Abstract
We developed the Michigan Body Map (MBM) as a self-report measure to assess body areas where chronic pain is experienced and to specifically quantify the degree of widespread body pain when assessing for centralized pain features (e.g., fibromyalgia-like presentation). A total of 402 patients completed the measure in five distinct studies to support the validation of the original and a revised version of the MBM. Administration is rapid 39–44 sec and errors for the original MBM were detected in only 7.2% of the possible body areas. Most errors underestimated the number of painful areas or represented confusion in determining the right versus left side. The MBM was preferred (p=0.013) and felt to better depict pain location (p=0.001) when compared to the Widespread Pain Index checklist of the 2011 Fibromyalgia Survey Criteria, but participants did not express any preference between the MBM and Brief Pain Inventory body map. Based on the data from the first three studies, a revised version of the MBM was created including a front and back body image and improved guidance on right-sidedness versus left. The revised MBM was preferred when compared to the original and more accurate in depicting painful body areas (p=0.004). Furthermore, the revised MBM showed convergent and discriminant validity with other self-report measures of pain, mood and function. In conclusion, the MBM demonstrated utility, reliability and construct validity. This new measure can be used to accurately assess for the distribution or widespreadedness of bodily pain as an element of the fibromyalgia survey score.
1. Introduction
Body maps have been used for many decades to assess the location of pain complaints. Self-report measures such as the Brief Pain Inventory (BPI) and McGill Pain Questionnaire allow patients to shade or note the areas on a body map where an individual experiences pain [12; 14; 15]. Beyond noting location in clinical settings, quantifying and analyzing body map data for research purposes has remained challenging, unstandardized, and infrequently reported. The inclusion of pain location in the 2011 Fibromyalgia Survey Criteria however piqued greater interest in this metric and spurred additional research into refining such measurement.
Fibromyalgia is a disorder characterized by widespread body pain, along with comorbid symptomatology [5]. The 2011 Fibromyalgia Survey Criteria include the assessment of pain in 19 specific body areas to assess for the presence of widespread pain [20; 21]. The survey was designed to assess widespread pain using the Widespread Pain Index (WPI). The areas from the WPI are then combined with the Symptom Severity scale to assess the presence and severity of fibromyalgia [20; 21]. The painful areas in the WPI are listed in word form only, are not alphabetized, and do not follow a particular anatomical order. In addition, the WPI areas are limited to the 19 areas used to evaluate fibromyalgia, thereby limiting wider use of the WPI in other areas of pain research and clinical care. For reference, the WPI checklist was previously published in the Appendix of Wolfe et al [20].
To facilitate collection of the survey-based criteria regarding widespread body pain, we created the Michigan Body Map (MBM; Supplemental Figure 1). The MBM is a graphic mannequin with the 19 areas from the WPI superimposed upon it in anatomically relevant locations. In addition, the MBM contains 16 additional areas for more general use. Our group has used the MBM to assess for widespread pain in a number of recent studies [1–4; 8–10; 17]. Despite its face validity, apparent ease of use, and straightforward scoring, more formal support for its validity has not yet been published.
This study was designed to have five nested sub-studies each supporting aspects of validation for the MBM. The first study was designed to assess face-validity and comprehension of the MBM. The second study assessed the test-retest reliability of the MBM. The third study was designed to compare the MBM to the WPI checklist from the 2011 Fibromyalgia Survey Criteria [20] and another commonly used body map, the Brief Pain Inventory [14]. This study was designed to help assess the accuracy of each of the three indices of pain location and to support the convergent validity of the MBM with other established measures of pain location. In addition, the third study assessed patient preferences when compared to these other measures of bodily pain. The fourth study used the data from the first three studies to compare the original MBM to a revised version based upon patient feedback from the first 3 studies. Finally, the fifth study assessed the discriminate validity of the revised MBM when compared to other validated self-report measures of pain, mood and function. We hypothesized that the original MBM would be easily understood and accurate (Studies 1 and 2) and would be preferred to the WPI checklist (Study 3). We hypothesized that the revised MBM would be preferred to and more accurate than the original MBM (Study 4). Furthermore, we hypothesized that the MBM would demonstrate good convergent validity when compared to other body maps (Study 3) and divergence from other measures of pain, mood and affect (Study 5).
2. Materials and Methods
2.1 Study setting and participants
These studies were approved by the Institutional Review Board (Ann Arbor, MI). In Studies 1–4, patients were recruited from the Spine Program (Department of Physical Medicine and Rehabilitation, University of Michigan Medical School, Ann Arbor, MI). Written informed consent was obtained for all patients. The data for Study 5 were collected as part of an Institutional Review Board-approved clinical care and research effort in the Back and Pain Center (Department of Anesthesiology, University of Michigan)[9], and written informed consent was waived.
For Studies 1–4, the body map measures were completed by the patient in the presence of a research assistant but without assistance from the research assistant. The completion time was assessed by the research assistant using a stopwatch. Following administration of the paper body maps, scripted interviews were used to assess personal experiences with the measures and to assess accuracy.
