Significance
How gene regulation orchestrates brain activities that lead to cognition and behavior remains a challenging question in biology. Alternative pre-mRNA splicing (AS) is a crucial mechanism that is extensively used in the brain to generate diverse and functionally distinct protein products from a limited number of eukaryotic genes, and can thereby switch neuron functions and rewire neural circuits for different behaviors. Here, we elucidate the function of an essential AS regulatory protein, P-element somatic inhibitor (PSI), in coordinating male courtship behavior in the fruit fly Drosophila melanogaster. We show that PSI fine-tunes the AS patterns of a dynamic network of neural gene transcripts and exerts precise control of male mating behavior. Our results provide important information into mechanisms for behavior control in animals.
Keywords: PSI, U1 snRNP, alternative pre-mRNA splicing, male courtship behavior
Abstract
Alternative pre-mRNA splicing (AS) is a critical regulatory mechanism that operates extensively in the nervous system to produce diverse protein isoforms. Fruitless AS isoforms have been shown to influence male courtship behavior, but the underlying mechanisms are unknown. Using genome-wide approaches and quantitative behavioral assays, we show that the P-element somatic inhibitor (PSI) and its interaction with the U1 small nuclear ribonucleoprotein complex (snRNP) control male courtship behavior. PSI mutants lacking the U1 snRNP-interacting domain (PSIΔAB mutant) exhibit extended but futile mating attempts. The PSIΔAB mutant results in significant changes in the AS patterns of ∼1,200 genes in the Drosophila brain, many of which have been implicated in the regulation of male courtship behavior. PSI directly regulates the AS of at least one-third of these transcripts, suggesting that PSI–U1 snRNP interactions coordinate the behavioral network underlying courtship behavior. Importantly, one of these direct targets is fruitless, the master regulator of courtship. Thus, PSI imposes a specific mode of regulatory control within the neuronal circuit controlling courtship, even though it is broadly expressed in the fly nervous system. This study reinforces the importance of AS in the control of gene activity in neurons and integrated neuronal circuits, and provides a surprising link between a pleiotropic pre-mRNA splicing pathway and the precise control of successful male mating behavior.
How gene regulation modulates neuronal activities leading to cognition and behavior is an important question in biology. Although many behavior-associated genes and neuronal cell types have been identified, a detailed understanding that links the molecular events of gene regulation to specific behaviors is still lacking. Alternative pre-mRNA splicing (AS) is a crucial gene regulatory mechanism that enables a single gene to generate functionally distinct messenger RNA transcripts (mRNAs) and protein products (1). The nervous system makes extensive use of AS to generate diverse and complex neural mRNA expression patterns that determine numerous neuronal cell types and functions (2). AS is regulated by the small nuclear ribonucleoprotein complexes (snRNPs) that compose the spliceosome for intron recognition and removal, as well as a large repertoire of non-snRNP RNA-binding proteins that affect decisions on splice site use (3). This dynamic and complex AS regulatory network modulates diverse neuronal functions, like synaptic transmission and signal processing, hence further impacting higher brain functions, such as cognition and behavioral control (4).
The Drosophila KH-domain RNA binding splicing factor P-element somatic inhibitor (PSI) is best known for regulating tissue-specific AS of the Drosophila P-element transposon transcripts to restrict transposition activity to germ-line tissues (5, 6). PSI directly interacts with the U1 snRNP through a 70-aa tandem direct repeat domain at the C terminus of the PSI protein (termed the “AB” domain) (7). Deletion of the AB domain in transgenic flies resulted in male sterility and male courtship defects (6). U1 snRNP, as an essential component of the spliceosome that binds to 5′ splice sites (5′SS), defines exon–intron boundaries, and initiates spliceosome assembly for intron removal (3). U1 snRNP further affects AS decisions and suppresses pre-mRNA premature cleavage and polyadenylation through binding to pseudo-5′SS (5′SS-like motifs that are not used for splicing) that are abundantly distributed throughout the transcriptome (8–10). It remains a mystery how U1 snRNP differentiates the vast number of functional 5′SS and pseudo-5′SS in the transcriptome that leads to functionally distinct AS patterns. In the case of Drosophila P-element transposon AS regulation, the PSI–U1 snRNP interaction enables PSI to modulate the competitive binding of U1 snRNP between the accurate 5′SS in the third intron and an upstream pseudo-5′SS in the transposon pre-mRNA, and thus influence the final AS decision (5, 6). It is possible that PSI may play a more general role in specifically localizing U1 snRNP to the transcriptome for AS regulation beyond the P-element transposon, and thus exert a more broad influence over fruit fly physiology.
Results
Disruption of the PSI–U1 snRNP Interaction in Drosophila Causes Aberrant Male Courtship Behavior.
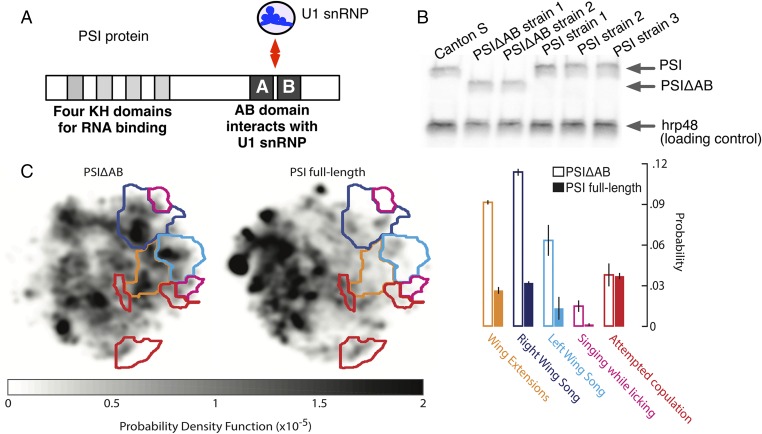
To evaluate how the PSI–U1 snRNP interaction affects Drosophila behavioral activities, we used an automated behavioral video tracking and analysis system to quantitatively assess the courtship behaviors of PSI-null mutant Drosophila strains carrying P-element transgenes expressing either the full-length PSI protein or AB domain-depleted PSI protein (PSIΔAB) (7, 11) (Fig. 1 A and B and Movies S1–S3). The transgenic PSI and PSIΔAB proteins are expressed at comparable levels to endogenous PSI in wild-type flies (Fig. 1B). We found that male PSIΔAB flies were significantly less effective in courting females than flies with the full-length PSI protein (Fig. 1C). Specifically, PSIΔAB males performed nearly all courtship behaviors two to three times more often but had the same number of copulating attempts, indicating that the females were less receptive to PSIΔAB male courtship (Fig. 1C). We noticed one particular deficit in courtship in PSIΔAB male flies: they appear to have difficulty initiating song production. These flies spent more time with their wings at a moderate level of extension (orange region in Fig. 1C and Movie S3) before each singing bout.
Fig. 1.
