Abstract
Objective
HIV-infected older adults (HOA) are at risk of functional decline. Interventions promoting physical activity that can attenuate functional decline and are easily translated into the HOA community are of high priority. We conducted a randomized, controlled clinical trial to evaluate whether a physical activity counseling intervention based on self-determination theory (SDT) improves physical function, autonomous motivation, depression and the quality of life (QOL) in HOA.
Methods
A total of 67 community-dwelling HOA with mild-to-moderate functional limitations were randomized to one of two groups: a physical activity counseling group or the usual care control group. We used SDT to guide the development of the experimental intervention. Outcome measures that were collected at baseline and final study visits included a battery of physical function tests, levels of physical activity, autonomous motivation, depression, and QOL.
Results
The study participants were similar in their demographic and clinical characteristics in both the treatment and control groups. Overall physical performance, gait speed, measures of endurance and strength, and levels of physical activity improved in the treatment group compared to the control group (p<0.05). Measures of autonomous regulation such as identified regulation, and measures of depression and QOL improved significantly in the treatment group compared to the control group (p<0.05). Across the groups, improvement in intrinsic regulation and QOL correlated with an improvement in physical function (p<0.05).
Conclusion
Our findings suggest that a physical activity counseling program grounded in SDT can improve physical function, autonomous motivation, depression, and QOL in HOA with functional limitations.
INTRODUCTION
The age distribution of the American population is currently experiencing a dramatic demographic shift towards that of older ages. This demographic shift is also apparent among HIV-infected individuals, who are living longer in part due to more effective anti-retroviral therapy (ART) and infections occurring later in life. The number of HIV-infected older adults (HOA) has significantly increased over the last two decades (Effros et al., 2008). HIV-infected individuals are identified by the CDC as being “older adults” at the relatively young age of 50 years old (“HIV Among People Aged 50 and Older,” para. 1). Some argue that HIV-infected adults experience signs of accelerated aging at a younger age compared to the general population (Pathai, Bajillan, Landay, & High, 2014). Individuals with HIV infection, for example, are at risk of complex chronic diseases with multiple comorbidities at an earlier age than uninfected individuals (Guaraldi et al., 2011). Further, 50 years and older happens to reflect the upper age range for HIV-infected individuals (Luther & Wilkin, 2007) as the life expectancy of infected individuals despite adequate ART is still lower than that of the general population (Hogg et al., 2008).
Studies have shown that this cohort can have reduced physical function and can experience higher prevalence of physical frailty characteristics at a younger age compared to uninfected individuals (Desquilbet et al., 2009; Oursler, Sorkin, Smith, & Katzel, 2006; Rees, Meister, Mohler, & Klotz, 2014; Shah et al., 2014). Some of the functional impairments seen in HIV-infected individuals are comparable in a physiological sense to those sometimes seen in 10–15 years older people who are uninfected (Desquilbet et al., 2007). We note that there is research demonstrating functional impairments in this population (Brothers et al., 2014). However, the research evaluating the level and intensity of physical activity (PA) engagement in this population is limited and inconclusive (Schuelter-Trevisol et al., 2012). One study reported that HIV-infected individuals engage in less vigorous physical activity than HIV-uninfected ones (Smit et al., 2006), while a separate study reported the opposite (Fillipas et al., 2008).
It is well known that even modest amounts of PA can improve physical function and quality of life (QOL) in older adults (Pahor et al., 2006). However, the majority of older adults do not exert enough PA to realize the benefits (Harvey, Chastin, & Skelton, 2013; Taylor, 2014). The promotion of PA has become a public health priority worldwide. Unfortunately, the HIV infected population is a challenging cohort with respect to behavior change. HIV-infected patients, and particularly HOA, tend to be socioeconomically disadvantaged, live in isolation because of their often limited support network, suffer from depression, experience negative perceptions in the community, and can have multiple comorbidities (Emlet, 2006; Lyons, Pitts, Grierson, Thorpe, & Power, 2010; Roger, Mignone, & Kirkland, 2013; Shippy & Karpiak, 2005). These factors can negatively impact HOA persons’ motivation and ability to comply with recommended behavior change such as engaging in PA.
Interestingly, findings from a recent study suggest that physical frailty in HIV-infected patients is potentially reversible and is positively associated with depression and inversely related with low PA (Rees, Meister, Mohler, & Klotz, 2014). In this study, essentially all of the participants who were frail and had low PA were also depressed. This study concluded that interventions aimed at increasing PA and stimulating emotional well-being are needed to prevent and ameliorate physical frailty in this target population. To date, the majority of research in HIV and frailty has focused on the understanding of biological and social determinants of HIV-related frailty and functional decline (Brothers et al., 2014). There has been limited research done with respect to identifying effective translational interventions aimed at preventing or ameliorating frailty in this group. Further research is warranted to develop evidence-based guidelines to promote PA, and thereby prevent functional decline in this challenging population.
Previous clinical trials conducted in the HIV-uninfected adult population suggest that the self-determination theory (SDT) is a suitable framework for understanding and promoting PA (Fortier, Duda, Guerin, & Teixeira, 2012; Teixeira, Carraca, Markland, Silva, & Ryan, 2012). SDT is a unique and effective theory of human motivation in which a patient’s needs for autonomy are acknowledged (Ryan & Deci, 2000; Teixeira, Carraca, Markland, Silva, & Ryan, 2012). The SDT environment encourages one to become self-determined (autonomous) to engage in activities, while at the same time internalizing the motivation to regulated behaviors, which may not be initially interesting or valued. To foster this internalization, a patient-centered approach is used. An approach that satisfies the psychological needs of relatedness, autonomy, and competence is encouraged (Deci & Ryan, 2012; Ryan & Deci, 2000; Teixeira, Carraca, Markland, Silva, & Ryan, 2012).
An intervention that fosters psychological need satisfaction for PA can boost emotional well-being and help alleviate some of the barriers that would normally prevent HOA from engaging in PA. Of note, SDT has been used for PA intervention clinical trials for adults and has been proven to be beneficial in improving PA (Teixeira, Carraca, Markland, Silva, & Ryan, 2012). The majority of the SDT-based PA intervention trials in adults had duration of about 3 months or less with the participants (Edmunds, Ntoumanis, & Duda, 2008; Fortier, Sweet, O’Sullivan, & Williams, 2007; Levy & Cardinal, 2004). In one 13-week study, the contact time between the study team and participant entailed three in-person counseling sessions followed by three telephone sessions (Fortier, Sweet, O’Sullivan, & Williams, 2007). This study found that the intervention was successful in changing autonomous motivation and increasing PA.
Similarly, a 10-week study with follow-up at one year found that it’s SDT based PA intervention resulted in increased PA engagement at follow-up (Van Hoecke et al., 2013). Another study with 30 intervention sessions over one year duration demonstrated increased autonomous motivation and higher PA engagement in the intervention group (Silva et al., 2010). Autonomous motivation was found to be a mediator of long-term engagement in PA (Silva et al., 2011). Moreover, a previous clinical trial has demonstrated that autonomous motivation is also a predictor of improved exercise capacity (Mildestvedt, Meland, & Eide, 2008). Taken together, these findings suggest that improvements in autonomous motivation can not only contribute to maintenance of PA, but also can potentially predict improvements in PA related physiologic outcomes. Therefore, the literature on SDT based PA interventions in adults provides evidence for using SDT-based interventions in improving PA levels and changing PA behavior. However, there is paucity of research using SDT-based intervention in evaluating the effect on physiological and biological markers of PA-related health outcomes such as physical function.
