Abstract
During visceral leishmaniasis (VL), T helper 1 (Th1)-based inflammation is induced to control intracellular parasites. Inflammation-based pathology has been shown to be dampened by interleukin 10 and eventual Programmed Death1 (PD1)-mediated T cell exhaustion. Cell type(s) responsible for the initiation of T cell-produced IL-10 during VL are unknown. CD19+, CD5−, CD1d−, IgDhi regulatory B cells from healthy controls produced IL-10 in absence of infection or stimulation in contrast to IgDlo/neg B cells. IgDhi B cells may have a de novo vs. induced regulatory program. IgDhi B cells increased three-fold in population size as VL progressed. B cells from VL dogs were necessary and sufficient to suppress T helper 1 (Th1) cell effector function. IgDhi B cells induced T cell and IgDlo B cell IL-10 production. Blockage of B cell-specific PD-L1 restored Th1 responses. IgDhi regulatory B cells represent a novel regulatory B cell which may precipitate T cell exhaustion during VL.
Introduction
Zoonotic visceral leishmaniasis (VL) without treatment is a fatal systemic disease. VL results in 500,000 annual new human cases and greater than 20,000 deaths per year. Leishmania infantum, the causative agent of VL in the New World and Mediterranean basin, has natural hosts that include dogs and humans (1). As VL patients or the animal model, dogs, progressed to clinical VL, there was impaired CD4+ T cell proliferation, IFN-γ production; T cell exhaustion (2–5). The canine model of VL, nearly identical in pathology to human disease, has proven to be valuable to understand immunologic causes of progressive clinical VL (2, 3, 6–9).
Immature-like B cells are a recently described regulatory B cell which produces substantial amounts of IL-10 in patients with autoimmune diseases (10). Other murine regulatory B cells had a CD5+, CD19hi, CD24hi surface phenotype; however naïve-like B cells do not share this distinction and expressed a non-activated, resting B cell phenotype of human B10 cells is not clearly defined (11, 12). Generally these regulatory B cells are predominantly characterized by their production of IL-10, do not express classical activation markers and have dual surface expression of IgM and IgD. Role(s) of regulatory surface receptors in the function(s) of these naïve-like B cells are not well established. CD19+, CD27−, IgM+, IgD+ B cells increase in frequency within whole blood during clinical Chagas’ disease (13) and P. falciparum cerebral malaria (14), suggesting a causal link between IgM+/IgD+ naïve-like B cells and persistence of intracellular protozoal infection. Despite these correlative findings, very little is known regarding the specific role of IgD+ IL-10 producing B cells in natural infection settings, or regulatory function(s) of IgDhi expressing cells. Insight into potential suppressive functions of this B cell subset will expand our understanding of immune regulatory roles of IgD+ B cells during chronic infection.
Studies of multiple autoimmune diseases, including lupus (15), rheumatoid arthritis (16), and chronic granulomatous disease (17), demonstrated that IL-10-producing B cells were critical for dampening inflammatory disease Induction or presence of functional IL-10 producing regulatory B cells had novel therapeutic capacity in these autoimmune diseases (18). Comparatively little is known about these regulatory B cells specifically alter progression of infectious diseases (19–22).
Infection with Leishmania (L.) infantum initially induces a robust Th1 immune response. This Th1 response is dampened by regulatory immune responses when infection was not controlled by the initial IFN-γ-based response (2, 3, 23, 24). It was demonstrated that during VL, T cell responses were characterized by IL-10 production and increased inhibitory receptor/ligand Programmed Death (PD)1/PDL1-expression leading to cellular exhaustion (2). Studies to date focused on CD4+ or CD8+ T cell regulation during VL. Whether regulatory T cell responses were initiated directly by the inflammatory environment during VL or if additional regulatory immune cells precipitate regulatory responses is unknown. Other studies characterized marginal zone B cell activation and IL-10 production of B cells in experimental L. donovani or L. major murine-infection to drive T cell development toward Th2-baised responses (25, 26). During natural, progressive infection, the presence of activated B cells within the spleen of L. infantum infected dogs correlated with abnormal germinal center formation (27). The phenotype and role of regulatory B cells as a source of IL-10 during VL and how PD1/PDL1 interactions may alter the function of regulatory B cells is not known.
Recent advances in our understanding of regulatory B cells suggested that these cells have a broad role in immune regulation (12). Regulatory B cells directly influence inflammatory T cell function (20). We hypothesized that these cells might therefore predicate activation of regulatory T cells during progressive VL. CD19+ IgDhi B cells expanded three-fold during progressive VL and were the predominant population of IL-10 producing B cells during clinical VL. IgDhi B cells consistently produced IL-10 in all collected control, subclinical, and clinical groups, indicating IL-10 production was a core function of these cells. IgDhi B10 B cells did not display typical surface markers of murine B regulatory cells (CD5+, CD19hi, CD24hi, CD1dhi). Instead these IL-10 producing B cells had a phenotype more similar to that observed in immature B cells of human patients during hepatitis B virus infection (19). IgDhi B cells induced IL-10 production in co-cultured T and IgDint/lo B cells. When magnetically-enriched B cells from L. infantum-infected dogs were co-cultured with enriched T cells, B cells were sufficient to suppress T cell function through IL-10 and PD1; conversely removal of IgDhi B cells preserved CD4+ T cell function despite presence of other APCs. Blockade of IL-10 and PD1 interactions on B cells produced significant recovery of T cell function (p<0.01). These novel regulatory B cells represent an important component of immune regulation during chronic L. infantum infection and greatly expand our understanding of non-experimentally induced regulatory B cells.
Materials and Methods
Animals
This study utilizes a cohort of US hunting dogs described in Boggiatto et al., 2010 and dogs collected for euthanasia for Leishmania-prevention efforts in Natal, Brazil (Figure 1). Supplemental table 1 provides the sex and repeated diagnostic information for all U.S. dogs used in this study. The average age of these dogs was 4.1 years, symptomatic animals range from 2–10 years of age, asymptomatic and endemic control animals trended slightly younger but not significantly so. Symptomatic dogs tend to have a history of tick-borne disease, roughly 15% of the overall cohort of dogs is seropositive for tick-borne disease, including Ehrlichia, Babesia and Borrelia. All dogs are routinely dewormed, although helminth infection is still possible. U.S. born, uninfected kennelmate dogs serve as endemic control animals to the infected animals in figures 2–6. Asymptomatic-infected dogs were Leishmania PCR-positive, had no to low serological response to specific Leishmania antigens and no clinical signs of disease; symptomatic animals were PCR-positive, had high serological levels and 3 or more specific signs of Leishmaniasis (Supplemental Table 1). The average age of the study population was 4.1 years old. For more information about the natural history of VL from birth in a subset of these dogs, see Vida et al, 2016 (54).
Figure 1.
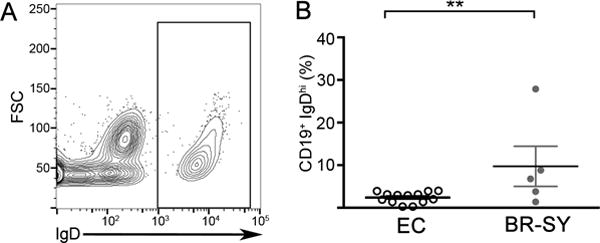
L. infantum infected dogs from Brazil display high levels of immunoglobulin D on the surface of their B cells suggesting the occurrence of a naïve-like B cell during chronic VL. (A) Representative flow cytometry plot of IgD expression on CD19+ B cells isolated from clinically symptomatic, L. infantum infected Brazilian dogs. (B) Quantification of CD19+, IgDhi populations in individuals. Endemic controls (EC), or Brazilian symptomatic (BR-SY) dogs. N=5, 1 experiment. Significance determined via one-way ANOVA ±SEM **p<0.01,
Figure 2.