Disruption of the normal PSI–U1 snRNP interaction causes defects in Drosophila male courtship behavior. (A) Diagram of the PSI protein, which is composed of four RNA-binding KH domains and the AB repeat domain that interacts with U1 snRNP. (B) Immunoblot of fly head extracts from PSI-null mutant flies that carry transgenes expressing PSIΔAB or full-length PSI proteins, with wild-type Canton S fly head extracts serving as positive control. Protein detected with specific antibody probes are indicated at the right. (C, Left) Behavioral space densities for the male PSIΔAB and full-length PSI fly strains in the presence of a Canton S young virgin female. Colored lines indicate regions of the space corresponding to behaviors associated with the male courtship ritual. (Right) Probability that a male fly of the PSIΔAB strain (open bars) or the full-length PSI strain (solid bars) performs each of several courtship behaviors in the presence of a young Canton S virgin female fly. Mean of seven individual flies of each strain, with ±SE shown. See also Movies S1–S3.
PSI Is Broadly Expressed in Both Male and Female Drosophila Brains.
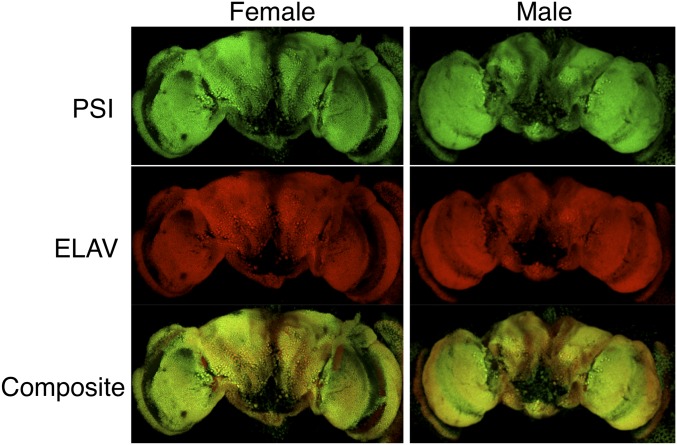
To investigate how the loss of the PSI–U1 snRNP interaction specifically affects male courtship behavior, we first examined the expression pattern of the PSI protein in both male and female fly brains. Using immunofluorescent antibody staining and confocal microscopy, we found that PSI is broadly expressed in the adult brain and its expression pattern overlaps extensively but not completely with the pan-neuronal marker protein ELAV (Fig. 2). No significant difference was detected between male and female fly brains for PSI protein expression (Fig. 2). Furthermore, PSI is expressed in the nucleus (Fig. S1A), consistent with its function as a pre-mRNA splicing factor. In larval brains, PSI is expressed broadly in both differentiated neurons as well as neuronal precursor cells, and PSI is also broadly expressed in Drosophila embryos (Fig. S1 B and C).
Fig. 2.
PSI protein is expressed broadly in both male and female adult Drosophila brains. Immunofluorescent staining and confocal microscopy imaging of the endogenous PSI protein in dissected Canton S adult female (Left) and male (Right) brains. Shown are stacks of consecutive confocal planes. PSI is shown in green (Top), ELAV is shown in red (Middle), and a composite of both patterns with overlap shown (Bottom). See also Fig. S1. (Magnification: 20×.)
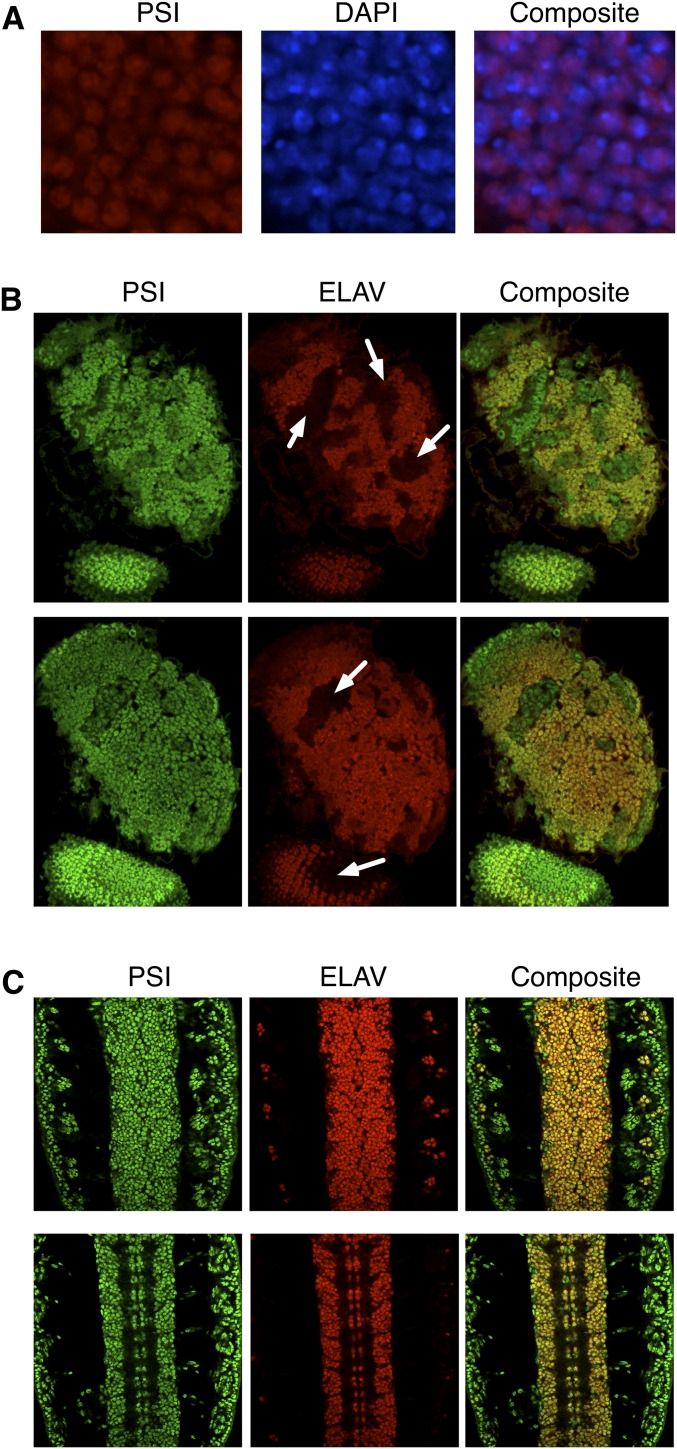
Fig. S1.
PSI protein is a nuclear protein and is expressed broadly in the nervous system of Drosophila larvae and embryos. (A) Immunostaining of endogenous PSI protein in an adult Canton S male brain. Samples are costained with the DNA-binding dye, DAPI. (B) Immunofluorescent staining and confocal imaging of endogenous PSI protein, costained with ELAV (a pan-neuronal marker) in a wild-type Canton S Drosophila third-instar larval brain. Shown are two single confocal planes of one brain lobe and an eye disk. White arrows indicate clusters of neuronal precursor cells. (C) Immunofluorescence staining and confocal imaging of endogenous PSI protein (costained with ELAV) in a Canton S Drosophila embryo. Ventral view, anterior is to the top. Two stacks of consecutive confocal planes are shown. (Magnification: A, 100×; B and C, 60×.)
The PSI–U1 snRNP Interaction Coordinates a Pre-mRNA Splicing Network in the Brain for Male Courtship Behavior Regulation.