A validity check was conducted when appropriate by verbally inquiring as to the presence or absence of pain in each of the 35 body areas after completion of the body maps. This verbal query was the standard against which errors in reporting were calculated. Right and left sides were specifically clarified when appropriate. Areas of discrepancy between the completed MBM and the verbal administration were clarified by the research assistant. Each of the body regions was assigned one point in both the original and revised MBM, thereby creating a score of 0–35. The 19 body areas of the WPI are included in the MBM. Given that one of the intended uses of the MBM was to calculate the 2011 Fibromyalgia Survey Criteria, the impact of discrepancies was determined by analyzing the differences in the WPI scored both with and without corrections.
2.2 Measures
2.2.1 The Michigan Body Map
The original Michigan Body Map (MBM) was a one-sided, front facing body image with 35 possible checkbox body areas where patients were asked to denote areas of chronic pain, which was defined in the measure as “persistent or recurrent pain present for the last 3 months or longer (chronic pain).” A separate checkbox was positioned off the body map to report “No Pain.” The MBM contained the 19 body areas in the WPI checklist described in the 2011 Survey Criteria for Fibromyalgia [20; 21], with an additional 16 body areas to allow for more generalized use. The right and left sides were noted on the top right and left of the body map, respectively (Supplemental Figure 1).
2.2.2 Comparison body maps
The MBM was compared to two other measures used to assess areas of body pain. The first was the WPI, which is a grid with a checklist of body areas without a corresponding body image. It is used as one of the two components used to calculate the fibromyalgia survey score [20; 21]. The second comparison body map was from the Brief Pain Inventory (BPI), which is a two-sided body image on which patients can draw and shade areas of pain but without pre-specified areas [14].
2.2.3 The revised Michigan Body Map
Study 4 (see specific methods below) compared a revised version of the MBM created to address the concerns identified with the original MBM. The measure contains a front- and backsided image, as well as specific notation of right and left for each of the sided body areas (Supplemental Figure 2).
2.2.4 Pain characterization measures
So as to assess construct validity, study 5 (see specific methods below) compared the MBM to other measures of pain, mood and function commonly used in studies of pain assessment, clinical treatment trials and symptom monitoring [19]. These measures included pain intensity and interference (BPI)[14], neuropathic pain descriptors (PainDETECT)[7], anxiety and depression (Hospital Anxiety and Depression Scale)[23], catastrophizing (Coping Strategies Questionnaire – Catastrophizing subscale)[13], and physical function (Oswestry Disability Index)[6].
2.3 Study 1: Comprehension and face validity of the assessment task
The first study was designed to assess patient comprehension of the tasks associated with completing the MBM, comprehension of the mannequin graphic, and the item wording. Following completion of the MBM, validity was assessed as previously described in the “Study Setting and Participant” section above. Participants were also queried about any difficulties they had comprehending the demands or nature of the assessment task. For Study 1, descriptive comprehension metrics were summarized and accuracy and error rates reported. A comparison between corrected and uncorrected fibromyalgia survey criteria scores was conducted using a dependent samples t-test.
2.4 Study 2: Test-retest reliability
The second study focused on the test-retest reliability of the MBM. As in the first study, the patients completed the MBM on their own. Following the first administration, accuracy was assessed. Patients then returned to the clinic for a retest 1–2 weeks later. Wilcoxon signed-rank test and dependent samples t-test were used to assess the test-retest reliability of the MBM. As in Study 1, the corrected and uncorrected WPI scores using the MBM at the two different time points were compared using a dependent samples t-test. Pearson’s correlation coefficient was calculated to assess the relationship between MBM-derived widespread pain index scores at each time point. Finally, the percentage agreement for each of the 35 individual body areas at the two time points was calculated.
2.5 Study 3: Comparison of MBM to the Widespread Pain Index checklist and the Brief Pain Inventory body map
Patients in the third study were randomly assigned to receive the MBM and either the Widespread Pain Index checklist from the 2011 Survey Criteria for Fibromyalgia [20] or the body map from the Brief Pain Inventory (BPI)[14]. The group assignment and order in which the measures were completed were determined using a block randomization schedule (MBM vs. WPI checklist; or MBM vs. BPI body map). Patients first filled out the two maps, and then accuracy was assessed. Correlations between the three body maps were used to assess convergent validity. The sum of 19 body areas in the MBM used to calculate the WPI were used to compute the correlation between MBM and WPI. The sum of all 35 body areas in MBM and BPI were used to compute the correlation between MBM and BPI.
Finally, patients were asked which of the two body maps were preferred, including an option for “no preference.” They were also asked which measure best allowed them to depict their pain, best allowed for distinguishing the left and right side, and was easiest to complete - again with an option for “no preference” between the two. Lastly, patients were asked if there were any areas where they had pain but were not able to indicate using either map. Dependent samples t-tests, chi-square goodness of fit tests, and McNemar’s exact test were used to compare preferences for MBM, WPI, and BPI body maps.
