Abstract
Chorioamnionitis is associated with preterm labor and fetal inflammatory response syndrome (FIRS), causing fetal organ injury and morbidity particularly in extremely premature infants. However, the effects of inflammation on the fetal immune system remain poorly understood, due to the difficulty of studying immune development in infants. Therefore, we used the model of intra-amniotic (IA) LPS administered at ~80% gestation in rhesus monkeys to cause chorioamnionitis and FIRS that is similar in human pathology. Importantly, the frequency of IL-17+ and IL-22+ CD4+ T-cells increased in the spleen of LPS-exposed fetuses, while Treg frequency decreased. These changes persisted for at least 48h. Notably, Th17 cytokines were predominantly expressed by FoxP3+CD4+ T-cells and not by their FoxP3− counterparts. Bi-functional IL17+FoxP3+ exhibited a phenotype of inflammatory Treg (RORcHigh/+, HeliosLow/−, IL-2+, IFNγ+ and IL-8+) compared to typical FoxP3+ cells. Diminished splenic Treg frequency in LPS-exposed fetuses was associated with inadequate Treg generation in the thymus. Mechanistically, the emergence of inflammatory Treg was largely dependent on IL-1 signaling. However, blockage of IL-1R signaling did not abolish the deleterious effects of LPS on Treg frequency in the thymus or spleen. Collectively, we demonstrate that a prenatal inflammatory environment leads to inadequate Treg generation in the thymus with a switch of splenic Treg towards an inflammatory phenotype. Both processes likely contribute to the pathogenesis of chorioamnionitis. Approaches to manipulate Treg numbers and function could thus be useful therapeutically to alleviate FIRS in preterm infants.
Introduction
Chorioamnionitis, which is inflammation of the fetal membranes and amniotic fluid, is associated with preterm labor and morbidity in extremely premature infants (1–3). Chorioamnionitis is most commonly caused by ascending infections, where the organisms from the lower genital tract gain access to the amniotic fluid inducing intrauterine inflammation and chorioamnionitis (4, 5). Fetal response to chorioamnionitis termed fetal inflammatory response syndrome (FIRS), is associated with fetal organ injury (6–9). In particular, fetuses and newborns exposed to chorioamnionitis can have alterations of T-cell immune responses, and thymic involution (10–16). However, due to limitations in the access to samples in humans, the effects of chorioamnionitis on fetal T-cells in tissues such as the spleen and thymus remain poorly understood. The Rhesus macaque is ideal to answer these questions. In these animals, intra-amniotic (IA) injection of Group B streptococci, IL-1β or TNFα induces chorioamnionitis characterized by massive infiltration of neutrophils in the decidua and elevated levels of inflammatory cytokines in the amniotic fluid and fetal lung (6, 17–19). These features reproduce closely what is observed in human chorioamnionitis.
Experimental chorioamnionitis caused by IA administration of IL-1β in monkeys causes profound changes in fetal lymphoid organs, notably increased IL-17+ T-cells and decreased regulatory T-cells (Treg) frequency in the spleen and lymph nodes (6). The Th17 subset of CD4+ T-cells promotes inflammation, notably by signaling chemokine production by endothelial cells, which result in the recruitment of inflammatory monocytes and neutrophils to the site of inflammation (20, 21). Th17 cells develop from naïve CD4+ T-cells in the periphery, and the key factors involved in this differentiation are the signal transducer and activator of transcription 3 (Stat3) and the retinoic acid receptor-related orphan receptors gamma (RORγ) or its human homolog RORc. The cytokine milieu strongly influences subset differentiation, with TGF-β, IL-6, IL-1β, IL-21 and IL-23 promoting pathogenic Th17 cells, whereas Th17 cells that develop in the presence of IL-6 and TGF-β are nonpathogenic (22). In contrast, Tregs are a subset of CD4+ T-cells critical for the maintenance of immunological self-tolerance (23). Tregs originate from two distinct pathways, either through thymic development or by peripheral differentiation from conventional, non-Treg, naive T-cells (Tcons) to become peripheral Treg.
The expression of the transcription factor forkhead box P3 (FoxP3) is essential for Treg development and function. TGF-β and IL-2 induce the transcription of Foxp3 (24, 25). Although Tregs are generally considered to be anti-inflammatory, Tregs have a greater plasticity than other CD4+ T-cells (26). In fact, under certain circumstances, such as in the presence of proinflammatory cytokines, naïve Treg can become inflammatory Tregs (27–29). Importantly for our studies, Tregs with the capacity to express proinflammatory cytokines, notably IL-17 and IFN-γ, have been described in cord blood of healthy neonates (28, 30).
Therefore, we used the LPS-induced chorioamnionitis model in the Rhesus macaque to ask how intrauterine inflammation influences the developing fetal immune system. Our hypothesis was that intrauterine inflammation affects Treg development in the fetal thymus as well as promotes the emergence of inflammatory Tregs in the fetal peripheral lymphoid organs. In addition, based on our previous results in this model (6), we also postulated that IL-1 is critical for these processes. We tested this second hypothesis by administering the cross-reactive recombinant human IL-R antagonist (IL-RA) together with LPS.
Materials and methods
Animals
All animal procedures were approved by the Institutional Animal Care and use Committee at the University of California, Davis. Normally cycling, adult female Rhesus macaques (Macaca mulatta) (n=28) were time mated. At approximately 130 days of gestation (about 80% of term gestation), the pregnant Rhesus received either 1 ml saline solution or 1mg of LPS (Sigma-Aldrich, St.Louis MO) in 1 ml saline solution by ultrasound guided IA injection. IA administration of LPS or saline was performed in multiparous macaques of similar weights and ages with fetus with similar fetal genders and gestational ages (Table I). Fetuses delivered surgically were euthanized with pentobarbital and fetal tissues were collected. There were no spontaneous deaths or preterm labor in the animals.
Table 1.
Description of the animals included in the study
| Controls (n=8) | LPS (16h)* (n=6) | LPS (48h)* (n=8) | LPS+ IL-1RA (16h+ 48h)* (n=6) | |
|---|---|---|---|---|
| Maternal age (Years) | 10.5± 2.0 | 9 ± 2.4 | 10.9± 2.7 | 10.2± 2.2 |
| Maternal weight (Kg) | 8.3± 1.5 | 8.4 ±1.7 | 8.7 ±0.6 | 12 ± 2 |
| Fetal Gestational age at delivery (days) | 132± 2.2 | 130 ± 0.75 | 132 ± 4.6 | 129 ± 1.7 |
| Fetal Birth Weight (g) (mean ± SEM) | 330 ± 20 | 333 ± 48 | 320 ± 49 | 358 ± 3 |
| Fetal Sex (% Female) | 62 | 50 | 87 | 17 |
Data are expressed as mean ± DS percentage.
Interval from IA injection to delivery.
In some animals, the IL-1 receptor antagonist (IL-1RA, Kineret® Sobi, Stockholm, Sweden) was given to the pregnant monkey IA (50mg) and SC (100mg) 1 and 3 hours before LPS, respectively (see Suppl. Fig. 5A). LPS animals that were delivered 48h after IA received a second IL-1RA treatment (IA and SC to the mother) 24h after the first treatment (see Suppl. Fig. 5B).
Cytokine measurement in the broncho-alveolar lavage fluid and recombinant IL-RA levels
Alveolar wash of the left fetal lung was used for measurements of IL-6, IL-8, TNFα and GM-CSF. Cytokine concentrations were determined by Luminex using non-human primate multiplex kit (Millipore Austin, TX) according to the manufacturer’s protocol. Human IL-1RA levels were measured in the fetal plasma using the human IL-1RA ELISA kit (R&D systems, Minneapolis, MN), which does not detect rhesus macaque IL-1RA.
Fetal cell isolation, culture and stimulation
The spleen and thymus were diced and dissociated into homogenous cell suspensions using a pestle. Cell suspensions were filtered through 70-μm cell strainers and washed in RPMI 1640culture media containing 10% FCS, 100 IU/ml penicillin, 100 IU/ml streptomycin, and 2 mmol/l glutamine. PBMCs were isolated from 10 ml heparinized fetal blood using Ficoll-Hypaque (GE Healthcare, Piscataway, NJ) gradient centrifugation within 3 h of collection. Red blood cell (RBC) lysis in splenocyte suspensions were with ammonium chloride/potassium carbonate/EDTA (Lonza BioWhittaker, Pittsburgh, PA).
Cells were rested in culture overnight at 37°C and 5% CO2, viability was consistently >90% (trypan blue exclusion test) the next day. For cytokine measurements, cells were stimulated with 50 ng/ml PMA (Sigma-Aldrich, St. Louis, MO) and 750 ng/ml ionomycin (Calbiochem, San Diego, CA) for 5 h, with 10 μg/ml brefeldin A (Sigma-Aldrich) and 1 μl/ml monensin 1000× (eBioscience, San Diego, CA) added for the last 4 h.
