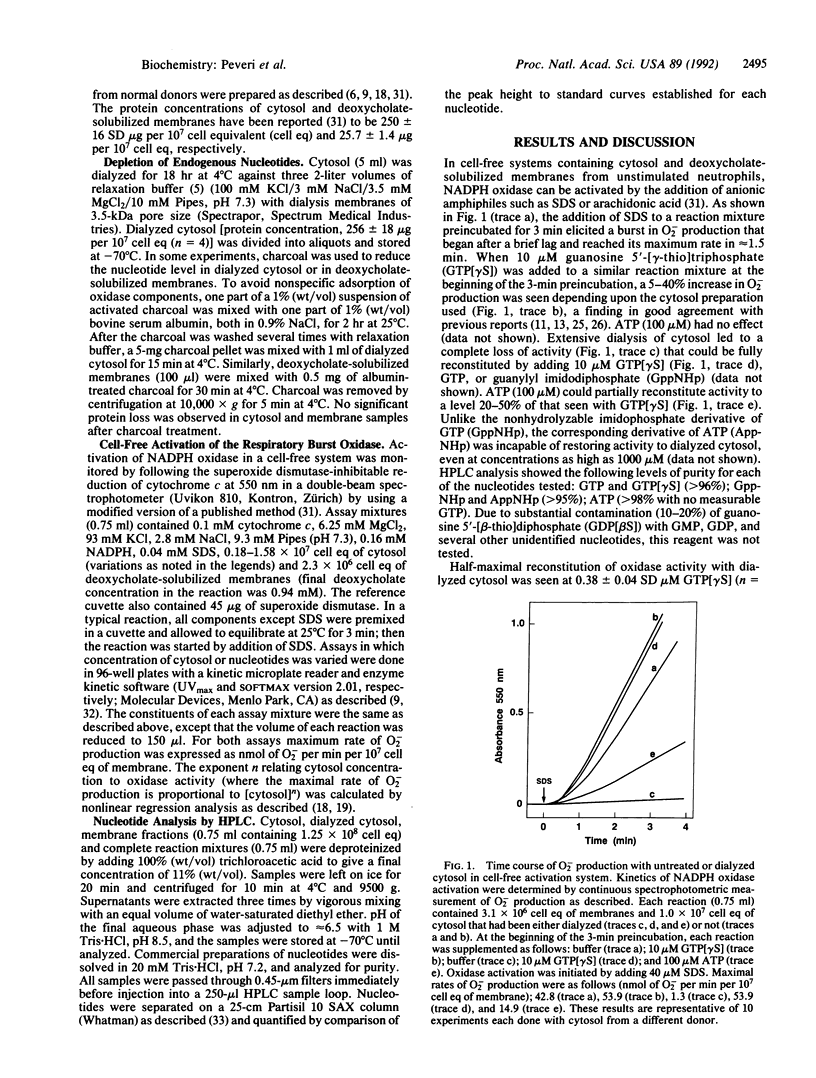
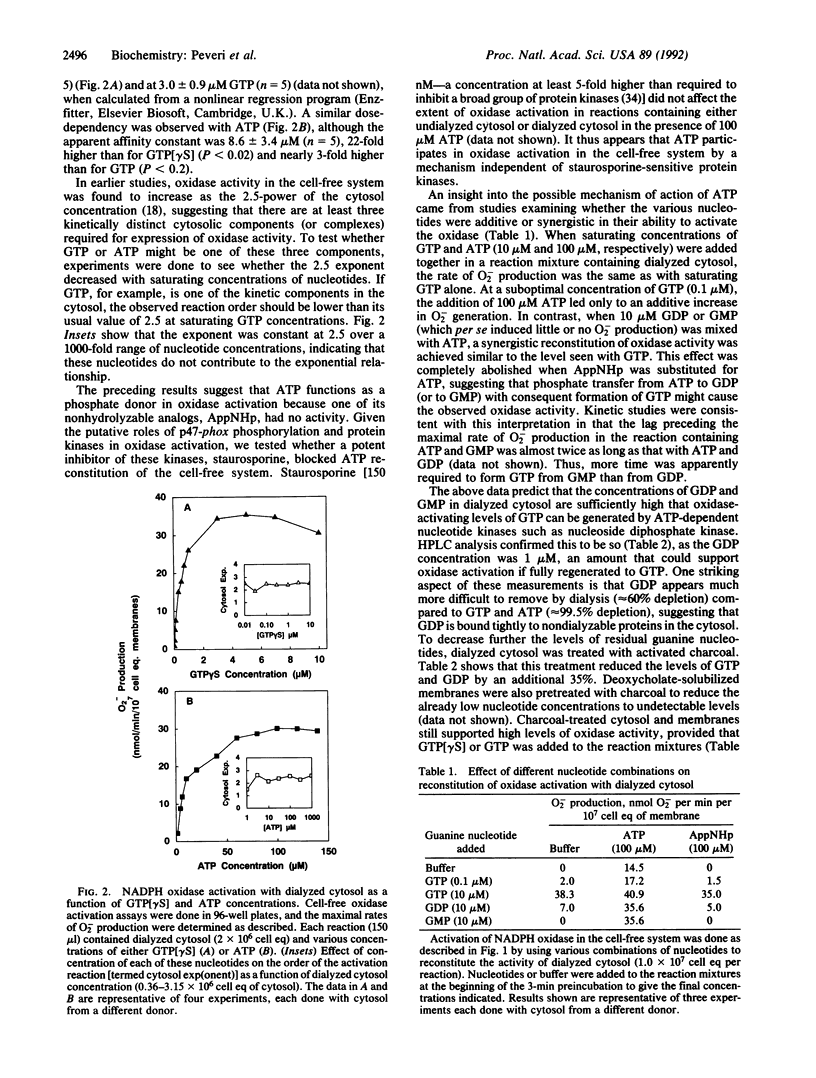
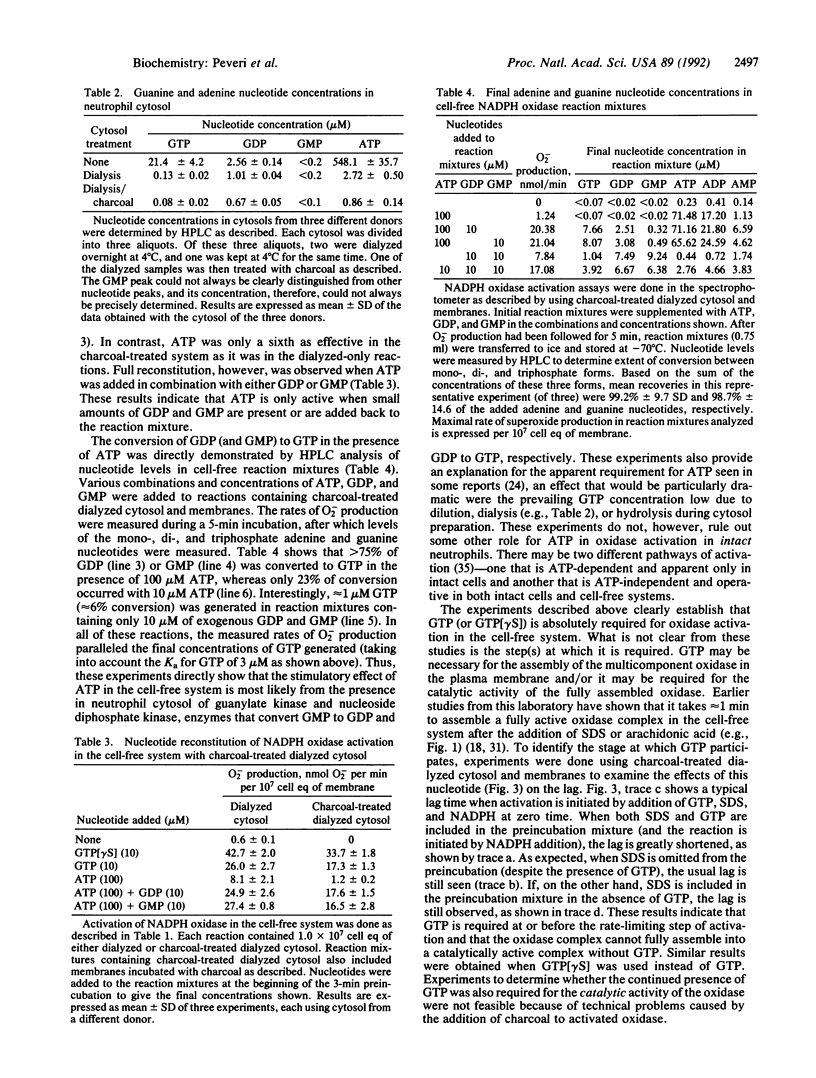
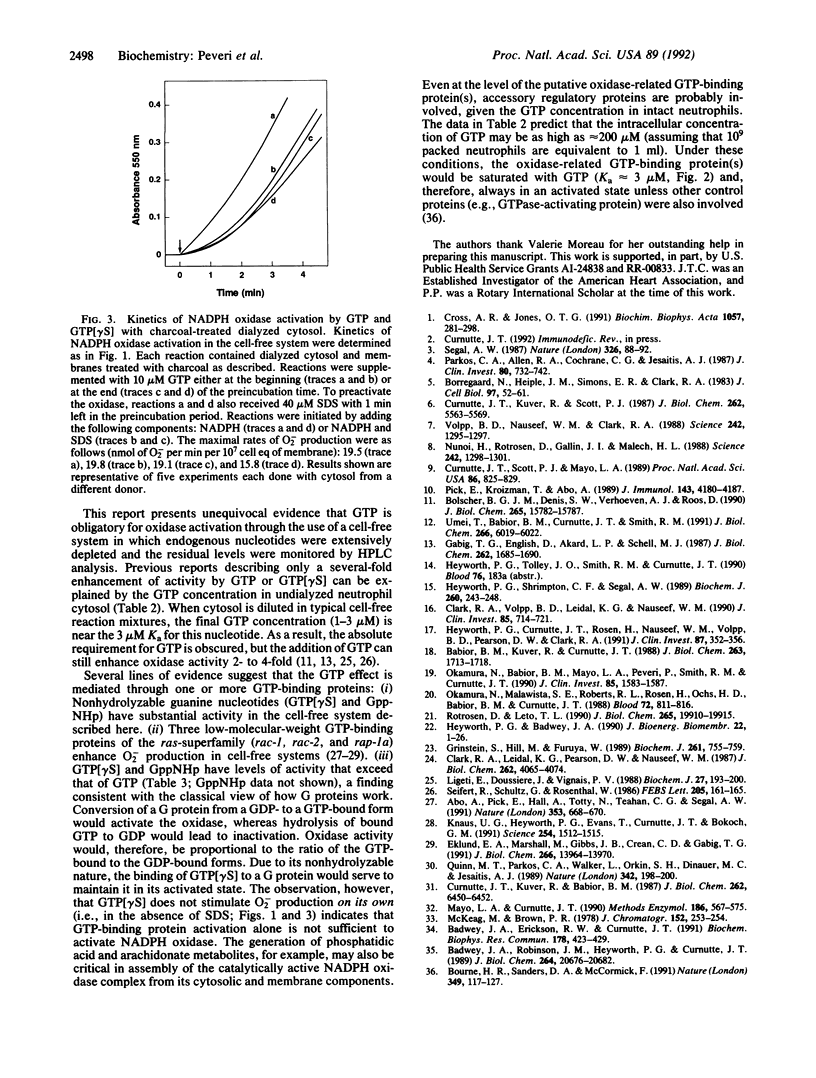
Abstract
Guanine and/or adenine nucleotides appear to be involved in the activation of the superoxide-generating NADPH oxidase of phagocytic cells. Their precise roles, however, are unclear, as much of the evidence for their involvement comes from experiments in which nucleotides have been added to complex systems already rich in both endogenous nucleotides and enzymes capable of interconverting them. To circumvent this problem we have examined the role of nucleotides in neutrophil NADPH oxidase activation by using a cell-free system in which adenine and guanine nucleotide concentrations were carefully controlled and monitored by (i) depletion of endogenous nucleotides by extensive dialysis and charcoal treatment; (ii) reconstitution of the depleted system with reagents analyzed for purity; and (iii) measurement of nucleotide levels in cytosol preparations and in oxidase reaction mixtures by HPLC analysis. In contrast to previous reports that have demonstrated only a several-fold enhancement of oxidase activity by GTP or its analogs, we have shown that oxidase activation was absolutely dependent upon GTP in reactions containing dialyzed cytosol in which the total endogenous nucleotide levels were reduced by greater than 99.5%. Kinetic studies revealed that GTP is required at or before the rate-limiting step in oxidase activation. Two nonhydrolyzable analogs of GTP, guanosine 5'-(gamma-thio)triphosphate and guanylyl imidodiphosphate, were even more active than GTP, suggesting the involvement of one or more GTP-binding proteins. In contrast, ATP was neither necessary nor sufficient for oxidase activation. If reaction mixtures were contaminated with GDP and/or GMP, however, ATP (but not its nonhydrolyzable analog adenylyl imidodiphosphate) could indirectly support oxidase activation by means of endogenous enzymes that catalyze the ATP-dependent conversion of GMP and GDP to GTP.