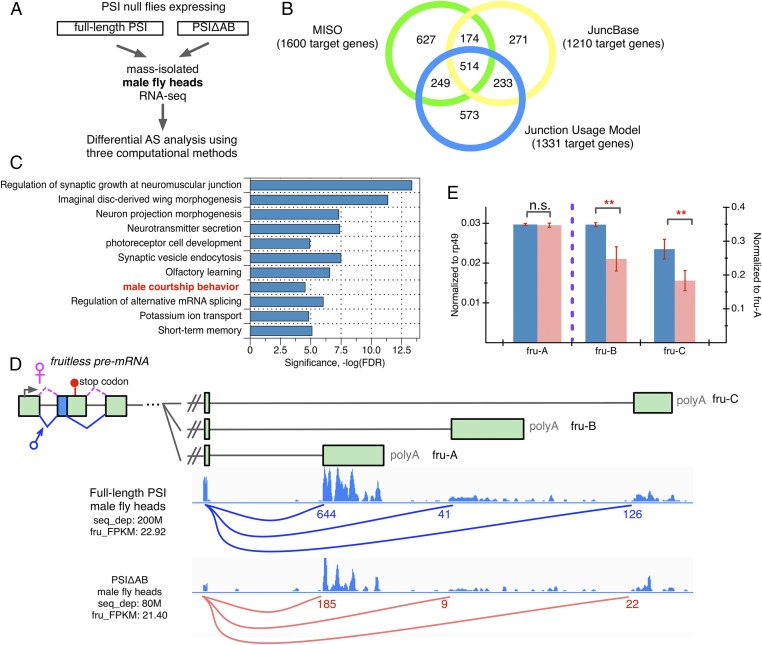
To address how the presence or absence of the U1 snRNP-interacting PSI AB domain can affect specific phenotypes related to Drosophila male courtship behavior, we compared neuronal pre-mRNA AS patterns between full-length PSI and mutant PSIΔAB transgenic flies. We prepared mRNA from mass-isolated Drosophila heads from PSI and PSIΔAB adult males and carried out standard mRNA-seq assays (Fig. 3A). To detect, compare, and quantitate differences in pre-mRNA splicing patterns between the full-length PSI and the PSIΔAB mutant, we used three different computational methods [MISO (12), JuncBase (13), and the Junction Use Model (JUM) (14)] (Fig. 3B, Fig. S2, and Datasets S1–S3). Among them, JUM does not depend on any prior knowledge of genome annotation and is especially important and useful here for AS analysis in the nervous system, where extensive and largely unannotated splicing events occur (Materials and Methods). This quantitative comparison revealed 1,170 genes (identified by at least two independent computational methods) whose mRNA transcript splicing patterns were altered in PSIΔAB flies versus the full-length PSI strain. Remarkably, Gene Ontology (GO) analysis (15) of these PSIΔAB splicing target genes showed functional enrichment, particularly in male courtship behavioral regulation (compared with the 12,163 expressed genes in fly heads), in addition to many neuronal functions, including dendrite/axon morphogenesis, neurotransmitter transport/reception, learning, and memory (Fig. 3C, Table 1, and Dataset S1). These results correlate well with the phenotypes that we observe in PSIΔAB flies and indicate that the PSI–U1 snRNP interaction plays a crucial role in maintaining normal male courtship behavior by ensuring correct AS patterns in the brain.
Fig. 3.
Disruption of the normal PSI-U1 snRNP interaction changes the AS of gene transcripts functionally enriched for male courtship behavioral regulation, including fruitless, the master courtship regulator gene. (A) Flowchart of the procedure used to compare the transcriptome-wide AS patterns in male fly heads from the PSIΔAB fly strain versus full-length PSI. (B) Summary of identified differentially spliced gene transcripts in PSIΔAB male fly heads versus full-length PSI strain by three independent computational methods, MISO (12) (green circle), JuncBase (13) (yellow circle), and JUM (14) (blue circle). See also Fig. S2 and Datasets S1–S3. (C) GO analysis of the AS target gene transcripts of PSIΔAB. Categories of related genes are listed at the left and enrichment significance (−log FDR) is indicated along the x axis. (D) The PSIΔAB mutant affects the AS of the three male-specific fruitless mRNA isoforms that are functionally distinct for maintaining normal male courtship behavior (cruciality fru-C > fru-B > fru-A) (17). Here the 5′ and 3′ ends of the fruitless pre-mRNA are shown. Green rectangles represent exons and black lines introns. The male-specific fruitless mRNA isoforms use an alternative 3′SS in the second exon and thus bypass a premature stop codon (shown by the stop sign) that is exclusively included in female fruitless isoforms, resulting in only male-specific transcripts encoding functional proteins. RNA-seq tracks (34) from PSIΔAB and full-length PSI male fly heads are shown below. Arcs represent the splice junctions specific for fru-A, fru-B, and fru-C, respectively, with the number of reads mapped to each junction shown. The overall expression of fruitless stays the same for PSIΔAB and wild-type samples, as shown by the fragments per kilobase per million (FPKM) value. (E) RT-qPCR verification of the AS of fru-A, -B, and -C in PSIΔAB male fly head (pink) versus full-length PSI (blue). RNA level of fru-A normalized to rp49 (y axis on the left) and level of fru-B and fru-C normalized to fru-A (y axis on the right). Mean of male head RNA samples from three separate PSIΔAB or full-length PSI fly lines with ±SE is shown. Significance was analyzed by one-way ANOVA test. n.s., not significant; **P value < 0.02, statistically significant.
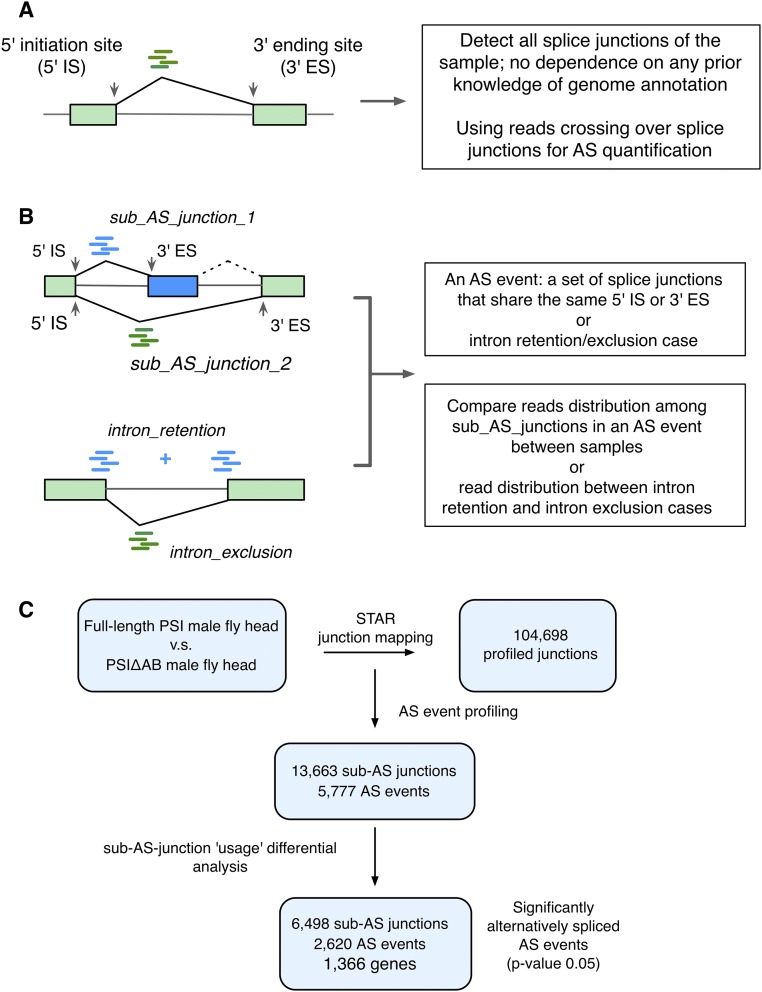
Fig. S2.