Physical function is an important health outcome because functional decline is associated with negative health outcomes such as increased morbidity, mortality, nursing home admissions, and poor QOL (Fried et al., 2001). In fact, one very recent study in HOA demonstrated that physical function is a predictor of QOL independent of age, comorbidities, and immune function (Erlandson et al., 2014) suggesting the importance of designing interventions that can improve physical function in this population.
The primary purpose of our study was to evaluate the effect of a PA counseling intervention grounded in SDT on physical function compared to the usual care in HOA. Our hypothesis was that physical function will improve in the treatment group compared to the control group. The secondary purpose of our study was to evaluate the effect of our intervention on PA specific needs of autonomy and competence, autonomous motivation for PA, depression, and QOL. Our hypothesis was that the above-mentioned variables will improve in the treatment group compared to the control group. Lastly, we explored whether changes in physical function are associated with changes in QOL and autonomous motivation. Our hypothesis was that changes in physical function will positively correlate with changes with QOL and autonomous motivation.
METHODS
Participants
Community-dwelling HOA were recruited from our urban University of Rochester Medical Center (URMC) hospital-based infectious disease clinic, which provides services to over 1,000 HIV-infected patients. Eligibility criteria for study enrollment included: HIV positive, age ≥45 years, stable antiretroviral therapy (ART), and functional limitations as measured by physical performance test score <36. Exclusion criteria included: any AIDS defining illness for six months prior to enrollment or comorbid medical conditions that would prevent the participant from engaging in PA. Participants with severe cardiopulmonary illness, severe anemia, significant orthopedic or neuromuscular limitations, renal failure, cirrhosis, significant cognitive or sensory limitations, untreated depression, unstable manic or psychotic disorder, and active malignancy were excluded. Participant medical histories were obtained from self-completed questionnaires and from electronic medical records. An informed consent was obtained from all participants prior to enrollment and the study was approved by the URMC’s Research Participants Review Board.
Study Design
Baseline Assessments
Assessment of Physical Function
The Physical Performance Test (PPT) is a global measure of physical function that assesses the ability to perform usual daily activities (Brown, Sinacore, Binder, & Kohrt, 2000; Shah et al., 2012; Villareal et al., 2011). The PPT includes seven standardized timed tasks (50-foot walk, putting on and removing a coat, picking up a penny, standing up from a chair, lifting a book, climbing one flight of stairs, and a progressive Romberg test) and two additional tasks (climbing four flights of stairs and performing a 360 degree turn). The score for each item ranges between 0 and 4, with a perfect score equal to 36 (Brown, Sinacore, Binder, & Kohrt, 2000; Shah et al., 2012; Villareal et al., 2011). A MicroFET 2, hand-held dynamometer was used to measure knee extension and flexion muscle strength (Schaubert & Bohannon, 2005). Isometric strength was determined by calculating the mean peak force from three trials. Dynamic balance was assessed as the time needed to complete an obstacle course whereby participants stand from a sitting position from a standard 18-inch high chair, walk forward 6 feet, step over a 2 × 2-inch obstacle, walk forward another 6 feet, ascend a 6-inch high curb, turn around, step down off the curb, and return to the chair as quickly and safely as possible (Brown, Sinacore, Binder, & Kohrt, 2000; Shah et al., 2012; Villareal et al., 2011). Aerobic capacity was evaluated by a six-minute walk test (Enright et al., 2003) in which participants were instructed to cover as much distance as possible in six minutes by walking laps over a 100-feet course. Finally, gait speed was measured as the time needed to walk 50 feet as quickly and safely as possible (Brown, Sinacore, Binder, & Kohrt, 2000; Shah et al., 2012; Villareal et al., 2011). All functional measures were performed by a single experienced technician who was blinded to the study groups.
Assessment of Physical Activity Level
Each participant’s level of PA was determined by the 12-item Physical Activities Scale for the Elderly (PASE) questionnaire (Washburn, Smith, Jette, & Janney, 1993). The PASE score is determined by questions related to leisure, household, and work-related activities (Washburn, McAuley, Katula, Mihalko, & Boileau, 1999). PASE assesses the frequency, duration, and intensity level (mild, moderate and strenuous) of activity over the previous week with higher scores indicating greater physical activity. We also used the Paffenburger Physical Activity Index to assess weekly energy expended through leisure time PA (Paffenbarger et al., 1993; Paffenbarger, Wing, & Hyde, 1995).
Assessment of Self-Determination for PA
This outcome was assessed using SDT based instruments. The 3-item Locus of Causality for PA scale assesses the participant’s autonomy regarding performing PA (Markland & Hardy, 1997; Silva et al., 2008). This scale indicates the extent to which the individual feels that he/she chooses to engage in PA rather than feeling that they are pressured to do so. Responses are scored on a 7-point Likert scale, ranging from 1 (not true at all) to 7 (very true), with higher scores indicating a greater perceived autonomy or a greater self-determination. Autonomous motivation or regulation for PA was also assessed using the Behavioral Regulation in Exercise Questionnaire-2 (Moustaka, Vlachopoulos, Vazou, Kaperoni, & Markland, 2010), which consists of two subscales: identified regulation (i.e. valuing the benefits of PA) and intrinsic motivation (i.e. experiencing enjoyment of PA). Each item is rated on a 5-point Likert scale ranging from 1 (not true at all for me) to 5 (very true for me) with higher scores indicating more autonomous motivation. The Perceived Competence scale is a 4-item scale that assesses perceived confidence regarding performing PA. Participants rate their degree of confidence on a 7-point Likert scale ranging from 1 (not at all true) to 7 (very true) (Williams, Grow, Freedman, Ryan, & Deci, 1996).
Assessment of Depression
The Beck Depression Inventory-II is a 21-item depression inventory designed to assess depressive symptomatology (Beck, Steer, Ball, & Ranieri, 1996). Scores on the individual items range from 0 to 3. Total scores can range between 0 and 63 with higher scores reflecting higher levels of depression.
Assessment of Health-Related QOL
The Short-Form Health Survey (36-item) is a patient-reported survey of patient health (Erlandson et al., 2014; Lyons, Perry, & Littlepage, 1994; McHorney, Ware, & Raczek, 1993). This survey was used to evaluate the participant’s perception of QOL in the domains of physical functioning, role limitations due to physical health, emotional well-being, social functioning and general health with higher scores indicating greater QOL.
Treatment and Control Interventions
Randomization
Participants were randomly assigned to either the treatment group or the usual care control group. We chose to stratify our participants by gender to balance our study design because there is a higher prevalence of HIV in men compared to women (“HIV Among Men in the United States,” para. 1). Assignment leveraged a computer generated randomization strategy stratified for gender. The randomization algorithm was maintained by a member of the research team who did not interact with the participants.