2.6 Study 4: Comparison of the original MBM to a revised version
Based upon patient feedback from Studies 1–3 (see Results below), a revised version of the MBM was created to improve accuracy. Specifically, the revised MBM contains front- and back-sided images side-by-side (Supplemental Figure 2). In addition, the revised MBM contains a “Rt” in front of right-sided body areas (e.g., Rt knee) and a “Lt” in front of left-sided body areas (e.g., Lt knee) as suggested by the patients in the initial studies.
In the fourth study, patients were administered both the original and the revised MBM in random order. After completing both body maps, patients were asked which body map was preferred, which best depicted their painful areas, was easiest to complete, and which best distinguished left- and right-sided body areas. For the revised MBM, patients were also asked if they understood what “lt” and “rt” stood for on the revised MBM. As in studies 1–3, a validity check was conducted.. A dependent samples t-test was used to compare the accuracy of the new MBM to the original.
2.7 Study 5: Assessment of discriminant validity for the revised MBM
In addition to completing the revised MBM, patients completed a packet of validated self-report measures of pain, mood, and function as part of a clinical care and research initiative [9]. These measures included the Brief Pain Inventory (BPI) Pain Severity and Interference scales [14], the PainDETECT[7], Oswestry Disability Index (ODI)[6], catastrophizing from the Coping Strategies Questionnaire (CSQ)[13], and the Hospital Anxiety and Depression Scales (HADS)[23]. Pairwise correlations were used to assess the discriminant validity of the revised MBM to other validated measures common to pain assessment. While Study 3 assesses convergent validity and expects to find the strongest relationships between the MBM and other metrics of pain location, Study 5 assesses divergent validity and expects to find positive but weaker relationships between the MBM and these other pain-related but separate constructs.
2.8 Statistical Considerations
Data were analyzed using Stata 13.1 (Stata Corp., 2013). For each of the studies, demographic and descriptive statistics are presented in Table 1. Sample sizes for Studies 1 – 3 were determined without the aid of an a priori power analysis. However, post hoc power calculations revealed that power of 99.8%, 81%, and 80% was achieved for each of the 3 studies, respectively. For Study 4, a power analysis was based on the chi-square goodness of fit test based on the data collected in Study 2. A sample size of 79 participants resulted in 80% power to detect a 67.5% to 32.5% preference for the new body map compared to the original body map. An a priori power analysis was not conducted for Study 5, rather all available new patient survey data featuring the new body map was included in the analysis. A post hoc power analysis of Study 5 revealed that that the sample size of 237 resulted in 80% to detect a correlation as low as r = 0.18.
Table 1.
Demographics and Michigan Body Map (MBM) completion time for all participants and broken down for each study.
|
|
||||||
|---|---|---|---|---|---|---|
| Overall | Study 1: Understanding and Accuracy | Study 2: Test-Retest | Study 3: Comparison to other Measures | Study 4: Original vs Revised MBM | Study 5: Discriminant Validity | |
|
| ||||||
| (n = 402) | (n = 25) | (n = 20) | (n = 40) | (n = 80) | (n = 237) | |
| Age | 51.07 (15.90) | 51.08 (16.77) | 49.2 (12.01) | 52.28 (17.75) | 52.59 (14.54) | 50.46 (16.60) |
| Female | 60.45% | 48.00% | 15.00% | 42.50% | 44.44% | 39.66% |
| Caucasian | 86.32% | 92.00% | 85.00% | 87.50% | 87.50% | 88.60% |
| Married | 52.23% | 36.00% | 45.00% | 45.00% | 55.00% | 56.52% |
| College graduate | 42.29% | 52.00% | 75.00% | 52.50% | 48.75% | 34.00% |
| Disability benefits | 17.91% | 16.00% | 0.00% | 22.50% | 15.00% | 22.93% |
|
| ||||||
| Completion time for MBM (seconds) | 39 [38] | 49 [43] | 29 [26] | 45 [39] | Original MBM 34.5 [29] |
--- |
| Revised MBM 44 [32.5]* | ||||||
Data presented as mean (standard deviation), median [interquartile range], or percentages as appropriate. Time of completion of MBM was not collected in Study 5.
When compared to the original MBM, the revised MBM took significantly more time to complete (p = 0.003)
3. Results
3.1 Demographics and completion time
The mean age across all five studies was 51.07 years (SD 15.9), and 60.45% of the patients were female. The demographic characteristics of all of the patients enrolled, as well as a breakdown between the different sub-studies, are included in Table 1. The median time to complete the original MBM measure across the studies was 39 [interquartile range (IQR) 38] seconds and for the revised MBM was 44 [IQR 32.5] seconds.
3.2 Study 1: Comprehension and face validity of the Michigan Body Map
Twenty-five patients were recruited for the study assessing comprehension and face validity of the MBM (Table 2). The majority of patients (95%) correctly identified pain that was chronic in nature (defined as 3 months or longer), and most patients (84%) stated that the MBM allowed them to indicate all of their areas of pain. Those that responded negatively to this item wanted to differentiate pain in the calf, clavicle, toes, and fingers, as well as more detail for the jaw.
Table 2.