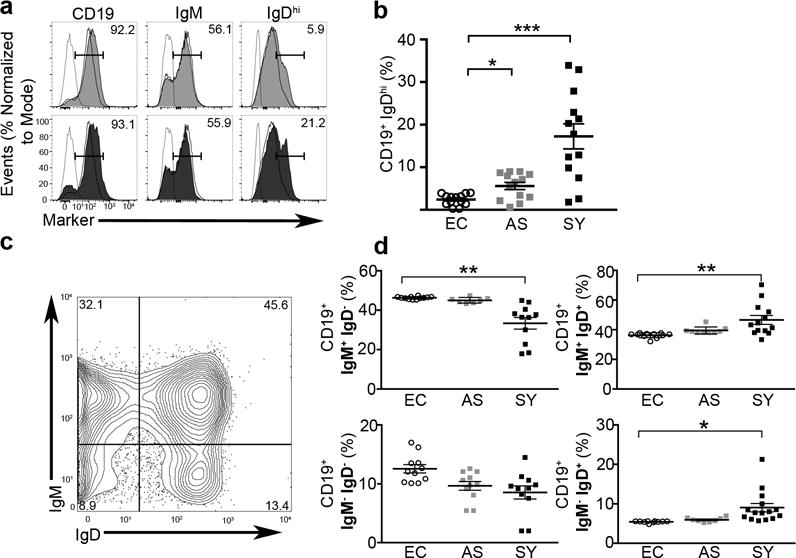
Immunoglobulin IgD significantly increased on the surface of B cells during visceral leishmaniasis. (A) Histogram of isotype (dashed), endemic control (open-solid), asymptomatic (grey) or symptomatic (black) magnetically selected B cells. Percentages of CD19 (left), IgM (center) or IgDhi (right). Histograms representative of n=7 per group and 3 experiments. (B) Quantification of CD19+, IgDhi populations in individuals. Endemic controls (EC), asymptomatic (AS), symptomatic (SY) dogs. N=13 per group from 5 experiments, except SY-BR where N=5, 1 experiment. Significance determined via one-way ANOVA ±SEM **p<0.01, ***p<0.001. (C) Relative expression of IgD and IgM in healthy endemic control dog. Representative of n=7 and 3 experiments. (D) Relative surface expression of IgM and IgD positive peripheral blood B cell populations. N=12 per group from 3 experiments. Statistical significance determined via one-way ANOVA with multiple comparisons was used for statistical analysis. Bars represent ±SEM *p<0.05, **p<0.01.
Figure 6.
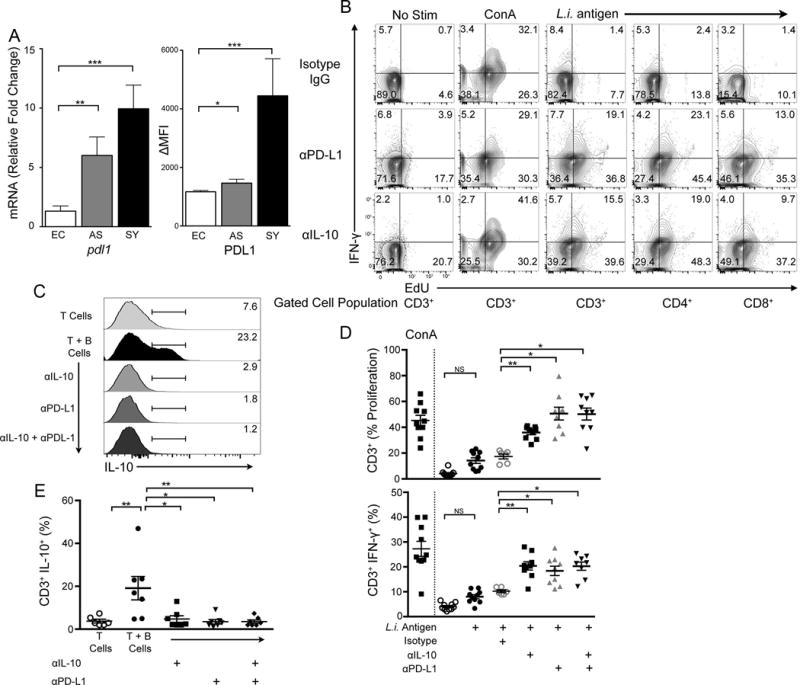
Inhibitory receptor PD-L1 and IL-10 drove B cell suppression of T cell function in ex vivo cells from VL symptomatic dogs. (A) mRNA expression of PDL1 via qPCR in B cells (left). MFI surface expression of PD-L1 on CD19+, IgDhi B cells (right) (B) Representative contour plots of IFN-γ vs. proliferation in non-stimulated, positive-control ConA or L. infantum antigen stimulated T cells after B cell co-culture. B cells treated with isotype IgG, anti-PD-L1 (αPD-L1), or anti-IL-10 (αIL-10) specific antibodies prior to incubation with indicated T cell populations. (C) Intracellular IL-10 expression in non-stimulated CD3+ T cells isolated from symptomatic VL group. Histograms represent intracellular IL-10 percent positive cells. (D) CD3+T cell responses to co-culture with anti-PDL1 or IL-10 antibody-treated B cells from symptomatic animals. Percentages normalized to isotype control. N=7 per group. (E) Population data of CD3+ T cells producing IL-10 from symptomatic dogs co-cultured with B cells. Percentages normalized to isotype control subtracted from endemic control. N=7 per group. One-way ANOVA with multiple comparisons ± SEM. p<0.05, **p<0.01, ***p<0.001.
Ethics Statement
All animal use for this submission was reviewed and approved by IACUC at Iowa State University protocol #10-06-6242-K and University of Iowa protocol #1307131, both AAALAC accredited Universities. This work adhered to the National Institutes of Health animal use policies for USDA animals (dogs).
Leishmania DNA Isolation
Isolation and qPCR was performed as previously described Esch et al. 2013. DNA from canine a blood samples was isolated using blood DNA isolation kit (QIAGEN, Valencia, CA) according to the manufacturer’s instructions. DNA concentration and purity was measured by a NanoDrop ND1000 spectrophotometer (Thermo Scientific, Wilmington, DE).
Diagnostic qPCR
PCR was performed as previously described in Esch et al. 2013. Briefly, L. infantum kinetoplast DNA (kDNA) was detected using specific primers and probes F 5′-CCGCCCGCCTCAAGAC, R 5′-TGCTGAATATTGGTGGTTTTGG, (Integrated DNA Technologies, Coralville, IA) and, Probe 5′-6FAM-AGCCGCGAGGACC-MGBNFQ (Applied Biosystems, Foster City, CA) (FAM: laser-activated reporter dye; MGBNFQ: 3′-minor-groove binder non-fluorescent quencher). BLAST analysis indicated that these primers and probe were specific for L. infantum, and qPCR analysis using L. major and L. amazonensis as the DNA target were negative for amplification with this primer and probe set. Leishmania SSU rRNA was identified using specific fluorogenic probe LEIS.P1 (5′-6-carboxyfluorescein [6-FAM]-CGGTTCGGTGTGTGGCGCC-3′) and flanking primers LEIS.U1 (5′-AAGTGCTTTCCCATCGCAACT-3′); and LEIS.L1 (5′-GACGCACTAAACCCCTCCAA-3′) (Applied Biosystems) as previously designed (28). Blood DNA samples were analyzed by qPCR in duplicate using a 96-well format of two concentrations, whole blood and a dilution 1:10. Amplification was performed using an ABI 7000 qPCR system (Applied Biosystems), Perfecta qPCR super Mix (Quanta Biosciences, Gaithersburg, MD), and Low ROX master mix (Quanta Biosciences). Primers were used at 775 nM and probe at 150 nM with thermocycling at 95 °C for three min, followed by 50 cycles at 95 °C for fifteen sec, and 60 °C for one min. Results were analyzed by ABI 7000 System SDS Software v1.2.3 (Applied Biosystems).
PBMC Isolation
Whole blood samples were separated into PBMC, as previously described in Boggiatto et al., 2010. Briefly, whole blood samples collected in EDTA-containing tubes were diluted 1:1 with PBS (Cellgro, Manassas, VA). Diluted whole blood was centrifuged at 1000 × g (Eppendorf, Hauppauge, NY) for 30 min at room temperature through Ficoll/Histopaque 1077 (Sigma-Aldrich, St. Louis, MO). PBMCs were counted by using a hemocytometer. PBMCs were washed twice in PBS and suspended in complete RPMI 1640 (RPMI 1640 supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 25 mM HEPES buffer, and 50 μM 2-mercaptoethanol). PMBCs were counted and adjusted to 5 × 105 cells per well on a 96 well plate.
