Abstract
Purpose
To determine the benefit of radiation therapy (RT) in resolution of neurologic symptoms and deficits and whether the type of RT fields influences central nervous system (CNS) control in adults with CNS leukemia.
Methods and Materials
A total of 163 adults from 1996 to 2012 were retrospectively analyzed. Potential associations between use of radiation and outcome were investigated by univariate and multivariate analysis.
Results
The median survival time was 3.8 months after RT. Common presenting symptoms were headache in 79 patients (49%), cranial nerve VII deficit in 46 (28%), and cranial nerve II deficit in 44 (27%). RT was delivered to the base of skull in 48 patients (29%), to the whole brain (WB) in 67 (41%), and to the craniospinal axis (CS) in 48 (29%). Among 149 patients with a total of 233 deficits, resolution was observed in 34 deficits (15%), improvement in 126 deficits (54%), stability in 34 deficits (15%), and progression in 39 deficits (17%). The 12-month CNS progression-free survival was 77% among those receiving CS/WB and 51% among those receiving base of skull RT (P = .02). On multivariate analysis, patients who did not undergo stem cell transplantation after RT and base of skull RT were associated with worse CNS progression-free survival.
Conclusions
Improvement or resolution of symptoms occurred in two thirds of deficits after RT. Comprehensive radiation to the WB or CS seems to offer a better outcome, especially in isolated CNS involvement.
Introduction
Leukemia involving the central nervous system (CNS) has traditionally resulted in a very poor prognosis, with efforts focused on preventing CNS relapse rather than treatment once relapse has occurred (1, 2). Without CNS prophylaxis, CNS involvement occurs in <5% of patients with acute myeloid leukemia (2) compared with 25% to 50% in patients with lymphoid leukemias. The use of radiation therapy (RT) has been marginalized over the past 2 decades with the introduction of upfront CNS penetrating chemotherapy with high-dose methotrexate and cytosine arabinoside. There is also the added concern about the unwarranted side effects of RT (3–6).
With the increasing number of patients who undergo multiple salvage regimens and thus survive multiple systemic relapses, CNS relapse and its treatment have emerged as important components of the disease course (7–10). Previous work has been done to clarify the symptoms of CNS leukemia and the benefit of RT; however, little information exists regarding extent of the optimal RT field (11–14). To control CNS disease, RT can be focused on the base of skull, the whole brain, or the entire craniospinal axis. The purpose of this study was to clarify the presentation of CNS leukemia, determine the benefit of RT in symptom resolution, and assess which factors, including the type of RT, influence CNS control in adults with CNS leukemia treated in the modern era.
Methods and Materials
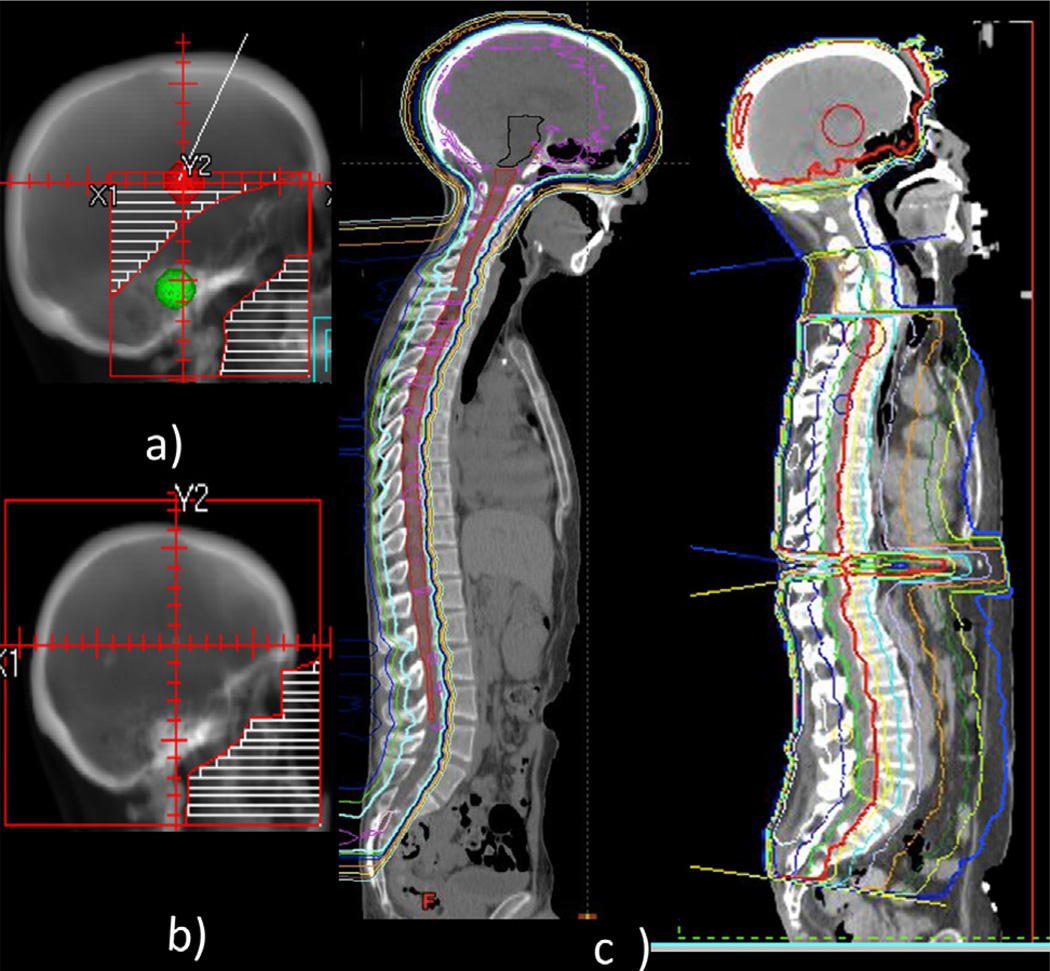