Results from Study 1- Assessment of understanding of Michigan Body Map.
| N (%) | |
|---|---|
| Pain present for 3 months or morea | |
| Yes | 19 (95%) |
| No | 1 (5%) |
| Accuracy of MBM vs Verbal Report | |
| All areas matched | 4 (16%) |
| 1 error (discrepancy) | 4 (16%) |
| 2 errors | 6 (24%) |
| 3 errors | 5 (20%) |
| 4+ errors | 6 (24%) |
| Left/Right identified correctlyb | |
| Always | 16(76.2%) |
| Errors present | 5 (23.8%) |
5 patients did not respond to this item
4 patients were not asked this question because their pain was not delineated left/right (e.g. head, neck, back pain)
Following the paper administration of the MBM, the patient was verbally queried about the presence of pain in each of the 35 body areas regardless of how they responded on the paper MBM. Using the verbal interview as the standard for accuracy, responses from the MBM were compared. Out of a total of 875 areas (35 area * 25 people), only 63 (7.2%) of the possible responses were mismatched between paper and verbal administrations. Of the 63 mismatched responses, 50 (79.4%) were unchecked on paper but reported in the verbal confirmation. This suggests that in this sample, the MBM was slightly underestimating the number of painful body areas. Twenty-two (34.9%) of the 63 mismatched responses were in patients who had misinterpreted the left and right side designations. The body areas with no mismatches included the face, neck, jaw, abdomen, groin, left lower arm. The body areas with the most mismatches (n = 5) were the lower back, right upper leg and left upper leg. Four (16%) participants had no discrepancies between written and verbal administrations and 15 (60%) had only 1 to 3 discrepant areas. There was no significant correlation between number of body areas checked and number of discrepant areas (r(24) = −0.039, p = 0.852). The majority of patients identified right/left correctly (16 of 21 correct, 76.2%). There are a total of 35 body areas on the MBM. Most patients (77.3%) felt that left versus right side was adequately indicated on the MBM (e.g., rated “somewhat” to “very clear”).
The MBM has more body areas (n = 35) than the Widespread Pain Index in the 2011 Fibromyalgia Survey Criteria. [20] Given that one of the major intended uses of the MBM is to calculate the widespread pain index component of the fibromyalgia survey score, the score was calculated for the cohort with and without the corrections made when determining discrepancies in completion. When left uncorrected, the mean fibromyalgia survey score for painful body regions was 3.9 (SD = 2.9). When corrected, the mean fibromyalgia survey score for painful body regions changed to 4.6 (SD = 2.6) (t(21) = 4.69, p < 0.001).
3.3 Study 2: Test-Retest Assessment of the MBM
Five people (25%) completed the MBM identically at both administrations and 25% (n = 5) had only one discrepant body part (M = 2.35, Median = 1.5, SD = 2.2). Percentage agreement for each body part from first administration to second ranged from 85% to 100% (Supplemental Table 1). The correlation between total number of body areas checked at each administration was positive and statistically significant (r(18)= 0.766, p = 0.001)γ. Again, the WPI score described by Wolfe et al. [20] to calculate the fibromyalgia survey score was not significantly influenced by these discrepancies when assessed using the MBM. The widespread pain index score derived from the MBM on the first administration (M = 3.1, SD = 1.86) did not significantly differ from the score on the second administration (M = 3.4, SD = 1.93) (t(19) = −1.24, p = 0.230). The correlation between the MBM-derived widespread pain index scores for each administration was positive and statistically significant (r(19) = 0.838, p < 0.001). The time to complete the MBM was similar between the initial and follow up administrations 1–2 weeks later (29 sec [IQR 26] vs 26 sec [IQR 22], Wilcoxon sign test, p = 0.359).
3.4 Study 3: Preference for the MBM was superior to the widespread pain index checklist and equivalent to the BPI body map
In this study, the high correlations between the MBM and the WPI (r(38) = 0.954, p < 0.001) and with the BPI body map (r(38) = 0.934, p < 0.001) supported excellent convergent validity. The time to complete the MBM was equivalent to the widespread pain index checklist [20] (MBM: 32 sec [24] vs WPI: 43 sec [35], Wilcoxon sign test, p = 0.815). Time to complete the MBM was also equivalent to the body map included in the BPI[14] (MBM: 52 sec [32] vs BPI: 52 sec [22], Wilcoxon sign test, p = 0.167).