The computational pipeline for analyzing differential AS profiles using the JUM method. See Materials and Methods for details. (A) The JUM uses reads that cross over splice junctions for AS detection and quantification. (B) The JUM defines a nonintron-retention AS event as a set of splice junctions that share the same 5′IS or 3′ES. The use of each subjunction (read distribution to each subjunction) in the AS event is then compared among different experimental samples. For intron retention, read distribution between the retention and intron-exclusion cases are compared among different biological samples. (C) Flowchart of the JUM computational analysis to identify significantly changed AS targets of PSIΔAB from RNA-seq samples of full-length PSI and PSIΔAB mutant fly heads.
Table 1.
List of JUM-identified gene transcripts that present significantly altered AS patterns in the transgenic PSIΔAB vs. full-length PSI male fly heads that have been implicated in the regulation of male courtship behavior in Drosophila
| Gene symbol | Gene full name | Type of AS changes |
| fne | Found in neurons | Alternative 5′SS |
| fru | Fruitless | Alternative 3′SS |
| cac | Cacophony | Cassette exon |
| dlg1 | Discs large 1 | Cassette exon |
| Alternative 5′SS | ||
| Adar | Adenosine deaminase acting on rna | Cassette exon |
| Alh | Alhambra | Alternative 3′SS |
| slo | Slowpoke | Cassette exon |
| Alternative 5′SS | ||
| para | Paralytic | Cassette exon |
| Alternative 5′SS | ||
| Alternative 3′SS | ||
| Intron retention | ||
| Nrg | Neuroglian | Alternative 3′SS |
| orb2 | cg43782 gene product from transcript cg43782-rh | Alternative 5′SS |
| Alternative 3′SS | ||
| CASK | Cask ortholog | Cassette exon |
| Alternative 5′SS | ||
| pros | Prospero | Alternative 5′SS |
| Fmr1 | cg 6203 gene product from transcript cg6203-rc | Alternative 5′SS |
| Moe | Moesin | Cassette exon |
| Alternative 5′SS | ||
| qtc | Quick-to-court | Alternative 5′SS |
| Cassette exon | ||
| CaMKII | Calcium/calmodulin-dependent protein kinase ii | Cassette exon |
| lig | Lingerer | Cassette exon |
| 5-HT7 | Serotonin receptor 7 | Alternative 5′SS |
| egh | Egghead | Alternative 5′SS |
Gene transcripts that also have enriched PSI iCLIP tags are indicated by boldface type. The specific AS patterns are shown for each gene.
Interestingly, the male-specific transcripts of the fruitless gene are among the prominent AS targets of the PSIΔAB mutant (Fig. 3D and Table 1). The fruitless gene is a well-known master transcriptional regulator that determines the development and function of the neural circuit for fly courtship behavior through the alternative, sex-specific splicing of its mRNA transcripts (16). The sex-specific AS of the second exon in the fruitless pre-mRNA ensures that only male-specific transcripts encode functional fruitless proteins that determine courtship behavior (16) (Fig. 3D). Specifically, AS further generates three functionally distinct male-specific fruitless transcripts (termed fru-A, fru-B, and fru-C), whose relative levels are critical in maintaining normal male courtship behavior (Fig. 3D) (17). Each of the three transcripts differs from the others by only the last coding exon that encodes distinct zinc finger DNA binding domains, respectively, so that the fru-A, fru-B, and fru-C proteins can each potentially affect the expression of distinct sets of downstream target genes (17). Among them, the fru-C mRNA isoform was found to be the most important for normal male courtship behavior, fru-B less crucial than fru-C, and fru-A the least important (17). Remarkably, the levels of fru-C and fru-B among the three isoforms significantly decreased in the PSIΔAB male fly heads compared with the full-length PSI, consistent with the observed courtship behavior defects (Fig. 3 D and E). Notably, fruitless is only expressed in a distinct set of ∼2,000 neurons in the Drosophila brain, which compose the regulatory modules of the neural circuit for courtship control (18). These results indicate that although the PSI protein is broadly expressed throughout the fly brain, it plays crucial roles in the functional processing of key regulatory transcripts (like fruitless) that are specific to a subset of functionally important neurons and thus can impact specific fly behaviors. Besides fruitless, splicing pattern changes for other gene transcripts were identified (Fig. 3C, Table 1, and Dataset S1) and could also have contribute to the physiology associated with the male courtship behavior defects.
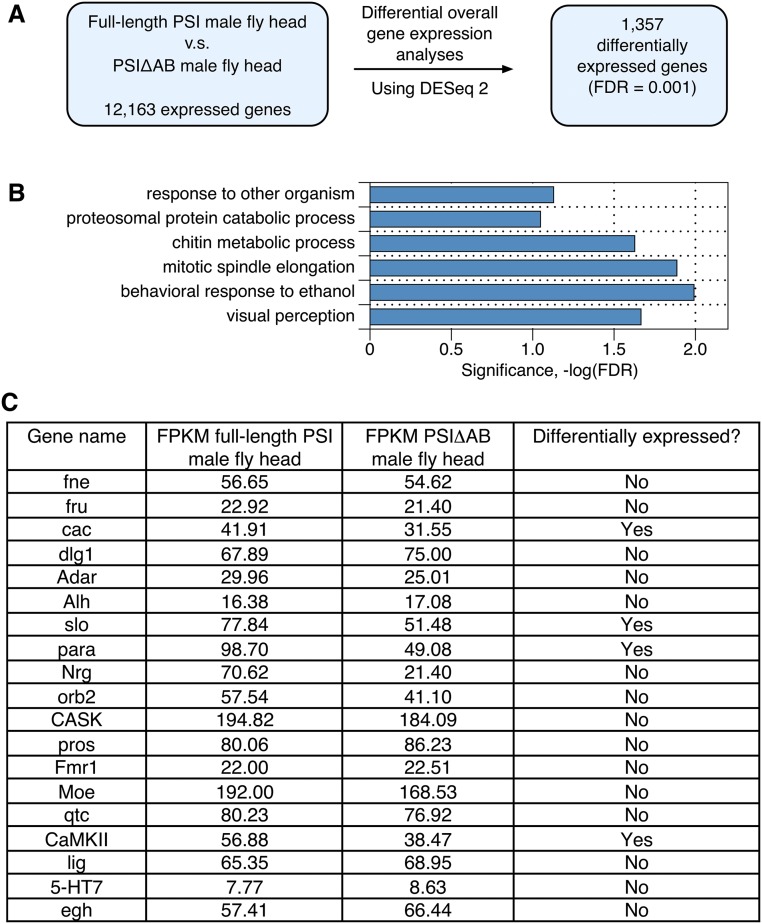
In addition, we investigated the overall gene-expression level changes in transcripts between the PSIΔAB mutant and wild-type fly heads and compared the results with the differential AS analyses using DESeq2 (19). The profile of differentially expressed genes is vastly different from the profile of differentially alternatively spliced gene transcripts (Fig. S3 and Dataset S4). The majority of courtship-associated gene transcripts that present significant AS pattern changes in the PSIΔAB mutant male heads maintain the same overall gene-expression transcript levels in the mutant and wild-type flies (Fig. S3C). GO analyses of the 1,357 identified differentially expressed genes showed only a slight functional enrichment in visual perception, mitotic spindle elongation, chitin metabolic processes, and behavioral responses to ethanol (Fig. S3B). Thus, we associate the phenotypic courtship behavior defects observed in the PSIΔAB mutant males mostly with the alternative pre-mRNA splicing changes in the fly brain.
