Abstract
Purpose of review
The review aims to provide a brief summary and evaluation of the current state of research that uses multiphoton fluorescence microscopy for intravital kidney imaging.
Recent findings
Direct visualization of the glomerular filter, proximal and distal tubule segments, and the renal vasculature in the living, intact kidney in zebrafish, mouse, and rat models with high temporal and spatial resolution provided new insights into the function of the normal and diseased kidney. New technical developments in fluorescence excitation and detection, in combination with transgenic animal models for cell function and fate mapping, and serial imaging of the same glomerulus in the same animal over several days further advanced the field of nephrology research, and the understanding of disease mechanisms.
Summary
Intravital multiphoton imaging has solved many critical technical barriers in kidney research and allowed the dynamic portrayal of the structure and function of various renal cell types in vivo. It has become a widely used research technique, with significant past achievements, and tremendous potential for future development and applications for the study and better understanding of kidney diseases.
Keywords: calcium, cell fate, glomerular filtration barrier, multiphoton microscopy, podocyte
INTRODUCTION
The last few years have seen the continuation of the trend of intravital imaging of the kidney using multiphoton microscopy (MPM) becoming more and more appreciated and used by a growing number of nephrology research laboratories worldwide. Owing to its ability to directly visualize dynamic changes in tissue morphology and function with subcellular resolution, and over time, intravital MPM imaging remained a preferred experimental technique for investigators to study renal physiology and disease. Over the past 15 years, several nephrology laboratories have applied this high power imaging technique and developed various modalities of MPM to quantitatively visualize renal glomerular, tubular, and vascular structures and their functions. These include single nephron glomerular filtration rate, blood flow, tubular flow, the concentrating and diluting mechanism, renin granular content, release, and tissue renin activity, cell and mitochondrial metabolism, cell migration and fate, intracellular processes, and parameters such as endocytosis, pH, calcium, and many others that can be directly and quantitatively visualized in the intact living kidney with high temporal, spatial resolution in noninvasive optical tissue sections [1,2,3▪,4–7,8▪▪,9–11]. These are significant and unique capabilities of this technology, and, currently, no other experimental methods provide this amazing morphological and functional detail of the mouse and rat kidney in vivo. The basic principles, advantages, and examples of using multiphoton excitation fluorescence microscopy have been reviewed elsewhere [3▪,5–7,8▪▪,10,12,13]. Here, we present a brief summary of the most recent intravital MPM imaging studies of the kidney, emphasizing the most important aspects and future directions of technical developments and research applications, and discussing the scientific breakthroughs that were made possible by the use of intravital MPM.
KEY POINTS.
Intravital imaging using MPM has become a widely used experimental technique in nephrology research.
Owing to its capabilities and versatility, MPM remains the first choice high power imaging method for studying the intact living kidney.
Direct visual clues on changes in cell and tissue morphology and function observed by MPM imaging in vivo have provided important insights into kidney disease mechanisms.
Multiphoton microscopy imaging of the glomerulus
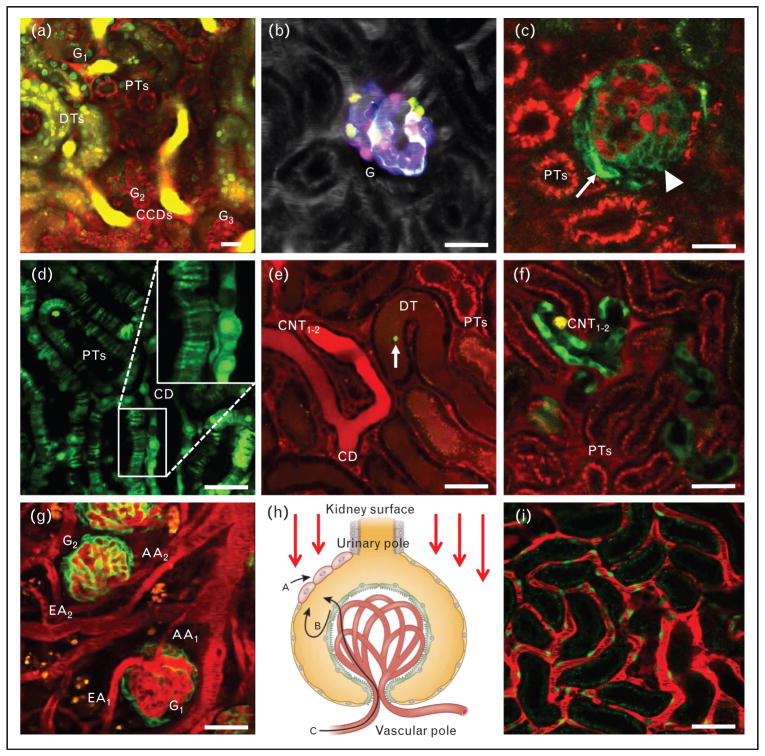
