Abstract
Neurotensin (NT) via its receptor 1 (NTR1) modulates development of colitis, decreases HIF-1α/PHD2 interaction, stabilizes and increases HIF-1α transcriptional activity and promotes intestinal angiogenesis. HIF-1α induces miR-210 expression while miR-210 is strongly up-regulated in response to NT in NCM460 human colonic epithelial cells overexpressing NTR1 (NCM460-NTR1). Here we examined whether NT activates a NTR1-HIF-1α-miR-210 cascade using in vitro (NCM460-NTR1 cells) and in vivo [transgenic mice overexpressing (HIF-1α-OE) or lacking HIF-1α (HIF-1α-KO) in intestinal epithelial cells and mice lacking NTR1 (NTR1-KO)] models. Pre-treatment of NCM460-NTR1 cells with the HIF-1α inhibitor PX-478 or silencing of HIF-1α (si-HIF-1α) attenuated miR-210 expression in response to NT. Intracolonic 2,4,6-trinitrobenzenesulfonic acid (TNBS) administration (2-day model) increased colonic miR-210 expression that was significantly reduced in NTR1-KO, HIF-1α-KO mice, and in wild-type (WT) mice pre-treated intracolonicaly with locked nucleic acid anti-miR-210 (LNA-anti-miR-210). In contrast, HIF-1α-OE mice showed increased miR-210 expression at baseline that was further increased following TNBS administration. HIF-1α-OE mice had also exacerbated TNBS-induced neovascularization compared to TNBS-exposed WT mice. TNBS-induced neovascularization was attenuated in HIF-1α-KO mice, or mice pre-treated intracolonicaly with anti-miR-210. Intracolonic anti-miR-210 also reduced colitis in response to TNBS (2 days). Importantly, miR-210 expression was increased in tissue samples from ulcerative colitis patients (UC). We conclude that NT exerts its proinflammatory and proangiogenic effects during acute colitis via an NTR1-PHD2/HIF-1α-miR-210 signaling pathway. Our results also demonstrate that miR-210 plays a proinflammatory role in the development of colitis.
Keywords: Human, Rodent, Endothelial cells, Acute Phase Reactants, Cytokines, Transcription Factors, Molecular Biology, Transgenic/Knockout Mice
Introduction
Hypoxia plays a central role in the progression of Inflammatory Bowel Disease (IBD) where intestinal mucosal tissue damage results during both acute and chronic inflammation1–3. Hypoxia inducible factor (HIF-1), is a key transcription factor in the response to hypoxia and is central in maintaining homeostasis during colitis4, 5. Importantly, several pro-inflammatory cytokines are able to activate HIF-1 in various cell types, including intestinal epithelial cells6, 7. We have recently reported that the neuropeptide neurotensin (NT) via its high affinity receptor neurotensin receptor 1 (NTR1) activates HIF-1α transcriptional activity and promotes intestinal angiogenesis by increasing colonic expression of vascular endothelial growth factor α (VEGFα)8. NT/NTR1 interactions play an important role in the development and progress of experimental colitis and IBD9–11, while colonic expression of both NT and NTR1 are up-regulated in both conditions9, 11. Several studies also indicate that NTR1 signaling is implicated in the pathophysiology of colitis via mechanisms that involve regulation of expression of inflammation-associated genes9, 10, 12, 13. A subgroup of these genes code for micro-RNAs (miRNAs), representing short (19–25 nucleotides), single-stranded RNA molecules, acting primarily as negative transcriptional regulators by binding to the 3′ un-translated regions (UTR) of transcripts14,15. Our recent results indicate that exposure of human colonic NCM460 epithelial cells overexpressing NTR1 (NCM460-NTR1) to NT altered expression of several miRNAs16, 17. Interestingly, expression of miR-210, a known downstream target of HIF-1α18–20, showed the highest increase in response to NT treatment, suggesting that an NTR1-dependent miR-210 signaling pathway may play a role in signaling pathways related to hypoxia during colitis.
Based on these considerations, we tested the hypothesis that NT-driven HIF-1α-miR-210 interactions may regulate progress of colitis and colitis-associated angiogenesis. To address this hypothesis we used NCM460-NTR1 colonic epithelial and colon cancer adenocarcinoma HCT-116 cells as well NTR1 deficient mice, intestinal epithelial cell-specific HIF-1α overexpressing mice21, as well as mice with specific intestinal epithelial disruption of HIF-1α22. The effect of intracolonic administration of LNA-anti-miRNA-210 in the development of experimental colitis was also examined.
Materials and Methods
Antibodies and Reagents
We used the following reagents: NT (Phoenix-Biotechnology, San Antonio, TX), HIF-1α inhibitor PX-478 (MedKoo, Chapel Hill, NC), LNA-anti-miR-210 (Exiqon, Woburn, MA), Lipofectamine 2000, lipofectamine RNAimax and OptiMEM (Life Technologies, Grand Island, NY), anti-HIF-1α monoclonal antibody LS-B145/10218 (Lifespan Biosciences, Seattle, WA), anti-β-Actin polyclonal antibody GTX30632 (GeneTex Inc. Irvine, CA).
Human IBD biopsy specimens
Total RNAs from colon tissues from patients with active Ulcerative colitis (UC), active Crohn’s disease (CD), and normal subjects (n=12 per group) were purchased from OriGene (Rockville, Maryland, USA). Biopsies were obtained through IRB protocols with documented patient consent, all from accredited US medical institutions (www.origene.com). cDNA conversion of RNA samples was performed as described below, and levels of miR-210 were determined by quantitative RT-PCR analysis.
Transduction of NCM460 Cells With NTR1 (NCM460-NTR1)
The human neurotensin receptor 1 (NTSR1) gene was isolated from pCR2.123 with Eco RV and inserted into lentiviral backbone CMV-IRES-GFP-PGK-Puro at the Eco RV site, 5’ to IRES. Lentiviral particles expressing NTRS1 were generated as we previously described16. After transduction, NCM460-NTR1 cells were maintained as described below.
Cellular Studies
Human colonic epithelial cells (NCM460-NTR1) and colonic cancer HCT-116 cells were maintained in M3D medium (Incell, San Antonio, TX) and McCoy5a (ATCC, Manassas, VA) respectively supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum, 1% L-glutamine, and 10 units/ml penicillin, and 100 µg/ml streptomycin at 37°C in air supplemented with 5% CO2. For HIF-1α silencing, NCM460-NTR1 cells were seeded (6-well plates, 3 × 105 cells per well) and 24 h later, were transfected with si-RNA against HIF-1α or siRNA-A control (Santa Cruz, Santa Cruz, CA) using LipofectamineTM RNAiMAX (Thermo Fisher Scientific, Waltham, MA); 48 h post-transfection, were treated as indicated with NT. For miR-210 silencing, HCT-116 or NCM460-NTR1 cells were transfected with antisense miR-210 (as-miR-210), using Lipofectamine RNAiMAX. Cells transfected with antisense control miRNA (as-miR-control) served as controls. Where indicated, NCM460-NTR1 cells were treated with NT (10−7 M, 6 h) +/− 18 h of pretreatment with PX-478 (40 × 10−6 M) or their vehicles (1% BSA in PBS and 0.9% NaCl in water respectively).
Transgenic mice
The NTR1 knockout mice Ntsr1tmDgen (designated NTR1-KO) were from the Jackson Laboratory and bred in our facility. Animals were backcrossed onto C57BL6/J background for five generations. We performed one additional backcross before intercrossing animals to generate littermate controls used in this study. The intestinal epithelium-specific HIF-1α overexpressing mice (designated HIF-1α-OE), the intestinal epithelium-specific HIF-1α knockout mice (designated Hif1α-KO) HIF-1αΔIE and littermate controls were previously described22 and were on C57BL/6 background. HIF-1α-OE and HIF1α-KO colitis experiments were performed by the group of Dr. Yatrik Shah at the University of Michigan and mouse colon cDNA and tissue samples were analyzed at UCLA. All animals were maintained in standard cages in a light- and temperature-controlled room, and were allowed standard chow and water ad libitum.
Colitis mouse models
Animal studies were approved by the institutional animal care and use committee. TNBS colitis was induced to 8–12 week old mice by 100 µl intracolonic enema of 250 mg/kg TNBS (Fluka, Ronkonkoma, NY)24. Control groups were injected with 100 µl of 30% ethanol intracolonicaly. Mice were returned to their cages and sacrificed 48 h post colitis by carbon dioxide. Colon tissues were isolated and dissected for further analysis.
qRT-PCR
Total RNA from mouse colons was isolated using standard TRIzol reagent protocol (Life Technologies, Carlsbad, CA) and cDNA was prepared using miRCURY LNA Universal RT microRNA PCR cDNA kit (Exiqon). qRT-PCR for micro-RNAs was performed using micro-RNA specific primers (Exiqon) and miRCURY LNA Universal RT microRNA PCR SYBR Green master mix (Exiqon). qRT-PCR for mRNAs of interest was performed using specific primers obtained (Applied Biosystems) according to the manufacturer's instructions.
