Significance
Life on Earth is substantially driven by a circuit of photosynthesis and glucose oxidation. Photosynthesizers capture sunlight and store its energy in the bonds of carbohydrates. Oxidation of carbohydrates provides organisms with a source of ATP and organic carbon for the synthesis of cellular building blocks. Our data provide strong evidence that the Entner–Doudoroff pathway of glucose degradation, which has been previously long overlooked, operates in cyanobacteria and plants. Phylogenetic analyses reveal that the cyanobacterial ancestor of plastids transferred this glycolytic route, via endosymbiotic gene transfer, to the plant lineage.
Keywords: glucose degradation, Entner–Doudoroff-pathway, Embden–Meyerhof–Parnas pathway, oxidative pentose phosphate pathway, endosymbiotic gene transfer
Abstract
Glucose degradation pathways are central for energy and carbon metabolism throughout all domains of life. They provide ATP, NAD(P)H, and biosynthetic precursors for amino acids, nucleotides, and fatty acids. It is general knowledge that cyanobacteria and plants oxidize carbohydrates via glycolysis [the Embden–Meyerhof–Parnas (EMP) pathway] and the oxidative pentose phosphate (OPP) pathway. However, we found that both possess a third, previously overlooked pathway of glucose breakdown: the Entner–Doudoroff (ED) pathway. Its key enzyme, 2-keto-3-deoxygluconate-6-phosphate (KDPG) aldolase, is widespread in cyanobacteria, moss, fern, algae, and plants and is even more common among cyanobacteria than phosphofructokinase (PFK), the key enzyme of the EMP pathway. Active KDPG aldolases from the cyanobacterium Synechocystis and the plant barley (Hordeum vulgare) were biochemically characterized in vitro. KDPG, a metabolite unique to the ED pathway, was detected in both in vivo, indicating an active ED pathway. Phylogenetic analyses revealed that photosynthetic eukaryotes acquired KDPG aldolase from the cyanobacterial ancestors of plastids via endosymbiotic gene transfer. Several Synechocystis mutants in which key enzymes of all three glucose degradation pathways were knocked out indicate that the ED pathway is physiologically significant, especially under mixotrophic conditions (light and glucose) and under autotrophic conditions in a day/night cycle, which is probably the most common condition encountered in nature. The ED pathway has lower protein costs and ATP yields than the EMP pathway, in line with the observation that oxygenic photosynthesizers are nutrient-limited, rather than ATP-limited. Furthermore, the ED pathway does not generate futile cycles in organisms that fix CO2 via the Calvin–Benson cycle.
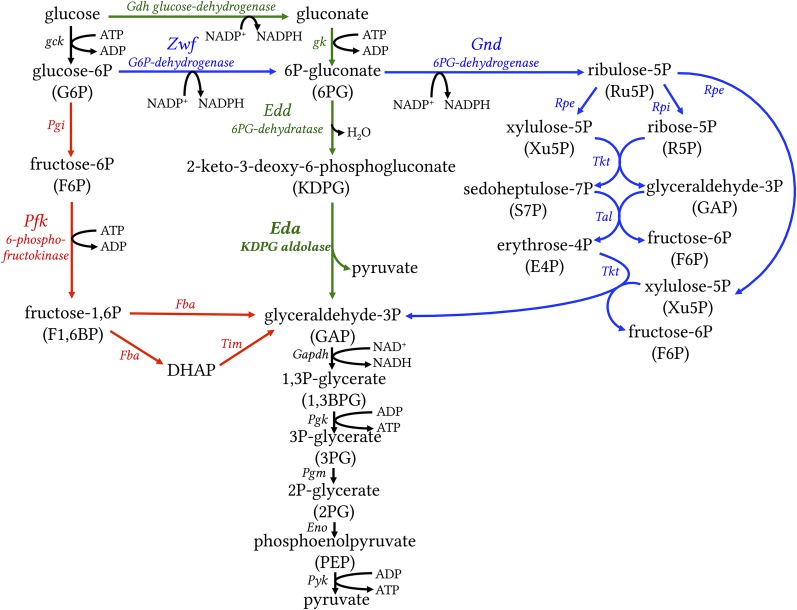
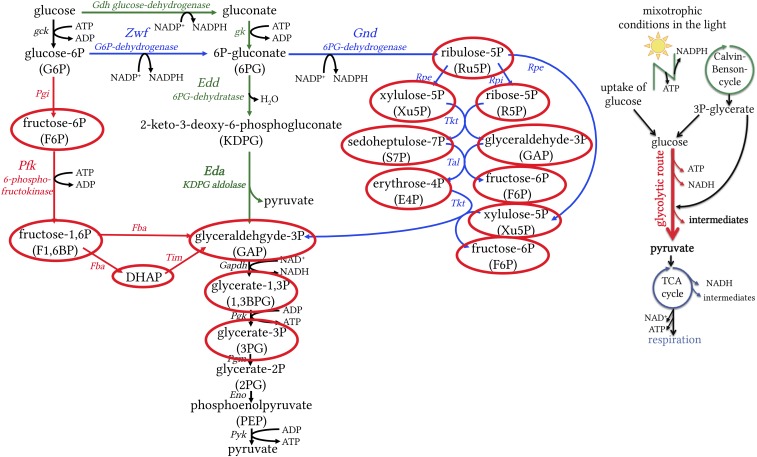
The breakdown of glucose is central for energy and biosynthetic metabolism throughout all domains of life. The Embden–Meyerhof–Parnas (EMP) pathway (glycolysis) and the oxidative pentose phosphate (OPP) pathway are the backbones of eukaryotic carbon and energy metabolism (1, 2). They generate ATP, NAD(P)H, and biosynthetic precursors for amino acids, nucleotides, and fatty acids. Prokaryotes, in contrast, exhibit a broad diversity in sugar oxidation pathways (3–5). These routes differ in ATP yield, in the enzymes and cofactors involved, and in the chemical intermediates of the pathways. The most common glycolytic routes in prokaryotes are the EMP, ED, and OPP pathways (Fig. 1). The key enzyme unique to the ED pathway is 2-keto-3-deoxygluconate-6-phosphate (KDPG) aldolase (Eda), whereas phosphofructokinase (PFK) is unique to the EMP pathway in the catabolic direction (3, 6). KDPG as a metabolite is exclusively found in the ED pathway (Fig. 1). The first two steps of the OPP pathway are catalyzed by glucose 6-phosphate-dehydrogenase (Zwf) and 6-phosphogluconate dehydrogenase (Gnd). As the pentose phosphate pathway can either run in its oxidative mode (OPP pathway) to oxidize carbohydrates or in its reductive mode (Calvin–Benson cycle) to fix CO2, it is tightly regulated. Zwf plays a central role in the fine-tuning of the OPP pathway and the Calvin–Benson cycle to prevent futile cycles (7–9). However, Zwf is not unique to the OPP pathway, as its product 6-P gluconate can also be further metabolized in the ED pathway. Cyanobacteria and plants are known to oxidize carbohydrates via either the EMP pathway or the OPP pathway (Fig. 1) (10–12).
Fig. 1.
The most common glycolytic routes in prokaryotes. EMP, often simply referred to as glycolysis, is shown in red with key enzyme PFK; the OPP pathway is shown in blue with enzyme Zwf; and the ED pathway is shown in green with key enzyme KDPG aldolase (Eda). Reactions that are shared by all pathways are shown in black. The EMP and OPP pathways are known to operate in cyanobacteria and plants. We show that in addition, the ED pathway operates in cyanobacteria and plants. Eda, KDPG aldolase; Edd, phosphogluconate dehydratase; Eno, enolase; Fba, fructose bisphosphate aldolase; Gdh, glucose dehydrogenase; Gk, gluconate kinase; Gnd, 6-phosphogluconate dehydrogenase; Pfk, 6-phosphofructokinase; Pgi, phosphoglucose isomerase; Pgk, phosphoglycerate kinase; Rpe, ribulose-5-phosphate epimerase; Rpi, ribulose-5-phosphate isomerase; Tal, transaldolase; Tim, triosephosphate isomerase; Tkt, transketolase; Zwf, glucose-6-phosphate dehydrogenase.