The main focus of recent in-vivo glomerular imaging studies has been the glomerular filtration barrier (GFB), and the podocyte in particular. This is well justified by their importance in the development of glomerular and renal diseases, delicate three-dimensional structure, and inaccessibility by other in-vivo research techniques. One important technical advance has been the application of MPM for imaging glomeruli in the intact living mouse kidney [9,14], which overcame the technical limitation and necessity to study the Munich-Wistar-Fromter rat model that features superficial glomeruli [2,4]. Although the structure and function of the rat glomerulus resemble the human condition, which is advantageous for translational studies, the availability of an arsenal of mouse genetic tools has made the mouse MPM model better suited for mechanistic studies. The accessibility of multiple mouse glomeruli for MPM imaging studies is demonstrated in Fig. 1a, and has also been confirmed by several laboratories [15–17,18▪,19,20]. Furthermore, significant improvements in imaging technology, mainly in fluorescence detection (availability of high sensitivity gallium arsenide phosphide and hybrid detectors) now allow routine MPM imaging of glomeruli in adult male mice, and not only in adolescent female mice as before. The application of long wavelength multiphoton excitation (up to 1300 nm) can further improve depth penetration and the routine in-vivo MPM imaging of deep rather than superficial glomeruli [21▪▪].
FIGURE 1.
Intravital multiphoton microscopy (MPM) images of glomeruli (a–c), tubules (d–f), and vasculature (g–i) in the intact C57BL6 mouse kidney. In all animals and images, plasma was labeled by Alexa594-albumin (red) given in i.v. bolus. (a), Deep optical section of three adjacent glomeruli (G1–3), round-shaped PTs, elongated DTs with intensely nucleic acid stain (Hoechst33342)-labeled cell nuclei (green), and cortical collecting ducts (CCDs) containing highly concentrated Lucifer Yellow, a freely filtered dye injected i.v. in bolus. (b), Multicolor labeling of podocytes in the transgenic Podocin-Confetti mouse model, either by genetically encoded membrane-targeted cyan fluorescent protein (CFP), nuclear green fluorescent protein (GFP), cytosolic yellow fluorescent protein (YPF) or cytosolic red fluorescent protein (RFP). Plasma label is shown in greyscale. (c), Spontaneous glomerulosclerosis in a Podocin-GCaMP3 mouse kidney, with high calcium (intense green) in a subset of podocytes that cluster (arrowhead) or invade the parietal Bowman’s capsule (arrow). Albumin uptake is visible in proximal tubule segments (PTs, red). (d), 2′,7′-Bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF)-loaded PTs and a CD featuring a striated pattern in PTs, and diffuse homogenous labeling of whole cells in the CD (magnified area shown in inset) in Sprague-Dawley rat kidney. (e), Podocyte injury-induced leakage of plasma albumin (red) into the renal tubular system in a Podocin-GFP mouse. High albumin uptake in PT segments, and the transit of high amounts of albumin are visible downstream the nephron in DT, and connecting tubules (CNT1–2) merging into CDs. A detached podocyte (green, arrow) is seen flowing downstream in the DT lumen. (f), MPM imaging of cytosolic calcium changes in tubular epithelial cells in vivo in kidney-specific cadherin-GCaMP3 mice. The genetically encoded calcium indicator GCaMP3 (green) is seen highly expressed in two adjacent connecting tubules (CNT1–2) and in some cells of PTs. (g), Studying renal cortical vascular responses in Podocin-GFP mice after unilateral ureter obstruction. Several glomeruli and their adjacent afferent (AA) and efferent arterioles (EA) are clearly visible. (h), Illustration of the model of glomerular imaging and cell fate tracking by the combination of genetic lineage tracing and serial MPM. Red arrows indicate the direction of fluorescence excitation light coming from the microscope objective at the kidney surface. Visual clues on the migration pattern and dynamics of the same genetically labeled single stem/progenitor cells and their progenies [e.g., unique single cell identity by RFP (red) expression] can help to determine if a new cell (and its identical color progeny) that appeared on the parietal layer of Bowman’s capsule originated from the renal interstitium (a), or the visceral podocyte layer (b), or from a circulating cell population (c). Modified with permission from [8▪▪]. (i), Peritubular capillaries in a receptor tyrosine kinase (Tie2)-GFP mouse kidney. Capillary endothelial cells are labeled green. Bars=20μm. CT, collecting duct; DT, distal tubules; PT, proximal tubules.