Immunohistochemisry
Colon tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Tissue sections were prepared by the Translational Pathology Core Laboratory (University of California, Los Angeles). Sections were blocked and incubated with a rabbit polyclonal von Willebrand Factor (vWF) antibody (Millipore, Billerica, MA) overnight at 4°C. After washing, sections were incubated with donkey anti-rabbit IgG and slides, stained with an ABC kit for color development (Santa Cruz, CA) and photographed under the microscope and computerized. Image analysis of vWF-stained cells was performed using the Scion Image Software as we described8. For histological scoring, sections were stained with H&E, photographed at multiple locations and analyzed by scoring specimens on a 0 to 10 scale for the following colitis parameters: mucosal integrity (0 to 6 scale), mucosal neutrophil infiltration (0 to 3 scale) and edema (0 to 1 scale)8.
In situ hybridization
For the localization of miR-210, paraffin embedded sections of mouse colon were used for in situ hybridization. Custom miRCURY LNA microRNA detection probes for miR-210 were designed and labeled with DIG at both 3’- and 5’-ends, and used according to the manufacturer’s instructions (Exiqon).
In vivo miRNA silencing
A modification of our recent published procedure was used17. Briefly, C57BL6 male mice (8–12 weeks old) received intracolonicaly vehicle or 10mg/kg LNA-anti-miR-210 (Exiqon) 48 and 24 h before induction of TNBS colitis (250 mg/kg). All intracolonic enemas were performed using a 3.5 cm long polyethylene cannula (Intramedic PE-20 tubing, Becton Dickinson, Parsippany, NJ, U.S.A.) attached to an insulin syringe and introduced into the colon reaching ~3 cm from the anus. Animals were sacrificed after 2 days and colon tissues were collected and dissected. Briefly, colons were cut 4 cm to 2 cm away from the anus and separated in three parts for RNA, protein isolation, and immunohistochemistry. The efficiency of miR-210 knockdown after LNA-anti-miR-210-treatment was evaluated by qPCR in mouse colon samples.
Immunoadsorption
Anti-HIF-1α monoclonal antibody LS-B145/10218 (Lifespan Biosciences, Seattle, WA) or non-specific human IgG (Sigma) were coupled (2 µg antibody/ 30 µl of the beads according to the manufacturer’s instructions) with Dynal magnetic beads (Dynal Biotech, Carlsbad, CA), pretreated with BSA (1% in PBS for 30 min at 4oC). Equal protein samples of cell lysates from NCM460-NTR1 cells treated +/− NT (10−7 M, 6 h) were brought to equal volumes and were subsequently incubated with anti-HIF-1α coupled beads (0.5 M NaCl, 30 µl of beads), or nonspecific IgG coupled beads for 16 hours at 4oC with gentle shaking. Antibody-bound material was eluted from the antibody with 2% SDS Laemmli sample buffer and kept as SDS Elute.
Gel electrophoresis and immunoblotting
Equal volumes of SDS Elutes were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis according to Laemmli, and transferred to polyvinylidene difluoride membranes in 25 mmol/L Tris, 192 mmol/L glycine. Equal volume of cell lysates incubated with the beads was also subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis to serve as control. Membranes were blocked (phosphate-buffered saline, 10% nonfat dry milk, 0.05% Tween-20) and probed with anti-PHD2 polyclonal antibody (ab4561, abcam, Cambridge, MA) and anti-β-Actin polyclonal antibody (GTX3063, GeneTex Inc. Irvine, CA) followed by corresponding horseradish peroxidase–labeled secondary antibodies (1:1000). Blots were developed with enhanced chemiluminescence reagent (PerkinElmer Life Sciences, Waltham, MA). Western blot bands were quantified using image analyzer LAS-4000 mini (Fujifilm). Data are represented by cropped images from the original membranes. Loading controls (β-actin) are derived from the same original membranes.
Statistical analysis
Quantitative results were expressed as means with standard errors of the means. The results were analyzed using Prism professional statistics software (Graphpad, San Diego, CA). Student's t test was used for intergroup comparisons.
Results
NT signaling induces inflammatory markers through miR-210 expression
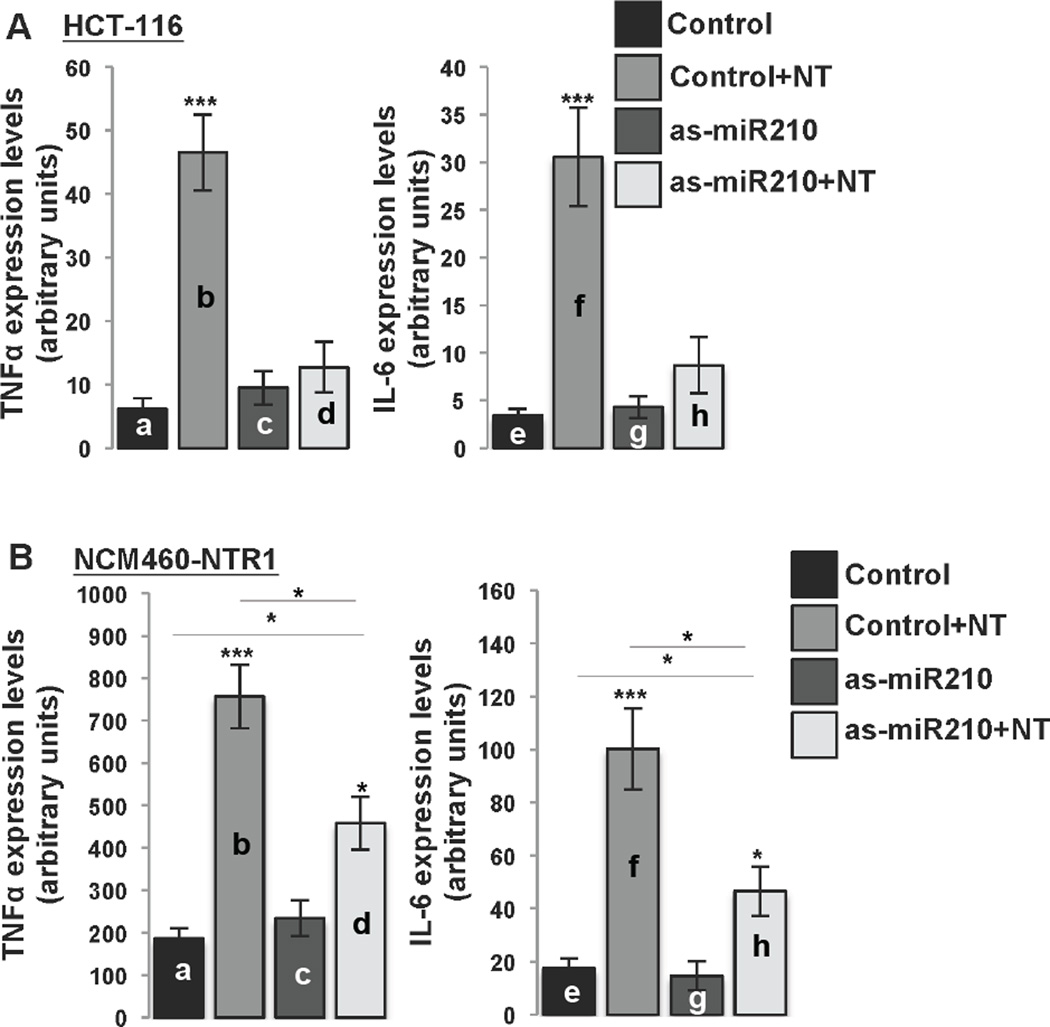
NTR1 signaling in human colonic epithelial cells stimulates expression of proinflammatory genes25, 26 and alters expression of several miRNAs, including miR-21016. We previously showed that NT exposure of NCM460-NTR1 and HCT-116 cells (that express high levels of endogenous NTR112), stimulates increased miR-210 expression16. Therefore, both cell lines were used in this part of the study. We first used LNA-anti-miR-210 to examine whether NT-induced cytokine expression in NCM460-NTR1 and in colonic adenocarcinoma HCT-116 cells is miR-210-dependent. We show that NT significantly induced TNFα and IL-6 expression in both HCT-116 cells (a vs b, p=0.0001 and e vs f, 0.0003; Fig. 1A) and NCM460-NTR1 cells (a vs b, p=0.0001 and a vs f, 0.0004; Fig. 1B). Transfection of HCT-116 cells with LNA-anti-miR-210 reduced NT-induced TNFα and IL-6 expression to levels comparable to untreated cells (d vs b vs a; Fig. 1A). Transfection of NCM460-NTR1 cells with LNA-anti-miR-210 also attenuated NT-induced TNFα and IL-6 expression as compared to NT treated controls (b vs d, p=0.0127 and f vs h, 0.0134; Fig. 1B). However, these levels were still significantly higher in NT-treated compared to control NCM460-NTR1 cells pretreated with LNA-anti-mir210 (c vs d, p=0.0137 and g vs h, p=0.0149; Fig. 1B). Pretreatment of NCM460-NTR1 cells with LNA-Scrambled had no effect on NT-induced cytokine expression (b vs d; Suppl. Fig. 1). Moreover, NT treatment of NCM460 cells which normally express very low levels of NTR1 had no effect on miR-210 levels of expression (a vs b; Suppl. Fig. 1C). These results suggest that in human colonocytes, induction of proinflammatory cytokine expression in response to NT stimulation is at least in part miR-210 dependent.
