Abstract
Study Design Retrospective study.
Objectives Assess demographics, ossification characteristics, surgical outcomes, and complications in patients with both diffuse idiopathic spinal hyperostosis (DISH) and ossification of the posterior longitudinal ligament (OPLL) compared with patients who only have OPLL.
Methods Clinical charts and radiographs of all patients treated surgically from February 2004 to July 2012 for cervical myeloradiculopathy due to DISH with OPLL or OPLL alone were reviewed retrospectively. All patients were observed for a minimum of 1 year. Pre- and postoperative Nurick grades were assessed for all patients.
Results Forty-nine patients underwent surgical treatment for cervical myeloradiculopathy due to OPLL, and 8 also had DISH (average 58.9 years, range 37 to 70). The DISH with OPLL group had a significantly higher proportion of subjects with diabetes mellitus (50 versus 9.8% in the OPLL-only group). Everyone in the DISH with OPLL group had continuous or mixed-type OPLL, whereas 78% of patients in the OPLL-only group had primarily segmental type. Operative treatments for patients in the DISH with OPLL group included laminoplasty, anterior decompression and fusion, and posterior laminectomy with fusion. By Nurick grade, 5 patients improved and 3 showed no change.
Conclusion Patients with both DISH and OPLL had a higher prevalence of diabetes mellitus and either continuous or mixed-type OPLL classifications. Surgical outcomes were mostly satisfactory; there was no aggravation of symptoms after surgery during the follow up period.
Keywords: cervical myeloradiculopathy, diffuse idiopathic spinal hyperostosis (DISH), ossification of posterior longitudinal ligament (OPLL), surgery
Introduction
Diffuse idiopathic skeletal hyperostosis (DISH) is a common disorder in Caucasian people, especially in the middle-aged or elderly, and is characterized by bony proliferation along the anterior side of the spine and at extraspinal locations with ossification of other joints.1 2 3 4 5 6 7 8 9 On the other hand, ossification of the posterior longitudinal ligament (OPLL) was initially recognized as specific to Asian people, but more recently the incidence has increased in Europe and the United States. Several previous reports discussed the genetic association between DISH and OPLL, but it remains unclear whether DISH and OPLL are genetically related.10 11 12 Several studies examining the cervical issues related to DISH have reported surgical treatments for dysphagia or spine trauma.13 14 15 16 17 Although a large number of publications have reported on surgical outcomes for cervical myelopathy due to OPLL, cervical myelopathy or radiculopathy due to DISH with OPLL is a rare disease, with only a few published case reports.18 19 20 21 Most physicians are not familiar with the full array of the clinical manifestations and surgical outcomes associated with cervical DISH with OPLL.
The purpose of this study was to assess the demographics, ossification characteristics, surgical outcomes, and complications in patients having DISH with OPLL compared with patients with OPLL but without DISH.
Materials and Methods
The Institutional Review Board at Washington University School of Medicine approved this study. We retrospectively reviewed the records of all patients with cervical myeloradiculopathy having DISH with OPLL or OPLL alone who were treated surgically at our institution from February 2004 to July 2012. The clinical charts and radiographs were reviewed for the demographics (age, gender, race, follow-up period, and diabetes mellitus), ossification characteristics, surgical outcomes, and complications such as pseudarthrosis, dural tear, infection, and paralysis. Using lateral radiographs or sagittal computed tomography (CT) images, we radiologically classified OPLL in the cervical spine into four types: segmental, continuous, mixed ,and other.22 Signal change in the spinal cord (high-intensity area) was recognized on both sagittal and axial views of T2-weighted magnetic resonance imaging (MRI). All patients were observed for a minimum of 1 year. The Nurick grade was assessed for all patients pre- and postoperatively.23 The patients who had incomplete clinical data and questionnaire and less than 1-year follow-up were excluded.
Data was analyzed using SPSS (version 20.0; IBM, Armonk, New York, United States). We performed univariate analyses to examine the relationship between the DISH with OPLL group and the OPLL-only group. We used the Mann-Whitney U test for non-normally distributed variables and the Fisher exact test for categorical variables. The threshold for significance was a p value of <0.05.
