Abstract
Study Design Biomechanical cadaveric study.
Objective Clinical studies indicate that using less-rigid fixation techniques in place of the standard all-pedicle screw construct when correcting for scoliosis may reduce the incidence of proximal junctional kyphosis and improve patient outcomes. The purpose of this study is to investigate whether there is a biomechanical advantage to using supralaminar hooks in place of pedicle screws at the upper-instrumented vertebrae in a multilevel thoracic construct.
Methods T7–T12 spines were biomechanically tested: (1) intact; (2) following a two-level pedicles screw fusion from T9 to T11; and after proximal extension of the fusion to T8–T9 with (3) bilateral supra-laminar hooks, (4) a unilateral hook + unilateral screw hybrid, or (5) bilateral pedicle screws. Specimens were nondestructively loaded while three-dimensional kinematics and intradiscal pressure at the supra-adjacent level were recorded.
Results Supra-adjacent hypermobility was reduced when bilateral hooks were used in place of pedicle screws at the upper-instrumented level, with statistically significant differences in lateral bending and torsion (p < 0.05 and p < 0.001, respectively). Disk pressures in the supra-adjacent segment were not statistically different among top-off techniques.
Conclusions The use of supralaminar hooks at the top of a multilevel posterior fusion construct reduces the stress at the proximal uninstrumented motion segment. Although further data is needed to provide a definitive link to the clinical occurrence of PJK, this in vitro study demonstrates the potential benefit of “easing” the transition between the stiff instrumented spine and the flexible native spine and is the first to demonstrate these results with laminar hooks.
Keywords: proximal junctional kyphosis, biomechanics, scoliosis, thoracic, spinal fusion, supra-laminar hooks, adjacent-segment disease
Introduction
Proximal junctional kyphosis (PJK) is a well-documented occurrence associated with multisegment arthrodesis procedures utilizing posterior instrumentation in the thoracic or thoracolumbar spine. Typically analyzed within the context of adolescent and adult spinal deformity surgery, it has been characterized radiographically by the presence of a proximal junction sagittal Cobb angle greater than 10 degrees and an increase in proximal junction sagittal Cobb angle of greater than 10 degrees compared with preoperative measurement.1 The prevalence of PJK is reportedly between 26 and 46%,1 2 3 4 5 with the majority of the incidences identified within 2 years postoperatively.6 Although PJK is often considered to be a radiographic finding with little to no clinical implications,1 7 it has recently been linked to inferior Scoliosis Research Society pain subscores,7 suggesting a need for further investigation.
In the years since it was first demonstrated as safe and suitable for the treatment of thoracic spinal deformities,8 9 10 rigid posterior fixation has continually increased in popularity and is now considered the gold standard for the correction of scoliosis.11 This growing trend toward increased construct stiffness and load-bearing posterior instrumentation has been proposed as a preeminent risk factor for PJK,4 12 in addition to old age, fusion to the sacrum, low bone mineral density, and poor sagittal alignment.4 6 13 14
Although few studies offer insight into the mechanical pathology of PJK, added stress at the proximal adjacent level to a long fusion construct has been suggested as a possible causative factor.15 The transition from a rigid pedicle screw–rod construct to the intact spine represents a drastic and immediate change in stiffness, which in theory subjects the adjacent motion segments to unnaturally large displacements and rotations. Based on this concept, several groups have investigated the use of less-rigid instrumentation at the terminus of a long construct to determine whether they can reduce hypermobility and stress concentration at adjacent levels. For example, using finite element modeling, Cahill et al predicted that a tapered rod at the uppermost end of a scoliosis construct would reduce the stress at the adjacent segment and the potential for implant damage.16 Similarly, Durrani et al reported that the use of posterior dynamic stabilization at the caudal-most level reduces the adjacent-level hypermobility and the potential for distal junctional kyphosis.17 Less-restrictive hook instrumentation has also been shown to reduce the risk of PJK clinically3 10 15 18; however, in vitro studies demonstrating their mechanical advantage in the thoracic spine is limited.
The purpose of this study was to determine the biomechanical effect of less-rigid fixation techniques at the top of a pedicle screw–rod construct in thoracic spinal fusion. Specifically, we examine the effect of progressively less-rigid proximal fixation techniques on the biomechanical behavior of the supra-adjacent uninstrumented spine in a multilevel thoracic fixation model. We hypothesize that supralaminar hooks provide a more gradual transition between the stiff fusion construct and the flexible native spine when compared with an all-pedicle screw construct alone, thereby reducing hypermobility and the biomechanical risk for PJK.
Materials and Methods
Specimen Preparation
Ten fresh-frozen cadaveric thoracic spines (5 men, 5 women; mean age of 52, range: 30 to 64) were procured from a Cedars-Sinai-approved tissue bank and stored at −30°C. The specimens were initially screened radiographically for osteophytes and inadequate disk height followed by bone mineral density determination via dual-energy X-ray absorptiometry. The raw values of bone mineral density (in grams per square centimeter) were averaged over the T8–T11 vertebrae and used to assess bone quality (Table 1). The specimens were kept frozen until testing, at which point they were thoroughly cleaned of nonstructural soft tissue while preserving the disk, facet joint capsules, and ligamentous structures. The cranial (T7) and caudal (T12) vertebrae were potted to approximately half-axial height in metal testing cups using two-part epoxy resin (BJB Enterprises, Tustin, California, United States), allowing rigid fixation to the testing machine. Wood screws were implemented to help anchor the vertebral bodies into the potting material. The specimens were kept moist using saline-soaked gauze whenever possible.