Growth Behavior of Mutants Missing Selected Enzymes of Known Glycolytic Routes in Synechocystis
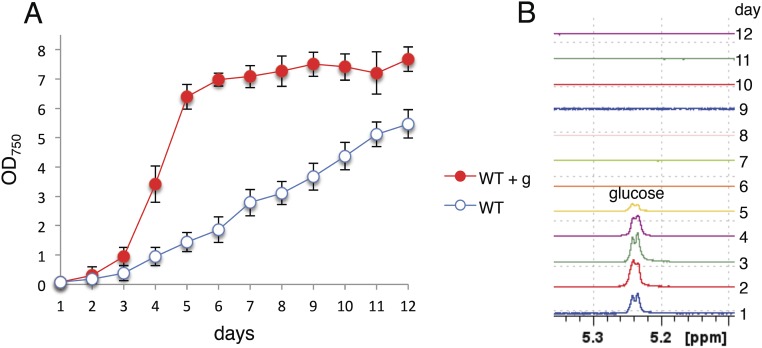
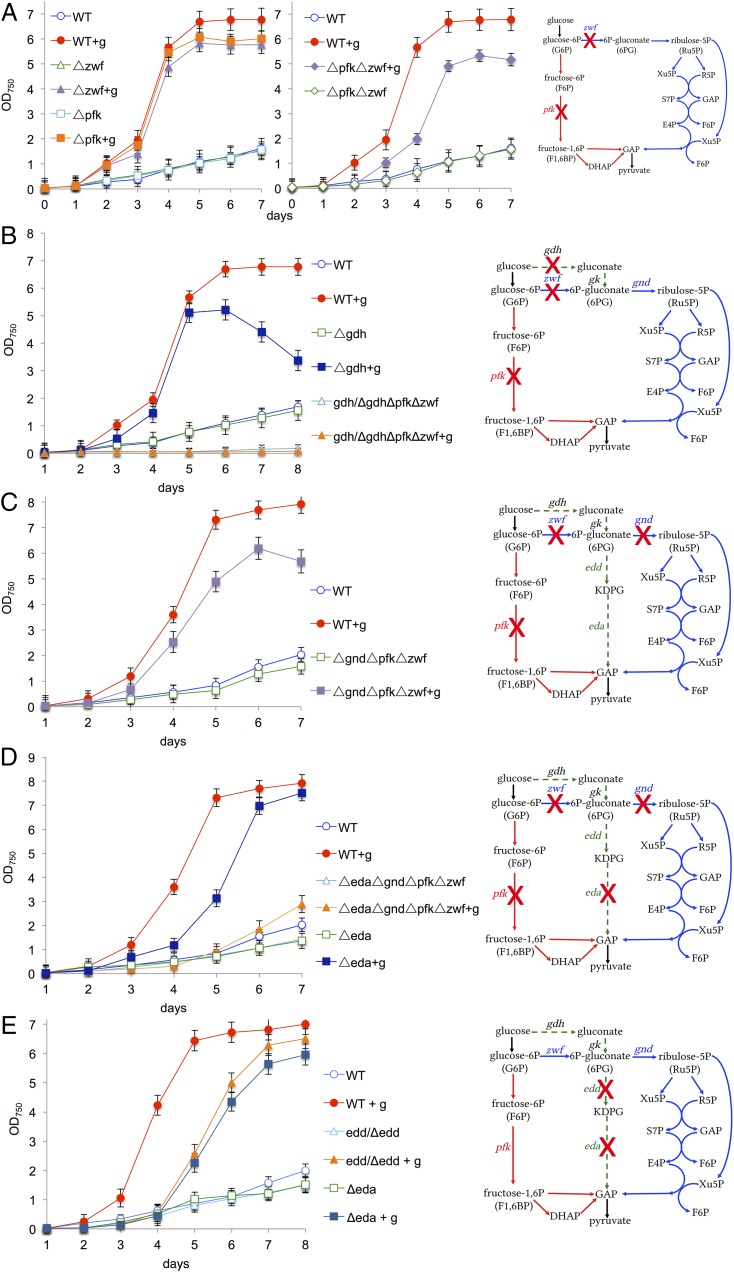
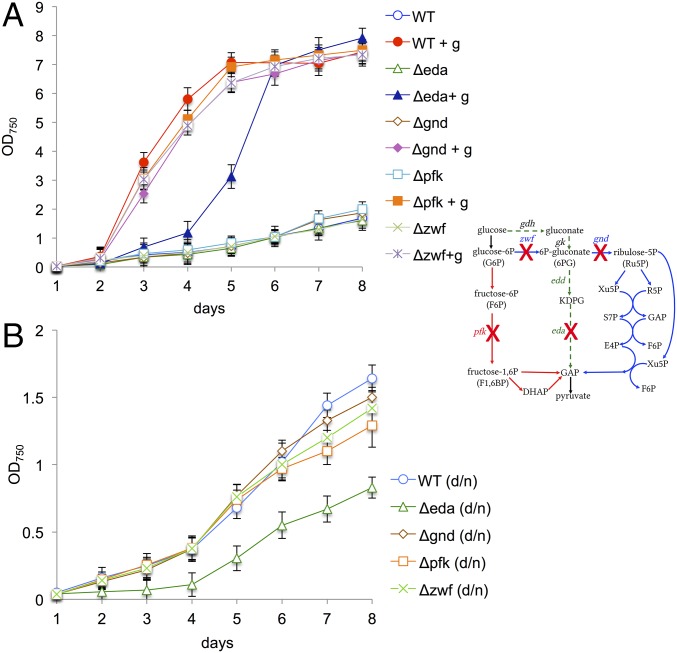
The cyanobacterium Synechocystis sp. PCC 6803 is able to assimilate glucose from its environment and to switch from autotrophy to mixotrophy, thereby significantly enhancing its growth (Fig. S1). To evaluate the physiological significance of known glycolytic routes in cyanobacteria, we deleted the genes for both isoforms of pfk - pfkB1 and pfkB2 - and zwf from the genome of Synechocystis (Fig. S2A). For clarity, the mutant ΔpfkB1ΔpfkB2 will be named Δpfk throughout this report. The Δpfk and Δzwf mutants and the ΔpfkΔzwf mutant exhibited enhanced growth under mixotrophic (light and glucose) compared with autotrophic (light) conditions (Fig. 2A), despite lacking essential enzymes for both the EMP pathway and the OPP pathway, indicating that this cyanobacterium has an additional route of glucose degradation. Synechocystis is thought to phosphorylate glucose via glucokinase to glucose-6P, which is then further oxidized either via the EMP pathway or the OPP pathway (Fig. 1) (13). We wondered whether the ΔpfkΔzwf mutant might bypass glucokinase via a concerted action of a glucose dehydrogenase (Gdh) and a gluconate kinase to form 6P-gluconate, which could then be further metabolized via Gnd in the OPP pathway (Figs. 1 and 2B). We found a gdh candidate (sll1709) in the Synechocystis genome (Fig. S3), which we deleted, resulting in the mutants Δgdh and gdh/ΔgdhΔpfkΔzwf. Whereas the putative gdh could be completely deleted from the WT, this was not possible in ΔpfkΔzwf, indicating that the gene is essential in this mutant (Fig. S2 B and C). Δgdh had an aberrant growth phenotype under mixotrophic conditions, and gdh/ΔgdhΔpfkΔzwf could no longer enhance its growth under mixotrophic conditions (Fig. 2B). To test whether 6P-gluconate is metabolized in the OPP pathway via Gnd, gnd was deleted from ΔpfkΔzwf to interrupt the assumed bypass at a later step in the reaction sequence (Fig. S2D). However, ΔgndΔpfkΔzwf grew under autotrophic conditions and still showed enhanced growth under mixotrophic conditions (Fig. 2C), strongly suggesting that Synechocystis possesses an additional, thus far unrecognized, pathway of glucose degradation.
Fig. S1.
Synechocystis assimilates glucose from its environment. (A) Growth of Synechocystis WT under autotrophic and mixotrophic (+g; 10 mM glucose) conditions in continuous light. Error bars represent the SD from three independent cultures, each measured in triplicate. Each growth experiment was repeated independently at least three times to ensure reproducibility. In the graph, the data of one growth experiment is shown. (B) Aliquots of the cultures were harvested, and the cell-free supernatant was analyzed via 1H-NMR, showing that the cells consumed glucose within the first 6 d. The signal of the anomeric H-atom is shown.
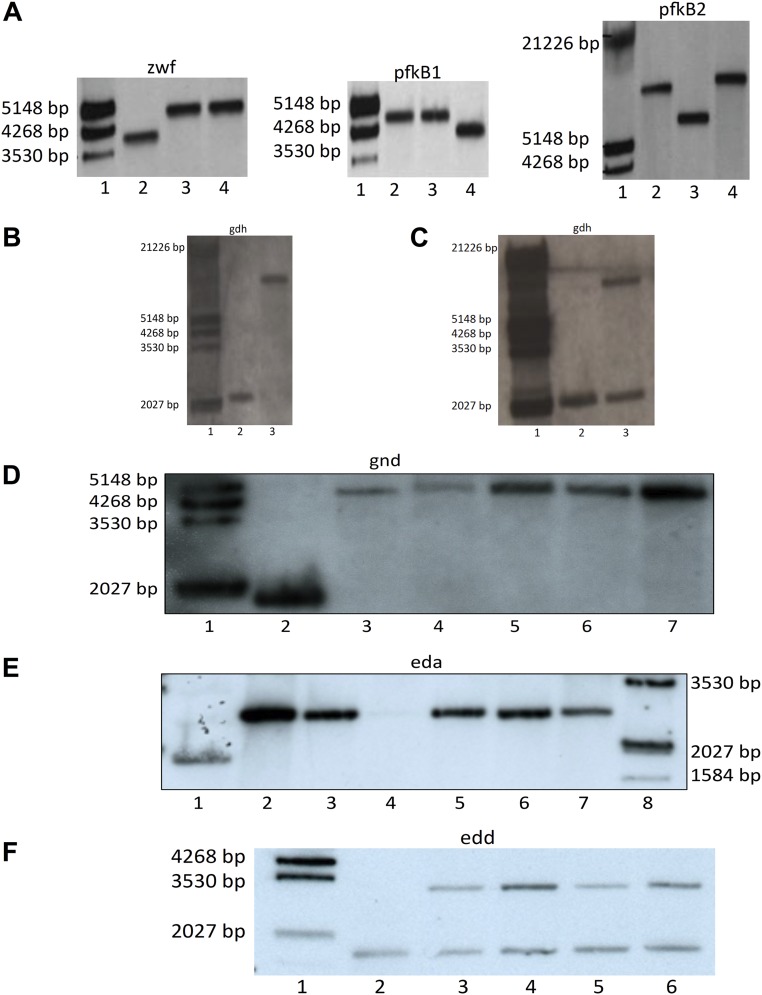
Fig. S2.