Decidua cell isolation, culture and stimulation
Purified decidua cell suspensions were prepared as previously described with some modifications (31). Briefly, the membranes were dissected from each placenta. Decidua parietalis was scraped from the underlying chorion, washed, and digested at 37°C with 125 mg/100 ml dispase II (Life Technologies, Grand Island, NY) plus 50 mg/100 ml collagenase A (Roche, Indianapolis, IN) in Dulbecco modified Eagle medium-F12 (DMEM-F12) medium with antibiotics. After 30 min, 0.4 mg/100 ml DNase I (Roche) was added to the cell suspension for an additional 30 min at 37°C on a shaking platform. Cell suspensions were filtered through 70-μm cell strainers twice, washed in PBS, and counted. Red blood cell lysis was performed using an ammonium chloride-potassium carbonate-ethylenediaminetetraacetic acid solution. Viability was > 90% by trypan blue exclusion test. Decidua suspensions were counted and plated at 1.0x106 cells/well in DMEM-F12 media overnight in 24-well plates. The next morning, cells were stimulated with PMA and ionomycin as described above.
Flow Cytometry
For multi-parameter flow-cytometry, a cocktail of conjugated antibodies that we validated for the Rhesus macaque was used to phenotype freshly isolated cell suspensions. The following antibodies were used: anti-CD3 (SP34-2), anti-Ki67 (B56), anti-IFNγ (B27), anti-IL-2 (5344.111), anti-CD1a (SK9), anti-CD7 (M-T701), anti-IL-8 (G265-8), anti-CD45 (D058.1283) (BD Biosciences); anti-FOXP3 (PCH101), anti-IL-17 (eBio64CAP17), anti-IL-22 (IL22JOP), anti-RORc (AFKJS-9), anti-CD8α (RPA-T8), anti-CD8β (SIDI8BEE), anti-CD27 (O323), anti-GATA-3 (TWAJ), (eBioscience, San Diego, CA); anti-CD34 (561), anti-CD4 (OKT4), anti-CD69 (FN50), anti-CCR6 (G034E3), anti-Helios (22F6) (BioLegend, San Diego, CA); anti-IL-1R (polyclonal), anti-T-bet (525803) (R&D Systems, Minneapolis, MN); anti-NKG2a (REA110) (Miltenyi-Biotec, Auburn, CA).
Fetal and maternal cell suspensions were treated with human IgG to block Fc-receptors and stained for surface markers. Cells were then washed and fixed with Fixation/Permeabilization Buffer (eBioscience) followed by Permeabilization Buffer (eBioscience). Cells then were stained for intracellular markers and analyzed on a FACS Fortessa 2 (BD Biosciences). Cells were gated based on forward- and side-scatter properties and the absence of fluorescence in the LIVE/DEAD® Fixable Dead Cell Stain kit (Invitrogen, Grand Island, NY). Flow cytometry analysis was performed using the FACSDiva software version 6.1.2 (BD Biosciences).
Statistical analyses
GraphPad Prism (GraphPad Software, La Jolla, California, USA) was used to graph and analyze data for statistical significance. Values from different measurements were expressed as median (range). Statistical differences between groups were analyzed using Mann-Whitney U-tests. Results were considered significantly different for p values ≤0.05. However, due to the limited number of samples per group, we also report trends (p values between 0.05 and 0.1).
Results
Intra-amniotic LPS alters fetal Th17 (IL-17+ and IL-22+ frequencies) and Treg responses
IA LPS induced a robust chorioamnionitis in the rhesus macaques, with a marked cellular infiltrate (mainly neutrophilic) at the interface between maternal and fetal tissues (Suppl. Fig. 1A–B). Inflammation also developed in fetal lungs, as demonstrated by the increased levels of IL-6, IL-8, TNF-α and GM-CSF at 16h and 48h after IA LPS, compared to untreated controls (Suppl. Fig. 1C). Inflammation in decidua and fetal lung was sustained at both 16h and 48h, without major differences between the 2 time points.
Based on our previous studies of the effect of IA IL-1β in rhesus macaques (6), we focused on fetal T-cell responses. Absolute counts of total CD4+ and CD8+ T-cells in the fetal blood and spleen were overall unchanged by LPS-exposure, with only a slight increase in the percentage of blood CD4+ T-cells at 16h (median of 54% vs a median of 45%, p=0.04). One notable effect of LPS exposure was the 2-fold increased proportion of CD4+ IL-17+ in the spleen (after a short polyclonal stimulation to reveal in vivo priming), which occurred at 16 and 48h post exposure (Fig. 1B). This increase was not evident in the PBMC (Fig. 1A). The frequency of CD4+ IL-22+ also increased in LPS-exposed fetuses, in both spleen and PBMC (Fig. 1C–D). In contrast to the increases seen in the CD4 subset, the frequency of IL-17+ and IL-22+ in CD8+ T-cells or NK cells (defined as CD3− CD8− NKG2A+) did not change in LPS-exposed fetuses (data not shown). Similarly, LPS did not alter the proportion of IFNγ+ and IL-2+ cells in any of these cellular subsets (data not shown). The immune profiles at 16h and 48h post-LPS were very similar, although the IL-22 response appeared to be maximal at 16h (Fig. 1D). The absolute number of splenic IL-17+ CD4+ T-cells was variable in control fetuses (median of 1.09 x106 cells/g tissue, range: 0.59 to 3.77). Although there was a 2-fold increase in the median absolute number of these cells 16h post-exposure (median: 2.44x106 cells/g spleen, range: 1.17 to 3.73), the difference was not significant due to the large variation in the control group. Similarly, there were no differences between controls and fetuses exposed to LPS for 48h (not shown). Similar results were observed when the absolute number of IL-22+CD4+ T-cells per g of spleen was calculated. Likewise, absolute numbers of CD8+ T-cells expressing either IL-17 or IL-22 were not changed by LPS exposure (data not shown).
Figure 1. Intra-amniotic LPS alter Th17 (IL-17 and IL-22) and Treg response by fetal CD4 T-cells.
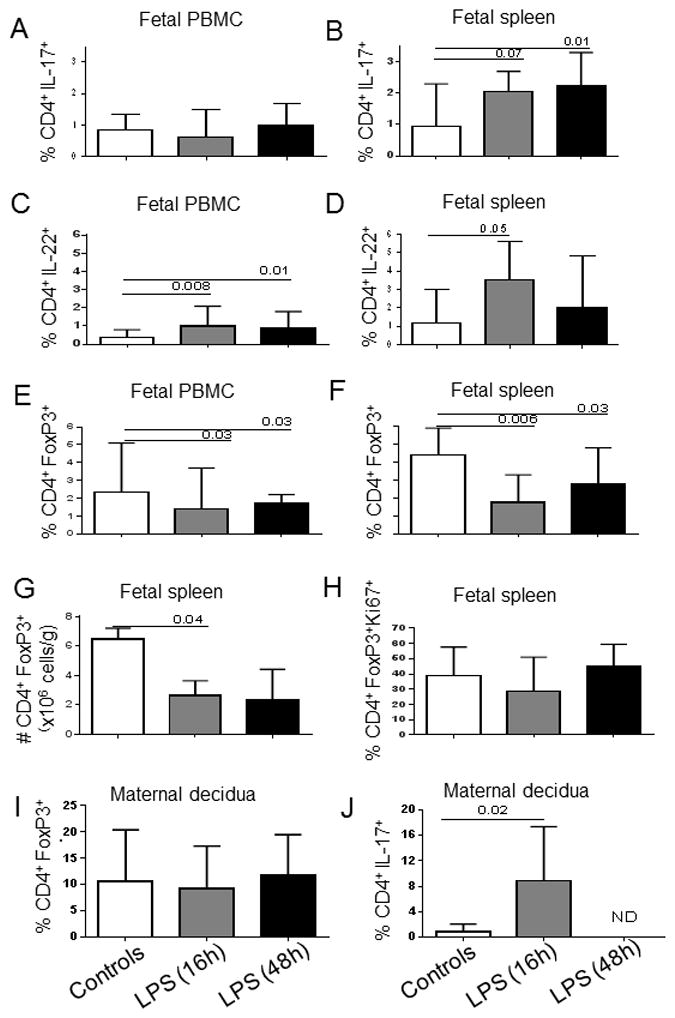
Bars show the median (range) percentage of CD3+CD4+ T-cells from PBMC and spleen expressing IL-17 (A, B), IL-22 (C, D) or FoxP3 (E, F). G) Absolute counts of splenic Treg (CD3+CD4+FoxP3+). H) Percentage of splenic Treg expressing Ki67 within the CD4+FoxP3+ population. Control group (n=8) and LPS-exposed fetuses for 16h (n=6) and 48h (n=8). I) Percentage of decidua Treg (CD3+CD4+FoxP3+) within the CD4+ T cell population. Decidua from unexposed dams (n=8) and from those exposed to IA LPS for 16h (n=4) and 48h (n=7). J) Percentages of decidua CD3+CD4+ T-cells expressing IL-17. Control group (n=4) and decidua from dams exposed to IA LPS for 16h (n=4). N.D.: Not done. Significant differences between groups were calculated using Mann-Whitney U tests test. Bars graphs show the median and interquartile range.
In contrast to the increased frequency of IL-17+ and IL-22+ CD4+ T-cells, the frequency of peripheral blood and spleen Treg (defined as the percentage of FoxP3+ cells within the CD3+CD4+ population; a representative gating strategy is shown in Suppl. Fig. 2A) significantly decreased 16h after IA LPS exposure compared to control animals, and this decrease persisted for 48h (Fig. 1E–F). Importantly, Treg absolute numbers were also significantly lower in LPS-exposed fetuses than in controls (Fig. 1G). Consequently, splenic Treg/IL-17 ratios were low in both groups of LPS-exposed fetuses (median of 4.8, 0.9 and 0.8 in controls, LPS 16h and LPS 48h, respectively, both p<0.009 versus controls). Low Treg numbers were not due to an effect of inflammation on Treg cell cycle, as LPS-mediated inflammation did not decrease Ki67 expression in Treg (Fig. 1H).
