded in the 1975–1981 period. (C) Population trajectory predicted by the matrix model with data from 2005 to 2013, but increasing the progression probability of infant palms by 2.8%.
Fig. S5.
Sensitivity response to changes in vital rates for infants. Sensitivity of population growth rate (λ) to increases in progression probability (A) and annual survivorship probability (B) of infant palms, based on the new matrix population model constructed from data gathered from 2005–2013. Open circles (○) indicate initial population growth rates and probabilities.
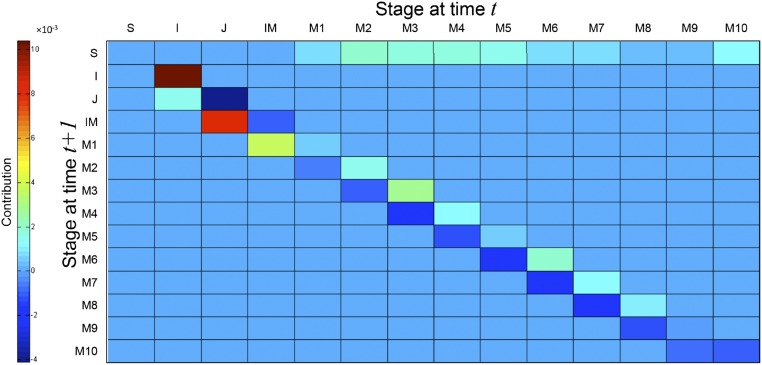
Fig. S6.
Life-table response experiment exploring the contribution of matrix elements to differences in population growth rate (λ) between the periods 1975–1981 and 2005–2013. The color scale represents negative (cold colors at bottom) to positive (hot colors at top) contribution values. The life stages as indicated in Fig. S2.
Overall, results from matrix modeling support the hypothesis that anthropogenic disturbances affected the demographic rates of A. mexicanum, resulting in a population explosion, and explain why the population age structure of A. mexicanum has become dominated by juveniles (Fig. 1B). We previously documented that waves of regeneration occur when a gap opens, creating a hump in the population age structure that diminishes with time in the absence of new gaps (22). In small islands of tropical forest formed by the creation of the Panama Canal, a few large-seeded canopy species dominated tree communities; apparently, such species increased because large mammal herbivores became extinct on those islands (13). This finding supports the observation of high-density patches of large-seeded plants (26) and our argument that defaunation has contributed to the explosion of A. mexicanum at the LTS.
Community-Level Consequences.
A mature A. mexicanum palm has a single hard stem [6-cm diameter at breast height (DBH), 0.5–7 m in height] that sustains a dense crown of 13–15 leaves (1.6 m long each) covering a mean area of 8 m2 (47). As a consequence, the population explosion of A. mexicanum resulted in reductions of space and other resources for trees in the understory. In 1976–1977, we conducted a census of all trees (DBH ≥ 3.3 cm) in our permanent plots, and we conducted a new census in 2013 (Methods). With these data, we assess whether the population increase of A. mexicanum affected tree diversity, species composition, or functional attributes of the forest.
Effects on tree abundance and diversity.
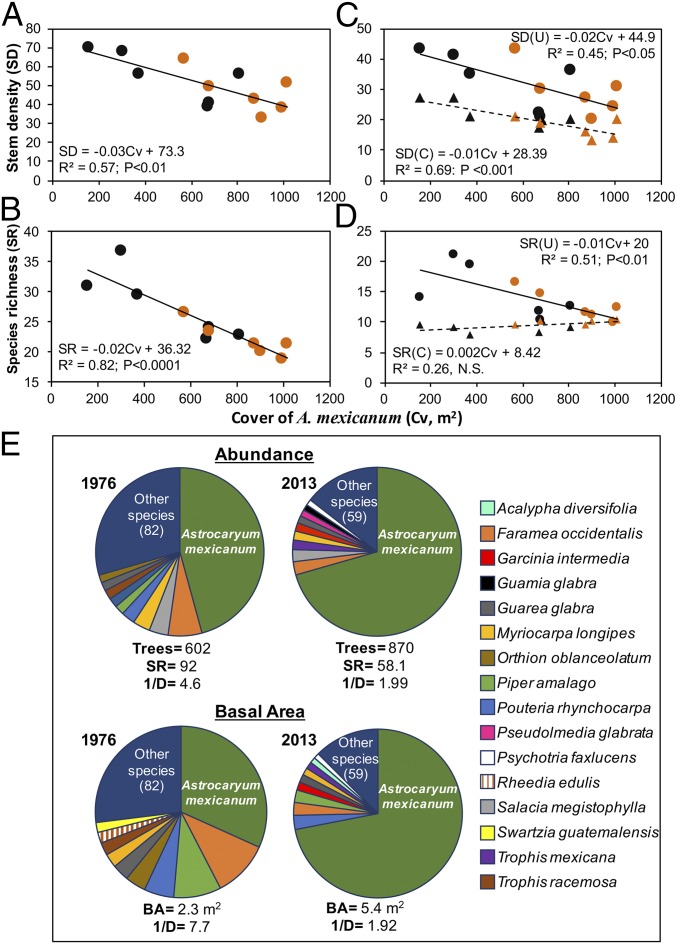
The number of tree stems declined over time as cover of A. mexicanum increased (Fig. 4A). Rarefied values of species richness (Methods) showed the same trend (Fig. 4B). A similar relationship was observed for stem density in both groups (Fig. 4C), when analyzing understory trees (≤10 m tall) separately from canopy trees. As expected, only understory trees showed a decline in species richness as the cover of A. mexicanum increased (Fig. 4D). Species diversity of canopy trees did not change over time. Among understory trees, the relative abundance of A. mexicanum (≥1.3 m tall) increased 25% from 1976 to 2013 (Fig. 4E), whereas other species suffered reductions of 22% in abundance and 37% in richness. The understory species diversity based on abundance (including A. mexicanum; Methods) decreased 57%. Considering basal area, these changes were even more pronounced (Fig. 4E).