(A) Southern blot with genomic DNA of Synechocystis WT and different mutants showing that Δpfk1ΔpfkB2Δzwf (ΔpfkΔzwf) is completely segregated. Dig-labeled λ-HindIII-marker (lane 1), WT (lane 2), ΔpforΔzwf (irrelevant to this study) (lane 3), and ΔpfkB1ΔpfkB2Δzwf (abbreviated for clarity as ΔpfkΔzwf throughout the rest of this article) (lane 4). The blots were hybridized with probes against zwf, pfkB1, and pfkB2, as indicated. Expected sizes for zwf probe: WT, 4,022 bp, and Δzwf, 4,633 bp; for pfkB1 probe: WT, 4,692 bp, and ΔpfkB1, 3,979 bp; and for pfkB2 probe: WT, 8,625 bp, and ΔpfkB2, 9,677 bp. (B) Southern blot with genomic DNA of Synechocystis WT and Δgdh, showing that Δgdh could be deleted completely from all genomic copies. Dig-labeled λ-HindIII-marker (lane 1), WT (lane 2), and Δgdh (lane 3). The blots were hybridized with a probe against gdh, as indicated. Expected size in the WT was 2,029 bp, and in Δgdh, it was 8,996 bp. (C) Southern blot with genomic DNA of Synechocystis WT and gdh/ΔgdhΔpfk Δzwf showing that gdh could not be deleted from all genomic copies in Δpfk Δzwf. Dig-labeled λ-HindIII-marker (lane 1), WT (lane 2), and gdh/ΔgdhΔpfk Δzwf (lane 3). The blots were hybridized with a probe against gdh, as indicated. Expected size in the WT was 2,029 bp, and in Δgdh, it was 8,996 bp. (D) Southern blot with genomic DNA of Synechocystis WT and different mutants showing that gnd could be deleted from all genomic copies in the respective mutants. Dig-labeled λ-HindIII-marker (lane 1), WT (lane 2), Δgnd (lane 3), ΔgndΔeda (lane 4), ΔpfkΔzwf Δgnd (lane 5), ΔpfkΔgnd (lane 6), and ΔpfkΔzwf Δgnd Δeda (lane 7). The blots were hybridized with a probe against gnd, as indicated. Expected size in the WT was 1,816 bp, and in Δgnd, it was 4,795 bp. (E) Southern blot with genomic DNA of Synechocystis WT and different mutants showing that eda could be deleted from all genomic copies in the respective mutants. WT (lane 1), Δeda (lane 2), Δeda (lane 3), Δeda Δgnd (lane 4), ΔpfkΔzwfΔeda (lane 5), ΔpfkΔzwfΔgndΔeda (lane 6), ΔpfkΔzwfΔgndΔeda (lane 7), and Dig-labeled λ-HindIII-marker (lane 8). The blots were hybridized with a probe against eda, as indicated. Expected size in the WT was 1,867 bp, and in Δeda, it was 2,696 bp. (F) Southern blot with genomic DNA of Synechocystis WT and different mutants showing that edd could not be deleted from all genomic copies in the respective mutants. Dig-labeled λ-HindIII-marker (lane 1), WT (lane 2), edd/Δedd (lane 3), edd/ΔeddΔzwf (lane 4), edd/ΔeddΔpfkΔzwf (lane 5), and edd/ΔeddΔpfkΔzwf Δgnd (lane 6). The blots were hybridized with probe against edd as indicated. Expected size in the WT was 1,420 bp, and in Δedd, it was 3,128 bp.
Fig. 2.
Growth of Synechocystis WT and different mutants (as indicated) under autotrophic and mixotrophic (+g) conditions in continuous light. (A–E) Curves are accompanied by simplified schemes of glycolytic routes: EMP pathway in red, OPP pathway in blue, and ED pathway in green. Crosses indicate deleted genes. Error bars represent the SD from three independent cultures, each measured in triplicate. Each growth experiment was repeated independently at least three times to ensure reproducibility. Thus, in total, nine cultures were measured. In the graph, the data of one growth experiment are shown.
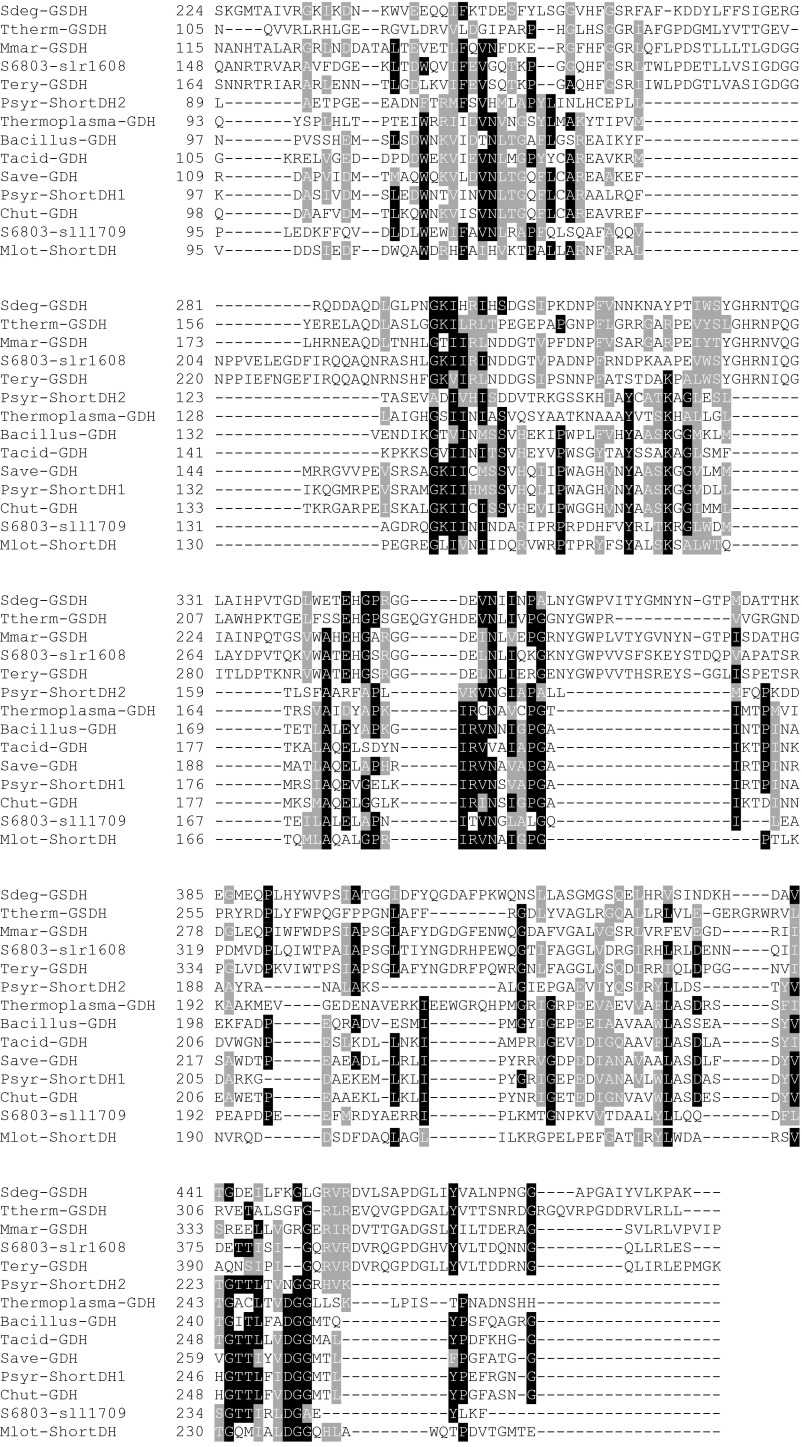
Fig. S3.
Alignment of Gdh, short-chain dehydrogenases, and glucose/sorbosone dehydrogenases with the two putative candidate proteins of Synechocystis sp. PCC 6803. The different proteins are from Saccharophagus degradans (Sdeg-GSDH), Thermus theromophilus Hb8 (Ttherm-GSDH), Maricaulis maris MCS10 (Mmar-GSDH), Trichodesmium erythraeum IMS101 (Tery-GSDH), Pseudomonas syringae pv. phaseolicola 1448A (Psyr-ShortDH2), Thermoplasma volcanium (Thermoplasma), Bacillus megaterium (Bacillus-GDH), Thermoplasma acidophilum (Tacid-GDH), Streptomyces avermitilis MA-4680 (Save-GDH), Pseudomonas syringae pv. syringae B728a (Psyr-ShortDH1), and Mesorhizobium loti MAFF303099 (Mlot-ShortDH). Structural information can be obtained from the Protein Data Bank (www.rcsb.org/) for the proteins of B. megaterium, Th. acidophilum, and T. thermophiles Hb8 under the IDs 1GCO, 3VTZ, and 2ISM, respectively. The genome of Synechocystis was searched for a Gdh. Two candidate genes could be identified. Sll1709 is annotated in the data bank cyanobase as Gdh and/or alternatively as 3-ketoacyl-acyl carrier protein reductase. It contains the Rossman fold typical for dehydrogenases binding NAD(P)+. The highest similarities are to the short-chain dehydrogenases (3-ketoacyl-acyl carrier protein reductase) of P. syringae and the Gdhs of a number of prokaryotes. The second candidate is the gene slr1608, which is annotated as a putative periplasmic Gdh in cyanobase. The closest homologs of this gene in other cyanobacteria are annotated as glucose/sorbosone dehydrogenases. These proteins do not contain a Rossmann-fold, so they do not use NAD(P)+ as substrate. According to the Signa lP tool of the Center for Biological Sequence Analysis prediction server (www.cbs.dtu.dk/services/), it is localized to the periplasm with a cleavage site between position 20 and 21 between the two Serin residues in the sequence ALS-SCG. Its localization indicates that it might reduce plastoquinone when oxidizing glucose. Deletion of sll1709 in ΔzwfΔpfk inhibited the ability of the mutant to enhance its growth on mixotrophic conditions (Fig. 2B).
Evidence for an Operating ED Pathway in the Cyanobacterium Synechocystis
A search of the Synechocystis genome for genes of the ED pathway uncovered candidates for 6PG-dehydratase (edd, slr0452) and the key enzyme KDPG aldolase (eda, sll0107) (Table S1).
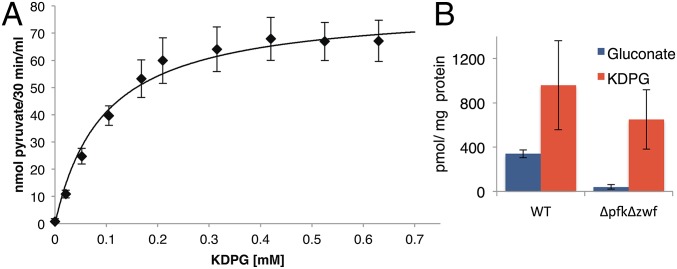
The candidate KDPG aldolase (Sll0107) of Synechocystis was tested for enzyme activity in cell-free homogenates. sll0107 was cloned for overexpression in Synechocystis into p2M2-Pcpc560ter (14). KDPG aldolase activity was not detected in cell-free homogenates of the Δeda mutant but was measured in cell-free homogenates of Δeda/cpc::edaSynechocystis, in which Sll0107 was overexpressed. In addition, Sll0107 was purified with a his-tag via a Ni-NTA column. Both enzyme assays showed the same biochemical characteristics. A KM value of 0.095 mM for the KDPG aldolase of Synechocystis was obtained (Fig. 3A and Fig. S4A). No activity was measurable when 2-keto-3-deoxygluconate was tested as a substrate in the same concentration range. This establishes that Sll0107 does not operate in a nonphosphorylating ED pathway (15) but is a functional KDPG aldolase of the phosphorylating ED pathway.
