Significance
Infants born prematurely suffer the greatest incidence of and impact from sepsis among all age groups. Therapeutic interventions aimed at reducing morbidity and mortality in this vulnerable population have been unsuccessful. Interleukin (IL)-18 is a proinflammatory member of the IL-1 superfamily. Serum IL-18 concentrations in uninfected premature infants are increased as compared with healthy adults. We show that IL-18 in the setting of sepsis results in gut injury, a potentiation of the host’s inflammatory response, increased bacteremia, and mortality mediated by IL-1 receptor 1 (IL-1R1)–dependent IL-17A produced by γδT and myeloid cells. The discovery of this novel IL-18/IL-1R1/IL-17A axis brings new hope for therapeutic interventions that target downstream IL-17A and ultimately reduce the increased mortality from sepsis in this understudied population.
Keywords: IL-18, IL-17, sepsis, neonate, pathogenesis
Abstract
Interleukin (IL)-18 is an important effector of innate and adaptive immunity, but its expression must also be tightly regulated because it can potentiate lethal systemic inflammation and death. Healthy and septic human neonates demonstrate elevated serum concentrations of IL-18 compared with adults. Thus, we determined the contribution of IL-18 to lethality and its mechanism in a murine model of neonatal sepsis. We find that IL-18–null neonatal mice are highly protected from polymicrobial sepsis, whereas replenishing IL-18 increased lethality to sepsis or endotoxemia. Increased lethality depended on IL-1 receptor 1 (IL-1R1) signaling but not adaptive immunity. In genome-wide analyses of blood mRNA from septic human neonates, expression of the IL-17 receptor emerged as a critical regulatory node. Indeed, IL-18 administration in sepsis increased IL-17A production by murine intestinal γδT cells as well as Ly6G+ myeloid cells, and blocking IL-17A reduced IL-18–potentiated mortality to both neonatal sepsis and endotoxemia. We conclude that IL-17A is a previously unrecognized effector of IL-18–mediated injury in neonatal sepsis and that disruption of the deleterious and tissue-destructive IL-18/IL-1/IL-17A axis represents a novel therapeutic approach to improve outcomes for human neonates with sepsis.
Four of every 10 neonates that develop sepsis die or experience major disability, even with timely antimicrobial therapy (1). Interleukin (IL)-18 is an effector of innate immunity and a proinflammatory cytokine canonically produced by the inflammasome from its inactive precursor form (2) and known to increase polymorphonuclear neutrophil (PMN) phagocytosis, reactive oxygen species (ROS) production, and bactericidal function (3). Uninfected prematurely born infants, who suffer the greatest degree of sepsis morbidity and mortality among neonates, can have elevated serum levels of IL-18 that exceed those in healthy uninfected adults (4, 5), but how this increase may predispose these infants to adverse infectious outcomes has not been defined.
In adults, IL-18 from endogenous or exogenous sources may be deleterious by inducing hypotension (6), generating acute lung injury (7), and driving substantial inflammation (8) leading to shock, organ failure, and death (9). In contrast, some studies have shown a critical positive role for IL-18 in the adult host response to Pseudomonas pneumonia (3, 10). The survival benefits of combined IL-18/IL-1β targeting were demonstrated recently in septic shock, but that study did not investigate the primary contributor to deleterious outcomes in murine sepsis, nor did they establish the mechanism of effect (11).
Here we demonstrate that IL-18–mediated inflammation induces IL-17A production in the neonate and that disruptions of IL-18, IL-1 receptor 1 (IL-1R1), or IL-17 signaling represent novel therapeutic approaches to improve outcomes for human neonates with sepsis. First, we demonstrate a significant improvement in survival in neonatal sepsis with IL-18 gene deletion and a dose-dependent increase in lethality caused by sepsis or endotoxemia with IL-18 administration. Exogenous IL-18 challenge given to mimic the preterm human condition dramatically enhanced the murine neonatal systemic inflammatory response to sepsis, disrupted small bowel architecture, and increased i.p. bacterial load and bacteremia but did not alter the function of myeloid cells or their recruitment to the peritoneal cavity. IL-18–mediated mortality in sepsis was independent of mature B and T cells or IL-1β but was dependent on intact IL-1R1 but not IL-1β signaling. We confirmed the relevance of these murine findings in human neonates in whom strong evidence of IL-18/IL-17 pathway activation was found via whole-blood genome-wide transcriptomic profiling. Exposure to IL-18 in murine neonates with sepsis dramatically increased early plasma IL-17A protein and IL-17A mRNA in the gut (myeloid and lymphoid origin) and lung (lymphoid origin). IL-17 mRNA production in IL-18–exposed septic neonatal mice was IL-1R1–dependent in the lung and gut. Finally, we show that genetic or pharmacologic targeting of IL-17 signaling prevented the enhancement of sepsis mortality by IL-18. These data bring renewed hope for a reduction in neonatal sepsis mortality and have broad implications for our understanding of IL-18–mediated immune responses in both human and animal biology.
Results
Interleukin-18 Is a Critical Mediator of Lethality in Neonatal Sepsis.
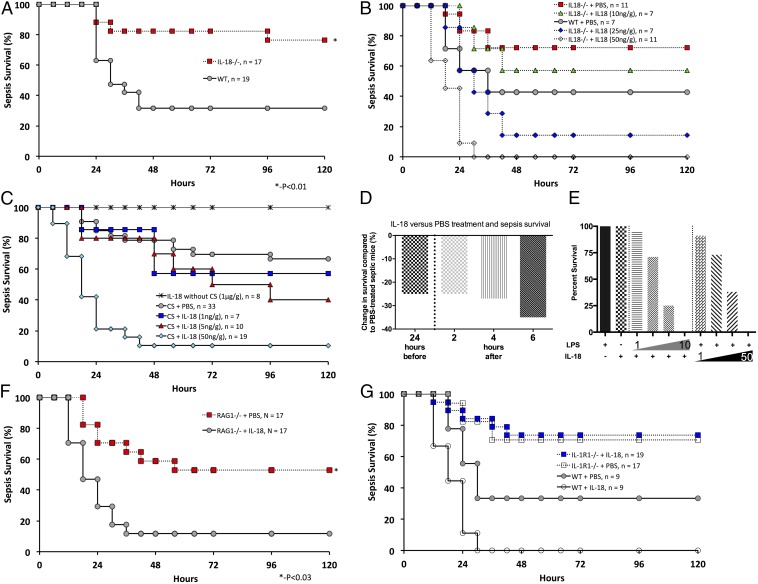
Survival among IL-18–null (IL-18−/−) neonatal mice was dramatically improved over WT neonates with polymicrobial sepsis (76% versus 32%, P < 0.05 by Fisher’s exact test) (Fig. 1A), indicating that endogenous IL-18 plays a critical adverse role in neonatal sepsis. This finding in neonates is in stark contrast to the absence of any effect of IL-18 deletion alone in adults after polymicrobial sepsis induced by cecal ligation and puncture (CLP) (12). Of note, sepsis modeling using cecal slurry (CS) results in a much more robust early systemic inflammatory response than seen with CLP; this robust response may contribute to the differences in survival seen between IL-18−/− neonates and adults (13). Administration of IL-18 protein to IL-18−/− neonates abrogated the advantage in sepsis survival (Fig. 1B).