The treatment group intervention, based on the SDT model of behavior change, consisted of a PA counseling program designed to maximize opportunities for personal decision-making, while giving the support needed to ensure proper education. We conducted 6 patient-centered counseling sessions based on the SDT model of behavior change (Ryan, 2008). The sessions were conducted with the patients in this group over the study duration. The first counseling session was a 60-minute face-to-face session by a physician and an experienced mental health therapist who were trained in SDT. During this first session, we established a rapport and took time to understand the individual’s interests, values, and behaviors. This information was used to assist participants with the behavior change process. Participants were encouraged to discuss barriers to PA and solutions to overcoming them, and when appropriate and desired, they were provided with potential strategies such as time management and self-monitoring. This autonomy supportive session also facilitated participants in setting appropriate PA goals for the following two weeks and an overall action plan. Every two weeks, participants were supported through follow-up telephone counseling sessions. These follow-up sessions were conducted by a graduate student in clinical investigation who was specifically trained in SDT and was also involved in the initial face to face counseling sessions. These 15–30 minute phone calls were used to provide positive feedback, re-evaluate and modify goals if necessary, and to provide autonomy support to facilitate persistence for participants chosen PA goals. To ensure proper education and support, participants in this group were offered an individually tailored PA prescription recommended by an experienced physical therapist that was aimed at improving endurance and strength. Consistent with the SDT model, participants took an active role in the customization of their own PA program. The first component of the program was a walking prescription that provided aerobic exercise of moderate intensity. Based on their values and goals, participants would choose a program of varying degrees of intensity. The second component of the program was a strength training prescription designed to provide moderate to intense resistance exercise targeting both the upper and lower body. The participants, if interested in this program, were given a set of 3 color-coded therapeutic resistance bands representing varying levels of resistance to perform the exercise program and were educated regarding the frequency and intensity of resistance training. They were asked to initiate a number of sets (1 set = 8–15 repetitions), which were customized for each individual for each of the 10 exercises at a moderately challenging level. The participant played an active role in customizing his or her PA program as they were given options to choose the exercises and their frequency and intensity. These exercises targeted the upper and lower body. If possible, the participants were encouraged to progressively increase intensity from their baseline sets and repetitions to a maximum of 4 sets of 15 repetitions for each exercise. The participants in the treatment group were asked not to discuss the study or intervention with other patients during the study period.
Participants assigned to the control group intervention were instructed to maintain their usual activities during the study period. This group did not receive any PA counseling or recommendations.
Evaluation of intervention
After 12 weeks, all assessments performed at baseline were repeated in the treatment and control groups.
Statistical Analysis
The number of participants enrolled in this study was based on the PPT data (primary outcome) we previously obtained (Villareal et al., 2011). We estimated that 28 participants would be needed to detect a clinically meaningful 1.7±2.0 (mean±SD) difference in PPT score change between groups with a > 80% power and a 0.05 significance level.
The analysis was performed using SAS 9.3. Baseline characteristics between groups were compared by using the t-test for unpaired samples for continuous variables and the Chi-square test for categorical variables. PROC MIXED was used to perform one-way repeated ANOVA to determine whether the change in outcomes between the treatment group and the control group was significantly different by examining the group × time effect. Demographic and clinical variables known to affect outcomes, including age, sex, race, alcohol use (yes or no), viral load detectable (yes or no), nadir CD4 cell count and CD4 cell count at baseline, were entered as covariates in the repeated ANOVA analysis. Significant within-group changes were studied by Contrast statement in PROC MIXED. Pearson correlation analysis was applied to investigate the association among residualized change scores (observed score at final – predicted score at final based on baseline score) in the physical functions variables and autonomous motivation and QOL variables. For our tests, p<0.05 was considered statistically significant. Results from repeated ANOVA are reported in Tables 2 to 4, as estimated population means (standard errors), and P-values for group × time effects.
Table 2.
Treatment Effects on Measures of Physical Function and Activity Levels by Repeated ANOVA Adjusting for Demographics
| Variables | Control (n=31) | Treatment (n=28) | Effect Size (SE) | P value |
|---|---|---|---|---|
| PPT score | 1.57 (0.60) | 0.01 | ||
| Baseline | 32.36 ± 0.58 | 32.13 ± 0.58 | ||
| Final | 33.68 ± 0.58* | 35.03 ± 0.58* | ||
| Gait Speed (m/s) | 0.12 (0.05) | 0.01 | ||
| Baseline | 1.31 ± 0.04 | 1.24 ± 0.04 | ||
| Final | 1.32 ± 0.04 | 1.38 ± 0.04* | ||
| 6-Min walk (m) | 32.99 (14.91) | 0.03 | ||
| Baseline | 514.74 ± 17.55 | 494.04 ± 18.00 | ||
| Final | 526.04 ± 17.55 | 538.34 ± 18.13* | ||
| Chair rise (sec.) | −2.36 (1.00) | 0.02 | ||
| Baseline | 13.34 ± .76 | 14.49 ± .77 | ||
| Final | 11.88 ± .76 | 10.67 ± .77* | ||
| Knee extension (kg) | 3.17 (1.04) | 0.004 | ||
| Baseline | 17.77 ± 1.04 | 16.66 ± 1.04 | ||
| Final | 18.02 ± 1.04 | 20.08 ± 1.03* | ||
| Knee flexion (kg) | 1.14 (0.77) | 0.15 | ||
| Baseline | 14.31 ± 0.75 | 14.32 ± 0.76 | ||
| Final | 14.57 ± 0.75 | 15.72 ± 0.75* | ||
| Obstacle course (sec.) | −0.94 (0.49) | 0.06 | ||
| Baseline | 10.29 ± 0.44 | 10.77 ± 0.44 | ||
| Final | 9.69 ± 0.44 | 9.24 ± 0.44* | ||
| PASE score | 56.99 (23.19) | 0.02 | ||
| Baseline | 164.07 ± 24.42 | 121.14 ± 24.38 | ||
| Final | 172.80 ± 24.42 | 186.87 ± 24.38* | ||
| Paff Index, kcal/week | 513.73 (255.91) | 0.05 | ||
| Baseline | 513.92 ± 162.34 | 636.75 ± 161.22 | ||
| Final | 756.33 ± 159.51 | 1392.89 ± 161.22* |
PPT, physical performances test score; PASE, Physical activity scale for the elderly; Paff index, Paffenbarger physical activity index; SE, Standard Error
Covariate adjustment for baseline age, sex, race, alcohol use, viral load detection, nadir CD4 count, and CD4 count
Note: Data are given as Mean ± S.E.
p<0.05 for the comparison of the value at the follow-up time with the within-group baseline value
Table 4.