Lymphocyte Isolation
To isolate T cells and B cells, negative magnetic selection was used under manufacturer’s instructions. Briefly, Human Pan T cell Isolation Kit (Miltenyi Biotec, Auburn, CA), or Human B cell Isolation Kit II (Miltenyi Biotec) was used to isolated untouched cell populations from peripheral canine blood. Blood was incubated with antibody cocktail containing biotinylated antibodies bound to a streptavidin-magnetic beads. Unwanted cells were labeled and removed using DEPLETE program on an Automax machine (Miltenyi Biotec). Antibody labeling of CD3 or CD19 was performed to determine purity and confirm isolation. For B cell depletion, LD columns were used for magnetic depletion using manufacturer’s instructions. Murine anti-CD19 microbeads (Miltenyi Biotec) were used to label B cells for removal and CD19+ cells were kept for IgD labeling. For IgD selection, CD19+ selected cells were labeled with anti-IgD [Clone IA6-2] (eBiosciences, San Diego, CA) and anti-mouse IgG magnetic beads (Miltenyi Biotec) were used per manufacturers instruction to select for high or low expressing cells as previously described in van der Vlugt et al. 2014. Anti-CD19, and Anti-IgD was used to confirm enrichment.
Flow Cytometry
To exclude the possibility of an increase in surface expression of IgD, doublets, dead cells (Live/Dead Fixable Violet, Life Technologies, Carlsbad, CA) and debris were excluded from all flow cytometry assays (Supplemental Fig. 1A–D). Cell labeling was performed as previously described in Esch et al. Florescence minus one (FMO) with isotype controls were used as previous for gating (Supplemental Fig. 1E). Briefly, Twenty-four hours prior to cell harvest, 5-ethynyl-2′-deoxyuridine (EdU; Life Technologies, Grand Island, NY) was added at 10 μM, and 10 μg/ml brefeldin A (Sigma-Aldrich) was added 6 h before harvest. Cells were harvested and washed prior to surface and intracellular labeling. Cells were labeled for flow cytometry at 4°C for 30 min in FACS buffer (PBS, 1% BSA) plus appropriate abs and fixed in BD Stabilizing Fixative (BD Biosciences, Franklin Lakes, NJ). Intracellular labeling followed permeabilization in 1% saponin solution (Life Technologies). Antibodies used were as follows: 1 μg/mL anti canine-CD4 [Clone YKIX302.9] (AbD Serotec, Raleigh, NC), 1 μg/mL anti-canine CD8 [Clone YCATE55.9 ] (AbD Serotec), 1 μg/mL anti-canine CD3 [Clone CA17.2A12] (AbD Serotec), 5 μg/mL anti-human IgD [Clone IA6-2], 1 μg/mL anti-canine IgM (AbD Serotec), 1 μg/mL anti-canine CD5 (AbD serotec), 0.5 μg/mL anti mouse-CD19 [Clone MB19-1] (eBioscience, San Diego, CA), 5 μg/mL anti-canine IFNγ, 5 μg/mL anti-human CD1d, 1 μg/mL anti-human PD-L1, 5 μg/mL anti-canine IL-4 (R&D Systems, Minneapolis, MN), 5 μg/mL anti-canine IL-10 (R&D Systems). Proliferation was measured using Pacific-Blue Click-IT EdU kit following manufacturer’s instruction (Life Technologies). Flow cytometry was performed on a BD LSR II and analyzed using FlowJo vX software.
Antigen and Mitogen Stimulation
Stimulation for ConA, L. infantum freeze-thawed (f-t) antigen and distemper antigen were performed as in Esch et al. 2013. Briefly, cells were stimulated with Con A (5 μg/ml) for 4 d, f-t L.i. (10 μg/ml) for 7 d, and distemper virus vaccine (Pfizer, Kalamazoo, MI), a non-Leishmania, positive control for 7 d.
Antibody Blocking
Antibody blockade was performed similar to previously described in Esch et al. 2013. Briefly, isolated B cells were treated with 10 μg/ml anti-human B7.H1 Ab (clone MIH-1; eBioscience), 10 μg/ml anti-canine IL-10 Ab (R&D Systems), both 5 μg/ml anti-human B7.H1 and 5 μg/ml anti-canine IL-10 Ab, or isotype for 4 h and washed, and returned to culture for Ag stimulation. Isogenic, enriched, T cells were added for co-culture.
Fluorescence Based Cell Sorting
Cells were labeled with anti-mouse-CD19, anti-human-IgD and anti-canine-IgM as described in the previous section. Cells were analyzed via FACS Diva II cell sorter (BD Biosciences) and gated for CD19+, IgDhi or CD19+, IgDneg/low cells. A total of 100,000 events was selected and segmented into appropriate tubes. The cells were either placed into culture or immediately lysed for RNA isolation with SDS-based lysis buffer component of Atrium™ Total RNA Isolation Kit (Bio-Rad, Hercules, CA) and stored at −80 °C.
RNA isolation, cDNA Synthesis and SYBR Green qPCR
RNA was isolated via RNeasy Mini Kit (Qiagen) or Atrium™ Total RNA Isolation Kit (Bio-Rad) and followed manufacturer’s instructions. RNase Out™ (Life Technologies, Carlsbad, CA) was added to avoid RNase-mediated RNA degradation. Samples were quantified via ND-1000 (Thermo Scientific) to standardize all samples to 200 ng of total RNA per reverse transcription reaction. iScript cDNA synthesis kit (Bio-Rad) was used per manufacturer protocol. Reverse transcription was performed in a Vapoprotect™ thermocycler (Eppendorf); 5 min at 25 °C, 6 min at 42 °C, 5 min at 72 °C and held at 4 °C. Primers were designed using the Primer-BLAST Tool (NCBI) with the Canis lupus familiaris genome for reference. iTaq Universal SYBR Green Supermix (Bio-Rad) was used for the qPCR reaction with master mix of primer sets created. Primers were used at a final concentration of 500 nM per well reaction. An ABI 7000 qPCR machine (Applied Biosystems) was used. Amplification conditions for all genes were the same: 5 min at 95 °C, 40 cycles of 15 s 95 °C and 1 min 60 °C (measure florescence step) and a dissociation step of 15 s 95 °C, 1 min 60 °C, 15 s 95 °C, 15 s 60 °C. CT values were generated using ABI PRISM SDS Software v1.2.3 (Applied Biosystems, Carlsbad, CA). CT values were calculated and normalized to endogenous control (GAPDH) and expressed relative to endemic control dogs using 2−ΔΔCT method as described by Livak and Schmittgen, 2001.
IL-10 ELISA
Canine specific IL-10 from cell supernatants were assayed per manufacturer’s instruction (R&D Systems) and as previously described in Esch et al., 2013. Plates were read with a Versa Max plate spectrophotometer (Sunnyvale, CA, USA) at 450 nm. The lower limit of detection was 2 pg/mL and upper limit was 1000 pg/mL.
IgD ELISA
Canine IgD serum quantification was performed according to manufacturer specifications. Briefly, the IgD coating antibody (Goat Anti-human IgD affinity purified, Bethyl Laboratories, Montgomery, TX) was diluted 1:5,000 in carbonate-bicarbonate buffer (Sigma-Aldrich). Dog serum samples were assayed undiluted. Human reference serum (Bethyl Laboratories) was used as a standard for quantification. Blocking was done with 1% bovine serum albumin (BSA) in phosphate buffered saline (PBS), and washes were done with PBS+0.01% Tween-20. The detection antibody (Goat Anti-human IgD HRP conjugated, Bethyl Laboratories) was diluted 1:20,000. The 1-Step Ultra TMB ELISA (Thermo Scientific) was warmed to room temp, added to each well, and incubated for 15 minutes. Two molar HCl was used as a stopping reagent. The plate was read on a Versa Max plate reader (Sunnyvale, CA, USA) at 450 nm. Quantification was done using Microsoft Excel. The limit of detection for IgD was 1 ng/mL-100 ng/mL.
Statistical analysis
Data were analyzed with GraphPad Prism6 software. Except where noted in the figure legend, statistical analysis performed utilizing one way-ANOVA with multiple comparison of means. *p<0.05, **p<0.01, ***p<0.001.
Results
Naïve-like regulatory B cells are present in progressive VL dogs from Brazil
Based on previous research that suggested a role for regulatory B cells during chronic infectious disease, we evaluated the presence of naïve-like regulatory B cells in the periphery of an endemic population of the L. infantum reservoir host, dogs. We selected dogs from Natal, Rio Grande do Norte, Brazil where L. infantum is endemic in both human and canine populations. Importantly, we observed a distinct circulating CD19+ IgDhi B cell within symptomatic, L. infantum-infected dogs (Fig. 1A). This population of B cells was significantly greater in proportion of total CD19+ cells compared to the population in uninfected control dogs (Fig. 1B). These B cells were present during chronic L. infantum infection and as they are also found during other chronic infections (11, 12), could be an important naïve-like regulatory cell.