Fig. 1.
(A) Sepsis survival for IL-18−/− (76%) and WT (32%) mice given an LD70 of CS (1.3 mg/g); *P < 0.05 by Fisher’s exact test. (B) IL-18−/− neonatal mice were concurrently challenged with an LD50 (1.0 mg/g) of CS and complementation with systemic (i.p.) IL-18 at the doses indicated or PBS. (C) Neonatal mice were concurrently challenged with an LD40 (0.9 mg/g) of CS and received IL-18 i.p. at the doses indicated or PBS. Doses of IL-18 up to 1 μg/g did not result in mortality or signs of illness. (D) WT neonatal mice were challenged with an LD70 (1.3 mg/g) of CS and received IL-18 at doses and times relative to CS challenge as indicated. Results are reported as the survival difference (%) between PBS-exposed and IL-18–exposed septic mice at 5 d after CS. Each experiment had a minimum of 10 mice per group; data shown represent combined data from at least two independent experiments. (E) I.p. administration of LPS (10 μg/g) or IL-18 (50 ng/g) alone did not result in neonatal mortality. IL-18 potentiated LPS-mediated mortality at all doses tested. (Left) Escalating LPS (1, 3, 5, 10 μg/g) with static IL-18 (50 ng/g); n ≥ 5 mice per group. (Right) Escalating IL-18 (5, 10, 25, 50 ng/g) with static LPS (10 μg/g); n ≥ 5 mice per group. All results represent combined data from at least two independent experiments. (F) RAG1−/− neonatal mice were concurrently challenged with an LD50 (1.0 mg/g) of CS and IL-18 (50 ng/g) or PBS (50 μL). RAG1−/− mice that received IL-18 had greater sepsis mortality than those that received PBS (53% vs. 12%, P = 0.03 by Fisher’s exact test). Results are representative of two independent experiments with similar results. (G) IL-1R1−/− neonatal mice were concurrently challenged with an LD70 (1.3 mg/g) of CS and either IL-18 (50 ng/g) or PBS (50 μL). IL-1R1−/− neonates did not demonstrate a decrease in sepsis survival following IL-18 administration versus PBS administration (74% vs. 71%). Results shown are combined data from two independent experiments with similar results.
Because uninfected preterm human neonates can manifest circulating concentrations of IL-18 notably higher than those in healthy adults (4), we tested the effect of increasing doses [1–50 ng/g body weight (BW)] of recombinant IL-18 in WT neonatal mice with concomitant septic challenge (Fig. 1C). IL-18 supplementation in the absence of sepsis had no effect on survival at doses up to and including 1 μg/g BW in healthy neonatal mice (Fig. 1C). Although the lowest dose of IL-18 (1 ng/g BW) decreased sepsis survival from 67% (in sham-treated septic neonates) to 57%, and highest dose of IL-18 (50 ng/g BW) decreased survival to 11% (P < 0.001 by Fisher exact test). This worsening outcome of neonatal sepsis by exogenous IL-18 in WT mice raised the question of the temporal effect of IL-18 on the progression of neonatal sepsis. Challenge with IL-18 24 h presepsis and 2, 4, and 6 h postsepsis had consistently adverse effects on survival (Fig. 1D). Because endotoxin (LPS) is the key virulence factor of Gram-negative bacteria, a common cause of lethal neonatal sepsis (14), we examined the impact of IL-18 administration on the outcome to LPS-induced endotoxic shock (Fig. 1E). Addition of IL-18 to LPS increased its lethality at all doses tested, indicating a deleterious potentiation of the inflammatory response to endotoxin. In contrast, challenging WT neonates with LPS alone at doses up to 10 μg/g BW was not associated with any mortality.
Because neonates possess an immature adaptive immune system (15), we hypothesized that the deleterious effects of IL-18 were through innate immune mechanisms. Of note, we previously showed that sepsis survival in neonates, in stark contrast to adults, is not exacerbated by deletion of the adaptive immune system [i.e., by recombination activating gene 1 (RAG1−/−) deletion] (16). We subsequently evaluated whether the deleterious effects of IL-18 would be maintained in RAG1−/− neonates. Consistent with our hypothesis, RAG1−/− neonates treated with IL-18 also demonstrated a decrease in survival compared with untreated neonates (12% versus 53%, P = 0.03 by Fisher’s exact test) (Fig. 1F), indicating that mature T or B cells are not required for IL-18–induced lethality.
Because IL-18 may increase IL-1β transcription alone (17) or in the setting of concordant inflammation (18), and the combination of IL-18 and IL-1β may be synergistically harmful (11), we examined the impact of IL-18 challenge in IL-1R1–null (IL-1R1−/−) neonatal mice with sepsis. In contrast to findings in WT mice, lethality in IL-1R1−/− neonates was not augmented by IL-18 administration (74% versus 71%) (Fig. 1G). However, IL-1β–null (IL-1β−/−) mice were not similarly protected from lethality associated with IL-18 and LPS administration (survival 16%, n = 38; WT survival 21%, n = 39, P = 0.77) or IL-18 administration during sepsis (survival 21%, n = 14; WT survival 15%, n = 13, P = 1.0) (SI Appendix, Fig. S1 A and B). Taken together, these findings illustrate that the increased IL-18–mediated mortality in sepsis is independent of mature B and T cells or IL-1β but is dependent on intact IL-1R1 signaling.