Fig. 4.
Temporal changes in structural attributes of tree assemblages related to the population explosion of A. mexicanum. Relationships between stem density (A) and rarefied species richness of trees (B; DBH ≥ 3.3 cm) vs. Cv of A. mexicanum; regression models are shown inside each panel. (C and D) Same relationships separating understory (U; stems with heights ≤10 m; circles and continuous line) and canopy (C; triangles and dashed lines) trees. Data for 1976 are shown in black symbols, and data for 2013 are shown in orange symbols. N.S., not significant. (E) Relative abundance and relative basal area of most abundant species in the understory (<10 m in height) in 1976 and 2013. In E, the number of trees, species richness (SR), basal area (BA), and inverse Simpson species diversity index (1/D) are shown for each year; for the year 2013, SR was rarefied using the same number of individuals that were recorded in 1976.
The reduction in abundance of canopy trees over time was likely caused by the increased fall of trees associated with forest fragmentation (discussed above). However, reductions in tree abundance and species diversity in the understory resulted from spatial exclusion by A. mexicanum. Based on data from tree censuses conducted in 2013 and a neighboring distance spatial analysis (Methods), trees showed a clumped spatial distribution and were significantly overdispersed relative to mature A. mexicanum (Table S1). Light limitation and seed rain interception by palms are two possible mechanisms that affect such a spacing process. A palm’s crown reduces light availability under its shadow by 5–10% (48) and intercepts significant amounts of leaf litter, including seeds falling from the forest canopy (49). Leaf area index (LAI) of A. mexicanum (Methods) increased, on average, from less than 0.5 m2/m2 in 1975 to 2.5 m2/m2 in 2013 (i.e., presently, there are more than two layers of palm crowns). Another study showed that abundance and species diversity of non-Astrocaryum seedlings exhibited a negative relationship with cover of A. mexicanum (50). Also, falling palm leaves physically damage and may kill tree seedlings and saplings, as shown for other understory palm species (51, 52).
Table S1.
Spatial neighborhood analysis assessing the exclusion effect of A. mexicanum on understory trees at the LTS
| Site | n | Tree/A. mexicanum | Tree/tree |
| A | 45 | 1.10 | 0.86 |
| AA | 36 | 0.99 | 1.00 |
| B | 22 | 1.17 | 0.62 |
| BB | 20 | 1.04 | 1.02 |
| C | 44 | 1.03 | 0.76 |
| CC | 57 | 1.33 | 0.88 |
| D | 50 | 1.18 | 0.75 |
| DD | 33 | 0.93 | 0.97 |
| Mean (SE) | 1.10 (0.045) | 0.86 (0.05) | |
Clark and Evans DI [modified by Donelly (61)] was calculated for each of eight permanent plots in 2013 considering trees with DBH ≤ 5 cm and stemmed palms. DI was calculated as the ratio between observed mean distance (ro) between a tree and a palm and between trees and the mean distance expected by random dispersion (re; Methods); DI > 1 indicates overdispersion, DI = 1 indicates random dispersion, and D < 1 indicates a clumped distribution. The last row shows the overall mean DI value (±SE). A parametric t test reveals a significant overdispersed spatial distribution between trees and palms (t = −2.22, df = 6, P < 0.05) and a clumped distribution between trees (t = 2.8, df = 6, P < 0.01).