Treatment Effects on Measures of Depression and Quality of Life (QOL) by Repeated ANOVA Adjusting for Demographics
| Variables | Control (n=31) | Treatment (n=28) | Effect Size (SE) | P value |
|---|---|---|---|---|
| Depression | −3.12 (1.36) | 0.03 | ||
| Baseline | 10.66 ± 2.17 | 13.16 ± 2.16 | ||
| Final | 10.18 ± 2.17 | 9.55 ± 2.16* | ||
| QOL domains | ||||
| Emotional Well-being | 10.97 (4.69) | 0.02 | ||
| Baseline | 79.15 ± 4.23 | 64.78 ± 4.23 | ||
| Final | 76.18 ± 4.23 | 72.78 ± 4.23* | ||
| Role Physical | 21.26 (9.40) | 0.03 | ||
| Baseline | 58.75 ± 8.85 | 57.69 ± 8.85 | ||
| Final | 57.13 ± 8.85 | 77.34 ± 8.85* | ||
| Physical Function | 12.08 (6.71) | 0.08 | ||
| Baseline | 71.94 ± 5.20 | 71.08 ± 5.23 | ||
| Final | 73.07 ± 5.20 | 84.29 ± 5.23* | ||
| General Health | 2.65 (4.11) | 0.52 | ||
| Baseline | 58.57 ± 5.01 | 59.80 ± 5.00 | ||
| Final | 55.38 ± 5.01 | 59.26 ± 5.00 | ||
| Social Functioning | 8.84 (6.44) | 0.18 | ||
| Baseline | 74.41 ± 5.42 | 67.22 ± 5.43 | ||
| Final | 73.60 ± 5.42 | 75.26 ± 5.43 |
Role Physical, Role limitations due to physical health; SE, Standard Error
Covariate adjustment for baseline age, sex, race, alcohol use, viral load detection, nadir CD4 count, and CD4 count
Note: Data are given as Mean ± S.E.
p<0.05 for the comparison of the value at the follow-up time with the within-group baseline value
RESULTS
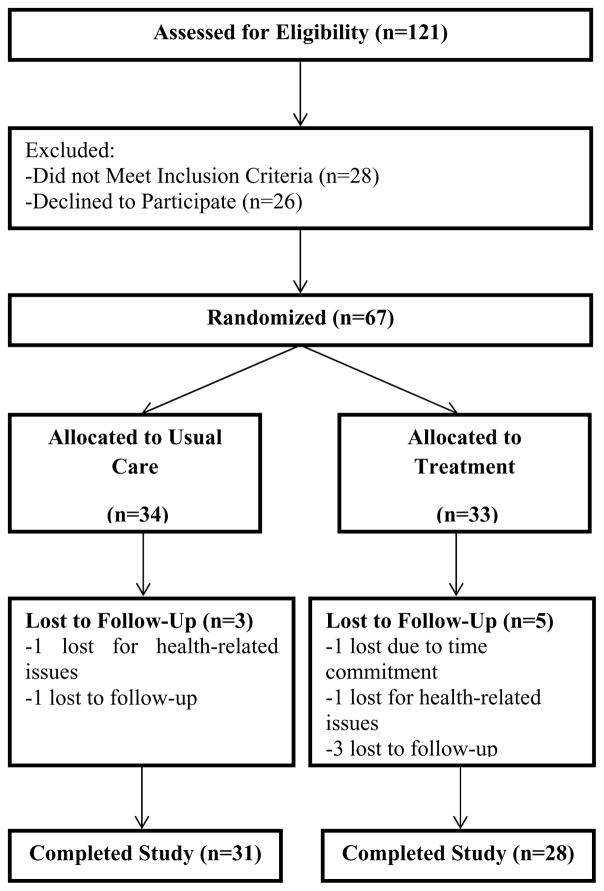
Figure 1 depicts a flow chart of study participation. A total of 121 participants were assessed for eligibility and screening. Of this total, 28 did not meet inclusion criteria, while 26 declined to participate further. The remaining 67 were randomized into two groups: 1) the treatment group (n=33) or 2) the control group (n=34). Eight participants were not able to complete the study for a variety of reasons; 5 participants from the treatment group and 3 participants from the control group (see Figure 1). Thus a total of 59 participants successfully completed the study and were included in the analyses. Ninety-three percent of participants in the treatment group participated in at least four out of six PA counseling sessions.
Figure 1.
Consort Diagram
Table 1 presents selected baseline demographic and clinical characteristics of the 59 participants who completed the study. Mean scores on these variables were similar between the treatment and control groups; none were statistically significantly different (p<0.05).
Table 1.
Demographic and Clinical Characteristics of Study Sample
| Variables | Control (n=31) | Treatment (n=28) | P value |
|---|---|---|---|
| Age, years | 56.23 ± 5.93 | 54.64 ± 6.07 | 0.32 |
| Gender | 0.96 | ||
| Male, n (%) | 19 (61.29) | 17 (60.71) | |
| Female, n (%) | 12 (38.71) | 11 (39.29) | |
| Race | 0.17 | ||
| White, n (%) | 9 (29.03) | 13 (46.43) | |
| Other, n (%) | 22 (70.97) | 15 (53.57) | |
| Education Level | 0.31 | ||
| Less than 12 Years, n (%) | 8 (25.81) | 6 (21.43) | |
| High School Graduate, n (%) | 10 (32.26) | 5 (17.86) | |
| More than High School, n (%) | 13 (41.94) | 17 (60.71) | |
| Currently employed, n (%) | 7 (22.58) | 8 (28.57) | 0.60 |
| HIV duration, years | 18.29 ± 7.33 | 17 ± 6.55 | 0.48 |
| Diabetes, n (%) | 3 (9.68) | 6 (21.43) | 0.21 |
| Hepatitis C Virus (HCV), n (%) | 10 (32.26) | 6 (21.43) | 0.35 |
| Hypertension, n (%) | 16 (51.61) | 12 (42.86) | 0.50 |
| Current Smoking | 0.71 | ||
| Smoker, n (%) | 17 (54.84) | 14 (50) | |
| Non-smoker, n (%) | 14 (45.16) | 14 (50) | |
| Alcohol use, n (%) | 10 (32.26) | 8 (28.57) | 0.76 |
| BMI, Kg/m2 | 26.54 ± 6.03 | 27.30 ± 0.01 | 0.60 |
| Waist circumference, cm | 91.91 ± 15.67 | 92.12 ± 13.42 | 0.96 |
| VACS Index | 30.03 ± 15.63 | 25.82 ± 15.2 | 0.30 |
| Depression Score | 11.26 ± 9.11 | 13.61 ± 10.12 | 0.35 |
| PASE Score | 153.50 ± 114.53 | 127.37 ± 98.98 | 0.35 |
| Peak Viral Load log copies/ml | 10.77 ± 1.78 | 10.58 ± 1.79 | 0.70 |
| Current Viral Load Detectable log copies/ml | 4.55 ± 2.36 | 4.29 ± 1.66 | 0.72 |
| CD4 Count | 678 ± 382 | 675 ± 675 | 0.98 |
| CD4 Nadir | 213 ± 178 | 242 ± 242 | 0.53 |
| CD4/8 Ratio | 0.82 ± 0.38 | 0.92 ± 0.63 | 0.44 |
| Current PI Use, n (%) | 12 (38.71) | 15 (53.57) | 0.25 |
| Current NRTI Use, n (%) | 31 (100.00) | 25 (89.29) | 0.06 |
| Current NNRTI Use, n (%) | 13 (41.94) | 10 (35.71) | 0.62 |
| Current II Use, n (%) | 8 (25.81) | 8 (28.57) | 0.81 |
VACS index, Veterans Aging Cohort Study Risk Index; PI, Protease Inhibitors; NRTI, Nucleoside/Nucleotide; Reverse Transcriptase Inhibitors; NNRTI, Non-Nucleoside Reverse Transcriptase Inhibitors; II, Integrase Inhibitors; PASE, Physical Activity Scale for the Elderly
Note: Data are given as Mean ± S.D.