Fig. S3.
Differential overall gene-expression level analyses in full-length PSI male fly heads and PSIΔAB male fly heads. (A) Flowchart of differential gene-expression level analyses in full-length PSI and PSIΔAB mutant fly heads using DESeq2. (B) GO analysis of the differentially expressed genes in PSIΔAB male fly head compared with full-length PSI. Categories of related genes are listed at the left and enrichment significance (−log FDR) is indicated along the x axis. (C) The FPKM values (normalized overall gene-expression level, fragments per kilobase per million) of all of the differentially alternatively spliced male courtship regulatory genes. Almost all of the gene transcripts maintain the same overall expression level in full-length PSI and PSIΔAB male fly heads.
Targeting of PSI to Key Courtship Behavior Regulatory Gene Transcripts Is Closely Associated with U1 snRNP Binding.
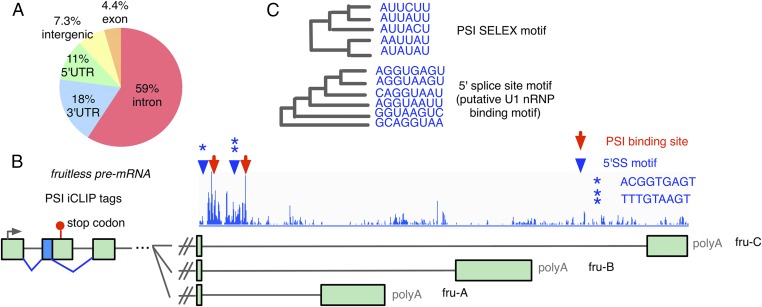
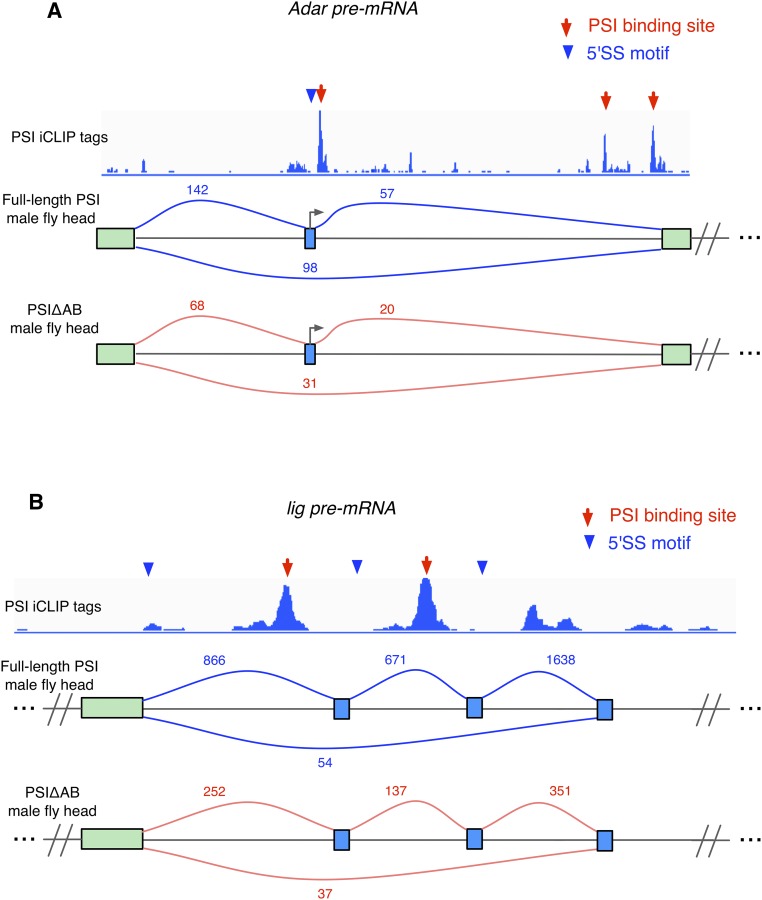
To test if the identified, differentially spliced, transcripts in PSIΔAB fly heads are direct targets bound by PSI, we performed PSI iCLIP (individual-nucleotide resolution cross-linking and immunoprecipitation) experiments (20) with nuclear extracts prepared from Drosophila Schneider Line-2 (S2) cells. Using a statistical method adapted from previous applications of HITS-CLIP (21) and iCLIP (22) (Materials and Methods), we identified 4,937 PSI binding sites in transcripts from 1,628 genes (Table S1 and Dataset S5). The majority (60%) of the PSI binding sites reside in introns (Fig. 4A). From the JUM-identified 1,331 genes whose transcripts are differentially spliced in PSIΔAB fly heads, 543 genes have transcripts with significantly enriched PSI iCLIP tags, indicating that they are directly bound by PSI. Remarkably, half of the male courtship regulatory genes that experience significant AS changes in PSIΔAB fly heads have enriched PSI iCLIP tags in their transcripts (Table 1). Interestingly, the fruitless pre-mRNA is a direct target of PSI. There are two prominent PSI binding sites that were identified near the common upstream 5′SS that is alternatively spliced to the three 3′ terminal exons from the male-specific fru-A, -B, and -C isoforms (Fig. 4B, red arrows). Furthermore, motif analysis in the vicinity of the identified PSI binding sites revealed significant enrichment for two major sequence motif categories: the A/CUU binding motif of PSI that had been previously identified by SELEX (23) and, remarkably, the 5′SS-like sequences (CAG/GTAAGT) that are known to be putative U1 snRNP binding sites (3, 8) (Fig. 4C). These results revealed a close association of PSI and U1 snRNP binding to the neuronal transcriptome. Indeed, two 5′SS or 5′SS-like motifs were identified that reside close to the two PSI binding sites in the fruitless pre-mRNA, upstream of the three 3′ terminal exons (Fig. 4B, blue arrowheads). This result indicates that PSI binding may affect U1 snRNP targeting to different 5′SS in fruitless pre-mRNA and affect interactions of U1 snRNP with the three alternative 3′SS to control AS of the fru-A, -B, and -C isoforms that are known to modulate fly courtship behavior. As in the case of PSI regulating Drosophila P-element transposon pre-mRNA splicing, it is likely that PSI plays a general role in selectively targeting U1 snRNP to specific sites in the nuclear transcriptome through its interaction with U1 snRNP, which may influence U1 snRNP-3′SS interactions and hence control the processing and functions of target gene transcripts that are crucial for various Drosophila activities. Besides fruitless, eight other differentially spliced male courtship regulatory gene transcripts also have significant enrichment of PSI CLIP tags that are within the alternative splicing regions, and close to 5′SS-like motifs (Table 1 and Fig. S4). These results further associate the binding of PSI to the transcriptome with the correct positioning of U1 snRNP, as well as the pre-mRNA alternative splicing decisions for maintaining normal animal physiology.
Table S1.