Increased IgD on the surface of B cells during clinical VL
Regulatory, naïve-like, B cells have been recently described as important for immune regulation during chronic autoimmune disease and have suggested roles during chronic infection (12). As regulatory B cells represent an important target for immune regulation and have unknown roles in progressive fatal, visceral leishmaniasis (VL), we assessed presence of regulatory IgD+ B cells during both asymptomatic (AS) L. infantum infection and symptomatic (SY) VL in the zoonotic reservoir host; dogs. Most IgD+ B cells co-express IgM (29). CD19+ B cells from three clinical states were analyzed for IgM and IgD surface expression (Fig. 2). These cells were IgDhi as detected by mean fluorescent intensity (Fig. 2A). Populations of IgDhi B cells were significantly increased roughly four-fold in the periphery of symptomatic, chronically infected dogs as compared to either uninfected-endemic controls or asymptomatic animals (Fig. 2B) (p<0.001). There were no significant differences between clinical groups for percentage of cells expressing surface CD19 or CD21 (data not shown). Normal canine circulating B cells have an IgM/IgD surface expression similar to that of healthy humans controls (Fig. 2C) (30). Ratios of percentages of IgM/IgD B cell populations shifted as L. infantum disease progressed, with significantly increased IgM−/IgD+ (p<0.05) or IgM+/IgD+ (p<0.01) expressing CD19+ cells; conversely, populations of percentages of IgM+/IgD− (p<0.01) cells decreased significantly in frequency. As the population size of the IgDhi cells increased with disease progression, we wanted to determine whether this occurred in parallel to increased secreted IgD. Serum IgD was secretion was limited, but significantly increased in clinical VL subjects compared to uninfected controls (p<0.05) (Supplemental Fig. 2).
To ensure that an increase in surface IgD was not due to activation of B cells, we compared ex vivo collected IgDhi B cells to CD19+ cells activated with TLR7/8 agonists. A significant shift in size, associated with activation, was observed only in the TLR7/8 agonist stimulated B cells (p<0.001) (Supplemental Fig. 2). There was no increase in surface expression of B cell activation markers CD24 or CD25 detected on IgDhi B cells compared to control (data not shown). Based on the increase in IgDhi B cell population with clinical progression this B cell subset may be important in contributing to cytokine-driven immunomodulation (31).
IgDhi B cells exhibited increased IL-10 production
Patients infected with HIV, Hepatitis B and Schistosoma haematobium had IgD+ B10 B cells which produced IL-10, correlated with immune suppression and/or pathogen survival (19, 21, 22). IL-10 has been shown to be an important factor in T cell suppression during chronic L. infantum infection (2, 23). A variety of immune cells are responsible for IL-10 secretion during chronic infection (32–34). To determine the contribution of IgDhi B cells to IL-10 production, IgDhi B cells were gated (Fig. 3A, left) and analyzed for intracellular IL-10 protein expression across peripheral blood from clinical groups (Fig. 3A). All IgDhi B cells had detectable intracellular IL-10, with no significant difference in the IL-10 producing IgDhi B cell population size from that of uninfected endemic control animals (Fig. 3B). Total CD19+ B cells were gated (Fig. 3C, left) for IL-10 expression across clinical groups (Fig. 3C) for comparison to IgDhi B cells (Fig. 3A). The percentage of the population of B cells positive for intracellular IL-10 increased four-fold over control and two-fold over asymptomatic dogs compared to cells from symptomatic animals (Fig. 3D). This increase was observed as the overall percentage of IgDhi B cells increased (Fig. 2B) suggesting that IgDhi B cells may be a predominant IL-10 producing B cell during symptomatic VL. Splenocytes isolated from symptomatic dogs also had a considerable population of IgDhi, IL-10 producing B cells indicating these cells were present in multiple reticuloendothelial sites (Fig. 3G, H). IgDint/lo or IgM+/IgD− cells marginally expressed intracellular IL-10 in control animals, but had four to tenfold increased production of IL-10 in symptomatic infection (Fig. 3I).
Figure 3.
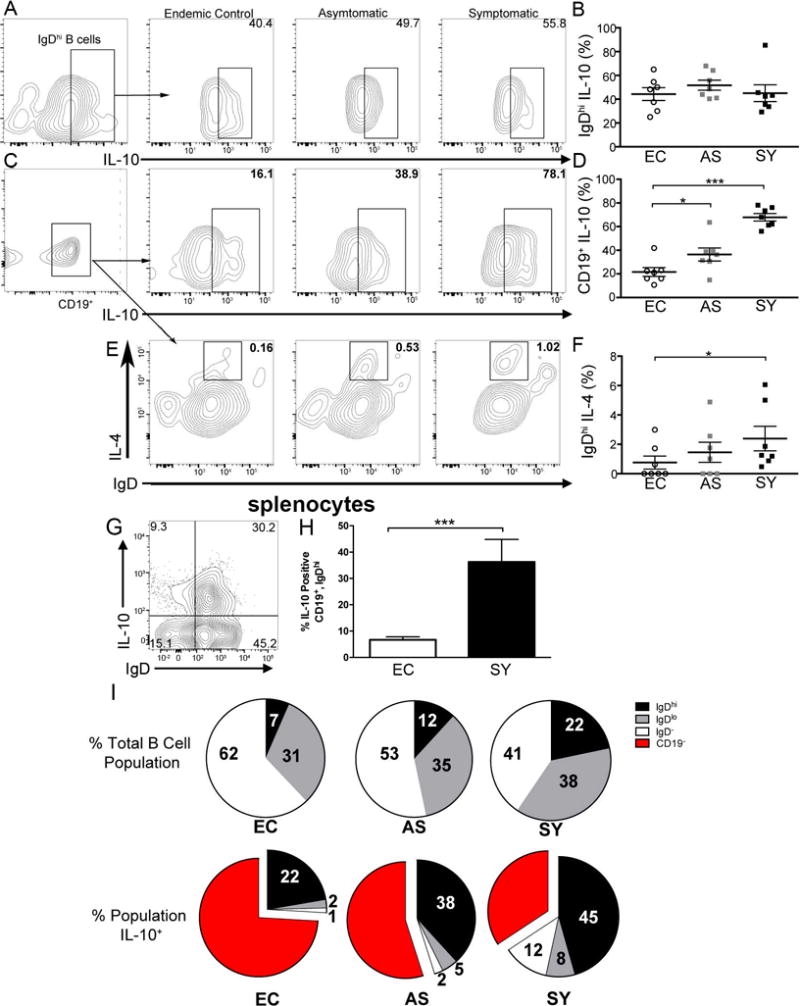
All IgDhi B cells have significantly increased IL-10 production. (A) CD19+ B cells gated for IgDhi and IL-10 via flow cytometry. Left panel (a) represents IgDhi gating scheme of CD19+ cells. Following panels are representative of IgDhi intracellular IL-10 from progressive clinical groups. Representative of n=7 per group and 5 experiments. (B) Percentage of CD19+, IgDhi, IL-10 positive cells per clinical group as represented in A. EC= endemic controls, AS= asymptomatic, SY= symptomatic. (C) Total CD19+ B cells gated for intracellular IL-10 via flow cytometry. Left represents CD19+ gate and following panels show representative IL-10 production in CD19+ cells from progressive clinical groups. (D) Quantification of CD19+, IL-10 percent positive cells per clinical group. Representative of n=7 per group and 5 experiments. (E) CD19+ cells analyzed for IgD and intracellular IL-4 via flow cytometry. Representative of n=7 per group and 2 experiments. (F) Quantification of CD19+, IgDhi, IL-4 percent positive cells per clinical group. Graph is representative of n=7 per group and 2 experiments. (G) Representative plot of IgD, IL-10 expression of splenic B cells from VL symptomatic dogs. (H) Graphical representation of percentage of IgDhi B cells within the spleen of L. infantum-infected symptomatic dogs. (I) IgD distribution across all CD19+ cells in the three clinical groups (top) and intracellular IL-10 production in IgD and B cell groups. All bars (B,D,F) represent ± SEM. One-way ANOVA with multiple comparisons was used for all statistical analysis. *p<0.05, ***p<0.001.
