Abstract
Objectives:
Marine organisms are a rich source of bioactive molecules with potential applications in medicine, biotechnology and industry; however, few bioactive compounds have been isolated from organisms inhabiting the Arabian Gulf and the Gulf of Oman. This study aimed to isolate and screen the anti-cancer activity of compounds and extracts from 40 natural products of marine organisms collected from the Gulf of Oman.
Methods:
This study was carried out between January 2012 and December 2014 at the Sultan Qaboos University, Muscat, Oman. Fungi, bacteria, sponges, algae, soft corals, tunicates, bryozoans, mangrove tree samples and sea cucumbers were collected from seawater at Marina Bandar Al-Rowdha and Bandar Al-Khayran in Oman. Bacteria and fungi were isolated using a marine broth and organisms were extracted with methanol and ethyl acetate. Compounds were identified from spectroscopic data. The anti-cancer activity of the compounds and extracts was tested in a Michigan Cancer Foundation (MCF)-7 cell line breast adenocarcinoma model.
Results:
Eight pure compounds and 32 extracts were investigated. Of these, 22.5% showed strong or medium anti-cancer activity, with malformin A, kuanoniamine D, hymenialdisine and gallic acid showing the greatest activity, as well as the soft coral Sarcophyton sp. extract. Treatment of MCF-7 cells at different concentrations of Sarcophyton sp. extracts indicated the induction of concentration-dependent cell death. Ultrastructural analysis highlighted the presence of nuclear fragmentation, membrane protrusion, blebbing and chromatic segregation at the nuclear membrane, which are typical characteristics of cell death by apoptosis induction.
Conclusion:
Some Omani marine organisms showed high anti-cancer potential. The efficacy, specificity and molecular mechanisms of anti-cancer compounds from Omani marine organisms on various cancer models should be investigated in future in vitro and in vivo studies.
Keywords: Anticancer Agents, Cancer Screening, Breast Cancer, Marine Organisms, Biological Products, Apoptosis, Oman
Advances in Knowledge
- This is the first study from Oman showing the anti-cancer potential of Omani marine organisms. Very few studies of this kind have been conducted in the Gulf Cooperative Council region.
- The greatest activity against the breast adenocarcinoma model was observed with the malformin A, kuanoniamine D, hymenialdisine and gallic acid compounds as well as the soft coral Sarcophyton sp. extract.
- Ultrastructural analysis and staining of the Michigan Cancer Foundation-7 cell line indicated the role of the apoptotic pathway in triggering cell death.
Application to Patient Care
- The results of this study indicate possibilities for the development of new treatments for breast adenocarcinomas and other cancers.
The marine ecosystem covers two-thirds of the Earth’s surface and has diverse environmental conditions, facilitating the specialisation and diversity of marine organisms; as such, these organisms are a rich source of as-yet-untapped bioactive molecules.1–3 Soft-body sessile marine organisms—such as algae, sponges, soft corals and tunicates—are important sources of bioactive compounds.4–6 This is due to the fact that sessile organisms often accumulate toxic and repellent compounds in their body, not only to compensate for their lack of mechanical defences and protective structures, but also to protect themselves from predators, pathogens and the accumulation of unwanted material on their surfaces.7 Few secondary metabolites have yet been isolated from marine organisms and transformed into pharmaceuticals, but it is expected that novel drugs will be isolated from marine organisms in the future.4,8–11
