Abstract
Intact, inactivated Streptococcus pneumoniae (Pn) [including the unencapsulated strain, R36A], markedly inhibits the humoral immune response to co-immunized heterologous proteins, a property not observed with several other intact Gram-positive or Gram-negative bacteria. In this study, we determined the nature of this immunosuppressive property. Since phosphorylcholine (PC), a major haptenic component of teichoic acid in the Pn cell wall, and lipoteichoic acid in the Pn membrane, was previously reported to be immunosuppresive when derived from filarial parasites, we determined whether R36A lacking PC (R36Apc-) was inhibitory. Indeed, although R36Apc- exhibited a markedly reduced level of inhibition of the IgG response to co-immunized cOVA, no inhibition was observed when using several other distinct PC-expressing bacteria or a soluble, protein-PC conjugate. Further, treatment of R36A with periodate, which selectively destroys PC residues, had no effect on R36A-mediated inhibition. Since R36Apc- also lacks choline-binding proteins (CBPs), that require PC for cell wall attachment, and since treatment of R36A with trypsin eliminated its inhibitory activity, we incubated R36A in choline chloride, which selectively strips CBPs from its surface. R36A lacking CBPs lost most of its inhibitory property, whereas the supernatant of choline chloride-treated R36A, containing CBPs, was markedly inhibitory. Co-immunization studies using cOVA and various Pn mutants, each genetically deficient in one of the CBPs, demonstrated that only Pn lacking the CBP, pneumococcal surface protein A (PspA), lost its ability to inhibit the IgG anti-cOVA response. These results strongly suggest that PspA plays a major role in mediating the immunosuppressive property of Pn.
INTRODUCTION
Pathogens have evolved numerous strategies for subverting immune-mediated control or clearance, through effects on both the innate and adaptive immune system (1, 2). For example, bacterial pathogens can alter downstream signaling by pattern recognition receptors (PRRs) (3), including a switch from production of pro-inflammatory cytokines to the production of IL-10, an anti-inflammatory, immunosuppressive cytokine (4). Bacteria can also promote immune deviation, resulting in a switch from Th1 or Th17 responses, which are host-protective, to a more Th2 phenotype, which allows microbial persistence (5). They also can express molecules that directly suppress T cell activation and proliferation (6, 7) and superantigens that alter T cell responses (8). At times, pathogens mimic the host's immune modulators to alter the immune response in their favor (9).
Pathogens may also interfere with immune responses to other antigenic challenges, including antibody responses to soluble, heterologous proteins. Thus, infection with the bacterium Salmonella typhimurium can delay the formation of germinal centers (GC) induced by haptenated proteins (10), whereas the bacterium Ehrlichia muris can inhibit NP-specific IgG responses to co-administered NP-chicken γ-globulin, by inhibiting the GC response (11). The protozoan Trypanosoma cruzi can induce suppressor T cells that inhibit trinitrophenol (TNP)-specific IgG responses to soluble TNP-conjugated proteins (12), whereas the protozoan Plasmodium chabaudi (13), as well as the foot-and-mouth disease virus (14) can suppress OVA-specific IgG responses to soluble OVA, associated with an inhibition of DC maturation and a resultant decrease in T cell stimulatory capacity.
In this regard, we previously reported an apparently novel mode of immunosuppression mediated by intact, inactivated Streptococcus pneumoniae (Pn). We observed that Pn strongly suppressed the IgG response to co-immunized, heteroglogous proteins, including chicken ovalbumin (cOVA) (15, 16). Specifically, the inhibition of induction of serum cOVA-specific IgG, in response to i.v. administered cOVA was associated with a marked reduction in the generation of specific CD4+ GC T follicular helper cells (Tfh) and GC B cells in the spleen, and antibody-secreting cells (ASC) in spleen and bone marrow, with no change in the percentages of T regulatory cells and only modest changes in early T cell proliferation (16). We further demonstrated that this inhibitory property was contained within the Pn cell wall. However, the identity of the relevant cell wall structure was not determined. Of note, the inhibitory effect of Pn appeared to be Pn-specific, in that neither intact, inactivated Streptococcus agalactiae nor Neissesria meningitis, capsular type C had any effect on the IgG anti-cOVA response (16).
Pn expresses a hapten, phosphorycholine (PC), which is covalently linked to its cell wall teichoic acid and membrane lipoteichoic acid, and which was absent from the particular strain of S. agalactiae or N. meningitidis used in our previous study. Previous reports demonstrated that PC, expressed on a secreted glycoprotein (ES-62), from the filarial nematode Acanthocheilonmea viteae, had immunosuppressive properties. In particular, it exhibited an anti-inflammatory property manifested as a downregulation of Th1-mediated autoimmunity and Th2-mediated hypersensitivity (17, 18). Proposed mechanisms of ES-62-mediated inhibition included desensitization of mast cell degranulation and induction of IL-10 from B-1 B cells, induction of an anti-inflammatory phenotype in macrophages and DC, inhibition of TCR- and BCR-mediated activation of T and B cells and induction of regulatory T cells (17, 18). In this regard, we began the current study to determine whether PC was responsible for the Pn-mediated immunosuppressive activity observed in our previous studies. Interestingly, although Pn lacking PC lost much, if not all, of its immunosuppressive activity, Pn-mediated inhibition was not due to a direct effect of PC, but to a PC-binding protein, pneumococcal surface protein A (PspA), that is shed from the Pn surface in the absence of PC expression.
MATERIALS AND METHODS
Mice
BALB/c mice were purchased from the National Cancer Institute (Frederick, MD). Homozygous DO11.10 mice × RAG-2−/− mice (BALB/c background) [from here on referred to as “DO11.10 mice”], in which all CD4+ T cells express a transgenic T cell receptor that encodes for a chicken cOVA peptide (amino acids 323-339), presented by MHC-IId, were purchased from Taconic Farms (Hudson, NY). For studies using NP-cOVA, BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and subsequently bred and maintained within the Biological Resource Center at National Jewish Health (NJH, Denver, CO). Mice were used between 7-12 weeks of age. These studies were conducted in accordance with the principles set forth in the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, revised 1996), and were approved by the Uniformed Services University of the Health Sciences and National Jewish Health Institutional Animal Care and Use Committees.
Reagents
cOVA (“Imject OVA”) was purchased from Thermo Scientific (Rockford, IL). (NP)19-OVA, (NP)26-BSA and PC-BSA were obtained from Biosearch Technologies (Novato, CA). Alum (Allhydrogel 2%) was obtained from Brenntag Biosector (Denmark). Indomethacin was obtained from Sigma (St. Louis, MO). PC covalently linked to keyhole limpet hemocyanin (KLH) and truncated (first 302 amino acids) recombinant PspA was made as previously described (19).
Bacterial strains
The unencapsulated mutant of D39 (S. pneumoniae, capsular type 2), [strain R36A]) was obtained from Dr. David Briles (University of Alabama at Birmingham, Birmingham, AL). S. pneumoniae strain T4R and the CBP mutants, were produced as previously described (20). Bacteria from frozen stocks were subcultured on BBL blood agar plates (VWR International, Bridgeport, NJ). Isolated colonies on blood agar were grown in Todd Hewitt Broth (BD Biosciences, San Jose, CA) to mid-log phase, collected, and heat-killed by incubation at 65°C for 2 h. Sterility was confirmed by subculture on blood agar plates. The concentration, in CFU/ml equivalents of heat-killed bacteria was estimated by measuring OD at 600nm after prior establishment of the relationship between actual CFU/ml and OD600nm. Bacteria were then aliquoted at 1010 CFU/ml in PBS and frozen at −20°C. The unencapsulated Neisseria meningitidis strain C311, was obtained from Dr. Michael Jennings (Griffith University, Southport, Queensland, Australia). Haemophilus influenzae type B strain Rd, was obtained from Dr. Jeff Weiser (University of Pennsylvania, PA). Erysipelothrix rhusiopathiae strain Fujisawa, was obtained from Yoshihiro Shimoji (National Institute of Animal Health, Tsukuba, Ibaraki, Japan).