IL-4 production is a hallmark of both cutaneous and visceral leishmaniasis implicated in supporting logarithmic parasite growth and disease progression (35, 36). Murine regulatory B cells were shown to produce IL-4 in conjunction with IL-10 after stimulation with immune regulatory receptor TIM-1 (37). Given the importance of IL-4 in driving non-healing immune responses to Leishmania, we investigated its expression in IgDhi B cells. As disease progressed, a small but significant (p<0.05) population IgDhi B cells expressed IL-4 (Fig. 3E, F).
Based on finding that these cells produced IL-10 in all three clinical settings, we wanted to establish whether IgDhi B cells had a different gene expression profile than that of IgDlo B cells. Human (and canine) immature regulatory B cell gene expression has not been well explored. We targeted known transcription factor or regulatory effector molecule transcripts to determine expression in peripheral blood from endemic control, asymptomatic and symptomatic L. infantum-infected dogs. We selected genes associated with regulatory B cells (38, 39), immune exhaustion (2, 40, 41), B cell associated/regulatory transcription factors (17, 23, 42). Regulatory B cell marker stim2 was decreased compared to IgDlo B cell controls (Supplemental Fig. 3). Transcription factor baff was decreased in asymptomatic dog IgDhi B cells compared to IgDlo B cells but was almost equivalently expressed in symptomatic dog IgDhi B cells compared to IgDlo B cells (Supplemental Fig. 3). B cell specific transcripts did not amplify in control T cell mRNA from matched canine blood samples (data not shown). The complete underlying transcriptional phenotype of IgDhi B cells requires more comprehensive study, these data demonstrate a unique transcriptional profile in IgDhi B cells compared to the remaining B cell population. IgDhi B cells support a cytokine environment propagated by yet unknown transcriptional control which may regulate pro-inflammatory T cell responses toward non-productivity.
B cells from VL symptomatic dogs suppressed CD4+ T cell Th1 function
IL-10 producing B cells acted to suppress T cell inflammatory functions in patients with lupus erythematosus and rheumatoid arthritis, (16, 18, 21). We hypothesized that this is true during progressive canine VL. To determine whether IL-10 producing B cells suppress T cell function during progressive VL, we co-cultured magnetic bead-enriched B cells from animals with symptomatic VL with isogenic T cells in the presence of antigen. L. infantum antigen-stimulated CD4+ T cells co-cultured with B cells from symptomatic dogs (Fig. 4A – left panel, black) had significant, four-fold smaller population of proliferative (Fig. 4B) and IFN-γ-producing CD4+ T cells (Fig. 4C) compared to CD4+ T cells cultured with B cells from immune-responsive, asymptomatic animals (Fig. 4A – left, grey). Conversly, IL-10 production was decreased in CD4+ T cells cultured with B cells from from asymptomatic (3.9%) compared to symptomatic dogs (15.9%). Symptomatic dog CD3+ T cells had a similar response (data not shown).
Figure 4.
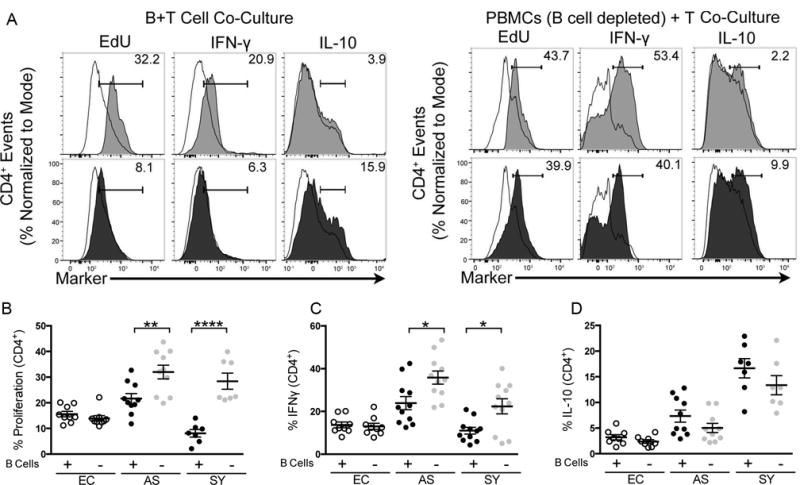
B cells isolated from VL symptomatic dogs sufficient and necessary to suppress CD4+ T cell TH1 function. (A) CD4+ T cell proliferation, IFN-γ production or IL-10 production in response to freeze-thawed (FT) L. infantum antigen when co-cultured with B cells (left) or co-cultured with B cell negative- PBMCs (right panel set). Histogram of responses by endemic control (open-solid line), asymptomatic (grey) or symptomatic (black) VL ex vivo CD4+ T cells. Representative of n=7 per group. (B,C,D) Percent CD4+ T cell population containing (B) EdU (proliferation), (C) IFNγ, and (D) IL-10 after co-culture with B cells (+) [5×105 cells/well] or co-cultured with B cell negative PBMCs [5×105 cells/well]. Graphs representative of n=7 per group. Percentages are normalized to isotype control and values subtracted from endemic control baseline. Statistical analysis performed utilizing one way-ANOVA with multiple comparison of the means. *p<0.05, **p<0.01, ****p<0.0001. EC = endemic controls, AS = asymptomatic and SY = symptomatic groups. All B cell co-culture was performed at 1:1 B/T cell ratios. Error bars represent ±SEM.
To determine the requirement for B cells in this suppressive response, B cells were depleted via magnetic labeling, and antigen-recall responses measured. In the absence of B cells, but presence of myeloid cells in PBMC co-culture, CD4+ T cells isolated from both asymptomatic and symptomatic animals robustly and significantly responded to L. infantum antigen (p<0.05) (Fig. 4A – right), particularly when compared to responses in the presence of CD19+ B cells. Symptomatic dog CD4+ T cells recovered a significant three-fold larger population of proliferative cells as compared to the proliferative population in the presence of regulatory B cells (p<0.0001) (Fig. 4B, black vs. grey). CD4+ T cells from symptomatic animals also regained a two-fold larger population of IFN-γ producing cells when B cells were depleted from culture (Fig. 4C) to similar levels as T cells isolated from asymptomatic dogs. IL-10 production from T cells trended down after co-culture with depleted B cells compared to culture without B cells in PBMC co-culture. Removal of B cells, a large portion of which produced IL-10, from co-cultured T cell functional assays recovered proliferative and IFN-γ responses in CD4+ T cells in both VL asymptomatic and symptomatic dogs.
IgDhi B cells induced IgDlo B cells to produce IL-10
The overall percentage of B cells producing IL-10 increased as VL progressed. We wanted to determine whether this increase could occur because IgDhi B cells induced IgDlo B cell IL-10 production. A similar phenomenon was described in IL-10-producing T regulatory cells (43, 44). IgDhi B cells were isolated from the blood of asymptomatic dogs through magnetic selection as described in van der Vlugt et al. (Fig. 5A) (22). Throughout the course of the experiment, IgDhi B cells maintained a mean population size of ~40% IL-10 producing cells (red), whereas the population of IgDlo B cells producing IL-10 (black) increased three-fold in size from 10 to ~30% by day 5, similar to production by IgDhi B cells (Fig. 5B). Conversly when IgDlo B cells were co-cultured with APCs depleted of IgDhi B cells, there was no increase in the mean population size producing intracellular IL-10 (black-dotted line). To determine if the amount of secreted IL-10 was increased in IgDhi and day 7 IgDlo/hi co-cultured cells as compared to between IgDlo B cells, supernatants collected at day 7 were assayed via ELISA for IL-10. IL-10 was secreted in significantly greater amounts by day 7 IgDhi or IgDlo/hi co-cultured B cells than from IgDlo B cells (Fig. 5C). Sorted IgDhi B cells influenced IgDlo/neg B cells to induce IL-10 demonstrating that IgDhi B cells may induce other B cell populations into IL-10 production as inflammation and VL progresses.
Figure 5.