Table 2 presents group comparisons on measures of physical function and PA levels. Overall physical performance (PPT total score), 6-min walk, gait speed, chair rise, knee extensor strength, PASE score, and weekly energy expenditure (Paff Index) all showed greater improvement in the treatment group compared to controls. The obstacle course demonstrated a trend of improvement in the treatment group as compared to the control group (p=0.06). There was no evidence to indicate greater improvement in knee flexor strength in treatment relative to control group. There were significant improvements in a majority of the measures for physical function and activity within the treatment group, but not in the control group (p<0.05).
Table 3 presents group comparisons of measures of self-determination for PA. Compared to the control group, the treatment group presented significantly higher scores in locus of causality and identified regulation (p<0.05). Our results did not show clear evidence of treatment effects on, perceived competence, or intrinsic regulation (p>0.05).
Table 3.
Treatment Effects on Measures of Self-Determination for Physical Activity (PA) by Repeated ANOVA Adjusting for Demographics
| Variables | Control (n=31) | Treatment (n=28) | Effect Size (SE) | P value |
|---|---|---|---|---|
| Locus of Causality for PA | 2.4 (1.16) | 0.04 | ||
| Baseline | 11.89 ± 1.01 | 12.15 ± 1.01 | ||
| Final | 12.60 ± 1.01 | 15.26 ± 1.01* | ||
| Perceived Competence | −0.18 (0.27) | 0.50 | ||
| Baseline | 5.78 ± 0.34 | 5.95 ± 0.34 | ||
| Final | 5.83 ± 0.34 | 5.82 ± 0.34 | ||
| Autonomous Regulation | ||||
| Identified Regulation | 0.44 (0.19) | 0.02 | ||
| Baseline | 2.60 ± 0.21 | 2.70 ± 0.21 | ||
| Final | 2.39 ± 0.21 | 2.92 ± 0.21 | ||
| Intrinsic Regulation | 0.31 (0.24) | 0.20 | ||
| Baseline | 2.39 ± 0.25 | 2.88 ± 0.25 | ||
| Final | 2.32 ± 0.25 | 3.13 ± 0.25 |
PA, physical activity; SE, Standard Error
Covariate adjustment for baseline age, sex, race, alcohol use, viral load detection, nadir CD4 count, and CD4 count
Note: Data are given as Mean ± S.E.
p<0.05 for the comparison of the value at the follow-up time with the within-group baseline value
Group comparisons on measures of depression and QOL are presented in Table 4. Compared to the control group, the treatment group exhibited significantly improved scores in depression and two QOL domains: emotional well-being and role limitations due to physical health (p<0.05). Physical functioning domain demonstrated a trend of improvement in the treatment group compared to the control group (p=0.08). With regard to general health and social functioning domains of QOL there were no differences between treatment and control groups.
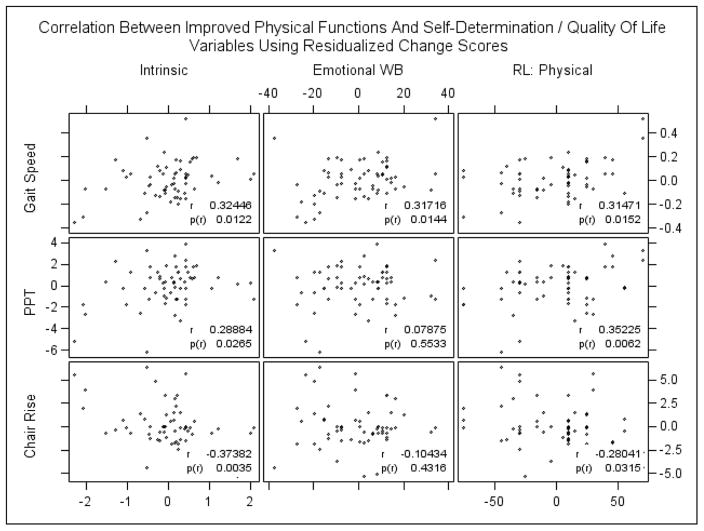
We found that the changes in intrinsic motivation, emotional well-being, and role limitations due to physical health correlated significantly with changes in physical functioning as measured by gait speed (see Figure 2). Furthermore, changes in intrinsic motivation and role limitations due to physical health also correlated significantly with changes in PPT and chair rise (p<0.05). There was no significant correlation between changes in identified regulation and physical function variables. We used residualized change scores to evaluate the correlation of changes in autonomous motivation and QOL domains with changes in physical function variables. The residualized change scores were obtained by regressing each outcome at final stage on their baseline values. In this way, we correct for regression to the mean. We initially fitted cohort-specific correlation models. However, the model fitting results suggested no significant difference in the correlations between the two groups. Therefore, we pooled all of the data and used Pearson correlation analysis to explore the relationships (see Figure 2).
Figure 2.
Intrinsic, Intrinsic Motivation; Emotional WB, Emotional well-being; RL: Physical, Role limitations due to physical health; PPT, physical performances test score.
DISCUSSION
The American population is experiencing a dramatic demographic shift towards older ages, and this includes HIV-infected individuals. The growing number of older adults with HIV is also due to the success of antiretroviral therapy which has extended the lifespan of those infected, an increasing incidence of HIV among older adults, and the overall aging of the population (Onen & Overton, 2009). The HOA cohort is a challenging population because these individuals often exhibit multiple psychosocial problems, such as poor socio-economic conditions, social isolation, HIV stigma, and depression (Emlet, 2006; Roger, Mignone, & Kirkland, 2013; Shippy & Karpiak, 2005), which can interfere with their ability to engage in PA. Moreover, HOA also experience increased comorbidity burden and functional limitations (Onen & Overton, 2009), thereby raising concerns among health care providers about their ability to age successfully.
Although it is known that even a modest amount of PA can improve physical function in older adults, the majority of older adults remains sedentary and has not incorporated this behavior change into their lifestyle (Harvey, Chastin, & Skelton, 2013). As a result, the promotion of PA, especially in older adults, has become a top priority in the public health agenda. The main purpose of this study was thus to evaluate an intervention that can be easily implemented within the HOA community to promote PA and improve physical function. The results suggest that a PA counseling intervention grounded in SDT can improve physical function, depression and QOL in HOA with functional limitations. This finding is especially important because there is a dramatic increase in the number of HOA who are at high risk of functional decline (Desquilbet et al., 2007; Shah et al., 2014). Further research is warranted to provide evidence-based guidelines to promote sustained PA in this population.
