Abstract
Diabetes mellitus is a non-communicable disease that occurs in both developed and developing countries. This metabolic disease affects all systems in the body, including the liver. Hyperglycaemia, mainly caused by insulin resistance, affects the metabolism of lipids, carbohydrates and proteins and can lead to non-alcoholic fatty liver disease, which can further progress to non-alcoholic steatohepatitis, cirrhosis and, finally, hepatocellular carcinomas. The underlying mechanism of diabetes that contributes to liver damage is the combination of increased oxidative stress and an aberrant inflammatory response; this activates the transcription of pro-apoptotic genes and damages hepatocytes. Significant involvement of pro-inflammatory cytokines—including interleukin (IL)-1β, IL-6 and tumour necrosis factor-α—exacerbates the accumulation of oxidative damage products in the liver, such as malondialdehyde, fluorescent pigments and conjugated dienes. This review summarises the biochemical, histological and macromolecular changes that contribute to oxidative liver damage among diabetic individuals.
Keywords: Diabetes Mellitus, Liver Diseases, Inflammation, Oxidative Stress
Diabetes mellitus (dm) is a major global public health problem with an escalating incidence and prevalence, particularly in developing and newly industrialised countries.1 Concern regarding this chronic disease is focused on serious DM-related complications which can affect multiple vital organ systems, thereby leading to more severe and irreversible pathological conditions such as nephropathy, retinopathy, vasculopathy, neuropathy and cardiovascular diseases, as well as hepatopathy.2 Research indicates that DM is associated with a number of liver abnormalities, such as abnormal glycogen deposition, non-alcoholic fatty liver disease (NAFLD), fibrosis, cirrhosis, hepatocellular carcinomas (HCCs), abnormal elevated hepatic enzymes, acute liver disease and viral hepatitis.3,4 Additionally, an excessive accumulation of fat in the liver may worsen insulin resistance and lead to severe metabolic dysfunction. A fatty liver and hyperglycaemia can destroy the hepatocytes and contribute to increased morbidity and mortality among diabetic patients.3 However, this subject requires further exploration and elucidation from various perspectives in order to promote understanding of the entire process involved. This review therefore analyses the various DM mechanisms which induce liver damage through the formation of a fatty liver. In addition, pathways of the histopathological and macromolecular changes that result in this complication are discussed.
Progression of Liver Damage in Diabetes Mellitus
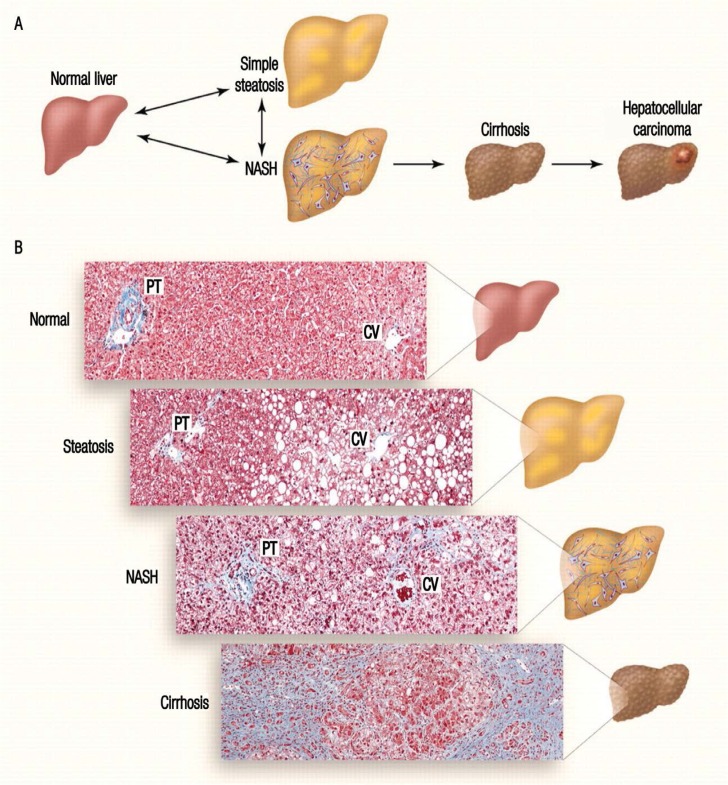
Several critical pathways have been identified as causing liver damage in diabetic patients. Insulin resistance, the main cause of hyperglycaemia and compensatory hyperinsulinaemia, is the predominant causative factor.5–7 As a collection of insulin-sensitive tissues, the liver is among the primary organs susceptible to the effects of hyperglycaemia-induced oxidative stress, which may lead to liver tissue injury.7–9 This is followed by derangement of protein, carbohydrate and lipid metabolism, thereby leading to increased oxidative stress and further triggering the inflammatory cascade.6,8,9 Both oxidative stress and inflammatory responses act as damaging agents in aggravating the pathological condition of DM.4,10 In some cases, DM causes excessive accumulation of fat cells in the liver resulting in a fatty liver and, consequently, NAFLD. Subsequently, 2–3% of NAFLD patients experience hepatic inflammation, necrosis and fibrosis, which are symptoms of a condition known as non-alcoholic steatohepatitis (NASH).2,10 Injured or fibrotic livers will then become cirrhotic, form HCCs and, eventually, go into liver failure [Figure 1].10–12
Figure 1:
Progression of liver damage. In diabetes mellitus, insulin resistance and hyperinsulinaemia cause non-alcoholic fatty liver disease and progress to non-alcoholic steatohepatitis (NASH) which manifests as inflammation and necrosis. Prolonged NASH will lead to liver fibrosis, known as cirrhosis, and finally hepatocellular carcinomas and end-stage liver disease.
NASH = non-alcoholic steatohepatitis; PT = portal triad; CV = central vein.