Effects on tree species composition.
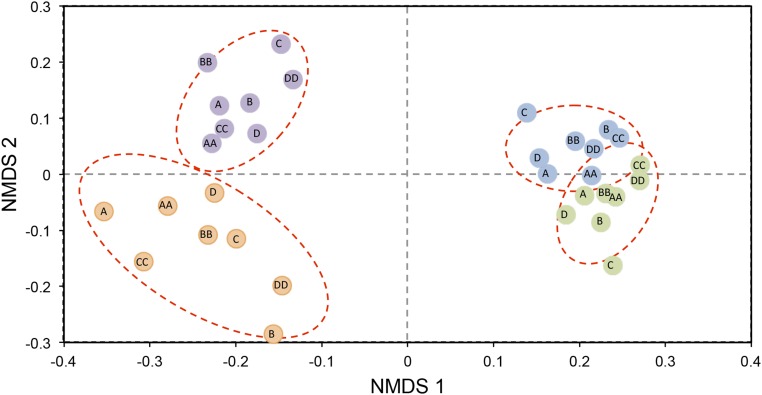
A nonparametric multidimensional ordination analysis (Methods) showed that composition of tree assemblages changed over time [multivariate ANOVA (MANOVA): F2,6 = 79.8, P < 0.001], with a change in the identity of codominant species in the understory. A. mexicanum displaced other species, becoming even more dominant in the understory as influenced by abundance or basal area (Fig. 4E). Also, species similarity of understory trees [as indicated by plot scores in nonmetric multidimensional scaling (NMDS) dimension 1] was negatively correlated with palm abundance, indicating an important role of A. mexicanum as an assemblage-structuring species (Fig. S7). Such a shift in assemblage composition and species dominance has been documented in systems where a very competitive species experiences predator release, as has been shown for invertebrate marine (53) and kelp forest systems (54). For canopy trees, we detected a significant change in composition, but much smaller than observed in the understory. These purported effects of population explosion on the forest will likely become obvious as changes observed in the understory trees propagate into the forest canopy. This hypothesis warrants further, long-term examination.
Fig. S7.
Temporal changes in composition of understory (trees ≤10 m in height) and canopy (trees >10 m in height) assemblages related to the population explosion of A. mexicanum. Results of the NMDS analysis show a significant segregation of understory and canopy assemblages over time. The stress value corresponding to this ordination was 0.14. Each data point represents a single plot in a particular year, and ellipses represent 2 SD of the centroid for a group. Orange points and ellipses correspond to understory trees in 1975, purple to understory trees in 2013, green to canopy trees in 1975, and blue to canopy trees in 2013. Labels indicate the studied sites as shown in Fig. 1. The scores on axis 1 for understory trees in 2013 were negatively correlated (Pearson coefficient) with palm abundance in 1976 (r = −0.71, P < 0.05) and 2013 (r = −0.75, P < 0.05).
The increase of palms has reduced the variability in species composition among plots, especially in the understory (Fig. S7). Other studies have shown that fragmentation and the extirpation of game animals are conductive to a floristic convergence in forest fragments (55, 56). In this context, our study documents how such disturbances may lead to a floristic homogenization of the forest understory due to the explosion of a single species. In the Los Tuxtlas region, it has been found that homogenization among fragments occurs when forest cover has been reduced severely in the landscape (57), but floristic divergence among fragments can also occur if different types or magnitudes of disturbance operate in different fragments (56).
Effects on Ecosystem Properties.
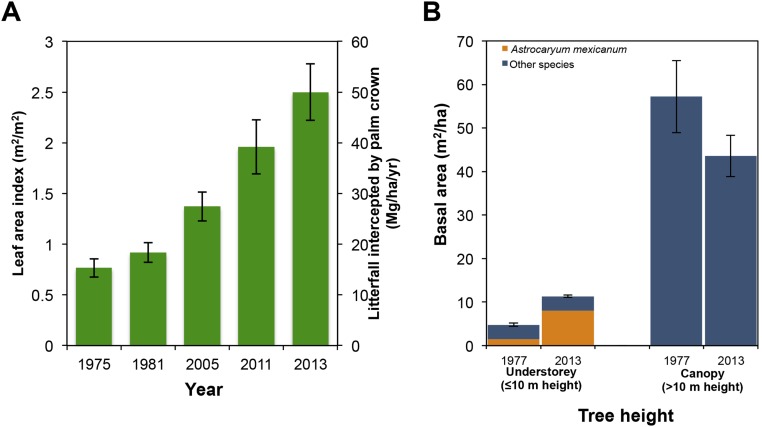
Palm crowns intercept considerable amounts of organic matter falling from the canopy (49). Using estimates of the amount of litter trapped by an average palm crown (Methods), we calculated that the amount of litterfall intercepted by the population of adult A. mexicanum increased 3.3-fold over the past four decades, from 18 to 59 Mg⋅ha−1⋅y−1 (Fig. S8A). In China, litter intercepted by understory vegetation decomposed at slower rates than did litter in the soil (58). Further research is needed to understand the implication of litter retention by A. mexicanum on nutrient flux rates and carbon cycling.