The initial studies with high power MPM imaging of the GFB used highly fluorescent molecular tracers of different sizes to label the filtrate, measure GFB permeability, and to visualize the podocytes in negative [2,9,19,22–26,27▪]. This approach also helped to shed light on new microanatomical details of the GFB, the presence and function of the subpodocyte space [24], its role in puromycin-induced focal segmental glomerulosclerosis [7,9], and angiotensin II-induced albumin vesicle transcytosis in podocytes [28▪▪]. In addition, unidentified migrating cells were seen in both in the parietal [29▪▪] and visceral layers of the Bowman’s capsule [9], which may play a role in the cellular remodeling of the GFB.
Positive identification of cells of the glomerulus and the GFB using genetically expressed fluorescent lineage tags (genetic cell fate mapping) in combination with intravital MPM imaging was another major technical advance [30▪▪,31▪▪]. This approach was able to unequivocally identify and visualize parietal epithelial cells and podocytes expressing green fluorescent protein (GFP) [31▪▪,32▪]. Further, the unique identification and tracking of the fate of single podocytes was made possible by the expression of multicolor fluorescent reporters, for example the Confetti construct of cyan, green, yellow, and red fluorescent protein (CFP/GFP/YFP/RFP) as shown in Fig. 1b [31▪▪]. Serial MPM imaging of the exact same glomerulus in the same kidney over several days shed light on the highly dynamic glomerular environment, the robust cellular remodeling of the glomerulus by migrating parietal epithelial cells and podocytes in disease conditions, including adriamycin nephropathy (which resembles human focal segmental glomerulosclerosis) and obstructive nephropathy. Other MPM studies used the genetically encoded calcium indicator GCaMP3 (a fusion protein containing the calmodulin-binding domain from the myosin light chain kinase also called M13 peptide, the circularly permutated green fluorescent protein, and the calmodulin) expressed in podocytes, and established the role of purinergic calcium signaling via purinergic receptor type Y2 receptors in primary and secondary (propagating) podocyte injury, cell clustering, and migration (Fig. 1c) [30▪▪]. Future studies with this serial MPM approach to study the cellular remodeling of the same glomerulus over several days will be essential to identify the renal progenitor cell types and their mechanisms involved in glomerular repair and regeneration.
Multiphoton microscopy imaging of renal tubules
Owing to the easily accessible S1–2 segments of the proximal tubule, which are close to the kidney surface, several research groups have used MPM imaging to visualize dynamic cellular processes in vivo in proximal tubule cells. Recent studies focused on proximal tubule albumin uptake, processing, transcytosis of filtered albumin, and elements of a reclamation pathway that minimizes urinary loss and catabolism of albumin, therefore prolonging its serum half-life [33]. Under different albumin loading conditions, investigators identified a complex molecular regulatory system that responded rapidly to different physiologic conditions to minimize alterations in serum albumin levels [34▪]. Figure 1e demonstrates the use of MPM for tracking the renal tubular processing of filtered albumin.