Reproduced with permission from Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: Old questions and new insights.12
Diagnosis of Liver Damage
Histological and Ultrastructural Changes
Studies have revealed the prominent histopathological changes in the livers of patients with DM.2,13 During the initiation of steatosis, the accumulation of fat in the vesicles displaces the cytoplasm of the liver. This condition is known as microvesicular steatosis, which then progresses to macrovesicular steatosis when excess lipids distort the nucleus. Besides glycogenated nuclei, other prominent features of steatosis include mild lymphocytic, neutrophilic and other inflammatory infiltrates. Steatosis usually takes place in acinar zone III.2,3 These features progress and worsen with steatohepatitis.3,7,14,15 Steatohepatitis and NASH can be diagnosed by the presence of hepatocellular steatosis, lobular inflammation and varying degrees of pericellular, perisinusoidal and periportal fibrosis, including cirrhosis.2,4 Other dominant features of steatohepatitis besides glycogenated nuclei are hepatocyte ballooning, varying degrees of hepatocyte necrosis and the presence of small, sparse and inconspicuous Mallory bodies. Degenerative changes are characterised by the vacuolation of hepatocytes in the centrizonal area of the cell and a discrepancy in the size of the nuclear and dilated sinusoidal spaces.2,4 Cirrhosis has three major histological features: (1) the formation of macro- and/or micronodular hepatocytes; (2) bridging of the fibrous septa; and (3) the disruption of the entire liver architecture. Under these conditions, steatosis and necroinflammatory mechanisms are difficult to observe.15–18
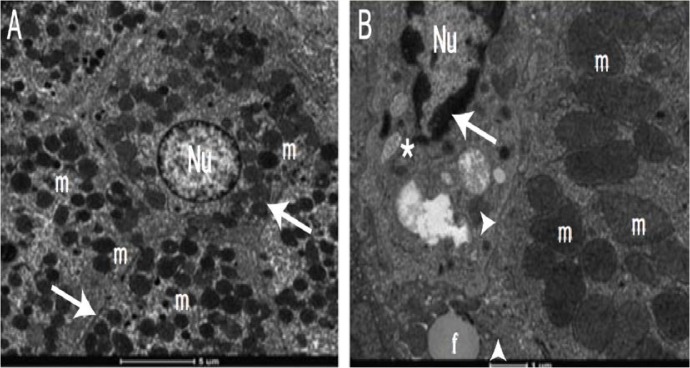
Ultrastructurally, the rough endoplasmic reticulum is significantly reduced in diabetic patients in comparison with the smooth endoplasmic reticulum.9 Moreover, DM causes a marked increase in mitochondrial volume fraction with highly diminished densities of mitochondrial cristae, pyknotic nuclei with damaged nuclear membranes and the extension of the perinuclear area [Figure 2].16–19 In addition, increased lipid accumulation and decreased glycogen content are also seen in the liver cells.16–19
Figure 2A&B:
Electron micrographs of a (A) healthy and (B) diabetic liver. Note the difference in appearance between the hepatocyte nuclei (Nu), organelles, mitochondria (m) and Disse’s space (arrows). In the diabetic liver, there is accumulation of homogenous substances in the nuclear chromatin (*), reduction in the number of organelles, loss of mitochondrial cristae (▲) and granular degeneration (►) in the hepatocytes.
Reproduced with permission from Lucchesi, AN, Cassettari LN, Spadella CT. Alloxan-induced diabetes causes morphological and ultrastructural changes in rat liver that resemble the natural history of chronic fatty liver disease in humans.19
Biochemical Changes
The spectrum of biochemical changes that typically occur in DM resemble those of liver diseases, from the secretion of abnormal liver enzymes to the development of HCCs or even end-stage liver failure.17,20 The initial and most important indicators in assessing liver injury are levels of plasma alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP) and γ-glutamyl transpeptidase (GGT).13,21,22 Research has shown an increase of AST and ALT in 50–80% of NAFLD patients; ALT levels were higher than AST levels for NAFLD patients, while patients with alcoholic fatty livers had higher AST than ALT levels.2 An increment in ALT levels may indicate fatty changes in the liver regardless of the presence of DM. Furthermore, serum ALT concentrations have been found to correlate with hepatic insulin resistance and later decline in hepatic insulin sensitivity compared with AST and GGT levels, which showed no connection with hepatic insulin.22 However, while ALP and GGT levels may be elevated, serum albumin and bilirubin levels are not.2,18,21 Hepatocytes work exclusively to synthesise serum albumin, but are not a good indicator of acute or mild hepatic dysfunction.13
Increased plasma triglyceride and low-density lipoprotein levels and decreased high-density lipoprotein levels are common characteristics of dyslipidaemia and can be observed in diabetic and steatosis patients.13,15 This is due to insulin resistance-related lipolysis which increases the circulating fatty acids taken up by the liver as an energy source. The accumulation of fatty acids disturbs the β-oxidation system in the hepatic mitochondria and leads to further infiltration of fats in the liver. The strong association between insulin resistance and steatosis makes DM an independent predictor for cirrhosis and HCCs among steatosis patients.13,15,17
Other Changes
The medical history and physical examination of a patient can reveal other laboratory abnormalities associated with liver damage, including hypoalbuminemia, prolonged prothrombin times and high levels of ferritin.13,23 In addition, a radiological assessment may identify fats through increased echogenicity on ultrasonography, hypodense fat on non-contrast computed tomography and fat content on magnetic resonance imaging.23
Mechanisms of Damage