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo A., Pick E., Hall A., Totty N., Teahan C. G., Segal A. W. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991 Oct 17;353(6345):668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kuver R., Curnutte J. T. Kinetics of activation of the respiratory burst oxidase in a fully soluble system from human neutrophils. J Biol Chem. 1988 Feb 5;263(4):1713–1718. [PubMed] [Google Scholar]
- Badwey J. A., Erickson R. W., Curnutte J. T. Staurosporine inhibits the soluble and membrane-bound protein tyrosine kinases of human neutrophils. Biochem Biophys Res Commun. 1991 Jul 31;178(2):423–429. doi: 10.1016/0006-291x(91)90124-p. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Robinson J. M., Heyworth P. G., Curnutte J. T. 1,2-dioctanoyl-sn-glycerol can stimulate neutrophils by different mechanisms. Evidence for a pathway that does not involve phosphorylation of the 47-kDa protein. J Biol Chem. 1989 Dec 5;264(34):20676–20682. [PubMed] [Google Scholar]
- Bolscher B. G., Denis S. W., Verhoeven A. J., Roos D. The activity of one soluble component of the cell-free NADPH:O2 oxidoreductase of human neutrophils depends on guanosine 5'-O-(3-thio)triphosphate. J Biol Chem. 1990 Sep 15;265(26):15782–15787. [PubMed] [Google Scholar]
- Borchardt R. T., Hegazi M. F., Schowen R. L. Determination of O-methylated metabolites of cathecholamines using high-performance liquid chromatography and electrochemical detection. J Chromatogr. 1978 May 11;152(1):253–259. doi: 10.1016/s0021-9673(00)85363-7. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Leidal K. G., Pearson D. W., Nauseef W. M. NADPH oxidase of human neutrophils. Subcellular localization and characterization of an arachidonate-activatable superoxide-generating system. J Biol Chem. 1987 Mar 25;262(9):4065–4074. [PubMed] [Google Scholar]