Results
The senior author performed cervical spine surgery on a total of 80 patients with OPLL and cervical myeloradiculopathy. Among these 80 patients, 31 patients were excluded because of the reasons described earlier. Finally, 49 patients with OPLL ended up being included. Of these 49 patients, 8 also had DISH (DISH with OPLL group): 6 men and 2 women with a mean age at surgery of 58.9 ± 12 years (range 37 to 70). The mean follow-up period was 37.3 months (range 12 to 71). In the DISH with OPLL group, 7 subjects were Caucasian and 1 was black; the OPLL-only group, 34 subjects were Caucasian, 6 were black, and 1 was Asian (p = 0.561). The types of OPLL in the DISH with OPLL group consisted of 4 continuous and 4 mixed types, and the OPLL-only group had 30 segmental, 3 continuous, 6 mixed, and 2 other types (p = 0.009).
The DISH with OPLL group had a significantly higher proportion of subjects with diabetes mellitus (DISH with OPLL group: 50%, 3 men and 1 woman; OPLL-only group: 9.8%, 2 men and 2 women; p = 0.017). Signal change on T2 MRI did not differ between the two groups (43 versus 36%, respectively; p = 0.518), and the fusion rate was high in both groups 83% (5/6) and 90% (28/31), respectively (p = 0.535; Table 1). The average preoperative Nurick grades for DISH with OPLL and OPLL-only groups were 2.6 and 1.3, respectively (p = 0.086). The postoperative Nurick grades were 1.9 and 0.6, respectively (p = 0.135). The operative treatment for patients in the DISH with OPLL group included laminoplasty in 2, anterior decompression and fusion in 1, and posterior laminectomy with fusion in 5 patients. Measured by Nurick grade, 5 patients improved (4 from grade 1 to 0, 1 from with grade 5 to 4) and 3 patients did not change (2 with grade 4, 1 with grade 5). In the DISH with OPLL group, 3 patients had complications intra- and postoperatively. One patient had deep wound infection after laminoplasty, which required debridement (case 5). Two patients had intraoperative dural tear complications (cases 6, 7), and one patient had pseudarthrosis after anterior surgery (case 6). No one in the DISH with OPLL group had an aggravation of their neurologic status at follow-up (Table 2).
Table 1. Clinical and radiologic data in patients with DISH with OPLL compared with patients with only OPLL.
| DISH with OPLL (n = 8) | OPLL only (n = 41) | p Value | |
|---|---|---|---|
| Age | 58.9 ± 12 (37–70) | 51.6 ± 9 (34–75) | 0.063 |
| Sex (M/F) | 6/2 | 21/20 | 0.200 |
| Follow-up (mo) | 26.5 ± 24 (12–71) | 37.3 ± 14 (12–71) | 0.304 |
| Race | 0.561 | ||
| Caucasian | 7 | 34 | |
| Black | 1 | 6 | |
| Asian | 0 | 1 | |
| Neurologic status | 0 | 1 | 0.119 |
| Radiculopathy | 0 | 5 | |
| Myelopathy | 5 | 0 | |
| Myeloradiculopathy | 3 | 36 | |
| OPLL type | 0.009 | ||
| Segmental | 0 | 30 | |
| Continuous | 4 | 3 | |
| Mixed | 4 | 6 | |
| Other | 0 | 2 | |
| DM | 50% (4/8) | 10% (4/41) | 0.017 |
| HIA on T2 MRI | 43% (3/7) | 36% (14/39) | 0.518 |
Abbreviations: DISH, diffuse idiopathic spinal hyperostosis; DM, diabetes mellitus; HIA, high-intensity area; MRI, magnetic resonance imaging; OPLL, ossification of the posterior longitudinal ligament.