Preparation of R36A lacking PC
PC-depleted R36A (R36Apc-) was prepared by growing R36A in a chemically defined medium (CDM) (21) and slowly adapting it to a very low choline chloride concentration (0.000001%), by gradually replacing choline chloride in the medium with ethanolamine (22). As a control, wild type (WT) R36A grown in CDM was also used for immunizations.
Depletion of CBPs from R36A and preparation of CBP-containing supernatant
R36A depleted of CBPs [R36Acbp-] was prepared by treating live R36A with 2% choline chloride (Sigma-Aldrich, St. Louis, MO) solution (23) prior to heat-inactivation. The resulting supernatant of choline chloride-treated R36A (R36Acbp- sup.) was concentrated and dialyzed using an Amicon Ultra-15 filter unit, 10,000 MWCO (Millipore Corp., Bedford, MA) and used for immunizations.
Treatment of R36A with trypsin
For trypsin treatment of R36A [R36A (trypsin)], 107 CFU heat killed R36A cells were incubated for 15min at 37°C with 100μg of trypsin (Merck Millipore, Billerica, MA) followed by incubation with 100μg of trypsin inhibitor, AEBSF (Merck Millipore, Billerica, MA). Cells were then washed 3 times with PBS and used for immunizations (24).
Destruction of PC on R36A by oxidation with periodate
Heat-killed R36A cells were washed with PBS followed by one wash in 100mM sodium acetate buffer, pH 5 (SAB). The bacterial pellet was then resuspended in 20mM sodium periodate in 100mM SAB at a concentration of 109 CFU R36A per ml. Bacteria were incubated in the dark at 4°C with mixing, for about 1h. Oxidation was blocked by incubating bacteria with glycerol for 30min at 4°C, followed by a wash at 4,000 rpm for 20 min. Bacteria were then washed twice with PBS and used for immunizations (25).
Sonication of R36A
Heat-killed R36Apc- was sonicated in an ultrasonic bath (VWR International), to break the chains (formed while grown in CDM), at a concentration of 109 CFU/ml for 15 minutes (operating frequency 35kHz). For a control, WT R36A was also sonicated similarly. Disruption of chains of bacteria was confirmed by microscopy.
SDS PAGE
2μl of R36Acbp- supernatant (equivalent to 6×107 CFU R36A) or 1×107 WT or trypsin-treated R36A, was mixed with NuPAGE sample reducing agent (10x) and NuPage LDS sample buffer (4x) (Life Technologies, Carlsbad, CA) to a final concentration of 1x in 25ul total sample volume. Samples were run on 4-12% gradient Bis-Tris precast gel in NuPAGE MOPS SDS running buffer (Life technologies), at 195V for 50min. The gel was then stained with Denville Blue™ protein stain (Denville Scientific Inc.).
Immunizations
Mice were immunized i.v. with 50 μg of cOVA or NP19-OVA adsorbed on 13μg of alum, in the presence or absence of 2×108 CFU heat-killed bacteria in PBS, unless otherwise mentioned. All secondary immunizations were performed in a similar manner, but in the absence of bacteria. Serum samples for measurement of Ag-specific IgG titers, at different time points, were prepared from blood obtained through the tail vein. For adoptive transfer studies with DO11.10 mice, 2.5 × 106 spleen cells (containing ~ 5×105 Tg T cells) from DO11.10 mice were injected i.v. into WT BALB/c mice, 24 h prior to immunization.
Measurement of antigen-specific serum titers by ELISA
Immulon 4 ELISA plates (Dynex Technologies, Chantilly, VA) were coated overnight with 5 μg/ml of cOVA or PspA, or 10 μg/ml of PC-KLH in PBS (50 μl/well) at 4°C. The plates were then blocked with PBS + 1% BSA (100 μl/well) for 2 h at 37°C. Three-fold serial dilutions of serum samples, starting at a 1/50 serum dilution, in PBS plus 1% BSA (50 μl/well) were then added and incubated overnight at 4°C followed by washing (3x) with PBS + 0.1% Tween-20. Alkaline phosphatase-conjugated polyclonal goat anti-mouse IgG Ab (200 ng/ml, 50 μl/well) in PBS plus 1% BSA was then added and plates were incubated at 37°C for 1 h. Plates where then washed with PBS + 0.1% Tween-20 and substrate (p-nitrophenyl phosphate, disodium; Sigma-Aldrich) was added at 1 mg/ml in TM buffer (1 M Tris + 0.3 mM MgCl2 [pH 9.8]) for color development. Color was read at an absorbance of 405 nm on a Multiskan Ascent ELISA reader (Labsystems, Finland).
Enumeration of NP-specific ASC by ELISPOT
NP-specific ASC were measured in 96-well flat bottom EIA/RIA high-binding plates (Costar, Corning, Sigma-Aldrich) coated overnight at 4°C with 2 μg/ml NIP15–BSA diluted in 0.05 M K2HPO4 (pH 8.0). Plates were washed 3x with PBS prior to blocking with warm PBS, 1% gelatin (Sigma-Aldrich) at 37°C for a minimum of 1 h. Plates were washed again 3x with PBS prior to incubation with cells. Single cell suspensions of splenocytes or bone marrow (harvested 2 wks post-immunization) were seeded in duplicate at 4-6 × 106 total viable cells per 100 μl in the first well, and 2-fold serial dilutions were carried out down the plate. Plates were incubated at 37°C in 5% CO2 for 5-6 h in RPMI Medium (Cellgro, Manassas, VA) supplemented with 10% heat-inactivated FBS (BioSource, Grand Island, NY), 2 mM GlutaMAX-I (Invitrogen), 100 U/ml Penicillin (Invitrogen, Grand Island, NY), 100 μg/ml Streptomycin (Invitrogen), and 0.05 mM 2-mercaptoethanol (Sigma-Aldrich). Following culture, cells were then lysed with 0.05% Tween 20 in dd H20 for 10 min at room temperature and subsequently washed 3x with PBS, 0.1% Tween 20. Secreted antibody was detected by incubating plates with an AP-conjugated goat anti-mouse IgG (SouthernBiotech, Birmingham, AL) diluted in 1% gelatin in PBS for 1 h at 37°C. After 3 washes with PBS/Tween 20, plates were developed overnight at 4°C with 1 mg/ml 5-Bromo-4-chloro-3-indolyl phosphate p-toluidine (BCIP; Sigma-Aldrich) salt substrate diluted in an alkaline buffer composed of 0.1 M 2-amino-2-methyl-1-propanol, 0.01% NaN3, 0.5 mM MgCl2, 0.007% Triton X-405, pH 10.25. Plates were washed 3x with deionized H2O, allowed to dry in the dark at room temperature, and scanned (Epson Perfection 2450 Photo Scanner). Developed spots were counted visually from the scanned images and the frequency of NP-specific ASCs per total number of cells plated was calculated.
Flow cytometric analysis
Individual samples of RBC-lysed spleen cells from 3-5 mice/group were stained using the following mouse-specific mAbs: Alexa Fluor 405-anti-CD4 (clone RM4-5) and allophycocyanin-anti-DO11.10 TCR (clone KJ1-26) [Invitrogen]; PE-Texas Red-anti-B220 (clone RA3-6B2), and FITC-anti-T and B cell activation antigen (clone GL7) [BD Biosciences, San Jose, CA]; PE-Cy7-anti-PD1 (clone 29F.1A12) [Biolegend, San Diego, CA]. Cells were analyzed using a LSR-II flow cytometer (BD Biosciences) and results were generated using FlowJo (Tree Star, Ashland, OR) and FACSDiva (BD Biosciences) software.