The institutional review board of our institution approved this study (DR10-0992). A retrospective analysis was conducted of 229 patients with pathologic or radiographic evidence of CNS leukemia treated with RT between January 1, 1996, and December 31, 2012. Patients under 18 years of age (n=66) were excluded. Pediatric patients were excluded because of disparities in treatment regimens among these patients. Available pathologic results were confirmed by a hematopathologist at our institution. Demographic information was obtained, including age, gender, race, and date of diagnosis. CNS disease at presentation or relapse was confirmed by any of the following: (1) the demonstration of blast cells within the cerebrospinal fluid (CSF) (ruling out blood contamination in patients with circulating blasts cells in peripheral blood at the time of the lumbar puncture) according to criteria set for the European Organization for Research and Treatment of Cancer study 58,881 (15); (2) computed tomography (CT) or magnetic resonance imaging (MRI) results strongly suggestive of leptomeningeal spread; and (3) new onset of neurologic deficits in the setting of presenting or relapsed leukemia that could not be explained by another cause. Treatment characteristics included the type of RT, previous systemic treatments, intrathecal (IT) treatments, and stem cell transplantation. Typical base of skull, whole brain, and craniospinal axis fields are shown in Figure 1.
Fig. 1.
Common radiation fields to treat central nervous system leukemia include base of skull (a), whole brain (b), or entire craniospinal axis (c) with proton (left) or photon (right).
Study endpoints
CNS progression was defined as positive CSF cytology, radiographic evidence of gross disease, or new onset of neurologic deficits after completion of RT for CNS disease.
Statistical analysis
Data analysis was performed with Stata/IC 12.0 statistical software. Pearson’s χ2 analysis was used to assess measures of univariate association in frequency tables. Unadjusted associations between treatment groups and outcomes were compared by survival analysis and the Kaplan-Meier log rank test. A P value ≤.05 was considered to be statistically significant. Statistical tests were based on a 2-sided significance level.
A Cox proportional hazards model was used for both univariate and multivariate analyses to assess the effect of patient characteristics and other prognostic factors of significance on the endpoint, which was CNS progression-free survival. All variables were assessed on a univariate basis, and factors with significance of .25 or less were assessed for multivariate analysis by the use of backward elimination. The estimated hazard ratio with 95% confidence interval is reported.
Results
A total of 163 patients met the inclusion criteria. Baseline patient and treatment characteristics are included in Table 1. A total of 106 patients were male (65%), and the median age was 35 years (range, 18–79 years). acute myeloid leukemia (AML), was present in 66 patients (41%), acute lymphoblastic leukemia (ALL), in 66 patients (41%) and chronic myeloid leukemia (CML)/chronic lymphocytic leukemia (CLL) in 28 patients (17%). Pathology via biopsy or positive CSF findings was detected in 59 patients (36%), with imaging findings positive in 26 patients (16%) and both via biopsy or positive CSF findings and imaging findings in 67 patients (41%). A total of 11 patients (7%) had neither pathology nor imaging findings consistent with CNS leukemia but had neurologic symptoms unexplained by other causes. CNS disease was present at initial disease presentation in 18 patients (11%).
Table 1.