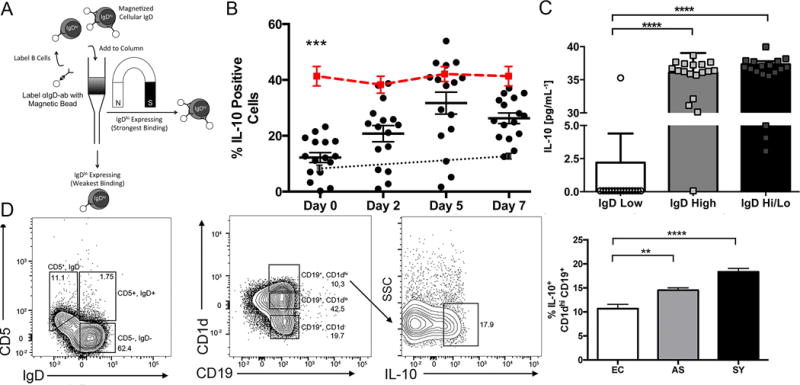
IgDhi B cells induced IgDlo B cells to produce IL-10. (A) Schema of magnetic IgDhi/IgDlo B cell separation. (B) IL-10 production in IgDhi (red line), IgDlo (black dots) and IgDlo-MHCII, with IgDhi B cell depleted co-culture (grey dots with dotted line). B cells were selected using CD19+ positive selection magnetic beads followed by labeling with anti-human-IgD magnetic beads. MHCII cells were isolated via adherence from total CD19−, cells. Dots represent results from individual dogs. (C) IL-10 secretion as measured by ELISA in IgDhi and co-cultured IgDhi/lo cells compared to only IgDlo cells after 7 days of culture. Dots represent results from individual asymptomatic VL dogs. Bars are ±SEM. N=18 per group. (D) CD5 vs. IgD expression, CD1d+ cells significantly increased IL-10 expression during progressive VL. ** p<0.01, *** p<0.001, **** p<0.0001 One-way ANOVA with multiple comparisons was used to for statistical analysis. Cells were incubated in a ratio of 10:1 (IgDlo:IgDhi) or (IgDlo:MHCII).
We wanted to evaluate if previously described, induced, CD1dhi regulatory B cells producing IL-10 were increased in the periphery of symptomatic, chronically L. infantum-infected dogs, which could represent a population of IgDlo B cells driven into IL-10 production. There was no increase in CD5+ expressing B cells across progressive VL groups (data not shown). The number of CD1dhi expressing B cells also did not increase population size in the periphery across VL progressive groups, however, IL-10 production of CD1dhi expressing B cells did increase as VL progressed (Fig 5D, right panel). It is possible that CD1dhi B cells were induced into IL-10 production by IgDhi B cells during progressive VL.
B cell PDL1 or IL-10 suppressed T cell function ex vivo from VL
We have demonstrated that B cells co-cultured with T cells suppressed L. infantum antigen-specific CD4+ T cell responses. Previous studies identified a role for B cell-produced IL-10 in T cell suppression during autoimmune disease (18, 19). Antigen-presenting cell (APC) expression of PDL1- has been demonstrated in multiple systems, including by our group in the canine VL model, to suppress T cell function (2). We examined the hypothesis that PD1/PDL-1 signaling may also be a mechanism of T cell suppression by B cells during VL. To ensure that pdl1 expression was present in canine B cells, we performed quantitative PCR to evaluate pdl1 mRNA expression from B cells isolated across the three clinical groups. mRNA expression of pdl1 increased five-fold from endemic control expression as L. infantum infection progressed to clinical disease (Fig. 6A, left panel). We also found significantly increased PDL1 surface expression on B cells by an increase in mean fluorescence intensity normalized to isotype (Fig. 6A, right panel). Based on this significant expression of PDL1 on the surface of B cells during clinical VL and the possibility that these interactions may mediate regulatory interactions as previously shown for T cells during VL (2), we wished to further examine whether abrogating B cell/T cell PDL1/PD1 interactions would recover T helper cell functions. We blocked IL-10, B cell PDL1, or both, during sorted culture with just magnetically-isolated B cells prior to T cell co-culture. After co-culture with B cells incubated with αPDL1 antibody blockade, CD3+, CD4+ and CD8+ T cells isolated from symptomatic dogs recovered both proliferative responses and IFNγ production in response to antigen stimulation compared to isotype control treatment of isolated B cells prior to co-culture (Fig. 6B, top). αIL-10 and αPDL1 independent blockade similarly recovered T cell function whereas dual blockade did not demonstrate an additive effect (Fig. 6B, D). As we previously demonstrated that T cells are a predominant source of IL-10 in VL in dogs (2), we wanted to determine if B cells would promote production of IL-10 in co-cultured T cells. B cell co-cultured T cells from symptomatic dogs had significant intracellular IL-10 production (p<0.01) (Fig. 6C) compared to isogenic T cells without B cell co-culture. Blocking B cell IL-10, PDL1 or both, abolished T cell IL-10 production (Fig. 6C). T cell proliferation and IFN-γ production were dramatically increased after antibody blockade of B cell specific PDL1 and IL-10. These results indicate the potential importance of PD1/PDL1 inhibitory receptor ligand interactions and IL-10 IgDhi B cells in dampening T cell proliferative and IFN-γ responses during chronic VL.
Discussion
Previous studies of regulatory B cells during Hepatitis B virus, HIV or Schistosoma infection demonstrated that IL-10 production from B cells was concurrent with immune suppression and/or pathogen persistence (19, 21, 22). Cells from VL patient splenic aspirates had higher IL-10 mRNA expression during progressive VL (23). Exact cellular sources of IL-10 were unknown. Mouse model studies of cutaneous leishmaniasis suggested CD4+, CD25+, FoxP3-expressing regulatory T cells (Tregs) as major contributors to the IL-10 cytokine pool during L. major infection (44). Treg cells were not significant contributors to IL-10 expression in human disease; depletion of CD25+ cells did not recover IFN-γ production (23). It is likely that other cells contribute to IL-10-mediated immune regulation during progressive human VL. Follicular dendritic cells (DCs) (45), natural killer cells (NK) (46), or T follicular helper cells (45, 47) were shown to be immune regulators of VL. IgDhi regulatory B cells may be an early inducer of additional regulatory cell populations. In agreement with data from others (23, 48), CD4+, IL-10+ T cells produced IL-10 after interaction with B cells from animals with progressive VL. A specific phenotype of these cells, or their specific role in regulation of T cell responses, has not previously been described in VL.
B regulatory cells are a recently described regulatory cell t shown to have a vital role in autoimmune disease regulation (11, 12). The role of IgD+ regulatory B cells during chronic infectious diseases had not been well-described, here regulatory IgDhi B10 B cells drove production of IL-10 during progressive VL and induced IL-10 production from both IgDlo/neg B cells and T cells. IgDhi B cells produced IL-10 in all settings, suggesting that these cells may constitutively express IL-10 and are not dependent on inflammatory induction, in sharp contrast to regulatory B cells from mice (20). IgDhi B cells were the only significant B cell type producing IL-10 during symptomatic VL. B cells from VL infected animals were both necessary and sufficient for suppression of T cell responses to antigenic stimulation. Suppression of T cell IFN-γ by B cells was mediated through PDL1 and IL-10 pathways as we observed marked improvement of IFN-γ production of CD3+, CD4+, and CD8+ T cells in response to L. infantum antigen after B cell PDL1/IL-10 antibody blockade. IgDhi B cells compared to IgDlo B cells demonstrated a differential transcriptional profile.
Previous reports of regulatory B cells underscored a role for inducible CD5+, IL-10 producing, B regulatory cells (20). IgDhi B cells found in VL expanded during prolonged inflammation similar to induced regulatory T cells (49), or murine regulatory B cells(50).5150 IgDhi B cells also produced IL-10 in healthy animals without inflammation, perhaps suggesting a regulatory B cell lineage which produces IL-10 under homeostatic conditions and expands under inflammation (Fig. 3). IgDhi B cells induced IgDlo/neg B cells to produce IL-10 (Fig. 5) underscoring their role in formation of inducible regulatory B cells similar to previously described CD5+ or CD1dhi B cell subsets, which were not IgDhi in our experimental setting (Fig. 5). Further studies are needed to explore ontogeny and roles for additional, induced, regulatory, B cell subsets during VL.
B cells have been shown to produce IL-10 after activation to promote homeostasis and immune regulation (51). L. infantum proteins were previously shown to activate B cells to produce IL-10 (52). IgDhi B cells had no differential increase in cell size ex vivo compared to TLR activated B cells and are likely to be immature and not classically activated. Instead these cells may have a non-antigen specific constitutive role. The targeted transcriptional profile of IgDhi B cells was substantially different than that of IgDlo B cells. Immune modulatory receptor TIM1, found on regulatory plasma cells (50), had only limited differenced in expression between IgDlo vs. IgDhi B cells or clinical groups indicating that IgDhi B cells do not share this trait with regulatory plasma cells. IgDhi B cell transcript expression of B cell transcription factors or importantly, regulatory markers, did not significantly change over disease progression and perhaps further highlights that IgDhi B cells are not an induced regulatory cell.