Fig. 3.
The cyanobacterium Synechocystis possesses an active KDPG aldolase and ED pathway. (A) Activity assay for KDPG aldolase (Eda) from Synechocystis. Concentration dependence of the conversion of KDPG to pyruvate. The KM was determined to 0.095 mM. (B) KDPG, the unique metabolite of the ED pathway, and gluconate were detected via IC-ESI-MSMS in mixotrophically grown Synechocystis WT and ΔpfkΔzwf cells. Error bars represent the SD from three replicates. Each experiment was repeated at least three times independently.
Fig. S4.
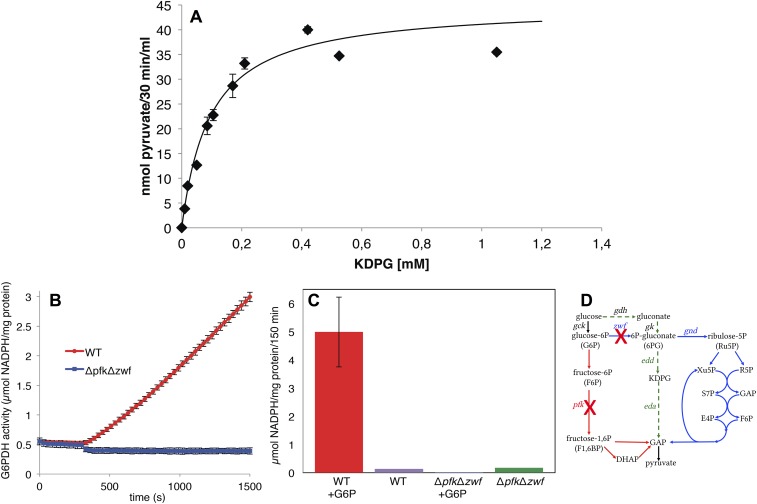
(A) Activity assay for purified KDPG aldolase (Eda) from Synechocystis. The enzyme was overexpressed in Synechocystis and purified with a his-tag via a Ni-NTA column from the cell extract. Concentration dependence of the conversion of KDPG to pyruvate. The KM was determined to be 0.095 mM. Error bars represent the SD from three replicates. (B and C) There is no glucose-6P dehydrogenase (G6PDH) activity in the ΔpfkΔzwf mutant. (B) Kinetic determination of G6PDH activity in WT and ΔpfkΔzwf upon addition of glucose-6P (G6P). (C) Endpoint determination of G6PDH activity in WT and ΔpfkΔzwf incubated for 150 min with and without glucose-6P (G6P). Error bars represent the SD from three replicates. Each experiment was repeated at least three times independently. (D) Simplified scheme of glycolytic routes: EMP pathway, red; OPP pathway, blue; ED pathway, green. KDPG found in ΔpfkΔzwf (Fig. 3B) thus has to originate from a pathway not involving the activity of glucose-6P dehydrogenase (Zwf).
Deletion mutants for sll0107 were constructed. To determine whether the ΔgndΔpfkΔzwf mutant bypasses the EMP and OPP pathway via the ED pathway, the KDPG aldolase (eda) was deleted both in this mutant and in the WT, resulting in ΔedaΔgndΔpfkΔzwf and Δeda (Fig. S2E). Δeda was impaired in its growth under mixotrophic conditions, but more important, ΔedaΔgndΔpfkΔzwf showed no enhanced growth under mixotrophic conditions (Fig. 2D). In contrast to eda (sll0107), the gene edd (slr0452) could not be completely deleted from all genome copies of Synechocystis (Fig. S2F). We hypothesize that the encoded enzyme might be involved in the synthesis of the branched chain amino acids valine and isoleucine, as well as the conversion of gluconate to KDPG, as it was shown for the dihydroxyacid dehydratase of the archaeon Sulfolobus solfataricus (16). However, the merodiploid mutant edd/Δedd had a similar growth phenotype as Δeda (Fig. 2E). Furthermore, gluconate, the metabolite of the assumed bypass via Gdh and gluconate kinase, was present in WT and ΔpfkΔzwf. Most important, KDPG, the metabolite unique to the ED pathway, could be detected in the WT and ΔpfkΔzwf (Fig. 3B) We checked the ΔpfkΔzwf mutant for any glucose-6P dehydrogenase (G6PDH) activity. As expected, no G6PDH activity was detectable, in contrast to the WT (Fig. S4 B and C). The KDPG in ΔpfkΔzwf thus has to originate from a pathway not involving Zwf. Collectively, this indicates that the assumed bypass is present, and it clearly establishes that the ED pathway operates in Synechocystis in vivo.
Physiological Significance of the ED Pathway in Synechocystis
To evaluate the physiological importance of the ED pathway compared with the EMP and OPP pathways in Synechocystis, Δeda, Δpfk, Δzwf, and Δgnd were grown under mixotrophic conditions in continuous light, and additionally under autotrophic conditions in a dark/night cycle, probably the most common condition encountered in nature. Under both these conditions, Synechocystis suffered most in growth when the ED pathway was interrupted compared with other glycolytic routes (Fig. 4 A and B).
Fig. 4.
Exclusively, deletion of KDPG aldolase delays growth under mixotrophic conditions in continuous light and autotrophic conditions in a day/night cycle. Growth of Synechocystis WT and single mutants, in which different key enzymes of glycolytic routes were deleted, as indicated in the simplified schemes: EMP pathway in red, OPP pathway in blue, and ED pathway in green. Crosses indicate deleted genes. (A) Cultures grown under autotrophic and mixotrophic (+g) conditions in continuous light. (B) Cultures grown under autotrophic conditions in day/night cycle (d/n). Error bars represent the SD from three independent cultures, each measured in triplicate. Each growth experiment was repeated independently at least three times to ensure reproducibility. In the graph, the data of one growth experiment is shown.
Occurrence of the ED Pathway in Cyanobacteria
The ED pathway has not previously been described in cyanobacteria, but BLAST searches revealed that KDPG aldolase is widespread among cyanobacteria (Table S1).
In fact, 92% of all sequenced cyanobacteria possess a KDPG aldolase, but only 57% possess PFK. 49% harbor both a KDPG aldolase and PFK, 8% possess only PFK, and 43% possess the KDPG aldolase but no PFK (Table S1). This distribution pattern indicates that the ED pathway is an important pathway of glucose degradation in cyanobacteria and is even more widespread than the EMP pathway in this group.
Occurrence of the ED Pathway in Moss, Fern, Algae, and Plants
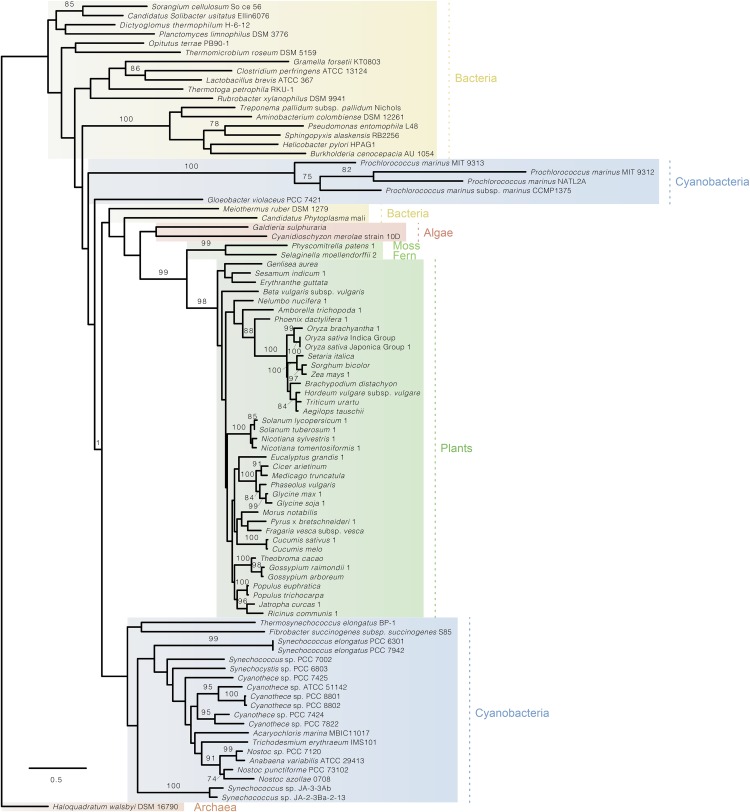
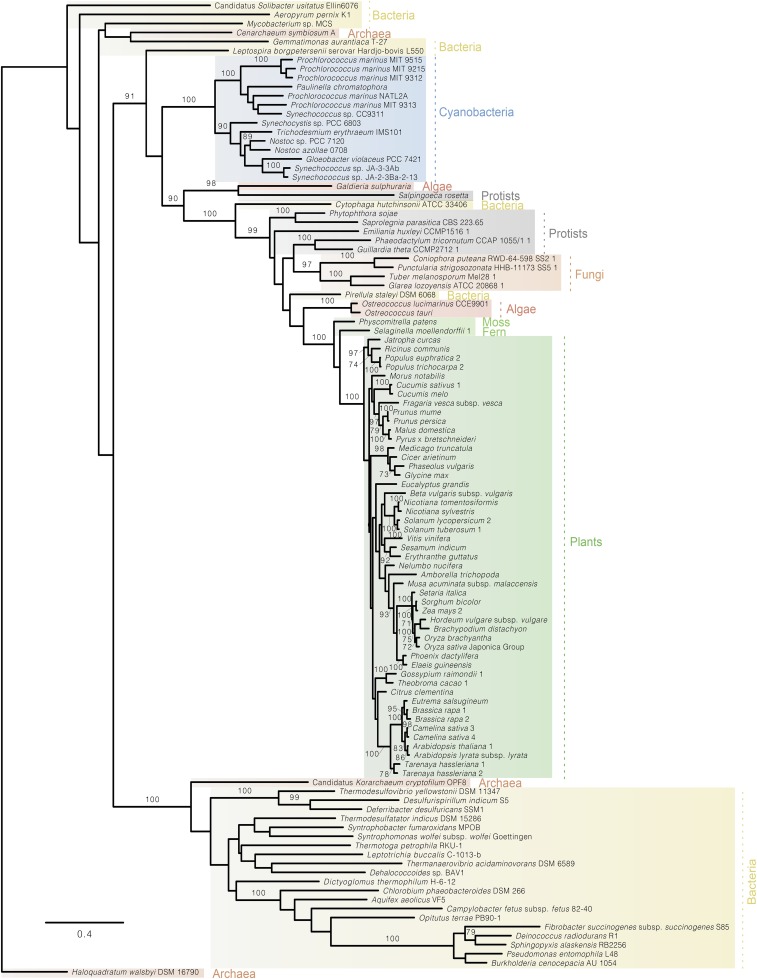
To check whether the ED pathway might also occur in other oxygenic photosynthesizers, Blast analyses were performed. Both Edd and KDPG aldolase were found to be present in moss and fern and widespread in algae and plants (Table S2 and Figs. S5 and S6). Phylogenetic analyses reveal that both plant enzymes branch with their homologs from cyanobacteria, indicating that they were acquired from the ancestors of plastids via endosymbiotic gene transfer (Figs. S5 and S6).