Figure 1.
(A) HCT-116 and (B) NCM460-NTR1 cells were transfected with as-miR-210 and/or NT (10−7 M, 6 h) 48 h following transfection (n=6, figure panels representative of three separate experiments). TNFα and IL-6 levels of expression were assessed using qPCR. Statistical analysis was performed using student’s t test. *p<0.05, **p<0.01, ***p<0.001
NT signaling increases miR-210 expression in human colonic epithelial cells via activation of HIF-1α
Several studies indicate that miR-210 gene-transcription is primarily regulated by HIF-1 transcriptional activity18–20 via a highly conserved HRE region within the miR-210 gene-promoter27. We have recently reported that NT exposure of NCM460-NTR1 cells does not promote HIF-1α levels of expression but rather causes HIF-1α protein stabilization and a subsequent increase in its transcriptional activity8. HIF-1α protein levels are primarily dependent on prolyl hydroxylation by prolyl hydroxylase 2 (PHD2) and subsequent proteolytic degradation28. Down-regulation of PHD2 activity is a potential means by which NT signaling results in HIF-1α accumulation at the protein level. NT treatment of NCM460-NTR1 cells did not alter PHD2 mRNA levels (data not shown), but caused a decrease in the amount of PHD2 co-immunoprecipitated with HIF-1α (Suppl. Fig. 2A), suggesting that NT attenuates PHD2/HIF-1α interaction, reducing the rate of HIF-1α protelytic degradation.
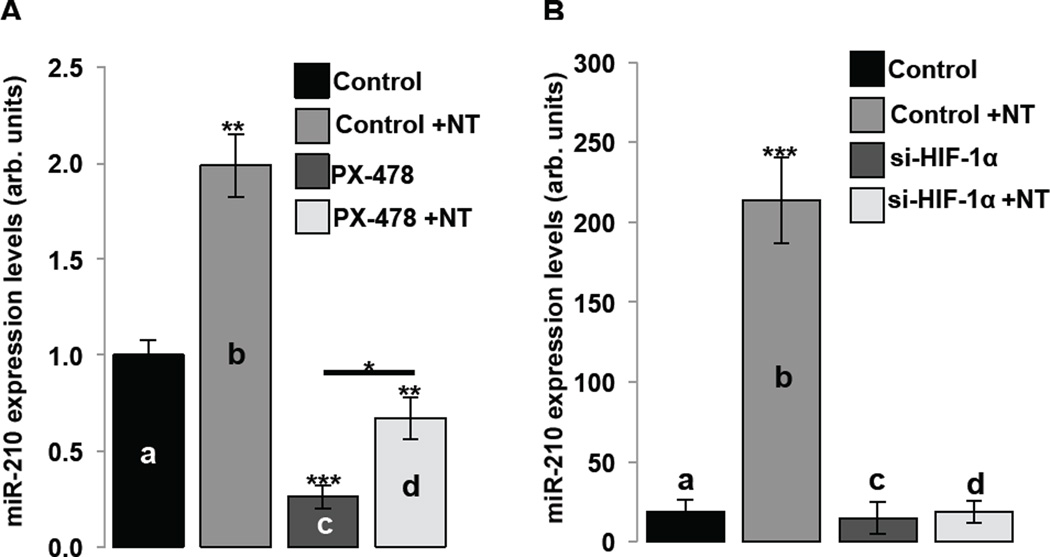
We next studied whether NT-induced up-regulation of miR-210 in human colonocytes depends on increased of HIF-1α levels and transcriptional activity. We blocked HIF-1α activity with either pharmacological inhibition or small interfering RNA (si-RNA) and confirmed inhibition by Western Blot analysis (Suppl. Fig. 2B). NT treatment significantly increased miR-210 levels of expression (a vs b, p=0.0057; Fig. 2A). On the other hand, pretreatment with PX-478 significantly reduced both basal (a vs c, p=0.0018; Fig. 2A) as well as NT-induced miR-210 expression (b vs d, p=0.0026; Fig. 2A). However, PX-478 did not completely reverse NT-induced miR-210 expression (c vs d, p=0.0309; Fig. 2A). We attribute this effect on incomplete inhibition of HIF-1α by the pharmacologic inhibitor PX-478 (Suppl. Fig. 2B). In a separate set of experiments, NT-treatment of NCM460-NTR1 cells again caused a significant increase in miR-210 levels of expression (a vs b, p=0.0001; Fig. 2B), that was abolished by pretreatment with si-HIF-1α (a vs c vs d; Fig. 2B). These data taken together with our recent results8 suggest that in human colonocytes, NT/NTR1 signaling decreases HIF-1α/PHD2 interaction, stabilizes HIF-1α, and increases its transcriptional activity, leading to increased miR-210 expression.
Figure 2.
NCM460-NTR1 cells were pretreated as indicated with (A) the HIF-1α inhibitor PX-478 (40 × 10−6 M, 18 h) and/or NT (10−7 M, 6 h) or (B) transfected with si-HIF-1α or siRNA-A control and/or NT (10−7 M, 6 h) 48 h following transfection. MiR-210 levels of expression were assessed using qPCR (n=6, figure panels representative of three separate experiments). Statistical analysis was performed using student’s t test. *p<0.05, **p<0.01, ***p<0.001
Colitis Induces HIF-1α-dependent miR-210 expression in mice
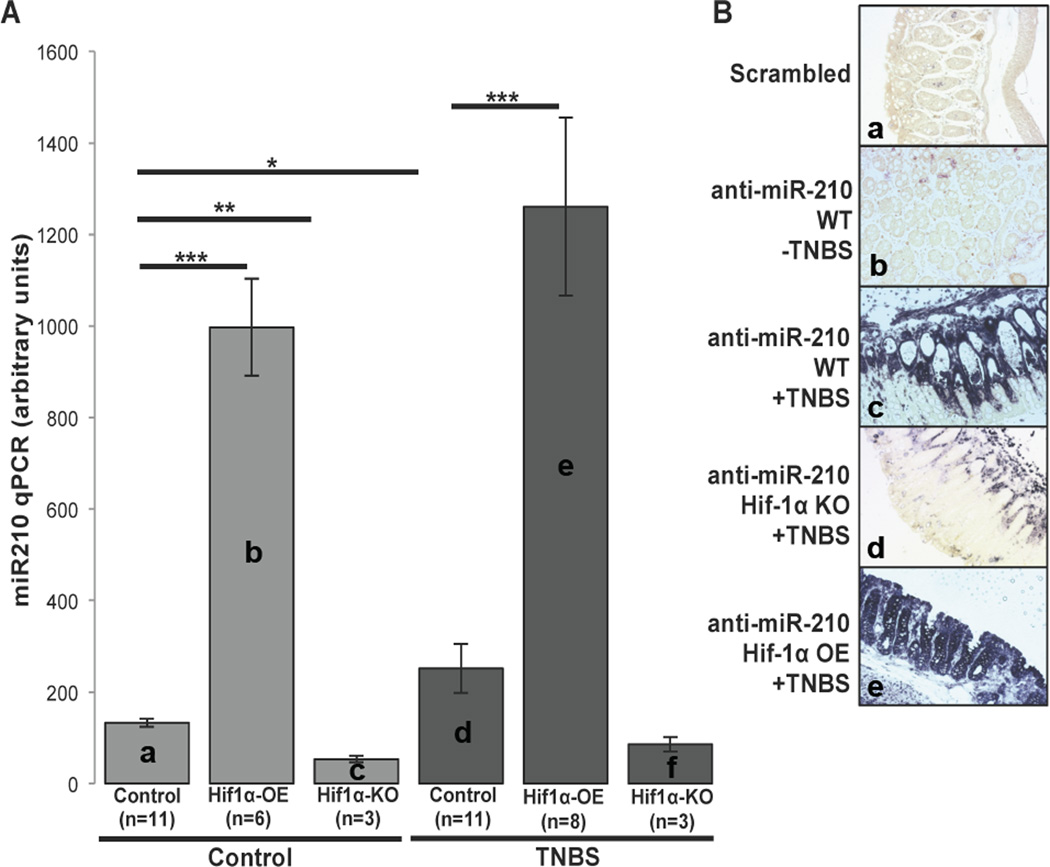
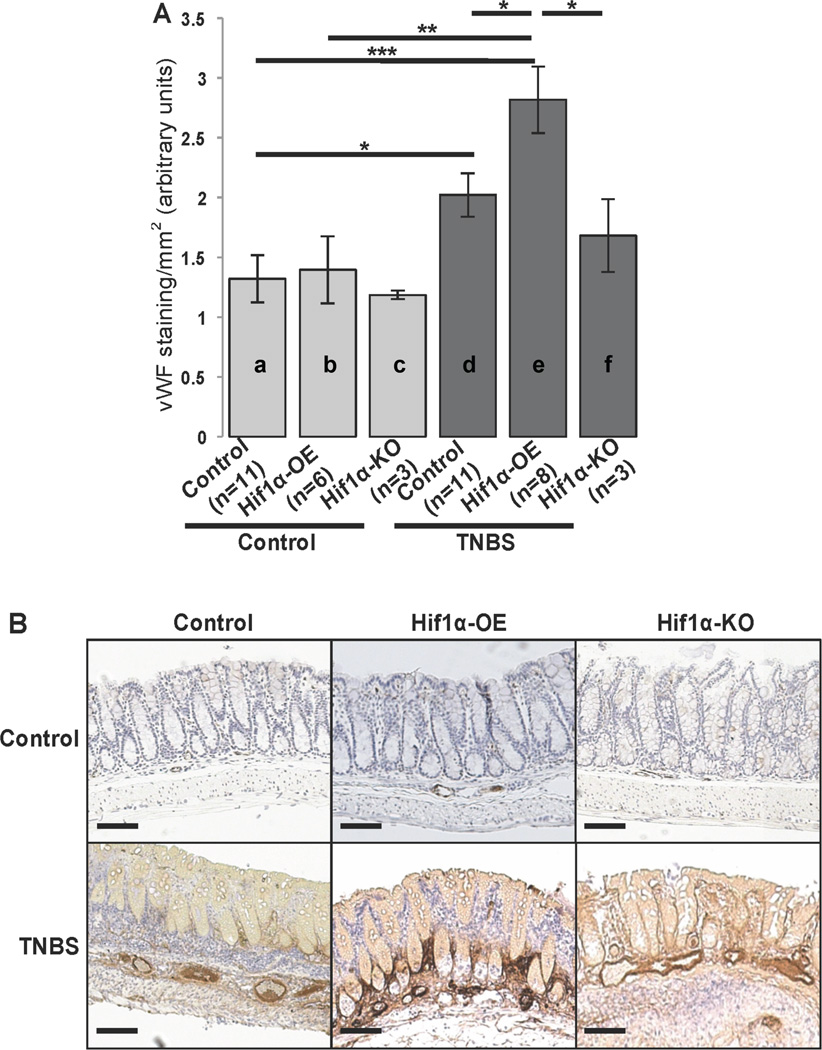
In order to investigate the role of HIF-1α transcriptional activity in colitis-induced miR-210 up-regulation in vivo, we employed experimental colitis in transgenic mouse models. We used intracolonic enema of TNBS in WT or mice that either overexpress (HIF-1α-OE) or lack HIF-1α expression (HIF-1α-KO) in their intestinal epithelium. Histologic analysis showed that TNBS administration caused histologic changes consistent with colitis in all three mouse models compared to ethanol-injected mice (Suppl. Fig. 3). We found no difference in the total histologic score following TNBS administration between the different mouse groups (d vs e vs f; Suppl. Fig. 3). However, compared to control littermates, HIF-1α-OE mice had increased colonic miR-210 expression both at baseline (a vs b, p=0.0001; Fig. 3A) and following TNBS exposure (d vs e, p=0.001; Fig. 3A). Interestingly, there was a slightly higher, but not statistically significant increase in mir-210 expression between control- and TNBS-treated HIF-1α-OE mice (b vs e; Fig. 3A). On the other hand, HIF-1α-KO mice showed reduced miR-210 expression at baseline (a vs c, p=0.0048; Fig. 3A), while TNBS treatment did not increase colonic expression of miR-210 (c vs f; Fig. 3A). These data suggest that in the mouse colon HIF-1α is capable of driving miR-210 expression.