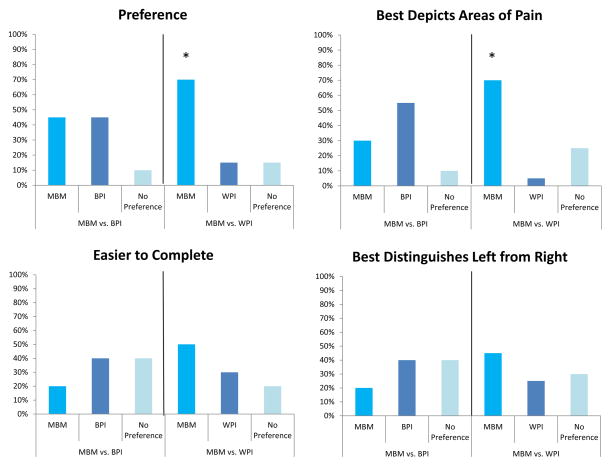
Patients preferred the MBM to the WPI checklist (Figure 1). Specific areas in which the MBM was deemed superior included patients’ overall preference (Figure 1A: 70% MBM, 15% WPI checklist, 15% no preference; χ2 (2) = 6.5, p = 0.039) and the measure that best depicted the areas of pain (Figure 1B: 70% MBM, 5% WPI checklist, 25% no preference; χ2 (2) = 9.7, p = 0.008). More patients (45%) felt that the MBM was superior to the WPI checklist in showing right- versus left-sided body areas of pain; however the result was not statistically significant (χ2 (2) = 2.6, p = 0.273). Only 25% of the patients felt that there was a painful area on their body not captured by the MBM, while 70% of patients felt that the WPI checklist was missing an area of their body pain (McNemar’s χ2 (1) = 7.36, p =0.012). There were no significant differences in patient preferences between the MBM and the BPI body map.
Figure 1. Comparisons between the Michigan Body Map (MBM) and the widespread pain index (WPI) from the 2011 Survey Criteria for Fibromyalgia and the body map of the Brief Pain Inventory (BPI).
Patients significantly preferred the MBM to the WPI and felt that the MBM better allowed for depiction of their painful body areas. Although not statistically significant patients described the MBM as easier to complete when compared to the WPI. There were no significant differences in the same assessments between the MBM and BPI body map.
Similar error rates to those reported in Study 1 were found in Study 3. We found that of the 1,400 body areas assessed (35 body areas* 40 participants), 128 (9.1%) were incorrect. Of the 128 incorrect, 99 (77.3%) were unchecked on the paper, but reported on the verbal administration. Additionally, 62 (48.4%) of the errors were observed in patients who had flipped left and right.
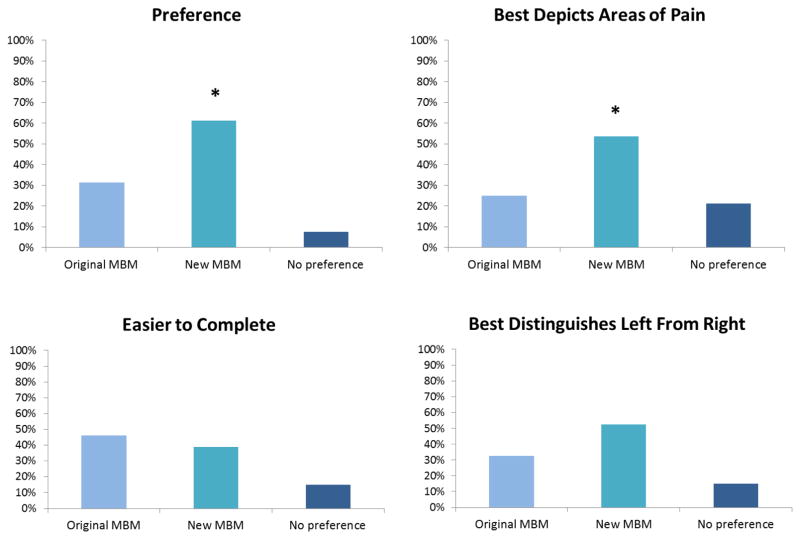
3.5 Study 4: A revised version of the MBM was more accurate and preferred to the original MBM
Based upon patient feedback from Studies 1–3 above, a revised version of the MBM was created to provide a front and back view, as well as to better distinguish right and left. As is noted in Figure 2, the revised version of the MBM was preferred to the original, and patients felt that it better allowed for depiction of their painful areas. When asked if they understand what the letters “lt” and “rt” represented, 78 (97.5%) indicated that they understood these markings represented “left” and “right,” respectively. There were no significant differences with respect to ease of completion or the patients’ feelings as to which body map better allowed for the distinction of right- versus left-sided body areas. The revised MBM took significantly longer to complete (44 seconds [IQR 32.5]) when compared to the original MBM 34.5 seconds [IQR 29] (Wilcoxon sign test, p = 0.002). The median difference of 10 seconds was not clinically significant, however.
Figure 2. Comparison of the original and revised Michigan Body Map (MBM).
When compared to the original MBM, the revised MBM was preferred and felt to better depict the patients’ painful body areas. The revised MBM was also felt to better represent right and left sided body areas. There were no differences in which body map was deemed easier to complete.
Again in this study, each of the body areas, whether checked or unchecked, were individually compared to verbal administration to assess accuracy. The new body map was significantly more accurate with only 1.6 (SD 1.99) mean errors compared to 2.5 (SD 2.71) mean errors with the original body map (t(79) = 2.97, p = 0.004). The difference in accuracy was not influenced by the order of administration of the new and original MBMs.