The results of this study are similar to other clinical trials in HIV-uninfected participants using interventions to promote PA based on SDT. Yet to our best knowledge this is the first study to evaluate this approach targeting the HOA population. Previous clinical trials have investigated the role of exercise in improving functional capacity (as measured by aerobic capacity and strength) in HIV-infected adults. Contrary to our study, these clinical trials targeted mostly young, healthy HIV-infected adults, and did not evaluate overall physical function (Dolan et al., 2006; Mutimura, Crowther, Cade, Yarasheski, & Stewart, 2008; Roos, Myezwa, van Aswegen, & Musenge, 2014; Terry et al., 2006). Examining overall physical performance and gait speed is important because it evaluates the ability of older adults to perform day-to-day activities and can measure the risk of frailty and mortality (Brown, Sinacore, Binder, & Kohrt, 2000; Studenski et al., 2011). An isolated measure of strength and aerobic capacity is usually considered insufficient to identify frailty and related loss of independence. Furthermore, prior studies have not used a counseling intervention grounded in SDT in their PA interventions. Using a theoretical model is critical because it can identify important barriers to sustained behavior change leading to improved PA (Fortier, Duda, Guerin, & Teixeira, 2012; Ryan & Deci, 2000).
This study was guided by the framework of the SDT, a theory of human motivation that is designed to produce behavior change (Deci & Ryan, 2012; Ryan & Deci, 2000). SDT draws a clear distinction between autonomous motivation (i.e. doing something willingly because it is consistent with values, personal commitments and intrinsic interests) and controlled forms of motivation (e.g., pleasing others or being pressured or coerced). Interestingly, we found that participants who showed improvement in intrinsic motivation also showed an improvement in gait speed. This finding suggests that this form of autonomous motivation for PA may be important to healthy aging because gait speed is considered as a marker of biological aging and an important predictor of survival (Studenski et al., 2011). This finding is also particularly relevant in the HOA cohort, as a recent longitudinal study demonstrated that HOA experience not only a lower gait speed, but also an accelerated decline in gait speed after the age of sixty compared to HIV-uninfected individuals (Schrack et al., 2014).
Our intervention was of 12-week duration. Other PA interventions in adults grounded in SDT also used a 12-week or less duration (Edmunds, Ntoumanis, & Duda, 2008; Fortier, Sweet, O’Sullivan, & Williams, 2007; Levy & Cardinal, 2004). This relatively short duration time was sufficient in demonstrating beneficial effects of moderate PA on physical function. Moreover, there is evidence to support that physiological adaptations to increase in PA can be seen as early as 6 weeks. (Murrock & Graor, 2014; Mustian et al., 2009). With respect to PA counseling sessions, participants in our study took part in 6 total sessions which included follow-up sessions on the telephone similar to that used in the study of Fortier (Fortier, Sweet, O’Sullivan, & Williams, 2007).
The goal of our intervention was to maximize opportunities for personal decision making, while giving the resources and support needed to ensure proper education and facilitate autonomous motivation which has been proven to result in behavior change (Ryan, 2008). In this respect, our intervention resulted in significant improvement in identified regulation for PA (i.e., valuing the benefits of PA), which is consistent with previous studies that targeted HIV uninfected participants (Silva et al., 2011; Silva et al., 2010; Van Hoecke et al., 2013). There was not a statistically significant improvement in intrinsic regulation for PA (i.e. experience the pleasure of PA), which might be due to the small sample size and/or the short duration of our intervention. Future studies with a larger sample size and longer duration are warranted to develop interventions than can foster both internalization and intrinsic regulation and lead to behavior change in this cohort.
Finally, our study did not show an improvement in PA-related perceived competence within the treatment group. We speculate that the lack of improvement in this measure was because the perceived competence scores were exaggerated at baseline in our participants, possibly from a lack of experiential knowledge regarding PA or from an initial social desirability effect. This finding was also reported in other clinical trials in HIV-uninfected participants using PA interventions grounded in SDT (Fortier, Duda, Guerin, & Teixeira, 2012; Fortier, Sweet, O’Sullivan, & Williams, 2007). Even so, our study did show a significant improvement with respect to participant’s autonomy and identified regulation for PA in the treatment group compared to the control group (Ryan & Deci, 2000).
We acknowledge several limitations of our study, and reiterate some of the strengths. First, the duration of the intervention was relatively short, yet we were able to detect significant improvements in functional outcome, demonstrating the efficacy of our brief intervention. Second, we were unable to collect weekly data on PA levels, however we were able to collect data on PA levels both pre and post intervention. Third, the control and the treatment group did not have the similar amount of contact time. Lastly, we did not use instruments that measure PA specific need satisfaction which have been previously used in SDT-based exercise psychology literature such as basic psychological need in exercise scale and psychological need satisfaction in exercise scale (Vlachopoulos & Michailidou, 2006; Wilson, 2006). Of note, other important studies in this field also have not measured PA-specific need satisfaction (Silva et al., 2010; Van Hoecke et al., 2013). The strengths of our study include the randomized controlled study design and low attrition rates. Our study population also represented a diverse minority population. This is important because not only are racial and ethnic minorities less likely to engage in PA compared to whites, but they are also at an increased risk of chronic diseases and related negative outcomes associated with sedentism (August & Sorkin, 2011).
In conclusion, our findings provide preliminary evidence that a PA counseling intervention based on SDT is not only feasible, but also successful in improving physical function, autonomous motivation, depression, and QOL in HOA with functional limitations. The dramatic shift in the age demographics of the people living with HIV signifies an urgent need for identifying clinical interventions that can be readily translated into the HOA community to foster sustained behavior change of increased PA and attenuate functional decline. The end goal is to promote healthy aging in this cohort and improve their QOL. Future large-scale studies of longer duration are warranted to validate and extend our results with this end goal in mind.
Acknowledgments
Funding source: National Institute of Aging K23AG043319-01A1, NIH grants P30 AI78498, AG020493 and Harford Foundation of Excellence. This study is registered at www.clinicaltrials.gov (NCT019840600).
We thank the participants, Alicia Tyrell, Joseph Guido, Dr. Diane Morse and Dr. Stephen Dewhurst for encouragement and support of this work.
Footnotes
Author Contributions: Study concept and design: KNS, WJH, RR; Acquisition of participants and data: ZM, YBY, DW, AEL, KNS, TNH. Analysis and interpretation of data: KNS, JMM, HY, ZM. Preparation of manuscript. KNS, JMM, ZM, YBY, HY, AEL, DW, RR.
