Abstract
Objective
Caregiver depressed mood and stress are associated with increased child asthma functional morbidity (AFM) and secondhand smoke exposure (SHSe), while social support (SS) reduces risk. This study extends previous literature by examining: (1) longitudinal patterns of pediatric AFM and SHSe and (2) how caregiver stress, depressed mood, and SS are related to child SHSe and AFM changes.
Methods
Participants were 334 caregivers who smoked, had a child with asthma, and who were enrolled in a smoking cessation induction/asthma intervention. SHSe and AFM were measured at baseline and 4, 6, and 12 months. All measures were caregiver self-report. We used an autoregressive latent trajectory model to examine the intercept, linear, and quadratic growth factors and autoregressive and cross-lagged effects of SHSe and AFM.
Results
After an asthma exacerbation, decreases in child AFM and SHSe were followed by respective increases over time. Child SHSe at 4-months and 6-months predicted subsequent child AFM. Autoregressive paths were only significant for AFM. Higher baseline caregiver depressed mood and stress predicted higher baseline child AFM, but not other growth factors. Higher baseline caregiver self-esteem SS was only associated with lower baseline child AFM and fewer increases in AFM across time. Exploratory analyses indicated higher baseline caregiver depressed mood and stress were associated with less favorable changes in child SHSe and AFM.
Conclusions
Caregiver depressed mood, stress, and SS should be considered when addressing pediatric SHSe and AFM. Caregiver support may be needed to maintain intervention gains.
Keywords: secondhand smoke exposure, asthma morbidity, social support, depression, stress
Introduction
Pediatric Asthma Functional Morbidity (AFM), or limitations in activity due to asthma symptoms, is exacerbated by several socio-environmental factors, including secondhand smoke exposure (SHSe; U.S. Department of Health and Human Services, 2006). SHSe is associated with heightened risk for asthma onset, symptom severity, and exacerbations among youth (U.S. Department of Health and Human Services, 2006). SHSe also impairs recovery from an asthma exacerbation (Otsuki, Rand, Butz, & Riekert, 2009).
Although SHSe has declined over the last 15 years, overall rates remain high: 40.6% of children aged 3-11 and 33.8% of children aged 12-19 are exposed to SHS (Homa et al., 2015). Rates of SHSe are higher (58.8%) among children aged 6-11 with asthma relative to same-aged children without asthma (44.7%) (Quinto, Kit, Lukacs, & Akinbami, 2013). Parental smoking, the most common source of SHSe (Ding et al., 2010), poses compounded risk for youth with asthma due to both the health effects and the intergenerational transmission of smoking uptake (U.S. Department of Health and Human Services, 2006).
Longitudinal studies demonstrate that reductions in SHSe are correlated with reductions in asthma morbidity (Gerald et al., 2009). A recent Cochrane systematic review reported mixed evidence for the efficacy of SHSe interventions for families of children with asthma, but also noted that the majority of the studies resulted in health improvements for children (Baxi et al., 2014). While there is evidence that reductions in SHSe improve pediatric AFM (Baxi et al., 2014; Gerald et al., 2009), the role of parental depressed mood, stress, and social support on changes in SHSe and AFM remains unclear. The present study extends the current literature by examining how caregiver depressed mood, perceived stress, and social support impact longitudinal changes in pediatric SHSe and AFM among children with asthma whose caregivers smoke. Depressed mood and stress were conceptualized as risk factors for asthma, and social support was conceptualized as a factor with the potential to enhance asthma outcomes.
Parental stress and depressed mood are two key modifiable risk factors for both SHSe and AFM. Higher levels of parental stress and depressed mood (Chen & Schreier, 2008; Pak & Allen, 2012) are associated with increased childhood asthma morbidity. Among families with children with asthma, higher rates of maternal stress and depressed mood (Pak & Allen, 2012) have been shown to be risk factors for child SHSe (Butz et al., 2011).
On the other hand, higher levels of parental social support are associated with a lower number of pediatric asthma-related emergency room visits (Rand et al., 2000), decreased asthma symptoms (Berz et al., 2007), and increased asthma control (Scheckner, Arcoleo, & Feldman, 2015). Higher levels of parental social support are also related to less parental perceived stress (Bloomberg & Chen, 2005), decreased depressed mood (Kingsbury et al., 2014), and increased parental smoking cessation (Tooley, Busch, McQuaid, & Borrelli, 2014). Pediatric AFM, SHSe, parental perceived stress, and parental depressed mood appear to be closely interrelated and parental social support appears to be a key resource factor as it is associated with lower risk for parental smoking, stress, and depressed mood and negative pediatric asthma outcomes.