The liver plays a vital role in regulating glucose levels in physiological and pathological states such as DM. In type 2 DM, insulin resistance in the liver will cause hyperglycaemia and further distortion of glucose metabolism. Even under severe hyperglycaemic conditions, the liver does not detect the availability of glucose and produces more via the combined action of a few enzymes such as glucose-6-phosphate dehydrogenase, fructose-1,6-diphosphate, hexokinase and glucokinase.2,13 The liver also regulates glucose homeostasis by modulating the expression of various kinds of genes encoding secretory proteins (i.e. hepatokines).24,25 Different types of hepatokines via different pathways activate either positive or negative feedback mechanisms to control the metabolism process.24 Three dominant hepatokines exist: fetuin-A, betatrophin/angiopoietin-like protein 8 (ANGPTL8) and fibroblast growth factor 21 (FGF21). Fetuin-A results in increased inflammation and insulin resistance by inhibiting the insulin receptor tyrosine kinase in skeletal muscle tissue and hepatocytes.24 In contrast, ANGPTL8 is known for its proliferation action on the beta cells of the pancreas while FGF21 is recognised as an insulin-sensitising hormone.25 These hepatokines significantly exacerbate the diabetic condition, especially in type 2 DM, by regulating subclinical inflammation.24
According to certain studies, the aberrant signal which promotes glucose production in the liver during DM supposedly also enhances fatty acid oxidation due to a lack of fuel demand.2,13 However, other research has found that the liver stops oxidising fatty acids and uses them instead to synthesise triglycerides which then accumulate abnormally in the liver.2,13,21 In type 1 DM, insulin deficiency upregulates hormone-sensitive lipase in the adipose tissues, subsequently leading to increased lipolysis and the circulation of free fatty acids, which subsequently accumulate in the liver. These processes enhance the hepatic uptake of very-low-density lipoproteins and synthesis of triglycerides.2,13,14 Concurrently, elevated glucagon levels inhibit hepatic triglyceride output. Therefore, accumulation of fat in the liver may be due to an imbalance in the uptake, synthesis, export and oxidation of free fatty acids in the liver.12,14,15 Aside from abnormalities in lipoprotein metabolism, an accumulation of hepatic fat in DM may be due to either hyperglycaemia-induced activation of the transcription factor carbohydrate-responsive element-binding protein and sterol regulatory element-binding protein 1c, the upregulation of the glucose transporter 2 protein with subsequent intrahepatic fat synthesis or a combination of these mechanisms.12
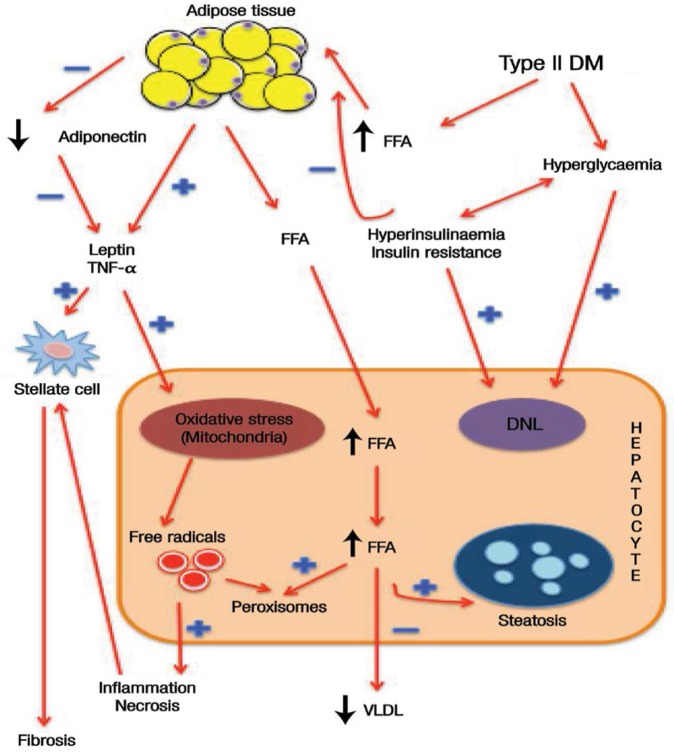
It is worth noting that an accumulation of adipocytes in the liver will cause hepatic insulin resistance.7 Environmental conditions and genetic traits also contribute to the phenotypic expression of insulin resistance; these factors act in combination with hyperinsulinaemia to increase fat mass and lipolysis and elevate the level of free fatty acids. At the same time, insulin signalling is reduced in a dose-dependent manner, eventually increasing hepatic glucose and lipotoxicity.7 However, modifications of adipocytokine secretion in hyperinsulinaemia occur by triggering gene expression of pro-inflammatory cytokines, such as leptin and tumour necrosis factor-α (TNF-α); these modifications are not only involved in the development of insulin resistance but also interfere with insulin signalling, thereby worsening DM.15 An increase in adipocytokines may contribute to mitochondrial oxidative stress and trigger the formation of free radicals and peroxisome.26 An increase in mitochondrial oxidative stress will also cause DNA mutation damage and trigger a series of deleterious effects on the mitochondrial respiratory chain by producing reactive oxygen species (ROS). This vicious cycle will escalate exponentially to an incompatible level, which will be countered by normal physiological feedback and thereby lead to liver damage.26,27 Hyperinsulinaemia may downregulate adiponectin, leading to hepatic lipogenesis and further decreasing free fatty acid oxidation.15 Thus, hyperinsulinaemia may act as a major contributor to the progression of liver damage.7 Inflamed and necrotic hepatocytes and adipocytokines will release several types of chemical mediators which will activate the stellate cells to produce connective tissue growth factor and collagen and cause an accumulation in the extracellular matrix, thereby favouring fibrosis [Figure 3].26
Figure 3:
Diagram showing the mechanism of liver damage in diabetes mellitus. Insulin resistance causes peripheral adipocytes to undergo lipolysis. Free fatty acids are then released to the bloodstream and eventually accumulate in the liver. At the same time, adipocytokines release tumour necrosis factor-α and leptin, worsening the hepatocyte damage by increasing oxidative stress in the mitochondria. The combined action of mitochondrial oxidative stress, hyperinsulinaemia and hyperglycaemia produce free radicals which in turn induce inflammation and cellular necrosis. Tissue inflammation stimulates the hepatic stellate cells to produce collagen, leading to fibrosis, cirrhosis and, finally, hepatocellular carcinomas.