In other studies, reduced proximal tubule tubular flow because of cell swelling and luminal obstruction was found in a model of septic acute kidney injury, which helped the better understanding of the mechanism of oliguria in the early phase of endotoxemia [35▪]. Megalin-mediated endocytic uptake of filtered lipotoxic substances in a high-fat diet was shown to cause proximal tubule cell injury and retrograde glomerular dysfunction [36▪]. In addition to imaging proximal tubule endocytosis, MPM has been very useful in studying alterations in mitochondrial structure and function, and the relevant aerobic and anaerobic metabolic pathways of both proximal and distal tubular epithelial cells in acute kidney injury [3▪,37]. These MPM imaging studies of many intracellular organelles were instrumental in uncovering several new proximal tubule mechanisms and their roles in a variety of kidney diseases.
New intravital MPM imaging approaches have been established to investigate cytosolic parameters of proximal tubule cells, including pH [38▪] and calcium [39▪]. MPM imaging of proximal tubule segments in the rat kidney loaded with the pH sensitive dye 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF) visualized the development of a high pH microdomain near the bottomof the brush border in response to an acute rise of blood pressure [38▪], which may be a new important mechanism in pressure natriuresis (inhibition of proximal tubule sodium reabsorption). BCECF loading of proximal tubules also reflected on major differences in tubular epithelial cell microanatomy between different tubule segments. Figure 1d demonstrates the typical striated pattern of proximal tubules on thin confocal optical sections when loaded with a pH dye, which was due to the uneven loading (and pH) of adjacent proximal tubule cells interdigitating via their many lateral cell processes. In contrast, collecting duct cells with continuous cytoplasm space show diffuse homogenous labeling (Fig. 1d). These anatomical differences need to be considered when analyzing intracellular vesicle movements in proximal tubule cells.
MPM imaging of cytosolic calcium changes in tubular epithelial cells in vivo has been established using transgenic rats [39▪] or mice expressing the genetically encoded calcium indicator GCaMP proteins. Basal levels, and ligand and drug-induced alterations in cell calcium levels in proximal tubule epithelial cells were measured successfully in a rat model, which opens new possibilities for future physiologic and pharmacologic investigations [39▪]. Figure 1f demonstrates the use of a Cre-lox-based mouse model for intravital calcium imaging of tubular epithelia, using kidney-specific cadherin-GCaMP3 mice [kidney-specific cadherin (Ksp)-DNA recombinase enzyme (Cre)/GCaMP3-fl mice, unpublished]. These GCaMP-based transgenic approaches have several advantages over earlier calcium imaging techniques that used classic fluorophores [40].
Vascular imaging
The afferent arteriole and the juxtaglomerular apparatus (JGA) are key anatomical structures that regulate renal hemodynamics and the rennin–angiotensin system. They are therefore important targets in a variety of nephrology studies. However, MPM imaging of the far side of the glomerulus, the vascular pole, and glomerular arterioles (Fig. 1g) has been technically challenging, because it requires the deepest optical sectioning through the highly light scattering glomerular capillaries (Fig. 1h). However, in-vivo imaging of the terminal, renin-positive part of the afferent arteriole has been successful in a few nephrons in both the rat and mouse kidney. Several MPM imaging studies helped to directly visualize and better understand the regulation of renin granular content in the JGA and collecting ducts in various conditions, including diabetes [4,10,14,41] and rennin–angiotensin system inhibitors [41,42], and the role of bulk ultrafiltration of plasma and fluid flow in the JGA [43]. Recent MPM studies of glomerular capillaries found increased blood flow, but constant shear stress in rats after nephrectomy because of increased capillary diameter and alterations in capillary blood rheology [44].
Perhaps the most widely used and characterized animal model in studying renal cortical blood flow, glomerular microcirculation, and vascular functions, is the unilateral ureteral obstruction model and the consequently developing hydronephrotic kidney, as established by Buhrle et al. [45]. This model has been used extensively for the past 25 years to study the renal vascular effects of a number of antihypertensive drugs on the afferent and efferent arteriole. The combination of this classic experimental model with intravital MPM imaging provides a new dimension for future renal hemodynamics studies, including single cell and subcellular resolution of morphological, intracellular calcium, membrane voltage changes of vascular smooth muscle cells [46], and the propagation of vascular signals along glomerular arterioles and nephron-to-nephron coupling [8▪▪]. Figure 1g demonstrates that glomerular hemodynamic parameters, vascular functions, including acute changes in glomerular, and afferent and efferent arteriole diameter can be directly visualized over time in this model. Commercially available mouse genetic tools (e.g., Cre-lox mouse models) will allow cell-type specific expression of fluorescent reporters (e.g., calcium-sensitive GCaMP proteins) in various vascular cell types, including smooth muscle, endothelial, and mesangial cells and podocytes [30▪▪], and their direct visualization with high temporal and spatial resolution using MPM imaging (Fig. 1g–i). Future studies using these visual experimental approaches will undoubtedly help to a better understanding of renal vascular (patho)physiology, and will be a tremendous aid in renal drug discovery, target validation, and testing. By tracking the same single, genetically labeled cell (by a unique color identifier) using serial MPM over several days, the origin, fate, and migration pattern of cells can be established (Fig. 1h).