For detection of PC or PspA on bacteria, 1 × 105 CFU heat-killed bacteria were incubated overnight at 4°C with 0.25 μg of mouse IgG2aκ anti-PC mAb (clone PCG2a2.A1) or mouse IgG2aκ anti-PspA mAb (clone DC10-IA5) in PBS plus 1% BSA. This was followed by washing with PBS (2x) and incubation with 0.5μg FITC-anti-mouse IgG2a (BD Biosciences, San Jose, CA) on ice for 30 min followed by 2 more washings with PBS. Bacteria were then analyzed by flow cytometry using a BD LSR-II flow cytometer. Bacteria incubated with only FITC-anti-mouse IgG2a were used as a negative control.
For studies to detect NP+ cells, the following mAbs were used: PE-Cy7-anti-B220 (clone RA3-6B2) and Allophycocyanin-Cy7-anti-IgD (clone 11.26c.2a) [BioLegend]; biotin-Igκ (clone 187.1, hybridoma), and PE-Cy5.5-CD11c (clone N418) [eBiosciences]. FITC-PNA (Vector Laboratories, Burlingame, CA) was used for detection of germinal center B cells and Alexa-647-OVA (Invitrogen) was used to exclude non-NP binding B cells. For detection of NP-specific B cells, cells were stained with PE-NP40 (Biosearch Technologies). The secondary reagent for detecting biotin-conjugated antibodies was Pacific Blue-streptavidin (Invitrogen). Flow cytometric analyses were performed by acquiring data on a Cyan analyzer (Dako, Denmark) and with FlowJo software (Tree Star).
Statistical analysis
Serum antigen-specific IgG titers were expressed as geometric means + SEM of the individual serum titers. Significance was determined by two-tailed Student's t test. Values of p<0.05 were considered statistically significant. Each experiment was performed at least twice to ascertain reproducibility.
RESULTS
The inhibitory effect of R36A on the cOVA-specific IgG response to cOVA is significantly reversed by depletion of phosphorylcholine (PC) from R36A
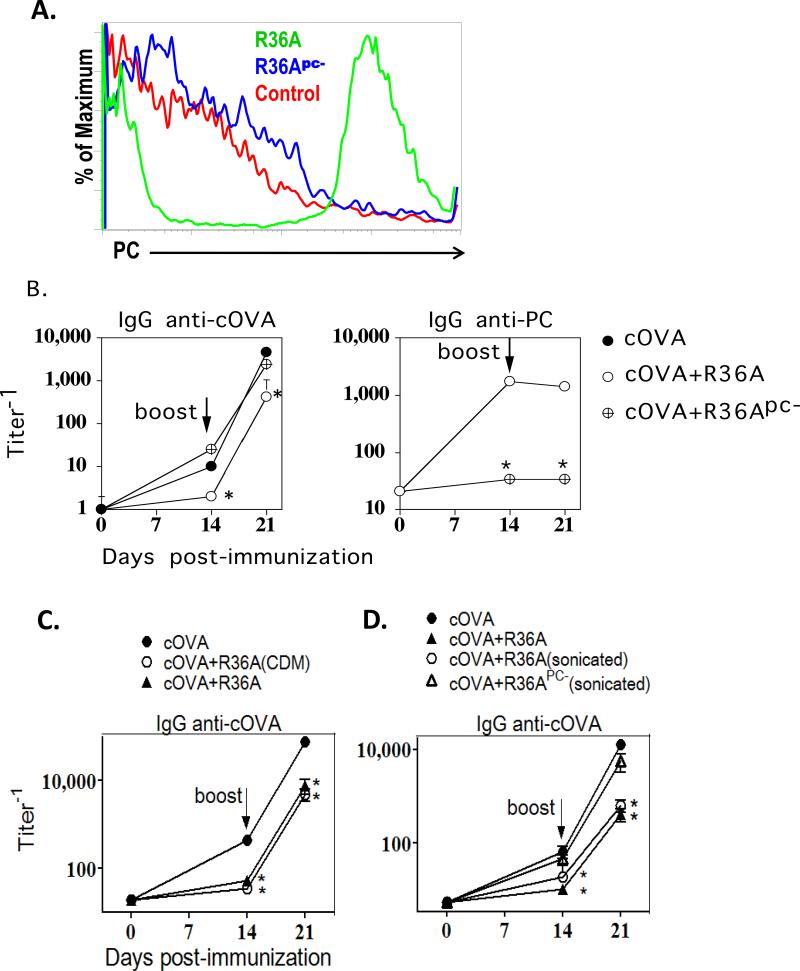
We previously demonstrated that several intact, inactivated encapsulated and unencapsulated strains of S. pneumoniae (including the unencapsulated strain R36A) suppress the IgG response to coimmunized heterologous proteins, including cOVA (15, 16). Although the suppressive activity was contained within the S. pneumoniae cell wall, its identity was not determined. S. pneumoniae, including strain R36A, expresses high levels of PC, which is covalently attached to the bacterial cell wall teichoic acid and membrane lipoteichoic acid (26, 27). Studies on the PC-containing filarial nematode glycoprotein, ES-62, have demonstrated a role for PC in ES-62-mediated immunosuppression (17). Of note, the strains of S. agalactiae and N. meningitidis, capsular type C used in a previous study (16), which did not inhibit the cOVA-specific IgG response, did not have any detectable surface expression of PC, as determined by flow cytometry (data not shown). Thus, we speculated that PC expression by R36A might account for the immunosuppressive activity of this bacterium. To determine this, we produced R36A that was markedly deficient in PC expression, by growing it in a chemically defined media (CDM) with gradual replacement of choline chloride with ethanolamine. As illustrated in Fig.1A, this R36A (referred to as R36Apc-) exhibited essentially no detectable PC expression by flow cytometry using an anti-PC mAb, in contrast to high expression of PC by R36A. Co-immunization of BALB/c mice with cOVA/alum + R36Apc- resulted in a cOVA-specific IgG response that was comparable to that observed in mice immunized with cOVA/alum alone, and was in distinct contrast to mice immunized with cOVA/alum + R36A (Fig. 1B, left panel), strongly suggesting that PC played a key role in the R36A-mediated inhibitory effect. As expected, mice immunized with R36Apc-, in contrast to R36A, failed to induce a detectable PC-specific IgG response (Fig. 1B, right panel).
Fig.1. Depletion of PC from R36A resulted in a significant reversal in its inhibitory effect on the cOVA-specific IgG response.
(A) PC expression by R36A or R36Apc- by flow cytometry (“control”, R36Apc- stained with FITC-anti IgG2a only [no primary anti-PC Ab]). BALB/c mice (7 per group) were immunized i.v. with 50 μg cOVA in alum: (B) with or without 2×108 CFU R36A or R36Apc-, (C) R36A (grown in standard THB medium) or CDM-grown R36A, or (D) R36A, sonicated R36A or sonicated R36Apc-. All mice were boosted i.v. with 50 μg cOVA + alum without bacteria, on d14. Serum titers of cOVA-specific and PC-specific IgG were measured by ELISA. Significance * p≤0.05
Growing bacteria in a different media can alter their cell wall composition and thus their properties (28, 29). R36Apc- was grown in CDM to efficiently control individual component concentration (in this case PC), whereas R36A used throughout this study was grown in THB medium. Thus, we grew R36A in CDM (containing PC) to determine whether it still inhibited the anti-cOVA IgG response. As shown in Fig. 1C, R36A grown in CDM inhibited the anti-cOVA IgG response similar to WT R36A grown in THB medium.