DM = diabetes mellitus; FFA = free fatty acids; TNF = tumour necrosis factor; DNL = de novo lipogenesis; VLDL = very-low-density lipoprotein.
In addition to the aforementioned factors, the expression of certain genes also influences the progression and severity of DM. For example, the most important cytochrome P450 member—cytochrome P450 family 2 subfamily E polypeptide 1 (CYP2E1)—is expressed at a higher concentration in the liver than in other organs. Indeed, the activity and expression of this protein increases in obese individuals and for those with NAFLD or NASH.28 Under these conditions, excessive metabolism of polyunsaturated fatty acids by CYP2E1 may yield cytotoxic products harmful to the liver. Furthermore, CYP2E1 is also involved in mitochondrial respiratory chain-induced ROS generation, which contributes to the increase in oxidative stress, thereby favouring the progression of NAFLD to NASH.28
Oxidative Stress-Mediated Damage
Oxidative stress is defined as an imbalance in the oxidant-to-antioxidant ratio, causing the generation of free radicals.9 The liver is the main detoxification organ of the body and plays an important role in controlling normal glucose homeostasis.6 The production of oxidants such as ROS-like superoxide anions, hydrogen peroxide and hydroxyl radicals by activated Kupffer cells has been identified as central to hepatic injuries.29 Kupffer cells, also known as hepatic macrophages, are one type of nonparenchymal cell that help maintain the integrity of liver cells. However, these phagocytic cells are also susceptible to the effects of oxidative stress produced by the surrounding cells and its own immune reactions.2,13
Excessive ROS production results in several deleterious events, including an irreversible oxidative modification of lipids, proteins and carbohydrates.28,30 In addition, it will induce apoptosis in hepatocytes and the release of inflammatory cytokines, thereby increasing the expression of adhesion molecules and the infiltration of leukocytes. A combination of all of these processes causes massive tissue destruction in the liver.16,29 However, the liver is equipped with potent antioxidants—such as superoxide dismutase (SOD), catalase (CAT) and the glutathione (GSH) enzyme family, including glutathione-S-transferases (GSTs) and glutathione peroxidases (GPXs)—so as not only to neutralise free radicals but also to protect the liver cells from oxidative damage.30,31 Previous research has proven that a decrease in SOD and CAT activities within a hyperglycaemic state leads to an increase in ROS, which eventually contributes to oxidation-induced liver damage.8,30,31
The liver also plays a pivotal role in the homeostasis of the GSH enzyme family. Different cell components such as the endoplasmic reticulum, mitochondria and nucleus consist of separate pools of GSH. Among these, mitochondrial GSH is more essential than cytoplasmic GSH in maintaining cell viability.31 Acting as the first line of defence as an endogenous antioxidant, the oxidation process takes place in the thiol group of GSH.31 Reduced levels of GSH in diabetic rat livers have been associated with depletion of decreased GST, GPX and glutathione reductase activity and with the accumulation of oxidative stress products, such as advanced glycation end-products (AGEs), protein oxidation products (POPs) and lipid peroxidation (LPO).32–34 However, measuring certain redox couples in cells—such as oxidised nicotinamide adenine dinucleotide (NAD)/reduced NAD and oxidised NAD phosphate (NADP)/reduced NADP—is difficult; as a result, the GSH-to-glutathione disulphide ratio in the liver is considered representative of a redox state.31
Oxidation Products
Carbohydrates
AGEs are complex heterogeneous molecules characterised by fluorescence, a brown colour and cross-linking reactions.33–35 Several distinct compounds exist such as pyrraline, pentosidine, imidazolone, N-carboxymethyllysine and methylglyoxal. The latter can yield irreversible AGEs which impair insulin signalling by forming a complex with insulin receptor substrate, thereby leading to conformational changes that affect the tyrosine phosphorylation and the docking function of these proteins.32 The formation of AGEs is closely related to the degree and duration of hyperglycaemia, the half-life of the protein and the permeability of the tissue to free glucose.32,34 Haemoglobin A1c is a product of glycosylated haemoglobin and has been widely used as an indicator of long-term elevated plasma glucose levels.33–35
Zuwała-Jagiełło et al. proved that the liver plays a major role in the clearance of plasma AGEs, which accumulate heavily in conditions of chronic liver damage.36 Moreover, AGE levels have been found to correlate positively with the severity of liver damage and decrease markedly after liver transplantation.36,37 However, hepatic stellate cells and myofibroblasts responsible for fibrogenesis in chronic liver disease will express the receptor for AGE (RAGE). The binding of AGE with RAGE will injure the structure of the extracellular matrix and induce a toxic effect by activating nuclear factor-kappa B (NF-κB) to stimulate the transcription of genes for cytokines and growth factors, further increasing expression of adhesive molecules and vascular permeability.9,32,34,35,38 Hence, AGE can be used as a clinical marker to evaluate liver function and diabetes severity.34–37
Proteins
Protein oxidation has been defined as “the covalent modification of a protein induced either directly by ROS or indirectly by reaction with secondary byproducts of oxidative stress”.38 This means that a massive production of free radicals may also give rise to the formation of protein-protein and protein-lipid cross-links, which in turn change the structure and function of the proteins by causing protein fragmentation and obstruction of the amino acid side chains.34,35,38 These changes lead to deleterious consequences such as inhibition of enzymatic and binding activities, increased susceptibility to aggregation, resistance to proteolysis, increased or decreased uptake by cells and altered immunogenicity.32,34,35,38 The same mechanism occurs when proteins are exposed to reactive nitrogen species (RNS). Protein nitration may disturb normal signalling pathways (e.g. the insulin signalling pathway), to detrimental effect; nitrated proteins also mediate hepatic injury by promoting the release of cytokines and chemokines through stimulation of Kupffer cells, thereby resulting in neutrophil infiltration and causing inflammatory tissue injury.39 Cysteine and methionine are among the most susceptible amino acids and can be modified by almost all oxidising species.36,38,40 Coincidently, cysteine is an important residue in many metabolic enzymes, kinases, phosphatases and transcription factors; oxidative modification of cysteine may therefore alter the functional properties and activity of proteins.36,38