Summary of PSI iCLIP data analysis in Drosophila S2 cells transcriptome-wide
| Sample | Read no. | Uniquely mapped reads, or tags (using Novoalign) | Unique tags (collapsed from PCR-bias) | Unique tags without any mutation | Significantly enriched cross-linking sites identified (P value 0.01) |
| PSI-iCLIP-rep1 | 3,962,179 | 2,637,621 (66.6%) | 1,446,095 | 1,175,219 | 2,930 |
| PSI-iCLIP-rep2 | 6,518,432 | 4,571,376 (70.1%) | 1,058,152 | 804,557 | 2,564 |
Fig. 4.
PSI binding to the transcriptome is closely associated with the U1 snRNP binding motifs. (A) Pie chart showing the distribution of iCLIP-identified PSI binding sites in different genomic regions. See also Table S1 and Dataset S5. (B) PSI iCLIP tag tracks are shown on the fruitless pre-mRNA. Red arrows showing identified enriched PSI binding sites and blue arrowheads showing putative 5′SS motifs. The sequences of the two putative 5′SS motifs are shown (blue stars). (C) Enriched 6-mer sequence motifs (Upper) and 8-mer motifs (Lower) near PSI iCLIP-derived binding sites transcriptome-wide.
Fig. S4.
PSI binding to courtship regulatory genes whose transcripts experience significant AS changes in PSIΔAB male fly heads compared with full-length PSI. PSI iCLIP tag tracks are shown for two additional courtship regulatory genes whose transcripts experience significant AS changes in PSIΔAB male fly head. (A) Adar pre-mRNA; (B) lig pre-mRNA. Red arrows show identified enriched PSI binding sites and blue arrowheads show putative 5′SS motifs.
Table S2.
Summary of the RNA-seq data analysis for transgenic PSIΔAB male fly heads versus full-length PSI male fly heads
| Sample | Paired-end read no. | Uniquely mapped, properly paired reads [using STAR (35)] |
| PSIΔAB rep1 male fly heads | 57,717,914 | 47,365,626 (82.06%) |
| PSIΔAB rep2 male fly heads | 86,608,558 | 59,485,684 (68.68%) |
| Full-length PSI male fly heads | 205,576,030 | 176,458,018 (85.84%) |
Discussion
AS patterns are often controlled by the interaction of RNA binding proteins (RBPs) with nascent pre-mRNA transcripts (24, 25). These RNA–protein interactions can determine where the spliceosomal U1 and U2 snRNPs bind to the transcriptome, and thus dictate AS decisions and constitute an important mechanism for gene regulation (3, 24, 25). RBPs, such as PSI (6) or TIA-1 (26–28), which directly interact with U1 snRNP, are good candidates for proteins controlling AS patterns in this manner, and changes in these RBP–snRNP associations can have profound phenotypic effects. For example, we show here that a subtle mutation that abolishes the PSI–U1 snRNP interaction dramatically changed the AS patterns of hundreds neuronal pre-mRNAs and resulted in highly abnormal male courtship behaviors. Given the diverse number of cell types, gene-expression patterns, and the extensive AS that occurs in animal nervous systems, we anticipate that AS regulation will play critical roles in both the normal physiological or disease states of neurons.
The PSI–U1 snRNP interaction may further play crucial roles in other pre-mRNA processing pathways. For example, U1 snRNP was recently ascribed a new function in regulating global mRNA 3′ end termination and suppression of premature pre-mRNA cleavage and polyadenylation near the 5′ ends of transcripts in humans, mice, and Drosophila through selective binding to 5′SS-like motifs, a process called telescripting (10). It has remained a mystery how U1 snRNP discriminates the numerous potential 5′SS sites across the transcriptome. PSI may be one example of RBP regulators that alter the binding of U1 snRNP to pre-mRNA sites through direct protein–protein interactions, and thus changing pre-mRNA splicing, polyadenylation, or other pre-mRNA processing patterns.
Our findings further reveal that even broadly expressed RBPs, such as PSI, can affect gene regulation in restricted subsets of neurons in the Drosophila brain that modulate specific behaviors, such as courtship and mating. The work presented here also provides the first identification of the PSI protein as a transacting RNA splicing factor controlling male-specific fruitless splicing.
Taken together, our results link the molecular interaction between PSI and U1 snRNP to specific phenotypic effects on Drosophila courtship behavior through the coordination of an AS program in the brain. We believe these results provide important insights into the mechanisms controlling gene activity in the nervous system, leading to the precise control of complex animal behaviors.
Materials and Methods
Preparation of RNA-seq Libraries from Drosophila Head RNA from Transgenic PSIΔAB and Full-Length PSI Fly Strain Males.
Drosophila heads were isolated from 50 mL of manually sorted and frozen males from the v16/v16 PSI mutant fly strain (PSI-null) carrying either the PSI genomic-cDNA wild-type or PSIΔAB transgenes (7), as described previously (29). Total RNA was isolated using Qiagen RNA Easy kits and polyA (+) RNA was purified using oligo (dT) Dynabeads (Life Technologies). Next, 100 ng of polyA (+) RNA was used to generate random-primed nondirectional cDNA libraries suitable for sequencing on the Illumina platform using the NEBNext mRNA Sample Prep kit (cat # E6110S; New England Biolabs). Libraries were size-selected on high-resolution agarose gels (Bio-Rad), analyzed on an Agilent Bioanalyzer and sequenced on an Illumina HiSeq2500 using 100-bp paired-end reads.
iCLIP Experiment on Nuclear RNA Binding of the PSI Protein.
Nuclear extracts from Drosophila Schneider Line-2 (S2) cells were prepared from UV-irradiated S2 cells, as described previously (20, 30). Standard iCLIP assays were performed as previously described (20, 30). UV–cross-linked nuclear extracts were subjected to immunoprecipitation with anti-PSI rabbit antibodies (31, 32) and processed for iCLIP exactly as described previously (22), except that the cDNA was electroeluted from the gel slices as described for PAR-CLIP (33). Libraries were prepared from the cyclized cDNA, as described previously (22), and sequenced on an Illlumina HiSEq. 2000 using 50-bp reads. For more details see SI Materials and Methods.
Brain Dissection and Immunostaining of Endogenous PSI Protein in the Fly Brain.
Embryos from an overnight collection, brains from third-instar larvae as well as 1-wk-old male and female flies were prepared and stained following standard procedures. Immunostaining with anti-PSI antibody (31, 32) and a rat anti-ELAV antibody (Developmental Studies Hybridoma Bank).
Behavioral Analysis.
Seven courting pairs from each of the PSIΔAB and PSI full-length strains were imaged for 17 min at 100 Hz, resulting in 700,000 frames per strain. Digital image analysis was used to segment the male fly from the images and a behavioral mapping analysis was performed as described previously (11). Representative videos from each of the discovered stereotyped behaviors were investigated and known courtship behaviors were categorized as described in the text.
Differential Alternative Splicing Analyses of the RNA-seq Data, PSI iCLIP, and Motif Analyses.
For differential alternative splicing analyses of the RNA-seq data, PSI iCLIP, and motif analyses, see SI Materials and Methods.
SI Materials and Methods
iCLIP Experiment on Nuclear RNA Binding of the PSI Protein.