Fig. S5.
Phylogenetic tree of KDPG aldolase (Eda) in archaea, bacteria, cyanobacteria, moss, fern, algae, and plants showing that the KDPG aldolases from eukaryotic photosynthesizers cluster with their homologs from cyanobacteria.
Fig. S6.
Phylogenetic tree of 6Pgluconate-dehydratase (Edd) in archaea, bacteria, cyanobacteria, protists, fungi, moss, fern, algae, and plants showing that Edds from eukaryotic photosynthesizers cluster with their homologs from cyanobacteria.
Evidence for an Operating ED Pathway in the Plant Barley (Hordeum vulgare)
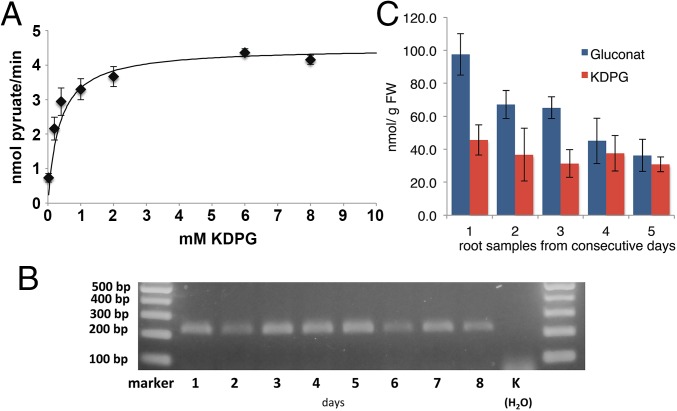
To test whether the putative plant KDPG aldolase is functional, the candidate gene BAJ87430 from barley (H. vulgare) was amplified from seedling cDNA. The protein was overexpressed in Escherichia coli, purified to apparent homogeneity via Ni-NTA, and subjected to a coupled enzyme test using lactate dehydrogenase. It had a KM value of 0.35 mM for the conversion of KDPG to GAP and pyruvate (Fig. 5A), close to the KM of the KDPG aldolase from other organisms (17, 18). In addition, 2-keto-3-deoxygluconate was tested as a substrate in the same concentration range to test whether the enzyme might participate in a nonphosphorylating ED pathway (15). No enzyme activity was detectable with 2-keto-3-deoxygluconate, indicating that higher plants, similar to cyanobacteria, possess a KDPG aldolase from the phosphorylating ED pathway. Glycolytic routes are especially important in periods of growth for the supply of ATP, NAD(P)H, and biosynthetic precursors. Therefore, mRNA was isolated from germinating barley seedlings during a period of 8 d and subjected to RT-PCR. The KDPG aldolase gene was expressed throughout this period during germination and early seedling development (Fig. 5B). Furthermore, KDPG, the metabolite unique to the ED pathway, was detected in roots of barley seedlings (Fig. 5C), indicating in vivo that this pathway operates in plants.
Fig. 5.
The plant barley (H. vulgare) possesses an active KDPG aldolase, which is expressed throughout early seedling development and an active ED pathway. (A) Activity assay for KDPG aldolase (Eda) from H. vulgare. Concentration dependence of the conversion of KDPG to pyruvate. The KM was determined to 0.35 mM. (B) RT-PCR was carried out with RNA from germinating H. vulgare seedlings collected over a period of 8 d with primers specific for the KDPG aldolase transcript (expected size with intron, 466 bp; without intron, 222 bp). (C) KDPG, the unique metabolite of the ED pathway and gluconate were detected via IC-ESI-MSMS in roots of H. vulgare seedlings. Error bars represent the SD from three replicates. Each experiment was repeated at least three times independently.
Collectively, our data thus indicate that the ED pathway operates in cyanobacteria and plants.
Discussion
Most enzymes involved in the EMP pathway are bidirectional, either serving the degradation of glucose (glycolysis) or working in the opposite direction in gluconeogenesis. However, PFK is unique to the oxidative direction of the EMP pathway (6), so its absence from the genome most likely results in the lack of this pathway, even if all other enzymes belonging to the EMP pathway are present. All open ocean species that thrive in nutrient-depleted habitats, such as Prochlorococcus, Crocosphaera, Synechococcus, and Trichodesmuim, possess a KDPG aldolase but are devoid of a PFK (Table S1). Those few cyanobacteria (8%) that lack a KDPG aldolase thrive in freshwater, which is generally richer in nutrients. The cyanobacterium Prochlorococcus was recently found to take up glucose from its environment (19). As its genome lacks PFK, the key enzyme of the EMP pathway, the authors concluded that Prochlorococcus metabolizes glucose in the light exclusively via the OPP pathway (19, 20). However, the OPP pathway is known to be down-regulated in the light and fine-tuned under mixotrophic conditions, so as to prevent a futile cycle with the Calvin–Benson cycle (21, 22). We therefore suggest that Prochlorococcus might oxidize glucose via the ED pathway under mixotrophic conditions, as shown for Synechocystis. If one molecule of glucose is metabolized via the EMP pathway, the cell gains two ATP and two NADH; if it is metabolized via the ED pathway, it gains one ATP, one NADH, and one NADPH. At first sight, the EMP pathway looks beneficial, as its ATP yield is higher. However, cyanobacteria in the open ocean should not be ATP-limited, as they perform photosynthesis, and light is unlikely to be a limiting resource in this habitat. The ED pathway has a higher thermodynamic driving force than the EMP pathway because of its lower ATP yield, and it furthermore requires 3.5 times less enzymatic protein to achieve the same glycolytic flux as the EMP pathways (3). Flamholz et al. thus argue that the choice between the EMP pathway and the ED pathway is a tradeoff between ATP yield and protein costs (3). Cyanobacteria, algae, and plants in general are nutrient-limited, rather than ATP-limited. Algae, plants, and cyanobacteria even possess non-energy-conserving bypasses in their respiratory chains (12, 23) that should enable the oxidation of carbohydrates for biosynthetic purposes when ATP is oversupplied. It therefore makes perfect sense that cyanobacteria rely on the ED pathway rather than on the EMP pathway, and that cyanobacteria from the open ocean, such as Prochlorococcus, that streamline both their genome and proteome to adapt to nutrient-depleted conditions (24, 25), even seem to omit the upper part of the EMP pathway. Further research is needed to substantiate these hypotheses.
Cyanobacteria have long been thought to degrade glucose mainly via the OPP pathway, rather than the EMP pathway (10, 13, 21, 26). Furthermore, cyanobacterial glycolytic routes were perceived of being foremost important under heterotrophic conditions in the dark (10, 13). However, evidence is accumulating that mixotrophy (uptake of organic carbon in the light) among phototrophic cyanobacteria and algae has been dramatically underestimated, especially in aquatic habitats (27, 28). Under these conditions, however, glycolytic routes have to run in parallel to the Calvin–Benson cycle. The diatom Phaeodactylum tricornutum was recently shown to harbor functional forms of Edd and Eda belonging to the ED pathway (29). In contrast to the ED pathway, both the EMP and OPP pathways share several intermediates with the Calvin–Benson cycle (Fig. S7). A simultaneous operation of Calvin–Benson cycle and either the OPP pathway or the EMP pathway might thus result in futile cycles that slow down CO2 fixation rates, thereby enhancing oxidative stress (26). This is not true for the ED pathway (Fig. S7) and might explain why Δeda compared with Δpfk, Δzwf, and Δgnd grew slower under mixotrophic conditions (Fig. 4A). In addition, the ED pathway provides cyanobacterial cells, obviously with a selective advantage under autotrophic conditions in a dark/night cycle (Fig. 4B), which might explain its high prevalence in photosynthesizing cells. The fact that the ED pathway is widespread in both cyanobacteria and plants indicates that it serves an important function. Otherwise, it would have been lost in evolution long ago. However, flux analyses are needed to quantify the relative contributions of EMP, OPP, and ED pathways to the degradation of carbohydrates in cyanobacteria and plants.
Fig. S7.
Both the EMP (red) and the OPP (blue) pathways share intermediates with the Calvin–Benson cycle (circled in red), but not the ED (green) pathway. The EMP and OPP pathways might thus form futile cycles under, for example, mixotrophic conditions when glycolytic route and CO2 fixation via the Calvin–Benson cycle run in parallel. This is not true for the ED pathway.
The present results show that cyanobacteria and plants possess an active ED pathway that was transferred from the cyanobacterial ancestors of plastids to the plant lineage. Oxygenic phototrophs thus possess three, instead of two, carbohydrate degradation pathways (Fig. 1), whereof the ED pathway is a previously overlooked glycolytic route of high physiological significance in cyanobacteria.
Materials and Methods
Full protocols are available in the SI Materials and Methods.
Growth Conditions Synechocystis.
For growth experiments, Synechocystis sp. PCC 6803 WT and mutants of this strain were grown in 250 mL BG11 medium in glass tubes at 28 °C under constant light (50 µE/m2/s). The cultures were aerated with filter-sterilized air. Mixotrophic cultures were supplemented with 10 mM (final concentration) glucose. For light/dark cycles, cultures were subjected to a cycle of 12 h darkness and 12 h light (50 µE/m2/s) under constant aeration.
Growth Conditions of H. vulgare for RT-PCR Experiments and Ion Chromatography Electron Spray Ionization Tandem Mass Spectrometry Analysis.