Another difference between R36A and R36Apc- is that R36Apc- grows in long chains due to a PC-dependent defect in the enzymatic cleavage of the daughter cells. To determine whether this difference in morphology accounts for the loss of R36A-mediated inhibition, we gently sonicated R36Apc-, using an ultrasonic bath, so as to break the chains but not lyse the cells. As a control we also sonicated R36A. We verified the disruption of chains of R36Apc- by viewing gram-stained bacteria microscopically (data not shown). Upon immunization, sonicated R36A inhibited the anti-cOVA IgG response, similar to untreated R36A, whereas sonicated R36Apc- did not (similar to R36Apc- in Fig. 1B), (Fig 1D). This confirms that chain formation by R36Apc-was not the cause of the reversal of inhibition.
R36Apc-, in contrast to R36A, does not inhibit the formation of GC Tfh, GC B cells, or ASC in response to immunization with cOVA
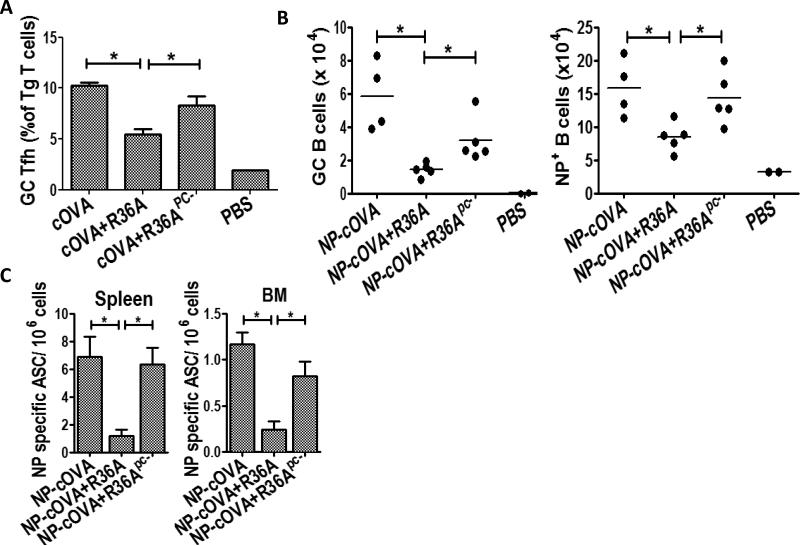
We previously demonstrated that R36A-induced suppression of the cOVA-specific IgG response to cOVA was associated with an inhibition in the formation of GC Tfh, GC B cells, and ASC (16). Consistent with the data illustrated in Fig. 1B (left panel), R36Apc-, in contrast to R36A, failed to inhibit the generation of GC Tfh cells on day 8 from adoptively transferred OVA peptide-specific CD4+ DO11.10 Tg T cells in response to cOVA/alum (Fig. 2A) or NP-specific GC B cells (Fig. 2B) or NP-specific ASC in spleen and bone marrow (Fig. 2C) on day 14 in response to NP-OVA/alum. These results confirm that the expression of PC by R36A is required for the R36A-mediated inhibition of the IgG response to cOVA.
Fig.2. R36Apc- did not inhibit the generation of GC Tfh, GC B cells or ASC in response to cOVA immunization.
(A) cOVA-specific Tg T cells from DO11.10 mice were adoptively transferred into BALB/c mice and 1 d later immunized i.v. with PBS only or 50 μg cOVA in alum with or without 2 × 108 CFU R36A or R36Apc- (3-5 mice/group). On d 8 following immunization gated CD4+ DO11.10 TCR+ Tg T cells from spleen cell suspensions were analyzed for GC Tfh cell phenotype (GL7hi PD1hi). (B) BALB/c mice (4-5/group) were immunized i.v. with PBS only or 50 μg NP-cOVA with or without 2 × 108 CFU R36A or R36Apc-. On d 14 post-immunization, NP-specific B cells (B220+ CD11C− NP+) were analyzed for isotype-switched GC NP+ B cells (IgD−/low PNA+) (C) Quantitation of NP-specific ASC from spleen and BM in response to NP-cOVA alone or with R36A or R36Apc- on d 14 post-immunization (4-5 mice/group). Significance * p≤0.05
PC is not directly involved in the R36A-mediated suppression of the cOVA-specific IgG response
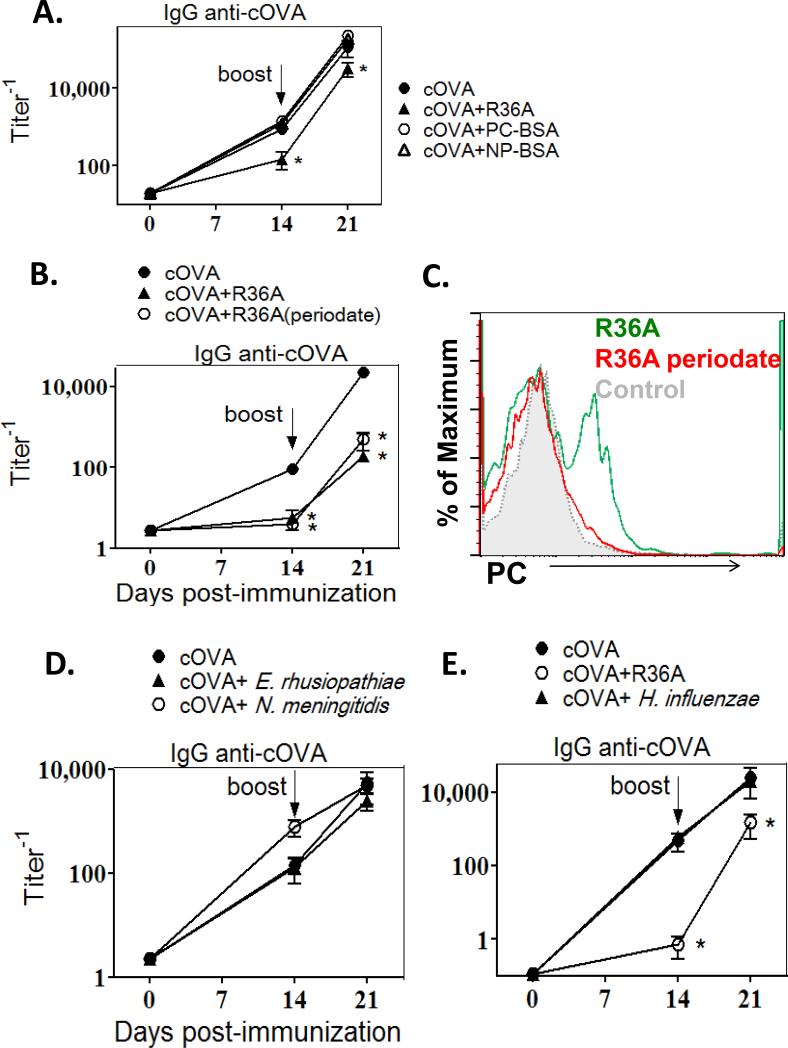
To explore a potential, direct role for PC in mediating inhibition of the IgG anti-cOVA response, we determined whether a soluble conjugate of PC and bovine serum albumin (PC-BSA) was inhibitory. Thus, mice were co-immunized with cOVA/alum and soluble PCBSA (or NP-BSA as a control). As shown in Fig. 3A, PC-BSA did not inhibit the anti-cOVA IgG response.
Fig. 3. Periodate-treated R36A significantly inhibited the anti-cOVA IgG response whereas soluble PC-BSA and other PC containing bacteria did not.