Demographic, tumor characteristics and treatment stratified by type of radiation therapy received (N=163)
| All patients | Base of skull | Whole brain | Craniospinal | ||
|---|---|---|---|---|---|
| Characteristic | n (%) | n (%) | n (%) | n (%) | P value |
| Total | 163 (100.0) | 48 (29.4) | 67 (41.1) | 48 (29.4) | |
| Age, y | |||||
| Median | 35 | 39.5 | 39 | 29.5 | |
| 18–29 | 66 (40.5) | 16 (33.3) | 26 (38.8) | 24 (50.0) | .31 |
| 30–39 | 26 (16.0) | 8 (16.7) | 8 (11.9) | 10 (20.8) | |
| 40–49 | 33 (20.2) | 11 (22.9) | 12 (17.9) | 10 (20.8) | |
| 50–59 | 18 (11.0) | 7 (14.6) | 10 (14.9) | 1 (2.1) | |
| 60–74 | 17 (10.4) | 5 (10.4) | 10 (14.9) | 2 (4.2) | |
| ≥75 | 3 (1.8) | 1 (2.1) | 1 (1.5) | 1 (2.1) | |
| Gender | |||||
| Male | 106 (65.0) | 36 (75.0) | 37 (55.2) | 33 (68.8) | .07 |
| Female | 57 (35.0) | 12 (25.0) | 30 (44.8) | 15 (31.3) | |
| Type | |||||
| AML | 66 (40.5) | 21 (43.8) | 31 (46.3) | 14 (29.2) | .22 |
| ALL | 66 (40.5) | 19 (39.6) | 22 (32.8) | 25 (52.1) | |
| CML/CLL | 28 (17.2) | 7 (14.6) | 14 (20.9) | 7 (14.6) | |
| Pathology results positive (CSF or biopsy) | |||||
| No | 37 (22.7) | 16 (33.3) | 13 (19.4) | 8 (16.7) | .11 |
| Yes | 126 (77.3) | 32 (66.7) | 54 (80.6) | 40 (83.3) | |
| Imaging positive (CT or MRI) | |||||
| No | 70 (42.9) | 24 (50.0) | 23 (34.3) | 23 (47.9) | .18 |
| Yes | 93 (57.1) | 24 (50.0) | 44 (65.7) | 25 (52.1) | |
| CNS disease at presentation | |||||
| No | 145 (89.0) | 42 (87.5) | 61 (91.0) | 42 (87.5) | .78 |
| Yes | 18 (11.0) | 6 (12.5) | 6 (9.0) | 6 (12.5) | |
| Prophylactic intrathecal chemotherapy | |||||
| No | 121 (74.2) | 33 (68.8) | 54 (80.6) | 34 (70.8) | .29 |
| Yes | 42 (25.8) | 15 (31.3) | 13 (19.4) | 14 (29.2) | |
| Previous systemic regimens | |||||
| 1 | 66 (40.5) | 29 (60.4) | 23 (34.3) | 14 (29.2) | .04 |
| 2 | 55 (33.7) | 10 (20.8) | 29 (43.3) | 16 (33.3) | |
| 3 | 28 (17.2) | 6 (12.5) | 11 (16.4) | 11 (22.9) | |
| 4 | 11 (6.7) | 3 (6.3) | 3 (4.5) | 5 (10.4) | |
| 5 | 3 (1.8) | 0 (0.0) | 1 (1.5) | 2 (4.2) | |
| Stem cell transplantation before RT | |||||
| No | 125 (76.7) | 35 (72.9) | 53 (79.1) | 37 (77.1) | .68 |
| Yes | 38 (23.3) | 13 (27.1) | 14 (20.9) | 11 (22.9) | |
| Bone marrow positive at CNS diagnosis | |||||
| No | 88 (54.0) | 31 (64.6) | 34 (50.7) | 23 (47.9) | .27 |
| Yes | 75 (46.0) | 17 (35.4) | 33 (49.3) | 25 (52.1) | |
| Intrathecal chemotherapy (No. of cycles) | |||||
| 0 | 20 (12.3) | 7 (14.6) | 9 (13.4) | 4 (8.3) | .10 |
| 1–5 | 44 (27.0) | 13 (27.1) | 17 (25.4) | 14 (29.2) | |
| 5–10 | 49 (30.1) | 18 (37.5) | 23 (34.3) | 8 (16.7) | |
| ≥10 | 50 (30.7) | 10 (20.8) | 18 (26.9) | 22 (45.8) | |
| Route of intrathecal chemotherapy | |||||
| None | 20 (12.3) | 7 (14.6) | 9 (13.4) | 4 (8.3) | .77 |
| LP | 127 (77.9) | 37 (77.1) | 52 (77.6) | 38 (79.2) | |
| Ommaya | 5 (3.1) | 1 (2.1) | 1 (1.5) | 3 (6.3) | |
| Both | 11 (6.7) | 3 (6.3) | 5 (7.5) | 3 (6.3) | |
| Consolidative RT | |||||
| No | 133 (81.6) | 47 (97.9) | 56 (83.6) | 30 (62.5) | <.001 |
| Yes | 30 (18.4) | 1 (2.1) | 11 (16.4) | 18 (37.5) | |
| RT dose, Gy | |||||
| <20 | 33 (20.2) | 8 (16.7) | 16 (23.9) | 9 (18.8) | .55 |
| 20–23.9 | 19 (11.7) | 4 (8.3) | 9 (13.4) | 6 (12.5) | |
| 24 | 82 (50.3) | 27 (56.3) | 34 (50.7) | 21 (43.8) | |
| >24 | 29 (17.8) | 9 (18.8) | 8 (11.9) | 12 (25.0) | |
| Cranial nerve symptoms | |||||
| No | 55 (33.7) | 3 (6.3) | 25 (37.3) | 27 (56.3) | <.001 |
| Yes | 108 (66.3) | 45 (93.8) | 42 (62.7) | 21 (43.8) | |
| Any CNS symptoms | |||||
| No | 41 (25.2) | 2 (4.2) | 17 (25.4) | 22 (45.8) | <.001 |
| Yes | 122 (74.8) | 46 (95.8) | 50 (74.6) | 26 (54.2) | |
| Stem cell transplantation after RT | |||||
| No | 104 (63.8) | 31 (64.6) | 49 (73.1) | 24 (50.0) | .04 |
| Yes | 59 (36.2) | 17 (35.4) | 18 (26.9) | 24 (50.0) | |
Abbreviations: ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia; CI = confidence interval; CLL = chronic lymphocytic leukemia; CML = chronic myeloid leukemia; CN = cranial nerve; CNS = central nervous system; CSF = cerebrospinal spinal fluid; CT = computed tomography; HR = hazard ratio; LP = lumbar puncture; MRI = magnetic resonance imaging; RT = radiation therapy.