Table 2. Demographic information for all patients with DISH and OPLL.
| Case no. | Age (y) | Sex | F/U (mo) | Race | OPLL type | HIA on T2 MRI | DM | Operative treatment | Fusion | Preoperative Nurick grade | Postoperative Nurick grade | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70 | M | 67 | Caucasian | Mixed | − | + | PSF (C2-T4) + LN+ FR | + | 1 | 0 | – |
| 2 | 68 | M | 24 | Caucasian | Continuous | + | − | PSF (C1–T1) + LN | + | 5 | 5 | – |
| 3 | 65 | M | 12 | Caucasian | Continuous | − | − | PSF (C2–T1) + LN | + | 1 | 0 | – |
| 4 | 63 | M | 71 | Caucasian | Mixed | n/a | + | PSF (C2–T2) + LN | + | 4 | 4 | – |
| 5 | 43 | F | 58 | Black | Continuous | + | − | Laminoplasty (C2–4) | 1 | 0 | Wound infection | |
| 6 | 37 | M | 26 | Caucasian | Mixed | − | + | PSF (C2–C7) + LN + FR | + | 5 | 4 | Dura tear |
| 7 | 58 | F | 15 | Caucasian | Continuous | + | + | ASF (C2–C5) with C3 CP | − | 4 | 4 | Dura tear, pseudarthrosis |
| 8 | 67 | M | 25 | Caucasian | Mixed | − | − | Laminoplasty (C3–C6) | 1 | 0 | – |
Abbreviations: ASF, anterior spinal fusion; CP, corpectomy; DISH, diffuse idiopathic spinal hyperostosis; DM, diabetes mellitus; FR, foraminotomy; F/U, follow-up; HIA, high-intensity area; LN, laminectomy; MRI, magnetic resonance imaging; n/a, not applicable; OPLL, ossification of the posterior longitudinal ligament; PSF, posterior spinal fusion.
Selected Case Examples
Case 4
A 63-year-old Caucasian man presented with numbness in his right arm and ulnar three digits, intrinsic muscle atrophy in his bilateral hand, and a balance problem. He had decreased sensation around both forearms and hands. He was able to walk with a cane, had a Nurick grade of 4, had diabetes mellitus, and did not have dysphagia. The CT sagittal image of the cervical spine showed DISH from C2 to T1 and mixed-type OPLL from C3 to C7 (Figs. 1A, B). He had a pacemaker, which precluded MRI. He underwent posterior spinal fusion (C2–T2) with laminectomy (C3–C7) and instrumentation. At 5-year follow-up, although his postoperative Nurick grade was 4, he still used a cane to walk, but his motor strength and sensation had improved. He had solidly fused from C2 to T2 on the lateral plain radiograph (Fig. 1C).
Fig. 1.
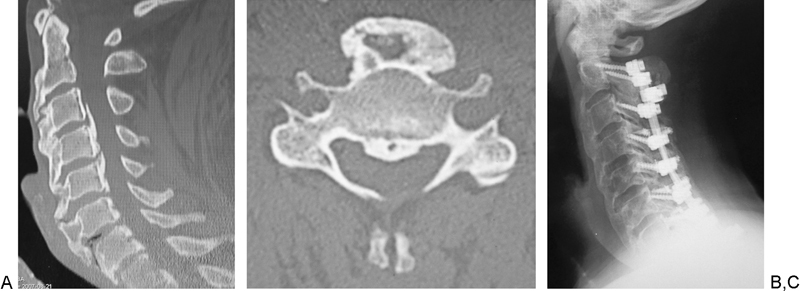
Case 4. Sagittal (A) and axial (B) computed tomography shows diffuse idiopathic spinal hyperostosis (C2–T1) and mixed-type ossification of the posterior longitudinal ligament (C3–C7). Postoperative plain lateral radiograph (C) shows postoperative posterior spinal instrumentation and fusion (C2–T2) with laminectomy (C3–C7).
Case 6
A 37-year-old Caucasian man had a history of thoracic decompression surgery performed elsewhere. He presented with arm pain, numbness, weakness, and a balance problem. He had weakness in his left hand and fingers and also in his legs. He had decreased sensation and numbness around the ulnar side of his forearms and ulnar digits bilaterally and had difficulty picking up small objects like coins or buttoning buttons. He had weakness in both upper extremities and left leg, making him unable to walk, thus he used a wheelchair (Nurick grade of 5). He also had diabetes mellitus. The CT sagittal and axial images of the cervical spine showed DISH (C2–C5 and C7–T2) and severe mixed-type OPLL (C1–C2, C4–C7; Figs. 2A, B). The T2-weighted sagittal MRI of the cervical spine revealed severe cord compression but no cord signal change (Fig. 2C). He underwent posterior spinal fusion (C3–C7) with laminectomy (C2–C7) and left C6–C7 foraminotomy with instrumentation. At the 2-year follow-up, his upper and lower extremity strength had improved bilaterally, which enabled him to walk with a walker. The sensation in his upper extremities had improved also (Nurick grade of 4). He had solid fusion from C2 to T2 on the lateral plain radiograph (Fig. 2D).