BALB/c mice (7/group) were immunized i.v. with 50μg cOVA in alum with or without: (A) 50μg PB-BSA or NP-BSA (as control) or 2 × 108 CFU R36A, (B) 2 × 108 CFU WT R36A or periodate-treated R36A, (D) 4×108 CFU E. rhusiopathiae or 2×108 CFU N. meningitidis, or (E) 2×108 CFU R36A or 5×108 CFU H. influenzae.. All mice were boosted i.v. with 50 μg cOVA + alum without bacteria, on d14. Serum titers of cOVA-specific IgG were measured by ELISA. (C) PC expression by R36A or R36A (periodate) by flow cytometry (“control”, R36A stained with FITC-anti IgG2a only [no primary anti-PC Ab]). Significance * p≤0.05.
To further explore a direct role for PC in mediating R36A-induced suppression, we treated bacteria with periodate, which oxidizes and destroys the exposed PC on the cell wall, without altering protein epitopes (30). Specifically, periodate treatment did not alter the expression of PspA on R36A, as demonstrated by flow cytometry using an anti-PspA mAb (data not shown). Co-immunization of cOVA with periodate-treated R36A, resulted in the inhibition of the anti-cOVA IgG response that was comparable to that observed with untreated R36A (Fig. 3B) further arguing against any direct role of PC in R36A-mediated suppression. Destruction of PC with periodate was confirmed by flow cytometry using an anti-PC mAb (Fig. 3C).
In a final set of experiments, we determined whether PC-expressing bacteria other than S. pneumoniae, also exhibit a suppressive property similar to R36A. Thus, we co-immunized mice with cOVA and Neisseria meningitidis (strain C311), Haemophilus influenzae (strain Rd), or Erysipelothrix rhusiopathiae (strain Fujisawa), which we demonstrated by flow cytometry to express PC (data not shown), and determined whether the cOVA-specific IgG responses were inhibited (Fig 3D and E). None of these three bacteria exhibited an inhibitory effect on the cOVA-specific IgG response. Collectively, these studies effectively rule out a direct role for PC in mediating S. pneumoniae-induced suppression of humoral immunity.
PC-binding proteins are responsible for the R36A-mediated inhibition of the IgG anti-cOVA response
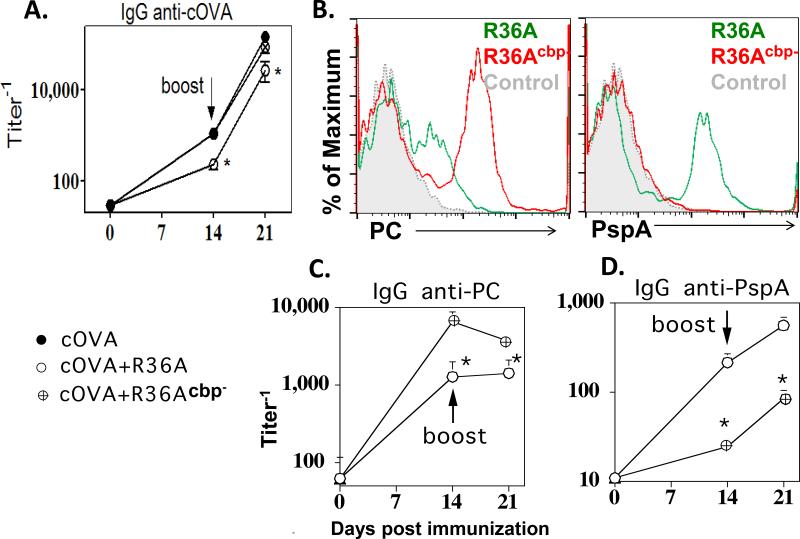
PC anchors a number of pneumococcal proteins to the cell wall (referred to as choline-binding proteins [CBPs]) via non-covalent interactions (31). In this regard, the inability of R36Apc- to inhibit the IgG anti-cOVA response to cOVA could be due to the loss of CBPs, and not due directly to the absence of PC. Of note, treatment of R36A with periodate destroys only the exposed PC residues, with the CBPs remaining bound to the cell wall, and thus could account for the continued inhibitory effect of periodate-treated R36A (25, 30). To determine a role for CBPs in R36A-mediated suppression, we treated R36A with choline chloride to detach CBPs from the cell wall through competitive binding. We refer to R36A depleted of CBPs as R36Acbp-. R36Acbp-, similar to R36Apc-, failed to inhibit the cOVA-specific IgG response to cOVA (Fig. 4A). PC staining of R36Acbp- using an anti-PC mAb was significantly higher than that of R36A likely due to the exposure of PC moieties that were initially blocked by bound CBPs (Fig. 4B, left panel), and the corresponding IgG anti-PC response following immunization with R36Acbp- was significantly higher relative to R36A (Fig. 4C). In contrast, choline chloride treatment of R36A essentially removed all detectable PspA, a major choline-binding protein, from the bacterial surface (Fig. 4B, right panel), which resulted in a minimal IgG anti-PspA response following immunization (Fig. 4D).
Fig. 4. Depletion of CBPs from R36A resulted in the loss of its inhibitory effect on the cOVA-specific IgG response.
BALB/c mice (7 per group) were immunized i.v. with 50 μg cOVA in alum with or without 2 × 108 CFU R36A or R36Acbp-. All mice were boosted i.v. with 50 μg cOVA in alum without bacteria, on d14. Serum titers of (A) cOVA-specific, (C) PC-specific and (D) PspA-specific IgG were measured by ELISA. (B) PC and PspA expression by R36A or R36Acbp- by flow cytometry (“control”, R36A stained with FITC-anti IgG2a only [no primary anti-PspA or anti-PC Ab]). Significance * p≤0.05.
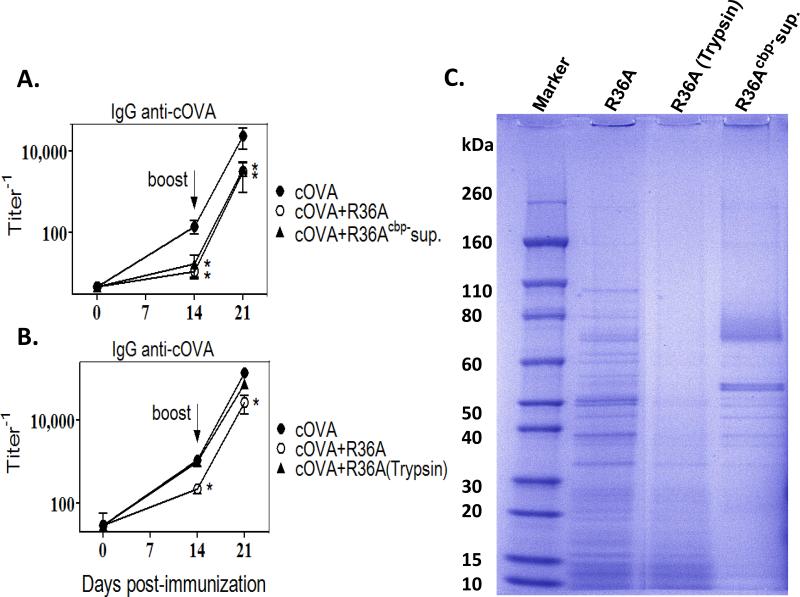
In a complementary set of experiments we determined whether the soluble CBPs released into the supernatant of choline chloride-treated R36A, would inhibit the IgG anti-cOVA response, when co-immunized with cOVA. R36Acbp- SN that was first dialyzed to remove choline chloride, and then concentrated, inhibited the IgG anti-cOVA response to the same degree as did intact R36A (Fig. 5A). To confirm that proteins were responsible for this inhibitory effect and not any other component released into the supernatant, we treated R36A with the protease trypsin and evaluated whether it still exhibited its immunosuppressive property. Trypsin-treated R36A [R36A(trypsin)] failed to inhibit the IgG anti-cOVA response (Fig. 5B), confirming that the inhibitory component in the supernatant is a protein. A marked loss of R36A proteins following treatment with trypsin was confirmed by SDS-PAGE electrophoresis (Fig. 5C). Further, an enrichment of several distinct bands of proteins in the CBP-containing supernatant relative to whole R36A, including a ~70kD band consistent with PspA, was also demonstrated (Fig. 5C), supporting the selective removal of CBPs from R36A using choline chloride. Collectively, these data strongly suggest that one or more CBPs are responsible for the R36A-mediated inhibition of the cOVA-specific IgG response.