Parental stress, depressed mood, and social support represent modifiable factors that have the potential to impact both smoking behavior and asthma. There is a paucity of information on how SHSe and AFM are related to one another over time, and how risk factors (i.e., depressed mood, stress) and resource factors (i.e., social support) may influence changes in SHSe and AFM over time. Specifically, the aims our study are to examine: (1) longitudinal changes in pediatric AFM and SHSe and their relationship to one another over time and (2) how possible risk factors (e.g., caregiver stress, depressed mood) and one protective factor (i.e., caregiver social support) are related to changes in SHSe and AFM using autoregressive latent trajectory analysis. It was hypothesized that SHSe would predict subsequent AFM and that caregivers with higher baseline social support, lower depressed mood, and lower perceived stress would have greater reductions in SHSe and AFM over time. Results from the present study may help to inform future intervention studies by identifying possible risk and resource factors that influence SHSe and AFM.
Methods
Participants
This is a secondary analysis of data from a smoking cessation induction study that included asthma education and motivational interviewing (N = 334; R01 HL062165-06, B. Borrelli, PI). Participants were primarily recruited from emergency departments and physician referrals. Caregivers of children with asthma were eligible for study participation if they met the following requirements: smoked ≥3 cigarettes per day for the last year and had smoked at least 100 cigarettes in their lifetime, were the primary caregiver of a child with asthma who was under the age of 18 and who had experienced an asthma exacerbation in the last two months necessitating urgent care (urgent care visit or hospitalization), ≥18 years of age, were not pregnant or planning to become pregnant, were reachable by telephone, were fluent in English, and were not enrolled in cessation treatment or using medication or nicotine replacement therapy to quit smoking. Families with target children who had other significant respiratory illnesses (e.g. cystic fibrosis) were excluded. Participants did not have to want to quit smoking to enroll in the study but had to be willing to discuss their smoking and have asthma education visits in their home.
The parent study was comprised of three study groups, one group of caregivers with healthy children and two groups of caregivers with children with asthma. Only participants from the two asthma groups were included in the present study. Of the 3,244 participants who were screened for asthma, 603 were eligible. Of those, 442 were enrolled and signed informed consent. Of those, 341 completed the core intervention home visits (asthma education and motivational interviewing for smoking). Asthma education was guidelines-based (National Asthma Education and Prevention Program, 2007); discussing smoking as a trigger served as a bridge to the smoking portion of the intervention.
Subsequent to the core intervention, participants were randomized to receive one of two different telephone-based interventions (6 calls over four months): one group received asthma education plus child wellness while the other group received asthma education plus a motivational intervention for smoking cessation. All participants who wanted to quit within 30 days were provided with an 8-week supply of Transdermal Nicotine Patch treatment at no cost. Additional details of the parent study can be found in (Borrelli et al., 2015). The Institutional Review Board at our institution approved the study and data were collected in Rhode Island and Massachusetts from 2008-2013. The sample for the current paper is drawn from the 341 participants who were randomized to the telephone-based interventions. As described later, seven cases were removed because they had outlying values for SHSe. Descriptive statistics on participant characteristics at baseline are provided in Table 1.
Table 1.
Participant Characteristics at Baseline.