Cancer remains one of the major causes of mortality worldwide; unsurprisingly, many research groups are currently focusing on finding novel anti-cancer drugs to enhance chemotherapy treatment and increase survival rates.12 A significant number of anti-cancer compounds have been isolated from marine organisms but only a few have been approved for treatment, for example the chemotherapy drugs cytosine arabinoside (isolated from the sponge Cryptotethya crypta) and eribulin (halichondrin B isolated from the sponge Halichondria okadai) and the anti-tumour drug trabectedin (ecteinascidin isolated from the tunicate Ecteinascidia turbinata).12–14 Bryostatin-1 is a macrolide compound produced by endosymbiotic bacteria of the bryozoan Bugula neritina which induces apoptosis at nanomolar concentrations in cancer cells and is enhanced by protein kinase C overexpression; it is currently in phase II clinical trials.13 Another product, didemnin B, was isolated from the tunicate Trididemnum solidum and revealed high activity against myelomas and breast, ovarian, cervical and lung cancers; unfortunately, it was excluded from any further consideration as an anti-cancer agent after it was found to be highly toxic.7,13
Data are limited regarding natural products from marine organisms and their application as traditional medications in the Arabian Gulf area, particularly Oman. While the activity of pure anti-cancer compounds isolated from marine organisms has been reported previously, it is possible that new anti-cancer compounds can be isolated from Omani organisms. Several novel natural products have been isolated from Spatoglossum variabile and Dictyota dichotoma algae gathered from the coast of the Arabian Sea in Karachi, Pakistan, but their pharmaceutical activity has not yet been investigated.15,16 An antifungal phenolic compound with aromatic unsaturation was produced from the bacterium Pseudomonas aeruginosa CMG1055, also isolated from the Arabian Sea coast of Pakistan.17 Gelliodes spp. and Spheciospongia spp.1 and spp.2 sponges from the Persian Gulf coast in Bushehr, Iran, were identified as the most active against a panel of bacterial pathogens such as Bacillus subtilis, Staphylococcus aureus, P. aeruginosa and Escherichia coli; unfortunately, no bioactive compounds were identified.18 In a previous study in Oman, researchers screened the anti-microbial, antidiatom and antilarval properties of Holothuria atra and H. edulis sea cucumbers collected from the Bandar Al-Khayran region; their findings suggested the presence of cytotoxic compounds.19 To the best of the authors’ knowledge, there is no information in the literature about anti-cancer compounds derived from marine organisms in Oman. Therefore, the aim of this study was to screen the anti-cancer activity of natural products and extracts of marine organisms collected from the Gulf of Oman.
Methods
This study was carried out between January 2012 and December 2014 at the Sultan Qaboos University, Muscat, Oman. A total of 40 natural products were obtained from different species of fungi, bacteria, marine sponges, algae, soft corals, tunicates, bryozoans, mangrove tree samples and sea cucumbers collected from seawater at Marina Bandar Al-Rowdha (23° 34.55′ North, 58° 36.27′ East) and Bandar Al-Khayran (23° 75′ North, 58° 75′ East) in Oman.20
Bacteria and fungi were isolated from the seawater using a marine broth (Oxoid Ltd., Basingstoke, UK) according to previously described methods.21 All macroorganisms were freeze-dried and extracted with methanol and ethyl acetate solvents (Sigma-Aldrich Corp., St. Louis, Missouri, USA). For microbes, cultures were centrifuged at 5,000 g and the bacterial cell pellets and fungi mycelium were extracted using the methanol and ethyl acetate solvents. All extracts were filtered using filtration paper (Whatman® grade 1 filtration paper, Sigma-Aldrich Corp.) and the solvents were removed by evaporation under reduced pressure using a rotary evaporator (Büchi Labortechnik AG, Flawil, Switzerland). The extracts were separated and purified using open-air silica for non-polar extracts or C18 columns for polar extracts. High-performance liquid chromatography was performed using a Shimadzu system (Shimadzu Corp., Kyoto, Japan) in order to further purify the fractions. Finally, the structure of pure secondary metabolites was elucidated on the basis of spectroscopic data, including infrared and ultraviolet radiation, high-resolution mass spectrometry and nuclear magnetic resonance spectroscopy. The novelty of the isolated compounds was assessed using the Royal Society of Chemistry MarinLit® database of marine natural products.22 The dry extracts and pure compounds were then re-dissolved either in dimethyl sulfoxide (DMSO; Sigma-Aldrich Corp.) or methanol.