- Clark R. A., Volpp B. D., Leidal K. G., Nauseef W. M. Two cytosolic components of the human neutrophil respiratory burst oxidase translocate to the plasma membrane during cell activation. J Clin Invest. 1990 Mar;85(3):714–721. doi: 10.1172/JCI114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T. Enzymic mechanisms of superoxide production. Biochim Biophys Acta. 1991 May 6;1057(3):281–298. doi: 10.1016/s0005-2728(05)80140-9. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Kuver R., Babior B. M. Activation of the respiratory burst oxidase in a fully soluble system from human neutrophils. J Biol Chem. 1987 May 15;262(14):6450–6452. [PubMed] [Google Scholar]
- Curnutte J. T., Kuver R., Scott P. J. Activation of neutrophil NADPH oxidase in a cell-free system. Partial purification of components and characterization of the activation process. J Biol Chem. 1987 Apr 25;262(12):5563–5569. [PubMed] [Google Scholar]
- Curnutte J. T., Scott P. J., Mayo L. A. Cytosolic components of the respiratory burst oxidase: resolution of four components, two of which are missing in complementing types of chronic granulomatous disease. Proc Natl Acad Sci U S A. 1989 Feb;86(3):825–829. doi: 10.1073/pnas.86.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund E. A., Marshall M., Gibbs J. B., Crean C. D., Gabig T. G. Resolution of a low molecular weight G protein in neutrophil cytosol required for NADPH oxidase activation and reconstitution by recombinant Krev-1 protein. J Biol Chem. 1991 Jul 25;266(21):13964–13970. [PubMed] [Google Scholar]
- Gabig T. G., English D., Akard L. P., Schell M. J. Regulation of neutrophil NADPH oxidase activation in a cell-free system by guanine nucleotides and fluoride. Evidence for participation of a pertussis and cholera toxin-insensitive G protein. J Biol Chem. 1987 Feb 5;262(4):1685–1690. [PubMed] [Google Scholar]
- Grinstein S., Hill M., Furuya W. Activation of electropermeabilized neutrophils by adenosine 5'-[gamma-thio]triphosphate (ATP[S]). Role of phosphatases in stimulus-response coupling. Biochem J. 1989 Aug 1;261(3):755–759. doi: 10.1042/bj2610755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth P. G., Badwey J. A. Protein phosphorylation associated with the stimulation of neutrophils. Modulation of superoxide production by protein kinase C and calcium. J Bioenerg Biomembr. 1990 Feb;22(1):1–26. doi: 10.1007/BF00762842. [DOI] [PubMed] [Google Scholar]