Fig. S8.
Ecosystem effects following the population explosion of A. mexicanum. (A) Temporal change in LAI (proportion of ground area covered by palm leaves) and amount of litter retention in A. mexicanum crowns per unit area; note that values of the LAI are shown on the right y axis and values for litter retention are shown on the left y axis. (B) Temporal change in mean basal area (vertical error bars represent 95% confidence intervals) of A. mexicanum as well as understory and canopy trees.
The population explosion of A. mexicanum also affects the dynamics of tree biomass. Using basal area as a surrogate for biomass, we estimated that biomass of understory trees in permanent plots (n = 8) increased 2.4-fold between 1976 and 2013 due to the population growth of A. mexicanum (Fig. S8B). The relative contribution of this palm to community basal area (trees with a DBH ≥ 3.3) increased 5.6-fold, from an average of 2.7% (±0.6, SE) in 1976 to 15.4% (±2.1) in 2013. Longevity of adult A. mexicanum is about 100 y, whereas longevity of canopy tree species is, on average, two- to fourfold longer (59). The replacement of tree biomass (with long residence periods) by palm biomass (with shorter residence periods) may have important effects on carbon dynamics, which needs to be explored by future studies.
Concluding Remarks.
How widespread are our observed fragmentation and defaunation consequences for tropical rainforest reserves? A recent worldwide survey has discussed that most reserves in the tropics exhibit signals of contemporaneous human disturbances (mainly fragmentation and defaunation) that are eroding biodiversity (5). As far as we know, no other study explicitly unravels the ecological causes of such processes. We clearly documented the effects of anthropic disturbances on the ecology of a dominant keystone, shade-tolerant plant with consequences for the structure and function of the forest ecosystem. Our study highlights the negative impact of local extinctions, as represented by the loss of trophic interactions on the maintenance of species diversity. Also, it provides insights about the cascading disruptions associated with human disturbances that characterize the Anthropocene, documenting that such effects even transpire in protected areas.
Methods
Permanent Plots.
Demographic data of palms representing all life cycle stages were gathered from 1975 to 1981 in eight permanent plots (20 × 30 m each) established in six closed-canopy and two gap forest sites (21, 27). More than 2,500 palms were tagged, measured, and monitored in yearly censuses for survival, growth, reproduction, and seedling recruitment. Reproduction and growth were monitored until 1986 (27). We returned to the same eight plots in 2005, 2011, and 2013, and registered all of the surviving palms and recruits and estimated size-specific survival, growth, and reproduction rates.
Estimation of Gap Opening Rates.
The stems of A. mexicanum are useful for dating fallen trees [up to 70 y old (28)]. Falling trees that open gaps in the forest canopy bend palms; surviving palms regain upright growth leaving a kink in the stem. Gap age can be estimated by dividing the length of the stem formed after the kink by the mean stem growth rate (4.8 cm⋅y−1). In the 2005–2013 censuses, we recorded bent palms in all permanent plots and used the function GOR = [G(Bx/Sx)/A] to estimate annual gap opening rate (GOR), where G is the gap area per bent stem (25 m2), Bx is the number of palms in a plot that were bent “x” years ago from the present, Sx is the yearly probability of survival of bent palms (0.978 ind/ind per year), and A is plot area (in our case, 4,800 m2).
Satiation of Seed Predators.
Combining all plots, we recorded total seed production for the years 1975, 1976, 1977, 1978, 1981, 1982, and 2005, as well as the total number of emerged seedlings in the following years. For each year, the probability of seed predation (PSP) was computed as 1 − (number of emerged seedling/number of seeds produced). To assess satiation of predators, PSP was regressed against the amount of available seeds using a logistic model of three parameters.
Effects on Community Structure.