Treatment characteristics
Treatment before RT consisted of 1 systemic regimen in 103 patients (63%), 2 regimens in 35 patients (22%) and 3 or more regimens in 25 patients (15%). A total of 20 patients (12%) did not receive IT chemotherapy, 121 patients (74%) received IT chemotherapy before the diagnosis of CNS disease; and 143 patients (88%) received IT chemotherapy after diagnosis of CNS disease. Among patients who received IT chemotherapy before the CNS diagnosis, all received IT chemotherapy after CNS diagnosis. A total of 38 patients (23%) underwent stem cell transplantation before RT. RT was delivered after the first evidence of CNS disease in 155 patients (95%) and with subsequent relapses in 8 patients (5%). RT was delivered to the base of skull in 48 patients (29%), to the whole brain in 67 patients (41%), and to the craniospinal axis in 48 patients (29%). A total of 36 patients (22%) experienced relapse within the CNS after RT.
CNS symptoms
The most common presenting symptoms at diagnosis of CNS leukemia included headache in 79 patients (48.5%), cranial nerve VII deficit in 46 patients (28.2%), cranial nerve II deficit in 44 patients (27.0%), extremity weakness in 26 patients (16), cranial nerve III deficit in 23 patients (14%), and cranial nerve VI deficit in 17 patients (10.4%) (Table 2). A single cranial nerve was involved in 70 patients (42.9%), 2 were involved in 25 patients (15.3%), and 3 or more were involved in 13 patients (8.0%). A total of 33 patients (20.3%) had only a positive lumbar puncture result without neurologic symptoms.
Table 2.
Neurologic symptoms on presentation with central nervous system leukemia (n = 163)
| Neurologic symptoms | n (%) |
|---|---|
| Headache | 79 (48.5) |
| Seizure | 6 (3.7) |
| Altered mental status | 6 (3.7) |
| Speech deficit | 8 (4.9) |
| Ataxia | 2 (1.2) |
| Extremity weakness | 26 (16.0) |
| Extremity numbness | 15 (9.2) |
| Urinary | (0.0) |
| CN II | 44 (27.0) |
| CN III | 23 (14.1) |
| CN IV | 3 (1.8) |
| CN V | 16 (9.8) |
| CN VI | 17 (10.4) |
| CN VII | 46 (28.2) |
| CN VIII | 8 (4.9) |
| CN IX/X | 4 (2.5) |
| CN XI | 0 (0.0) |
| CN XII | 3 (1.8) |
| No. of CN affected | |
| None | 55 (33.7) |
| One | 70 (42.9) |
| Two | 25 (15.3) |
| Three | 9 (5.5) |
| Four | 3 (1.8) |
| Five | 1 (0.6) |
Abbreviation: CN = cranial nerve.
Resolution of symptoms
Overall, among those with complete follow-up data regarding symptom resolution (149 patients with a total of 233 deficits), resolution was observed in 34 deficits (16%), improvement in 126 deficits (54%), stability in 34 deficits (15%), and progression in 39 deficits (17%) (Table 3). Headache resolved in 9 patients (16%), improved in 32 patients (58%), was stable in 8 patients (15%), and progressed in 6 patients (11%). Among those with cranial nerve II deficits, symptoms resolved in 5 patients (12%), improved in 22 patients (52%), were stable in 7 patients (17%), and progressed in 8 patients (19%). Among those with cranial nerve VII deficits, symptoms resolved in 7 patients (18%), improved in 16 patients (42%), were stable in 5 patients (13%), and progressed in 10 patients (26%).
Table 3.