Another hot topic in nephrology research, and an area where MPM imaging can be very useful in further development, is the role of vascular endothelium in kidney diseases. In the past, intravital imaging studies provided visual clues on the role of endothelial fenestrations of the terminal afferent arteriole (nanopores) [43], and the glomerular capillary endothelial surface layer (glycocalyx) in vascular dysfunction, and albumin leakage [23]. Also, the density and function of peritubular capillaries, which are easily accessible on the kidney surface for intravital MPM imaging, are critically important in the context of kidney disease. The role of peritubular capillary rarefaction, which causes kidney ischemia and accelerated nephron injury and interstitial fibrosis, is well established in the progression of chronic kidney disease. Rouleaux formation by red blood cells, leukocyte sticking and rolling in peritubular capillaries, and the leakiness of these blood vessels, have been visualized by intravital imaging studies in several experimental models of acute renal injury [1,47] which helped to evolve our understanding of the mechanisms of tubular epithelium-vascular endothelium crosstalk, inflammatory cell interactions, and the development of effective therapeutic strategies. Other MPM studies visualized renal erythropoietin producing cells that tightly associate with the capillary endothelium, and found that erythropoietin synthesis can be restored after renal injury by activating the hypoxia signaling pathway [48▪]. Figure 1i illustrates the high detail imaging of peritubular capillaries, endothelial cells, and various blood cells that can be achieved by using intravital MPM.
CONCLUSION
Intravital MPM imaging of the rat and mouse kidney has become a routine nephrology research technique available in most basic biomedical research facilities and is now being used worldwide. It has provided tremendous contributions to our better understanding of the structure and functions of the normal and diseased kidney, and the many pathological mechanisms of kidney disease. Constant improvements in laser, optics, and microscopy technology, the development of new MPM imaging modalities, in combination with genetic animal models, will further advance its use for nephrology research and, perhaps, for clinical diagnostics.
Acknowledgments
Financial support and sponsorship
This work was supported in part by US National Institutes of Health grants DK64324 and DK100944, by the American Heart Association grant 15GRNT23040039, and by the American Diabetes Association grant 4–15-CKD-56 to J.P.-P. We thank James Burford for his assistance with intravital imaging.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Ashworth SL, Sandoval RM, Tanner GA, Molitoris BA. Two-photon microscopy: visualization of kidney dynamics. Kidney Int. 2007;72:416–421. doi: 10.1038/sj.ki.5002315. [DOI] [PubMed] [Google Scholar]
- 2.Dunn KW, Sandoval RM, Kelly KJ, et al. Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am J Physiol Cell Physiol. 2002;283:C905–C916. doi: 10.1152/ajpcell.00159.2002. [DOI] [PubMed] [Google Scholar]
- 3▪.Hall AM, Molitoris BA. Dynamic multiphoton microscopy: focusing light on acute kidney injury. Physiology (Bethesda) 2014;29:334–342. doi: 10.1152/physiol.00010.2014. The review study summarizes the applications of MPM to study novel mechanisms and treatments in different forms of acute kidney injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang JJ, Toma I, Sipos A, et al. Quantitative imaging of basic functions in renal (patho)physiology. Am J Physiol Renal Physiol. 2006;291:F495–F502. doi: 10.1152/ajprenal.00521.2005. [DOI] [PubMed] [Google Scholar]
- 5.Molitoris BA, Sandoval RM. Intravital multiphoton microscopy of dynamic renal processes. Am J Physiol Renal Physiol. 2005;288:F1084–F1089. doi: 10.1152/ajprenal.00473.2004. [DOI] [PubMed] [Google Scholar]
- 6.Peti-Peterdi J. Multiphoton imaging of renal tissues in vitro. Am J Physiol Renal Physiol. 2005;288:F1079–F1083. doi: 10.1152/ajprenal.00385.2004. [DOI] [PubMed] [Google Scholar]