Much like AGEs, research has shown that POP serum concentrations increase with the progression of chronic diseases and are exacerbated in the presence of DM.37,40,41 Therefore, POPs are distinct biomarkers for oxidative stress. Previous studies have reported that the liver plays an important role in the elimination of POPs.42,43 Zuwała-Jagiełło et al. demonstrated a significant increase of POPs in a group of cirrhotic patients; POP levels were proportional to the severity of the disease.36
Lipids
LPO occurs when polyunsaturated fatty acids interact with oxygen and is often used as a marker of tissue oxidative stress.44 Various aldehydes derived from lipid hydroperoxides can be measured to quantify LPO as they reflect the extent of damage associated with oxidative stress at the tissue and cellular levels. These aldehydes have longer half-lives than reactive free radicals and can diffuse from their site of origin, circulate freely in the blood vessels or incorporate with lipoproteins, thereby reaching and attacking target sites.44 Notably, LPO causes a prominent increase in malondialdehyde and thiobarbituric acid-reactive substances in the livers of people with DM.3 The formation of these products alters the transbilayer fluidity gradient of plasma membranes and damages the membrane components. It also affects membrane-bound enzymes and receptor activity and leads to inflammation and cell necrosis.31,37 In NAFLD patients, LPO triggers the release of inflammatory mediators such as TNF-α via the activation of adipocytokine and other pathways.44–46 This vicious cycle activates hepatic stellate cells and further leads to the proliferation and synthesis of collagenous substances, which favours the formation of fibrosis; this is an essential step in all types of chronic liver damage processes.45
Inflammation-Mediated Damage
Inflammation plays a vital role in host defence by releasing pro-inflammatory cytokines to protect the host from injury, including TNF-α, interleukin (IL)-1β and IL-6. Chronic inflammatory conditions cause tissue damage, fibrosis and loss of cellular function. Growing evidence links a low-grade chronic inflammatory state to DM complications, particularly liver-related complications.43 These conditions are further exacerbated by the combined action of a complex regulatory network of cells and mediators designed to resolve inflammatory responses, mainly macrophages. The resulting increase in circulatory cytokines will then aggravate insulin resistance; however, anti-inflammatory drugs reverse these complex reactions.14,47,48 Thus, inflammation might constitute a major causal factor for non-communicable diseases such as DM, rather than an associated risk factor. For example, IL-1β together with TNF-α is responsible for the development of DM types 1 and 2 via inhibition of glucose-induced insulin secretion and impairment of β-cell function, thereby decreasing the biosynthesis of insulin and inducing apoptotic cell death.48 In addition, IL-1β may also stimulate proliferation of T and B cells and induce the release of adhesion molecules, in addition to triggering the production of other cytokines and pro-inflammatory mediators, thereby having a detrimental effect on the liver.29,49,50 The biological response to IL-1β is mostly shown through the activation of the c-Jun N-terminal kinase (JNK) pathway and the nuclear translocation of NF-κB.49
Signalling Mechanisms Contributing to Damage
Nuclear Factor-Kappa B
First described as a nuclear factor specific to B cells bound to the B site of the κ-light chain gene enhancer, NF-κB was then found to be expressed in other cell types, including the liver epithelium, where it regulates the proliferation and survival of hepatocytes during regeneration and development.31,51 This protein complex may be activated by the release of pro-inflammatory molecules such as IL-1β, IL-6, TNF-α and C-reactive protein.8,50 Other non-inflammatory states and mediators may also trigger NF-κB activation, such as free fatty acids, hyperglycaemia, obesity, protein kinase C and oxidative stress.8,50,52 The activation of NF-κB may trigger different types of responses depending on stimuli received, such as pro-apoptotic gene activation or cell regeneration. Hence, the activation of NF-κB in DM may initiate a series of deleterious events via the further elevation of pro-inflammatory cytokines.31,50,51 Moreover, NF-κB may also directly and indirectly promote the production of ROS and RNS and increase LPO, AGEs and POPs, thereby increasing liver tissue damage.14 These products are thought to be generated through the phosphorylation-induced ubiquitination and subsequent proteolysis of inhibitor of NF-κB kinase subunit-α and through TNF-α- or lymphotoxin-β-mediated pathways.51,52 It has been postulated that inappropriate activation of NF-κB increases the likelihood of hepatic steatosis progression to cirrhosis, thereby causing further liver damage.51 Therefore, NF-κB is not only an appropriate target for DM treatment but also for the treatment of hepatocellular injuries.9
Mitogen-Activated Protein Kinase
Mitogen-activated protein kinases (MAPKs) are signalling transducers which transmit external stimuli to the nucleus by dual phosphorylation of the Thr183 and Tyr185 antibodies. The MAPK families consist of three different signalling cascades converging on extracellular signal-regulated kinases, JNKs and p38 MAPKs, which exert different types of reactions upon stimulation.52 Activated by cytokines and growth factors, extracellular signal-regulated kinases play a central role in regulating cell growth and differentiation. Meanwhile, JNK and p38 MAPKs, also known as stress-activated MAPKs, are triggered by pro-inflammatory cytokines such as TNF-α and IL-1β as well as environmental and genotoxic stressors.52 Stimulation of stress-activated MAPKs will exert various cellular functions, such as apoptosis, necrosis, survival, proliferation, migration and inflammation.10 In DM, MAPK cascades are activated by osmotic perturbation caused by hyperglycaemia, oxidative stress and AGEs.8 Studies have shown that prolonged exposure to oxidative stress because of high levels of liver TNF-α will activate the pro-apoptotic function of JNKs; detrimentally affecting the hepatocytes.52 Thus, JNKs are a promising therapeutic target for individuals with liver injuries.