Symptomatic outcomes of neurologic signs or symptoms after radiation therapy (n = 149)*
| Resolve | Improve | Stable | Progress | ||
|---|---|---|---|---|---|
| Outcome | n (%) | n (%) | n (%) | n (%) | Total |
| CN II | 5 (11.9) | 22 (52.4) | 7 (16.7) | 8 (19.0) | 42 |
| CN III | 1 (6.7) | 9 (60.0) | 2 (13.3) | 3 (20.0) | 15 |
| CN IV | 1 (33.3) | 2 (66.7) | 0 (0.0) | 0 (0.0) | 3 |
| CN V | 3 (21.4) | 7 (50.0) | 3 (21.4) | 1 (7.1) | 14 |
| CN VI | 5 (31.3) | 6 (37.5) | 2 (12.5) | 3 (18.8) | 16 |
| CN VII | 7 (18.4) | 16 (42.1) | 5 (13.2) | 10 (26.3) | 38 |
| CN VIII | 0 (0.0) | 4 (57.1) | 2 (28.6) | 1 (14.3) | 7 |
| CN IX/X | 0 (0.0) | 1 (33.3) | 1 (33.3) | 1 (33.3) | 3 |
| CN XII | 1 (33.3) | 1 (33.3) | 0 (0.0) | 1 (33.3) | 3 |
| Extremity weakness |
2 (9.1) | 15 (68.2) | 2 (9.1) | 3 (13.6) | 22 |
| Extremity numbness |
0 (0.0) | 11 (73.3) | 2 (13.3) | 2 (13.3) | 15 |
| Headache | 9 (16.4) | 32 (58.2) | 8 (14.5) | 6 (10.9) | 55 |
| Total | 34 (14.6) | 126 (54.1) | 34 (14.6) | 39 (16.7) | 233 |
Abbreviation: CN = cranial nerve.
Sufficient follow-up information was available for 149 patients with a total of 233 deficits.
Survival outcomes
The median survival time for all patients was 3.8 months after RT. The 12-month overall survival was 33% among those with negative bone marrow results at the time of CNS diagnosis, compared with 23% among those with positive bone marrow results (P = .005).
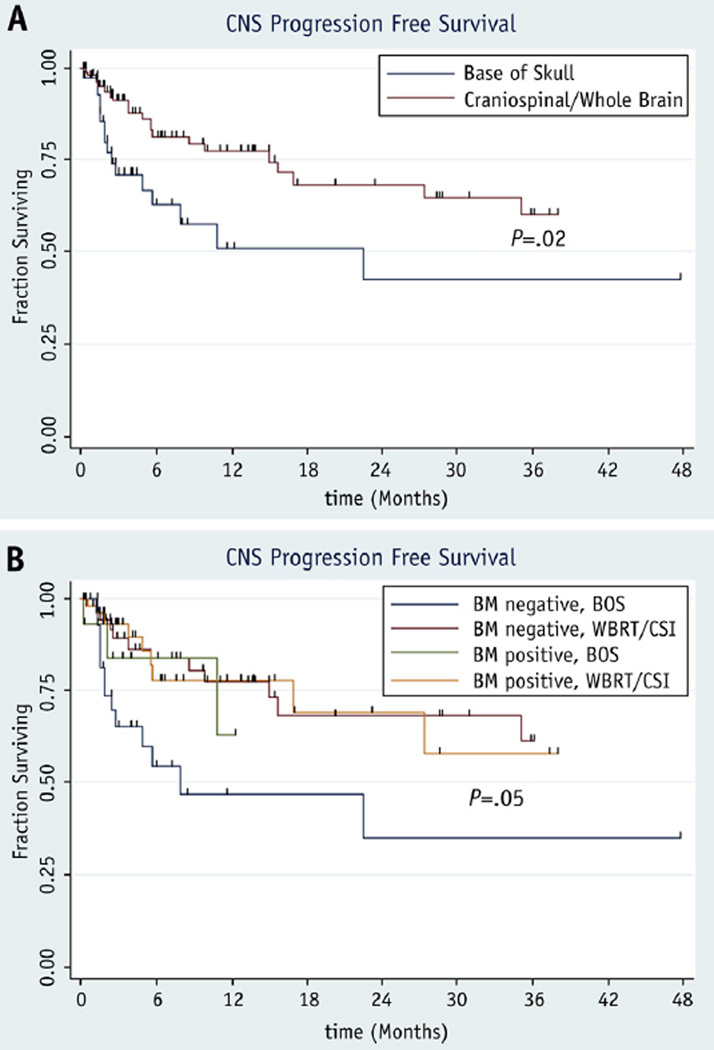
The 12-month CNS progression-free survival after RT was 77% among those receiving craniospinal axis/whole brain RT and 51% among those receiving base of skull RT (P = .02) (Fig. 2a). Patients with negative bone marrow results who received base of skull RT had 12-month CNS progression-free survival of 47%, compared with 77% among those with negative bone marrow results who received craniospinal axis/whole brain RT, 63% among those with positive bone marrow results who received base of skull RT, and 78% among those with positive bone marrow results who received craniospinal axis/whole brain RT (P = .05) (Fig. 2b).
Fig. 2.
Kaplan-Meier progression-free survival curves comparing patients receiving base of skull radiation therapy with those treated by craniospinal or whole brain radiation therapy among all patients (a) and stratified by bone marrow status (b). BM = bone marrow; BOS = base of skull radiation therapy; CNS = central nervous system; CSI = craniospinal radiation therapy; WBRT = whole brain radiation therapy.
Univariate analysis of patient characteristics and treatment factors on overall survival and CNS progression-free survival are shown in Supplement E1 (available at www.redjournal.org). Cranial nerve symptoms, any CNS symptoms, base of skull RT, and no stem cell transplantation after RT were associated with worse CNS progression-free survival. Older age, CML/CLL compared with ALL, cranial nerve symptoms, any CNS symptoms, fewer than 10 IT treatments, and no stem cell transplantation after RT were associated with worse overall survival.