Table 1. Summary of specimen information.
| Specimen no. | Age (y) | Sex | Average BMD (g/cm2) | COD |
|---|---|---|---|---|
| 1 | 34 | F | 0.94575 | Breast cancer (with Mets) |
| 2 | 59 | F | 0.76125 | Colon cancer |
| 3 | 55 | F | 0.605 | Lymphoma |
| 4 | 58 | M | 1.491 | Heart attack |
| 5 | 30 | F | 0.7725 | Pancreatitis |
| 6 | 64 | F | 1.03225 | Biphenotypic acute leukemia |
| 7 | 54 | M | 0.85025 | End-stage liver disease |
| 8 | 52 | M | 0.82975 | ALS |
| 9 | 58 | M | 1.83925 | Lung cancer (with Mets) |
| 10 | 51 | M | 1.00675 | NS |
Abbreviations: ALS, amyotrophic laterals sclerosis; BMD, bone mineral density; COD, cause of death; Mets, metastases; NS, not specified.
Biomechanical Testing
Biomechanical testing was conducted on an MTS Bionix Testing System augmented with a biaxial spine simulator to impose bending rotations (MTS Bionix 370.02, MTS Systems Corp., Eden Prairie, Minnesota, United States; Fig. 1). Forces and moments were measured using a load cell (Mini45, ATI Industrial Automation, Apex, North Carolina, United States) rigidly attached directly below the caudal end of the specimen. The specimens were placed onto the testing machine preliminary forces, and torques were offset manually by adjusting the rotation angles or translational position of the caudal vertebrae, allowing the test to begin from a neutral posture.
Fig. 1.

Specimen mounted on MTS testing machine (MTS Bionix 370.02, MTS Systems Corp., Eden Prairie, Minnesota, United States), equipped with “spine pin” infrared rigid body markers and intervertebral disk pressure sensor needle.
The specimens were nondestructively loaded in extension, flexion, left and right lateral bending, and torsion with a maximum bending moment of 4 N · m applied after three preload cycles.19 20 Loading was initially imposed using displacement-control at a rate of 0.5 degrees per second to prevent rapid movements through the neutral zone, followed by load-control at 0.2 N · m/s until maximum bending moment was reached and held constant over a 10-second hold.
Each specimen was tested five times using the above protocol starting with the uninstrumented intact spine, followed by a two-level posterior fusion between T9 and T11 utilizing a standard pedicle screw–rod (cobalt-chrome alloy) fixation technique. Afterward, three different “top-off” constructs were instrumented immediately cranial (T8–T9) to the two-level fusion and tested in a semirandom order: bilateral supralaminar hooks, a hook–screw hybrid (hook on the left side, screw on the right), and bilateral pedicle screws (Fig. 2). Bilateral hooks and bilateral pedicle screws alternated between first and last top-off construct, and the hybrid test was always the second construct tested to eliminate any unnecessary damage to the specimen. All instrumentation was performed by a single fellowship-trained spinal surgeon (F.L.A.). Caution was used when inserting the supralaminar hooks to ensure the ligamentous complex was not disrupted. High resolution X-rays (LX-60, Faxitron X-Ray, LLC Lincolnshire, Illinois, United States) were taken after each surgical procedure to ensure proper placement of the pedicle screws and hooks.
Fig. 2.
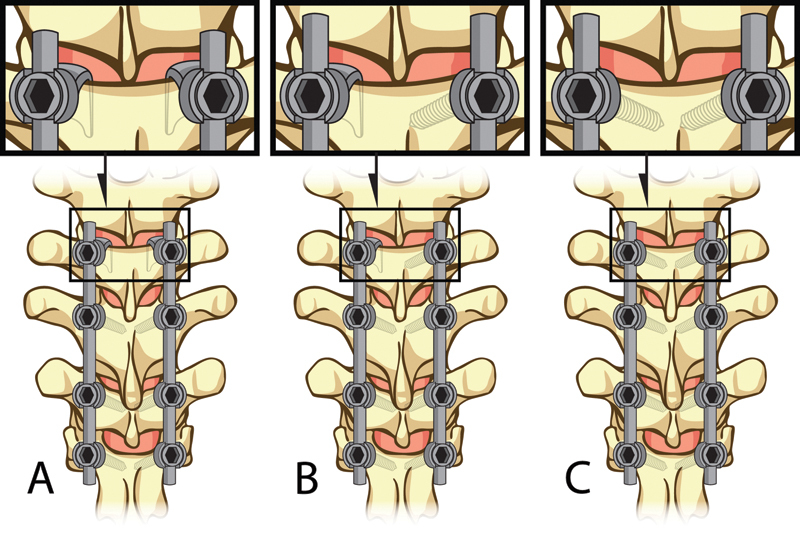
Illustration depicting each of the three upper-instrumented vertebrae anchor type: (A) bilateral supra-laminar hooks, (B) hybrid with a supra-laminar hook on the left and a unilateral screw on the right, and (C) bilateral pedicle screws.