Nuclei were isolated from suspension cultures of S2 cells by hypotonic lysis and Dounce homogenization. Nuclei were resuspended in isotonic buffer and irradiated on ice with 150 mJ/cm2 at 254 nm in a Stratalinker 2400. Lysis buffer and high-salt wash buffer used were as follows. Lysis buffer: 50 mM Tris⋅HCl, pH 7.4, 100 mM NaCl, 1% Igepal CA-630, 0.1% SDS, 0.5% sodium deoxycholate; on the day of iCLIP: 1/100 volume of protease inhibitor mixture. High-salt wash: 50 mM Tris⋅HCl, pH 7.4, 1 M NaCl, 1 mM EDTA, 1% Igepal CA-630, 0.1% SDS, 0.5% sodium deoxycholate.
Protein Immunoblotting.
Drosophila head proteins were extracted with SDS-gel sample buffer. Proteins were separated on SDS/PAGE minigels and electroblotted to nitrocellulose membranes. Nitrocellulose membranes were probed with polyclonal rabbit anti-PSI and anti-hrp48 primary antibodies (31, 32) diluted in 1× PBST with 5% nonfat milk. A 1:10,000 dilution of goat anti-rabbit hrp-conjugated IgG (Bio-Rad) was use for detection by ECL (Bio-Rad) and imaged on a Bio-Rad ChemDoc station.
RNA Extraction and qRT-PCR.
Drosophila heads were isolated from manually sorted and flash-frozen male flies of the appropriate genotype. RNA was extracted using Qiazol (Qiagen), as per the manufacturer’s instructions. cDNA was obtained from 500 ng of total RNA using the QuantiTect kit (Invitrogen). The following real-time PCR primers were designed using the software Primer3 (SourceForge): primers for fru-A—F-acgttgcccactctataccg and R-ctctgcggtcgatggatatt; primers for fru-B—F-gccttttcgaaccattttga and R-cgactgcgaaaatgctgtta; and primers for fru-C—F-agttgcgagtcgaggagaag and R-ccttcgaaacggtttgttgt.
Computational Analysis of the Differentially Alternatively Spliced Pre-mRNA Profiles in PSIΔAB and Full-Length PSI Male Fly Heads.
RNA-seq reads were mapped to the Drosophila genome (dm3) using STAR (35) with the two-pass mode for the most sensitive novel junction discovery and mapping reads to splice junctions. Of the reads, 70–86% were mapped uniquely to the genome and are properly paired.
MISO and JuncBase analyses were performed as described previously (12, 13). The JUM (14) analysis is described in detail below. Among the three methods, MISO provides robust statistical comparison of differential AS only on known AS events (MISO provides a pre-annotated AS event database as part of the package). JuncBase can analyze both known and novel AS events, but users have to provide a pre-annotated exon coordinate file. Here we provided a de novo assembled genome annotation file created by Cufflinks (36). Both current versions of MISO and JuncBase perform pairwise comparisons between samples but do not take biological replicates into the statistics construction. We developed a computational method called the Junction Use Model (JUM) to identify differentially alternatively spliced pre-mRNA transcripts in different biological samples. The JUM method detects both known and novel splicing junctions in the biological samples, with no dependence on any prior knowledge of genome annotation. Here, JUM uses RNA-seq reads that cross over splice junctions for AS quantification, as these reads are the most direct evidence for the existence of the splice junctions (Fig. S2A). For each junction, we define the last nucleotide position of the upstream exon as the 5′ initiation site (5′IS) of that junction, and the first nucleotide position of the downstream exon as the 3′ ending site (3′ES) of that junction. In a nonintron-retention case, we define an AS event as a set of splice junctions that share the same 5′IS or the same 3′ES, with each splice junction in the set defined as a sub–AS-junction of that AS event (Fig. S2B, Upper). To quantify differential splicing of the AS event between different conditions, we counted reads that were mapped to each sub–AS-junction in each condition and compared the read distribution in sub–AS-junctions among different biological samples. In an intron-retention case, an AS event is defined as the combination of the intron-retention and intron-exclusion cases (Fig. S2B, Lower). We then counted and compared reads that were mapped to the splice junction (evidence for intron exclusion) and the reads that map to the two exon–intron junctions right upstream and downstream of the intron (evidence for intron inclusion) in each condition. The above definition for an AS event and its quantification covers all seven categories of AS types. For the statistical analysis of the differential AS, JUM applies the core algorithm developed previously (37) to incorporate variability among biological replicates to generate robust statistical tests. Such a method of statistics has proven to be beneficial in the many applications of differential gene-expression and AS analysis packages, such as EdgeR (38), DESeq. (19), and DEXSeq. (39).
Note that using the JUM method that does not depend on any prior knowledge of genome annotation is crucial for AS analysis in the nervous system, where extensive and largely unannotated splicing events occur. For example, the AS of fruitless male-specific mRNA transcripts fru-A, fru-B, and fru-C is not included in the pre-annotated AS database of MISO, and can only be detected using novel AS event finding algorithms, such as JUM.
Computational Analyses of the PSI iCLIP Data.
Reverse transcription following the iCLIP procedure mostly terminates at the covalently cross-linked nucleotide in the target RNA fragment. However, occasionally the reaction will extend across the RNA-protein cross-linking site and usually leave behind a mutation at the cross-linking site, be it deletion, substitution, or insertion. We first performed cross-link–induced mutation sites analysis, as described previously (21), to identify all unique iCLIP tags that contain deletions, insertions, or substitutions. We then filtered these reads out and only used unique iCLIP tags that do not contain any mutations and define the −1 site of each tag as the cross-linking site. Finally, we performed permutation-based procedures, as described previously (40), to profile the cross-linking sites that have statistically enriched iCLIP tags compared with the background in the transcriptome.
Motif Analyses of Sequences in the Vicinity of PSI Binding Sites Identified by iCLIP.
Sequences 15-bp upstream and downstream of each identified single-nucleotide PSI cross-linking site are taken for motif analyses. The occurrence of all possible k-mers (k = 6–10) were counted in these PSI binding-region sequences, as well as in the whole Drosophila genome as background. The enrichment of each k-mer in the PSI-binding regions was then calculated by performing Fisher’s exact test.
Supplementary Material
Acknowledgments
Transgenic PSI wild-type and PSIΔAB mutant male flies in the PSI deletion v16/v16 mutant background were outcrossed and sorted while D.C.R. was on sabbatical at the Howard Hughes Medical Institute Janelia Research Campus. We thank Todd Laverty and the Janelia Drosophila core facility for fly food and microscope space; Arnim Jenett and the FlyLight Project and the imaging core facility at the Howard Hughes Medical Institute Janelia Research Campus for help with high-resolution confocal imaging; and Mike Levine for critical comments on the manuscript. This work was supported by National Institutes of Health (NIH) Grants R01 GM094890 and R01 GM097352 (to D.C.R.) and R01 GM098090 (to J.W.S.); Center for RNA Systems Biology at University of California, Berkeley, NIH Grant P50102706 (J. Cate, PI); NIH Grant T32 HG003284 (to U.K.); and a fellowship from the Deutsche Forschungsgemeinschaft (V.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE79916).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600936113/-/DCSupplemental.