All trees with a DBH ≥ 3.3 cm were recorded, taxonomically identified, and measured (DBH) in the eight permanent plots in 1976 (47) and 2013. Stem density (excluding A. mexicanum) and species richness were calculated for each plot and census, considering all trees and categorizing them as understory (≤10 m in height) and canopy trees. Species richness was rarefied with the same number of trees across plots and years using the EcoSimR package, version 1.2, in R (60). We calculated total crown cover (Cv) of A. mexicanum palms per plot, multiplying mean crown area per palm (6.3 ± 0.8 m2) by the number of palms taller than 1.3 m; then, we used linear regression models to assess the influence of Cv on assemblage density and richness, considering all plots and years.
Effects on Community Composition.
Based on the Bray–Curtis index, we used NMDS analysis to assess the change in tree composition in the eight study plots between the years 1976 and 2013 in the understory (trees <10.1 m in height) and the canopy. For this analysis, we constructed a matrix, in which the columns contain the species recorded in all plots and years (excluding A. mexicanum), the rows contain the plots subdivided in years (1976, 2013) and canopy position, and the cells contain the species basal area. Finally, the two main dimensions from NMDS were used as dependent variables in a MANOVA and corresponding post hoc Bonferroni tests to evaluate differences in composition related to canopy position of years.
Spatial Pattern Analysis.
For each study plot (n = 8), we computed the spatial dispersion index (DI) of Clark and Evans as modified by Donelly (61) to include edge plot effects, considering distances between understory trees (with stems with 1–5 cm DBH) and their nearest adult A. mexicanum palms (T-Am) and distances among neighboring trees (T-T). For each case, we computed the observed mean distance (ro) and the mean distance expected by chance (re). The latter was calculated as: 1/2A + [0.051 + (0.041/n−0.5) (L/n)], where A is the plot area, n the number of trees, and L the longitude of the total plot boundary. The DI was calculated as the ro-to-re ratio, indicating a random pattern when equal to 1, an overdispersed pattern when DI > 1, and an aggregated pattern when DI < 1. Finally, to assess whether the DI departed significantly from 1, a t test of the form (1 − DIm)/s.e. was used, where DIm is the mean DI over all plots (n = 8, df = 7) and s.e. is the SE.
LAI.
For each of six permanent plots in closed-canopied sites, LAI was calculated as LAI = C × N/A, where C is the mean Cv per palm, N is the number of adult palms (1- to 700-cm stem length) per plot, and A is the plot area. The LAI was calculated for each census (1975, 1981, 2005, 2011, and 2013). The LAI was expressed as yearly averages (±SE).
Litter Intercepted by the A. mexicanum Crowns.
Based on another study (49), the mean annual dry weight of litterfall trapped by the crown of a mature palm was estimated to be 0.0142 Mg. For different years and plots, the litter production trapped by the palm population was estimated by multiplying this figure by the number of mature palms in each plot.
Exclusion of Vertebrates.
In June 1988, a set of 32 1-m2 quadrats was randomly established (2-m minimum distance between quadrats) in each of five forest sites (25 × 25 m each, 0.5-km distance between nearest sites) at the LTS. At each site, 16 quadrats were randomly selected to exclude vertebrates using a metallic mesh (1.2 × 1.2 m and 1-m height) with a 0.5-inch aperture; the other quadrats were unmodified and served as a control treatment. Over the following 42 mo, we conducted 12 censuses of seedlings of A. mexicanum. To assess the effect of exclusion on seedling density (log-transformed), we combined the data from the 16 quadrats corresponding to each treatment per site and applied a repeated-measures ANOVA, assessing the treatment × time interaction term (n = 5).
Matrix Modeling.
With data gathered during 2005–2013 in our original closed-canopy plots, we constructed a new matrix population model (NPM; Fig. S3B). Given that we do not have direct estimations of new demographic rates for infants (newborn seedlings and 1- to 8-y-old palms with bifid leaves), those rates recorded in the period 1975–1981 were used. To assess the impact on λ of a plausible increase in the progression probability for infants, we used a sensitivity analysis with a manual perturbation approach (46). In this analysis, the progression or survival probabilities for infants were systematically increased, starting with the probabilities recorded during 1975–1981. Finally, we conducted a retrospective one-way life-table response experiment (LTRE) (46) to assess the effects on λ caused by the demographic changes experienced by A. mexicanum between the periods 1975–1981 and 2005–2013. For this experiment, we computed differences between our original population transition matrix (corresponding to the period 1975–1981) and the new one (NPM; corresponding to the period 2005–2013), weighted by a mean sensitivity matrix, according to the following formula:
Here, λt+1 is the population growth rate observed in the period 2005–2013 (t+1), λt is the population growth rate observed in the period 1975–1981 (t), ai,j corresponds to the transition probabilities in the matrix at time t and t+1, and represents stage-specific sensitivity values calculated on the average transition matrix of the two periods (A†).