3.6 Study 5: The Michigan Body Map demonstrates convergent and discriminant validity when compared to other validated measures of pain, mood and function
A total of 237 new patients to the pain clinic completed the revised MBM along with validated measures of pain severity, pain interference, physical functioning, and affect. These were the Brief Pain Inventory (BPI) Pain Severity and Interference scales, the Pain DETECT scale, Oswestry Disability Index (ODI), the Catastrophizing Subscale from the Coping Strategies Questionnaire (CSQ), and the Hospital Anxiety and Depression Scales (HADS). Pairwise correlations were conducted between these scales and the total number of body areas on the Michigan Body Map (see Table 3). The correlations between the MBM and each of the pain-related constructs were all significant and positive but of relatively lower magnitude when compared to the correlations with other measures of pain location from Study 3 (e.g., each correlation in Study 5 was less than r = 0.41). Correlations of this magnitude suggest that less than 17% of the variance in each of these other scales overlaps with the MBM measure. Thus, in assessing pain wide-spreadedness, the MBM is assessing a somewhat unique construct that has positive associations with other metrics of pain. The 19 selected body areas for the WPI score demonstrated weaker, but similar correlations (data not shown). Study 5 in conjunction with Study 3, lends support to both the convergent and divergent validity of the MBM.
Table 3.
The Michigan Body Map demonstrated discriminant validity from other validated self-report measures of pain, mood and function.
| Correlation coefficient | R-squared | p-value | |
|---|---|---|---|
| BPI Pain Severity | 0.275 | 0.075 | < 0.001 |
| BPI Pain Interference | 0.323 | 0.104 | < 0.001 |
| PainDETECT | 0.384 | 0.148 | < 0.001 |
| ODI | 0.406 | 0.165 | < 0.001 |
| Catastrophizing | 0.154 | 0.024 | 0.024 |
| HADS Depression | 0.133 | 0.018 | 0.048 |
| HADS Anxiety | 0.183 | 0.034 | 0.006 |
Pearson correlation coefficients of the correlation between the new Michigan Body Map and measures of pain severity, pain interference, physical functioning, and affect provide evidence of discriminant validity. BPI = Brief Pain Inventory; HADS = Hospital Anxiety and Depression Scale; ODI = Oswestry Disability Index.
4. Discussion
The Michigan Body Map is a quick, reliable measure of widespread body pain
In this series of five studies assessing 407 patients, we supported the validity of the Michigan Body Map in a mixed sample of chronic pain patients. To our knowledge, this was the first study to provide support for the validly of using a body map as a means of quantifying widespread body pain in the service of assessing fibromyalgia-like or centralized pain symptoms. The median time for completion was 39 [IQR 38] seconds. The accuracy of the measure was good in most cases. When errors occurred, verbal inquiry revealed that most were errors of omission, thereby showing that the degree of widespread body pain could be underestimated using this method. Whereas inaccuracies are not desired, it is likely preferable that the symptoms be under- rather than over-estimated. More importantly, there were no significant differences in the WPI scores when derived using the MBM between the corrected and uncorrected forms. Therefore, the underestimation of painful areas would not be expected to significantly influence the fibromyalgia survey score when used in this manner.
Given the increased clinical and research interest in centralized pain conditions, it is important to ensure that the measures used to assess widespread body pain are valid. Most body maps have never had formal validation, although there have been some preliminary studies in adolescent and elderly populations [16; 18]. Generally, body maps have been found to be easy for patients to use and informative in that the location of the pain can be accurately communicated. Yet, body maps and manikins are not easily scored in a reliable manner. Most body maps require that patients shade in areas in which they experience pain; however, it is difficult to score body maps in this way. For example, at what point does low back pain become hip pain? How these distinctions are made is critical for accurately quantifying pain location - especially for the assessment of specific body areas in order to meet diagnostic criteria (e.g., 2011 Fibromyalgia Survey Criteria [5; 20]). In a study of trained raters, complete scoring agreement was observed in only 78% of cases (kappa >0.60) indicating room for improvement over the status quo [11]. A major objective of the validation work herein was to address some of these specific concerns.
The Michigan Body Map was preferred to the Widespread Pain Index (WPI) checklist
The WPI body checklist was described by Wolfe et al. [21; 22] as a way to quantify the number of painful body areas (0–19 possible areas). The MBM contains exactly the same body areas plus an additional 16 areas to allow for more general use. Patients preferred the MBM to the WPI checklist (Figure 1), and the MBM was felt to better depict painful body areas. We therefore suggest that the MBM be used when assessing the fibromyalgia survey score.
Although the BPI body map was equally preferred, the MBM allows for the calculation of widespread body pain
The two-sided body map included in the Brief Pain Inventory is a classic measure for assessing painful body areas, but there were no statistical differences in preference between the BPI and the MBM. The BPI body map does not, however, allow for quantification of widespread body pain. Moreover, the BPI body map leaves ambiguity as to the specific body area(s) noted by the patient. The advantage of the MBM was the inclusion of the named body area to eliminate ambiguity. Although some patients still marked and shaded outside of the checkboxes, it occured less frequently with the MBM than with the other body maps.
The revised Michigan Body Map was preferred to the original version
Using the information from the first three studies, we created a revised MBM (Supplemental Figure 2). The addition of a front- and back-sided body image, as well as better distinction of right and left, was preferred by patients (Figure 2). This also led to significant improvements in the accuracy. While the revised MBM was more accurate, the MBM-derived WPI score was not significantly different between the original and revised MBM. As such, data collected between the two versions can reasonably be combined for study purposes.