The Deleterious Mechanisms of IL-18 in Neonatal Sepsis.
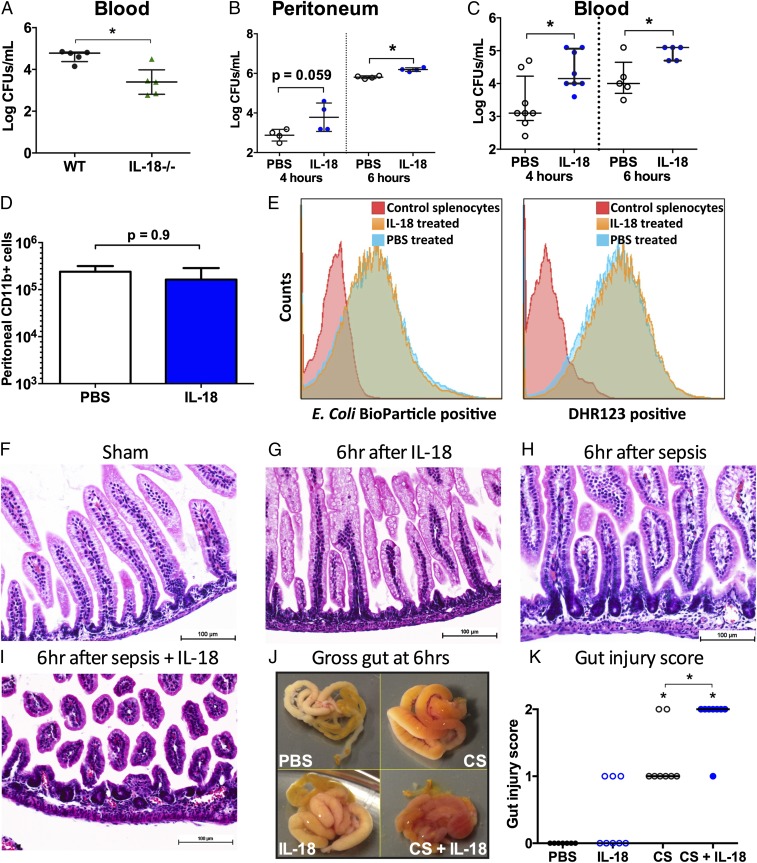
The enhancement of phagocytic cell function by IL-18 in infected adult mice with burns (3) led us to examine whether mortality in neonates was associated with changes in host protective immunity and whether bacterial clearance was modified in IL-18 deficiency. Surprisingly, we found that IL-18−/− mice with sepsis had significantly less bacteremia at 16 h postsepsis (before any mortality) than septic WT mice (Fig. 2A). Administration of IL-18 to septic neonatal mice did not improve protective immunity but significantly raised i.p. bacterial load at 6 h postsepsis (P < 0.05) (Fig. 2B) and bacteremic load at 4 and 6 h postsepsis (P < 0.05) (Fig. 2C). Of note, IL-18 treatment did not impair the local recruitment (Fig. 2D) or function (phagocytosis/ROS production) of neonatal myeloid (CD11b+) cells (Fig. 2E), which were predominantly PMNs (>80% Ly6G+CD11b+), consistent with our previous report (19).
Fig. 2.
(A) Reduction in bacterial colonization at 16 h (before any mortality in either group) in IL-18−/− mice compared with WT mice. *P < 0.05 by Wilcoxon signed-rank test. (B) IL-18–challenged WT mice had greater peritoneal bacterial loads than PBS-treated mice at 6 h and a trend toward greater load at 4 h. Results from individual mice in each group and median with interquartile range shown. *P < 0.05 by Wilcoxon signed-rank test. (C) IL-18–exposed septic WT neonatal mice showed a significant increase in bacteremia at 4 and 6 h postsepsis compared with PBS-treated septic controls. *P < 0.05 by Wilcoxon signed-rank test. (D and E) Because peritoneal and blood bacterial loads were increased following IL-18 challenge concurrent with sepsis, we examined peritoneal CD11b+ effector cell recruitment (D; median with range shown) and function (E; mean fluorescence intensity is shown on the x axis) for septic mice treated with either PBS or IL-18. Neonatal peritoneal washes were pooled from 10 animals per group for each of the three independent experiments. Functional results shown are representative of three independent experiments. (F–I) H&E staining (magnification: 200×) of neonatal small bowel (ileum) from healthy sham (PBS)-treated animals (F), 6 h after IL-18 exposure alone (G), 6 h after sepsis (H), and 6 h after sepsis with IL-18 challenge (I). (J and K) Gross appearance of small bowel from each group 6 h after exposure as shown (J) and gut injury score for each respective group (K). Results shown are representative of findings in at least three animals per group from at least two independent experiments.
Because the small bowel is especially sensitive to sepsis-induced organ injury (20), we examined the gross phenotype and histology of the small bowel to determine whether IL-18 exaggerates sepsis-associated disruption of the intestinal architecture. In contrast to ileum in healthy and IL-18–treated neonates (Fig. 2 F–G and J–K), the ileum after sepsis showed gross evidence of injury (Fig. 2J) as well as histologic evidence of progressive separation of the villi from the thickened basement membrane without substantial changes in villus structure (Fig. 2 H and K). IL-18 delivery to neonatal mice with sepsis caused substantially greater disruption of villous architecture and hemorrhage (Fig. 2 I–K) that was readily apparent on gross examination (Fig. 2J). Thus, delivery of IL-18 aggravated the disrupted small bowel architecture in the setting of sepsis.
Human Neonates with Sepsis Exhibit IL-18 and IL-17 Signaling in Peripheral Blood.