Acknowledgments
We thank the LTS (Institute of Biology, Universidad Nacional Autónoma de México) for provision of fieldwork facilities. We thank J. Rodríguez-Velázquez, E. González-Soriano, S. Sinaca, X. García-Orth, W. Gudiño, A. González-Rodríguez, and C. Ramos for technical and field assistance. We are grateful to M. Willig and W. Laurance for reviewing the manuscript and to J. Wright and P. Balvanera for making valuable comments to an early draft. This study was supported by grants from the Mexican National Council for Science and Technology.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 5150.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1602893113/-/DCSupplemental.
References
- 1.Achard F, et al. Determination of deforestation rates of the world’s humid tropical forests. Science. 2002;297(5583):999–1002. doi: 10.1126/science.1070656. [DOI] [PubMed] [Google Scholar]
- 2.Ranney JW, Bruner MC, Levenson J. In: Forest Island Dynamics in Man-Dominated Landscapes. Burgess RL, Sharpe DM, editors. Springer-Verlag; New York: 1981. pp. 67–95. [Google Scholar]
- 3.Laurance WF, et al. Effects of forest fragmentation on recruitment patterns in Amazonian tree communities. Conserv Biol. 1998;12(2):460–464. [Google Scholar]
- 4.Laurance WF, et al. Rain forest fragmentation and the structure of Amazonian liana communities. Ecology. 2001;82(1):105–116. [Google Scholar]
- 5.Laurance WF, et al. Averting biodiversity collapse in tropical forest protected areas. Nature. 2012;489(7415):290–294. doi: 10.1038/nature11318. [DOI] [PubMed] [Google Scholar]
- 6.Turner IM, T Corlett R. The conservation value of small, isolated fragments of lowland tropical rain forest. Trends Ecol Evol. 1996;11(8):330–333. doi: 10.1016/0169-5347(96)10046-x. [DOI] [PubMed] [Google Scholar]
- 7.Peres CA. Synergistic effects of subsistence hunting and habitat fragmentation on Amazonian forest vertebrates. Conserv Biol. 2001;15(6):1490–1505. [Google Scholar]
- 8.Abernethy KA, Coad L, Taylor G, Lee ME, Maisels F. Extent and ecological consequences of hunting in Central African rainforests in the twenty-first century. Philos Trans R Soc Lond B Biol Sci. 2013;368(1625):20120303. doi: 10.1098/rstb.2012.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terborgh J, et al. Ecological meltdown in predator-free forest fragments. Science. 2001;294(5548):1923–1926. doi: 10.1126/science.1064397. [DOI] [PubMed] [Google Scholar]
- 10.Dirzo R, et al. Defaunation in the Anthropocene. Science. 2014;345(6195):401–406. doi: 10.1126/science.1251817. [DOI] [PubMed] [Google Scholar]
- 11.Laurance WF. Edge effects in tropical forest fragments: Application of a model for the design of nature reserves. Biol Conserv. 1991;57(2):205–219. [Google Scholar]
- 12.Wright SJ. The myriad consequences of hunting for vertebrates and plants in tropical forests. Perspect Plant Ecol Evol Syst. 2003;6(1):73–86. [Google Scholar]
- 13.Leigh EG, Wright SJ, Herre EA, Putz FE. The decline of tree diversity on newly isolated tropical islands: A test of a null hypothesis and some implications. Evol Ecol. 1993;7(1):76–102. [Google Scholar]
- 14.Phillips OL. The changing ecology of tropical forests. Biodivers Conserv. 1997;6(2):291–311. [Google Scholar]
- 15.Melo FP, Arroyo-Rodríguez V, Fahrig L, Martínez-Ramos M, Tabarelli M. On the hope for biodiversity-friendly tropical landscapes. Trends Ecol Evol. 2013;28(8):462–468. doi: 10.1016/j.tree.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Laurance WF, Delamônica P, Laurance SG, Vasconcelos HL, Lovejoy TE. Rainforest fragmentation kills big trees. Nature. 2000;404(6780):836. doi: 10.1038/35009032. [DOI] [PubMed] [Google Scholar]
- 17.Gagnon PR, et al. Growth of an understory herb is chronically reduced in Amazonian forest fragments. Biol Conserv. 2011;144(2):830–835. [Google Scholar]
- 18.Richards PW. The Tropical Rain Forest: An Ecological Study. 2nd Ed Cambridge Univ Press; Cambridge, UK: 1996. [Google Scholar]
- 19.Woodroffe R, Ginsberg JR. Edge effects and the extinction of populations inside protected areas. Science. 1998;280(5372):2126–2128. doi: 10.1126/science.280.5372.2126. [DOI] [PubMed] [Google Scholar]
- 20.González-Soriano E, Dirzo R, Vogt RC. 1997. Historia Natural de Los Tuxtlas (CONABIO and Universidad Nacional Autónoma de México, Mexico City). Spanish.