Clinical Implications, Management and Treatment
The treatment of DM remains a challenging issue. Researchers are exploring safe and effective medications to overcome the detrimental effects of insulin resistance-related metabolic derangement, including hyperglycaemia, hyperinsulinaemia, hyper-lipidaemia, oxidative stress, inflammation, atherosclerosis and other complications.53 For patients with NAFLD and NASH, no specific treatments yet exist apart from diet and lifestyle modifications to reduce body weight and prevent further injury.23,54 However, combined pharmacological therapy is recommended to improve insulin sensitivity in the liver (metformin and pioglitazone) and its periphery (thiazolidinediones), together with other drugs such as betaine, atorvastatin, losartan and orlistate.7,13,53 However, the clinical value of these treatments are very subjective. Patients taking these drugs should be closely monitored due to possible contraindications with DM medications and the vulnerable condition of the liver during the drug detoxification process.54
Antioxidant therapy is a potential future therapeutic strategy; increasing antioxidant levels in patients with DM-induced liver damage may hopefully counter the effects of oxidative stress and inflammation, thereby reducing the severity of diabetic complications. A few plant-based products and vitamins have been investigated as ways of protecting against and possibly reversing liver damage believed to be caused by oxidative stress and inflammation.48 Vitamin E and betaine are just a few of the antioxidants which have shown good clinical implications in the reduction of liver disease severity and the protection of the liver from DM-induced damage [Table 1].4,8,9,16,18,30,55–57
Table 1:
Comparative analysis of previous studies investigating the effectiveness of antioxidant supplements in preventing diabetes-induced liver damage
| Author and year of study | Study design | Antioxidant source | Dose | Duration | Protective effect |
|---|---|---|---|---|---|
| Welt et al.16 2004 | In vivo | Ginkgo biloba extract | 100 mg/kg/day | 4 months | Antioxidative |
| Palsamy et al.9 2010 | In vivo | Resveratrol | 5 mg/kg/day | 30 days | Anti-inflammatory, antiglycative and antioxidative |
| Parveen et al.30 2010 | In vivo | Pinus pinaster bark | 10 mg/kg/day | 4 weeks | Anti-diabetic and antioxidative |
| Hamden et al.18 2008 | In vivo | 17β-estradiol | 1 μg/kg/day | 4 weeks | Anti-apoptotic, anti-diabetic and antioxidative |
| Manna et al.8 2010 | In vitro and in vivo | Arjunolic acid | 20 mg/kg/day | 4 weeks | Anti-inflammatory, anti-apoptotic, anti-diabetic, antiglycative and antioxidative |
| Kim et al.55 2009 | In vivo | Kangen-karyu extract | 50, 100 and 200 mg/kg/day | 20 days | Anti-inflammatory, anti-diabetic, antiglycative and antioxidative |
| Maritim et al.56 2003 | In vivo | α-lipoic acid | 10 mg/kg/day | 14 days | Antioxidative |
| Jain et al.57 2009 | In vivo | L-cysteine | 1 mg/kg/day | 8 weeks | Anti-inflammatory, anti-diabetic, antiglycative and antioxidative |
| Guven et al.4 2006 | In vivo | Melatonin | 200 μg/kg/day | 9 weeks | Antioxidative |
Conclusion
Liver damage is a serious complication among patients with DM. Insulin resistance, which is aggravated by oxidative stress and aberrant inflammatory signals, has become one of the main factors contributing to liver damage. Insulin resistance subsequently leads to more chronic and potentially fatal conditions such as cirrhosis and end-stage liver failure. Antioxidants from plant-based and natural products with strong anti-diabetic, anti-inflammatory and antiglycative properties are emerging as a future therapy. As opposed to conventional medications, antioxidants may be an alternative and beneficial way to prevent and treat this life-threatening disease.
References
- 1.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/s0140-6736(11)60679-x. [DOI] [PubMed] [Google Scholar]
- 2.Reid AE. Non-alcoholic fatty liver disease. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology/diagnosis/management. 8th ed. St Louis, Missouri, USA: Saunders; 2006. pp. 1772–99. [Google Scholar]
- 3.Levinthal GN, Tavill AS. Liver disease and diabetes mellitus. Clin Diabetes. 1999;17:73. [Google Scholar]
- 4.Guven A, Yavuz O, Cam M, Ercan F, Bukan N, Comunoglu C, et al. Effects of melatonin on streptozotocin-induced diabetic liver injury in rats. Acta Histochem. 2006;108:85–93. doi: 10.1016/j.acthis.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Larter CZ, Farrell GC. Insulin resistance, adiponectin, cytokines in NASH: Which is the best target to treat? J Hepatol. 2006;44:253–61. doi: 10.1016/j.jhep.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Leclercq IA, Da Silva Morais A, Schroyen B, Van Hul N, Geerts A. Insulin resistance in hepatocytes and sinusoidal liver cells: Mechanisms and consequences. J Hepatol. 2007;47:142–56. doi: 10.1016/j.jhep.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: A metabolic pathway to chronic liver disease. Hepatology. 2005;42:987–1000. doi: 10.1002/hep.20920. [DOI] [PubMed] [Google Scholar]