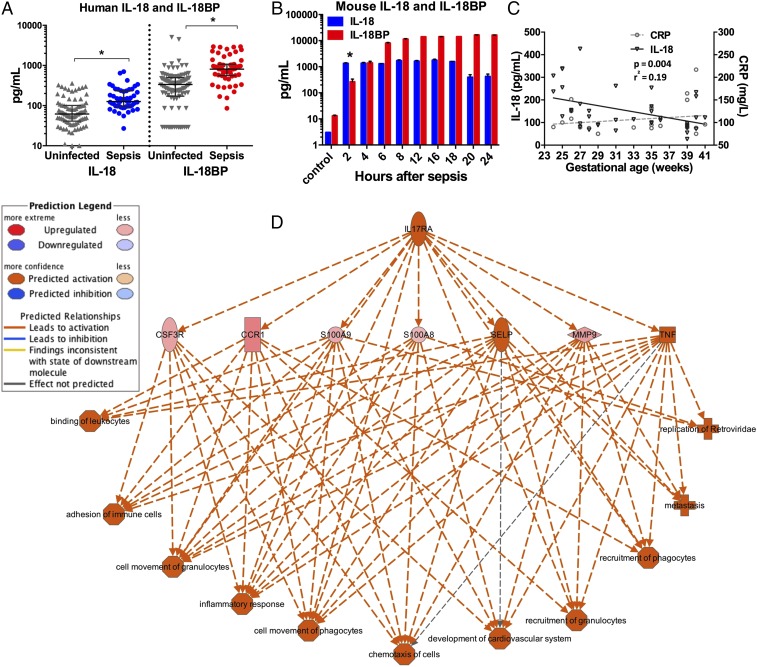
To verify the potential translational value of these experimental findings, we examined our recently collected transcriptomic analysis of the host response to sepsis in neonates (21). Among hospitalized infants prospectively evaluated for sepsis, plasma levels of both IL-18 and IL-18 binding protein (IL-18BP) were significantly elevated in those that developed sepsis (n = 37) compared with those determined not to have sepsis (n = 31) (Fig. 3A). Clinical characteristics of the neonates studied are shown in SI Appendix, Table S1.
Fig. 3.
(A and B) Plasma IL-18 and IL-18BP from human (A) and murine (B) neonates. *P < 0.05 by Wilcoxon signed-rank test. Individual values and median and interquartile ranges are shown. (C) Relationship between gestational age and plasma IL-18/CRP concentrations among septic human neonates. Linear regression for IL-18 (slope −6.8 ± 2.2, P = 0.004) and CRP (slope 2.3 ±2.2, P = 0.32). (D) Ingenuity Pathway Analysis was used to examine the expression of genes from neonates with sepsis with ≥1.5-fold change over uninfected infants. IL-17RA emerged as the most critical regulatory network with a consistency score of 23.4.
Because preterm neonates (born before 37 wk completed gestation) suffer the majority of the mortality with neonatal sepsis, we examined the relationship between plasma IL-18, C-reactive protein (CRP), and birth gestational age among patients in our human cohort (21). We identified an inverse correlation between gestational age and plasma IL-18 in infants with sepsis and a comparable degree of systemic inflammation (P = 0.004, r2 = 0.19) (Fig. 3C). These findings demonstrate that IL-18 signaling is activated with neonatal sepsis and that preterm septic neonates harbor relatively more IL-18 in circulating blood than their term counterparts with similar systemic inflammation.
To identify the key canonical networks in human neonates with sepsis, we examined whole-blood genome-wide expression on peripheral blood from infants with sepsis and compared the results with those in uninfected infants (21). Average gene expression was elevated for IL-18 (1.5-fold), IL-18R1 (4.5-fold), and IL-18RAP (threefold) among neonates with sepsis (P < 0.05 for each compared with uninfected infants). To garner the potential downstream mechanism(s) by which IL-18 may induce its detrimental effects, we used Ingenuity Pathway Analysis software to identify potential regulatory networks. Intriguingly, the IL-17 receptor pathway emerged as a top regulator effect network among neonates with sepsis, a finding that suggests that alterations in IL-17 signaling may have occurred in these infants (Fig. 3D). Taken together, these data support a previously unexplored transcriptomic link between IL-18 and IL-17 in the peripheral blood of human neonates, and especially preterm neonates, with sepsis.
IL-18 Delivery Amplifies IL-17A Production in Neonatal Sepsis.
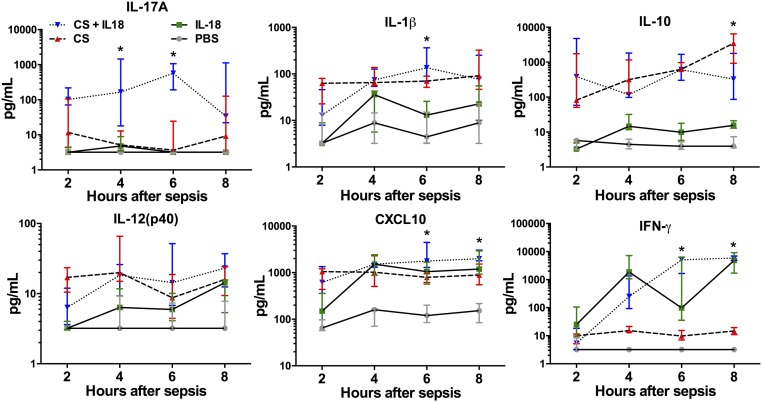
We verified from human neonatal blood analysis that IL-17 signaling represents a deleterious effector in neonatal sepsis. Following exposure to IL-18, polymicrobial sepsis, or polymicrobial sepsis with IL-18, plasma IL-17A from neonatal mice emerged as the earliest and most synergistically induced protein (33- and 143-fold increases at 4 and 6 h, respectively) among the inflammatory cytokines measured in septic mice treated with IL-18 as compared with septic controls (P < 0.05 by two-way ANOVA) (Fig. 4). We found no significant changes in plasma IL-12p40 (Fig. 4), IL-12p70, or IL-6 (SI Appendix, Fig. S1C), but IL-1β was increased at 6 h, and IL-10 was reduced at 8 h in septic mice administered IL-18 as compared with septic controls. Predictably, IL-18 exposure, in combination with sepsis, induced a significant increase in IFN-γ and CXCL10 at 6 and 8 h over sepsis alone (22). A similar induction of CXCL10 and IFN-γ were demonstrated when IL-18 (50 ng/g BW) was administered to healthy neonates, and no mortality was generated.
Fig. 4.
Plasma inflammatory response following sham injection (50 μL PBS) and after treatment with IL-18 (50 ng/g), CS (LD70), or CS plus IL-18. All plasma inflammatory mediator measurements occurred before mortality in any group. Values are shown as medians with interquartile ranges. n = 5 animals per group per time point. *P < 0.05 for CS + IL-18 vs. CS alone by two-way ANOVA.