Figure 3.
(A) HIF-1α-OE, HIF-1α-KO and control littermates (n=3 up to n=11) were treated as indicated with TNBS and colon samples were used to assess miR-210 levels of expression by qPCR. Statistical analysis was performed using student’s t test. *p<0.05, **p<0.01, ***p<0.001 (B) Representative images of in situ hybridization of miR-210 of colon tissues from TNBS-treated control, HIF-1α-OE and HIF-1α-KO mice and a non-treated control mouse (n=3 up to n=11). Scale: 50 µm.
We next examined localization of miR-210 in the colonic mucosa by in situ hybridization. We found that while miR-210 expression was not readily evident in control mouse colon tissue sections (b; Fig. 3B), but was highly expressed in TNBS-exposed colon. Mir-210 was expressed in both the epithelial and sub-epithelial colonic mucosa areas, but was primarily present in epithelial vs sub-epithelial areas (c; Fig. 3B). HIF-1α-KO mouse colon exposed to TNBS showed a much more modest increase in miR-210 expression, primarily confined in the colonic submucosa (d; Fig. 3B). As expected, miR-210 expression levels were very high in HIF-1α-OE mice exposed to TNBS, primarily in colonic epithelial cells (e; Fig. 3). Thus, miR-210 expression in colonic epithelial cells is largely dependent on HIF-1α transcriptional activity.
Intracolonic enema of LNA-anti-miRNA attenuates target miRNA expression in mouse colon
In order to evaluate the functional importance of an NT/NTR1/HIF-1α/miR-210 signaling axis in vivo, we silenced miR-210 expression in mouse colon by intracolonic enema of LNA-anti-miR-210 (Suppl. Fig. 4A & B)17. Colonic miR-210 expression was significantly reduced in LNA-anti-miR-210 treated mice (a vs b, p=0.0112; Suppl. Fig. 4C) while the corresponding levels of two arbitrarily chosen miRNAs, let7a and let7b, remained unaffected (c vs d and e vs f; Suppl. Fig. 4C), suggesting that colonic miR-210 in vivo silencing is relatively specific.
In vivo silencing of miR-210 attenuates colitis and affects colitis marker expression
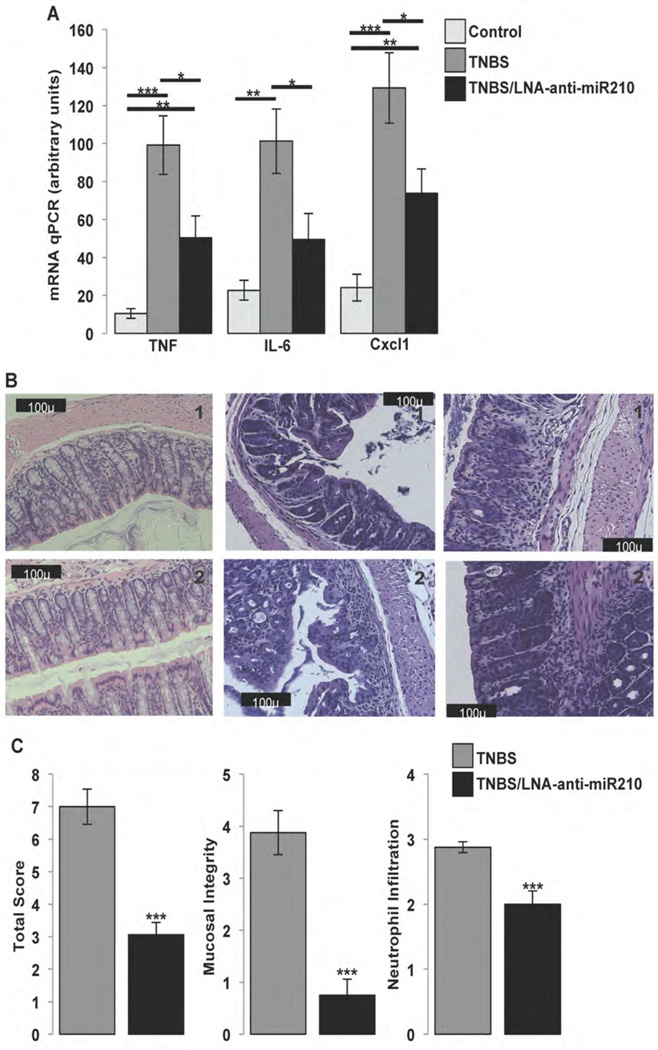
NT stimulates expression of interleukins 6 and 8 (IL-6 and IL-8) and TNFα in colonocytes in vitro29 and during colitis in vivo10. To evaluate the role of miR-210 during colitis, we assessed mRNA expression of these cytokines in WT mice that received intracolonic enema of LNA-anti-miR-210 and subsequently subjected to TNBS colitis. As expected, TNBS increased TNFα, IL-6 and the murine analogue of IL-8, Cxcl1 compared to untreated controls (a vs b, p=0.0005, d vs e, p=0.0023 and g vs h, p=0.0007; Fig. 4A). Importantly, pretreatment of mice with LNA-anti-miR-210 significantly attenuated mRNA expression of these cytokines following TNBS treatment (b vs c, p=0.0349, e vs f, p=0.045 and h vs i, p=0.0392; Fig. 4A) to levels comparable, or lower than those of untreated controls (a vs c, p=0.009, d vs f, not significant and g vs i, p=0.0095; Fig. 4A). On the other hand, intracolonic enema of LNA-scrambled control in combination with TNBS treatment did not affect expression of these cytokines compared to TNBS alone (Suppl. Fig. 4D). Histological scoring of colitis of mice treated with TNBS revealed that mice pretreated with LNA-anti-miR-210 exhibited significantly lower colitis score associated with improved mucosal integrity and neutrophil infiltration scores (p=0.0001, 0.0001 and 0.0004 respectively; Fig. 4B & C), compared to mice exposed to LNA-scrambled control. These results suggest a pro-inflammatory role for miR-210 in experimental colitis.