A breast adenocarcinoma cell line, Michigan Cancer Foundation (MCF)-7 (ATCC® HTB-22™, American Type Culture Collection, Manassas, Virginia, USA), and a control line of human fibroblasts were cultured in Dulbecco’s Modified Eagle medium (DMEM; Gibco®, Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA). This was supplemented with 10% fetal bovine serum (FBS) and a 1% anti-biotic antimycotic cocktail (Gibco®, Thermo Fisher Scientific Inc.) containing 10,000 units/mL of penicillin, 10,000 µg/mL of streptomycin and 25 µg/ mL of amphotericin B. The cells were maintained in a humidified incubator at 37 °C with a 5% carbon dioxide atmosphere. Extracts or solutions of pure compounds (1 µL) were applied to each well of a 96-well plate (Nunc™ MicroWell™ plate, Thermo Fisher Scientific Inc.). The MCF-7 cells or human fibroblasts were then seeded in the 96-well plate at a density of 1,500 cells per well in 100 μL of DMEM supplemented with 10% FBS before being incubated for 24 hours. At the end of the experiment, the cells were observed with an inverted microscope and the status of the cells was determined. The experiment was repeated three times.
Due to its activity and availability, the crude extract of Sarcophyton sp. was selected for further analysis to determine the mode of its anti-cancer action. The MCF-7 cells were treated for 24 hours with different concentrations (12.5, 25.0, 50.0, 100.0, 150.0, 200.0, 300.0, 350.0 and 400.0 μg/mL) of the crude extract prepared in DMEM, without phenol red solution or FBS. Cells treated with DMSO were included as negative controls for each concentration. Cells were seeded in a six-well plate and treated with the determined inhibitory concentration 50% (IC50) of Sarcophyton sp. extract on attainment of 70–80% confluence. The cells were subsequently stained with Hoechst dye in order to identify the mode of cell death induced by the bioactive extracts of Sarcophyton sp. in the MCF-7 breast cancer model. Treated cells were harvested, suspended in 40 μL of Hoechst-formalin solution (ratio: 1:50) and incubated overnight in the dark at 4 °C. Hoechst-stained cells were then mounted on a glass slide and examined under the microscope using the 4′,6-diamidino-2-phenylindole filter (excitation: 350 nm; emission: 461 nm). Cells treated with DMSO were included as a negative control. Following the cell viability assay, electron microscopy analysis of the treated cells was used to elucidate the mechanism by which the extracts destroyed the cells. The MCF-7 cell lines were visualised using a transmission electron microscope (TEM; JEOL, Peabody, Massachusetts, USA).23 Briefly, the cell samples were fixed, dehydrated using an alcohol series and embedded in epoxy resin. Ultra-thin sections were obtained using an ultramicrotome and stained with uranyl acetate and Reynolds’ lead citrate.
Results
A total of eight pure compounds and 32 extracts of marine organisms in Oman were investigated, with 22.5% showing strong or medium anti-cancer activity against the MCF-7 cells, including 62.5% of the compounds and 12.5% of the extracts [Table 1]. The greatest anti-cancer activity was observed for malformin A, kuanoniamine D, hymenialdisine and gallic acid compounds, as well as the soft coral Sarcophyton sp. extract. Medium activity was observed for the aaptamin compound and the bryozoan Schizoporella unicornis, gorgonian coral Acanthogorgia sp. and sponge Mycale sp. extracts. No quantifiable activity on the control human fibroblast cells was detected.