Fig. 5. Supernatant containing CBPs inhibited the cOVA-specific IgG response to cOVA, whereas trypsinized R36A did not.
BALB/c mice (7 per group) were immunized i.v. with 50 μg cOVA in alum with or without (A) 2×108 CFU R36A or supernatant of choline chloride-treated R36A (R36Acbp- sup.) equivalent to 2×108 CFU R36A or (B) 2×108 CFU R36A or trypsin treated R36A [R36A (trypsin)]. All mice were boosted i.v. with 50 μg cOVA in alum without bacteria, on d 14. Serum titers of cOVA-specific IgG were measured by ELISA. (C) Lysates from R36A cells, R36A (trypsin) and R36Acbp- sup., electrophoresed on SDS-PAGE. Significance * p≤0.05.
The choline binding protein, PspA plays a major role in R36A-mediated inhibition of the IgG anti-cOVA response
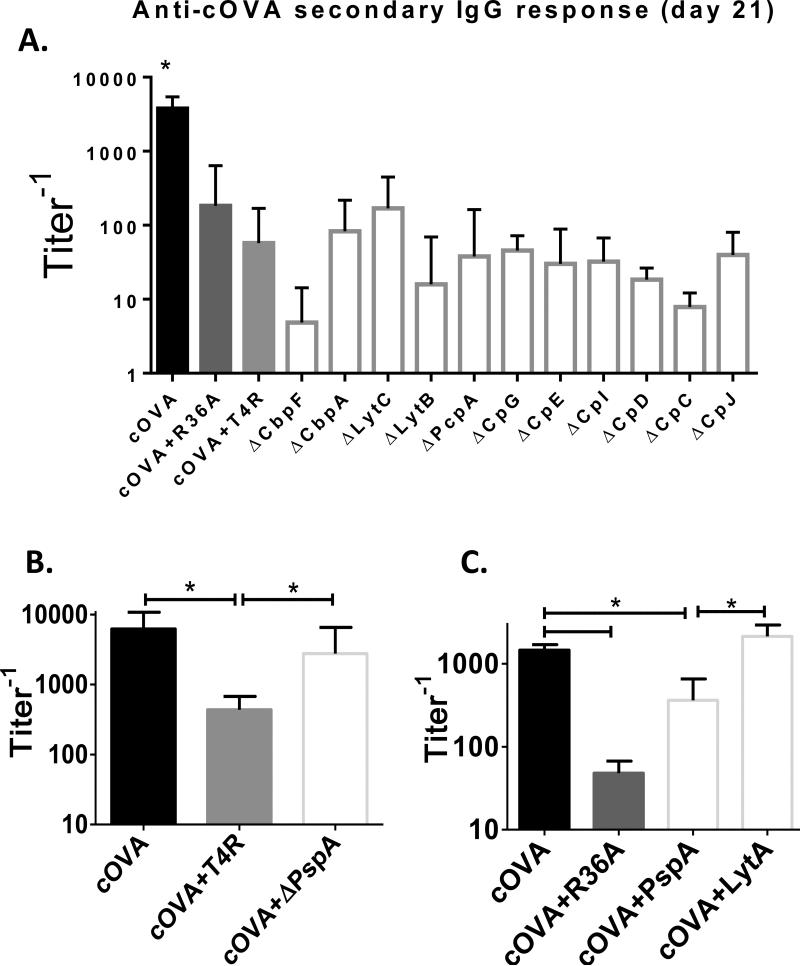
We next wished to determine whether the inhibitory property of the R36A-derived CBP preparation was shared by all CBPs, perhaps related to their conserved choline-binding domains, or was perhaps a unique property of one or few CBPs. To determine this, we used a library of S. pneumoniae (T4R strain) mutants, each one genetically deficient in a single CBP (Fig. 6A). All S. pneumoniae mutants inhibited the IgG anti-cOVA response, to a degree similar to that observed with either wild-type T4R or R36A, with the exception of the strain deficient for PspA, (ΔPspA T4R) where the IgG anti-cOVA response following co-immunization with cOVA was not significantly different than that observed following cOVA alone (Figs. 6A, B). A further study demonstrated that co-immunization of cOVA with another CBP, a full-length recombinant Pn-derived LytA protein consisting of the choline-binding domain, resulted in no inhibition of the IgG anti-cOVA response at the doses used (Fig. 6C), thus arguing against a role for the choline-binding domain by itself as the inhibitory moiety. Of note, a truncated, recombinant PspA consisting of the first 302 amino acids, and lacking the choline-binding domain partially inhibited the IgG anti-cOVA response to cOVA, but not to the extent mediated by intact R36A (Fig. 6C) or the preparation of natural CBPs (Fig. 5A) suggesting that full-length and/or natural PspA may be required for full inhibitory activity. Unfortunately, technical considerations have historically prevented multiple attempts to purify usable quantitates of full-length recombinant or natural PspA (David Briles, personal communication), including in our own laboratory. Nevertheless, our collective results strongly implicate PspA as the major mediator of R36A-induced inhibition of humoral immunity to co-immunized heterologous proteins.
Fig. 6. All CBP- Pn mutants, except for the ΔPspA Pn mutant, inhibited the cOVA-specific IgG response.
BALB/c mice (7 per group) were immunized i.v. with 50 μg cOVA in alum with or without (A) 2×108 CFU R36A or T4R or T4R mutants for the respective CBPs listed in the graph. (B) 2×108 CFU T4R or T4R PspA mutant (ΔPspA). (C) 2×108 intact R36A, 50 μg recombinant, truncated PspA, or 50 μg recombinant full-length LytA. All mice were boosted i.v. with 50 μg cOVA in alum without bacteria, on d 14. Serum titers of cOVA-specific IgG were measured by ELISA. Significance * p<0.05.
DISCUSSION
We previously demonstrated that intact, inactivated Pn strongly inhibits the IgG response to co-immunized cOVA, as well as other heterologous proteins, by suppressing the formation of specific GC Tfh, GC B cells, and ASC (15, 16). This inhibitory effect of Pn occurs early, only during the first 24 hours following co-immunization with protein, and its effect is transient (16). Further, Pn exerts its action only locally within the secondary lymphoid organ in which the humoral response to the co-immunized protein is occurring (16). The inclusion of a pro-inflammatory TLR agonist such as CpG-ODN, with the co-immunized protein that was adsorbed to alum, could partially counteract this Pn-mediated inhibitory effect. Although we previously demonstrated that this inhibitory moiety was localized to the Pn cell wall, its identity remained unknown.