After stratification, H. vulgare (Golden Promise) seeds were grown on moist nutrient-free vermiculite at 21 °C under continuous light (250 µE/m2/s). Whole seedlings were collected every 24 h from day 1 to day 8 for RT-PCR experiments, and roots from seedlings were collected from day 2 to day 6 for Ion chromatography electron spray ionization tandem mass spectrometry (IC-ESI-MSMS) analysis.
Generation of Synechocystis Deletion Mutants.
Constructs for the deletion mutants were made by PCR fusion, as described (30). The primers used are shown in Table S3. In principle, an antibiotic resistance cassette was fused to approximately 200 bp directly up- and downstream of the gene to be deleted. The constructs were ligated in pCRII-TOPO (Invitrogen), sequenced, and transformed into Synechocystis to replace the respective gene by the antibiotic resistance cassette. Mutants were checked by Southern hybridization (Fig. S2) (30).
Extraction and Identification of Metabolites via IC-ESI-MSMS Measurements.
Metabolites were extracted from Synechocystis and barley (H. vulgare) roots via methanol:chloroform:water. The separation and detection of metabolites was carried out using an ion chromatography system (Dionex Thermo Fisher) connected to a triple quadrupole mass spectrometer QQQ6490 (Agilent Technologies). Quantification was performed with authentic standards at different concentrations.
Overexpression and Purification of KDPG Aldolase of Synechocystis sp. PCC 6803.
For homologous overexpression of the KDPG aldolase (sll0107), ORF was cloned into p2M2-Pcpc560ter (14) with a C-terminal His6-tag (for primers, see Table S3) and transformed into Δeda, resulting in the mutant Δeda/cpc::edaSynechocystis. To purify the enzyme cells of the overexpression strain, Δeda/cpc::edaSynechocystis were harvested, resuspended in 100 mM Tris at pH 8.0 and 150 mM NaCl, and broken with glass beads (diameter, 0.17–0.18 mm) by vortexing 10 min at 4 °C. After centrifugation at 800 × g for 1 min at 4 °C, the liquid phase was removed and centrifuged at 1,300 × g for 10 min at 4 °C. The resulting homogenate was checked for unbroken cells under the microscope and, if necessary, centrifuged a second time at 1,300 × g for 10 min at 4 °C to remove unbroken cells. The homogenate was applied on a Ni-NTA column. After it completely entered the column, it was washed four times with two column volumes (cvs) wash buffer (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole at pH 8). Subsequently, the protein was eluted from the column by applying six times 0.5 cvs elution buffer (50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole at pH 8) and collecting the eluate in 0.5-cv fractions.
Overexpression and Purification of KDPG Aldolase of H. vulgare (Golden Promise).
cDNA from a barley seedling was subjected to a PCR with primers specific to the KDPG aldolase of H. vulgare (BAJ87430; AK356212) (Table S3).
The ORF was fused to a C-terminal His6-tag and ligated into the pMAL-pIII (NEB), resulting in pMALedaH.vulgareHis. This vector was transformed into E. coli [BL21(pLys3)] and induced with 0.4 mM IPTG for 24 h. After harvesting, the cells were resuspended in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole at pH 8) and incubated with 1 mg/mL lysozyme for 30 min on ice. Cells were lysed by six 10-s ultrasound bursts on ice with 10-s intermittent cooling periods. To remove cell debris, the homogenate was centrifuged at 20,000 × g for 15 min, and the supernatant was loaded on a Ni-NTA column. The column was run as described earlier for the purification of the KDPG aldolase from Synechocystis.
KDPG Aldolase (Eda) in Vitro Measurements.
KDPG aldolase activity was measured by the production of pyruvate from KDPG. The isolated KDPG aldolases from Synechocystis and barley were used in 1-mL assays that contained 10 mM NADH, 5 U lactate dehydrogenase, and KDPG in a range from 0.02 to 8 mM in 100 mM Tris, 150 mM NaCl at pH 8 at 32 °C. The measurements were started by the addition of KDPG, and the consumption of NADH was followed at 366 nm.
In addition, KDPG aldolase from Synechocystis was also measured directly in cell homogenates of the overexpression strain Δeda/cpc::edaSynechocystis. In that case, homogenates were prepared by breaking the cells with glass beads, as described for the purification of the KDPG aldolase from Synechocystis (see above).
The total extract was used directly for KDPG aldolase measurements. Aliquots of the extract were incubated with different KDPG concentrations (0.02–0.7 mM) for 30 min. After 30 min, the samples were centrifuged for 5 min at 20,000 × g to remove the membranes. Subsequently, the amount of pyruvate generated was determined by measuring the absorption change at 366 nm after adding 10 mM NADH and 5 U lactate dehydrogenase. The extract of the mutant Δeda was used as a control and showed in all cases no production of pyruvate.
Glucose-6P Dehydrogenase (zwf) in Vitro Measurements.
Glucose-6P dehydrogenase activity was measured in Synechocystis WT and ΔpfkΔzwf by the conversion of NADP to NADPH on addition of glucose-6P. A kinetic and an endpoint determination were carried out.
Phylogenetic Analyses.
Homologous amino acid sequences were selected by an all-against-all bidirectional best BLAST hit approach. Pairwise hits were clustered into families using MCL (31). Multiple sequence alignments were performed with MAFFT (32), and phylogenetic trees were generated with PhyML (33).
SI Materials and Methods
Culture and Growth Conditions Synechocystis.
Synechocystis sp. PCC 6803 WT and mutants of this strain were kept frozen at −80 °C. For growth experiments, cells were spread on BG11 (34) agar plates supplemented with antibiotic in continuous light. For precultures, 50 mL BG11 supplemented with antibiotic was inoculated and kept on a shaker (GFL 3020) (100 rpm) in continuous light. The cells were harvested and washed twice and resuspended in fresh BG11 medium to remove the antibiotic. Aliquots of these resuspended cells were used to inoculate 250 mL BG11 medium in glass tubes that were left at 28 °C under constant light (50 µE/m2/s). The cultures were aerated with filter-sterilized ambient air. Mixotrophic cultures were supplemented with 10 mM (final concentration) glucose. For a dark/night cycle, cultures were subjected to a cycle of 12 h darkness and 12 h light (50 µE/m2/s) under constant aeration. In each growth experiment, three glass tubes were grown in parallel. Each experiment was repeated at least three times independently.
Aliquots were taken under sterile conditions every 24 h to measure optical density photometrically at 750 nm. Cultures were checked for contaminations on a regular basis under the light microscope and by plating aliquots on LB plates.
Growth Conditions of H. vulgare for RT-PCR Experiments and IC-MSMS Analysis.
H. vulgare (Golden Promise) seeds were sown on moist, nutrient-free vermiculite and kept at 4 °C for 36 h in darkness. The seeds were then transferred to a climate chamber at 21 °C under continuous light (250 µE/m2/s). Seven hours after the transfer into light (day 1), 20 whole seedlings were collected every 24 h for 8 d for RT-PCR experiments and immediately frozen in liquid N2 and stored at −80 °C for further RNA isolation. The first sampled seeds had already reached germination state 05 (radicle emerged); on day 8, the young plants had reached seedling development stage 11 (second leaf visible), according to the BBCH scale (35). Development of the seedlings stopped after day 8 because of nutrient limitation. For IC-ESI-MSMS analysis, roots from seedlings were collected from day 2 to day 6.
Southern Hybridization to Check the Synechocystis Mutants.
Synechocystis holds several copies of its genome. Therefore, Southern blots were carried out to check whether genes had been completely deleted from all genomic copies. Genomic DNA of the WT and the respective mutants was digested with HindIII, blotted, and hybridized with Dig-labeled probes against a region close to the deleted gene, such that a discrimination between WT and the mutated form was possible. The expected sizes for detected bands for the gene in its original (WT) and its modified (Δ) form are indicated in the respective figure legends (Fig. S2).
Extraction of Metabolites for IC-ESI-MSMS Measurements.
Synechocystis cells were cultivated in 250 mL BG11 in glass tubes. An equivalent of 20 mL cells with an OD750nm of 6 was transferred in precooled tubes on ice and centrifuged 1 min at 8,228 × g at −9 °C. Pellets were frozen in N2 for at least 5 min, resuspended in 300 µL cold (4 °C) 80% methanol, and agitated 15 min at 70 °C to break cells and inactivate enzymes. Samples were placed on ice. Cells were further broken with glass beads (0.17–0.18 mm) by vortexing 3 × 3 min with intermittent cooling on ice. After centrifugation at 800 × g for 1 min at 4 °C, the liquid phase was removed and centrifuged at 1,300 × g for 10 min at 4 °C. The supernatant was further centrifuged for 15 min at 20,817 × g at 4 °C. The sample was mixed with chloroform (1/3 volume of the supernatant) and agitated 5 min at 37 °C. Phase separation was induced by the addition of H2O (two times the volume of chloroform). The samples were centrifuged 5 min at 20,817 × g. The upper polar fraction containing the metabolites was freeze-dried overnight and resuspended in H2O for analysis.
To extract metabolites from barley (H. vulgare) roots, 100 mg frozen fresh plant tissue was used. One milliliter extraction buffer containing chloroform/methanol in a ratio of 1:1 (V/V) was added. The mixture allows the extraction of lipophilic and hydrophilic substances together. Samples were mixed thoroughly and placed on a vortex at 4 °C while shaking carefully for at least 20 min, and 300 µL HPLC-grade water was added to each sample and mixed carefully. Subsequently, samples were centrifuged at 20,817 × g at 4 °C for 10 min, and the supernatant was transferred in a new tube and dried in a speed vac (Christ RVC2-33IR) for 2 h at 35 °C. The pellet was resuspended in 100–200 µL ultrapure water and used immediately for the measurement of desired compounds.
Zwf in Vitro Measurements.
Cells of Synechocystis WT and ΔpfkΔzwf were harvested, resuspended in 100 mM Tris at pH 8.0 and 150 mM NaCl, and broken with glass beads (diameter, 0.17–0.18 mm) by vortexing 10 min at 4 °C. After centrifugation at 800 × g for 1 min at 4 °C, the liquid phase was removed and centrifuged at 1,300 × g for 10 min at 4 °C. The resulting homogenate was centrifuged at 20,817 × g at 4 °C. Protein contents were determined via a Bradford assays. BSA was used as a standard.