- 8.Manna P, Das J, Ghosh J, Sil PC. Contribution of type 1 diabetes to rat liver dysfunction and cellular damage via activation of NOS, PARP, IkappaBalpha/NF-kappaB, MAPKs, and mitochondria-dependent pathways: Prophylactic role of arjunolic acid. Free Radic Biol Med. 2010;48:1465–84. doi: 10.1016/j.freeradbiomed.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 9.Palsamy P, Sivakumar S, Subramanian S. Resveratrol attenuates hyperglycemia-mediated oxidative stress, proinflammatory cytokines and protects hepatocytes ultrastructure in streptozotocin-nicotinamide-induced experimental diabetic rats. Chem Biol Interact. 2010;186:200–10. doi: 10.1016/j.cbi.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Romagnoli M, Gomez-Cabrera MC, Perrelli MG, Biasi F, Pallardó FV, Sastre J, et al. Xanthine oxidase-induced oxidative stress causes activation of NF-kappaB and inflammation in the liver of type I diabetic rats. Free Radic Biol Med. 2010;49:171–7. doi: 10.1016/j.freeradbiomed.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 11.Porepa L, Ray JG, Sanchez-Romeu P, Booth GL. Newly diagnosed diabetes mellitus as a risk factor for serious liver disease. CMAJ. 2010;182:E526–31. doi: 10.1503/cmaj.092144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: Old questions and new insights. Science. 2011;332:1519–23. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawford JM, Iacobuzio-Donahue C. Liver and biliary tract. In: Kumar V, Abbas AK, Fausto N, Aster JC, editors. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia, Pennsylvania, USA: Saunders; 2009. pp. 833–90. [Google Scholar]
- 14.Al-Hussaini AA, Sulaiman NM, Alzahrani MD, Alenizi AS, Khan M. Prevalence of hepatopathy in type 1 diabetic children. BMC Pediatr. 2012;12:160. doi: 10.1186/1471-2431-12-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regnell SE, Lernmark Å. Hepatic steatosis in type 1 diabetes. Rev Diabet Stud. 2011;8:454–67. doi: 10.1900/rds.2011.8.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welt K, Weiss J, Martin R, Dettmer D, Hermsdorf T, Asayama K, et al. Ultrastructural, immunohistochemical and biochemical investigations of the rat liver exposed to experimental diabetes and acute hypoxia with and without application of Ginkgo extract. Exp Toxicol Pathol. 2004;55:331–45. doi: 10.1078/0940-2993-00337. [DOI] [PubMed] [Google Scholar]
- 17.Moscatiello S, Manini R, Marchesini G. Diabetes and liver disease: An ominous association. Nutr Metab Cardiovasc Dis. 2007;17:63–70. doi: 10.1016/j.numecd.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Hamden K, Carreau S, Boujbiha MA, Lajmi S, Aloulou D, Kchaou D, et al. Hyperglycaemia, stress oxidant, liver dysfunction and histological changes in diabetic male rat pancreas and liver: Protective effect of 17 beta-estradiol. Steroids. 2008;73:495–501. doi: 10.1016/j.steroids.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 19.Lucchesi AN, Cassettari LN, Spadella CT. Alloxan-induced diabetes causes morphological and ultrastructural changes in rat liver that resemble the natural history of chronic fatty liver disease in humans. J Diabetes Res. 2015;2015:494578. doi: 10.1155/2015/494578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mavrogiannaki AN, Migdalis IN. Nonalcoholic fatty liver disease, diabetes mellitus and cardiovascular disease: Newer data. Int J Endocrinol. 2013;2013:450639. doi: 10.1155/2013/450639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J. Harrison’s Principles of Internal Medicine. 18th ed. Vol. 2. New York, USA: McGraw-Hill; 2011. [Google Scholar]
- 22.Nannipieri M, Gonzales C, Baldi S, Posadas R, Williams K, Haffner SM, et al. Liver enzymes, the metabolic syndrome, and incident diabetes: The Mexico City diabetes study. Diabetes Care. 2005;28:1757–62. doi: 10.2337/diacare.28.7.1757. [DOI] [PubMed] [Google Scholar]
- 23.Elsheikh E, Henry LL, Younossi ZM. Current management of patients with nonalcoholic fatty liver disease. Expert Rev Endocrinol Metab. 2013;8:549–58. doi: 10.1586/17446651.2013.846212. [DOI] [PubMed] [Google Scholar]
- 24.Stefan N, Sun Q, Fritsche A, Machann J, Schick F, Gerst F, et al. Impact of the adipokine adiponectin and the hepatokine fetuin-A on the development of type 2 diabetes: Prospective cohort- and cross-sectional phenotyping studies. PLoS One. 2014;9:e92238. doi: 10.1371/journal.pone.0092238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iroz A, Couty JP, Postic C. Hepatokines: Unlocking the multi-organ network in metabolic diseases. Diabetologia. 2015;58:1699–1703. doi: 10.1007/s00125-015-3634-4. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Compean D, Jaquez-Quintana JO, Gonzalez-Gonzalez JA, Maldonado-Garza H. Liver cirrhosis and diabetes: Risk factors, pathophysiology, clinical implications and management. World J Gastroenterol. 2009;15:280–8. doi: 10.3748/wjg.15.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shokolenko I, Venediktova N, Bochkareva A, Wilson GL, Alexeyev MF. Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res. 2009;37:2539–48. doi: 10.1093/nar/gkp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung TM, Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J Hepatol. 2013;58:395–8. doi: 10.1016/j.jhep.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Wei Y, Chen P, de Bruyn MD, Zhang W, Bremer E, Helfrich W. Carbon monoxide-releasing molecule-2 (CORM-2) attenuates acute hepatic ischemia reperfusion injury in rats. BMC Gastroenterol. 2010;10:42. doi: 10.1186/1471-230X-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parveen K, Khan MR, Mujeeb M, Siddigui WA. Protective effects of Pycnogenol on hyperglycemia-induced oxidative damage in the liver of type 2 diabetic rats. Chem Biol Interact. 2010;186:219–27. doi: 10.1016/j.cbi.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 31.Han D, Hanawa N, Saberi B, Kaplowitz N. Mechanisms of liver injury: III - Role of glutathione redox status in liver injury. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1–7. doi: 10.1152/ajpgi.00001.2006. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed N. Advanced glycation endproducts: Role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Horiuchi S. The liver is the main site for metabolism of circulating advanced glycation end products. J Hepatol. 