Table 1:
Pure compounds* and extracts isolated from Omani marine organisms and their activity against the breast cancer Michigan Cancer Foundation-7 cell line
| Isolate | Organism | Species | Phylum | Solvent | ACA† | |
|---|---|---|---|---|---|---|
| Compound | Aaptamin | Sponge | Hemiasterella sp. | Porifera | DMSO | Medium |
| Hymenidin | Sponge | Halichondria sp. | Porifera | DMSO | Weak | |
| 2-Bromoaldisine | Sponge | Thethya sp. | Porifera | DMSO | None | |
| Hymenialdisine | Sponge | Hemiasterella sp. | Porifera | DMSO | Strong | |
| Malformin A | Fungus | Aspergillus niger | Ascomycota | DMSO | Strong | |
| Kojic acid | Fungus | Aspergillus sp. | Ascomycota | MeOH | None | |
| Kuanoniamine D | Tunicate | Didemnum sp. | Chordata | DMSO | Strong | |
| Gallic acid | Mangrove tree | Avicennia marina | Tracheophyta | MeOH | Strong | |
| Extract | Methanol | Soft coral | Sarcophyton sp. | Cnidaria | DMSO | None |
| Ethyl acetate | Soft coral | Sarcophyton sp. | Cnidaria | DMSO | Strong | |
| Methanol | Soft coral | Sinularia sp. | Cnidaria | DMSO | None | |
| Ethyl acetate | Soft coral | Sinularia sp. | Cnidaria | DMSO | None | |
| Methanol | Soft coral | Cladiella sp. | Cnidaria | DMSO | Weak | |
| Ethyl acetate | Soft coral | Cladiella sp. | Cnidaria | DMSO | Weak | |
| Methanol | Soft coral | Scleronephthya sp. | Cnidaria | DMSO | None | |
| Ethyl acetate | Soft coral | Scleronephthya sp. | Cnidaria | DMSO | Weak | |
| Methanol | Soft coral | Dendronephthya sp. | Cnidaria | DMSO | None | |
| Ethyl acetate | Soft coral | Dendronephthya sp. | Cnidaria | DMSO | None | |
| Methanol | Tunicate | Phallusia nigra | Chordata | DMSO | None | |
| Ethyl acetate | Tunicate | Phallusia nigra | Chordata | DMSO | None | |
| Methanol | Sponge | Chondrosia sp. | Porifera | DMSO | None | |
| Ethyl acetate | Sponge | Chondrosia sp. | Porifera | DMSO | None | |
| Ethyl acetate | Fungus | Penicillium sp. | Ascomycota | DMSO | None | |
| Methanol | Fungus | Penicillium sp. | Ascomycota | DMSO | None | |
| Methanol | Sea cucumber | Holothuria edulis | Echinodermata | DMSO | Weak | |
| Ethyl acetate | Sea cucumber | Holothuria atra | Echinodermata | DMSO | Weak | |
| Methanol | Gorgonian coral | Acanthogorgia sp. | Cnidaria | DMSO | Weak | |
| Ethyl acetate | Gorgonian coral | Acanthogorgia sp. | Cnidaria | DMSO | Medium | |
| Methanol | Bryozoan | Schizoporella unicornis | Bryozoa | DMSO | None | |
| Ethyl acetate | Bryozoan | Schizoporella unicornis | Bryozoa | DMSO | Medium | |
| Methanol | Sponge | Mycale sp. | Porifera | DMSO | Medium | |
| Ethyl acetate | Sponge | Mycale sp. | Porifera | DMSO | None | |
| Methanol | Bacterium | Halomonas sp. | Proteobacteria | DMSO | None | |
| Ethyl acetate | Bacterium | Halomonas sp. | Proteobacteria | DMSO | None | |
| Methanol | Bacterium | Marinobacter sp. | Proteobacteria | DMSO | None | |
| Ethyl acetate | Bacterium | Marinobacter sp. | Proteobacteria | DMSO | None | |
| Methanol | Green alga | Ulva sp. | Chlorophyta | MeOH | None | |
| Ethyl acetate | Green alga | Ulva sp | Chlorophyta | MeOH | None | |
| Ethyl acetate | Bryozoan | Bugula sp. | Bryozoa | MeOH | None | |
| Methanol | Bryozoan | Bugula sp. | Bryozoa | MeOH | Weak | |
| Control | - | - | - | DMSO and MeOH | None |
ACA = anti-cancer activity; DMSO = dimethyl sulfoxide; MeOH = methanol.
All compounds were dissolved either in MeOH or DMSO prior to the experiments and MeOH solutions were also evaporated beforehand. Correspondent MeOH and DMSO controls were included.