Fig. 2.
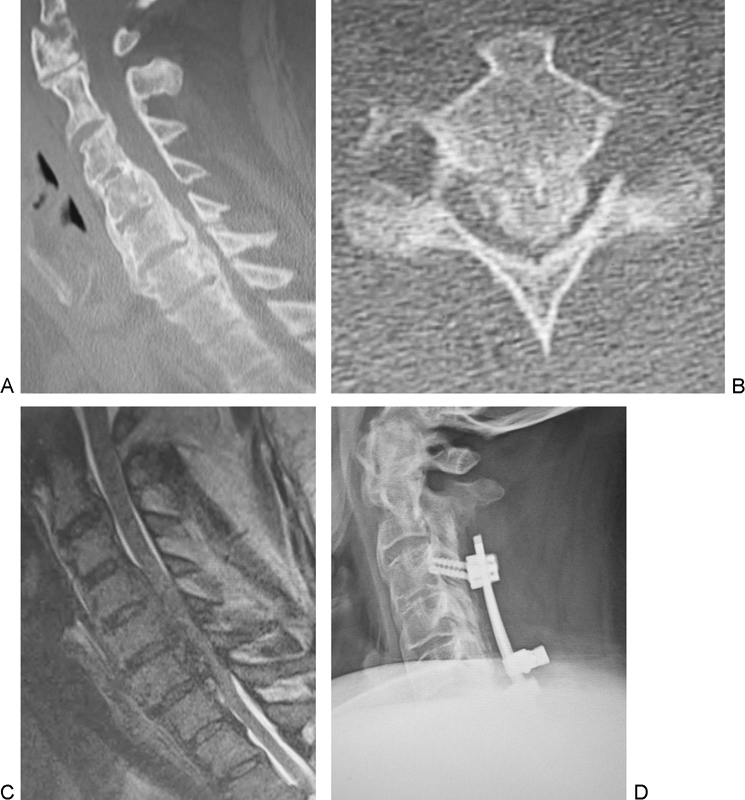
Case 6. Sagittal (A) and axial (B) computed tomography shows diffuse idiopathic spinal hyperostosis (C2–C5, C7–T2) and mixed-type ossification of the posterior longitudinal ligament(C1–C2, C4–C7). Sagittal image on T2-weighted magnetic resonance imaging (C) shows severe cord compression. Postoperative plain lateral radiograph (D) shows postoperative posterior spinal instrumentation and fusion (C3–C7) with laminectomy (C2–C7).
Discussion
In our study, of the 49 OPLL patients who underwent surgery, 8 (16%) had DISH with cervical myeloradiculopathy. The types of OPLL in the DISH with OPLL group consisted of 4 continuous and 4 mixed types, and the OPLL-only group had 30 segmental, 3 continuous, 6 mixed, and 2 other types. Patients with DISH with OPLL had a higher prevalence of diabetes mellitus. The operative treatment for patients in the DISH with OPLL group included laminoplasty in 2, anterior decompression and fusion in 1, and posterior laminectomy with fusion in 5 patients. Surgical outcomes were mostly satisfactory, and there was no aggravation of symptoms after surgery during the follow-up period.