Glucose-6P dehydrogenase activity was measured by the conversion of NADP to NADPH on addition of glucose-6P. A kinetic and an endpoint determination were carried out. Aliquots of cell-free homogenates of Synechocystis WT and ΔpfkΔzwf containing 90 µg protein were measured in 1-mL assays that contained 1 mM NADP and 3 mM glucose 6P in 100 mM Tris, 150 mM NaCl at pH 8 at 30 °C. The measurements were started by the addition of glucose-6P, and the consumption of NADP was followed at 340 nm. For the endpoint measurement, an additional assay was incubated for 150 min, and the absorption change was determined at 340 nm.
RT-PCR in H. vulgare.
Frozen H. vulgare seedlings were ground to a powder in the presence of liquid nitrogen, using a pestle and mortar. RNA was isolated with TRIfast-reagent (Peqlab) according to the manufacturer´s instructions. Genomic DNA was digested, and 1 µg RNA was transcribed to cDNA with QuantiTec-Reverse-Transcription kit (Qiagen) with oligo-dT-primers according to the manufacturer´s instructions. cDNA was diluted 1:3 in 40 µL water, and 1 µL cDNA was subjected to PCR. The primers for the detection of KDPG mRNA were constructed to bind in two adjacent exons so the product would span the intron, to discriminate between genomic DNA with introns and spliced mRNA without intron (Table S3). The expected PCR product size, including intron, is 466 bp; for a PCR product without intron, 222 bp were expected.
NMR Measurements.
For NMR analyses, 2-mL aliquots of 250-mL cultures grown in glass tubes were taken every 24 h and centrifuged for 15 min at 20,000 × g. The cell-free supernatants were directly frozen at −20 °C. After thawing, 550 μL cell-free supernatant was transferred into an NMR tube, and 50 μL D2O (Euroiso-top), including trimethylsilylpropanoic as the internal reference compound, was added. The signal of the internal trimethylsilylpropanoic standard was set to 0 ppm. 1d-1H-NMR spectra using the excitation sculpting sequence for water resonance suppression were recorded on a Bruker DRX 500 MHz NMR spectrometer controlled by Topspin 1.3 software at 300 K, using a 90° pulse width of 14.2 μs, a spectral width of 16 ppm, a 2-s acquisition time, 128 scans, and a repetition delay of 2 s.
Identification of Metabolites via IC-ESI-MSMS.
The separation and detection of metabolites was carried out using an ion chromatography system (Dionex Thermo Fisher) connected to a triple quadrupole mass spectrometer QQQ6490 (Agilent Technologies). Separated compounds were ionized at atmospheric pressure with electrospray and directed to the mass spectrometer via Jetstream spraying. The control of the complete system, recording of the spectra, and data acquisition were performed with the Chromeleon software, release 7.0 (Dionex GmbH), and MassHunter software, release B.07.01. To detect gluconate and KDPG, an ICS5000 system (Dionex Thermo Fisher) was used, including a gradient pump Detector Compartment (DC), an autosampler, and a conductivity detector. Separation of the metabolites was carried out using a high-capacity ion exchange column (AS11-HC, anion column high capacity; 250 × 2 mm) connected to a guard column of the same material (AG 11-HC; 10 × 2 mm) and an anion trap column-1, which is placed between the eluents and separation columns to remove the anions present in the solutions. The gradient was accomplished with purest water (buffer A; Millipore) and a concentrated potassium solution EGCIII KOH (buffer B; Dionex), and the corresponding gradient was produced using an eluent generator version SP at a flow rate of 0.32 mL per minute and heated at 35 °C during the whole measurement. The gradient was produced by changes of the buffer B as follows: 0–4 min at 4%, 4–10 min at 15%, 10–18 min at 80%, and 18–25 min at 4%. The duration of the run is 25 min.
ESI-MS/MS analysis was conducted in the negative ionization mode, and the following parameters were used: desolvation temperature, 250 °C; nitrogen gas of 720 L/h, with a heater temperature of 250 °C; capillary voltage, 3.5 KV; and different dwell times between 40 and 200 s. Collision energy differed among the compounds and was in the range between 6 and 50 eV for different masses. Deprotonated ions [M-H]− were monitored with a span of 1 amu. Multiple-reactions monitoring was performed to identify individual compounds accurately. This allows minimization of parallel monitoring and enhancing the sensitivity. First, we measured various concentrations of pure standards including gluconate and KDPG between 0.1 and 1,000 pmol on column and found that detection limits for gluconate and KDPG were 0.1 and 0.5 pmol, respectively.
To test the reliability and the recovery rate for the detected metabolites, we used five independent replicates of barley material spiked with external standards, five samples without standards, and an additional five samples without plant material and external standards. All samples were subjected to the same extraction protocol described, and metabolites were measured by ion chromatography combined with triple quad MS. We found the following recovery rates (average ± SE; n = 5): gluconate, 91.4 ± 8.3, and KDPG, 76.4 ± 5.5. Pure standards for gluconate and KDPG were prepared, and specific masses were determined using a MassHunter Optimizer 7.1.7109.0. The following specific masses were obtained: gluconate (129, 74.9, 58.9, and 57) and KDPG (177.04). Out of the detected masses, 74.9 (gluconate) and 177.04 (KDPG) were used for monitoring and quantification of the compounds. Quantification was performed with authentic standards at different concentrations.
Phylogenetic Analyses of Eda.
Homologous amino acid sequences for eda from H. vulgare among viridiplantae, rhodophyta, and metazoa in the National Center for Biotechnology Information (NCBI) nr database were collected using the BLAST (41) engine at NCBI on May 29 and June 1, 2015, with an expectation threshold not greater than 10−10. Homologous sequences among prokaryotes were collected using BLASTp version 2.2.29+ against the prokaryotic genomes in RefSeq March 2012, with an expectation threshold not greater than 10−10 and −max_target_sEqs. 5000. Prochlorococcus marinus str. MIT 9312 eda sequence was added to the dataset. An all-against-all bidirectional best BLAST hit approach (36) was performed, and global alignments were calculated for remaining pairs with the needle from EMBOSS version 6.6.0 (37). Only pairs with a global amino acid identity of at least 20% were kept. Eukaryotic and prokaryotic pairs were clustered separately with MCL version 14-137 (31), based on global sequence identity with option –I 5.0. The largest resulting cluster from each group was retained. Only one representative of each prokaryotic taxonomic group was retained, and for Synechocystis sp. PCC 6803, only one strain was retained. For cyanobacteria, all strains in the cluster were retained. Uncultured termite group1 bacterium was also discarded. Sequences from P. marinus sp. MIT 9313 and NATL2A were added to the dataset. In the eukaryotic cluster, the following redundant in-paralogues were discarded because they grouped with the remaining copy in the respective organism: ERM99071.1 (Amborella trichopoda), XP_003534460.1 (Glycine max), XP_012068735.1 (Jatropha curcas), KCW65854.1 (Eucalyptus grandis), XP_002987165.1 (Selaginella moellendorffii), XP_010251919.1 (Nelumbo nucifera), XP_010312181.1 (Solanum lycopersicum), and XP_011655527.1 (Cucumis sativus). Prokaryotic and eukaryotic sequences were joined and aligned with mafft version 7.164b (32) and conserved alignment region extracted using extractalign from EMBOSS version 6.6.0 (37) with option –regions 107–394. Alignment was recoded into PHYLIP format, using clustalw version 2.1 (38) with –convert and –output = phylip. Best fit model was estimated using ProtTest version 3.4 (39) with –LG –WAG –I –G –IG –F –AICC –S 2 –s BEST. One hundred phylogenetic bootstrap sample trees were calculated using seqboot from PHYLIP version 3.695 (40) and PhyML version 20120412 (33), with –m LG –f m –v e –c 4 –a e –s BEST –o tlr, and bootstrap values calculated with consense from PHYLIP version 3.695 (40) were transferred to the tree based on the original alignment.
Phylogenetic Analyses of Phosphogluconate Dehydratase.
Homologous amino acid sequences for Phosphogluconate dehydratase (edd) from P. tricornutum CCAP 1055/1 among eukaryotes in the RefSeq database were collected using the BLAST (41) engine at NCBI in July 2015 with an expectation threshold not greater than 10−10. Homologous sequences among prokaryotes were collected using blastp version 2.2.29+ against the prokaryotic genomes in RefSeq March 2012 with an expectation threshold not greater than 10−10 and –max_target_sEqs. 5000. An all-against-all bidirectional best BLAST hit approach (36) was performed on all prokaryotic and eukaryotic sequences together, and global alignments were calculated for remaining pairs with the needle from EMBOSS version 6.6.0 (37). Only pairs with a global amino acid identity of at least 25% were kept. Eukaryotic and prokaryotic pairs were clustered separately with MCL version 14-137 (31), based on global sequence identity, with –I 5.0. The largest resulting cluster from each group was retained. Only one representative of each prokaryotic taxonomic group was retained, with the exception of cyanobacteria, where more strains were sampled. Uncultured termite group1 bacterium was discarded. In the eukaryotic cluster, the following redundant possible in-paralogue was discarded because it grouped with the remaining copy in the organism: NP_001189959.1 (Arabidopsis thaliana). The following sequences were discarded because of possible sequence contamination: XP_005971683.1, XP_005965752.1, XP_005981413.1, XP_005966131.1, XP_005981131.1 (Pantholops hodgsonii), XP_007460116.1 (Lipotes vexillifer), and XP_003390882.1 (Amphimedon queenslandica). The following possible paraloguous sequences were also discarded: XP_004252330.1 (S. lycopersicum), XP_006346583.1 (Solanum tuberosum), and NP_001170508.1 (Zea mays). Prokaryotic and eukaryotic sequences were joined and aligned with mafft version 7.164b (32) and conserved alignment region extracted using extractalign from EMBOSS version 6.6.0 (37) with –regions 110–980. Alignment was recoded into PHYLIP format, using clustalw version 2.1 (38) with –convert and –output = phylip. One hundred phylogenetic bootstrap sample trees were calculated using seqboot from PHYLIP version 3.695 (40) and PhyML version 20120412 (33), with options –m LG –f m –v e –c 4 –a e –s BEST –o tlr, and bootstrap values calculated with consense from PHYLIP version 3.695 (40) were transferred to the tree based on the original alignment.