Since IA LPS injection might also affect maternal Treg frequency and cytokine production, we measured the frequency of Treg in the decidua, which is of maternal origin. As shown in Fig. 1I, Treg frequencies were not altered in the decidua from LPS-exposed dams, as compared to control dams. Treg absolute numbers in the decidua were also unchanged (median: 0.03x106 cell/g, 0.02x106 cell/g and 0.04x106 cell/g in controls, LPS-exposed dams at 16 and 48h, respectively, p>0.9). However, similar to what occurred in fetal tissues, the proportion of decidua CD4+ T-cells that expressed IL-17 was significantly increased in the dams exposed to LPS for 16h compared to controls (Fig. 1J). The IL-17 expression by decidua CD8+ T-cells did not change (data not shown). For technical reasons, IL-17 expression could not be analyzed in the decidua of the dams that were exposed to LPS for 48h.
Th17 cytokines are predominantly expressed by FoxP3+ CD4+ T cells in LPS-exposed fetuses
To define which subsets within fetal CD4 T-cells predominantly express Th17 cytokines, we next compared the expression of IL-17 and IL-22 in FoxP3− and FoxP3+ CD4+ T-cells (see Suppl. Fig. 2B for a representative gating strategy). In control animals, the frequency of cells expressing IL-17 and IL-22 was higher in the FoxP3+ subset than in their FoxP3− counterparts (Fig. 2A–D). Notably, the frequency of these IL-17-expressing FoxP3+CD4+ cells significantly increased in both groups of LPS-exposed fetuses compared to controls (Fig. 2A), while IL-17 expression remained unchanged in FoxP3− cells (Fig. 2B). In contrast, both FoxP3+ and FoxP3− CD4+ T-cells expressed IL-22 more frequently in LPS-exposed fetuses than in controls (Fig. 2C–D). Consequently, CD4+ T-cells expressing both IL-17 and IL-22 were more prevalent in the FoxP3+ subset than in the FoxP3− cells, and their frequency increased after LPS exposure (Fig. 2E–F). Accordingly, the frequency of RORc+FoxP3+ CD4+ T-cells was increased in fetuses exposed to LPS for 16h compared to controls (median: 20% vs 12%; p=0.09), and this trend persisted in fetuses exposed for 48h (22% vs. 12%; p=0.07).
Figure 2. Increased Th17 cytokines induced by LPS are predominantly from fetal FoxP3+ Treg.
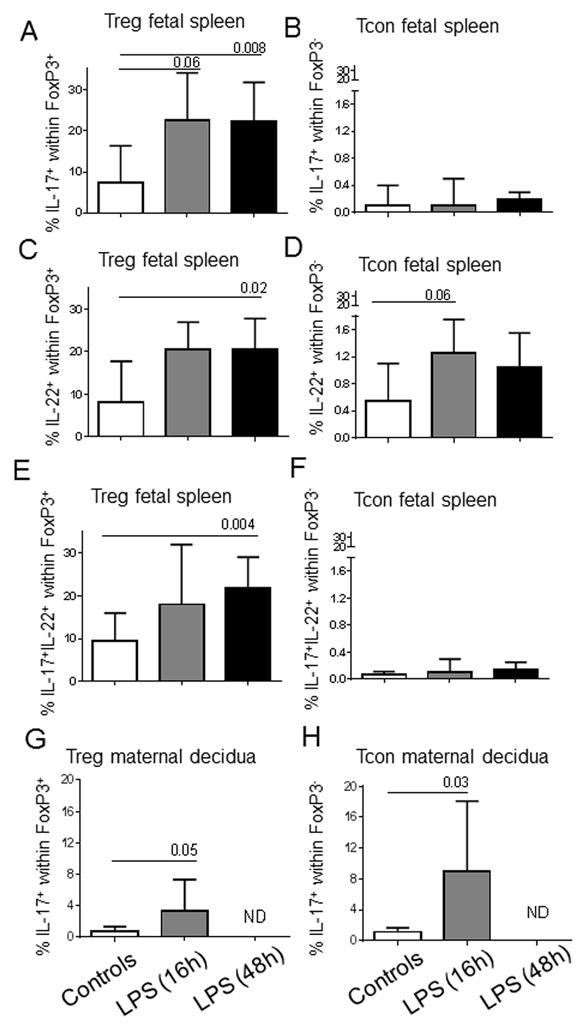
(A) to (F): Bars show the median (range) frequency of Treg (FoxP3+) and Tcon (FoxP3−) within the fetal spleen CD4+ T cell population that expressed IL-17 (A, B), IL-22 (C, D), or both IL-17 and IL-2 (E, F). Control group (n=8) and LPS-exposed fetuses for 16h (n=6) and 48h (n=8) were compared using Mann-Whitney U tests. (G) to (H): Frequency of Treg (FoxP3+) and Tcon (FoxP3−) within the maternal decidua CD4+ T cell population that expressed IL-17. Control group (n=4) and decidua from dams exposed to IA LPS for 16h (n=4) were compared using Mann-Whitney U tests. N.D.: Not done
In contrast to what occurred in fetal tissues, the increased frequency of IL-17+ CD4+ T-cells in the maternal decidua cells was mainly due to a higher proportion of these cells in the conventional FoxP3− CD4+ T-cells than that in control dams, although the frequency of bifunctional IL-17+FoxP3+ CD4+ T-cells also modestly increased in the decidua of LPS-exposed dams (Fig. 2G–H).
Fetal bifunctional IL17+FoxP3+ cells have a pro-inflammatory phenotype compared to typical FoxP3+ cells
We next performed a thorough phenotypic analysis of both FoxP3+ subsets (the IL-17+FoxP3+ and the IL-17-FoxP3+ subset) in controls and LPS-exposed fetuses. We did similar comparisons for the IL-17+ and IL-17− FoxP3− subsets (gating strategy in suppl. Fig. 2C). IL-17+FoxP3+ cells tended to express slightly lower FoxP3 MFI than that of IL-17−FoxP3+ cells (Fig. 3A). As expected, IL-17+ Treg exhibited a higher expression of RORc but a lower expression of Helios than the typical IL-17− Treg (Fig. 3B–C). This difference was also marked when percentages of RORc+ or Helios+ cells were analyzed in both Treg populations (median RORC+: 78% vs 2% in IL17+ vs IL17− Treg, respectively, p= <0.0001; median Helios+: 19% and 86% in IL17+ Treg and IL17− Treg, respectively, p= <0.0001). In addition, IL-17+ FoxP3+ expressed significantly more IL-2, IFN-γ, IL-8 and the cell cycle marker Ki67 than the IL-17−FoxP3+ cells (Fig. 3D–G), confirming that neonates have two subsets of Tregs, which differ in their capacity to produce pro-inflammatory cytokines. Importantly, there was no notable difference in the profile of IL-17+ or IL-17− FoxP3+ in LPS-exposed fetuses and control fetuses (compare in Fig. 3 the similar distribution of stars –representing the controls- with that of triangles or circles –depicting LPS-exposed fetuses for 16h or 48h, respectively).
Figure 3. Bifunctional IL17+ Treg exhibited a pro-inflammatory phenotype compared to typical Treg.
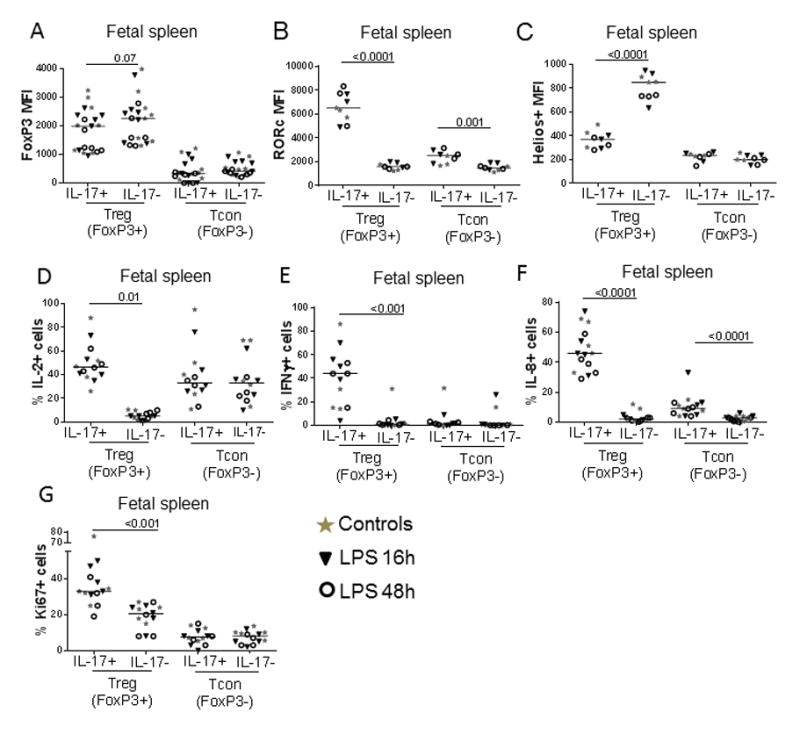
Graphs show the phenotype of splenic IL-17+ or IL-17− Treg (defined as CD3+CD4+FoxP3+), as well as that of IL-17+ or IL-17− Tcon counterparts (defined as CD3+CD4+FoxP3−). Panels display A) FoxP3 MFI, B) RORc MFI, C) Helios MFI, D)% of IL-2+, E) % of IFN-γ+, F) % of IL-8+ and G) % of Ki67+ cells in each subset. Each dot represents a single animal, with controls displayed as stars, and LPS-exposed animals displayed as either triangles (16h time point) or circles (48h time point). Bars show the median for each analysis, with all groups combined. P values correspond to Mann-Whitney tests.