The Michigan Body Map is a distinct self-report measure of a distinct pain-related construct
Most commonly, pain is assessed by intensity or by quality along with other measures of cognition, affect and function. Pain location (e.g., wide-spreadedness) is less often assessed but appears to be a unique pain-related construct sharing only 17% overlapping variance with these other aspects of pain. The strong convergence of the MBM with other measures of pain location and its weaker but positive relationships with other pain-related constructs support the construct validity of the MBM.
Strengths and Limitations
The large size (n = 407) and structured multi-study approach were strengths of this study. Another strength was our comparison of the MBM to other commonly used methods of assessing pain location. In particular, this study demonstrated that the MBM was preferred to the originally described way of assessing widespread body pain in the 2011 Fibromyalgia Survey Criteria [20]. We therefore believe that the MBM can and perhaps should be used in lieu of the WPI checklist when assessing for features of fibromyalgia or centralized pain. The final strength of this study was the use of the data from the first three sub-studies to create a new measure to address the problems in the original MBM.
There are some limitations to this study. First, the data were collected at two tertiary care pain clinics at the same institution, and therefore the data may not be generalizable to other populations. In addition, the measure may be more obvious to pain patients who would have been more likely to have been exposed to body maps to assess for painful areas. Our group has experience with the administration of the measure in non-pain cohorts perioperatively, and we have not found problems. Lastly, we did not collect test-retest data for the revised MBM. Given the test-retest data for the original MBM (Study 2) and the improved accuracy from the revised MBM when compared to the original MBM (Study 4), we would anticipate the test-retest accuracy to be good for the revised MBM.
Future Directions
Despite the favorable data supporting the use of the MBM in clinical care and research, there are still likely opportunities to improve the measure. Whereas widespread body pain is a hallmark symptom for centralized pain and important for its detection [5], the dichotomous nature of assessing painful areas does not allow for adequate assessment of change over time. Interventions or medications may improve pain in a specific body area; however, the some pain may persist even if to a lesser degree. A body map that assesses the presence of pain in specific body areas and also allows for an assessment of the severity may better guide clinical decision-making and care. The challenge, however, is maintaining a measure that is brief and simple to better allow for application in clinical care. Pen-and-paper measures are still widely used; however, an electronic format will likely be superior for assessing severity in the specific body areas or in body zones. Further validation would be required for such an approach.
Conclusions
In conclusion, the original and revised MBM are both valid measures for assessing painful body areas and for calculating widespread body pain in the context of assessing the fibromyalgia survey criteria.
Supplementary Material
The original MBM is a one-sided self-report measure of up to 35 body areas of chronic pain with an option for “No pain.”
Based on the data collected in Studies 1–3, a revised version of the MBM was created. The named body areas are the same as the original MBM; however, the revised version has a front and back body image and notation of right and left on each appropriate body area.
Acknowledgments
Funding: The study was funded by the Department of Anesthesiology, University of Michigan Medical School, Ann Arbor, MI.
Drs. Brummett, Hassett and Moser had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Moser conducted and is responsible for the data analysis.
The authors thank the physicians, nurses and staff of the Spine Program (Physical Medicine and Rehabilitation) and the Back and Pain Center (Department of Anesthesiology) for their assistance in this study. The authors also thank Kate Brummett, B.A. for assistance in the creation of the original Michigan Body Map.
Footnotes
Disclosures: Dr. Brummett receives research funding from Neuros Medical Inc. (Willoughby Hills, Ohio). Dr. Clauw is a consultant for Pfizer, Inc. (New York, New York, USA); Johnson and Johnson (New Brunswick, New Jersey, USA); Forest Pharmaceuticals (New York, New York, USA); Merck (Whitehouse Station, New Jersey, USA); Nuvo Research, Inc. (Mississauga, Ontario, Canada); Eli Lilly, Inc. (Indianapolis, Indiana, USA); Grunenthal Pharma Ltd. (Dublin, Ireland); Jazz Pharmaceuticals, Inc. (Palo Alto, California, USA). Dr. Clauw also receives research funding from Merck Pharmaceuticals, Cerephex, and Forest Pharmaceuticals. Dr. Hassett has received research funding from Bristol-Myers (Princeton, New Jersey, USA) and is a consultant for Lexicon Pharmaceuticals (The Woodlands, Texas, USA). Dr. Williams is a consultant for Community Health Focus, Inc. (Orange County, CA, USA). There are otherwise no relevant disclosures.
Data from one participant was removed from this correlation because the participant had exceptionally more difficulty than the others and was thus removed to more accurately reflect the overall group’s reliability on this measure. When this outlier data point is included in the analysis, the correlation is still significant at r(19) = 0.586, p = 0.007).