References
- 1.Wang ET, et al. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456(7221):470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q, Lee JA, Black DL. Neuronal regulation of alternative pre-mRNA splicing. Nat Rev Neurosci. 2007;8(11):819–831. doi: 10.1038/nrn2237. [DOI] [PubMed] [Google Scholar]
- 3.Wahl MC, Will CL, Lührmann R. The spliceosome: Design principles of a dynamic RNP machine. Cell. 2009;136(4):701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Raj B, Blencowe BJ. Alternative splicing in the mammalian nervous system: Recent insights into mechanisms and functional roles. Neuron. 2015;87(1):14–27. doi: 10.1016/j.neuron.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Siebel CW, Fresco LD, Rio DC. The mechanism of somatic inhibition of Drosophila P-element pre-mRNA splicing: Multiprotein complexes at an exon pseudo-5′ splice site control U1 snRNP binding. Genes Dev. 1992;6(8):1386–1401. doi: 10.1101/gad.6.8.1386. [DOI] [PubMed] [Google Scholar]
- 6.Labourier E, Adams MD, Rio DC. Modulation of P-element pre-mRNA splicing by a direct interaction between PSI and U1 snRNP 70K protein. Mol Cell. 2001;8(2):363–373. doi: 10.1016/s1097-2765(01)00311-2. [DOI] [PubMed] [Google Scholar]
- 7.Labourier E, Blanchette M, Feiger JW, Adams MD, Rio DC. The KH-type RNA-binding protein PSI is required for Drosophila viability, male fertility, and cellular mRNA processing. Genes Dev. 2002;16(1):72–84. doi: 10.1101/gad.948602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buratti E, Baralle D. Novel roles of U1 snRNP in alternative splicing regulation. RNA Biol. 2010;7(4):412–419. doi: 10.4161/rna.7.4.12153. [DOI] [PubMed] [Google Scholar]
- 9.Kaida D, et al. U1 snRNP protects pre-mRNAs from premature cleavage and polyadenylation. Nature. 2010;468(7324):664–668. doi: 10.1038/nature09479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berg MG, et al. U1 snRNP determines mRNA length and regulates isoform expression. Cell. 2012;150(1):53–64. doi: 10.1016/j.cell.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berman GJ, Choi DM, Bialek W, Shaevitz JW. Mapping the stereotyped behaviour of freely moving fruit flies. J R Soc Interface. 2014;11(99):20140672. doi: 10.1098/rsif.2014.0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz Y, Wang ET, Airoldi EM, Burge CB. Analysis and design of RNA sequencing experiments for identifying isoform regulation. Nat Methods. 2010;7(12):1009–1015. doi: 10.1038/nmeth.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks AN, et al. Conservation of an RNA regulatory map between Drosophila and mammals. Genome Res. 2011;21(2):193–202. doi: 10.1101/gr.108662.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q, Moore MJ, Adelmant G, Marto JA, Silver PA. PQBP1, a factor linked to intellectual disability, affects alternative splicing associated with neurite outgrowth. Genes Dev. 2013;27(6):615–626. doi: 10.1101/gad.212308.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z. GOrilla: A tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics. 2009;10:48. doi: 10.1186/1471-2105-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demir E, Dickson BJ. fruitless splicing specifies male courtship behavior in Drosophila. Cell. 2005;121(5):785–794. doi: 10.1016/j.cell.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 17.von Philipsborn AC, et al. Cellular and behavioral functions of fruitless isoforms in Drosophila courtship. Curr Biol. 2014;24(3):242–251. doi: 10.1016/j.cub.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto D, Koganezawa M. Genes and circuits of courtship behaviour in Drosophila males. Nat Rev Neurosci. 2013;14(10):681–692. doi: 10.1038/nrn3567. [DOI] [PubMed] [Google Scholar]
- 19.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huppertz I, et al. iCLIP: Protein-RNA interactions at nucleotide resolution. Methods. 2014;65(3):274–287. doi: 10.1016/j.ymeth.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore MJ, et al. Mapping Argonaute and conventional RNA-binding protein interactions with RNA at single-nucleotide resolution using HITS-CLIP and CIMS analysis. Nat Protoc. 2014;9(2):263–293. doi: 10.1038/nprot.2014.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.König J, et al. iCLIP reveals the function of hnRNP particles in splicing at individual nucleotide resolution. Nat Struct Mol Biol. 2010;17(7):909–915. doi: 10.1038/nsmb.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amarasinghe AK, MacDiarmid R, Adams MD, Rio DC. An in vitro-selected RNA-binding site for the KH domain protein PSI acts as a splicing inhibitor element. RNA. 2001;7(9):1239–1253. doi: 10.1017/s1355838201010603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee Y, Rio DC. Mechanisms and regulation of alternative pre-mRNA splicing. Annu Rev Biochem. 2015;84:291–323. doi: 10.1146/annurev-biochem-060614-034316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen M, Manley JL. Mechanisms of alternative splicing regulation: insights from molecular and genomics approaches. Nat Rev Mol Cell Biol. 2009;10(11):741–754. doi: 10.1038/nrm2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Förch P, Puig O, Martínez C, Séraphin B, Valcárcel J. The splicing regulator TIA-1 interacts with U1-C to promote U1 snRNP recruitment to 5′ splice sites. EMBO J. 2002;21(24):6882–6892. doi: 10.1093/emboj/cdf668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu Y, et al. Dynamic regulation of alternative splicing by silencers that modulate 5′ splice site competition. Cell. 2008;135(7):1224–1236. doi: 10.1016/j.cell.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izquierdo JM, et al. Regulation of Fas alternative splicing by antagonistic effects of TIA-1 and PTB on exon definition. Mol Cell. 2005;19(4):475–484. doi: 10.1016/j.molcel.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Emery P. RNA extraction from Drosophila heads. Methods Mol Biol. 2007;362:305–307. doi: 10.1007/978-1-59745-257-1_20. [DOI] [PubMed] [Google Scholar]
- 30.Bradley T, Cook ME, Blanchette M. SR proteins control a complex network of RNA-processing events. RNA. 2015;21(1):75–92. doi: 10.1261/rna.043893.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siebel CW, Kanaar R, Rio DC. Regulation of tissue-specific P-element pre-mRNA splicing requires the RNA-binding protein PSI. Genes Dev. 1994;8(14):1713–1725. doi: 10.1101/gad.8.14.1713. [DOI] [PubMed] [Google Scholar]
- 32.Siebel CW, Admon A, Rio DC. Soma-specific expression and cloning of PSI, a negative regulator of P element pre-mRNA splicing. Genes Dev. 1995;9(3):269–283. doi: 10.1101/gad.9.3.269. [DOI] [PubMed] [Google Scholar]
- 33.Hafner M, et al. PAR-CliP—A method to identify transcriptome-wide the binding sites of RNA binding proteins. J Vis Exp. 2010;(41):pii 2034. doi: 10.3791/2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson JT, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29(1):24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobin A, et al. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trapnell C, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28(5):511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson MD, Smyth GK. Moderated statistical tests for assessing differences in tag abundance. Bioinformatics. 2007;23(21):2881–2887. doi: 10.1093/bioinformatics/btm453. [DOI] [PubMed] [Google Scholar]
- 38.Robinson MD, McCarthy DJ, Smyth GK. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anders S, Reyes A, Huber W. Detecting differential usage of exons from RNA-seq data. Genome Res. 2012;22(10):2008–2017. doi: 10.1101/gr.133744.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weyn-Vanhentenryck SM, et al. HITS-CLIP and integrative modeling define the Rbfox splicing-regulatory network linked to brain development and autism. Cell Reports. 2014;6(6):1139–1152. doi: 10.1016/j.celrep.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.