Three-dimensional vertebral kinematics was measured via an optical infrared camera system (Optotrak Certus, Northern Digital Inc., Waterloo, Ontario, Canada). Infrared emission triads were mounted on all six vertebral bodies, and movements were tracked relative to a predefined anatomical set of axes. Code written with MATLAB software (vR2009b, The MathWorks Inc., Natick, Massachusetts, United States) converted the resultant motion into 3-2-1 Euler angles that were subsequently translated to range of motion (ROM) data in the sagittal, coronal, and transverse planes, relative to the initial neutral position.
Intervertebral disk pressure was measured using a custom pressure sensor array (Humanetics Innovative Solutions, Plymouth, Michigan, United States). Three small pressure sensors were embedded in a 13-gauge needle that was inserted anterior to posterior into the proximal adjacent (T7–T8) disk. Radiographic measurements were taken prior to testing to determine the appropriate penetrative length and trajectory of the needle to ensure all three sensors were contained centered within the nucleus pulposus.
Data Analysis
ROM over the entire specimen (T7–T12), at the upper-instrumented segment (T8–T9), and at the supra-adjacent uninstrumented segment (T7–T8) was calculated. The intervertebral disk pressure at the T7–T8 motion segment was calculated as the average across the pressure sensors embedded in the needle. Hypermobility, defined as the additional ROM above that measured in the intact spine, was analyzed at the supra-adjacent (T7–T8) segment. Outcomes between the various top-off methods (hooks, hybrid, and screws) were compared with the corresponding intact and two-level fusion construct values using repeated-measures analysis of variance followed by the Tukey-Kramer post hoc test. The level for statistical significance was set at p ≤ 0.05 (SAS statistical software, SAS Institute Inc., Cary, North Carolina, United States).
Results
Total specimen (T7–T12) ROM decreased significantly after instrumenting a two-level fusion from T9 to T11 for all bending modes (p < 0.0001) and was further reduced after topping-off with a third level of instrumentation at T8–T9 using hooks, screws, and the hybrid construct, in all but extension (Fig. 3).
Fig. 3.
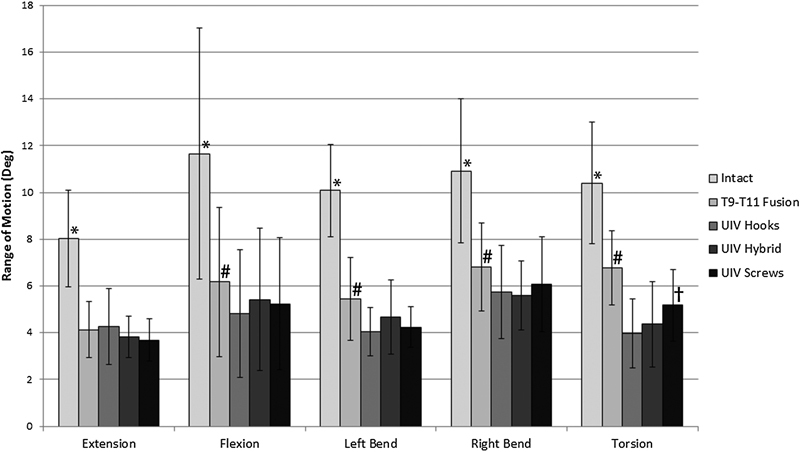
Mean angular range of motion over the entire specimen (T7–T12) was significantly reduced after instrumenting the two fusion from T9 to T11 (*p < 0.0001) and was further reduced in all bending modes, except for extension after the fusion was extended to T8–T9 (UIV) with either hooks, hybrid, or pedicle screws (# p < 0.05). Average total specimen motion was significant greater in torsion when bilateral hooks were used in comparison with hybrid and bilateral pedicle screws († p < 0.05). Errors bars represent ± 1 standard deviation. Abbreviation: UIV, upper-instrumented vertebrae.
The changes in ROM at individual motion segments, specifically the top-off level (T8–T9) and the supra-adjacent segment (T7–T8), were analyzed as a percentage of total motion to normalize the differences in flexibility among the specimens. The percent of total motion at both T7–T8 and T8–T9 significantly increased from intact in all bending modes after the addition of a pedicle screw–rod construct between T9 and T11 (p < 0.05; Figs. 4 and 5). When the fusion was proximally extended to include T8–T9 with any of the three constructs (hooks, hybrid, screws), there was a significant increase in stiffness at the top-off motion segment (Fig. 5). Differences in upper-instrumented vertebrae (UIV) stiffness between top-off constructs were most apparent during extension and torsion. In torsion, stiffness at the top-off level (T8–T9) was reduced by 6% when the hooks were implemented compared with either a hybrid or all-screw construct (Fig. 6). This decrease in stiffness at the uppermost instrumented level resulted in a more gradual transition to the proximal uninstrumented spine, shown by a reduction in hypermobility at supra-adjacent level (Fig. 6). In extension, although not statistically significant, hooks resulted in the least amount of supra-adjacent hypermobility (17 ± 11.5%) compared with the hybrid (19 ± 8.2%) and bilateral screw (23 ± 8.3%) construct (Fig. 7). Similarly, in flexion hypermobility at the supra-adjacent level was 15 ± 7.5% with bilateral hooks and increased to 16 ± 6.0% and 18 ± 6.4%, respectively, with the hybrid construct and bilateral screws. Both right and left lateral bending displayed significantly higher levels of hypermobility at T7–T8 with screws (21 ± 4.4% and 18.0 ± 6.0%, respectively) compared with the hybrid construct (18.7 ± 4.7% and 16.33 ± 5.8%) and bilateral hooks (16 ± 5.7% and 15 ± 5.5%). The most significant jump in superadjacent hypermobility was during torsion with bilateral screws requiring T7–T8 to carry 27.9% (±8.6%) more of the total ROM compared with hooks (12 ± 8.9%) and the hybrid construct (15 ± 12%).