- 7.Peti-Peterdi J, Burford JL, Hackl MJ. The first decade of using multiphoton microscopy for high-power kidney imaging. Am J Physiol Renal Physiol. 2012;302:F227–F233. doi: 10.1152/ajprenal.00561.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8▪▪.Peti-Peterdi J, Kidokoro K, Riquier-Brison A. Novel in vivo techniques to visualize kidney anatomy and function. Kidney Int. 2015;88:44–51. doi: 10.1038/ki.2015.65. The review article is the latest comprehensive summary and evaluation of the current state-of-the-art of intravital imaging of the kidney using MPM. It provides a detailed review of imaging approaches of the glomerulus, the rat and mouse kidney, transgenic models, calcium imaging, and unconventional uses of multiphoton lasers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peti-Peterdi J, Sipos A. A high-powered view of the filtration barrier. J Am Soc Nephrol. 2010;21:1835–1841. doi: 10.1681/ASN.2010040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peti-Peterdi J, Toma I, Sipos A, Vargas SL. Multiphoton imaging of renal regulatory mechanisms. Physiology (Bethesda) 2009;24:88–96. doi: 10.1152/physiol.00001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sipos A, Toma I, Kang JJ, et al. Advances in renal (patho)physiology using multiphoton microscopy. Kidney Int. 2007;72:1188–1191. doi: 10.1038/sj.ki.5002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn KW, Young PA. Principles of multiphoton microscopy. Nephron Exp Nephrol. 2006;103:e33–e40. doi: 10.1159/000090614. [DOI] [PubMed] [Google Scholar]
- 13.Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- 14.Toma I, Kang JJ, Sipos A, et al. Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J Clin Invest. 2008;118:2526–2534. doi: 10.1172/JCI33293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devi S, Li A, Westhorpe CL, et al. Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat Med. 2013;19:107–112. doi: 10.1038/nm.3024. [DOI] [PubMed] [Google Scholar]
- 16.Hohne M, Ising C, Hagmann H, et al. Light microscopic visualization of podocyte ultrastructure demonstrates oscillating glomerular contractions. Am J Pathol. 2013;182:332–338. doi: 10.1016/j.ajpath.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Khoury CC, Khayat MF, Yeo TK, et al. Visualizing the mouse podocyte with multiphoton microscopy. Biochem Biophys Res Commun. 2012;427:525–530. doi: 10.1016/j.bbrc.2012.09.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18▪.Kistler AD, Caicedo A, Abdulreda MH, et al. In vivo imaging of kidney glomeruli transplanted into the anterior chamber of the mouse eye. Sci Rep. 2014;4:3872. doi: 10.1038/srep03872. Report of an interesting, unusual approach to study the structure and function of transplanted glomeruli in the mouse eye. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakano D, Kobori H, Burford JL, et al. Multiphoton imaging of the glomerular permeability of angiotensinogen. J Am Soc Nephrol. 2012;23:1847–1856. doi: 10.1681/ASN.2012010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiessl IM, Bardehle S, Castrop H. Superficial nephrons in BALB/c and C57BL/6 mice facilitate in vivo multiphoton microscopy of the kidney. PLoS One. 2013;8:e52499. doi: 10.1371/journal.pone.0052499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21▪▪.Schuh CD, Haenni D, Craigie E, et al. Long wavelength multiphoton excitation is advantageous for intravital kidney imaging. Kidney Int. 2015 doi: 10.1038/ki.2015.323. Epub ahead of print The first study on the use of long wavelength (>1300 nm) excitation fluorescence imaging of the living mouse kidney, which allows third harmonic generation and deep imaging of glomeruli. [DOI] [PubMed] [Google Scholar]
- 22.Russo LM, Sandoval RM, McKee M, et al. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int. 2007;71:504–513. doi: 10.1038/sj.ki.5002041. [DOI] [PubMed] [Google Scholar]
- 23.Salmon AH, Ferguson JK, Burford JL, et al. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol. 2012;23:1339–1350. doi: 10.1681/ASN.2012010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salmon AH, Toma I, Sipos A, et al. Evidence for restriction of fluid and solute movement across the glomerular capillary wall by the subpodocyte space. Am J Physiol Renal Physiol. 2007;293:F1777–F1786. doi: 10.1152/ajprenal.00187.2007. [DOI] [PubMed] [Google Scholar]