Anti-cancer activity was classified as either none (no activity), weak (<1,000 µg/mL), medium (100–1,000 µg/mL) or strong (>100 µg/mL).
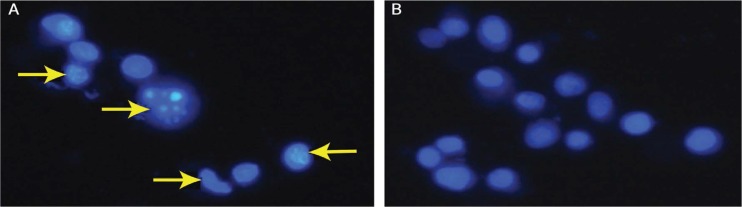
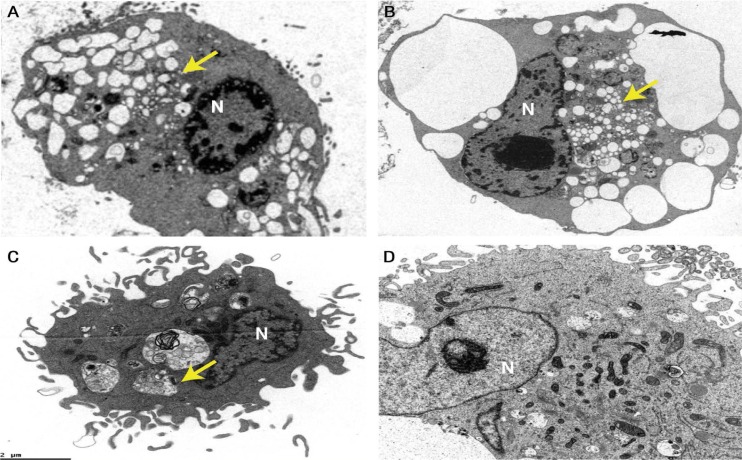
Treatment of MCF-7 cells with different concentrations of Sarcophyton sp. extract indicated a gradual increase in anti-cancer bioactivity as observed from escalating levels of cell death with increasing treatment concentration. The IC50 was 97 µg/mL; concentrations of cell death induction were most potent at doubled IC50. The presence of fragmented nuclei was observed in treated cells in comparison to the negative control cells [Figure 1]. This highlighted the role of the apoptotic pathway in triggering cell death. Furthermore, the TEM ultrastructural analysis of the Sarcophyton sp.-treated cells indicated the presence of cell membrane blebbing, intense vacuo-lisation and chromatic segregation at the periphery of the nucleus [Figure 2]. These changes confirmed the activation of apoptosis in the treated MCF-7 cells.
Figure 1A & B:
Hoechst stains at ×40 magnification of Michigan Cancer Foundation-7 breast cancer cells treated with (A) 97 µg/mL of Sarcophyton sp. extract for 24 hours and (B) dimethyl sulfoxide as a negative control. Note the presence of nuclear fragmentation in the cells treated with the Sarcophyton sp. extract (arrows).
Figure 2A–D:
Transmission electron microscopy ultrastructural analysis at ×4,000 magnification of (A–C) Michigan Cancer Foundation (MCF)-7 breast cancer cells treated with 97 µg/mL of Sarcophyton sp. extract for 24 hours and (D) control cells treated with solvents. Intensive blebbing, vacuolisation and chromatic segregation (arrows) due to apoptosis were observed in the MCF-7 cells.
N = nucleus.