- 21.Piñero D, Martínez-Ramos M, Sarukhán J. A population model of Astrocaryum mexicanum and a sensitivity analysis of its finite rate of increase. J Ecol. 1984;72(3):977–991. [Google Scholar]
- 22.Sarukhán J, Piñero D, Martínez-Ramos M. In: Studies on Plant Demography: A Festschrift for John L. Harper. White J, editor. Academic; Oxford: 1985. pp. 17–31. [Google Scholar]
- 23.Piñero D, Martínez-Ramos M, Mendoza A, Álvarez-Buylla E, Sarukhán J. Demographic studies in Astrocaryum mexicanum and their use in understanding community dynamics. Principes. 1986;30:108–116. [Google Scholar]
- 24.Mendoza E, Fay J, Dirzo R. A quantitative analysis of forest fragmentation in Los Tuxtlas, southeast Mexico: Patterns and implications for conservation. Rev Chil Hist Nat. 2005;78:451–467. [Google Scholar]
- 25.Dirzo R, Miranda A. Contemporary neotropical defaunation and forest structure, function, and diversity—a sequel to John Terborgh. Conserv Biol. 1990;4:444–447. [Google Scholar]
- 26.Dirzo R, Miranda A. In: Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions. Price P, Lewinson T, Fernandes G, Benson W, editors. Wiley; New York: 1991. pp. 273–287. [Google Scholar]
- 27.Martínez-Ramos M. In: Plant Population Ecology. Davy D, Hutchings M, Watkinson AR, editors. Blackwell Scientific; Oxford: 1988. pp. 293–313. [Google Scholar]
- 28.Hixon MA, Carr MH. Synergistic predation, density dependence, and population regulation in marine fish. Science. 1997;277(5328):946–949. [Google Scholar]
- 29.Martínez-Ramos M, Álvarez-Buylla E, Sarukhán J, Piñero D. Treefall age determination and gap dynamics in a tropical forest. J Ecol. 1988;76(3):700–716. [Google Scholar]
- 30.Piñero D, Sarukhán J. Reproductive behaviour and its individual variability in a tropical palm, Astrocaryum mexicanum. J Ecol. 1982;70(2):461–472. [Google Scholar]
- 31.Svenning J. Crown illumination limits the population growth rate of a neotropical understorey palm (Geonoma macrostachys, Arecaceae) Plant Ecol. 2002;159(2):185–199. [Google Scholar]
- 32.Martínez-Ramos M, Anten NPR, Ackerly DD. Defoliation and ENSO effects on vital rates of an understorey tropical rain forest palm. J Ecol. 2009;97(5):1050–1061. [Google Scholar]
- 33.Caswell H. Matrix Population Models: Construction, Analysis and Interpretation. 2nd Ed Sinauer; Sunderland, MA: 2001. [Google Scholar]
- 34.Stearns SC. Life-history tactics: A review of the ideas. Q Rev Biol. 1976;51(1):3–47. doi: 10.1086/409052. [DOI] [PubMed] [Google Scholar]
- 35.Brewer S, Rejmánek M. Small rodents as significant dispersers of tree seeds in a neotropical forest. J Veg Sci. 1999;10:165–174. [Google Scholar]
- 36.Sánchez-Cordero V, Martínez-Gallardo R. Postdispersal fruit and seed removal by forest-dwelling rodents in a lowland rainforest in Mexico. J Trop Ecol. 1998;14(2):139–151. [Google Scholar]
- 37.Rodríguez-Velázquez J. 1994. Efecto del mosaico de regeneración y la densidad sobre la remoción post-dispersión de las diásporas de la palma tropical Astrocaryum mexicanum Liebm. PhD dissertation (Universidad Nacional Autónoma de México, Mexico City). Spanish.