Figure 4.
(A) Control (C57BL6/J) mice untreated (control) or received as indicated intracolonic enema of LNA-anti-miR-210 (10mg/kg), 48 h and 24 h before receiving as indicated intracolonic enema of TNBS (250mg/kg). 48 h later mice (n=5) where sacrificed, colon tissues where collected for RNA isolation and expression levels of the inflammatory markers TNFα, IL-6, and Cxcl1 were assessed by qPCR. (B) Representative (2) images from H&E staining of colon tissues from C57BL6/J mice treated as indicated with TNBS (250mg/kg) with or without intracolonic administration of LNA-anti-miR-210 (10mg/kg). Scale: 100 µm. (C) Total histological score, mucosal integrity and neutrophil infiltration of colon tissues from C57BL6/J mice treated with TNBS (250mg/kg) with or without intracolonic administration of LNA-anti-miR-210 (10mg/kg) (n=8). Statistical analysis was performed using student’s t test. *p<0.05, **p<0.01, ***p<0.001
HIF-1α expression affects colitis-associated angiogenesis
We have previously shown that NTR1 promotes intestinal angiogenesis in vivo, while NT induces the expression of VEGFα in NCM460-NTR1 cells in vitro8. To assess angiogenesis during colitis in the presence or absence of HIF-1α, we used endothelial cell staining with vWF in colon tissue sections collected from HIF-1α-OE, HIF-1α-KO and control littermates following intracolonic exposure to TNBS or control. As expected, angiogenesis was significantly increased in TNBS-treated WT mice as compared to controls (a vs d, p=0.019; Fig. 5A & B). Importantly, this increase was exacerbated in TNBS treated HIF-1α-OE mice compared to untreated WT and HIF-1α-OE mice (a vs e, p=0.0004 and b vs e, p=0.0058; Fig. 5A). A comparison between TNBS treated WT and HIF-1α-OE mouse colon also indicated a significant increase in vWF-positive cells in HIF-1α-OE mice (d vs e, p=0.0264; Fig. 5A). On the other hand, TNBS-treated HIF-1α-KO mice showed significantly lower angiogenesis compared to TNBS-treated HIF-1α-OE mice (e vs f, p=0.0422; Fig. 5A), while they showed no significant difference when compared to healthy HIF-1α-KO mice (c vs f; Fig. 5A). These data indicate that HIF-1α transcriptional activity is required for colitis-induced colonic angiogenesis.
Figure 5.
HIF-1α-OE, HIF-1α-KO and control littermates were treated as indicated with TNBS (250mg/kg). Mice were sacrificed 48 h later and colon tissues were fixed in formalin, embedded in paraffin and endothelial cells where stained with vWF antibody (n=3 to 11). (A) Relative colon tissue angiogenesis (vWF positive cells/mm2) quantified by AxioVision (Carl Zeiss microscopy) in HIF-1α-OE, HIF-1α-KO and control littermates treated as indicated with TNBS (250mg/kg). Statistical analysis was performed using student’s t test. *p<0.05 (B) Representative images from vWF stained colon tissues sections. Scale: 100 µm.
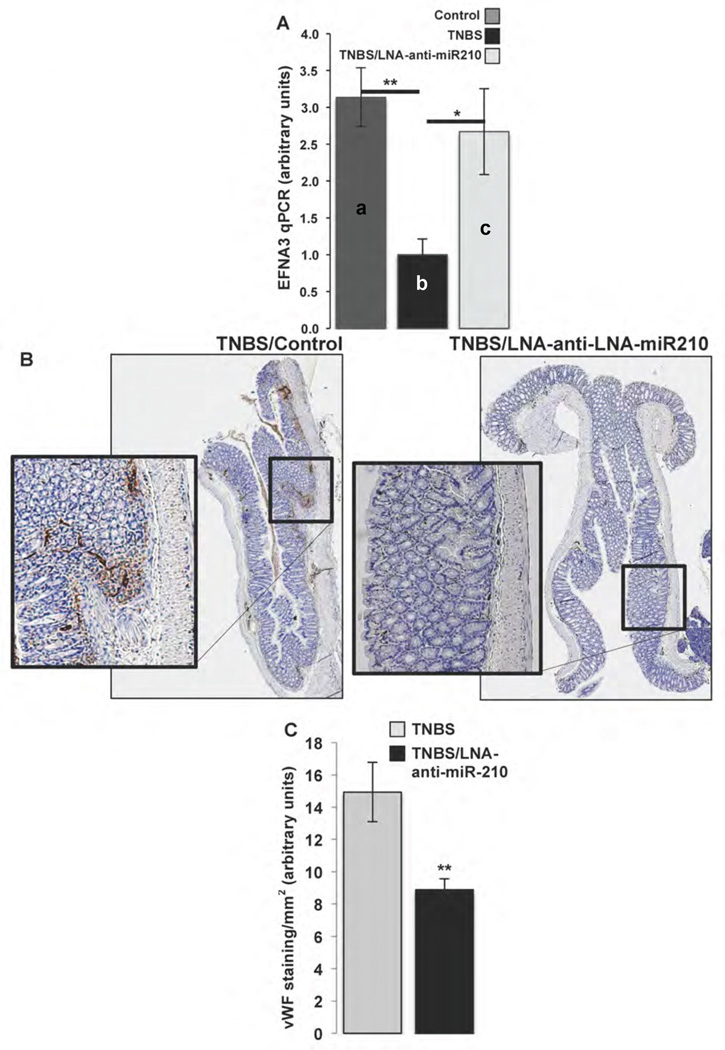
In vivo silencing of miR-210 affects colitis-induced angiogenesis
Mir-210 is also known to target and inhibit the expression of ephrin-A3 (EFNA3), a receptor protein-tyrosine kinase that blocks angiogenesis30. Using qRT-PCR analysis we found that EFNA3 mRNA expression is significantly decreased in colons of TNBS treated mice compared to control littermates (a vs b, p=0.004; Fig. 6A). Importantly, EFNA3 mRNA expression levels were restored in colons of LNA-anti-miR-210-treated mice (b vs c, p=0.0381; Fig. 6A). Moreover, endothelial cell staining showed significantly reduced angiogenesis in TNBS-treated mice pretreated with intracolonic enema of LNA-anti-miR-210 (p=0.0081; Fig. 6B & C). Collectively, these results suggest that miR-210 exerts its pro-inflammatory effects, at least in part, by regulating angiogenesis.
Figure 6.
C57BL6 mice received as indicated intracolonic enema of LNA-anti-miR-210 (10mg/kg), 48 h and 24 h before mice receiving as indicated intracolonic enema of TNBS (250mg/kg). 48 h later mice (n=8) where sacrificed, colon tissues where collected either for RNA isolation and qPCR or were fixed in formalin, embedded in paraffin and endothelial cells where stained with vWF antibody. (A) EFNA3 expression levels were assessed by qPCR. (B) Representative longitudinal section microphotographs (reconstructed from ×5 magnification microphotographs, AxioVission, Carl Zeiss microscopy) of vWF stained colon tissues. (C) Relative colon tissue angiogenesis (vWF positive cells/mm2) quantified by AxioVision (Carl Zeiss microscopy). Statistical analysis was performed using student’s t test. *p<0.05, **p<0.01
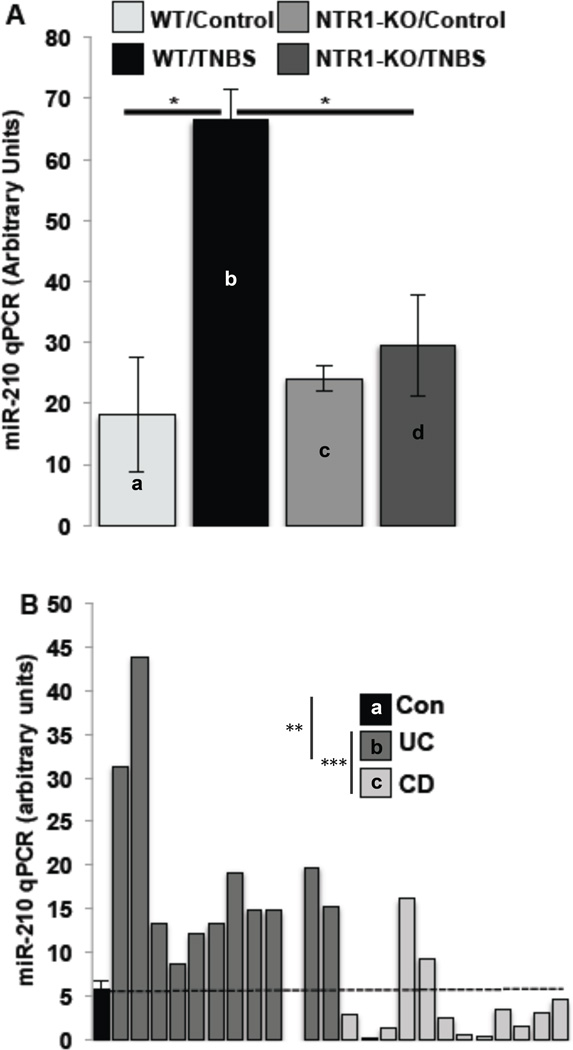
Colonic miR-210 expression during colitis is NTR1-dependent manner
To directly asses the relationship of miR-210 expression with NTR1 signaling we compared the expression of miR-210 in the colon of NTR1-KO and control littermates. TNBS-treatment of control mice caused a significant increase in the colonic miR-210 expression (a vs b, p=0.0109; Fig. 7A) that was significantly reduced in TNBS treated NTR1-KO mice (b vs d, p=0.0194; Fig. 7A), suggesting that increased miR-210 expression during colitis is NTR1-dependent.