- Heyworth P. G., Curnutte J. T., Nauseef W. M., Volpp B. D., Pearson D. W., Rosen H., Clark R. A. Neutrophil nicotinamide adenine dinucleotide phosphate oxidase assembly. Translocation of p47-phox and p67-phox requires interaction between p47-phox and cytochrome b558. J Clin Invest. 1991 Jan;87(1):352–356. doi: 10.1172/JCI114993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth P. G., Shrimpton C. F., Segal A. W. Localization of the 47 kDa phosphoprotein involved in the respiratory-burst NADPH oxidase of phagocytic cells. Biochem J. 1989 May 15;260(1):243–248. doi: 10.1042/bj2600243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus U. G., Heyworth P. G., Evans T., Curnutte J. T., Bokoch G. M. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991 Dec 6;254(5037):1512–1515. doi: 10.1126/science.1660188. [DOI] [PubMed] [Google Scholar]
- Ligeti E., Doussiere J., Vignais P. V. Activation of the O2(.-)-generating oxidase in plasma membrane from bovine polymorphonuclear neutrophils by arachidonic acid, a cytosolic factor of protein nature, and nonhydrolyzable analogues of GTP. Biochemistry. 1988 Jan 12;27(1):193–200. doi: 10.1021/bi00401a029. [DOI] [PubMed] [Google Scholar]
- Mayo L. A., Curnutte J. T. Kinetic microplate assay for superoxide production by neutrophils and other phagocytic cells. Methods Enzymol. 1990;186:567–575. doi: 10.1016/0076-6879(90)86151-k. [DOI] [PubMed] [Google Scholar]
- Nunoi H., Rotrosen D., Gallin J. I., Malech H. L. Two forms of autosomal chronic granulomatous disease lack distinct neutrophil cytosol factors. Science. 1988 Dec 2;242(4883):1298–1301. doi: 10.1126/science.2848319. [DOI] [PubMed] [Google Scholar]
- Okamura N., Babior B. M., Mayo L. A., Peveri P., Smith R. M., Curnutte J. T. The p67-phox cytosolic peptide of the respiratory burst oxidase from human neutrophils. Functional aspects. J Clin Invest. 1990 May;85(5):1583–1587. doi: 10.1172/JCI114608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N., Malawista S. E., Roberts R. L., Rosen H., Ochs H. D., Babior B. M., Curnutte J. T. Phosphorylation of the oxidase-related 48K phosphoprotein family in the unusual autosomal cytochrome-negative and X-linked cytochrome-positive types of chronic granulomatous disease. Blood. 1988 Aug;72(2):811–816. [PubMed] [Google Scholar]