- 38.Chinchilla FA. Seed predation by mammals in forest fragments in Monteverde, Costa Rica. Rev Biol Trop. 2009;57(3):865–877. doi: 10.15517/rbt.v57i3.5499. [DOI] [PubMed] [Google Scholar]
- 39.Sánchez-Cordero V, Fleming TH. Biology of the Heteromyidae. Special Publication 10. American Society of Mammalogists; Provo, UT: 1993. pp. 596–616. [Google Scholar]
- 40.Eguiarte L, et al. Direct and indirect estimates of neighborhood and effective population size in a tropical palm, Astrocaryum mexicanum. Evolution. 1993;47(1):75–87. doi: 10.1111/j.1558-5646.1993.tb01200.x. [DOI] [PubMed] [Google Scholar]
- 41.Brewer S. Predation and dispersal of large and small seeds of a tropical palm. Oikos. 2001;92(2):245–255. [Google Scholar]
- 42.Gálvez D, Kranstauber B, Kays RW, Jansen PA. Scatter hoarding by the Central American agouti: A test of optimal cache spacing theory. Anim Behav. 2009;78(6):1327–1333. [Google Scholar]
- 43.Mendoza A, Piñero D, Sarukhán J. Effects of experimental defoliation on growth, reproduction and survival of Astrocaryum Mexicanum. J Ecol. 1987;75(2):545–554. [Google Scholar]
- 44.Crompton D. 2007. The effects of harvesting on the population structure of Astrocaryum mexicanum and its potential for understory cultivation. PhD dissertation (University of Hawaii, Manoa, HI)
- 45.Arellano PJJ, Aguirre PMA. 2002. Montecarlo Versión 1.0. (Instituto de Ecología, Universidad Nacional Autónoma de México, Campus Morelia, Michoacán, Mexico) Spanish.
- 46.Caswell H. Analysis of life table response experiments I. Decomposition of effects on population growth rate. Ecol Modell. 1989;46(3):221–237. [Google Scholar]
- 47.Piñero D, Sarukhán J, González E. Estudios demográficos en plantas. Astrocaryum mexicanum Liebm. 1. Estructura de las Poblaciones. Bol Soc Bot Mex. 1977;37:69–118. Spanish. [Google Scholar]
- 48.Pérez-Ishiwara JR. 1990. Caracterización del microclima lumínico y sus efectos en el comportamiento reproductivo de una palma tropical. PhD dissertation (Universidad Nacional Autónoma de México, México DF, México). Spanish.
- 49.Álvarez-Sánchez J, Guevara S. Litter interception on Astrocaryum mexicanum Liebm. (Palmae) in a tropical rain forest. Biotropica. 1999;31(1):89–92. [Google Scholar]
- 50.Dyer GA. 1990. Interferencia lumínica de Astrocaryum mexicanum Liebm. (Palmae) en el sotobosque e implicaciones sobre la comunidad de árboles jóvenes de Los Tuxtlas, Veracruz. PhD dissertation (Universidad Nacional Autónoma de México, Mexico City). Spanish.
- 51.Vandermeer JH. Notes on density dependence in Welfia georgii Wedl. ex Burret (Palmae) a lowland rainforest species in Costa Rica. Brenesia. 1977;10/11:9–15. [Google Scholar]
- 52.Clark DB, Clark DA. The role of physical damage in the seedling mortality regime of a neotropical rain forest. Oikos. 1989;55(2):225–230. [Google Scholar]
- 53.Estes JA, Tinker MT, Williams TM, Doak DF. Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science. 1998;282(5388):473–476. doi: 10.1126/science.282.5388.473. [DOI] [PubMed] [Google Scholar]
- 54.Paine RT. Food web complexity and species diversity. Am Nat. 1966;100(910):65. [Google Scholar]
- 55.Laurance WF, et al. Rain forest fragmentation and the proliferation of successional trees. Ecology. 2006;87(2):469–482. doi: 10.1890/05-0064. [DOI] [PubMed] [Google Scholar]
- 56.Laurance WF, et al. Habitat fragmentation, variable edge effects, and the landscape-divergence hypothesis. PLoS One. 2007;2(10):e1017. doi: 10.1371/journal.pone.0001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arroyo-Rodríguez V, et al. Plant β-diversity in fragmented rain forests: Testing floristic homogenization and differentiation hypotheses. J Ecol. 2013;101(6):1449–1458. [Google Scholar]
- 58.He X, Lin Y, Han G, Ma T. Litterfall interception by understorey vegetation delayed litter decomposition in Cinnamomum camphora plantation forest. Plant Soil. 2013;372:207–219. [Google Scholar]
- 59.Martínez-Ramos M, Álvarez-Buylla E. How old are tropical rain forest trees? Trends Plant Sci. 1998;3(10):400–405. [Google Scholar]
- 60.Gotelli NJ, Ellison AM. 2013 EcoSimR 1.00. Available at www.uvm.edu/∼ngotelli/EcoSim/EcoSim.html. Accessed May 15, 2014.
- 61.Donelly K. In: Simulation Methods in Archaeology. Hodder I, editor. Cambridge Univ Press; London: 1986. pp. 91–95. [Google Scholar]