Fig. 4.
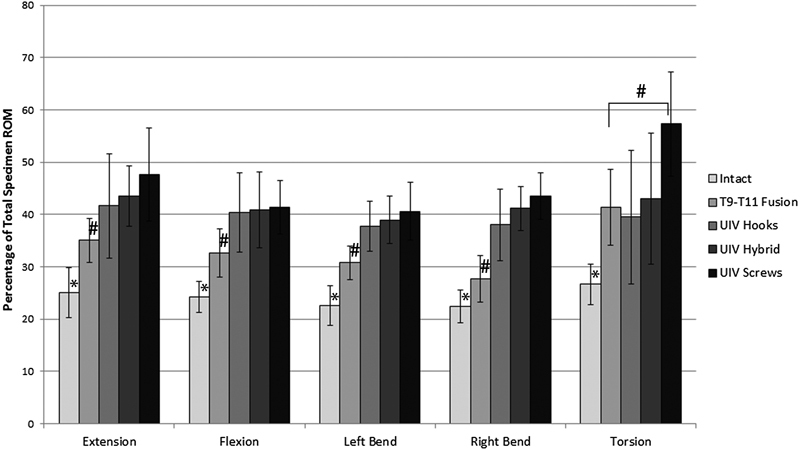
Average ROM at proximal-adjacent segment (T7–T8) as a percentage of total ROM increased after the addition of a two-level fusion from T9 to T11 (*p < 0.001) and further increased after extending the fusion to T8–T9 in all bending modes except torsion with hooks and hybrid technique (# p < 0.05). Statistical differences among the three top-off techniques are indicated separately in Fig. 7. Abbreviations: ROM, range of motion; UIV, upper-instrumented vertebrae.
Fig. 5.
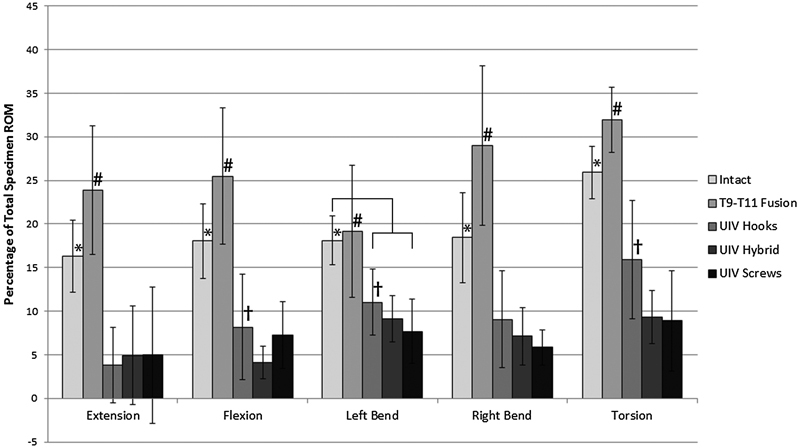
Average ROM at UIV (T8–T9) as a percentage of total ROM. After instrumenting a two-level fusion from T9 to T11, ROM at T8–T9 increased significantly, as expected, for all but lateral bending (*p < 0.05). After instrumenting the UIV with bilateral hooks, hybrid hook–screw, and bilateral screws, there was a significant decrease in the percent of motion carried at the segment (# p < 0.01). Hooks allowed significantly more motion at the UIV when compared with hybrid in flexion, and both hybrid and hooks in left bending and torsion († p < 0.05). Abbreviations: ROM, range of motion; UIV, upper-instrumented vertebrae.
Fig. 6.
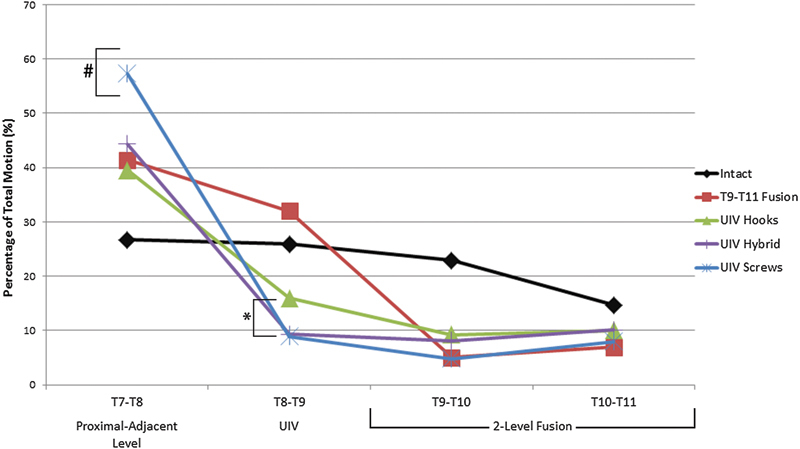
Average rotation at each motion segment during torsion for all constructs as a percentage of total specimen motion. Hooks significantly decreased the stiffness at the UIV (T8–T9, *p < 0.05) and reduced the percent of motion carried through the proximal adjacent level when compared with an all-pedicle screw construct (# p < 0.05). Abbreviation: UIV, upper-instrumented vertebrae.