DISH is characterized by ossification of the entheses, ligaments, and joint capsules.1 2 3 4 5 6 7 8 9 The spine is selectively affected with bony bridging across the intervertebral disk spaces seen in anteroposterior and lateral radiographs. In addition, OPLL also involves the posterior aspect of the vertebral bodies and disks, predominantly of the cervical spine.20 Although OPLL has come to be recognized as a subtype of DISH, it is not yet clear whether DISH and OPLL are genetically related.12 DISH is most commonly observed in individuals over the age of 50 with a reported prevalence between 2.5 and 28%.24 25 On the other hand, the incidence of OPLL is 2.4% in the Asian population and 0.16% in non-Asian populations.26 27 The Japanese literature shows the incidence of DISH with OPLL as 25% (27/109 cases of OPLL).28 Diabetes has been recognized as one of the risk factors associated with both DISH and OPLL.29 30 In the OPLL-only group, 9 to 27% of patients had accompanying diabetes mellitus.31 32 Similarly, hyperglycemia is the most common laboratory abnormality reported in patients with DISH, and the prevalence was reported at 12 to 50%.33 In our study, diabetes was more prevalent in the DISH with OPLL group (50%) compared with the OPLL-only group (10%).
Four types of frankly ossified OPLL have been described. The segmental type (39%) is located behind the vertebrae, the continuous type (27%) extends between the vertebrae crossing the intervening disk spaces, the mixed type (29%) includes both the continuous and segmental variants, and the “other” form (5%) is localized to the disk spaces with added retrovertebral expansion.34 In our study, the OPLL classification in the OPLL group had a similar distribution. On the other hand, patients in the DISH with OPLL group had only continuous or mixed-type ossification forms. Considering the high prevalence of diabetes and the high distribution of continuous and mixed ossification types in the DISH with OPLL group, patients with both DISH and OPLL might have a greater ossifying tendency.
Whether treatment by direct anterior resection or indirect posterior decompression of OPLL (with or without fusion) is superior remains controversial.35 36 37 38 39 40 41 Surgical alternatives are based on the severity of myelopathy, the presence or absence of lordosis or kyphosis, and the coexistence of disk disease, congenital stenosis, and spondylosis along with the recognized type of OPLL.36 Epstein and Hollingsworth first reported a patient presenting with cervical myelopathy due to OPLL with a coexistent ossification of the anterior longitudinal ligament (OALL). The patient had a successful outcome after a central corpectomy and a combined excision of the symptomatic OALL and segmental OPLL with grafting and instrumentation.42 Afterward, the same author reported two different surgical strategies for managing simultaneous DISH and OPLL resulting in dysphagia or myelopathy in two geriatric patients. Although DISH and OPLL may coexist in geriatric patients, only those with dysphagia should undergo DISH resection, and others demonstrating myelopathy should have laminectomy only.19 On the other hand, Chacko and Daniel reported the effectiveness of oblique corpectomy as a surgical option in patients with asymptomatic OALL in the setting of progressive myelopathy due to OPLL with intrinsic stability as a result of their OALL. They mentioned that this technique avoids a multilevel central corpectomy that is associated with significant instability often requiring reconstructive procedures.18 In this study, 7 patients (88%) had a posterior procedure, 5 of whom had posterior instrumented procedures. Most cases in our study had both anterior and posterior ossifications that were thick, extensive, and continuous; the senior author considered posterior decompression and fusion surgery to be safer and more effective for these cases compared with anterior surgery. For patients with cervical myeloradiculopathy due to DISH with OPLL who have no kyphotic deformity of the cervical spine, no dysphagia, and no autofusion of the spine, we recommend surgical posterior fusion using instrumentation to prevent any persistent motion that may then allow the patient full recovery from all of the neurologic deficits.
There are clear limitations to our study. First, the retrospective nature of this study may impact overall accuracy. Second, multiple surgical procedures were adopted in our cases, thus there was no standard surgical protocol. However, these cases are rare and therefore we should consider the surgical procedures on a case-by-case basis. Third, this study has a small number of patients with DISH with OPLL. However, previous reports about cervical myeloradiculopathy due to DISH with OPLL were only case reports, and our study introduced more information on the clinical features and radiologic manifestations of this rare entity.
Conclusion
DISH with OPLL is not common; in the current study, 8/49 patients with OPLL (16%) had DISH with cervical myeloradiculopathy. There was a high prevalence of diabetes mellitus compared with the OPLL group. Patients in the DISH with OPLL group had only continuous or mixed-type OPLL proliferation. Surgical outcomes were mostly satisfactory; no symptoms were aggravated after surgery during the follow-up period.