Discussion
Numerous studies have indicated the benefits of marine flora and fauna extracts in various fields, including improvement in the prognosis of several diseases such as cancer.24–26 The complexity, poor prognosis and patient-, type- and stage-specificity of cancer requires the investigation and identification of novel compounds with effective clinical utility. In this study, the aim was to investigate the anti-cancer activity of extracts and compounds isolated from marine organisms in Oman. Almost a quarter of the investigated extracts and compounds showed medium or strong anti-cancer activity. The strongest activity against the MCF-7 breast adenocarcinoma model was observed for the malformin A, kuanoniamine D, hymenialdisine and gallic acid compounds and the soft coral Sarcophyton sp. extract. Hagimori et al. observed that malformin can disrupt the cell cycle at the G2 checkpoint in cancer cells.27 Similarly, kuanoniamine A and C isolated from sponges has been found to inhibit the growth of tumour and non-tumour cell lines, as well as an oestrogen-dependent breast cancer cell line.28 Smith et al. revealed that hymenialdisine has anti-cancer properties against human colorectal carcinomas.29 Furthermore, gallic acid has been shown as a potent compound with antimicrobial, anti-oxidant, quorum-sensing inhibitory and anti-cancer properties.30–32
In the current study, the tested compounds had no measurable effects on human fibroblast cells. This may suggest that these compounds are non-toxic, although alternatively this could indicate that fibroblasts are very resistant compared to the MCF-7 breast cancer cell line. This observation is in agreement with earlier findings regarding the resistance of fibroblasts to several toxic compounds.32 Moreover, MCF-10A cells are not considered a good control model since they have a transformed phenotype and are abnormal epithelial cells.33 New epithelial non-transformed cells are commercially available; however, they are difficult to handle and propagate.33 As such, there is a need for future in vivo studies to be carried out in order to assess the toxicity of marine natural products using immunosuppressed mice.
Analysis of the anti-cancer activity of Sarcophyton sp. extracts in the present study indicated the potency of this extract in inducing cell death in the MCF-7 breast cancer model at the IC50 of 97 µg/mL. Moreover, TEM microscopic analysis and Hoechst staining indicated the presence of fragmented nuclei within the treated cells. Typical characteristics of cell death induction by apoptosis were noted, such as nuclear fragmentation, membrane blebbing and increased vacuolisation. The anti-cancer activity of soft coral extracts has been previously reported.34,35 For example, diterpenes from the soft coral Xenia elongata were found to induce apoptosis in a genetically engineered mouse cell line which was D3-deficient in the BAK1 and BAX genes.35 The preliminary results of the current study therefore indicate the potential benefit of Sarcophyton sp. extracts in cancer treatment. An in-depth analysis of the molecular effects, specificity and efficacy of the extract for breast, ovarian, colon and prostate cancers is required through further in vitro and in vivo studies; this may potentially lead to the development of new treatments for breast adenocarcinomas and other cancers.
Conclusion
The results of this study highlight the anti-cancer potential of various marine organisms in Oman. The active anti-cancer compound extracted from Sarcophyton sp. is of particular interest and should be isolated in future experiments. The compound should be tested on in vitro models of various cancers to determine the specificity of its anti-cancer activity. Finally, the molecular mechanism and pathways activated in response to treatment with this compound should be investigated. Such an investigation will provide possibilities for the development of new cancer treatments.