2002;36:123–5. doi: 10.1016/S0168-8278(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 34.Yagmur E, Tacke F, Weiss C, Lahme B, Manns MP, Keifer P, et al. Elevation of Nepsilon-(carboxymethyl)lysine-modified advanced glycation end products in chronic liver disease is an indicator of liver cirrhosis. Clin Biochem. 2006;39:39–45. doi: 10.1016/j.clinbiochem.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed N, Thornalley PJ, Lüthen R, Häussinger D, Sebekova K, Schinzel R, et al. Processing of protein glycation, oxidation and nitrosation adducts in the liver and the effect of cirrhosis. J Hepatol. 2004;41:913–19. doi: 10.1016/j.jhep.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Zuwała-Jagiełło J, Pazgan-Simon M, Simon K, Warwas M. Elevated advanced oxidation protein products levels in patients with liver cirrhosis. Acta Biochim Pol. 2009;56:679–85. [PubMed] [Google Scholar]
- 37.Sebeková K, Kupcová V, Schinzel R, Heidland A. Markedly elevated levels of plasma advanced glycation end products in patients with liver cirrhosis: Amelioration by liver transplantation. J Hepatol. 2002;36:66–71. doi: 10.1016/S0168-8278(01)00232-X. [DOI] [PubMed] [Google Scholar]
- 38.Shacter E. Quantification and significance of protein oxidation in biological samples. Drug Metab Rev. 2000;32:307–26. doi: 10.1081/DMR-100102336. [DOI] [PubMed] [Google Scholar]
- 39.Abdelmegeed MA, Song BJ. Functional roles of protein nitration in acute and chronic liver diseases. Oxid Med Cell Longev. 2014;2014:149627. doi: 10.1155/2014/149627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witko-Sarsat V, Friendlander M, Nguyen Khoa T, Capeillère-Blandin C, Nguyen AT, Canteloup S, et al. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol. 1998;161:2524–32. [PubMed] [Google Scholar]
- 41.Nazratun N, Mahmood AA, Kuppusamy UR, Ahmad TS, Tan SY. Diabetes mellitus exacerbates advanced glycation end product accumulation in the veins of end-stage renal failure patients. Vasc Med. 2006;11:245–50. doi: 10.1177/1358863x06072202. [DOI] [PubMed] [Google Scholar]
- 42.Iwao Y, Anraku M, Hiraike M, Kawai K, Nakajou K, Kai T, et al. The structural and pharmacokinetic properties of oxidized human serum albumin, advanced oxidation protein products (AOPP) Drug Metab Pharmacokinet. 2006;21:140–6. doi: 10.2133/dmpk.21.140. [DOI] [PubMed] [Google Scholar]
- 43.Svistounov D, Smedsrød B. Hepatic clearance of advanced glycation end products (AGEs): Myth or truth? J Hepatol. 2004;41:1038–40. doi: 10.1016/j.jhep.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 44.McNuff MA, Omoruyi FO, Morrison EY, Asemota HN. Hepatic function enzymes and lipid peroxidation in streptozotocin-induced diabetic rats fed bitter yam (Dioscorea polygonoides) steroidal sapogenin extract. Diabetol Croat. 2003;32:17–23. [Google Scholar]
- 45.Moreira RK. Hepatic stellate cells and liver fibrosis. Arch Pathol Lab Med. 2007;131:1728–34. doi: 10.5858/2007-131-1728-HSCALF. [DOI] [PubMed] [Google Scholar]
- 46.Mahmoud AM, Ashour MB, Abdel-Moneim A, Ahmed OM. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J Diabetes Complications. 2012;26:483–90. doi: 10.1016/j.jdiacomp.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Ugochukwu NH, Figgers CL. Dietary caloric restriction modifies inflammatory responses in the livers of streptozotocin-induced diabetic rats. Nutr Res. 2006;26:221–6. doi: 10.1016/j.nutres.2006.05.003. [DOI] [Google Scholar]
- 48.Seven A, Guzel S, Seymen O, Civelek S, Bolayirli M, Uncu M, et al. Effects of vitamin E supplementation on oxidative stress in streptozotocin induced diabetic rats: Investigation of liver and plasma. Yonsei Med J. 2004;45:703–10. doi: 10.3349/ymj.2004.45.4.703. [DOI] [PubMed] [Google Scholar]
- 49.Banerjee M, Saxena M. Interleukin-1 (IL-1) family of cytokines: Role in type 2 diabetes. Clin Chim Acta. 2012;413:1163–70. doi: 10.1016/j.cca.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 50.Martínez-Flórez S, Gutiérrez-Fernández B, Sánchez-Campos S, González-Gallego J, Tuñón MJ. Quercetin attenuates nuclear factor-kappaB activation and nitric oxide production in interleukin-1beta-activated rat hepatocytes. J Nutr. 2005;135:1359–65. doi: 10.1093/jn/135.6.1359. [DOI] [PubMed] [Google Scholar]
- 51.Arsura M, Cavin LG. Nuclear factor-kappaB and liver carcinogenesis. Cancer Lett. 2005;229:157–69. doi: 10.1016/j.canlet.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 52.Nakagawa H, Maeda S. Molecular mechanisms of liver injury and hepatocarcinogenesis: Focusing on the role of stress-activated MAPK. Patholog Res Int. 2012;2012:172894. doi: 10.1155/2012/172894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeFronzo RA, Eldor R, Abdul-Ghani M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care. 2013;36:S127–38. doi: 10.2337/dcS13-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adams LA, Angulo P. Treatment of non-alcoholic fatty liver disease. Postgrad Med J. 2006;82:315–22. doi: 10.1136/pgmj.2005.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim HY, Okamoto T, Yokozawa T. Beneficial effects of Chinese prescription Kangen-karyu on diabetes associated with hyperlipidemia, advanced glycation endproducts, and oxidative stress in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2009;124:263–9. doi: 10.1016/j.jep.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 56.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 57.Jain SK, Velusamy T, Croad JL, Bull R. L-cysteine supplementation lowers blood glucose, glycated hemoglobin, CRP, MCP-1, oxidative stress and inhibits NFkappaB activation in the livers of Zucker diabetic rats. Free Radic Biol Med. 2009;46:1633–38. doi: 10.1016/j.freeradbiomed.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]