- Parkos C. A., Allen R. A., Cochrane C. G., Jesaitis A. J. Purified cytochrome b from human granulocyte plasma membrane is comprised of two polypeptides with relative molecular weights of 91,000 and 22,000. J Clin Invest. 1987 Sep;80(3):732–742. doi: 10.1172/JCI113128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick E., Kroizman T., Abo A. Activation of the superoxide-forming NADPH oxidase of macrophages requires two cytosolic components--one of them is also present in certain nonphagocytic cells. J Immunol. 1989 Dec 15;143(12):4180–4187. [PubMed] [Google Scholar]
- Quinn M. T., Parkos C. A., Walker L., Orkin S. H., Dinauer M. C., Jesaitis A. J. Association of a Ras-related protein with cytochrome b of human neutrophils. Nature. 1989 Nov 9;342(6246):198–200. doi: 10.1038/342198a0. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Leto T. L. Phosphorylation of neutrophil 47-kDa cytosolic oxidase factor. Translocation to membrane is associated with distinct phosphorylation events. J Biol Chem. 1990 Nov 15;265(32):19910–19915. [PubMed] [Google Scholar]
- Segal A. W. Absence of both cytochrome b-245 subunits from neutrophils in X-linked chronic granulomatous disease. Nature. 1987 Mar 5;326(6108):88–91. doi: 10.1038/326088a0. [DOI] [PubMed] [Google Scholar]
- Seifert R., Rosenthal W., Schultz G. Guanine nucleotides stimulate NADPH oxidase in membranes of human neutrophils. FEBS Lett. 1986 Sep 1;205(1):161–165. doi: 10.1016/0014-5793(86)80886-9. [DOI] [PubMed] [Google Scholar]
- Umei T., Babior B. M., Curnutte J. T., Smith R. M. Identification of the NADPH-binding subunit of the respiratory burst oxidase. J Biol Chem. 1991 Apr 5;266(10):6019–6022. [PubMed] [Google Scholar]
- Volpp B. D., Nauseef W. M., Clark R. A. Two cytosolic neutrophil oxidase components absent in autosomal chronic granulomatous disease. Science. 1988 Dec 2;242(4883):1295–1297. doi: 10.1126/science.2848318. [DOI] [PubMed] [Google Scholar]