Acknowledgments
This study was funded by an internal grant from the Sultan Qaboos University (#IG/AGR/FISH/12/01). The authors would like to acknowledge the support given to them by Sultan Qaboos University.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Fenical W, Jensen PR. Developing a new resource for drug discovery: Marine actinomycete bacteria. Nat Chem Biol. 2006;2:666–73. doi: 10.1038/nchembio841. [DOI] [PubMed] [Google Scholar]
- 2.Cooper EL, Yao D. Diving for drugs: Tunicate anticancer compounds. Drug Discov Today. 2012;17:636–48. doi: 10.1016/j.drudis.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Blunt JW, Copp BR, Keyzers RA, Munro MH, Prinsep MR. Marine natural products. Nat Prod Rep. 2013;30:237–323. doi: 10.1039/c2np20112g. [DOI] [PubMed] [Google Scholar]
- 4.Proksch P, Edrada RA, Ebel R. Drugs from the seas: Current status and microbiological implications. Appl Microbiol Biotechnol. 2002;59:125–34. doi: 10.1007/s00253-002-1006-8. [DOI] [PubMed] [Google Scholar]
- 5.Thoms C, Schupp P. Biotechnological potential of marine sponges and their associated bacteria as producers of new pharmaceuticals (part I) J Inter Biotech Law. 2005;2:217–20. doi: 10.1515/jibl.2005.2.5.217. [DOI] [Google Scholar]
- 6.Penesyan A, Kjelleberg S, Egan S. Development of novel drugs from marine surface associated microorganisms. Mar Drugs. 2010;8:438–59. doi: 10.3390/md8030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paul VJ, Arthur KE, Ritson-Williams R, Ross C, Sharp K. Chemical defenses: From compounds to communities. Biol Bull. 2007;213:226–51. doi: 10.2307/25066642. [DOI] [PubMed] [Google Scholar]
- 8.Schwartsmann G, Ratain MJ, Cragg GM, Wong JE, Saijo N, Parkinson DR, et al. Anticancer drug discovery and development throughout the world. J Clin Oncol. 2002;20:47S–59S. doi: 10.1200/JCO.2002.07.122. [DOI] [PubMed] [Google Scholar]
- 9.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–77. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 10.Abraham I, El Sayed K, Chen ZS, Guo H. Current status on marine products with reversal effect on cancer multidrug resistance. Mar Drugs. 2012;10:2312–21. doi: 10.3390/md10102312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terlau H, Olivera BM. Conus venoms: A rich source of novel ion channel-targeted peptides. Physiol Rev. 2004;84:41–68. doi: 10.1152/physrev.00020.2003. [DOI] [PubMed] [Google Scholar]
- 12.Sawadogo WR, Schumacher M, Teiten MH, Cerella C, Dicato M, Diederich M. A survey of marine natural compounds and their derivatives with anti-cancer activity reported in 2011. Molecules. 2013;18:3641–73. doi: 10.3390/molecules18043641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simmons TL, Andrianasolo E, McPhail K, Flatt P, Gerwick WH. Marine natural products as anticancer drugs. Mol Cancer Ther. 2005;4:333–42. [PubMed] [Google Scholar]
- 14.Hussain SM, Fareed S, Ansari S, Khan MS. Marine natural products: A lead for anti-cancer. Indian J Geomarine Sci. 2012;41:27–39. [Google Scholar]
- 15.Hayat S, Atta-ur-Rahman, Chouldhary MI, Khan KM, Abbaskhan A. Two new cinnamic acid esters from marine brown alga Spatoglossum variabile. Chem Pharm Bull (Tokyo) 2002;50:1297–9. doi: 10.1248/cpb.50.1297. [DOI] [PubMed] [Google Scholar]
- 16.Ali MS, Pervez MK, Ahmed F, Saleem M. Dichotenol-A, B and C: The C-16 oxidized seco-dolastanes from the marine brown alga Dictyota dichotoma (Huds.) lamour. Nat Prod Res. 2004;18:543–9. doi: 10.1080/14786410310001622059. [DOI] [PubMed] [Google Scholar]
- 17.Uzair B, Ahmed N, Mohammad FV, Ahmad VU. Detection, isolation and partial characterization of antifungal compound(s) produced by Pseudomonas aeruginosa CMG1055. J Chem Soc Pak. 2008;30:649–53. [Google Scholar]
- 18.Safaeian S, Hosseini H, Asadolah AA, Farmohamadi S. Antimicrobial activity of marine sponge extracts of offshore zone from Nay Band Bay, Iran. J Mycol Med. 2009;19:11–16. doi: 10.1016/j.mycmed.2008.11.003. [DOI] [Google Scholar]
- 19.Dobretsov S, Al-Mammari IM, Soussi B. Bioactive compounds from Omani sea cucumbers. J Agricultural Mar Sci. 2009;14:49–53. [Google Scholar]
- 20.Riguera R. Isolating bioactive compounds from marine organisms. J Mar Biotech. 1997;5:187–93. [Google Scholar]
- 21.Dobretsov SV, Qian PY. Effect of bacteria associated with the green alga ulva reticulata on marine micro- and macrofouling. Biofouling. 2002;18:217–28. doi: 10.1080/08927010290013026. [DOI] [Google Scholar]
- 22.Royal Society of Chemistry MarinLit: A database of the marine natural products literature. From: pubs.rsc.org/marinlit/ Accessed: Jan 2016.