Our results support previous studies which demonstrated the role of PDL1 in B-cell function and antibody responses under experimentally-induced chronic inflammatory conditions in mice (41). PDL1 has been described as an important ligand in controlling immune function during experimental (53) and natural VL (2). PD1/PDL1 interactions drove CD4+ T cell exhaustion characterized by reduced proliferation and IFN-γ production mediated by adherent antigen presenting cells (2). We posit that B cells also provided PDL1 driven T cell IL-10 production. PDL1 represents a potential target of B cell directed immune therapy during progressive VL. Antibodies targeted against PDL1 and PD1 inhibitory pathways of lupus and cancer improved T cell responses and are being evaluated by clinical trials (33). Targeting these cells through immune therapy may also provide clinically relevant applications in immunotherapy during additional persistent infectious diseases.
We have demonstrated increased presence of a novel and critical regulatory B10 B cell which highly expressed IgD during progressive VL. These cells produced IL-10, induced both other B cells and T cells to produce IL-10 and suppressed IFN-γ through PDL1/PD1. IgDhi B10 B cells represent a novel, unexplored, B cell immune regulatory cell and signify a potential new target in chronic infectious disease therapy.
Supplementary Material
Acknowledgments
The flow cytometry facility utilized by this study is funded through user fees and the generous financial support of the Carver College of Medicine, Holden Comprehensive Cancer Center, and Iowa City Veteran’s Administration Medical Center. We would like to thank the foxhound owners for study participation. We would also like to thank Dr. Tara Grinnage-Pulley, Dr. Kevin Esch, Dr. Mandy Larson, Ben Scott, and Hailie Fowler for their generous help in sample collection.1
Footnotes
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30CA086862, supported by the National Center for Research Resources of the National Institutes of Health under Award Number 1S10 RR027219, funded by the National Institutes of Health Tropical Medicine Research Center grant AI030639, a grant from the Morris Animal Foundation and start-up funds from the University of Iowa, College of Public Health, Department of Epidemiology.
Author Contributions
R.G.S. designed the experiments, collected the samples, performed: cell isolations, flow cytometry and FACS collection, primer design, qPCR, ELISA; analyzed the data and wrote the manuscript. I.M.L performed: the primer design, qPCR and ELISA. A.J.S. collected the samples, performed the diagnostic qPCR and serology testing. T. W, J.H. and M.W, aided in experimental design and writing of the manuscript. S.M.B.J., C. deO.M.A. and J.F.V.C. collected samples, performed FACS analysis and aided in manuscript preparation. C.A.P. collected the samples, designed the experiments, analyzed the data and wrote the manuscript.
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Kaye PM, Aebischer T. Visceral leishmaniasis: immunology and prospects for a vaccine. Clin Microbiol Infect. 2011;17:1462–1470. doi: 10.1111/j.1469-0691.2011.03610.x. [DOI] [PubMed] [Google Scholar]
- 2.Esch KJ, Juelsgaard R, Martinez PA, Jones DE, Petersen CA. Programmed death 1-mediated T cell exhaustion during visceral leishmaniasis impairs phagocyte function. Journal of immunology (Baltimore, Md.: 1950) 2013;191:5542–5550. doi: 10.4049/jimmunol.1301810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boggiatto PM, Ramer-Tait AE, Metz K, Kramer EE, Gibson-Corley K, Mullin K, Hostetter JM, Gallup JM, Jones DE, Petersen CA. Immunologic indicators of clinical progression during canine Leishmania infantum infection. Clin Vaccine Immunol. 2010;17:267–273. doi: 10.1128/CVI.00456-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez-Ruiz J, Salaiza-Suazo N, Carrada G, Escoto S, Ruiz-Remigio A, Rosenstein Y, Zentella A, Becker I. CD8 cells of patients with diffuse cutaneous leishmaniasis display functional exhaustion: the latter is reversed, in vitro, by TLR2 agonists. PLoS Negl Trop Dis. 2010;4:e871. doi: 10.1371/journal.pntd.0000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortiz M, Mon C, Herrero JC, Oliet A, Rodriguez I, Ortega O, Gallar P, Hinostroza J, Cobo G, del Alamo M, Jimenez J, Torres R, Digiogia C, San Martin J, Vigil AI, Blanco J. Glomerulonephritis and cryoglobulinemia: first manifestation of visceral leishmaniasis. Clin Nephrol. 2015;83:370–377. doi: 10.5414/CN108195. [DOI] [PubMed] [Google Scholar]
- 6.Esch KJ, Schaut RG, Lamb IM, Clay G, Morais Lima AL, do Nascimento PR, Whitley EM, Jeronimo SM, Sutterwala FS, Haynes JS, Petersen CA. Activation of Autophagy and Nucleotide-Binding Domain Leucine-Rich Repeat-Containing-Like Receptor Family, Pyrin Domain-Containing 3 Inflammasome during Leishmania infantum-Associated Glomerulonephritis. The American journal of pathology. 2015;185:2105–2117. doi: 10.1016/j.ajpath.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen CA. New means of canine leishmaniasis transmission in north america: the possibility of transmission to humans still unknown. Interdiscip Perspect Infect Dis. 2009;2009:802712. doi: 10.1155/2009/802712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen CA. Leishmaniasis, an emerging disease found in companion animals in the United States. Top Companion Anim Med. 2009;24:182–188. doi: 10.1053/j.tcam.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson-Corley KN, Hostetter JM, Hostetter SJ, Mullin K, Ramer-Tait AE, Boggiatto PM, Petersen CA. Disseminated Leishmania infantum infection in two sibling foxhounds due to possible vertical transmission. The Canadian veterinary journal. La revue veterinaire canadienne. 2008;49:1005–1008. [PMC free article] [PubMed] [Google Scholar]
- 10.Iwata Y, Matsushita T, Horikawa M, DiLillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, Hall RP, St Clair EW, Tedder TF. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov EI, Spolski R, Poe JC, Leonard WJ, Tedder TF. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491:264–268. doi: 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Candando KM, Lykken JM, Tedder TF. B10 cell regulation of health and disease. Immunological reviews. 2014;259:259–272. doi: 10.1111/imr.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez ER, Olivera GC, Quebrada Palacio LP, Gonzalez MN, Hernandez-Vasquez Y, Sirena NM, Moran ML, Ledesma Patino OS, Postan M. Altered distribution of peripheral blood memory B cells in humans chronically infected with Trypanosoma cruzi. PloS one. 2014;9:e104951. doi: 10.1371/journal.pone.0104951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asito AS, Moormann AM, Kiprotich C, Ng’ang’a ZW, Ploutz-Snyder R, Rochford R. Alterations on peripheral B cell subsets following an acute uncomplicated clinical malaria infection in children. Malaria journal. 2008;7:238. doi: 10.1186/1475-2875-7-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Lee HJ, Yoo IS, Kang SW, Lee JH. Regulatory B cells are inversely associated with disease activity in rheumatoid arthritis. Yonsei medical journal. 2014;55:1354–1358. doi: 10.3349/ymj.2014.55.5.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saussine A, Tazi A, Feuillet S, Rybojad M, Juillard C, Bergeron A, Dessirier V, Bouhidel F, Janin A, Bensussan A, Bagot M, Bouaziz JD. Active chronic sarcoidosis is characterized by increased transitional blood B cells, increased IL-10-producing regulatory B cells and high BAFF levels. PloS one. 2012;7:e43588. doi: 10.1371/journal.pone.0043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Correale J, Equiza TR. Regulatory B cells, helminths, and multiple sclerosis. 2014 Methods in molecular biology (Clifton, NJ)1190:257–269. doi: 10.1007/978-1-4939-1161-5_18. [DOI] [PubMed] [Google Scholar]