Figure 7.
MiR-210 levels of expression in (A) colon samples from WT or NTR1-KO mice (n=7), treated as indicated with TNBS (48 h) and in (B) human colon samples (n=12 per group) from healthy subjects (shown as mean +/− SEM) and patients with UC or Crohn’s Disease (CD) (shown as individual values), were assessed by qPCR. Statistical analysis was performed using student’s t test. *p<0.05
Colonic miR-210 expression is increased in the colon of UC patients
To confirm and extend our animal model findings in IBD tissues, we assessed the expression levels of miR-210 in colon specimens obtained from UC and CD patients. We found that miR-210 mRNA expression is significantly increased in colon tissue samples from UC, but not CD patients compared to control samples (a vs b, p=0.0027 and b vs c, p=0.0009; Fig. 7B). Importantly, all UC colon samples showed higher miR-210 expression than the mean level of miR-210 expression detected in control samples (Fig. 7B). In contrast CD patients’ colonic samples showed no significant changes in colonic miR-210 levels compared to controls.
Discussion
NT and NTR1 expression is increased in both human and animal intestine during experimental colitis and IBD9, 11. NT/NTR1 pro-inflammatory signaling in colonic epithelial cells23, 26, 31, 32 is linked to the development and progress of colitis9, 10, 33, by mechanisms related, at least in part, to increased angiogenesis, via a NT-regulated HIF-1α -dependent pathway at the colonocyte level8. We have recently presented evidence supporting the ability of NT to differentially regulate expression of miRNAs in human colonocytes16, with miR-210 showing the highest up-regulation in response to NT16. We now show increased expression of miR-210 in colonic biopsies of UC patients as well as in the colon of mice with experimental colitis (Fig. 7A & B), and present evidence that increased miR-210 expression during colitis is NTR1-dependent (Fig. 7A). Most importantly, we show that intracolonic silencing of miR-210 attenuates experimental colitis (Fig. 4B & C), inhibits miR-210-driven angiogenesis (Fig. 5B & C), and reduces inflammatory cytokine expression (Figs. 1A & B and 4A). Lastly, using two genetically modified HIF-1α mouse models we present direct in vivo evidence suggesting the importance of epithelial HIF-1α activity in the regulation of miR-210 expression in the colon (Figs. 3 and 5).
Our results demonstrate increased expression of miR-210 in human (UC) and experimental colitis. ΜiR-210 is frequently referred to as “hypoxamir” since its expression is increased in a variety of cell types during hypoxia in response to HIF-1α transcriptional activity34 and has been the focus of several laboratories, since hypoxia is a common hallmark of inflammation35. Expression of mir-210 is decreased in the gastric mucosa during chronic inflammation with Helicobacter pylori36, but increased in murine macrophages treated with lipopolysaccharide (LPS), leading to diminished LPS-induced pro-inflammatory cytokine expression, suggesting an anti-inflammatory role for miR-21037. In acute kidney tissue injury, circulating levels of miR-210 are significantly up-regulated and correlated with patient mortality suggesting, a pro-inflammatory role for this miRNA38. MiR-210 was also shown to have a pro-inflammatory role by promoting inflammatory cytokine expression in CD-4+ T cells from psoriasis vulgaris patients39. Together, our results from the intestine and intestinal epithelial cells compared to results from other organs (stomach or skin) and different cells (macrophages or T-cells) indicate that regulation of miR-210 expression is tissue- and cell type-specific and it differs during acute or chronic inflammation. Interestingly, miR-155, which is also highly up-regulated in response to NT signaling16, targets HIF-1α expression and provides a negative-feedback loop for the resolution of HIF-1α activity in cells exposed to prolonged hypoxia, leading to oscillatory behavior of HIF-1α-dependent transcription40. Studies examining the role of miR-155 in the context of HIF-1α and NT are certainly warranted.
NT signaling causes the stabilization, nuclear translocation and transcriptional activity of HIF-1α8, an important transcription factor for miR-210 expression18–20. During colitis, NT and NTR1 expression is increased9, 11 affecting HIF-1α transcriptional activity8. In the present study we found that colonic miR-210 expression is increased in WT mice with experimental colitis (Fig. 7A), as well as in mice overexpressing HIF-1α in intestinal epithelial cells both at base line and during colitis (b & e; Fig. 3). Consistent with these findings, HIF-1α epithelial cell specific deficient mice had reduced miR-210 levels at base line and during colitis (c & f; Fig. 3). Using NTR1 deficient mice we also present direct evidence that the regulation of miR-210 during colitis is NTR1-dependent (Fig. 7A). In support of our in vivo findings, NT treatment of colonic epithelial cells resulted in increased miR-210 expression in a HIF-1α-dependent manner (Fig. 2). The mechanism of NT-mediated HIF-1α activation8 involves, at least in part, a decrease in PHD2-HIF-1α interaction (Suppl. Fig. 2A). De novo synthesized cytoplasmic HIF-1α is rapidly hydroxylated by PHDs (primarily PHD2), a recognition signal for binding of von Hippel-Lindau (pVHL) ubiquitin E3 ligase complex and subsequent ubiquitination and degradation of HIF-1α41. Attenuation of PHD2/HIF-1α interaction leads to accumulation of HIF-1α at the protein level, its nuclear translocation and subsequent miR-210 up-regulation (Fig. 2), through HIF-1α transcriptional activity42–44.
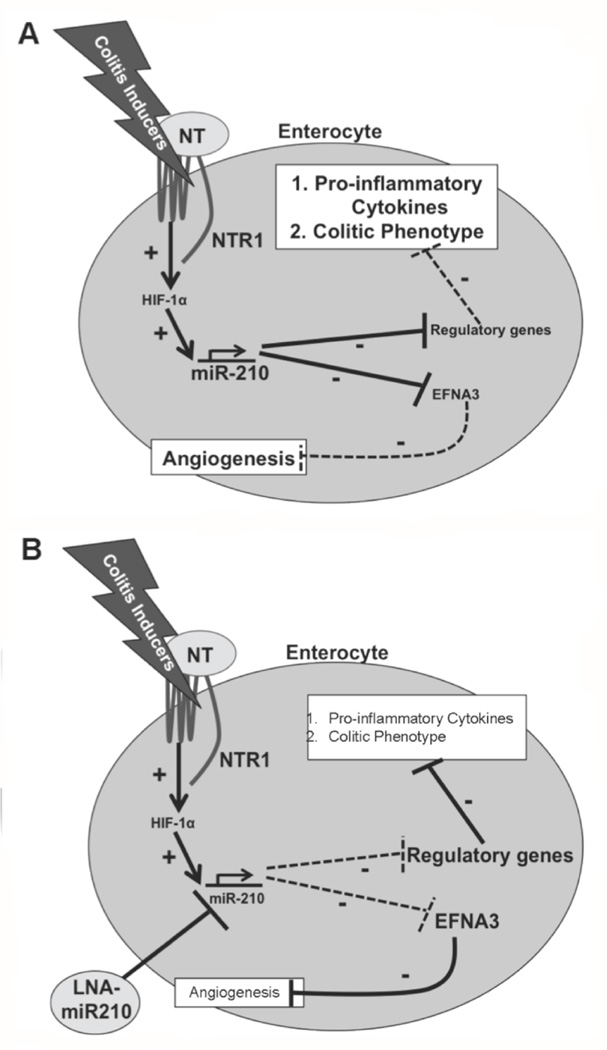
Using in vitro silencing with LNA-anti-miR-210 in two different colonic epithelial cell lines we found that miR-210 reduces NT-induced TNFα and IL-6 expression (Fig. 1), both NF-κB and MAPK-driven, pro-inflammatory cytokines45–48. Consistent with these results, signaling of NTR1 to both NF-κB- and MAPK-driven pathways has also been reported25, 32, while miR-210 is also linked to NF-κB and MAPK activation49, 50. Importantly in vivo intracolonic silencing of miR-210 reduced colonic levels of both TNFα and IL-6 in mice with TNBS-induced colitis (Fig. 4A), and decreased histologic colitis scores (Fig. 4B & C), confirming our cellular findings and supporting a pro-inflammatory role for this miRNA in colitis pathophysiology (Fig. 8).