On multivariate analysis, patients who did not receive stem cell transplantation after RT and base of skull RT were associated with worse CNS progression-free survival (Table 4).
Table 4.
Results of Cox multivariate analysis with central nervous system progression-free survival as endpoint (N = 163)
| Therapy | HR | 95% CI | P |
|---|---|---|---|
| Stem cell transplantation after radiation therapy | |||
| Yes | 1 | ||
| No | 3.89 | 1.85–8.17 | <.001 |
| Radiation type | |||
| CS | 1 | ||
| WB | 1.12 | 0.48–2.62 | .80 |
| BOS | 2.84 | 1.22–6.60 | .02 |
Abbreviations: BOS = base of skull radiation therapy; CI = confidence interval; CS = craniospinal radiation therapy; HR = hazard ratio; WB = whole brain radiation therapy.
Discussion
These results represent the largest series of patients with leukemia treated with RT for CNS disease. We found headache, facial nerve, optic nerve, extremity weakness, oculomotor nerve deficit, and abducens nerve deficit to be the most common neurologic manifestations of CNS leukemia, with improvement or resolution in about two thirds of deficits after RT. Patients who received craniospinal axis/whole brain RT had improved CNS progression-free survival. Interestingly, patients with isolated CNS involvement (ie negative bone marrow results) benefited the most from a comprehensive radiation field compared with patients who presented with both CNS and bone marrow involvement.
Strategies to prevent CNS relapse include prophylactic IT chemotherapy, RT, systemic therapy, or a combination of these treatments (1). The Children’s Cancer Group showed that intensive chemotherapy with IT methotrexate during induction, consolidation, and delayed intensification was equivalent to whole brain RT (18 Gy) in preventing CNS relapse in children with ALL (16). However, these results cannot be extrapolated to patients with CNS disease because IT chemotherapy has a maximal effect only in sterilizing subclinical disease in patients with normal CSF flow. One study of 17 patients with leptomeningeal disease found 11 deaths among the 13 patients with abnormal CSF flow, presumably caused by obstruction from gross disease, compared with no deaths among the patients with normal CSF flow (17). They also noted methotrexate toxicity in the 2 patients with delayed flow studies. In vivo studies have shown that IT chemotherapy given by lumbar puncture has low cranial concentrations (18, 19). Nevertheless, in our study, patients who received fewer than 10 IT treatments had worse overall survival on univariate analysis. As opposed to IT chemotherapy, which depends on normal CSF flow, modern RT is able to deliver a relatively homogeneous dose regardless of physiologic deviations. If indeed the chemotherapy delivery to the brain is compromised with CNS disease, intensive local cranial RT may be of benefit, and may explain our findings that patients who received only base of skull RT experienced worse outcomes compared with more comprehensive treatment.
These findings are consistent with previous reports of RT use for CNS leukemia. One institution published a series of 28 leukemia patients treated for cranial nerve palsies without radiographic findings who received RT. Only 2 patients received base of skull RT; the remainder received whole brain RT. The researchers noted resolution of symptoms in 14 patients (50%), improvement in 8 patients (29%), and stable or progressive disease in 4 patients (14%) (12). Gray et al (11) reported on 20 patients with cranial nerve deficits treated with whole brain RT for CNS leukemia or lymphoma to a dose of 10 to 30 Gy in 2- to 3-Gy fractions and noted a 95% response rate and a 44% complete response rate at 3 months.
Over the past century, RT has undergone tremendous technologic advancement, which now allows delivery of an effective dose to the target while minimizing dose to surrounding normal tissues. Historically, orthovoltage radiation was limited by low penetration and high surface dose, leading to significant toxicity and to dose inhomogeneity compared with modern linear accelerator—based RT (20). Over the past several decades, advances in diagnostic imaging with CT and MRI have enabled better target delineation. Treatment planning has moved from 2-dimensional planning with x-ray imaging and hand calculations to 3-dimensional CT-based planning with computer-assisted calculations (21). These advances allow us to accurately determine the precise dose to the target and adjacent normal structures (22). In addition, innovations in image guidance assure proper daily target coverage while minimizing dose to adjacent structures (23). More recent advancements such as proton therapy have allowed craniospinal axis RT to be delivered with reduced toxicity (hematologic, nausea, vomiting, weight loss, and esophagitis) (24).
The neurocognitive effects of the standard regimen for CNS leukemia of 24 Gy in 12 fractions is poorly understood, given that most whole brain RT regimens for solid tumor brain metastasis consist of a higher total dose and higher doses per fraction. Some studies have actually shown an improvement in neurocognitive outcomes after whole brain RT in solid tumors, resulting from a reduced intracranial tumor burden; thus, isolating the effects of radiation alone on cognitive outcomes becomes very challenging (25–29). In addition, the neurocognitive effects of chemotherapy can further confound this analysis (30).