In the current study we began by determining whether Pn expression of PC, a hapten abundantly expressed within the cell wall through covalent attachment to teichoic acid, was the inhibitory moiety. This was based on several reports demonstrating an immunosuppressive effect of PC when expressed on a secreted glycoprotein (ES-62), from the filarial nematode Acanthocheilonmea viteae (17, 18). We demonstrate that although Pn lacking PC loses most, if not all, of its inhibitory effect, the inhibitory moiety is not PC itself, but a choline-binding protein (CBP), PspA, that requires non-covalent binding to PC in order to remain in the Pn cell wall. Of note, previous reports have shown that PspA is expressed on all Pn strains (32, 33), is itself highly immunogenic in mice and elicits protective antibodies against live Pn challenge (34). The role of PspA as an inhibitor of humoral immunity was elucidated in the current study by first conclusively demonstrating that the inhibitory activity was trypsin-sensitive, and contained within the CBPs expressed by Pn, and then through comprehensive testing of genetic CBP-deficient Pn strains, with only ΔPspA Pn losing its inhibitory property. The inability of a full-length recombinant Pn-derived LytA protein, containing the choline-binding domain to inhibit the IgG anti-cOVA response to cOVA, argues against this domain by itself as sufficient to mediate inhibition, but does not rule out a requirement for its presence within the full-length PspA. In this regard, a truncated, recombinant PspA expressing only the first 302 amino acids, and thus lacking the choline-binding domain, had only partial inhibitory activity at the doses used, relative to intact R36A or the preparation of natural CBPs. Unfortunately, significant technical difficulties have precluded the production of full-length recombinant or natural PspA in usable quantities, by our or other laboratories (David Briles, personal communication), and thus could not be directly tested for its predicted inhibitory activity. The possibility that heat treatment of intact R36A altered PspA to make it immunosuppressive, is ruled out by the experiment demonstrating the immunosuppressive nature of the supernatant obtained from the choline chloride-treated R36A. Thus, live bacteria were treated with choline chloride and the supernatant obtained after this treatment (consisting of all the CBPs including PspA) was injected into mice. This supernatant (which was never subjected to any heat treatment) exhibited inhibition on a scale similar to that seen with heat killed intact R36A.
The ability of Pn to inhibit IgG responses to co-immunized heterologous proteins appears to be relatively unique to Pn, in that various strains of intact S. agalactiae, N. meningitidis, H. influenzae, and E. rhusiopathiae were non-inhibitory, the latter 3 also expressing PC. Although, several bacterial species can express PC, they have not been reported to have choline-binding proteins. Pn undergoes spontaneous phase variation between a transparent and an opaque phenotype (13) with the transparent phenotype being more capable of adhering and colonizing the nasopharynx, and the opaque phenotype being more phagocytosis-resistant and predominant in blood (11, 12). In this regard, the expression of CBPs is phase variable, with PspA being expressed in higher amounts in opaque variants, whereas some others such as CbpA and LytA being increased in the transparent variants (35). This ability might enable Pn to regulate its immunosuppressive activity differentially during different phases of infection. However, the exact mechanism by which PspA is regulating the early phase of the humoral immune response that leads to subsequent suppression of Ag-specific GC Tfh, GC B cells, and Ab responses, remains to be determined. One property of PspA that might be relevant in this regard, is its ability to interfere with complement deposition on the Pn surface, thus acting as a virulence factor that prevents complement-mediated bacterial clearance (36, 37). Complement plays an early key role in promoting antibody responses, and its production by macrophages within secondary lymphoid organs can be regulated post-immunization (38, 39). Regardless of the mechanism of Pn-mediated inhibition, our data suggest that the presence of Pn either as a colonizing agent or during overt infection may down-regulate the development of antibody responses to heterologous proteins expressed or secreted by other pathogens, or Pn itself. However, the Pn-mediated inhibition of the IgG responses to co-immunized proteins can be at least partly overcome under certain circumstances, such as in the presence of a strong innate stimulus (e.g. CpG-ODN) as we previously demonstrated (16), or when the co-immunized protein is highly immunogenic (i.e. tetanus toxoid) [unpublished].
ACKNOWLEDGMENTS
Authors acknowledge Kateryna Lund and Karen M. Wolcott for their technical assistance with flow cytometry.
Supported by: N.I.H. 2R01-AI49192 (CMS) and USUHS Dean's Research and Education Endowment Fund (CMS).
Abbreviations
- CBP
choline-binding protein
- R36APC-
- cOVA
chicken ovalbumin
- GC
germinal center
- PC
phosphorylcholine
- Pn
Streptococcus pneumoniae
- PspA
Pneumococcal surface protein A
- R36A
unencapsulated Streptococcus pneumoniae, serotype 2
- R36Apc-
R36A lacking PC
- R36ACBP-
R36A depleted of phosphorylcholine-binding proteins
- Tfh
T follicular helper cell
Footnotes
The opinions expressed herein are those of the authors, and are not necessarily representative of those of the Uniformed Services University of the Health Sciences (USUHS), the Department of Defense (DOD), or the United States Army, Navy, or Air Force.
REFERENCES
- 1.Hajishengallis G, Lambris JD. Microbial manipulation of receptor crosstalk in innate immunity. Nat. Rev. Immunol. 2011;11:187–200. doi: 10.1038/nri2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finlay BB, McFadden G. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell. 2006;124:767–82. doi: 10.1016/j.cell.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 3.Tato CM, Hunter CA. Host-Pathogen Interactions: Subversion and utilization of the NF-kB pathway during infection. Infect. Immun. 2002;70:3311–7. doi: 10.1128/IAI.70.7.3311-3317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sing A, Rost D, Tvardovskaia N, Roggenkamp A, Wiedemann A, Kirschning CJ, Aepfelbacher M, Heesemann J. Yersinia V-antigen exploits Toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 2002;196:1017–24. doi: 10.1084/jem.20020908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergman MP, Engering A, Smits HH, van Vliet SJ, van Bodegraven AA, Wirth H-P, Kapsenberg ML, Vandenbroucke-Grauls CMJE, van Kooyk Y, Appelmelk BJ. Helicobacter pylori modulates the T helper Cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J. Exp. Med. 2004;200:979–90. doi: 10.1084/jem.20041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gebert B, Fischer W, Weiss E, Hoffmann R, Haas R. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science. 2003;301:1099–102. doi: 10.1126/science.1086871. [DOI] [PubMed] [Google Scholar]
- 7.Boulton IC, Gray-Owen SD. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat. Immunol. 2002;3:229–36. doi: 10.1038/ni769. [DOI] [PubMed] [Google Scholar]
- 8.Kotb M, Norrby-Teglund A, McGeer A, El-Sherbini H, Dorak MT, Khurshid A, Green K, Peeples J, Wade J, Thomson G, Schwartz B, Low DE. An immunogenetic and molecular basis for differences in outcomes of invasive group A streptococcal infections. Nat. Med. 2002;8:1398–404. doi: 10.1038/nm1202-800. [DOI] [PubMed] [Google Scholar]
- 9.Finlay BB, McFadden G. Anti-Immunology: Evasion of the host immune system by bacterial and viral pathogens. Cell. 2006;124:767–82. doi: 10.1016/j.cell.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham AF, Gaspal F, Serre K, Mohr E, Henderson IR, Scott-Tucker A, Kenny SM, Khan M, Toellner KM, Lane PJ, Maclennan IC. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J. Immunol. 2007;178:6200–7. doi: 10.4049/jimmunol.178.10.6200. [DOI] [PubMed] [Google Scholar]