Sponsor’s Role: None
References
- August KJ, Sorkin DH. Racial/ethnic disparities in exercise and dietary behaviors of middle-aged and older adults. Journal of General Internal Medicine. 2011;26(3):245–250. doi: 10.1007/s11606-010-1514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Brothers TD, Kirkland S, Guaraldi G, Falutz J, Theou O, Johnston BL, Rockwood K. Frailty in people aging with human immunodeficiency virus (HIV) infection. Journal of Infectious Diseases. 2014;210(8):1170–1179. doi: 10.1093/infdis/jiu258. [DOI] [PubMed] [Google Scholar]
- Brown M, Sinacore DR, Binder EF, Kohrt WM. Physical and performance measures for the identification of mild to moderate frailty. J Gerontol A Biol Sci Med Sci. 2000;55(6):M350–M355. doi: 10.1093/gerona/55.6.m350. [DOI] [PubMed] [Google Scholar]
- Deci EL, Ryan RM. Self-determination theory in health care and its relations to motivational interviewing: a few comments. Int J Behav Nutr Phys Act. 2012;9:24. doi: 10.1186/1479-5868-9-24. 1479-5868-9-24 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, Margolick JB. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci. 2007;62(11):1279–1286. doi: 10.1093/gerona/62.11.1279. 62/11/1279 [pii] [DOI] [PubMed] [Google Scholar]
- Desquilbet L, Margolick JB, Fried LP, Phair JP, Jamieson BD, Holloway M, Jacobson LP. Relationship between a frailty-related phenotype and progressive deterioration of the immune system in HIV-infected men. J Acquir Immune Defic Syndr. 2009;50(3):299–306. doi: 10.1097/QAI.0b013e3181945eb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan SE, Frontera W, Librizzi J, Ljungquist K, Juan S, Dorman R, … Grinspoon S. Effects of a supervised home-based aerobic and progressive resistance training regimen in women infected with human immunodeficiency virus: a randomized trial. Arch Intern Med. 2006;166(11):1225–1231. doi: 10.1001/archinte.166.11.1225. 166/11/1225 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds J, Ntoumanis N, Duda JL. Testing a self-determination theory-based teaching style intervention in the exercise domain. European Journal of Social Psychology. 2008;38(2):375–388. doi: 10.1002/ejsp.463. [DOI] [Google Scholar]
- Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, … High KP. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47(4):542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlet CA. “You’re awfully old to have this disease”: experiences of stigma and ageism in adults 50 years and older living with HIV/AIDS. Gerontologist. 2006;46(6):781–790. doi: 10.1093/geront/46.6.781. [DOI] [PubMed] [Google Scholar]
- Enright PL, McBurnie MA, Bittner V, Tracy RP, McNamara R, Arnold A, Newman AB. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123(2):387–398. doi: 10.1378/chest.123.2.387. [DOI] [PubMed] [Google Scholar]
- Erlandson KM, Allshouse AA, Jankowski CM, Mawhinney S, Kohrt WM, Campbell TB. Relationship of physical function and quality of life among persons aging with HIV infection. AIDS. 2014;28(13):1939–1943. doi: 10.1097/qad.0000000000000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillipas S, Bowtell-Harris CA, Oldmeadow LB, Cicuttini F, Holland AE, Cherry CL. Physical activity uptake in patients with HIV: who does how much? International Journal of STD and AIDS. 2008;19(8):514–518. doi: 10.1258/ijsa.2007.007237. [DOI] [PubMed] [Google Scholar]
- Fortier MS, Duda JL, Guerin E, Teixeira PJ. Promoting physical activity: development and testing of self-determination theory-based interventions. Int J Behav Nutr Phys Act. 2012;9:20. doi: 10.1186/1479-5868-9-20. 1479-5868-9-20 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier MS, Sweet SN, O’Sullivan TL, Williams GC. A self-determination process model of physical activity adoption in the context of a randomized controlled trial. Psychology of Sport and Exercise. 2007;8:741–757. [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, … McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, … Palella F. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53(11):1120–1126. doi: 10.1093/cid/cir627. cir627 [pii]; [DOI] [PubMed] [Google Scholar]
- Harvey JA, Chastin SF, Skelton DA. Prevalence of sedentary behavior in older adults: a systematic review. International Journal of Environmental Research and Public Health. 2013;10(12):6645–6661. doi: 10.3390/ijerph10126645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIV Among Men in the United States. n.d Retrieved June 5, 2015, from http://www.cdc.gov/hiv/risk/gender/men/index.html?s_cid=tw_drmermin-00103.
- HIV Among People Aged 50 and Older. n.d Retrieved June 5, 2015, from http://www.cdc.gov/hiv/risk/age/olderamericans.
- Hogg R, Lima V, Sterne JA, Grabar S, Battegay M, Bonarek M, … May M. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293–299. doi: 10.1016/s0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy SS, Cardinal BJ. Effects of a self-determination theory-based mail-mediated intervention on adults’ exercise behavior. Am J Health Promot. 2004;18(5):345–349. doi: 10.4278/0890-1171-18.5.345. [DOI] [PubMed] [Google Scholar]
- Luther VP, Wilkin AM. HIV infection in older adults. Clin Geriatr Med. 2007;23(3):567–583. vii. doi: 10.1016/j.cger.2007.02.004. S0749-0690(07)00018-3 [pii]; [DOI] [PubMed] [Google Scholar]
- Lyons A, Pitts M, Grierson J, Thorpe R, Power J. Ageing with HIV: health and psychosocial well-being of older gay men. AIDS Care. 2010;22(10):1236–1244. doi: 10.1080/09540121003668086. 924492411 [pii]; [DOI] [PubMed] [Google Scholar]
- Lyons RA, Perry HM, Littlepage BN. Evidence for the validity of the Short form 36 Questionnaire (SF-36) in an elderly population. Age and Ageing. 1994;23(3):182–184. doi: 10.1093/ageing/23.3.182. [DOI] [PubMed] [Google Scholar]
- Markland D, Hardy L. On the factorial and construct validity of the Intrinsic Motivation Inventory: conceptual and operational concerns. Res Q Exerc Sport. 1997;68(1):20–32. doi: 10.1080/02701367.1997.10608863. [DOI] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- Mildestvedt T, Meland E, Eide GE. How important are individual counselling, expectancy beliefs and autonomy for the maintenance of exercise after cardiac rehabilitation? Scand J Public Health. 2008;36(8):832–840. doi: 10.1177/1403494808090633. [DOI] [PubMed] [Google Scholar]
- Moustaka FC, Vlachopoulos SP, Vazou S, Kaperoni M, Markland D. Initial validity evidence for the Behavioural Regulation in Exercise Questionnaire – 2 among Greek exercise participants. European Journal of Psychological Assessment. 2010;26:269–276. [Google Scholar]
- Murrock CJ, Graor CH. Effects of dance on depression, physical function, and disability in underserved adults. J Aging Phys Act. 2014;22(3):380–385. doi: 10.1123/japa.2013-0003. [DOI] [PubMed] [Google Scholar]
- Mustian KM, Peppone L, Darling TV, Palesh O, Heckler CE, Morrow GR. A 4-week home-based aerobic and resistance exercise program during radiation therapy: a pilot randomized clinical trial. J Support Oncol. 2009;7(5):158–167. [PMC free article] [PubMed] [Google Scholar]