The fact that marrow status affected the CNS progression-free survival indicates that we can use this information to clinically guide the choice of radiation field to use. Presumably, patients with negative marrow results are still chemotherapy sensitive, and in this group CNS involvement represents the sanctuary location of the disease and thus was not adequately addressed by either high-dose or IT chemotherapy. Subsequently, the use of a comprehensive field of radiation can be indicated. This also explains why patients who went on to undergo stem cell transplantation seemed to benefit from comprehensive radiation, given that they need negative marrow to proceed with stem cell transplantation. It is worth noting that patients with positive marrow who received base of skull RT had an unexpectedly high CNS progression-free survival because the majority died shortly after finishing RT with a short follow-up and inability to assess their CNS status.
Although the results of the current study are compelling, several limitations exist. Although our single-institution series did show a statistically significant difference between craniospinal axis/whole brain RT and base of skull RT in CNS progression-free survival, the small numbers and retrospective nature of this study limit its interpretation. There are also other variables that we could not fully account for and that potentially influence progression-free survival, most notably the heterogeneity of prior chemotherapy and the biology of the disease. In addition, decisions regarding optimal management were determined by the individual treating clinicians and could have been affected by significant selection bias. The strength of this series lies in the large numbers, high-quality pathologic and radiographic review, and long-term follow-up information.
In conclusion, we found that RT can palliate neurologic symptoms, with improvement or resolution in about two thirds of deficits. Comprehensive RT fields with whole brain/craniospinal axis contribute to a better outcome, particularly when the marrow is negative at the onset of CNS disease. Patients with a short life expectancy may be treated with base of skull RT only for symptomatic relief; however, those with a longer life expectancy or who go on to receive stem cell tranplantation may be better served with more comprehensive treatment to the craniospinal axis to obtain longer CNS control.
Supplementary Material
Summary.
Headache, cranial nerve VII, cranial nerve II, and cranial nerve III involvement are the most common neurologic manifestations of CNS leukemia. Improvement or resolution is observed in about two thirds of deficits after radiation therapy. On multivariate analysis, patients who did not undergo stem cell transplantation after RT and base of skull RT were associated with worse CNS progression-free survival.
Acknowledgments
Supported in part by a training fellowship from the Keck Center for Interdisciplinary Bioscience Training of the Gulf Coast Consortia (Grant No. T15 LM007093) to G. V. Walker.
Footnotes
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Wellwood J, Taylor K. Central nervous system prophylaxis in haematological malignancies. Intern Med J. 2002;32:252–258. doi: 10.1046/j.1445-5994.2002.00214.x. [DOI] [PubMed] [Google Scholar]
- 2.Shihadeh F, Reed V, Faderl S, et al. Cytogenetic profile of patients with acute myeloid leukemia and central nervous system disease. Cancer. 2012;118:112–127. doi: 10.1002/cncr.26253. [DOI] [PubMed] [Google Scholar]
- 3.Kantarjian HM, O’Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18:547–561. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 4.Omura GA, Moffitt S, Vogler WR, et al. Combination chemotherapy of adult acute lymphoblastic leukemia with randomized central nervous system prophylaxis. Blood. 1980;55:199–204. [PubMed] [Google Scholar]
- 5.Gustafsson G, Schmiegelow K, Forestier E, et al. Improving outcome through two decades in childhood ALL in the Nordic countries: The impact of high-dose methotrexate in the reduction of CNS irradiation. Nordic Society of Pediatric Haematology and Oncology (NOPHO) Leukemia. 2000;14:2267–2275. doi: 10.1038/sj.leu.2401961. [DOI] [PubMed] [Google Scholar]
- 6.Jabbour E, Thomas D, Cortes J, Kantarjian HM, O’Brien S. Central nervous system prophylaxis in adults with acute lymphoblastic leukemia: current and emerging therapies. Cancer. 2010;116:2290–2300. doi: 10.1002/cncr.25008. [DOI] [PubMed] [Google Scholar]
- 7.Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9:257–268. doi: 10.1016/S1470-2045(08)70070-6. [DOI] [PubMed] [Google Scholar]
- 8.Kantarjian HM, Thomas D, Ravandi F, et al. Defining the course and prognosis of adults with acute lymphocytic leukemia in first salvage after induction failure or short first remission duration. Cancer. 2010;116:5568–5574. doi: 10.1002/cncr.25354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanulla M, Schrappe M. Treatment of childhood acute lymphoblastic leukemia. Semin Hematol. 2009;46:52–63. doi: 10.1053/j.seminhematol.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 11.Gray JR, Wallner KE. Reversal of cranial nerve dysfunction with radiation therapy in adults with lymphoma and leukemia. Int J Radiat Oncol Biol Phys. 1990;19:439–444. doi: 10.1016/0360-3016(90)90555-x. [DOI] [PubMed] [Google Scholar]