- 11.Racine R, Jones DD, Chatterjee M, McLaughlin M, Macnamara KC, Winslow GM. Impaired germinal center responses and suppression of local IgG production during intracellular bacterial infection. J. Immunol. 2010;184:5085–93. doi: 10.4049/jimmunol.0902710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reed SG, Roters SB, Goidl EA. Spleen cell-mediated suppression of IgG production to a non-parasite antigen during chronic Trypanosoma cruzi infection in mice. J. Immunol. 1983;131:1978–82. [PubMed] [Google Scholar]
- 13.Millington OR, Di Lorenzo C, Phillips RS, Garside P, Brewer JM. Suppression of adaptive immunity to heterologous antigens during Plasmodium infection through hemozoin-induced failure of dendritic cell function. J. Biol. 2006;5:5. doi: 10.1186/jbiol34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostrowski M, Vermeulen M, Zabal O, Geffner JR, Sadir AM, Lopez OJ. Impairment of thymus-dependent responses by murine dendritic cells infected with foot-and-mouth disease virus. J. Immunol. 2005;175:3971–9. doi: 10.4049/jimmunol.175.6.3971. [DOI] [PubMed] [Google Scholar]
- 15.Chattopadhyay G, Chen Q, Colino J, Lees A, Snapper CM. Intact bacteria inhibit the induction of humoral immune responses to bacterial-derived and heterologous soluble T cell-dependent antigens. J. Immunol. 2009;182:2011–9. doi: 10.4049/jimmunol.0802615. [DOI] [PubMed] [Google Scholar]
- 16.Saumyaa S, Arjunaraja, Pujanauski L, Colino J, Torres RM, Snapper CM. Immunosuppressive property within the Streptococcus pneumoniae cell wall that inhibits generation of T follicular helper, germinal center, and plasma cell response to a coimmunized heterologous protein. Infect. Immun. 2013;81:3426–33. doi: 10.1128/IAI.00688-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harnett MM, Melendez AJ, Harnett W. The therapeutic potential of the filarial nematode-derived immunodulator, ES-62 in inflammatory disease. Clin. Exp. Immunol. 2009;159:256–67. doi: 10.1111/j.1365-2249.2009.04064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grabitzki J, Lochnit G. Immunomodulation by phosphocholine--biosynthesis, structures and immunological implications of parasitic PC-epitopes. Mol. Immunol. 2009;47:149–63. doi: 10.1016/j.molimm.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 19.Wu ZQ, Vos Q, Shen Y, Lees A, Wilson SR, Briles DE, Gause WC, Mond JJ, Snapper CM. In vivo polysaccharide-specific IgG isotype responses to intact Streptococcus pneumoniae are T cell dependent and require CD40-and B7-ligand interactions. J. Immunol. 1999;163:659–67. [PubMed] [Google Scholar]
- 20.Gosink KK, Mann ER, Guglielmo C, Tuomanen EI, Masure HR. Role of novel choline binding proteins in virulence of Streptococcus pneumoniae. Infect. Immun. 2000;68:5690–5. doi: 10.1128/iai.68.10.5690-5695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van de Rijn I, Kessler R. Growth characteristics of group A streptococci in a new chemically defined medium. Infect. Immun. 1980;27:444–8. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Briles DE, King JD, Gray MA, McDaniel LS, Swiatlo E, Benton KA. PspA, a protection-eliciting pneumococcal protein: immunogenicity of isolated native PspA in mice. Vaccine. 1996;14:858–67. doi: 10.1016/0264-410x(96)82948-3. [DOI] [PubMed] [Google Scholar]
- 23.Colino J, Chattopadhyay G, Sen G, Chen Q, Lees A, Canaday DH, Rubtsov A, Torres R, Snapper CM. Parameters underlying distinct T cell-dependent polysaccharide-specific IgG responses to an intact gram-positive bacterium versus a soluble conjugate vaccine. J. Immunol. 2009;183:1551–9. doi: 10.4049/jimmunol.0900238. [DOI] [PubMed] [Google Scholar]
- 24.Hammerschmidt S, Talay SR, Brandtzaeg P, Chhatwal GS. SpsA, a novel pneumococcal surface protein with specific binding to secretory Immunoglobulin A and secretory component. Mol. Microbiol. 1997;25:1113–24. doi: 10.1046/j.1365-2958.1997.5391899.x. [DOI] [PubMed] [Google Scholar]
- 25.Casal J, Jado I, Fenoll A, Perez A, Torano A. Periodate oxidation of R36A pneumococci greatly enhances production of hybridomas secreting anti-protein antibodies. Microb. Pathogenesis. 1998;24:111–6. doi: 10.1006/mpat.1997.0175. [DOI] [PubMed] [Google Scholar]
- 26.Jennings HJ, Lugowski C, Young NM. Structure of the complex polysaccharide C-substance from Streptococcus pneumoniae type 1. Biochemistry. 1980;19:4712–9. doi: 10.1021/bi00561a026. [DOI] [PubMed] [Google Scholar]
- 27.Cosenza H, Kohler H. Specific inhibition of plaque formation to phosphorylcholine by antibody against antibody. Science. 1972;176:1027–9. doi: 10.1126/science.176.4038.1027. [DOI] [PubMed] [Google Scholar]
- 28.van de Rijn I, Kessler RE. Growth characteristics of group A streptococci in a new chemically defined medium. Infect. Immun. 1980;27:444–8. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van de Rijn I. Quantitative analysis of cell walls of nutritionally variant streptococci grown under various growth conditions. Infect. Immun. 1985;49:518–22. doi: 10.1128/iai.49.3.518-522.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodward MP, Young WW, Jr, Bloodgood RA. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J. Immunol. Methods. 1985;78:143–53. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]
- 31.AlonsoDeVelasco E, Verheul AF, Verhoef J, Snippe H. Streptococcus pneumoniae: virulence factors, pathogenesis, and vaccines. Microbiol. Rev. 1995;59:591–603. doi: 10.1128/mr.59.4.591-603.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Briles DE, Tart RC, Wu H-Y, Ralph BA, Russell MW, McDaniel LS. Systemic and mucosal protective immunity to pneumococcal surface protein. Ann. N. Y. Acad. Sci. 1996;797:118–26. doi: 10.1111/j.1749-6632.1996.tb52954.x. [DOI] [PubMed] [Google Scholar]
- 33.Ogunniyi AD, Folland RL, Briles DE, Hollingshead SK, Paton JC. Immunization of mice with combinations of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect. Immun. 2000;68:3028–33. doi: 10.1128/iai.68.5.3028-3033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Briles DE, Tart RC, Swiatlo E, Dillard JP, Smith P, Benton KA, Ralph BA, Brooks-Walter A, Crain MJ, Hollingshead SK, McDaniel LS. Pneumococcal diversity: considerations for new vaccine strategies with emphasis on pneumococcal surface protein A (PspA). Clin. Microbiol. Rev. 1998;11:645–57. doi: 10.1128/cmr.11.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenow C, Ryan P, Weiser JN, Johnson S, Fontan P, Ortqvist A, Masure HR. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 1997;25:819–29. doi: 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]
- 36.Ren B, Szalai AJ, Hollingshead SK, Briles DE. Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface. Infect. Immun. 2004;72:114–22. doi: 10.1128/IAI.72.1.114-122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren B, Szalai AJ, Thomas O, Hollingshead SK, Briles DE. Both family 1 and family 2 PspA proteins can inhibit complement deposition and confer virulence to a capsular serotype 3 strain of Streptococcus pneumoniae. Infect. Immun. 2003;71:75–85. doi: 10.1128/IAI.71.1.75-85.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gadjeva M, Verschoor A, Brockman MA, Jezak H, Shen LM, Knipe DM, Carroll MC. Macrophage-derived complement component C4 can restore humoral immunity in C4-deficient mice. J. Immunol. 2002;169:5489–95. doi: 10.4049/jimmunol.169.10.5489. [DOI] [PubMed] [Google Scholar]
- 39.Fischer MB, Ruger B, Vaculik C, Becherer A, Wadsak W, Yanagida G, Losert UM, Chen J, Carroll MC, Eibl MM. The presence of MOMA-2(+) macrophages in the outer B cell zone and protection of the splenic micro-architecture from LPS-induced destruction depend on secreted IgM. Eur. J. Immunol. 2007;37:2825–33. doi: 10.1002/eji.200636996. [DOI] [PubMed] [Google Scholar]