In FoxP3− cells, the IL-17+ subset also contained a slightly higher frequency of IL-8+ and expressed higher RORc MFI cells than their IL-17− counterparts, but the difference was much less marked than that found in the FoxP3+ subset (Fig 3B and 3F). Both FoxP3− subsets expressed similar high levels of IL-2 and low expression of FoxP3, Helios, IFN-γ and Ki67 (Fig. 3A, B, D, E, G). Again, the expression pattern of IL-17+ or IL-17− FoxP3− cells was very similar in controls and LPS-exposed fetuses.
We also analyzed the phenotype of CD4+ T-cells in unstimulated conditions, using RORc expression to differentiate the subsets (Suppl. Fig. 2D shows the gating strategy). As shown in Suppl. Fig. 2E, RORc+ FoxP3+ and FoxP3− cells expressed higher levels of CCR6 than their RORc− counterparts. In 2 animals from each group, we also found that both RORc+ Treg and Tcon expressed more T-bet than their RORc− counterparts (Suppl. Fig 2G). IL-1R and GATA-3 expression were similar in both Treg subsets (Suppl. Fig 2F and 2H), while RORc+FoxP3− cells exhibited a higher expression of IL-1R and GATA-3 than RORc− FoxP3− cells. Again, expression profiles were very similar in LPS-exposed fetuses and controls in 2–5 animals from each group
Intra-amniotic LPS alters fetal thymic T cell development
To analyze the effect of LPS-induced inflammation on thymic T-cell development, we designed a flow cytometry panel to identify different thymocyte subsets, spanning from early hematopoietic stem cell precursors (HSC), double negative 3 (DN3), double positive (DP) thymocytes, to late subsets such as CD4 and CD8 single positive (CD4SP and CD8SP) thymocytes (32–35). The gating strategy and representative examples of staining are shown in Suppl. Fig. 3A and 3B. We also included an anti-FoxP3 Ab in the panel to quantify thymic Treg.
LPS-induced chorioamnionitis did not change fetal thymus weight (data not shown), but altered the frequency of several thymocyte subsets. In particular, IA LPS increased the frequency of total DP at 16h (p=0.03) and 48h (p=0.06) compared to controls (Fig. 4A). Alteration in the DP subset was mainly due to the increased proportion of cells at the DPII stage (Fig. 4A). Frequencies of total HSC and DN3 did not change in LPS-exposed fetuses (data not shown). IA LPS also increased the frequency of total CD4SP thymocytes, due to a slight increase in the CD4SPII subset and increases in the most abundant subset, the CD4SPV (Fig. 4B). In contrast, LPS exposure did not change CD8SP thymocyte frequency (data not shown). Absolute counts of DP and CD4SP thymocytes were also higher in LPS-exposed fetuses, at both 16h and 48h after exposure (data not shown).
Figure 4. Intra-amniotic LPS alters T-cell development in fetal thymus.
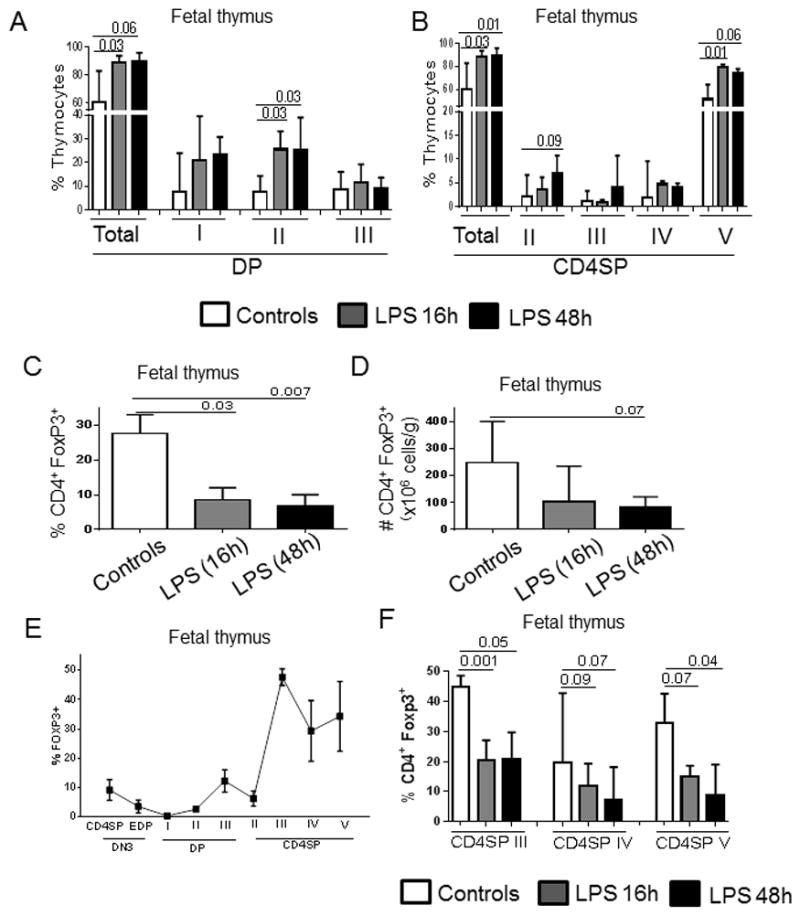
Bars show the median and range: A) the percentage of total double positive (DP) thymocytes and each DP subset (I, II, III); B) the percentage of total CD4 single positive (CD4SP) thymocytes and each CD4SP subset (II, III, IV and V); C) the percentage and D) absolute number of fetal Treg defined as the % of FoxP3+ cells within the total CD4SP thymocytes. E) FoxP3 detection in each stage of thymic development in control fetuses. F) Percentage of fetal Treg defined as the % of FoxP3+ cells within CD4SP III, IV and V stages in control and LPS-exposed fetuses. Control group (n=5) and fetuses exposed to LPS for 16h (n=5) and 48h (n=5) were compared using Mann-Whitney U tests.
To analyze whether LPS-mediated alterations in early and late T-cell development also affected thymic Treg generation, we quantified FoxP3 expression in total CD4SP thymocytes. As shown in Fig. 4C, thymic Treg frequency diminished in both groups of LPS-exposed fetuses compared to that in controls. Similarly, thymic Treg absolute counts decreased by approximately 3-fold 48h post LPS (Fig. 4D). Treg are present in the last three CD4 stages of CD4SP (CD4SP III, IV and V) (Fig. 4E), and LPS exposure decreased their proportion in all these stages (Fig. 4F). Notably, in contrast to splenic CD4+ T-cells, we did not detect RORc expression in any of the thymic CD4SP subsets, either FoxP3+ or FoxP3− (Suppl. Fig 3C, figure from one representative of 4 donors in each group).
LPS-induced fetal immune alterations are partially inhibited by IL-1R blockade
We previously reported that a single IA exposure of IL-1β induced fetal inflammation in Rhesus monkeys (6). We thus explored whether the blockade of IL-1R signaling by recombinant IL-1RA could abolish the effect of LPS exposure on the fetal immune system (36). Based on our previous experiments in the sheep, IL-1RA administered IA reached the fetal mucosal compartment, lung and gastrointestinal tract, but not the fetal or maternal blood compartment (37). In contrast, systemic treatment given to the dam provided adequate blood levels both in the dam and the fetus (38). Therefore, to maximize the blockade within the dam and the fetus, IL-1RA was given both IA and systemically to the pregnant animals (the experimental design is given in Suppl. Fig. 4A–B). Since the immune profile was similar for animals exposed to LPS for 16h or 48h (see Fig 1–3), we combined these two time points in our analyses. Importantly, using a specific ELISA, we could detect human IL-1RA in the cord blood of the 6 treated fetuses (median: 862 ng/ml, range: 113 to 1,477 ng/ml). These doses were in the therapeutic ranges described in either infants with severe auto-inflammatory conditions (39, 40) or infant monkeys (41). As expected based on the effect of IL-1RA in the sheep model of chorioamnionitis (37, 42), IL-1RA significantly decreased neutrophilic recruitment to the decidua (Fig. 5A) as well as the levels of IL-6 and TNF-α in the alveolar wash, compared to LPS-exposed fetuses (Fig. 5B, C). However, levels of IL-8 and GM-CSF in the alveolar washes did not decrease in IL-1RA-treated animals (data not shown).
Figure 5. IA LPS-induced immune alterations were partially inhibited by IL-1 receptor blockage.