References
- 1.Brummett CM, Goesling J, Tsodikov A, Meraj TS, Wasserman RA, Clauw DJ, Hassett AL. Prevalence of the fibromyalgia phenotype in patients with spine pain presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis Rheum. 2013;65(12):3285–3292. doi: 10.1002/art.38178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brummett CM, Janda AM, Schueller CM, Tsodikov A, Morris M, Williams DA, Clauw DJ. Survey Criteria for Fibromyalgia Independently Predict Increased Postoperative Opioid Consumption after Lower-extremity Joint Arthroplasty: A Prospective, Observational Cohort Study. Anesthesiology. 2013;119(6):1434–1443. doi: 10.1097/ALN.0b013e3182a8eb1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brummett CM, Lohse AG, Tsodikov A, Moser SE, Meraj TS, Goesling J, Hooten M, Hassett AL. Aberrant analgesic response to medial branch blocks in patients with characteristics of fibromyalgia. Reg Anesth Pain Med. 2015;40(3):249–254. doi: 10.1097/AAP.0000000000000235. [DOI] [PubMed] [Google Scholar]
- 4.Brummett CM, Urquhart AG, Hassett AL, Tsodikov A, Hallstrom BR, Wood NI, Williams DA, Clauw DJ. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67(5):1386–1394. doi: 10.1002/art.39051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clauw DJ. Fibromyalgia: A Clinical Review. JAMA. 2014;311(5):1547–1555. doi: 10.1001/jama.2014.3266. [DOI] [PubMed] [Google Scholar]
- 6.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976) 2000;25(22):2940–2952. doi: 10.1097/00007632-200011150-00017. discussion 2952. [DOI] [PubMed] [Google Scholar]
- 7.Freynhagen R, Baron R, Gockel U, Tolle TR. PainDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–1920. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- 8.Hassett AL, Hilliard PE, Goesling J, Clauw DJ, Harte SE, Brummett CM. Reports of chronic pain in childhood and adolescence among patients at a tertiary care pain clinic. J Pain. 2013;14(11):1390–1397. doi: 10.1016/j.jpain.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Hassett AL, Wasserman R, Goesling J, Rakovitis K, Shi B, Brummett CM. Longitudinal assessment of pain outcomes in the clinical setting: development of the “APOLO” electronic data capture system. Reg Anesth Pain Med. 2012;37(4):398–402. doi: 10.1097/AAP.0b013e3182524672. [DOI] [PubMed] [Google Scholar]
- 10.Janda AM, As-Sanie S, Rajala B, Tsodikov A, Moser SE, Clauw DJ, Brummett CM. Fibromyalgia survey criteria are associated with increased postoperative opioid consumption in women undergoing hysterectomy. Anesthesiology. 2015;122(5):1103–1111. doi: 10.1097/ALN.0000000000000637. [DOI] [PubMed] [Google Scholar]
- 11.Lacey RJ, Lewis M, Jordan K, Jinks C, Sim J. Interrater reliability of scoring of pain drawings in a self-report health survey. Spine (Phila Pa 1976) 2005;30(16):E455–458. doi: 10.1097/01.brs.0000174274.38485.ee. [DOI] [PubMed] [Google Scholar]
- 12.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1(3):277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 13.Swartzman LC, Gwadry FG, Shapiro AP, Teasell RW. The factor structure of the Coping Strategies Questionnaire. Pain. 1994;57(3):311–316. doi: 10.1016/0304-3959(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 14.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5(2):133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Uden A, Astrom M, Bergenudd H. Pain drawings in chronic back pain. Spine (Phila Pa 1976) 1988;13(4):389–392. doi: 10.1097/00007632-198804000-00002. [DOI] [PubMed] [Google Scholar]
- 16.von Baeyer CL, Lin V, Seidman LC, Tsao JC, Zeltzer LK. Pain charts (body maps or manikins) in assessment of the location of pediatric pain. Pain Manag. 2011;1(1):61–68. doi: 10.2217/pmt.10.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wasserman RA, Brummett CM, Goesling J, Tsodikov A, Hassett AL. Characteristics of chronic pain patients who take opioids and persistently report high pain intensity. Reg Anesth Pain Med. 2014;39(1):13–17. doi: 10.1097/AAP.0000000000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiner D, Peterson B, Keefe F. Evaluating persistent pain in long term care residents: what role for pain maps? Pain. 1998;76(1–2):249–257. doi: 10.1016/s0304-3959(98)00059-1. [DOI] [PubMed] [Google Scholar]
- 19.Williams DA. The importance of psychological assessment in chronic pain. Curr Opin Urol. 2013;23(6):554–559. doi: 10.1097/MOU.0b013e3283652af1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38(6):1113–1122. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62(5):600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe F, Hauser W, Hassett AL, Katz RS, Walitt BT. The development of fibromyalgia--I: Examination of rates and predictors in patients with rheumatoid arthritis (RA) Pain. 2011;152(2):291–299. doi: 10.1016/j.pain.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 23.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The original MBM is a one-sided self-report measure of up to 35 body areas of chronic pain with an option for “No pain.”
Based on the data collected in Studies 1–3, a revised version of the MBM was created. The named body areas are the same as the original MBM; however, the revised version has a front and back body image and notation of right and left on each appropriate body area.