Fig. 7.
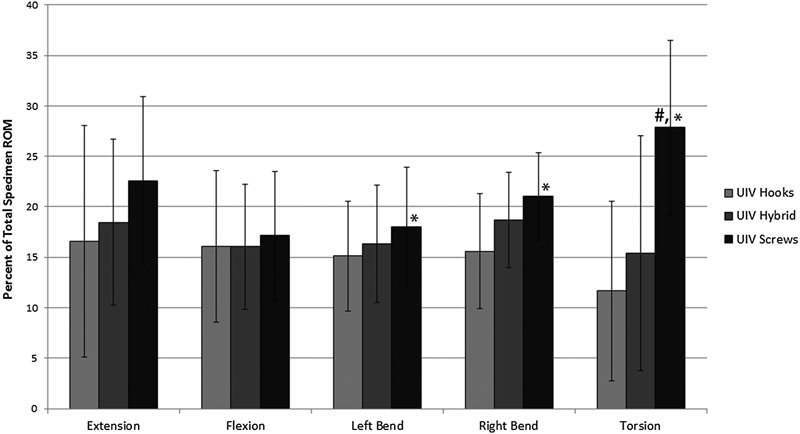
Average increase in percent of total motion at the proximal supra-adjacent level (T7–T8), normalized to the intact specimen. Screws significantly increased the percent of total motion carried by T7–T8 when compared with bilateral hooks (*p < 0.05) and the hybrid construct in torsion (# p < 0.05). Abbreviations: ROM, range of motion; UIV, upper-instrumented vertebrae.
Data from the three anterior–posterior pressure sensors within the intradiscal needle was averaged and used to compare the supra-adjacent disk pressure between the surgical constructs tested. Changes in supra-adjacent disk pressure were most notable during sagittal plane motion (Fig. 8). In extension, there was a significant drop in pressure from intact specimens with the addition of a two-level fusion construct at T9–T11 (p < 0.001), which was further reduced when the fusion was extended to a third level (p < 0.001) with no statistical difference between the three top-off constructs. Supra-adjacent disk pressure decreased in flexion after the two-level fusion (p < 0.05) and significantly increased with all three top-off constructs after the fusion was extended, again with no statistical difference among the three top-off technique.
Fig. 8.
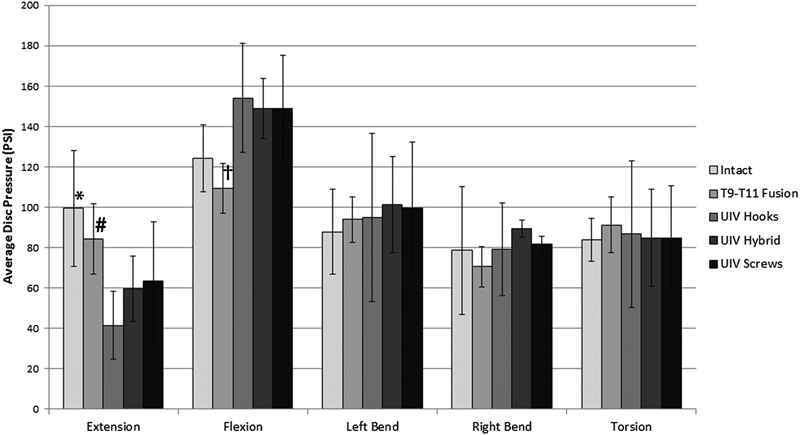
Changes in intradiscal pressure recorded in the supra-adjacent motion segment. There were no significant changes in pressure in torsion or left and right bending. In extension, there was a significant decrease in pressure at T7–T8 after a two-level fusion was added from T9–T11 (*p < 0.001), which decreased again after the UIV (T8–T9) was instrumented with all three constructs tested (# p < 0.001). In flexion there also was a decrease in pressure after adding a two-level fusion which increase above the intact levels after the topping off the fusion at T8–T9 († p < 0.001). Abbreviation: UIV, upper-instrumented vertebrae.
Discussion
Characterization of PJK as a true pathologic condition rather than just a radiographic finding has sparked research interest into the adjacent-level effects of various fixation techniques.1 2 3 5 6 7 8 10 In a recent retrospective review, Helgeson et al revealed a significant increase in PJK with an all-pedicle screw construct compared with an all-hook or hybrid construct and, based on their results, recommended using hooks at the UIV.15 Similarly, Hassanzadeh et al reported a reduced rate of PJK when topping off a long thoracic construct with transverse process hooks,18 which was mechanically validated in a recent study by Thawrani et al.21 Our biomechanical investigation into supralaminar hooks is in agreement with these studies and provides a biomechanical rationale for the use of less rigid proximal fixation with the intent of reducing the risk for PJK.