| Caregiver Characteristics | |
|---|---|
| Demographics | |
| Age [M (SD)] | 34.01 (9.88) |
| Female [n (%)] | 267 (79.9%) |
| Race/Ethnicity[n (%)] | |
| White, Non-Hispanic | 158 (47.3) |
| Black, Non-Hispanic | 80 (24.0%) |
| Hispanic | 54 (16.2%) |
| Other | 42 (12.6%) |
| Completed education beyond high school [n (%)] | 129 (38.7%) |
| Income below $25,000 [n (%)] | 220 (70.8%) |
| Cigarettes smoked per day [M (SD)] | 14.09 (10.16) |
| Fagerstrom Test for Nicotine Dependence[M (SD)] | 4.10 (2.33) |
| Other smokers lived in the home [n (%)] | 131 (43.4%) |
| Total home smoking ban in place [n (%)] | 214 (64.1%) |
|
| |
| Child Characteristics | |
|
| |
| Demographics | |
| Age [M (SD)] | 5.07 (4.48) |
| Female [n (%)] | 140 (41.9%) |
| Asthma-related variables | |
| Number of doctor visits during the past year [M (SD)] | 5.97 (10.78) |
| Number of emergency room visits during the past year [M (SD)] | 3.02 (4.66) |
| Asthma symptom days in the past month [M (SD)] | 9.56 (10.03) |
| Uses a controller medication [n (%)] | 204 (62%) |
Measures
Assessments for SHSe and AFM were completed at baseline, 4-months, 6-months, and 12-months. Demographic (age, race, gender, education, income) and smoking history variables (cigarettes smoked per day, behavioral measure of nicotine dependence (Fagerstrom Test for Nicotine Dependence; Heatherton et al., 1991), home smoking ban (no smoking allowed anywhere in the home), presence of other smokers in the home) were assessed via caregiver self-report.
Secondhand Smoke Exposure (SHSe)
Self-reported SHSe was assessed with a structured caregiver interview that measured child SHSe from the target caregiver and others. SHSe was measured by assessing the number of cigarettes a child was exposed to during the past week. As part of the standardized scoring of this measure, a composite score was computed that measured the child's total SHSe from all people and all places during the past week. The composite score has been used in multiple studies and has demonstrated good reliability and validity (Matt et al., 2000). Higher scores indicate higher SHSe. Self-reported SHSe was used as the dependent variable because there were a greater number of assessment points which allowed for an examination of non-linear change. Objective SHSe was measured with passive nicotine air monitors (i.e., dosimeters). These monitors have good validity in homes (Leaderer & Hammond, 1991) and have been validated in an intercomparison study (Caka et al., 1990). Baseline assessments of objective SHSe and self-reported SHSe were correlated, r = .34, p < .001. Participants received $20 for questionnaire completion and $5 for returning the dosimeters in good condition.
Asthma Functional Morbidity (AFM)
AFM was measured by the Asthma Assessment Form, an adaptation of the Functional Severity Scale (Rosier et al., 1994), which assesses asthma symptoms and resultant limitations in general activity, school attendance, and sports participation during the past month. This scale has good internal consistency (α = .72-.86; Koinis-Mitchell, Kopel, Salcedo, McCue, & McQuaid, 2014). The range of scores is 0-4 with lower scores indicating fewer limitations.
Social Support
Social support was measured with the 16- item Interpersonal Support Evaluation List (Cohen, Mermelstein, Kamarck, & Hoberman, 1985) which includes four social support subscales: appraisal (someone to talk to about problems, belonging (people to do things with), tangible (material support), and self-esteem (positive feedback from others). The 16-item version is derived from the 40-item version and has good reliability (Payne et al., 2012). We replaced one item (an item that implies that the participant has a car) with another item from the 40-item version (an item about having someone mail an important letter at the post office for you) to account for the possibility that our urban sample might not own cars.
Depressed Mood
The 20-item Center for Epidemiologic Studies Depression Scale (CES-D) was used to assess depressive symptoms and has demonstrated good reliability and validity (Radloff, 1977). The clinical cut-off indicative of significant depressed mood is 16 (Lewinsohn, Seeley, Roberts, & Allen, 1997). Higher scores indicate higher depressive symptoms.
Perceived Stress
Perceived stress was measured via the 4-item Perceived Stress Scale which assesses perceived environmental and experiential stress (Cohen, Kamarck, & Mermelstein, 1983). This scale has good reliability and validity (Cohen et al., 1983). Higher scores indicate higher perceived stress.
Data Analysis
Analyses were performed with Mplus (version 7; Muthén & Muthén, 2012). Outliers were identified via graphical techniques and seven cases with outlying values for SHSe were removed. SHSe was non-normally distributed with large variance so a square-root transformation was used to bring this variable towards normality. For all subsequent analyses, maximum likelihood estimation with robust standard errors was used and standardized results are presented. Analyses were conducted via three main steps.