Cells in the Lamina Propria and the Lung Parenchyma Produce IL-17A.
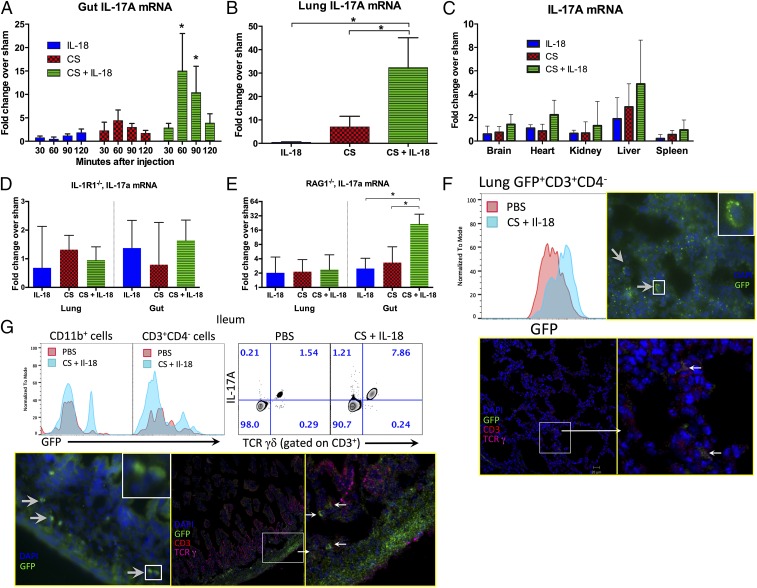
The plasma IL-17A response that we observed following treatment of septic mice with IL-18 was early and robust. Hence, we searched for potential sources of IL-17A in neonates by examining tissues commonly injured by sepsis. We found that the small bowel was a significant source (15-fold over sham treatment) of IL-17A mRNA at 60 and 90 min postsepsis in septic neonatal mice treated IL-18 as compared with either IL-18 treatment or sepsis alone (P < 0.05 by two-way ANOVA) (Fig. 5A). The identification of the small bowel as a prominent source of IL-17A expression complements our evidence of significant intestinal injury in murine neonates linked to potential microbial translocation in IL-18–potentiated neonatal sepsis. Similar to IL-17A mRNA production in the small bowel, IL-17A mRNA was significantly elevated in lung (32-fold) over septic controls and IL-18 administration alone (Fig. 5B) but was not significantly altered in the brain, heart, kidney, liver or spleen of septic neonatal mice that received IL-18 as compared with mice treated with IL-18 or sepsis alone (Fig. 5C). Because IL-1R1−/− mice were protected from the deleterious effects of IL-18 with sepsis, we also examined IL-17A mRNA production in the gut and lung from IL-18–treated septic neonatal IL-1R1−/− mice. In contrast to WT mice, IL-1R1−/− mice demonstrated minimal IL-17A mRNA production following IL-18 treatment and sepsis (Fig. 5D). These results are consistent with previous reports that IL-18 can drive IL-1 and that blockade of both IL-18 and IL-1 is associated with an increase in sepsis survival (11) and substantially bridge the gaps in our mechanistic understanding of how IL-18 participates in this inflammatory pathway.
Fig. 5.
(A) Small bowel (ileum) was harvested from neonatal mice at the time points shown following exposure to IL-18 (50 ng/g), CS (LD70), or CS + IL-18 and were examined for IL-17A mRNA by RT-PCR. *P < 0.05 by two-way ANOVA (CS + IL-18 versus CS alone or IL-18 alone). Data are shown as medians with interquartile ranges and are representative of technical duplicates and three independent experiments. (B) IL-17A mRNA was measured in lung 60 min after exposure to IL-18 alone, CS, or CS + IL-18 (*P < 0.05 by two-way ANOVA). Data shown are medians with interquartile ranges and are representative of technical duplicates and three independent experiments. (C) Organs shown were harvested from WT neonatal mice (P5–7) 60 min after exposure to IL-18 alone (50 ng/g), CS, or CS + IL-18 and were examined for IL-17A mRNA by RT-PCR. (D) Lung and gut were harvested from IL-1R1−/− neonates 60 min after stimulation as shown and were examined for IL-17A mRNA by RT-PCR. (E) Lung and gut were harvested from RAG1−/− neonates 60 min after stimulation as shown and were examined for IL-17A mRNA by RT-PCR. Data are shown as medians with interquartile ranges and are representative of technical duplicates and three independent experiments. (F) Lungs from IL-17A-GFP reporter neonatal mice were harvested 2 h after stimulation with PBS or sepsis (LD70) + IL-18 (50 ng/g). The histogram shows an overlay of the CD3+GFP+ cells indicating an increase in the IL-17A GFP reporter induced by sepsis + IL-18. Increased GFP expression occurred exclusively in CD3+CD4− cells. Immunocytochemistry revealed that the GFP+ cells in the lung coexpressed TCRγ and CD3. White boxes indicate areas shown at higher magnification in the Inset (Upper) and on the Right (Lower). (G) An increase in GFP+ cells that expressed either CD3+CD4−TCRγδ+ or CD11b+ was identified in the ileum and localized to the lamina propria in septic neonates treated with IL-18. White boxes indicate areas shown at higher magnification.
To identify the cellular sources of IL-17A mRNA in the gut, we first examined septic RAG1−/− neonates treated with IL-18. Although the IL-17A transcript from the intestine of RAG1−/− neonates remained robust, IL-17A expression was greatly attenuated in the lung as compared with WT mice (Fig. 5E). Using an IL-17A-GFP reporter mouse, we identified only CD3+CD4− cells in the lung parenchyma that coexpressed GFP (Fig. 5F and SI Appendix, Fig. S1H). Immunocytochemistry revealed that the GFP+ cells in the lung coexpressed TCRγ and CD3. In ileum, both CD11b+- and CD3+CD4−TCRγ+-expressing GFP+ cells localized to the lamina propria in septic neonates treated with IL-18 (Fig. 5G). Enriched PMNs from healthy murine neonatal bone marrow (SI Appendix, Fig. S1D) produced IL-18 mRNA when exposed to LPS or the combination of LPS and ATP (SI Appendix, Fig. S1E). IL-17A mRNA and p38 phosphorylation were increased with exposure to IL-18, LPS, or the combination of LPS and IL-18 (SI Appendix, Fig. S1 F and G).