Disclosures Ryoji Tauchi, none Sang-Hun Lee, Consultant: Medtronic Colleen Peters, none Shiro Imagama, none Naoki Ishiguro, none K. Daniel Riew, Grant: AOSpine, Cerapedics, Medtronic; Speakers' bureau: AOSpine, NASS; Royalties: Biomet, Medtronic, Osprey, Medyssey; Stocks: Expanding Orthopedics, Amedica, Benvenue, Nexgen Spine, Osprey, Paradigm Spine, Spinal Kinetics, Spineology, Vertiflex, PSD, Medyssey; Travel expenses: AOSpine, NASS, SRS, Broadwater, Selby Spine; Board membership: CSRS, AOSpine International, Global Spine Journal, Spine Journal, NASS
Note
This paper was designed and submitted acting on guidelines of the Institutional Review Board of Washington University School of Medicine.
References
- 1.Littlejohn G O. Philadelphia, PA: Mosby Elsevier; 2011. Diffuse idiopathic skeletal hyperostosis; pp. 1801–1806. [Google Scholar]
- 2.Bywaters E GL, Doyle F H, Oakley N. Senile hyperostotic ankylosing spondylosis (Forestier and Querol) in diabetes mellitus. Arthritis Rheum. 1966;9:495 (Abstract). [Google Scholar]
- 3.Julkunen H, Heinonen O P, Pyörälä K. Hyperostosis of the spine in an adult population. Its relation to hyperglycaemia and obesity. Ann Rheum Dis. 1971;30(6):605–612. doi: 10.1136/ard.30.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris J, Carter A R, Glick E N, Storey G O. Ankylosing hyperostosis. I. Clinical and radiological features. Ann Rheum Dis. 1974;33(3):210–215. doi: 10.1136/ard.33.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Resnick D, Shaul S R, Robins J M. Diffuse idiopathic skeletal hyperostosis (DISH): Forestier's disease with extraspinal manifestations. Radiology. 1975;115(3):513–524. doi: 10.1148/15.3.513. [DOI] [PubMed] [Google Scholar]
- 6.Resnick D, Niwayama G. Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH) Radiology. 1976;119(3):559–568. doi: 10.1148/119.3.559. [DOI] [PubMed] [Google Scholar]
- 7.Utsinger P D. Diffuse idiopathic skeletal hyperostosis. Clin Rheum Dis. 1985;11(2):325–351. [PubMed] [Google Scholar]
- 8.Rogers J, Waldron T. DISH and the monastic way of life. Int J Osteoarchaeol. 2001;11(5):357–365. [Google Scholar]
- 9.Maat G JR, Mastwijk R W, Van der Velde E A. Skeletal distribution of degenerative changes in vertebral osteophytosis, vertebral osteoarthritis and DISH. Int J Osteoarchaeol. 1995;5:289–298. [Google Scholar]
- 10.Wiberg C, Klatt A R, Wagener R. et al. Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J Biol Chem. 2003;278(39):37698–37704. doi: 10.1074/jbc.M304638200. [DOI] [PubMed] [Google Scholar]
- 11.Tsukahara S, Miyazawa N, Akagawa H. et al. COL6A1, the candidate gene for ossification of the posterior longitudinal ligament, is associated with diffuse idiopathic skeletal hyperostosis in Japanese. Spine (Phila Pa 1976) 2005;30(20):2321–2324. doi: 10.1097/01.brs.0000182318.47343.6d. [DOI] [PubMed] [Google Scholar]
- 12.Havelka S, Veselá M, Pavelková A. et al. Are DISH and OPLL genetically related? Ann Rheum Dis. 2001;60(9):902–903. [PMC free article] [PubMed] [Google Scholar]
- 13.Bransford R J, Koller H, Caron T. et al. Cervical spine trauma in diffuse idiopathic skeletal hyperostosis: injury characteristics and outcome with surgical treatment. Spine (Phila Pa 1976) 2012;37(23):1923–1932. doi: 10.1097/BRS.0b013e31825b17fc. [DOI] [PubMed] [Google Scholar]
- 14.Mader R. Clinical manifestations of diffuse idiopathic skeletal hyperostosis of the cervical spine. Semin Arthritis Rheum. 2002;32(2):130–135. doi: 10.1053/sarh.2002.33726. [DOI] [PubMed] [Google Scholar]