Figure 8.
(A) Schematic representation of NT/NTR1 signaling in a colitic flare involving the transcription factor HIF-1α and miR-210. (+) indicate stimulation while (−) indicate inhibition of downstream targets. Solid arrows indicate intracellular effects while doted arrows indicate effects intercellular communication. (B) Schematic representation of the anti-inflammatory role of in vivo silencing of miR-210.
Histologic colitis scores of transgenic mice overexpressing or lacking HIF-1α did not show any difference on the degree of colitis compared to wild type, TNBS-exposed mice (Suppl. Fig. 3) suggesting that in the acute 2 day colitis model used in our study HIF-1α expression alone is not sufficient to affect colitis phenotype. This is in contrast to a previous mouse TNBS colitis study showing that decreased epithelial cell expression of HIF-1α correlated with more severe clinical symptoms, while increased epithelial cell expression of HIF-1α was protective51. Differences in the TNBS treatment duration and dose may account for the colitis phenotype differences between our study and the study by Karhausen and colleagues51. Karhausen et al51 used a chronic TNBS colitis model where mice were pre-sensitized with TNBS and were then treated with intracolonic TNBS enema (2.5 mg / mouse of 20 g, 7 days), while in our study an acute model of TNBS-induced experimental colitis (5 mg / mouse of 20 g, 2 days) was used.
We show that NT-induced HIF-1α promotes neovascularization through the up-regulation of miR-210 that in turn negatively regulates the expression of genes that inhibit angiogenesis. One such miR-210 target is the angiogenesis blocker EFNA330. Indeed, our results show attenuated EFNA3 expression during TNBS colitis that was reversed in LNA-anti-miR-210 pre-treated mice (Fig. 6). As expected, TNBS-induced colonic angiogenesis was attenuated in HIF-1α-KO mice and increased in HIF-1α-OE mice (Fig. 5). These results suggest that NT, at least in part, promotes colonic angiogenesis via a HIF-1α-miR-210-EFNA3 pathway (Fig. 8).
To our knowledge, this is the first report indicating HIF-1α as the main transcription factor responsible for NT-induced miR-210 up-regulation. Moreover, our studies using a significant number of transgenic mice elucidate an important mechanism by which NT exerts its pro-inflammatory and angiogenic effects by promoting miR-210 expression in intestinal epithelial cells. Our results point to miR-210 as a potential novel target treatment of colonic inflammation while the relative expression levels of mir-210 and/or its downstream target EFNA3, may serve as potential biomarkers for UC diagnosis.
Supplementary Material
Acknowledgments
Source of Support: Supported by NIH DK60729, P50 DK64539, DK 47373, P30 DK41301, Animal Models Core (CP), and CA148828 (YMS); Fellowship Grant Awards from the Crohn’s and Colitis Foundation of America, Inc (KB, IKML & XX). Support was also provided by the Blinder Research Foundation for Crohn’s Disease, and the Eli and Edythe Broad Chair (CP), Research Scholar Award from American Gastroenterological Association (XX).
We would like to thank Dr. Sean P. Colgan, University of Colorado for his guidance and for providing mouse tissue samples used for preliminary experiments, which lead to this study and the Translational Pathology Core Laboratory (TPCL) at the University of California at Los Angeles for processing colonic tissue sections.
Abbreviations
- as
antisense
- HIF
hypoxia inducible factor
- IBD
inflammatory bowel disease
- miR
microRNA
- NT
neurotensin
- NTR1
neurotensin receptor 1 (high affinity)
- si
small interfering
- TNBS
2,4,6-trinitrobenzene sulfonic acid
- UC
ulcerative colitis
- UTR
un-translated regions
- VEGF
vascular endothelial growth factor
- vWF
von Willebrand Factor
References
- 1.Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. Journal of molecular medicine. 2007;85:1295–1300. doi: 10.1007/s00109-007-0277-z. [DOI] [PubMed] [Google Scholar]
- 2.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nature reviews. Gastroenterology & hepatology. 2010;7:281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. The Journal of experimental medicine. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semenza GL. Angiogenesis in ischemic and neoplastic disorders. Annual review of medicine. 2003;54:17–28. doi: 10.1146/annurev.med.54.101601.152418. [DOI] [PubMed] [Google Scholar]
- 5.Feinman R, Deitch EA, Watkins AC, Abungu B, Colorado I, Kannan KB, Sheth SU, Caputo FJ, Lu Q, Ramanathan M, Attan S, Badami CD, Doucet D, Barlos D, Bosch-Marce M, Semenza GL, Xu DZ. HIF-1 mediates pathogenic inflammatory responses to intestinal ischemia-reperfusion injury. American journal of physiology. Gastrointestinal and liver physiology. 2010;299:G833–G843. doi: 10.1152/ajpgi.00065.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scharte M, Jurk K, Kehrel B, Zarbock A, Van Aken H, Singbartl K. IL-4 enhances hypoxia induced HIF-1alpha protein levels in human transformed intestinal cells. FEBS letters. 2006;580:6399–6404. doi: 10.1016/j.febslet.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 7.Hellwig-Burgel T, Stiehl DP, Wagner AE, Metzen E, Jelkmann W. Review: hypoxia-inducible factor-1 (HIF-1): a novel transcription factor in immune reactions. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2005;25:297–310. doi: 10.1089/jir.2005.25.297. [DOI] [PubMed] [Google Scholar]
- 8.Bakirtzi K, West G, Fiocchi C, Law IK, Iliopoulos D, Pothoulakis C. The neurotensin-HIF-1alpha-VEGFalpha axis orchestrates hypoxia, colonic inflammation, and intestinal angiogenesis. The American journal of pathology. 2014;184:3405–3414. doi: 10.1016/j.ajpath.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castagliuolo I, Wang CC, Valenick L, Pasha A, Nikulasson S, Carraway RE, Pothoulakis C. Neurotensin is a proinflammatory neuropeptide in colonic inflammation. The Journal of clinical investigation. 1999;103:843–849. doi: 10.1172/JCI4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koon HW, Kim YS, Xu H, Kumar A, Zhao D, Karagiannides I, Dobner PR, Pothoulakis C. Neurotensin induces IL-6 secretion in mouse preadipocytes and adipose tissues during 2,4,6,-trinitrobenzensulphonic acid-induced colitis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8766–8771. doi: 10.1073/pnas.0903499106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brun P, Mastrotto C, Beggiao E, Stefani A, Barzon L, Sturniolo GC, Palu G, Castagliuolo I. Neuropeptide neurotensin stimulates intestinal wound healing following chronic intestinal inflammation. American journal of physiology. Gastrointestinal and liver physiology. 2005;288:G621–G629. doi: 10.1152/ajpgi.00140.2004. [DOI] [PubMed] [Google Scholar]
- 12.Maoret JJ, Anini Y, Rouyer-Fessard C, Gully D, Laburthe M. Neurotensin and a non-peptide neurotensin receptor antagonist control human colon cancer cell growth in cell culture and in cells xenografted into nude mice. International journal of cancer. Journal international du cancer. 1999;80:448–454. doi: 10.1002/(sici)1097-0215(19990129)80:3<448::aid-ijc19>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 13.Tasuta M, Iishi H, Baba M, Taniguchi H. Enhancement by neurotensin of experimental carcinogenesis induced in rat colon by azoxymethane. British journal of cancer. 1990;62:368–371. doi: 10.1038/bjc.1990.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKenna LB, Schug J, Vourekas A, McKenna JB, Bramswig NC, Friedman JR, Kaestner KH. MicroRNAs control intestinal epithelial differentiation, architecture, and barrier function. Gastroenterology. 2010;139:1654–1664. 1664, e1. doi: 10.1053/j.gastro.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakirtzi K, Hatziapostolou M, Karagiannides I, Polytarchou C, Jaeger S, Iliopoulos D, Pothoulakis C. Neurotensin signaling activates microRNAs-21 and-155 and Akt, promotes tumor growth in mice, and is increased in human colon tumors. Gastroenterology. 2011;141:1749–1761. e1. doi: 10.1053/j.gastro.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Law IK, Bakirtzi K, Polytarchou C, Oikonomopoulos A, Hommes D, Iliopoulos D, Pothoulakis C. Neurotensin-regulated miR-133alpha is involved in proinflammatory signalling in human colonic epithelial cells and in experimental colitis. Gut. 2014 doi: 10.1136/gutjnl-2014-307329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, Calin GA, Ivan M. A microRNA signature of hypoxia. Molecular and cellular biology. 2007;27:1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang X, Le QT, Giaccia AJ. MiR-210--micromanager of the hypoxia pathway. Trends in molecular medicine. 2010;16:230–237. doi: 10.1016/j.molmed.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devlin C, Greco S, Martelli F, Ivan M. miR-210: More than a silent player in hypoxia. IUBMB life. 2011;63:94–100. doi: 10.1002/iub.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue X, Ramakrishnan S, Anderson E, Taylor M, Zimmermann EM, Spence JR, Huang S, Greenson JK, Shah YM. Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology. 2013;145:831–841. doi: 10.1053/j.gastro.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell metabolism. 2009;9:152–164. doi: 10.1016/j.cmet.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao D, Keates AC, Kuhnt-Moore S, Moyer MP, Kelly CP, Pothoulakis C. Signal transduction pathways mediating neurotensin-stimulated interleukin-8 expression in human colonocytes. The Journal of biological chemistry. 2001;276:44464–44471. doi: 10.1074/jbc.M104942200. [DOI] [PubMed] [Google Scholar]