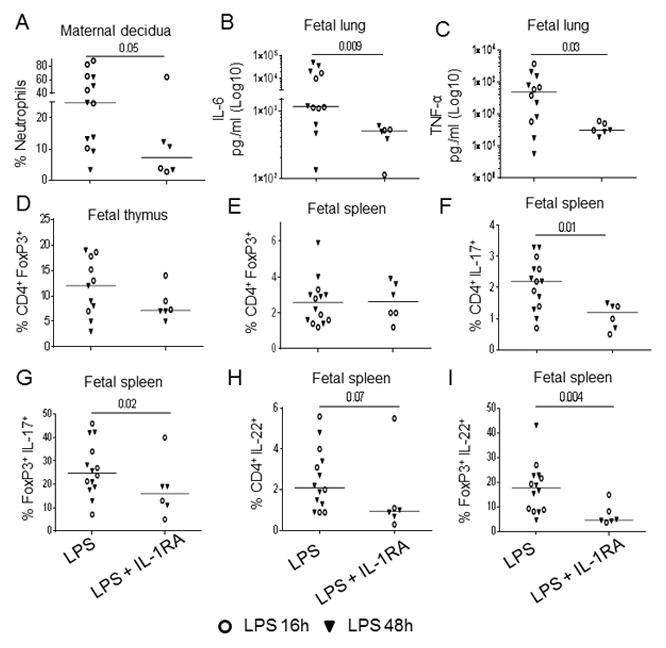
Fetuses were exposed to IA LPS in presence or absence of IL-1RA (given both systemically and intra-amniotically, as displayed in Suppl. Fig. 4). In all figures, each dot represents a single animal, with triangles and circles corresponding to the 16h and 48h time point, respectively. Bars show the median for each analysis, with both time points combined. P values correspond to Mann-Whitney tests. Figures show: the % neutrophils in the decidua (A); and the concentration of IL-6 (B) and TNFα (C) in fetal alveolar washes. (D–E) Dot figures show the frequency of D) thymic Treg (% of FoxP3+ cells within CD4SP thymocytes) and E) splenic Treg (% of FoxP3+ cells within CD3+CD4+ T-cells). F–I) Figures show the frequency in spleen of F) total IL-17+ within CD4+ T-cells, G) IL-17+ within FoxP3+CD4+ T-cells, H) total IL-22+ within CD4+ T-cells, I) IL-22+ within FoxP3+CD4+ T-cells.
In terms of immune alterations, IL-1RA blockade did not abolish the deleterious effect of LPS on Treg frequency in the thymus (Fig. 5D) or spleen (Fig. 5E). It also did not reverse the LPS effect on Treg absolute numbers (data not shown). However, the frequency of IL-17+ and IL-22+ CD4+ T-cells was significantly decreased in IL-1RA-treated animals (Fig 5F and 5H), and thus, the Treg/IL-17 ratios increased after IL-1RA treatment (median: 0.9 vs 2.6, in LPS vs LPS+IL-1RA-treated animals respectively, p= 0.02). Most importantly, the percentage of bifunctional IL-17+FoxP3+ and IL-22+FoxP3+ CD4+ T-cells was significantly decreased in IL-1RA-treated animals (Fig. 5G and 5I).
Discussion
More than 50% of very preterm births are associated with intrauterine inflammation or chorioamnionitis, which is often subclinical (43–45). The progression of inflammation to the fetal compartment is associated with an increased risk for long-term morbidities including bronchopulmonary dysplasia and necrotizing enterocolitis (46–49). Both preterm birth and ascending infections during pregnancy increase the risk of asthma later in infancy and epidemiological studies suggest that maternal infection during pregnancy contributes to the development of neurological diseases including autism, schizophrenia, and cerebral palsy (50–54). In experimental animal models of chorioamnionitis, skin, gut, and lung inflammation, as well as brain abnormalities are also evident in exposed fetuses (rev. in (7)). However, the consequences for the developing immune system of in utero exposure to cytokines and microbial products remain poorly understood. Our goal was to extensively analyze the consequences of LPS-induced chorioamnionitis in fetal rhesus macaques, because the ontogeny of their immune system is very similar to human fetal development.
One of our findings in this model is that Treg frequency and absolute number decreased in both the spleen and PBMC of LPS-exposed fetuses compared with controls. These results confirm our previous findings in fetal lambs and nonhuman primates, in which IA LPS or IL-1α decreased Treg in fetal lymphoid tissues, including spleen, lymph nodes and gut (6, 55). The similar expression of Ki67 in Treg from LPS-exposed fetuses and controls suggests that LPS-inflammation did not decrease Treg frequency through inhibition of cell cycle. We thus explored whether chorioamnionitis altered Treg thymic development. To our knowledge, this is the first detailed study on this topic. Severe alterations were found at several development stages, with a notable increase in the percentages and absolute counts of DP and CD4SP. We did not find gross thymic involution or increased frequency of total thymic CD3+ and CD4+ cells, as previously described (56–59). This discrepancy could be related to differences in timing, as previous studies analyzed the thymus 5–7d post LPS exposure. Importantly, we found a significant reduction of thymic Treg generation, which is in agreement with the diminished expression of thymic FoxP3+ cells in LPS-exposed lambs (56, 58). Of note, we had a more detailed panel than previously used, which allowed for a more granular analysis of subsets, particularly that of thymic Tregs. Our data thus suggest that chorioamnionitis specifically decreases the thymic generation of Tregs, which could be an underlying mechanism for reduced Treg frequency in the periphery.
Characterizing splenic fetal FoxP3+CD4+ T-cells, we found that they are able to express more proinflammatory cytokines (notably IL-17) after short re-stimulation than their FoxP3− counterparts, including in unexposed fetuses. These bifunctional fetal IL-17+ Treg cells shared many phenotypic characteristics of Th17 cells, such as the transcription factor RORc (27, 60) which mediates IL-17 promoter activation (61, 62). Notably, these fetal macaque IL-17+ Treg did not express the Ikaros transcription factor family member, Helios (28), which is interesting because expression of Helios was recently shown in murine models to lock in the Treg phenotype, increasing FoxP3 expression while inhibiting IL-17 production (63). The altered expression of these transcription factors in the inflammatory Treg could thus be associated with their tendency to generate more Th1/Th17-type cytokines. Of note, a similar subset of Treg, e.g. capable of producing proinflammatory cytokines such as IFN-γ and IL-17, had been described in the cord bloods from healthy neonates (27, 30). Fetal CD4+ T-cells also have an increased expression of molecules important for Th17 differentiation and maintenance (such as RORc, STAT3 and IL-23R) (64, 65). These data are also in agreement with the fact that in vitro differentiation into Th17 cells occurs more readily in naive CCR6+ Treg than in CCR6+ conventional T-cells (27), which has been linked to the higher levels of expression of IL-1 and IL-2 receptors by Treg (66, 67). Furthermore, we found that dual-functional IL-17+FoxP3+ cells were the main T-cell subset capable of expressing the potent neutrophil chemoattractant CXCL8 (IL-8) (68). High levels of IL-8 are found in human neonates with infections (69), although the cellular source of this IL-8 has not been identified. Taken together, our data thus strongly suggest that the fetal Treg pool is heterogeneous, with the presence of the conventional Treg and a population of proinflammatory Treg that express transcription factors associated with Th17 and Th1 subsets, and is able to secrete cytokines (IL-2, IFNγ and IL-8) when stimulated. Future single cell analyses of the fetal Treg pool will be needed to better understand the Treg lineage commitment during the fetal development.
Importantly, we also report for the first time that chorioamnionitis significantly increased the proportion of these inflammatory Treg, particularly in the spleen. These IL-17+/RORC+ FoxP3+ T-cells were present in the spleen but not in the thymus, which is in agreement with previous studies in humans (29). Supporting the hypothesis that these cells are generated extra-thymically, splenic IL-17+ Treg did not express Helios, which was originally described as a specific marker of thymic-derived Treg (70). Despite some controversies, Helios remains the best marker to identify thymic Treg in humans and nonhuman primates (rev by (71)). Our results are also reminiscent to what has been described in other inflammatory conditions such as inflammatory bowel disease, colon cancer and rheumatoid arthritis (72–76). The functionality of this bi-functional Treg is still not definitively settled (27). Unfortunately, it is difficult to investigate the suppressive function of Treg in the macaque, in particular to compare the IL-17+ Treg and IL-17− Treg subsets, due to the small size of the fetal organs in these outbred animals and the low frequency of this subset (2–3% of CD4+ T-cells) in the macaque. However, it should be noted that we have previously shown that exposure to severe chorioamnionitis, which resembles our animal model, decreased the suppressive capacity of human cord blood Treg (77). Further studies of human CB in neonates exposed to severe chorioamnionitis will be needed to further link overall Treg functionality with the increased proportion of inflammatory Treg.