- 15.McCafferty R R, Harrison M J, Tamas L B, Larkins M V. Ossification of the anterior longitudinal ligament and Forestier's disease: an analysis of seven cases. J Neurosurg. 1995;83(1):13–17. doi: 10.3171/jns.1995.83.1.0013. [DOI] [PubMed] [Google Scholar]
- 16.Meyer P R Jr. Diffuse idiopathic skeletal hyperostosis in the cervical spine. Clin Orthop Relat Res. 1999;(359):49–57. doi: 10.1097/00003086-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Westerveld L A, Verlaan J J, Oner F C. Spinal fractures in patients with ankylosing spinal disorders: a systematic review of the literature on treatment, neurological status and complications. Eur Spine J. 2009;18(2):145–156. doi: 10.1007/s00586-008-0764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chacko A G, Daniel R T. Multilevel cervical oblique corpectomy in the treatment of ossified posterior longitudinal ligament in the presence of ossified anterior longitudinal ligament. Spine (Phila Pa 1976) 2007;32(20):E575–E580. doi: 10.1097/BRS.0b013e31814b84fe. [DOI] [PubMed] [Google Scholar]
- 19.Epstein N E Simultaneous cervical diffuse idiopathic skeletal hyperostosis and ossification of the posterior longitudinal ligament resulting in dysphagia or myelopathy in two geriatric North Americans Surg Neurol 2000535427–431., discussion 431 [DOI] [PubMed] [Google Scholar]
- 20.Griffiths I D, Fitzjohn T P. Cervical myelopathy, ossification of the posterior longitudinal ligament, and diffuse idiopathic skeletal hyperostosis: problems in investigation. Ann Rheum Dis. 1987;46(2):166–168. doi: 10.1136/ard.46.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Resnick D, Guerra J Jr, Robinson C A, Vint V C. Association of diffuse idiopathic skeletal hyperostosis (DISH) and calcification and ossification of the posterior longitudinal ligament. AJR Am J Roentgenol. 1978;131(6):1049–1053. doi: 10.2214/ajr.131.6.1049. [DOI] [PubMed] [Google Scholar]
- 22.The Investigation Committee on OPLL of the Japanese Ministry of Public Health and Welfare . The ossification of the posterior longitudinal ligament of the spine (OPLL) Nippon Seikeigeka Gakkai Zasshi. 1981;55(4):425–440. [PubMed] [Google Scholar]
- 23.Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95(1):87–100. doi: 10.1093/brain/95.1.87. [DOI] [PubMed] [Google Scholar]
- 24.Boachie-Adjei O, Bullough P G. Incidence of ankylosing hyperostosis of the spine (Forestier's disease) at autopsy. Spine (Phila Pa 1976) 1987;12(8):739–743. doi: 10.1097/00007632-198710000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Mata S, Chhem R K, Fortin P R, Joseph L, Esdaile J M. Comprehensive radiographic evaluation of diffuse idiopathic skeletal hyperostosis: development and interrater reliability of a scoring system. Semin Arthritis Rheum. 1998;28(2):88–96. doi: 10.1016/s0049-0172(98)80041-3. [DOI] [PubMed] [Google Scholar]
- 26.Tsuyama N. Ossification of the posterior longitudinal ligament of the spine. Clin Orthop Relat Res. 1984;(184):71–84. [PubMed] [Google Scholar]
- 27.Wang M Y, Thambuswamy M. Ossification of the posterior longitudinal ligament in non-Asians: demographic, clinical, and radiographic findings in 43 patients. Neurosurg Focus. 2011;30(3):E4. doi: 10.3171/2010.12.FOCUS10277. [DOI] [PubMed] [Google Scholar]
- 28.Ehara S, Shimamura T, Nakamura R, Yamazaki K. Paravertebral ligamentous ossification: DISH, OPLL and OLF. Eur J Radiol. 1998;27(3):196–205. doi: 10.1016/s0720-048x(97)00164-2. [DOI] [PubMed] [Google Scholar]