- 25.Schiessl IM, Castrop H. Angiotensin II AT2 receptor activation attenuates AT1 receptor-induced increases in the glomerular filtration of albumin: a multiphoton microscopy study. Am J Physiol Renal Physiol. 2013;305:F1189–F1200. doi: 10.1152/ajprenal.00377.2013. [DOI] [PubMed] [Google Scholar]
- 26.Yu W, Sandoval RM, Molitoris BA. Rapid determination of renal filtration function using an optical ratiometric imaging approach. Am J Physiol Renal Physiol. 2007;292:F1873–F1880. doi: 10.1152/ajprenal.00218.2006. [DOI] [PubMed] [Google Scholar]
- 27▪.Schiessl IM, Kattler V, Castrop H. In vivo visualization of the antialbuminuric effects of the angiotensin-converting enzyme inhibitor enalapril. J Pharmacol Exp Ther. 2015;353:299–306. doi: 10.1124/jpet.114.222125. The work directly visualized the effects of the most common therapeutic strategy for kidney disease, and evaluated the contribution of glomerular vs. tubular factors of albuminuria development. [DOI] [PubMed] [Google Scholar]
- 28▪▪.Schiessl IM, Hammer A, Kattler V, et al. Intravital imaging reveals angiotensin II-induced transcytosis of albumin by podocytes. J Am Soc Nephrol. 2016;27:731–744. doi: 10.1681/ASN.2014111125. Epub ahead of print. Visualization of albumin containing vesicles and their transport (transcytosis) in podocytes that provide a new mechanism for glomerular albumin processing in conditions of kidney injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29▪▪.Peti-Peterdi J, Burford JL, Hackl MJ. Can kidney regeneration be visualized? Nephron Exp Nephrol. 2014;126:86. doi: 10.1159/000360673. Application of intravital multiphoton imaging techniques for studying kidney tissue remodeling and regeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30▪▪.Burford JL, Villanueva K, Lam L, et al. Intravital imaging of podocyte calcium in glomerular injury and disease. J Clin Invest. 2014;124:2050–2058. doi: 10.1172/JCI71702. Intravital imaging of cell calcium changes in a disease model for the first time. The work identified purinergic calcium signaling in podocytes as the primary culprit in primary and secondary (propagating) podocyte injury, and in the development of glomerular disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31▪▪.Hackl MJ, Burford JL, Villanueva K, et al. Tracking the fate of glomerular epithelial cells in vivo using serial multiphoton imaging in new mouse models with fluorescent lineage tags. Nat Med. 2013;19:1661–1666. doi: 10.1038/nm.3405. Combination of intravital imaging with transgenic mouse models using fluorescent cell lineage tags. Development and first application of serial multiphoton imaging of the same glomerulus in the same kidney over several days, during the development of glomerular disease. This technique has tremendous potential for future applications to study renal tissue remodeling and regeneration by stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32▪.Endlich N, Simon O, Gopferich A, et al. Two-photon microscopy reveals stationary podocytes in living zebrafish larvae. J Am Soc Nephrol. 2014;25:681–686. doi: 10.1681/ASN.2013020178. Zebrafish model of intravital imaging of GFP-labeled podocytes in glomeruli for several hours, which showed stationary podocytes in the normal kidney (no migration such as in injury models) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickson LE, Wagner MC, Sandoval RM, Molitoris BA. The proximal tubule and albuminuria: really! J Am Soc Nephrol. 2014;25:443–453. doi: 10.1681/ASN.2013090950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34▪.Wagner MC, Campos-Bilderback SB, Chowdhury M, et al. Proximal tubules have the capacity to regulate uptake of albumin. J Am Soc Nephrol. 2016;27:482–494. doi: 10.1681/ASN.2014111107. Epub ahead of print. Investigation of a molecular network in proximal tubule cells, which is important in the regulation of albumin transcytosis (recycling) depending on albumin load. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35▪.Nakano D, Doi K, Kitamura H, et al. Reduction of tubular flow rate as a mechanism of oliguria in the early phase of endotoxemia revealed by intravital imaging. J Am Soc Nephrol. 2015;26:3035–3044. doi: 10.1681/ASN.2014060577. The first work of a new, very productive multiphoton imaging lab, describing the early structural and functional changes in proximal tubule cells after septic acute kidney injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪.Kuwahara S, Hosojima M, Kaneko R, et al. Megalin-mediated tubuloglomerular alterations in high-fat diet-induced kidney disease. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2015020190. Epub ahead of print Intravital imaging of the endocytic uptake of filtered lipotoxic substances in proximal tubule cells in response to high-fat diet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall AM, Rhodes GJ, Sandoval RM, et al. In vivo multiphoton imaging of mitochondrial structure and function during acute kidney injury. Kidney Int. 2013;83:72–83. doi: 10.1038/ki.2012.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38▪.Brasen JC, Burford JL, McDonough AA, et al. Local pH domains regulate NHE3 mediated Na+ reabsorption in the renal proximal tubule. Am J Physiol Renal Physiol. 2014;307:F1249–F1262. doi: 10.1152/ajprenal.00174.2014. A combined mathematical modeling and intravital imaging experimental work which identified a high pH subapical microdomain in proximal tubule cells which develops in response to elevated blood pressure, and inhibits sodium reabsorption. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39▪.Szebenyi K, Furedi A, Kolacsek O, et al. Visualization of calcium dynamics in kidney proximal tubules. J Am Soc Nephrol. 2015;26:2731–2740. doi: 10.1681/ASN.2014070705. An exciting transgenic rat model to visualize cell calcium changes in proximal tubule cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Svenningsen P, Burford JL, Peti-Peterdi J. ATP releasing connexin 30 hemi-channels mediate flow-induced calcium signaling in the collecting duct. Front Physiol. 2013;4:292. doi: 10.3389/fphys.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang JJ, Toma I, Sipos A, et al. The collecting duct is the major source of prorenin in diabetes. Hypertension. 2008;51:1597–1604. doi: 10.1161/HYPERTENSIONAHA.107.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang JJ, Toma I, Sipos A, et al. Imaging the renin-angiotensin system: an important target of antihypertensive therapy. Adv Drug Deliv Rev. 2006;58:824–833. doi: 10.1016/j.addr.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Rosivall L, Mirzahosseini S, Toma I, et al. Fluid flow in the juxtaglomerular interstitium visualized in vivo. Am J Physiol Renal Physiol. 2006;291:F1241–F1247. doi: 10.1152/ajprenal.00203.2006. [DOI] [PubMed] [Google Scholar]
- 44.Ferrell N, Sandoval RM, Bian A, et al. Shear stress is normalized in glomerular capillaries following (5/6) nephrectomy. Am J Physiol Renal Physiol. 2015;308:F588–F593. doi: 10.1152/ajprenal.00290.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buhrle CP, Hackenthal E, Helmchen U, et al. The hydronephrotic kidney of the mouse as a tool for intravital microscopy and in vitro electrophysiological studies of renin-containing cells. Lab Invest. 1986;54:462–472. [PubMed] [Google Scholar]
- 46.Marsh DJ, Toma I, Sosnovtseva OV, et al. Electrotonic vascular signal conduction and nephron synchronization. Am J Physiol Renal Physiol. 2009;296:F751–F761. doi: 10.1152/ajprenal.90669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Molitoris BA. Therapeutic translation in acute kidney injury: the epithelial/endothelial axis. J Clin Invest. 2014;124:2355–2363. doi: 10.1172/JCI72269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48▪.Souma T, Nezu M, Nakano D, et al. Erythropoietin Synthesis in Renal Myofibroblasts Is Restored by Activation of Hypoxia Signaling. J Am Soc Nephrol. 2016;27:428–438. doi: 10.1681/ASN.2014121184. [Epub ahead of print]. Intravital imaging of erythropoietin producing cells and their stimulation after injury in transgenic mouse models. [DOI] [PMC free article] [PubMed] [Google Scholar]