In addition to providing a more gradual biomechanical transition between the upper-most instrumented and uninstrumented vertebral segments, both supralaminar or transverse process spinal hooks have the theoretical advantage of causing less disruption of the adjacent-segment facet capsule compared with pedicle screws. Minimizing the damage to the adjacent-segment facet complex may also account for the reduced incidence of PJK with hooks compared with pedicle screws. Additionally, bone mineral density has been shown to be highly correlated with pedicle screw pullout strength.22 Hooks, on the other hand, rely strictly on the spinal geometric features and are affected minimally by cancellous bone quality as they engage the outer cortical surface and may therefore provide a more advantageous means of fixation.23 24
The popularity of transverse process hooks compared with laminar hooks at the UIV is likely based on ease of placement of transverse process hooks, as well the concern of spinal canal compromise with laminar hooks.25 To the authors' knowledge, there has been no direct biomechanical comparison of these two fixation techniques, although it seems intuitive that a properly placed laminar hook would have higher pullout strength than a transverse process hook. This increased strength would certainly be evident after attempted pedicle screw placement at the UIV, which has been found to significantly weaken the compressive load capabilities of the transverse process.26 Additionally, laminar hooks can be used to augment the pedicle screw strength, if required, at the same level.27
Variations in our ROM data between bending modes at the proximal level when hooks are implemented can be explained by the mechanical design and orientation of the supralaminar hooks. Laminar hooks are rigidly fixed to the rods and arch over the superior aspect of the lamina. This design provides strong resistance during flexion as the hooks engage and pull downward on the lamina as the spine attempts to rotate forward. In contrast, during extension, torsion, and to some degree lateral bending, the mechanical resistance to motion is primarily from friction between the bone and the hook, which provides less opposition to applied bending moments, and is reflected in the percent of total ROM at the supra-adjacent segment (Fig. 7), where all three top-off constructs have similar performance during flexion but in all other bending modes hooks display a reduced level of stress, or hypermobility at T7–T8.
Our study, like many biomechanical studies, is burdened by several limitations due to the use of cadaveric tissue. Although the small number of specimens limited our statistical ability, a reduction in hypermobility at the UIV was demonstrated when hooks were used in place of pedicle screws. In an effort to minimize specimen damage, the hybrid construct was always tested second and bilateral hooks and bilateral pedicle screws alternated between first and third. We believe this testing scheme was the best because it equally distributed any variation between the hooks and screws, counterbalancing the two most clinically relevant UIV anchor types. Another limitation to our study was the location chosen to analyze PJK. Most clinicians associate PJK with the upper thoracic (UT) spine, making our use of the lower thoracic (LT) spine arguably less clinically relevant. We chose this location for two reasons: (1) the UT spine was typically more degenerated in our human specimens, and (2) disk space in the upper levels was too narrow to accommodate the 13-gauge needle used to measure disk pressure. In our research, we found most studies indicate PJK can occur at any level,1 3 13 with some studies suggesting it is actually more frequent in the LT spine.28 29 Fujimori et al recently published a retrospective study comparing outcome measures between choosing the UT (T1–T6) or LT (T7–T12) spine for the location of the UIV in patients with adult spinal deformity.28 In their analysis, the authors found the incidence of PJK was higher in the LT spine compared with the UT spine (LT = 41%, UT = 32%, difference not significant). Of those cases, 25% in the LT group required revision surgery versus 20% in the UT group. The authors suggested the UT segments are more stabilized due to the rib cage and scapulae, whereas the LT spine is biomechanically more vulnerable and therefore more susceptible to PJK. Likewise, Scheer et al concluded that patients who have fixation extending to the UT spine are less likely to develop PJK and have a lower revision rate for PJK than those with fixation terminating in the thoracolumbar region.29 Again, suggesting rates of PJK are just as common, if not more common, in the LT spine. Finally, with only a two-level fusion below the UIV, the length of our construct was much shorter than a typical deformity construct, which could arguably affect our results. Thawrani et al used longer thoracic segments to analyze transverse process hooks at the UIV21; in comparison, our results show that the proximal anchor type does not affect the fused segments below, suggesting that a shorter construct can adequately demonstrate changes in stiffness and ROM.
Conclusions
The present study is the first to investigate the biomechanical performance of supralaminar hooks when used to fix the UIV at the top of a multisegment thoracic pedicle screw–rod construct in vitro. Based on our results, placing bilateral laminar hooks at the UIV in a multilevel thoracic fixation model reduces hypermobility at the proximal, uninstrumented spine when compared with the more conventional all-pedicle screw technique. All bending modes trended in favor of this conclusion, with lateral bending and torsion being statistically significant. This decrease in motion and resultant stress at the supra-adjacent level provides biomechanical support for recent clinical findings that suggest all-pedicle screw constructs can lead to inferior patient outcomes as a result of higher rates of PJK when compared with less-rigid fixation at the UIV.3 7
Our biomechanical analysis adds to growing literature of fixation methods designed to “ease” the transition between the stiff instrumented spine and the flexible native spine and is the first to demonstrate these results with laminar hooks. Our data provides a mechanical rationale for considering hooks at the UIV of a multilevel thoracic fusion, although further studies are needed to confirm that these results translate to a reduction in the clinical incidence of PJK and improved patient outcomes.