Supplementary Material
Acknowledgments
We thank Rüdiger Schulz for continuous support and for the scientific freedom he provides in his laboratory; Claudia Marquardt, Lennard Basenau, and Melanie Roggendorf for technical assistance; Christoph Wittmann, Judith Becker, and Tal Dagan for helpful discussions; members of the Karin Krupinska laboratory (Ulrike Voigt, Anke Schäfer, Mirl Trösch, Julien Hollmann, and Karin Krupinska) for helpful advice on experiments with H. vulgare and for providing access to equipment; and Mirl Trösch for cDNA from H. vulgare. K.G. thanks Dietrich Ober for being a wonderful mentor and we thank the reviewers for their attention to our article. This work was supported by grants from the Deutsche Forschungsgemeinschaft (Gu1522/1-1), Fazit-Stiftung, Deutsche Bundesstiftung Umwelt, and the cluster of Excellence “The Future Ocean” (CP1343).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1521916113/-/DCSupplemental.
References
- 1.Müller M, et al. Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiol Mol Biol Rev. 2012;76(2):444–495. doi: 10.1128/MMBR.05024-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stincone A, et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol Rev Camb Philos Soc. 2014;90(3):927–963. doi: 10.1111/brv.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flamholz A, Noor E, Bar-Even A, Liebermeister W, Milo R. Glycolytic strategy as a tradeoff between energy yield and protein cost. Proc Natl Acad Sci USA. 2013;110(24):10039–10044. doi: 10.1073/pnas.1215283110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bräsen C, Esser D, Rauch B, Siebers B. Carbohydrate metabolism in Archaea: Current insights into unusual enzymes and pathways and their regulation. Microbiol Mol Biol Rev. 2014;78(1):89–175. doi: 10.1128/MMBR.00041-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siebers B, Schönheit P. Unusual pathways and enzymes of central carbohydrate metabolism in Archaea. Curr Opin Microbiol. 2005;8(6):695–705. doi: 10.1016/j.mib.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Bennett BD, et al. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat Chem Biol. 2009;5(8):593–599. doi: 10.1038/nchembio.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pelroy RA, Rippka R, Stanier RY. Metabolism of glucose by unicellular blue-green algae. Arch Mikrobiol. 1972;87(4):303–322. doi: 10.1007/BF00409131. [DOI] [PubMed] [Google Scholar]
- 8.Née G, Zaffagnini M, Trost P, Issakidis-Bourguet E. Redox regulation of chloroplastic glucose-6-phosphate dehydrogenase: A new role for f-type thioredoxin. FEBS Lett. 2009;583(17):2827–2832. doi: 10.1016/j.febslet.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 9.Cossar JD, Rowell P, Stewart WDP. Thioredoxin as a modulator of glucose-6-phosphate dehydrogenase in a N2-fixing cyanobacterium. Microbiology. 1984;130:991–998. [Google Scholar]
- 10.Knowles VL, Plaxton WC. From genome to enzyme: Analysis of key glycolytic and oxidative pentose-phosphate pathway enzymes in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2003;44(7):758–763. doi: 10.1093/pcp/pcg086. [DOI] [PubMed] [Google Scholar]
- 11.Johnson X, Alric J. Central carbon metabolism and electron transport in Chlamydomonas reinhardtii: Metabolic constraints for carbon partitioning between oil and starch. Eukaryot Cell. 2013;12(6):776–793. doi: 10.1128/EC.00318-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plaxton WC, Podesta FE. The functional organization and control of plant respiration. Crit Rev Plant Sci. 2006;25(2):159–198. [Google Scholar]
- 13.Jansen T, et al. Characterization of trophic changes and a functional oxidative pentose phosphate pathway in Synechocystis sp PCC 6803. Acta Physiol Plant. 2010;32(3):511–518. [Google Scholar]
- 14.Zhou J, et al. Discovery of a super-strong promoter enables efficient production of heterologous proteins in cyanobacteria. Sci Rep. 2014;4:4500. doi: 10.1038/srep04500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reher M, Fuhrer T, Bott M, Schönheit P. The nonphosphorylative Entner-Doudoroff pathway in the thermoacidophilic euryarchaeon Picrophilus torridus involves a novel 2-keto-3-deoxygluconate- specific aldolase. J Bacteriol. 2010;192(4):964–974. doi: 10.1128/JB.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S, Lee SB. Catalytic promiscuity in dihydroxy-acid dehydratase from the thermoacidophilic archaeon Sulfolobus solfataricus. J Biochem. 2006;139(3):591–596. doi: 10.1093/jb/mvj057. [DOI] [PubMed] [Google Scholar]
- 17.Fong S, Machajewski TD, Mak CC, Wong C. Directed evolution of D-2-keto-3-deoxy-6-phosphogluconate aldolase to new variants for the efficient synthesis of D- and L-sugars. Chem Biol. 2000;7(11):873–883. doi: 10.1016/s1074-5521(00)00035-1. [DOI] [PubMed] [Google Scholar]
- 18.Scopes RK. Use of differential dye-ligand chromatography with affinity elution for enzyme purification: 2-keto-3-deoxy-6-phosphogluconate aldolase from Zymomonas mobilis. Anal Biochem. 1984;136(2):525–529. doi: 10.1016/0003-2697(84)90256-2. [DOI] [PubMed] [Google Scholar]
- 19.Muñoz-Marín MdelC, et al. Prochlorococcus can use the Pro1404 transporter to take up glucose at nanomolar concentrations in the Atlantic Ocean. Proc Natl Acad Sci USA. 2013;110(21):8597–8602. doi: 10.1073/pnas.1221775110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gómez-Baena G, et al. Glucose uptake and its effect on gene expression in prochlorococcus. PLoS One. 2008;3(10):e3416. doi: 10.1371/journal.pone.0003416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pelroy RA, Bassham JA. Photosynthetic and dark carbon metabolism of glucose by unicellular blue-green algae. Arch Mikrobiol. 1972;86(1):25–38. doi: 10.1007/BF00412397. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi H, Uchimiya H, Hihara Y. Difference in metabolite levels between photoautotrophic and photomixotrophic cultures of Synechocystis sp. PCC 6803 examined by capillary electrophoresis electrospray ionization mass spectrometry. J Exp Bot. 2008;59(11):3009–3018. doi: 10.1093/jxb/ern157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullineaux CW. Co-existence of photosynthetic and respiratory activities in cyanobacterial thylakoid membranes. Biochim Biophys Acta. 2014;1837(4):503–511. doi: 10.1016/j.bbabio.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Steglich C, et al. The challenge of regulation in a minimal photoautotroph: Non-coding RNAs in Prochlorococcus. PLoS Genet. 2008;4(8):e1000173. doi: 10.1371/journal.pgen.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dufresne A, et al. Genome sequence of the cyanobacterium Prochlorococcus marinus SS120, a nearly minimal oxyphototrophic genome. Proc Natl Acad Sci USA. 2003;100(17):10020–10025. doi: 10.1073/pnas.1733211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narainsamy K, Cassier-Chauvat C, Junot C, Chauvat F. High performance analysis of the cyanobacterial metabolism via liquid chromatography coupled to a LTQ-Orbitrap mass spectrometer: Evidence that glucose reprograms the whole carbon metabolism and triggers oxidative stress. Metabolomics. 2013;9(1):21–32. [Google Scholar]
- 27.Moore LR. More mixotrophy in the marine microbial mix. Proc Natl Acad Sci USA. 2013;110(21):8323–8324. doi: 10.1073/pnas.1305998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zubkov MV, Tarran GA. High bacterivory by the smallest phytoplankton in the North Atlantic Ocean. Nature. 2008;455(7210):224–226. doi: 10.1038/nature07236. [DOI] [PubMed] [Google Scholar]
- 29.Fabris M, et al. The metabolic blueprint of Phaeodactylum tricornutum reveals a eukaryotic Entner-Doudoroff glycolytic pathway. Plant J. 2012;70(6):1004–1014. doi: 10.1111/j.1365-313X.2012.04941.x. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann D, Gutekunst K, Klissenbauer M, Schulz-Friedrich R, Appel J. Mutagenesis of hydrogenase accessory genes of Synechocystis sp. PCC 6803. Additional homologues of hypA and hypB are not active in hydrogenase maturation. FEBS J. 2006;273(19):4516–4527. doi: 10.1111/j.1742-4658.2006.05460.x. [DOI] [PubMed] [Google Scholar]
- 31.Enright AJ, Van Dongen S, Ouzounis CA. An efficient algorithm for large-scale detection of protein families. Nucleic Acids Res. 2002;30(7):1575–1584. doi: 10.1093/nar/30.7.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30(14):3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guindon S, et al. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst Biol. 2010;59(3):307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 34.Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology. 1979;111:1–61. [Google Scholar]
- 35.Lancashire PD, et al. A uniform decimal code for growth stages of crops and weeds. Ann Appl Biol. 1991;119(3):561–601. [Google Scholar]
- 36.Tatusov RL, Koonin EV, Lipman DJ. A genomic perspective on protein families. Science. 1997;278(5338):631–637. doi: 10.1126/science.278.5338.631. [DOI] [PubMed] [Google Scholar]
- 37.Rice P, Longden I, Bleasby A. (EMBOSS: The european molecular biology open software suite. Trends Genet. 2000;16(6):276–277. doi: 10.1016/s0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- 38.Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 39.Abascal F, Zardoya R, Posada D. ProtTest: Selection of best-fit models of protein evolution. Bioinformatics. 2005;21(9):2104–2105. doi: 10.1093/bioinformatics/bti263. [DOI] [PubMed] [Google Scholar]
- 40.Felsenstein J. Inferring phylogenies from protein sequences by parsimony, distance, and likelihood methods. Methods Enzymol. 1996;266:418–427. doi: 10.1016/s0076-6879(96)66026-1. [DOI] [PubMed] [Google Scholar]
- 41.Altschul SF, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allard J, Grochulski P, Sygusch J. Covalent intermediate trapped in 2-keto-3-deoxy-6- phosphogluconate (KDPG) aldolase structure at 1.95-A resolution. Proc Natl Acad Sci USA. 2001;98(7):3679–3684. doi: 10.1073/pnas.071380898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.