- 23.Skalli O, Boykins LG, Coons L. Electron microscopy. In: Dobretsov S, Williams DN, Thomason JC, editors. Biofouling Methods. London, UK: Wiley-Blackwell; 2014. pp. 26–43. [DOI] [Google Scholar]
- 24.Lee JC, Hou MF, Huang HW, Chang FR, Yeh CC, Tang JY, et al. Marine algal products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int. 2013;13:55. doi: 10.1186/1475-2867-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sithranga Boopathy N, Kathiresan K. Anticancer drugs from marine flora: An overview. J Oncol. 2010;2010:214186. doi: 10.1155/2010/214186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper EL, Albert R. Tunicates: A vertebrate ancestral source of antitumor compounds. In: Kim SK, editor. Handbook of Anticancer Drugs from Marine Origin. Geneva, Switzerland: Springer; 2015. pp. 383–96. [DOI] [Google Scholar]
- 27.Hagimori K, Fukuda T, Hasegawa Y, Omura S, Tomoda H. Fungal malformins inhibit bleomycin-induced G2 checkpoint in Jurkat cells. Biol Pharm Bull. 2007;30:1379–83. doi: 10.1248/bpb.30.1379. [DOI] [PubMed] [Google Scholar]
- 28.Kijjoa A, Wattanadilok R, Campos N, Nascimento MS, Pinto M, Herz W. Anticancer activity evaluation of kuanoniamines A and C isolated from the marine sponge Oceanapia sagittaria, collected from the Gulf of Thailand. Mar Drugs. 2007;5:6–22. doi: 10.3390/md502006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith V, Raynaud F, Workman P, Kelland LR. Characterization of a human colorectal carcinoma cell line with acquired resistance to flavopiridol. Mol Pharmacol. 2001;60:885–93. doi: 10.1124/mol.60.5.885. [DOI] [PubMed] [Google Scholar]
- 30.Choubey S, Varughese LR, Kumar V, Beniwal V. Medical importance of gallic acid and its ester derivatives: A patent review. Pharm Pat Anal. 2015;4:305–15. doi: 10.4155/ppa.15.14. [DOI] [PubMed] [Google Scholar]
- 31.Singh BN, Singh BR, Singh RL, Prakash D, Dhakarey R, Upadhyay G, et al. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem Toxicol. 2009;47:1109–16. doi: 10.1016/j.fct.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 32.Wang K, Zhu X, Zhang K, Zhu L, Zhou F. Investigation of gallic acid induced anticancer effect in human breast carcinoma MCF-7 cells. J Biochem Mol Toxicol. 2014;28:387–93. doi: 10.1002/jbt.21575. [DOI] [PubMed] [Google Scholar]
- 33.Imbalzano KM, Tatarkova I, Imbalzano AN, Nickerson JA. Increasingly transformed MCF-10A cells have a progressively tumor-like phenotype in three-dimensional basement membrane culture. Cancer Cell Int. 2009;9:7. doi: 10.1186/1475-2867-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Byju K, Anuradha V, Vasundhara G, Nair SM, Kumar NC. In vitro and in silico studies on the anticancer and apoptosis-inducing activities of the sterols identified from the soft coral, Subergorgia reticulata. Pharmacogn Mag. 2014;10:S65–71. doi: 10.4103/0973-1296.127345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrianasolo EH, Haramaty L, White E, Lutz R, Falkowski P. Mode of action of diterpene and characterization of related metabolites from the soft coral, Xenia elongata. Mar Drugs. 2014;12:1102–15. doi: 10.3390/md12021102. [DOI] [PMC free article] [PubMed] [Google Scholar]