- Mutimura E, Crowther NJ, Cade TW, Yarasheski KE, Stewart A. Exercise training reduces central adiposity and improves metabolic indices in HAART-treated HIV-positive subjects in Rwanda: a randomized controlled trial. AIDS Res Hum Retroviruses. 2008;24(1):15–23. doi: 10.1089/aid.2007.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onen NF, Overton ET. HIV and aging: two converging epidemics. Mo Med. 2009;106(4):269–273. [PubMed] [Google Scholar]
- Oursler KK, Sorkin JD, Smith BA, Katzel LI. Reduced aerobic capacity and physical functioning in older HIV-infected men. AIDS Res Hum Retroviruses. 2006;22(11):1113–1121. doi: 10.1089/aid.2006.22.1113. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS, Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. New England Journal of Medicine. 1993;328(8):538–545. doi: 10.1056/nejm199302253280804. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. 1978. American Journal of Epidemiology. 1995;142(9):889–903. doi: 10.1093/oxfordjournals.aje.a117736. discussion 887–888. [DOI] [PubMed] [Google Scholar]
- Pahor M, Blair SN, Espeland M, Fielding R, Gill TM, Guralnik JM, … Studenski S. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61(11):1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2014;69(7):833–842. doi: 10.1093/gerona/glt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees HC, Meister E, Mohler MJ, Klotz SA. HIV-Related Frailty Is Not Characterized by Sarcopenia. J Int Assoc Provid AIDS Care. 2014 doi: 10.1177/2325957414553848. [DOI] [PubMed] [Google Scholar]
- Roger KS, Mignone J, Kirkland S. Social aspects of HIV/AIDS and aging: a thematic review. Can J Aging. 2013;32(3):298–306. doi: 10.1017/s0714980813000330. [DOI] [PubMed] [Google Scholar]
- Roos R, Myezwa H, van Aswegen H, Musenge E. Effects of an education and home-based pedometer walking program on ischemic heart disease risk factors in people infected with HIV: a randomized trial. Journal of Acquired Immune Deficiency Syndromes. 2014;67(3):268–276. doi: 10.1097/qai.0000000000000299. [DOI] [PubMed] [Google Scholar]
- Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol. 2000;55(1):68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- Ryan RM, Patrick H, Deci EL, Williams GC. Facilitating health behaviour change and its maintenance: Interventions based on self-determination theory. The European Health Psychologist. 2008;10:2–5. [Google Scholar]
- Schaubert KL, Bohannon RW. Reliability and validity of three strength measures obtained from community-dwelling elderly persons. Journal of Strength and Conditioning Research. 2005;19(3):717–720. doi: 10.1519/r-15954.1. [DOI] [PubMed] [Google Scholar]
- Schrack JA, Althoff KN, Erlandson KM, Schrack JA, Althoff KN, Erlandson KM, … Erlandson KM. Accelerated longitudinal gait speed decline in HIV-infected older adults. 5th International Workshop on HIV and Aging; Baltimore. 2014. p. Abstract 12. [Google Scholar]
- Schuelter-Trevisol F, Wolff FH, Alencastro PR, Grigoletti S, Ikeda ML, Brandao AB, … Fuchs SC. Physical activity: do patients infected with HIV practice? How much? A systematic review. Curr HIV Res. 2012;10(6):487–497. doi: 10.2174/157016212802429794. [DOI] [PubMed] [Google Scholar]
- Shah K, Hilton TN, Myers L, Pinto JF, Luque AE, Hall WJ. A New Frailty Syndrome: Central Obesity and Frailty in Older Adults with the Human Immunodeficiency Virus. J Am Geriatr Soc. 2012 doi: 10.1111/j.1532-5415.2011.03819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah KN, Majeed Z, Yang H, Guido JJ, Hilton TN, Polesskaya O, … Luque AE. Functional limitations and adipokines in HIV-infected older adults. J Frailty Aging. 2014;4(1):41–46. [PMC free article] [PubMed] [Google Scholar]
- Shippy RA, Karpiak SE. The aging HIV/AIDS population: fragile social networks. Aging Ment Health. 2005;9(3):246–254. doi: 10.1080/13607860412331336850. [DOI] [PubMed] [Google Scholar]
- Silva MN, Markland D, Carraca EV, Vieira PN, Coutinho SR, Minderico CS, … Teixeira PJ. Exercise autonomous motivation predicts 3-yr weight loss in women. Med Sci Sports Exerc. 2011;43(4):728–737. doi: 10.1249/MSS.0b013e3181f3818f. [DOI] [PubMed] [Google Scholar]
- Silva MN, Markland D, Minderico CS, Vieira PN, Castro MM, Coutinho SR, … Teixeira PJ. A randomized controlled trial to evaluate self-determination theory for exercise adherence and weight control: rationale and intervention description. BMC Public Health. 2008;8:234. doi: 10.1186/1471-2458-8-234. 1471-2458-8-234 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MN, Vieira PN, Coutinho SR, Minderico CS, Matos MG, Sardinha LB, Teixeira PJ. Using self-determination theory to promote physical activity and weight control: a randomized controlled trial in women. J Behav Med. 2010;33(2):110–122. doi: 10.1007/s10865-009-9239-y. [DOI] [PubMed] [Google Scholar]
- Smit E, Crespo CJ, Semba RD, Jaworowicz D, Vlahov D, Ricketts EP, … Tang AM. Physical activity in a cohort of HIV-positive and HIV-negative injection drug users. AIDS Care. 2006;18(8):1040–1045. doi: 10.1080/09540120600580926. [DOI] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, … Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. Physical activity is medicine for older adults. Postgraduate Medical Journal. 2014;90(1059):26–32. doi: 10.1136/postgradmedj-2012-131366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira PJ, Carraca EV, Markland D, Silva MN, Ryan RM. Exercise, physical activity, and self-determination theory: a systematic review. Int J Behav Nutr Phys Act. 2012;9:78. doi: 10.1186/1479-5868-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry L, Sprinz E, Stein R, Medeiros NB, Oliveira J, Ribeiro JP. Exercise training in HIV-1-infected individuals with dyslipidemia and lipodystrophy. Med Sci Sports Exerc. 2006;38(3):411–417. doi: 10.1249/01.mss.0000191347.73848.80. 00005768-200603000-00003 [pii] [DOI] [PubMed] [Google Scholar]
- Van Hoecke AS, Delecluse C, Opdenacker J, Lipkens L, Martien S, Boen F. Long-term effectiveness and mediators of a need-supportive physical activity coaching among Flemish sedentary employees. Health Promot Int. 2013;28(3):407–417. doi: 10.1093/heapro/das025. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, … Shah K. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364(13):1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachopoulos SP, Michailidou S. Development and Initial Validation of a Measure of Autonomy, Competence, and Relatedness in Exercise: The Basic Psychological Needs in Exercise Scale. Measurement In Physical Education and Exercise Science. 2006;10(3):179–201. [Google Scholar]
- Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. Journal of Clinical Epidemiology. 1999;52(7):643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. Journal of Clinical Epidemiology. 1993;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- Williams GC, Grow VM, Freedman ZR, Ryan RM, Deci EL. Motivational predictors of weight loss and weight-loss maintenance. Journal of Personality and Social Psychology. 1996;70(1):115–126. doi: 10.1037//0022-3514.70.1.115. [DOI] [PubMed] [Google Scholar]
- Wilson PM, Rogers WT, Rodgers WM, Wild TC. The psychological need satisfaction in exercise scale. Journal of Sport & Exercise Psychology. 2006;28(3):231–251. [Google Scholar]