Targeting of IL-17A Prevents the Enhancement of Sepsis Mortality by IL-18.
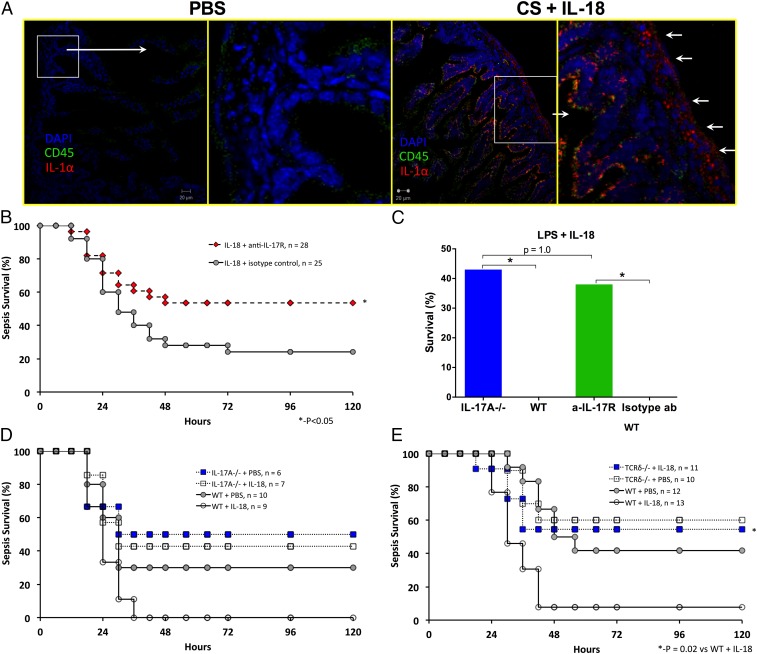
Our data indicated that increased IL-17A expression was associated with the deleterious effects of IL-18 in neonatal sepsis. Thus, blockade of IL-17A signaling may have potential therapeutic benefit. Studies in adult mice have produced conflicting results on the impact of targeting IL-17 in sepsis and did not examine upstream IL-18 (23, 24). Immunohistochemistry of the intestine of sham (PBS)-treated neonatal mice reveals very limited mature IL-1α and CD45 (Fig. 6A). In contrast, septic neonatal mice treated with IL-18 (50 ng/g) demonstrated clear increases in mature IL-1α (in the same region where the IL-17A-GFP signal was noted) and CD45 expression. Next, we demonstrated that, similar to IL-18, IL-17A potentiates LPS mortality in a dose-dependent manner (Fig. 1E and SI Appendix, Fig. S1I). Next, we disrupted the IL-18/IL-17A axis by targeting the IL-17 receptor A (IL-17RA) and assessed its impact on neonatal sepsis mortality. A single dose of anti–IL-17RA (10 μg/g BW) significantly ablated the deleterious effects of IL-18 on sepsis mortality as compared with similar mice treated with isotype control (54% vs. 24%, P < 0.05 by Fisher’s exact test) (Fig. 6B). IL-17RA blockade (in WT mice) or IL-17A deletion also had a similar effect on IL-18–potentiated endotoxin-induced mortality (Fig. 6C). We recognize that blockade of IL-17RA also may reduce IL-17F signaling. However, as predicted by our prior experimental results (Fig. 6 B and C), the increase in sepsis mortality associated with IL-18 treatment was completely abrogated in IL-17A−/− mice (Fig. 6D). Although there was a significant reduction in IL-18–potentiated sepsis mortality following IL-17 receptor blockade, the degree of rescue was not equivalent to the magnitude of increased mortality created with IL-18. These data suggest that the impact of IL-18 on sepsis mortality is multifactorial, including the potential role of nonculturable bacteria released from injured intestine, but certainly includes a robust inflammatory response predominated by IL-17A. Finally, we found mice lacking TCRδ, which are deficient in production of deleterious IL-17A by γδT cells, were protected from IL-18–potentiated sepsis (Fig. 6E). Overall, targeting IL-17 provides substantial protection during sepsis associated with the inflammatory stress that is enhanced in the presence of IL-18, as is the case in the most preterm and at-risk infants (Fig. 3C) (4).
Fig. 6.
(A) Immunohistochemistry of intestine of sham (PBS)-treated neonatal mice revealed limited mature IL-1α and CD45 expression. White boxes indicate the areas shown at higher magnification on the right. In contrast, septic neonatal mice treated with IL-18 (50 ng/g) demonstrated clear increases in mature IL-1α (white arrows) and CD45 expression. (B) Single-dose IL-17R blockade (10 μg per mouse i.p.) was associated with a significant decrease in sepsis mortality among IL-18–challenged septic neonates (*P < 0.05 by Fisher’s exact test). (C) IL-17A deletion (IL-17A−/−) or single-dose antibody-mediated IL-17 receptor blockade (anti–IL-17R) in WT mice reduced IL-18–augmented LPS mortality (*P < 0.05 by Fisher’s exact test). (D) IL-17A−/− neonatal mice were less susceptible to sepsis than WT mice and also were protected from the negative impact of IL-18 challenge concurrent with sepsis. (E) TCRδ−/− mice were less susceptible to sepsis than WT mice and also were protected from the negative impact of IL-18 challenge concurrent with sepsis.
Discussion
Our understanding of the newborn immune system is complicated by its immaturity, the presence of suppressive factors not present in adults, and its reliance on distinct host defense mechanisms that are either not present or not required for protective immunity in adults. Uncontrolled activation of inflammatory cascades by the immature neonatal immune system contributes to the development of catastrophic injury in neonatal inflammatory disease, including sepsis, respiratory distress syndrome, hypoxic brain injury, and necrotizing enterocolitis (NEC). Defining molecular mechanisms of disease that contribute to poor outcome in neonatal sepsis is paramount for the development of novel therapeutics. However, mechanistic analyses are lacking, particularly throughout development. Although we focused our studies on neonates because circulating IL-18 levels are much higher in uninfected human neonates than in adults, our mechanistic findings have broad implications for IL-18–mediated inflammatory responses in general.