- 19.Das A, Ellis G, Pallant C, Lopes AR, Khanna P, Peppa D, Chen A, Blair P, Dusheiko G, Gill U, Kennedy PT, Brunetto M, Lampertico P, Mauri C, Maini MK. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. Journal of immunology (Baltimore, Md: 1950) 2012;189:3925–3935. doi: 10.4049/jimmunol.1103139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42:607–612. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Siewe B, Stapleton JT, Martinson J, Keshavarzian A, Kazmi N, Demarais PM, French AL, Landay A. Regulatory B cell frequency correlates with markers of HIV disease progression and attenuates anti-HIV CD8(+) T cell function in vitro. Journal of leukocyte biology. 2013;93:811–818. doi: 10.1189/jlb.0912436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Vlugt LE, Zinsou JF, Ozir-Fazalalikhan A, Kremsner PG, Yazdanbakhsh M, Adegnika AA, Smits HH. Interleukin 10 (IL-10)-producing CD1dhi regulatory B cells from Schistosoma haematobium-infected individuals induce IL-10-positive T cells and suppress effector T-cell cytokines. The Journal of infectious diseases. 2014;210:1207–1216. doi: 10.1093/infdis/jiu257. [DOI] [PubMed] [Google Scholar]
- 23.Nylen S, Maurya R, Eidsmo L, Manandhar KD, Sundar S, Sacks D. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25+ (Foxp3) regulatory T cells in human visceral leishmaniasis. The Journal of experimental medicine. 2007;204:805–817. doi: 10.1084/jem.20061141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gautam S, Kumar R, Singh N, Singh AK, Rai M, Sacks D, Sundar S, Nylen S. CD8 T cell exhaustion in human visceral leishmaniasis. The Journal of infectious diseases. 2014;209:290–299. doi: 10.1093/infdis/jit401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ronet C, Hauyon-La Torre Y, Revaz-Breton M, Mastelic B, Tacchini-Cottier F, Louis J, Launois P. Regulatory B cells shape the development of Th2 immune responses in BALB/c mice infected with Leishmania major through IL-10 production. Journal of immunology (Baltimore, Md: 1950) 2010;184:886–894. doi: 10.4049/jimmunol.0901114. [DOI] [PubMed] [Google Scholar]
- 26.Bankoti R, Gupta K, Levchenko A, Stager S. Marginal zone B cells regulate antigen-specific T cell responses during infection. Journal of immunology (Baltimore, Md: 1950) 2012;188:3961–3971. doi: 10.4049/jimmunol.1102880. [DOI] [PubMed] [Google Scholar]
- 27.Silva JS, Andrade AC, Santana CC, Santos LQ, Oliveira CI, Veras PS, Vassallo J, dos-Santos WL. Low CXCL13 expression, splenic lymphoid tissue atrophy and germinal center disruption in severe canine visceral leishmaniasis. PloS one. 2012;7:e29103. doi: 10.1371/journal.pone.0029103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wortmann G, Sweeney C, Houng HS, Aronson N, Stiteler J, Jackson J, Ockenhouse C. Rapid diagnosis of leishmaniasis by fluorogenic polymerase chain reaction. Am J Trop Med Hyg. 2001;65:583–587. doi: 10.4269/ajtmh.2001.65.583. [DOI] [PubMed] [Google Scholar]
- 29.Geisberger R, Lamers M, Achatz G. The riddle of the dual expression of IgM and IgD. Immunology. 2006;118:429–437. doi: 10.1111/j.1365-2567.2006.02386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20:332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andreani G, Ouellet M, Menasria R, Gomez AM, Barat C, Tremblay MJ. Leishmania infantum amastigotes trigger a subpopulation of human B cells with an immunoregulatory phenotype. PLoS Negl Trop Dis. 2015;9:e0003543. doi: 10.1371/journal.pntd.0003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee S, Mukherjee B, Mukhopadhyay R, Naskar K, Sundar S, Dujardin JC, Roy S. Imipramine exploits histone deacetylase 11 to increase the IL-12/IL-10 ratio in macrophages infected with antimony-resistant Leishmania donovani and clears organ parasites in experimental infection. J Immunol. 2014;193:4083–4094. doi: 10.4049/jimmunol.1400710. [DOI] [PubMed] [Google Scholar]
- 34.Ansari NA, Kumar R, Gautam S, Nylen S, Singh OP, Sundar S, Sacks D. IL-27 and IL-21 are associated with T cell IL-10 responses in human visceral leishmaniasis. Journal of immunology (Baltimore, Md: 1950) 2011;186:3977–3985. doi: 10.4049/jimmunol.1003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar R, Bumb RA, Salotra P. Correlation of parasitic load with interleukin-4 response in patients with cutaneous leishmaniasis due to Leishmania tropica. FEMS Immunol Med Microbiol. 2009;57:239–246. doi: 10.1111/j.1574-695X.2009.00607.x. [DOI] [PubMed] [Google Scholar]
- 36.Miralles GD, Stoeckle MY, McDermott DF, Finkelman FD, Murray HW. Th1 and Th2 cell-associated cytokines in experimental visceral leishmaniasis. Infect Immun. 1994;62:1058–1063. doi: 10.1128/iai.62.3.1058-1063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, Chalasani G, Sayegh MH, Najafian N, Rothstein DM. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011;121:3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vadasz Z, Haj T, Toubi E. The role of B regulatory cells and Semaphorin3A in atopic diseases. Int Arch Allergy Immunol. 2014;163:245–251. doi: 10.1159/000360477. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg MV, Drake CG. LAG-3 in Cancer Immunotherapy. Curr Top Microbiol Immunol. 2011;344:269–278. doi: 10.1007/82_2010_114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nduati E, Gwela A, Karanja H, Mugyenyi C, Langhorne J, Marsh K, Urban BC. The plasma concentration of the B cell activating factor is increased in children with acute malaria. J Infect Dis. 2011;204:962–970. doi: 10.1093/infdis/jir438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters N, Sacks D. Immune privilege in sites of chronic infection: Leishmania and regulatory T cells. Immunol Rev. 2006;213:159–179. doi: 10.1111/j.1600-065X.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- 44.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 45.Smelt SC, Engwerda CR, McCrossen M, Kaye PM. Destruction of follicular dendritic cells during chronic visceral leishmaniasis. J Immunol. 1997;158:3813–3821. [PubMed] [Google Scholar]
- 46.Bogdan C. Natural killer cells in experimental and human leishmaniasis. Front Cell Infect Microbiol. 2012;2:69. doi: 10.3389/fcimb.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodrigues V, Laforge M, Campillo-Gimenez L, Soundaramourty C, Correia-de-Oliveira A, Dinis-Oliveira RJ, Ouaissi A, Cordeiro-da-Silva A, Silvestre R, Estaquier J. Abortive T follicular helper development is associated with a defective humoral response in Leishmania infantum-infected macaques. PLoS Pathog. 2014;10:e1004096. doi: 10.1371/journal.ppat.1004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh K, Nordstrom T, Morgelin M, Brant M, Cardell LO, Riesbeck K. Haemophilus influenzae resides in tonsils and uses immunoglobulin D binding as an evasion strategy. The Journal of infectious diseases. 2014;209:1418–1428. doi: 10.1093/infdis/jit593. [DOI] [PubMed] [Google Scholar]
- 49.Man K, Kallies A. Synchronizing transcriptional control of T cell metabolism and function. Nat Rev Immunol. 2015;15:574–584. doi: 10.1038/nri3874. [DOI] [PubMed] [Google Scholar]
- 50.Matsumoto M, Baba A, Yokota T, Nishikawa H, Ohkawa Y, Kayama H, Kallies A, Nutt SL, Sakaguchi S, Takeda K, Kurosaki T, Baba Y. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity. 2014;41:1040–1051. doi: 10.1016/j.immuni.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 51.Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol. 2015;15:441–451. doi: 10.1038/nri3857. [DOI] [PubMed] [Google Scholar]
- 52.Cabral SM, Silvestre RL, Santarem NM, Tavares JC, Silva AF, Cordeiro-da-Silva A. A Leishmania infantum cytosolic tryparedoxin activates B cells to secrete interleukin-10 and specific immunoglobulin. Immunology. 2008;123:555–565. doi: 10.1111/j.1365-2567.2007.02725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gigley JP, Bhadra R, Moretto MM, Khan IA. T cell exhaustion in protozoan disease. Trends Parasitol. 2012;28:377–384. doi: 10.1016/j.pt.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vida B, Toepp A, Schaut RG, Esch KJ, Juelsgaard R, Shimak RM, Petersen CA. Immunologic progression of canine leishmaniosis following vertical transmission in United States dogs. Vet Immunol Immunopathol. 2016;169:34–38. doi: 10.1016/j.vetimm.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