Mechanistically, chorioamnionitis, which increases the levels of many of the pro-inflammatory cytokines implicated in the switch of Treg towards a Th17 and a pro-inflammatory phenotype (27–29), could act by promoting the preferential survival of fetal inflammatory Treg, and/or by inducing them. Our data suggest the occurrence of both mechanisms. On one hand, the absolute number of inflammatory Treg, and their overall phenotype, did not change in LPS-exposed fetuses, whereas the total number of Treg decreased, which suggests a preferential survival of these cells in the fetal inflammatory milieu. On the other hand, IL1-RA treatment normalized the frequency of inflammatory Treg in LPS-exposed fetuses, while it did not prevent the deleterious effect of LPS on Treg absolute numbers, which suggests that a better survival of inflammatory Treg cannot completely explain the complex changes occurring in LPS-exposed fetuses. Further studies will be needed to understand the relative contribution of each mechanism to the chorioamnionitis-induced changes of the fetal Treg pool. Our results also implicate IL-1β as a key cytokine for the augmentation of these inflammatory FoxP3+ cells in the context of in utero inflammation. Mechanistically, IL-1β could directly signal Treg, or act through the increased expression of other inflammatory cytokines potentially involved in the induction of a Th17 profile, such as IL-6. Indeed, IL-1β given IA causes a massive increase of many of these cytokines in the amniotic fluid of pregnant ewes, which is decreased by IL-RA treatment (37). Molecular mechanisms underlying the changes in the FoxP3+ cell phenotype by chorioamnionitis have not been elucidated, but they could involve the regulation of hypoxia-inducible factor 1α (HIF-1α), through direct or indirect signaling by IL-1β. Indeed, IL-1β upregulates the expression of HIF-1α in murine CD4+ T-cells, which attenuates Treg development by binding FoxP3 and targeting it for proteasomal degradation, while it enhances Th17 development through direct transcriptional activation of RORc (78). However, as noted above, IL1-RA did not prevent the deleterious effect of LPS on Treg absolute numbers, in agreement with our previous data in the sheep model (79, 80). IL-1RA treatment also did not restore thymic Treg frequency, which suggests that thymic dysregulation, occurring through yet unidentified mechanisms, might represent a key pathway in the overall decreased Treg frequency in the periphery.
We also determined the effect of LPS IA challenge on the decidua, which is of maternal origin. In contrast to fetal tissues, Treg frequencies and absolute numbers in the decidua were not altered in LPS-exposed dams. These data confirm our previous findings in IA IL-1β-challenged animals (19). Similarly, a previous study of endotoxin-induced preterm birth in mice showed a reduction of Treg in the uterine tissues, but no changes in the decidua (81). Of note, a recent study reported that Treg are poorly suppressive in women who give birth prematurely (82). There could therefore be a decrease in decidua Treg functionality in the context of inflammation, which should be analyzed in future studies. In contrast to the unchanged Treg frequency, we found that the frequency of IL-17+ CD4+ T-cells significantly increased on the maternal side, but interestingly, these maternal IL-17+ T-cells were mainly FoxP3−. This result in line with the hypothesis that the Treg present in tissues of adult animals have a lower intrinsic plasticity than neonatal Tregs, because they are mainly memory cells (64).
Taken together, our results suggest that fetal inflammation during chorioamnionitis leads to inadequate Treg generation in the fetal thymus, as well as a switch of the remaining splenic Tregs towards a proinflammatory phenotype. Both mechanisms likely contribute to the FIRS, which develops in the context of severe chorioamnionitis. Manipulating fetal Treg number and/or function may thus prove useful to prevent the inflammatory sequelae in chorioamnionitis-exposed neonates. IL-1RA could be useful, as it decreased the frequency of inflammatory Tregs, but additional therapeutic strategies might be needed, as IL-1RA did not prevent fetal Treg depletion. Of interest, prophylactic IL-2 treatment in fetal sheep prevented intestinal inflammation and increased intestinal Treg frequency (83). Future experiments will thus be required to evaluate whether the combination of IL-2 and IL-1RA might completely block the deleterious effect of chorioamnionitis on the fetal immune system.
Supplementary Material
Acknowledgments
This work was partially supported by a Burroughs-Wellcome grant (to C.A.C.) and March of Dimes grant- Innovation catalyst grant (to S.G.K.), as well as funds from the CCHMC Perinatal Institute. The Eunice Kennedy Shriver National Institute of Child Health and Human Development diversity supplement (ROIHD078127) supported C.M.J. The Center for Excellence in Molecular Hematology Grant 1P30DK090971-01 and the Digestive Health Center Grant AR47363 partially supported Luminex and flow cytometry analyses. The California National Primate Research Center is supported by the NIH Office of Infrastructure Programs Grant P51OD011107.
The authors want to thank the CNPRC staff for its outstanding technical support, and most especially Mrs. Sarah Davis and Sona Santos for their invaluable help in animal management and autopsy. We also want to thank Manuel Alvarez and Paranthaman Senthamaraikannan for excellent technical support.
Footnotes
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Gravett MG, Rubens CE P Global Alliance to Prevent and T. Stillbirth Technical. A framework for strategic investments in research to reduce the global burden of preterm birth. Am J Obstet Gynecol. 2012;207:368–373. doi: 10.1016/j.ajog.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Erdemir G, Kultursay N, Calkavur S, Zekioglu O, Koroglu OA, Cakmak B, Yalaz M, Akisu M, Sagol S. Histological chorioamnionitis: effects on premature delivery and neonatal prognosis. Pediatr Neonatol. 2013;54:267–274. doi: 10.1016/j.pedneo.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Romero R, Grivel JC, Tarca AL, Chaemsaithong P, Xu Z, Fitzgerald W, Hassan SS, Chaiworapongsa T, Margolis L. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol. 2015 doi: 10.1016/j.ajog.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 5.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 6.Kallapur SG, Presicce P, Senthamaraikannan P, Alvarez M, Tarantal AF, Miller LM, Jobe AH, Chougnet CA. Intra-Amniotic IL-1beta Induces Fetal Inflammation in Rhesus Monkeys and Alters the Regulatory T Cell/IL-17 Balance. J Immunol. 2013;191:1102–1109. doi: 10.4049/jimmunol.1300270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kallapur SG, Presicce P, Rueda CM, Jobe AH, Chougnet CA. Fetal immune response to chorioamnionitis. Semin Reprod Med. 2014;32:56–67. doi: 10.1055/s-0033-1361823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strunk T, Doherty D, Jacques A, Simmer K, Richmond P, Kohan R, Charles A, Burgner D. Histologic chorioamnionitis is associated with reduced risk of late-onset sepsis in preterm infants. Pediatrics. 2012;129:e134–141. doi: 10.1542/peds.2010-3493. [DOI] [PubMed] [Google Scholar]
- 9.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65:S194–202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 10.De Felice C, Latini G, Del Vecchio A, Toti P, Bagnoli F, Petraglia F. Small thymus at birth: a predictive radiographic sign of bronchopulmonary dysplasia. Pediatrics. 2002;110:386–388. doi: 10.1542/peds.110.2.386. [DOI] [PubMed] [Google Scholar]
- 11.De Felice C, Toti P, Santopietro R, Stumpo M, Pecciarini L, Bagnoli F. Small thymus in very low birth weight infants born to mothers with subclinical chorioamnionitis. J Pediatr. 1999;135:384–386. doi: 10.1016/s0022-3476(99)70140-x. [DOI] [PubMed] [Google Scholar]
- 12.Toti P, De Felice C, Stumpo M, Schurfeld K, Di Leo L, Vatti R, Bianciardi G, Buonocore G, Seemayer TA, Luzi P. Acute thymic involution in fetuses and neonates with chorioamnionitis. Hum Pathol. 2000;31:1121–1128. doi: 10.1053/hupa.2000.16676. [DOI] [PubMed] [Google Scholar]
- 13.Di Naro E, Cromi A, Ghezzi F, Raio L, Uccella S, D’Addario V, Loverro G. Fetal thymic involution: a sonographic marker of the fetal inflammatory response syndrome. Am J Obstet Gynecol. 2006;194:153–159. doi: 10.1016/j.ajog.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 14.El-Haieg DO, Zidan AA, El-Nemr MM. The relationship between sonographic fetal thymus size and the components of the systemic fetal inflammatory response syndrome in women with preterm prelabour rupture of membranes. BJOG. 2008;115:836–841. doi: 10.1111/j.1471-0528.2008.01715.x. [DOI] [PubMed] [Google Scholar]
- 15.Yinon Y, Zalel Y, Weisz B, Mazaki-Tovi S, Sivan E, Schiff E, Achiron R. Fetal thymus size as a predictor of chorioamnionitis in women with preterm premature rupture of membranes. Ultrasound Obstet Gynecol. 2007;29:639–643. doi: 10.1002/uog.4022. [DOI] [PubMed] [Google Scholar]