Increased IL-18 expression has been demonstrated in premature neonates with brain injury (25) or sepsis (26) and NEC (27). Intriguingly, gut IL-17A mRNA was increased in experimental NEC in baboons (28), and recently IL-17A protein was shown to impair enterocyte tight junctions, increase enterocyte apoptosis, and reduce enterocyte proliferation leading to NEC in mice (29), highlighting the potential role of the IL-18/IL-17A axis in other neonatal inflammatory diseases. The deleterious role of excessive IL-17A, produced by many different cellular sources including a subset of γδT cells that can produce IL-17A rapidly via an IL-1R1–dependent pathway (30, 31), is well established in the pathogenesis of severe inflammatory and ischemic-injury models, including sepsis (23, 32, 33). However, the connection we have shown between IL-18 and downstream IL-1R1–dependent IL-17A production in the setting of sepsis clarifies a significant prior gap in our knowledge and has identified another potential therapeutic avenue. Although IL-18 and IL-17A play important roles in protective host defense, the actions of these and other cytokines during sepsis can become excessive and lead to death. Although long-term blockade would likely increase the risk of secondary or nosocomial infection, our data indicate that short-term blockade could prove life saving in the vulnerable neonatal population. We have presented data that point to an exaggerated inflammatory response predominated by IL-17A as the primary mechanism of increased mortality related to IL-18 exposure, but it remains plausible that LPS and IL-18 may have caused extraintestinal spread of bacteria that was not detected by standard culture techniques and that was responsible for increased mortality (34). Further studies are warranted to uncover other contributory mechanisms to IL-18–mediated injury.
Because IL-22 may promote the production of inactive pro–IL-18 (35), it is possible that human neonates may have a developmental excess of IL-22–producing cells that contribute to the large amount of circulating IL-18. Recently, IL-22 mRNA was shown to be modestly elevated (2- to 2.5-fold) above a fetal baseline in the intestine of humans with NEC and also elevated 1- to 1.25-fold above levels in control mice in neonatal mice with NEC (29). Because IL-18 plays a strongly deleterious role in the setting of sepsis, further studies are needed to determine the factors, including IL-22, contributing toward IL-18 production in neonates.
Our findings that deleterious effects of IL-18 require IL-1R1 signaling but are independent of IL-1β suggest that IL-1α signaling may play a prominent role in this pathway. Pro–IL-1α is ubiquitous in the cytoplasm and nuclei of healthy cells of skin, mucosal epithelium, liver, lung, kidney, platelets, and endothelium and serves as an alarmin that can signal via autocrine or juxtacrine pathways (36). Because IL-17A appears to be a significant end result of increased IL-18 and IL-1α signaling, pharmaceutical targeting of the IL-17 network may emerge as the preferred candidate for improving neonatal sepsis survival (37). The contributions of other modulators of the IL-18 system, such as IL-37, which binds IL-18R1 and inhibits IL-18 signaling, and IL-1α neutralization are additional opportunities for future preclinical investigation (38).
Materials and Methods
Mice.
The Institutional Animal Care and Use Committee at Vanderbilt University approved all studies before their initiation. Specific pathogen-free male and female C57BL/6 mice (WT and genetically modified) between 6 and 8 wk of age were purchased from The Jackson Laboratory and were allowed to equilibrate to their environment for a minimum of 7 d before any breeding or experimental use. Pups aged 5–7 d (P5–7) were considered neonates (13, 16, 19, 39). IL-1β−/− mice on a B6 background were the generous gift Scott Berceli (University of Florida, Gainesville, FL) and have been described previously (40). Mixed-sex litters were used for all neonatal studies.
Human Subjects and Sample Processing.
The Institutional Review Boards at Vanderbilt University and Duke University approved all studies before their initiation. Informed consent was obtained before sample collection. All patients and controls were previously reported in microarray-focused study that addressed a hypothesis different from those of the current report (21).
Additional materials and methods are included in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Dorina Avram for her assistance. Support for this work was provided by the NIH/National Institute of General Medical Sciences Grants GM106143 (to J.L.W.), T32 GM008554 and F31DK107321 (to C.S.W.), GM099771 and GM108025 (to H.R.W.), and GM097531 (to L.L.M); NIH/National Institute of Diabetes and Digestive and Kidney Diseases Grants DK097335 (to S.J.M.) and DK090146 (to D.J.M.); NIH/Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant HD061607 (to J.-H.W.); the University of Florida Department of Pediatrics, Vanderbilt Immunotherapy Program, The Children’s Miracle Network, The Gerber Foundation, Vanderbilt University Department of Pediatrics, and Vanderbilt Turner–Hazinski Awards.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.T.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1515793113/-/DCSupplemental.