Acknowledgments
The authors would like to thank Lea Kanim for her assistance with the statistical analysis. We would also like to thank Jeremy Kalma for his illustrative work. Research support was provided by Stryker Spine, Allendale, New Jersey, United States.
Footnotes
Disclosures Melodie F. Metzger, Grant: Stryker Spine Samuel T. Robinson, none Mark T. Svet, none John C. Liu, Consultant: Medtronic Frank L. Acosta, Grant: Stryker Spine; Consultant: Nuvasive
References
- 1.Glattes R C, Bridwell K H, Lenke L G, Kim Y J, Rinella A, Edwards C II. Proximal junctional kyphosis in adult spinal deformity following long instrumented posterior spinal fusion: incidence, outcomes, and risk factor analysis. Spine (Phila Pa 1976) 2005;30(14):1643–1649. doi: 10.1097/01.brs.0000169451.76359.49. [DOI] [PubMed] [Google Scholar]
- 2.Kim Y J, Bridwell K H, Lenke L G, Kim J, Cho S K. Proximal junctional kyphosis in adolescent idiopathic scoliosis following segmental posterior spinal instrumentation and fusion: minimum 5-year follow-up. Spine (Phila Pa 1976) 2005;30(18):2045–2050. doi: 10.1097/01.brs.0000179084.45839.ad. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y J, Bridwell K H, Lenke L G, Glattes C R, Rhim S, Cheh G. Proximal junctional kyphosis in adult spinal deformity after segmental posterior spinal instrumentation and fusion: minimum five-year follow-up. Spine (Phila Pa 1976) 2008;33(20):2179–2184. doi: 10.1097/BRS.0b013e31817c0428. [DOI] [PubMed] [Google Scholar]
- 4.Anderson A L, McIff T E, Asher M A, Burton D C, Glattes R C. The effect of posterior thoracic spine anatomical structures on motion segment flexion stiffness. Spine (Phila Pa 1976) 2009;34(5):441–446. doi: 10.1097/BRS.0b013e318198c62d. [DOI] [PubMed] [Google Scholar]
- 5.Lee G A, Betz R R, Clements D H III, Huss G K. Proximal kyphosis after posterior spinal fusion in patients with idiopathic scoliosis. Spine (Phila Pa 1976) 1999;24(8):795–799. doi: 10.1097/00007632-199904150-00011. [DOI] [PubMed] [Google Scholar]
- 6.Kim H J Lenke L G Shaffrey C I Van Alstyne E M Skelly A C Proximal junctional kyphosis as a distinct form of adjacent segment pathology after spinal deformity surgery: a systematic review Spine (Phila Pa 1976) 201237(22, Suppl):S144–S164. [DOI] [PubMed] [Google Scholar]
- 7.Kim H J, Bridwell K H, Lenke L G. et al. Proximal junctional kyphosis results in inferior SRS pain subscores in adult deformity patients. Spine (Phila Pa 1976) 2013;38(11):896–901. doi: 10.1097/BRS.0b013e3182815b42. [DOI] [PubMed] [Google Scholar]
- 8.Kim Y J Lenke L G Bridwell K H Cho Y S Riew K D Free hand pedicle screw placement in the thoracic spine: is it safe? Spine (Phila Pa 1976) 2004293333–342., discussion 342 [DOI] [PubMed] [Google Scholar]
- 9.Suk S I, Kim W J, Lee S M, Kim J H, Chung E R. Thoracic pedicle screw fixation in spinal deformities: are they really safe? Spine (Phila Pa 1976) 2001;26(18):2049–2057. doi: 10.1097/00007632-200109150-00022. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y J, Lenke L G, Cho S K, Bridwell K H, Sides B, Blanke K. Comparative analysis of pedicle screw versus hook instrumentation in posterior spinal fusion of adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2004;29(18):2040–2048. doi: 10.1097/01.brs.0000138268.12324.1a. [DOI] [PubMed] [Google Scholar]
- 11.Lenke L G, Kuklo T R, Ondra S, Polly D W Jr. Rationale behind the current state-of-the-art treatment of scoliosis (in the pedicle screw era) Spine (Phila Pa 1976) 2008;33(10):1051–1054. doi: 10.1097/BRS.0b013e31816f2865. [DOI] [PubMed] [Google Scholar]
- 12.Rhee J M, Bridwell K H, Won D S, Lenke L G, Chotigavanichaya C, Hanson D S. Sagittal plane analysis of adolescent idiopathic scoliosis: the effect of anterior versus posterior instrumentation. Spine (Phila Pa 1976) 2002;27(21):2350–2356. doi: 10.1097/00007632-200211010-00008. [DOI] [PubMed] [Google Scholar]
- 13.Yagi M, King A B, Boachie-Adjei O. Incidence, risk factors, and natural course of proximal junctional kyphosis: surgical outcomes review of adult idiopathic scoliosis. Minimum 5 years of follow-up. Spine (Phila Pa 1976) 2012;37(17):1479–1489. doi: 10.1097/BRS.0b013e31824e4888. [DOI] [PubMed] [Google Scholar]
- 14.Maruo K, Ha Y, Inoue S. et al. Predictive factors for proximal junctional kyphosis in long fusions to the sacrum in adult spinal deformity. Spine (Phila Pa 1976) 2013;38(23):E1469–E1476. doi: 10.1097/BRS.0b013e3182a51d43. [DOI] [PubMed] [Google Scholar]