- 16.Jeppesen DL. The size of the thymus: an important immunological diagnostic tool? Acta Paediatr. 2003;92:994–996. [PubMed] [Google Scholar]
- 17.Gravett MG, Haluska GJ, Cook MJ, Novy MJ. Fetal and maternal endocrine responses to experimental intrauterine infection in rhesus monkeys. Am J Obstet Gynecol. 1996;174:1725–1731. doi: 10.1016/s0002-9378(96)70203-x. discussion 1731–1723. [DOI] [PubMed] [Google Scholar]
- 18.Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195:1578–1589. doi: 10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 19.Presicce P, Senthamaraikannan P, Alvarez M, Rueda CM, Cappelletti M, Miller LA, Jobe AH, Chougnet CA, Kallapur SG. Neutrophil recruitment and activation in decidua with intra-amniotic IL-1beta in the preterm rhesus macaque. Biol Reprod. 2015;92:56. doi: 10.1095/biolreprod.114.124420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rendon JL, Choudhry MA. Th17 cells: critical mediators of host responses to burn injury and sepsis. J Leukoc Biol. 2012;92:529–538. doi: 10.1189/jlb.0212083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubino SJ, Geddes K, Girardin SE. Innate IL-17 and IL-22 responses to enteric bacterial pathogens. Trends Immunol. 2012;33:112–118. doi: 10.1016/j.it.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 22.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 23.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 24.Chen Q, Kim YC, Laurence A, Punkosdy GA, Shevach EM. IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+ T cells in vivo. J Immunol. 2011;186:6329–6337. doi: 10.4049/jimmunol.1100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hori S. Lineage stability and phenotypic plasticity of Foxp3(+) regulatory T cells. Immunol Rev. 2014;259:159–172. doi: 10.1111/imr.12175. [DOI] [PubMed] [Google Scholar]
- 27.Valmori D, Raffin C, Raimbaud I, Ayyoub M. Human RORgammat+ TH17 cells preferentially differentiate from naive FOXP3+Treg in the presence of lineage-specific polarizing factors. Proc Natl Acad Sci U S A. 2010;107:19402–19407. doi: 10.1073/pnas.1008247107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mercer F, Khaitan A, Kozhaya L, Aberg JA, Unutmaz D. Differentiation of IL-17-producing effector and regulatory human T cells from lineage-committed naive precursors. J Immunol. 2014;193:1047–1054. doi: 10.4049/jimmunol.1302936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, Bover L, Hanabuchi S, Khalili J, Marinova E, Zheng B, Littman DR, Liu YJ. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pesenacker AM, Bending D, Ursu S, Wu Q, Nistala K, Wedderburn LR. CD161 defines the subset of FoxP3+ T cells capable of producing proinflammatory cytokines. Blood. 2013;121:2647–2658. doi: 10.1182/blood-2012-08-443473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keelan JA, Wong PM, Bird PS, Mitchell MD. Innate inflammatory responses of human decidual cells to periodontopathic bacteria. Am J Obstet Gynecol. 2010;202:471 e471–411. doi: 10.1016/j.ajog.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 32.Spits H. Development of alphabeta T cells in the human thymus. Nat Rev Immunol. 2002;2:760–772. doi: 10.1038/nri913. [DOI] [PubMed] [Google Scholar]
- 33.Plum J, De Smedt M, Leclercq G, Taghon T, Kerre T, Vandekerckhove B. Human intrathymic development: a selective approach. Semin Immunopathol. 2008;30:411–423. doi: 10.1007/s00281-008-0135-2. [DOI] [PubMed] [Google Scholar]
- 34.Vanhecke D, Verhasselt B, De Smedt M, Leclercq G, Plum J, Vandekerckhove B. Human thymocytes become lineage committed at an early postselection CD69+ stage, before the onset of functional maturation. J Immunol. 1997;159:5973–5983. [PubMed] [Google Scholar]
- 35.Vanhecke D, Verhasselt B, De Smedt M, De Paepe B, Leclercq G, Plum J, Vandekerckhove B. MHC class II molecules are required for initiation of positive selection but not during terminal differentiation of human CD4 single positive thymocytes. J Immunol. 1997;158:3730–3737. [PubMed] [Google Scholar]
- 36.Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol. 1992;167:1041–1045. doi: 10.1016/s0002-9378(12)80035-4. [DOI] [PubMed] [Google Scholar]
- 37.Kallapur SG, Nitsos I, Moss TJ, Polglase GR, Pillow JJ, Cheah FC, Kramer BW, Newnham JP, Ikegami M, Jobe AH. IL-1 mediates pulmonary and systemic inflammatory responses to chorioamnionitis induced by lipopolysaccharide. Am J Respir Crit Care Med. 2009;179:955–961. doi: 10.1164/rccm.200811-1728OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nadeau-Vallee M, Quiniou C, Palacios J, Hou X, Erfani A, Madaan A, Sanchez M, Leimert K, Boudreault A, Duhamel F, Rivera JC, Zhu T, Noueihed B, Robertson SA, Ni X, Olson DM, Lubell W, Girard S, Chemtob S. Novel Noncompetitive IL-1 Receptor-Biased Ligand Prevents Infection- and Inflammation-Induced Preterm Birth. J Immunol. 2015;195:3402–3415. doi: 10.4049/jimmunol.1500758. [DOI] [PubMed] [Google Scholar]
- 39.Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, Laxer R, Tedgard U, Cowen EW, Pham TH, Booty M, Estes JD, Sandler NG, Plass N, Stone DL, Turner ML, Hill S, Butman JA, Schneider R, Babyn P, El-Shanti HI, Pope E, Barron K, Bing X, Laurence A, Lee CC, Chapelle D, Clarke GI, Ohson K, Nicholson M, Gadina M, Yang B, Korman BD, Gregersen PK, van Hagen PM, Hak AE, Huizing M, Rahman P, Douek DC, Remmers EF, Kastner DL, Goldbach-Mansky R. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360:2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, Kim HJ, Brewer C, Zalewski C, Wiggs E, Hill S, Turner ML, Karp BI, Aksentijevich I, Pucino F, Penzak SR, Haverkamp MH, Stein L, Adams BS, Moore TL, Fuhlbrigge RC, Shaham B, Jarvis JN, O’Neil K, Vehe RK, Beitz LO, Gardner G, Hannan WP, Warren RW, Horn W, Cole JL, Paul SM, Hawkins PN, Pham TH, Snyder C, Wesley RA, Hoffmann SC, Holland SM, Butman JA, Kastner DL. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355:581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fox E, Jayaprakash N, Pham TH, Rowley A, McCully CL, Pucino F, Goldbach-Mansky R. The serum and cerebrospinal fluid pharmacokinetics of anakinra after intravenous administration to non-human primates. J Neuroimmunol. 2010;223:138–140. doi: 10.1016/j.jneuroim.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baergen R, Benirschke K, Ulich TR. Cytokine expression in the placenta. The role of interleukin 1 and interleukin 1 receptor antagonist expression in chorioamnionitis and parturition. Arch Pathol Lab Med. 1994;118:52–55. [PubMed] [Google Scholar]
- 43.Lahra MM, Jeffery HE. A fetal response to chorioamnionitis is associated with early survival after preterm birth. Am J Obstet Gynecol. 2004;190:147–151. doi: 10.1016/j.ajog.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 44.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sanchez PJ, O’Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID, 3rd, Watterberg KL, Saha S, Das A, Higgins RD H Eunice Kennedy Shriver National Institute of Child and N. Human Development Neonatal Research. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dammann O, Allred EN, Leviton A, Shen-Schwarz S, Heller D, Genest DR, Collins MH. Fetal vasculitis in preterm newborns: interrelationships, modifiers, and antecedents. Placenta. 2004;25:788–796. doi: 10.1016/j.placenta.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Ambalavanan N, Carlo WA, D’Angio CT, McDonald SA, Das A, Schendel D, Thorsen P, Higgins RD. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics. 2009;123:1132–1141. doi: 10.1542/peds.2008-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, Kim BI. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1997;177:825–830. doi: 10.1016/s0002-9378(97)70276-x. [DOI] [PubMed] [Google Scholar]
- 48.Bhatia AM, Stoll BJ, Cismowski MJ, Hamrick SE. Cytokine levels in the preterm infant with neonatal intestinal injury. Am J Perinatol. 2014;31:489–496. doi: 10.1055/s-0033-1353437. [DOI] [PubMed] [Google Scholar]
- 49.Benkoe T, Baumann S, Weninger M, Pones M, Reck C, Rebhandl W, Oehler R. Comprehensive evaluation of 11 cytokines in premature infants with surgical necrotizing enterocolitis. PLoS One. 2013;8:e58720. doi: 10.1371/journal.pone.0058720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Getahun D, Strickland D, Zeiger RS, Fassett MJ, Chen W, Rhoads GG, Jacobsen SJ. Effect of chorioamnionitis on early childhood asthma. Arch Pediatr Adolesc Med. 2010;164:187–192. doi: 10.1001/archpediatrics.2009.238. [DOI] [PubMed] [Google Scholar]