- 12.Ha CS, Chung WK, Koller CA, et al. Role of radiation therapy to the brain in leukemic patients with cranial nerve palsies in the absence of radiological findings. Leuk Lymphoma. 1999;32:497–503. doi: 10.3109/10428199909058407. [DOI] [PubMed] [Google Scholar]
- 13.Lange CP, Brouwer RE, Brooimans R, et al. Leptomeningeal disease in chronic lymphocytic leukemia. Clin Neurol Neurosurg. 2007;109:896–901. doi: 10.1016/j.clineuro.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Mayadev JS, Douglas JG, Storer BE, et al. Impact of cranial irradiation added to intrathecal conditioning in hematopoietic cell transplantation in adult acute myeloid leukemia with central nervous system involvement. Int J Radiat Oncol Biol Phys. 2011;80:193–198. doi: 10.1016/j.ijrobp.2010.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sirvent N, Suciu S, Rialland X, et al. Prognostic significance of the initial cerebro-spinal fluid (CSF) involvement of children with acute lymphoblastic leukaemia (ALL) treated without cranial irradiation: Results of European Organization for Research and Treatment of Cancer (EORTC) Children Leukemia Group study 58881. Eur J Cancer. 2011;47:239–247. doi: 10.1016/j.ejca.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Tubergen DG, Gilchrist GS, O’Brien RT, et al. Prevention of CNS disease in intermediate-risk acute lymphoblastic leukemia: Comparison of cranial radiation and intrathecal methotrexate and the importance of systemic therapy: A Childrens Cancer Group report. J Clin Oncol. 1993;11:520–526. doi: 10.1200/JCO.1993.11.3.520. [DOI] [PubMed] [Google Scholar]
- 17.Mason WP, Yeh SD, DeAngelis LM. 111Indium-diethylenetriamine pentaacetic acid cerebrospinal fluid flow studies predict distribution of intrathecally administered chemotherapy and outcome in patients with leptomeningeal metastases. Neurology. 1998;50:438–444. doi: 10.1212/wnl.50.2.438. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro WR, Young DF, Mehta BM. Methotrexate: Distribution in cerebrospinal fluid after intravenous, ventricular and lumbar injections. N Engl J Med. 1975;293:161–166. doi: 10.1056/NEJM197507242930402. [DOI] [PubMed] [Google Scholar]
- 19.Bleyer WA, Poplack DG. Intraventricular versus intralumbar methotrexate for central-nervous-system leukemia: Prolonged remission with the Ommaya reservoir. Med Pediatr Oncol. 1979;6:207–213. doi: 10.1002/mpo.2950060304. [DOI] [PubMed] [Google Scholar]
- 20.Bernier J, Hall EJ, Giaccia A. Radiation oncology: A century of achievements. Nat Rev Cancer. 2004;4:737–747. doi: 10.1038/nrc1451. [DOI] [PubMed] [Google Scholar]
- 21.Bucci MK, Bevan A, Roach M., 3rd Advances in radiation therapy: Conventional to 3D, to IMRT, to 4D, and beyond. CA Cancer J Clin. 2005;55:117–134. doi: 10.3322/canjclin.55.2.117. [DOI] [PubMed] [Google Scholar]
- 22.Howell RM, Scarboro SB, Taddei PJ, et al. Methodology for determining doses to in-field, out-of-field and partially in-field organs for late effects studies in photon radiotherapy. Phys Med Biol. 2010;55:7009–7023. doi: 10.1088/0031-9155/55/23/S04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verellen D, De Ridder M, Linthout N, et al. Innovations in image-guided radiotherapy. Nat Rev Cancer. 2007;7:949–960. doi: 10.1038/nrc2288. [DOI] [PubMed] [Google Scholar]
- 24.Brown AP, Barney CL, Grosshans DR, et al. Proton beam craniospinal irradiation reduces acute toxicity for adults with medulloblastoma. Int J Radiat Oncol Biol Phys. 2013;86:277–284. doi: 10.1016/j.ijrobp.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Bentzen SM, Renschler M, et al. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25:1260–1266. doi: 10.1200/JCO.2006.09.2536. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence YR, Li XA, el Naqa I, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76:S20–S27. doi: 10.1016/j.ijrobp.2009.02.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinvorth S, Welzel G, Fuss M, et al. Neuropsychological outcome after fractionated stereotactic radiotherapy (FSRT) for base of skull meningiomas: A prospective 1-year follow-up. Radiother Oncol. 2003;69:177–182. doi: 10.1016/s0167-8140(03)00204-4. [DOI] [PubMed] [Google Scholar]
- 28.Steinvorth S, Wenz F, Wildermuth S, et al. Cognitive function in patients with cerebral arteriovenous malformations after radiosurgery: Prospective long-term follow-up. Int J Radiat Oncol Biol Phys. 2002;54:1430–1437. doi: 10.1016/s0360-3016(02)03800-2. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong C, Ruffer J, Corn B, et al. Biphasic patterns of memory deficits following moderate-dose partial-brain irradiation: Neuropsychologic outcome and proposed mechanisms. J Clin Oncol. 1995;13:2263–2271. doi: 10.1200/JCO.1995.13.9.2263. [DOI] [PubMed] [Google Scholar]
- 30.Buizer AI, de Sonneville LM, Veerman AJ. Effects of chemotherapy on neurocognitive function in children with acute lymphoblastic leukemia: A critical review of the literature. Pediatr Blood Cancer. 2009;52:447–454. doi: 10.1002/pbc.21869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.