- 29.Denko C W, Malemud C J. Body mass index and blood glucose: correlations with serum insulin, growth hormone, and insulin-like growth factor-1 levels in patients with diffuse idiopathic skeletal hyperostosis (DISH) Rheumatol Int. 2006;26(4):292–297. doi: 10.1007/s00296-005-0588-8. [DOI] [PubMed] [Google Scholar]
- 30.Kiss C, Szilágyi M, Paksy A, Poór G. Risk factors for diffuse idiopathic skeletal hyperostosis: a case-control study. Rheumatology (Oxford) 2002;41(1):27–30. doi: 10.1093/rheumatology/41.1.27. [DOI] [PubMed] [Google Scholar]
- 31.Kobashi G, Washio M, Okamoto K. et al. High body mass index after age 20 and diabetes mellitus are independent risk factors for ossification of the posterior longitudinal ligament of the spine in Japanese subjects: a case-control study in multiple hospitals. Spine (Phila Pa 1976) 2004;29(9):1006–1010. doi: 10.1097/00007632-200405010-00011. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto K, Kobashi G, Washio M. et al. Dietary habits and risk of ossification of the posterior longitudinal ligaments of the spine (OPLL); findings from a case-control study in Japan. J Bone Miner Metab. 2004;22(6):612–617. doi: 10.1007/s00774-004-0531-1. [DOI] [PubMed] [Google Scholar]
- 33.Sencan D, Elden H, Nacitarhan V, Sencan M, Kaptanoglu E. The prevalence of diffuse idiopathic skeletal hyperostosis in patients with diabetes mellitus. Rheumatol Int. 2005;25(7):518–521. doi: 10.1007/s00296-004-0474-9. [DOI] [PubMed] [Google Scholar]
- 34.Morimoto T, Matsuyama T, Hirabayashi H, Sakaki T, Yabuno T. Expansive laminoplasty for multilevel cervical OPLL. J Spinal Disord. 1997;10(4):296–298. [PubMed] [Google Scholar]
- 35.Chiba K, Ogawa Y, Ishii K. et al. Long-term results of expansive open-door laminoplasty for cervical myelopathy—average 14-year follow-up study. Spine (Phila Pa 1976) 2006;31(26):2998–3005. doi: 10.1097/01.brs.0000250307.78987.6b. [DOI] [PubMed] [Google Scholar]
- 36.Epstein N. Diagnosis and surgical management of cervical ossification of the posterior longitudinal ligament. Spine J. 2002;2(6):436–449. doi: 10.1016/s1529-9430(02)00394-7. [DOI] [PubMed] [Google Scholar]
- 37.Epstein N E. Laminectomy for cervical myelopathy. Spinal Cord. 2003;41(6):317–327. doi: 10.1038/sj.sc.3101477. [DOI] [PubMed] [Google Scholar]
- 38.Hale J J Gruson K I Spivak J M Laminoplasty: a review of its role in compressive cervical myelopathy Spine J 20066(6, Suppl):289S–298S. [DOI] [PubMed] [Google Scholar]
- 39.Houten J K Cooper P R Laminectomy and posterior cervical plating for multilevel cervical spondylotic myelopathy and ossification of the posterior longitudinal ligament: effects on cervical alignment, spinal cord compression, and neurological outcome Neurosurgery 20035251081–1087., discussion 1087–1088 [PubMed] [Google Scholar]
- 40.Jain S K Salunke P S Vyas K H Behari S S Banerji D Jain V K Multisegmental cervical ossification of the posterior longitudinal ligament: anterior vs posterior approach Neurol India 2005533283–285., discussion 286 [DOI] [PubMed] [Google Scholar]
- 41.Saunders R L, Pikus H J, Ball P. Four-level cervical corpectomy. Spine (Phila Pa 1976) 1998;23(22):2455–2461. doi: 10.1097/00007632-199811150-00022. [DOI] [PubMed] [Google Scholar]
- 42.Epstein N E Hollingsworth R Ossification of the cervical anterior longitudinal ligament contributing to dysphagia. Case report J Neurosurg 199990(2, Suppl):261–263. [DOI] [PubMed] [Google Scholar]