- 51.Lee SM, Park JW, Kim BJ, Park CW, Park JS, Jun JK, Yoon BH. Acute histologic chorioamnionitis is a risk factor for adverse neonatal outcome in late preterm birth after preterm premature rupture of membranes. PLoS One. 2013;8:e79941. doi: 10.1371/journal.pone.0079941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Armstrong-Wells J, Donnelly M, Post MD, Manco-Johnson MJ, Winn VD, Sebire G. Inflammatory predictors of neurologic disability after preterm premature rupture of membranes. Am J Obstet Gynecol. 2015;212:212 e211–219. doi: 10.1016/j.ajog.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Limperopoulos C, Bassan H, Sullivan NR, Soul JS, Robertson RL, Jr, Moore M, Ringer SA, Volpe JJ, du Plessis AJ. Positive screening for autism in ex-preterm infants: prevalence and risk factors. Pediatrics. 2008;121:758–765. doi: 10.1542/peds.2007-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR. The maternal interleukin-17a pathway in mice promotes autismlike phenotypes in offspring. Science. 2016 doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolfs TG, Kallapur SG, Polglase GR, Pillow JJ, Nitsos I, Newnham JP, Chougnet CA, Kroon E, Spierings J, Willems CH, Jobe AH, Kramer BW. IL-1alpha mediated chorioamnionitis induces depletion of FoxP3+ cells and ileal inflammation in the ovine fetal gut. PLoS One. 2011;6:e18355. doi: 10.1371/journal.pone.0018355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kunzmann S, Glogger K, Been JV, Kallapur SG, Nitsos I, Moss TJ, Speer CP, Newnham JP, Jobe AH, Kramer BW. Thymic changes after chorioamnionitis induced by intraamniotic lipopolysaccharide in fetal sheep. Am J Obstet Gynecol. 2010;202:476 e471–479. doi: 10.1016/j.ajog.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee AJ, V, Lambermont A, Pillow JJ, Polglase GR, Nitsos I, Newnham JP, Beilharz MW, Kallapur SG, Jobe AH, Kramer BW. Fetal responses to lipopolysaccharide-induced chorioamnionitis alter immune and airway responses in 7-week-old sheep. Am J Obstet Gynecol. 2011;204:364 e317–324. doi: 10.1016/j.ajog.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 58.Kuypers E, Collins JJ, Jellema RK, Wolfs TG, Kemp MW, Nitsos I, Pillow JJ, Polglase GR, Newnham JP, Germeraad WT, Kallapur SG, Jobe AH, Kramer BW. Ovine fetal thymus response to lipopolysaccharide-induced chorioamnionitis and antenatal corticosteroids. PLoS One. 2012;7:e38257. doi: 10.1371/journal.pone.0038257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuypers E, Wolfs TG, Collins JJ, Jellema RK, Newnham JP, Kemp MW, Kallapur SG, Jobe AH, Kramer BW. Intraamniotic Lipopolysaccharide Exposure Changes Cell Populations and Structure of the Ovine Fetal Thymus. Reprod Sci. 2013;20:946–956. doi: 10.1177/1933719112472742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ayyoub M, Deknuydt F, Raimbaud I, Dousset C, Leveque L, Bioley G, Valmori D. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci U S A. 2009;106:8635–8640. doi: 10.1073/pnas.0900621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ichiyama K, Yoshida H, Wakabayashi Y, Chinen T, Saeki K, Nakaya M, Takaesu G, Hori S, Yoshimura A, Kobayashi T. Foxp3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. J Biol Chem. 2008;283:17003–17008. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- 62.Ruan Q, Kameswaran V, Zhang Y, Zheng S, Sun J, Wang J, DeVirgiliis J, Liou HC, Beg AA, Chen YH. The Th17 immune response is controlled by the Rel-RORgamma-RORgamma T transcriptional axis. J Exp Med. 2011;208:2321–2333. doi: 10.1084/jem.20110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim HJ, Barnitz RA, Kreslavsky T, Brown FD, Moffett H, Lemieux ME, Kaygusuz Y, Meissner T, Holderried TA, Chan S, Kastner P, Haining WN, Cantor H. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science. 2015;350:334–339. doi: 10.1126/science.aad0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Black A, Bhaumik S, Kirkman RL, Weaver CT, Randolph DA. Developmental regulation of Th17-cell capacity in human neonates. Eur J Immunol. 2012;42:311–319. doi: 10.1002/eji.201141847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang D, Sun YY, Bhaumik SK, Li Y, Baumann JM, Lin X, Zhang Y, Lin SH, Dunn RS, Liu CY, Shie FS, Lee YH, Wills-Karp M, Chougnet CA, Kallapur SG, Lewkowich IP, Lindquist DM, Murali-Krishna K, Kuan CY. Blocking lymphocyte trafficking with FTY720 prevents inflammation-sensitized hypoxic-ischemic brain injury in newborns. J Neurosci. 2014;34:16467–16481. doi: 10.1523/JNEUROSCI.2582-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tran DQ, Andersson J, Hardwick D, Bebris L, Illei GG, Shevach EM. Selective expression of latency-associated peptide (LAP) and IL-1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood. 2009;113:5125–5133. doi: 10.1182/blood-2009-01-199950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bonacci B, Edwards B, Jia S, Williams CB, Hessner MJ, Gauld SB, Verbsky JW. Requirements for growth and IL-10 expression of highly purified human T regulatory cells. J Clin Immunol. 2012;32:1118–1128. doi: 10.1007/s10875-012-9701-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Himmel ME, Crome SQ, Ivison S, Piccirillo C, Steiner TS, Levings MK. Human CD4+ FOXP3+ regulatory T cells produce CXCL8 and recruit neutrophils. Eur J Immunol. 2011;41:306–312. doi: 10.1002/eji.201040459. [DOI] [PubMed] [Google Scholar]
- 69.Gibbons D, Fleming P, Virasami A, Michel ML, Sebire NJ, Costeloe K, Carr R, Klein N, Hayday A. Interleukin-8 (CXCL8) production is a signatory T cell effector function of human newborn infants. Nat Med. 2014;20:1206–1210. doi: 10.1038/nm.3670. [DOI] [PubMed] [Google Scholar]
- 70.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yadav M, Stephan S, Bluestone JA. Peripherally induced tregs - role in immune homeostasis and autoimmunity. Front Immunol. 2013;4:232. doi: 10.3389/fimmu.2013.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ueno A, Jijon H, Chan R, Ford K, Hirota C, Kaplan GG, Beck PL, Iacucci M, Fort Gasia M, Barkema HW, Panaccione R, Ghosh S. Increased prevalence of circulating novel IL-17 secreting Foxp3 expressing CD4+ T cells and defective suppressive function of circulating Foxp3+ regulatory cells support plasticity between Th17 and regulatory T cells in inflammatory bowel disease patients. Inflamm Bowel Dis. 2013;19:2522–2534. doi: 10.1097/MIB.0b013e3182a85709. [DOI] [PubMed] [Google Scholar]
- 73.Wang T, Sun X, Zhao J, Zhang J, Zhu H, Li C, Gao N, Jia Y, Xu D, Huang FP, Li N, Lu L, Li ZG. Regulatory T cells in rheumatoid arthritis showed increased plasticity toward Th17 but retained suppressive function in peripheral blood. Ann Rheum Dis. 2015;74:1293–1301. doi: 10.1136/annrheumdis-2013-204228. [DOI] [PubMed] [Google Scholar]
- 74.Kryczek I, Wu K, Zhao E, Wei S, Vatan L, Szeliga W, Huang E, Greenson J, Chang A, Rolinski J, Radwan P, Fang J, Wang G, Zou W. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol. 2011;186:4388–4395. doi: 10.4049/jimmunol.1003251. [DOI] [PubMed] [Google Scholar]
- 75.Blatner NR, Mulcahy MF, Dennis KL, Scholtens D, Bentrem DJ, Phillips JD, Ham S, Sandall BP, Khan MW, Mahvi DM, Halverson AL, Stryker SJ, Boller AM, Singal A, Sneed RK, Sarraj B, Ansari MJ, Oft M, Iwakura Y, Zhou L, Bonertz A, Beckhove P, Gounari F, Khazaie K. Expression of RORgammat marks a pathogenic regulatory T cell subset in human colon cancer. Sci Transl Med. 2012;4:164ra159. doi: 10.1126/scitranslmed.3004566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hovhannisyan Z, Treatman J, Littman DR, Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140:957–965. doi: 10.1053/j.gastro.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rueda CM, Wells CB, Gisslen T, Jobe AH, Kallapur SG, Chougnet CA. Effect of chorioamnionitis on regulatory T cells in moderate/late preterm neonates. Hum Immunol. 2015;76:65–73. doi: 10.1016/j.humimm.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wolfs TG, Kallapur SG, Knox CL, Thuijls G, Nitsos I, Polglase GR, Collins JJ, Kroon E, Spierings J, Shroyer NF, Newnham JP, Jobe AH, Kramer BW. Antenatal ureaplasma infection impairs development of the fetal ovine gut in an IL-1-dependent manner. Mucosal Immunol. 2013;6:547–556. doi: 10.1038/mi.2012.97. [DOI] [PubMed] [Google Scholar]
- 80.Shah TA, Hillman NH, Nitsos I, Polglase GR, Pillow JJ, Newnham JP, Jobe AH, Kallapur SG. Pulmonary and systemic expression of monocyte chemotactic proteins in preterm sheep fetuses exposed to lipopolysaccharide-induced chorioamnionitis. Pediatr Res. 2010;68:210–215. doi: 10.1203/PDR.0b013e3181e9c556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arenas-Hernandez M, Romero R, St Louis D, Hassan SS, Kaye EB, Gomez-Lopez N. An imbalance between innate and adaptive immune cells at the maternal-fetal interface occurs prior to endotoxin-induced preterm birth. Cell Mol Immunol. 2015 doi: 10.1038/cmi.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schober L, Radnai D, Schmitt E, Mahnke K, Sohn C, Steinborn A. Term and preterm labor: decreased suppressive activity and changes in composition of the regulatory T-cell pool. Immunol Cell Biol. 2012;90:935–944. doi: 10.1038/icb.2012.33. [DOI] [PubMed] [Google Scholar]
- 83.Nikiforou M, Vanderlocht J, Chougnet CA, Jellema RK, Ophelders DR, Joosten M, Kloosterboer N, Senden-Gijsbers BL, Germeraad WT, Kramer BW, Wolfs TG. Prophylactic Interleukin-2 Treatment Prevents Fetal Gut Inflammation and Injury in an Ovine Model of Chorioamnionitis. Inflamm Bowel Dis. 2015;21:2026–2038. doi: 10.1097/MIB.0000000000000455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