- 24.Castagliuolo I, Morteau O, Keates AC, Valenick L, Wang CC, Zacks J, Lu B, Gerard NP, Pothoulakis C. Protective effects of neurokinin-1 receptor during colitis in mice: role of the epidermal growth factor receptor. British journal of pharmacology. 2002;136:271–279. doi: 10.1038/sj.bjp.0704697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao D, Kuhnt-Moore S, Zeng H, Wu JS, Moyer MP, Pothoulakis C. Neurotensin stimulates IL-8 expression in human colonic epithelial cells through Rho GTPase-mediated NF-kappa B pathways. American journal of physiology. Cell physiology. 2003;284:C1397–C1404. doi: 10.1152/ajpcell.00328.2002. [DOI] [PubMed] [Google Scholar]
- 26.Zhao D, Zhan Y, Zeng H, Koon HW, Moyer MP, Pothoulakis C. Neurotensin stimulates interleukin-8 expression through modulation of I kappa B alpha phosphorylation and p65 transcriptional activity: involvement of protein kinase C alpha. Molecular pharmacology. 2005;67:2025–2031. doi: 10.1124/mol.104.010801. [DOI] [PubMed] [Google Scholar]
- 27.Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK, Story M, Le QT, Giaccia AJ. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Molecular cell. 2009;35:856–867. doi: 10.1016/j.molcel.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. The EMBO journal. 2003;22:4082–4090. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao D, Bakirtzi K, Zhan Y, Zeng H, Koon HW, Pothoulakis C. Insulin-like growth factor-1 receptor transactivation modulates the inflammatory and proliferative responses of neurotensin in human colonic epithelial cells. The Journal of biological chemistry. 2011;286:6092–6099. doi: 10.1074/jbc.M110.192534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fasanaro P, D'Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. The Journal of biological chemistry. 2008;283:15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao D, Zhan Y, Zeng H, Koon HW, Moyer MP, Pothoulakis C. Neurotensin stimulates expression of early growth response gene-1 and EGF receptor through MAP kinase activation in human colonic epithelial cells. International journal of cancer. Journal international du cancer. 2007;120:1652–1656. doi: 10.1002/ijc.22407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao D, Zhan Y, Koon HW, Zeng H, Keates S, Moyer MP, Pothoulakis C. Metalloproteinase-dependent transforming growth factor-alpha release mediates neurotensin-stimulated MAP kinase activation in human colonic epithelial cells. The Journal of biological chemistry. 2004;279:43547–4354. doi: 10.1074/jbc.M401453200. [DOI] [PubMed] [Google Scholar]
- 33.Bugni JM, Rabadi LA, Jubbal K, Karagiannides I, Lawson G, Pothoulakis C. The neurotensin receptor-1 promotes tumor development in a sporadic but not an inflammation-associated mouse model of colon cancer. International journal of cancer. Journal international du cancer. 2012;130:1798–1805. doi: 10.1002/ijc.26208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan YC, Banerjee J, Choi SY, Sen CK. miR-210: the master hypoxamir. Microcirculation. 2012;19:215–223. doi: 10.1111/j.1549-8719.2011.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kokura S, Yoshida N, Yoshikawa T. Anoxia/reoxygenation-induced leukocyte-endothelial cell interactions. Free radical biology & medicine. 2002;33:427–432. doi: 10.1016/s0891-5849(02)00852-3. [DOI] [PubMed] [Google Scholar]
- 36.Kiga K, Mimuro H, Suzuki M, Shinozaki-Ushiku A, Kobayashi T, Sanada T, Kim M, Ogawa M, Iwasaki YW, Kayo H, Fukuda-Yuzawa Y, Yashiro M, Fukayama M, Fukao T, Sasakawa C. Epigenetic silencing of miR-210 increases the proliferation of gastric epithelium during chronic Helicobacter pylori infection. Nature communications. 2014;5:4497. doi: 10.1038/ncomms5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi J, Qiao Y, Wang P, Li S, Zhao W, Gao C. microRNA-210 negatively regulates LPS-induced production of proinflammatory cytokines by targeting NF-kappaB1 in murine macrophages. FEBS letters. 2012;586:1201–1207. doi: 10.1016/j.febslet.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 38.Lorenzen JM, Kielstein JT, Hafer C, Gupta SK, Kumpers P, Faulhaber-Walter R, Haller H, Fliser D, Thum T. Circulating miR-210 predicts survival in critically ill patients with acute kidney injury. Clinical journal of the American Society of Nephrology : CJASN. 2011;6:1540–1546. doi: 10.2215/CJN.00430111. [DOI] [PubMed] [Google Scholar]
- 39.Zhao M, Wang LT, Liang GP, Zhang P, Deng XJ, Tang Q, Zhai HY, Chang CC, Su YW, Lu QJ. Up-regulation of microRNA-210 induces immune dysfunction via targeting FOXP3 in CD4(+) T cells of psoriasis vulgaris. Clinical immunology. 2014;150:22–30. doi: 10.1016/j.clim.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Bruning U, Cerone L, Neufeld Z, Fitzpatrick SF, Cheong A, Scholz CC, Simpson DA, Leonard MO, Tambuwala MM, Cummins EP, Taylor CT. MicroRNA-155 promotes resolution of hypoxia-inducible factor 1alpha activity during prolonged hypoxia. Molecular and cellular biology. 2011;31:4087–4096. doi: 10.1128/MCB.01276-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Molecular pharmacology. 2006;70:1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 42.Chan SY, Loscalzo J. MicroRNA-210: a unique and pleiotropic hypoxamir. Cell cycle. 2010;9:1072–1083. doi: 10.4161/cc.9.6.11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCormick RI, Blick C, Ragoussis J, Schoedel J, Mole DR, Young AC, Selby PJ, Banks RE, Harris AL. miR-210 is a target of hypoxia-inducible factors 1 and 2 in renal cancer, regulates ISCU and correlates with good prognosis. British journal of cancer. 2013;108:1133–1142. doi: 10.1038/bjc.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cicchillitti L, Di Stefano V, Isaia E, Crimaldi L, Fasanaro P, Ambrosino V, Antonini A, Capogrossi MC, Gaetano C, Piaggio G, Martelli F. Hypoxia-inducible factor 1-alpha induces miR-210 in normoxic differentiating myoblasts. The Journal of biological chemistry. 2012;287:44761–44771. doi: 10.1074/jbc.M112.421255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Molecular and cellular biology. 1990;10:2327–2234. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Molecular and cellular biology. 1990;10:1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong Z, Qi X, Fidler IJ. Tyrosine phosphorylation of mitogen-activated protein kinases is necessary for activation of murine macrophages by natural and synthetic bacterial products. The Journal of experimental medicine. 1993;177:1071–1077. doi: 10.1084/jem.177.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chauhan D, Kharbanda S, Uchiyama H, Urashima M, Fragoso R, Sen J, Kufe DW, Anderson KC. Identification of upstream signals regulating interleukin-6 gene expression during in vitro treatment of human B cells with pokeweed mitogen. Blood. 1994;84:2243–2252. [PubMed] [Google Scholar]
- 49.Kopriva SE, Chiasson VL, Mitchell BM, Chatterjee P. TLR3-induced placental miR-210 down-regulates the STAT6/interleukin-4 pathway. PloS one. 2013;8:e67760. doi: 10.1371/journal.pone.0067760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou H, Chen JX, Yang CS, Yang MQ, Deng Y, Wang H. Gene regulation mediated by microRNAs in response to green tea polyphenol EGCG in mouse lung cancer. BMC genomics. 2014;15(Suppl 11):S3. doi: 10.1186/1471-2164-15-S11-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. The Journal of clinical investigation. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glover LE, Colgan SP. Hypoxia and metabolic factors that influence inflammatory bowel disease pathogenesis. Gastroenterology. 2011;140:1748–1755. doi: 10.1053/j.gastro.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanaka T, Nangaku M. Angiogenesis and hypoxia in the kidney. Nature reviews. Nephrology. 2013;9:211–222. doi: 10.1038/nrneph.2013.35. [DOI] [PubMed] [Google Scholar]
- 54.Cullberg KB, Olholm J, Paulsen SK, Foldager CB, Lind M, Richelsen B, Pedersen SB. Resveratrol has inhibitory effects on the hypoxia-induced inflammation and angiogenesis in human adipose tissue in vitro. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2013;49:251–257. doi: 10.1016/j.ejps.2013.02.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.