References
- 1.Brocklehurst P, et al. INIS Collaborative Group Treatment of neonatal sepsis with intravenous immune globulin. N Engl J Med. 2011;365(13):1201–1211. doi: 10.1056/NEJMoa1100441. [DOI] [PubMed] [Google Scholar]
- 2.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 3.Kinoshita M, et al. Enhancement of neutrophil function by interleukin-18 therapy protects burn-injured mice from methicillin-resistant Staphylococcus aureus. Infect Immun. 2011;79(7):2670–2680. doi: 10.1128/IAI.01298-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maheshwari A, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Cytokines associated with necrotizing enterocolitis in extremely-low-birth-weight infants. Pediatr Res. 2014;76(1):100–108. doi: 10.1038/pr.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grobmyer SR, et al. Elevation of IL-18 in human sepsis. J Clin Immunol. 2000;20(3):212–215. doi: 10.1023/a:1006641630904. [DOI] [PubMed] [Google Scholar]
- 6.Robertson MJ, et al. Clinical and biological effects of recombinant human interleukin-18 administered by intravenous infusion to patients with advanced cancer. Clin Cancer Res. 2006;12(14 Pt 1):4265–4273. doi: 10.1158/1078-0432.CCR-06-0121. [DOI] [PubMed] [Google Scholar]
- 7.Dolinay T, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med. 2012;185(11):1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joshi VD, Kalvakolanu DV, Hasday JD, Hebel RJ, Cross AS. IL-18 levels and the outcome of innate immune response to lipopolysaccharide: Importance of a positive feedback loop with caspase-1 in IL-18 expression. J Immunol. 2002;169(5):2536–2544. doi: 10.4049/jimmunol.169.5.2536. [DOI] [PubMed] [Google Scholar]
- 9.Kinoshita M, Seki S, Ono S, Shinomiya N, Hiraide H. Paradoxical effect of IL-18 therapy on the severe and mild Escherichia coli infections in burn-injured mice. Ann Surg. 2004;240(2):313–320. doi: 10.1097/01.sla.0000133354.44709.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schultz MJ, et al. Interleukin-18 impairs the pulmonary host response to Pseudomonas aeruginosa. Infect Immun. 2003;71(4):1630–1634. doi: 10.1128/IAI.71.4.1630-1634.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanden Berghe T, et al. Simultaneous targeting of IL-1 and IL-18 is required for protection against inflammatory and septic shock. Am J Respir Crit Care Med. 2014;189(3):282–291. doi: 10.1164/rccm.201308-1535OC. [DOI] [PubMed] [Google Scholar]
- 12.Moreno SE, et al. IL-12, but not IL-18, is critical to neutrophil activation and resistance to polymicrobial sepsis induced by cecal ligation and puncture. J Immunol. 2006;177(5):3218–3224. doi: 10.4049/jimmunol.177.5.3218. [DOI] [PubMed] [Google Scholar]
- 13.Wynn JL, et al. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock. 2007;28(6):675–683. doi: 10.1097/SHK.0b013e3180556d09. [DOI] [PubMed] [Google Scholar]
- 14.Stoll BJ, et al. National Institute of Child Health and Human Development Very low birth weight preterm infants with early onset neonatal sepsis: The predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002-2003. Pediatr Infect Dis J. 2005;24(7):635–639. doi: 10.1097/01.inf.0000168749.82105.64. [DOI] [PubMed] [Google Scholar]
- 15.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4(7):553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 16.Wynn JL, et al. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112(5):1750–1758. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puren AJ, Fantuzzi G, Gu Y, Su MS, Dinarello CA. Interleukin-18 (IFNgamma-inducing factor) induces IL-8 and IL-1beta via TNFalpha production from non-CD14+ human blood mononuclear cells. J Clin Invest. 1998;101(3):711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalor SJ, et al. Caspase-1-processed cytokines IL-1beta and IL-18 promote IL-17 production by gammadelta and CD4 T cells that mediate autoimmunity. J Immunol. 2011;186(10):5738–5748. doi: 10.4049/jimmunol.1003597. [DOI] [PubMed] [Google Scholar]
- 19.Cuenca AG, et al. Critical role for CXC ligand 10/CXC receptor 3 signaling in the murine neonatal response to sepsis. Infect Immun. 2011;79(7):2746–2754. doi: 10.1128/IAI.01291-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shrum B, et al. A robust scoring system to evaluate sepsis severity in an animal model. BMC Res Notes. 2014;7:233. doi: 10.1186/1756-0500-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wynn JL, et al. Postnatal age is a critical determinant of the neonatal host response to sepsis. Mol Med. 2015;21:496–504. doi: 10.2119/molmed.2015.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okamura H, et al. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect Immun. 1995;63(10):3966–3972. doi: 10.1128/iai.63.10.3966-3972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flierl MA, et al. Adverse functions of IL-17A in experimental sepsis. FASEB J. 2008;22(7):2198–2205. doi: 10.1096/fj.07-105221. [DOI] [PubMed] [Google Scholar]
- 24.Freitas A, et al. IL-17 receptor signaling is required to control polymicrobial sepsis. J Immunol. 2009;182(12):7846–7854. doi: 10.4049/jimmunol.0803039. [DOI] [PubMed] [Google Scholar]
- 25.Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. BJOG. 2005;112(Suppl 1):16–18. doi: 10.1111/j.1471-0528.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 26.Kingsmore SF, et al. Identification of diagnostic biomarkers for infection in premature neonates. Mol Cell Proteomics. 2008;7(10):1863–1875. doi: 10.1074/mcp.M800175-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Héninger E, et al. Genetic variants of the interleukin-18 promoter region (-607) influence the course of necrotising enterocolitis in very low birth weight neonates. Eur J Pediatr. 2002;161(7):410–411. doi: 10.1007/s00431-002-0968-y. [DOI] [PubMed] [Google Scholar]
- 28.Namachivayam K, et al. Smad7 inhibits autocrine expression of TGF-β2 in intestinal epithelial cells in baboon necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2013;304(2):G167–G180. doi: 10.1152/ajpgi.00141.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egan CE, et al. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest. 2015;126(2):495–508. doi: 10.1172/JCI83356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paget C, et al. CD3bright signals on γδ T cells identify IL-17A-producing Vγ6Vδ1+ T cells. Immunol Cell Biol. 2015;93(2):198–212. doi: 10.1038/icb.2014.94. [DOI] [PubMed] [Google Scholar]
- 31.Duan J, Chung H, Troy E, Kasper DL. Microbial colonization drives expansion of IL-1 receptor 1-expressing and IL-17-producing gamma/delta T cells. Cell Host Microbe. 2010;7(2):140–150. doi: 10.1016/j.chom.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, et al. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120(1):331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gelderblom M, et al. Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood. 2012;120(18):3793–3802. doi: 10.1182/blood-2012-02-412726. [DOI] [PubMed] [Google Scholar]
- 34.Nowarski R, et al. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell. 2015;163(6):1444–1456. doi: 10.1016/j.cell.2015.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muñoz M, et al. Interleukin-22 induces interleukin-18 expression from epithelial cells during intestinal infection. Immunity. 2015;42(2):321–331. doi: 10.1016/j.immuni.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mease PJ, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370(24):2295–2306. doi: 10.1056/NEJMoa1315231. [DOI] [PubMed] [Google Scholar]
- 38.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: Back to the future. Immunity. 2013;39(6):1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gentile LF, et al. Protective immunity and defects in the neonatal and elderly immune response to sepsis. J Immunol. 2014;192(7):3156–3165. doi: 10.4049/jimmunol.1301726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turrin NP, Rivest S. Tumor necrosis factor alpha but not interleukin 1 beta mediates neuroprotection in response to acute nitric oxide excitotoxicity. J Neurosci. 2006;26(1):143–151. doi: 10.1523/JNEUROSCI.4032-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.