- 15.Helgeson M D, Shah S A, Newton P O. et al. Evaluation of proximal junctional kyphosis in adolescent idiopathic scoliosis following pedicle screw, hook, or hybrid instrumentation. Spine (Phila Pa 1976) 2010;35(2):177–181. doi: 10.1097/BRS.0b013e3181c77f8c. [DOI] [PubMed] [Google Scholar]
- 16.Cahill P J, Wang W, Asghar J. et al. The use of a transition rod may prevent proximal junctional kyphosis in the thoracic spine after scoliosis surgery: a finite element analysis. Spine (Phila Pa 1976) 2012;37(12):E687–E695. doi: 10.1097/BRS.0b013e318246d4f2. [DOI] [PubMed] [Google Scholar]
- 17.Durrani A, Jain V, Desai R. et al. Could junctional problems at the end of a long construct be addressed by providing a graduated reduction in stiffness? A biomechanical investigation. Spine (Phila Pa 1976) 2012;37(1):E16–E22. doi: 10.1097/BRS.0b013e31821eb295. [DOI] [PubMed] [Google Scholar]
- 18.Hassanzadeh H, Gupta S, Jain A. et al. Type of anchor at the proximal fusion level has a significant effect on the incidence of proximal junctional kyphosis and outcome in adults after long posterior spinal fusion. Spine Deformity. 2013;1(4):299–305. doi: 10.1016/j.jspd.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Goel V K Panjabi M M Patwardhan A G Dooris A P Serhan H; American Society for Testing and Materials. Test protocols for evaluation of spinal implants J Bone Joint Surg Am 20068802103–109. [DOI] [PubMed] [Google Scholar]
- 20.Wilke H J, Wenger K, Claes L. Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. Eur Spine J. 1998;7(2):148–154. doi: 10.1007/s005860050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thawrani D P, Glos D L, Coombs M T, Bylski-Austrow D I, Sturm P F. Transverse process hooks at upper instrumented vertebra provide more gradual motion transition than pedicle screws. Spine (Phila Pa 1976) 2014;39(14):E826–E832. doi: 10.1097/BRS.0000000000000367. [DOI] [PubMed] [Google Scholar]
- 22.Halvorson T L, Kelley L A, Thomas K A, Whitecloud T S III, Cook S D. Effects of bone mineral density on pedicle screw fixation. Spine (Phila Pa 1976) 1994;19(21):2415–2420. doi: 10.1097/00007632-199411000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Hackenberg L, Link T, Liljenqvist U. Axial and tangential fixation strength of pedicle screws versus hooks in the thoracic spine in relation to bone mineral density. Spine (Phila Pa 1976) 2002;27(9):937–942. doi: 10.1097/00007632-200205010-00010. [DOI] [PubMed] [Google Scholar]
- 24.Cordista A, Conrad B, Horodyski M, Walters S, Rechtine G. Biomechanical evaluation of pedicle screws versus pedicle and laminar hooks in the thoracic spine. Spine J. 2006;6(4):444–449. doi: 10.1016/j.spinee.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Polly D W Jr, Potter B K, Kuklo T, Young S, Johnson C, Klemme W R. Volumetric spinal canal intrusion: a comparison between thoracic pedicle screws and thoracic hooks. Spine (Phila Pa 1976) 2004;29(1):63–69. doi: 10.1097/01.BRS.0000105525.06564.56. [DOI] [PubMed] [Google Scholar]
- 26.Brown B S, McIff T E, Glattes R C, Burton D C, Asher M A. The effect of starting point placement technique on thoracic transverse process strength: an ex vivo biomechanical study. Scoliosis. 2010;5:14. doi: 10.1186/1748-7161-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun E, Alkalay R, Vader D, Snyder B D. Preventing distal pullout of posterior spine instrumentation in thoracic hyperkyphosis: a biomechanical analysis. J Spinal Disord Tech. 2009;22(4):270–277. doi: 10.1097/BSD.0b013e31816a6887. [DOI] [PubMed] [Google Scholar]
- 28.Fujimori T, Inoue S, Le H. et al. Long fusion from sacrum to thoracic spine for adult spinal deformity with sagittal imbalance: upper versus lower thoracic spine as site of upper instrumented vertebra. Neurosurg Focus. 2014;36(5):E9. doi: 10.3171/2014.3.FOCUS13541. [DOI] [PubMed] [Google Scholar]
- 29.Scheer J K, Lafage V, Smith J S. et al. Maintenance of radiographic correction at 2 years following lumbar pedicle subtraction osteotomy is superior with upper thoracic compared with thoracolumbar junction upper instrumented vertebra. Eur Spine J. 2015;24 01:S121–S130. doi: 10.1007/s00586-014-3391-y. [DOI] [PubMed] [Google Scholar]