Step 1: Identify the Best Fitting Model of Longitudinal Changes in SHSe and AFM
Three different multivariate analyses were used to explore different hypothesized patterns of change in SHSe and AFM and identify the best fitting model for our data: autoregressive/cross-lagged model, latent growth curve model, and autoregressive latent trajectory model (ALT). The models are different in that the autoregressive/cross-lagged model allowed for examination of autoregressive relationships and cross-lagged relationships. The ALT model allowed examination of constant changes over time (i.e., trajectories) along with the autoregressive and cross-lagged relationships. In the ALT model, the first time-point was treated as endogenous because we aimed to examine overall changes in SHSe and AFM. Each analysis controlled for study group, baseline home smoking ban, and seasonality.
Model fit was estimated with the χ2 likelihood ratio test, Comparative Fit Index (CFI), Tucker-Lewis Index (TLI), Standardized Root Mean Square Residual (SRMR), Root Mean Square Error of Approximation (RMSEA), and Bayesian Information Criterion (BIC; Geiser, 2013). More constrained ALT models were tested in a step-wise procedure and the following constraints were individually added: fixing the slope and quadratic growth parameter variances to zero, excluding the time-specific correlations, constraining the time-specific correlations to be equal, and lastly constraining the autoregressive parameters to be equal (Bollen & Curran, 2004).
Step 2: Add Individual Baseline Predictors (Caregiver Social Support, Depressed Mood, and Perceived Stress) Separately to the ALT Model
After the best fitting ALT model was identified (step 1), a series of analyses were conducted in which each baseline predictor (caregiver social support, depressed mood, and perceived stress) was added separately to the ALT model (steps 2a, 2b and 2c).
Step 3: Refit the ALT Model in the Derived Predictor Subgroups
If the predictor was significant in the model (in step 2), a subgroup analysis was conducted to explore whether estimates (i.e., growth factors) for SHSe and AFM differed depending on the baseline level of the significant predictor. For depressed mood, the groups were defined by the clinical cut-off of 16 with individuals with scores of 16 or higher in the higher depressed mood group (Lewinsohn et al., 1997). Median splits were used to identify the cut-points for group membership for social support (median for self-esteem social support = 11) and perceived stress (median = 7). Groups were divided so that the higher groups included scores above the median.
Results
Step 1: Longitudinal Changes in SHSe and AFM
Correlations and descriptive statistics are provided in Table 2. At baseline, objective and self-reported SHSe were significantly correlated, r = .34, p < .001. Separate latent growth curve models for SHSe and AFM were tested and the best fitting growth curve for each process was quadratic. The results from the multivariate autoregressive/cross-lagged, latent growth curve, and ALT models are presented in Table 3. Overall, the multivariate ALT model fit best, suggesting that the changes in SHSe and AFM are best represented by a model that includes overall changes across time (i.e., trajectories), autoregressive, and cross-lagged effects. In the best fitting model, slope/quadratic variances were constrained to zero and time-specific correlations and autoregressive parameters were constrained to be equal (labeled model 12 in Table 3).
Table 2.
Correlation matrix and descriptive statistics.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Baseline AFM | - | .40** | .34** | .40** | .13* | .05 | .06 | .01 | -.04 | .18b* | .07 | -.14* | .24** | .16** |
| 2. 4-month AFM | - | .49** | .44** | .02 | .03 | -.01 | -.09 | -.04 | .14b* | .11 | -.05 | .24** | .17** | |
| 3. 6-month AFM | - | .47** | -.03 | .003 | .08 | .03 | -.004 | .22b** | .17** | -.14* | .28** | .14* | ||
| 4. 12-month AFM | - | .06 | .01 | .15* | .10 | -.05 | .26b** | .07 | -.15* | .30** | .19** | |||
| 5. Baseline SHSe | - | .46** | .38** | .36** | -.46** | .05b | .004 | -.09 | .12* | .08 | ||||
| 6. 4-month SHSe | - | .46** | .44** | -.25** | .09b | -.04 | -.14* | .09 | -.01 | |||||
| 7. 6-month SHSe | - | .56** | -.26** | .08b | .13* | -.14* | .15* | .12* | ||||||
| 8. 12-month SHSe | - | -.22** | .05b | .02 | -.07 | .05 | .05 | |||||||
| 9. Home smoking ban | - | .07a | .03a | .11* | -.09 | .03 | ||||||||
| 10. Seasonality | - | .12a | .05b | .11b | .03b | |||||||||
| 11. Study Group | - | .002 | .03 | .05 | ||||||||||
| 12. Total Social Support Score | - | -.55** | -.48** | |||||||||||
| 13. Depressed Mood | - | .67** | ||||||||||||
| 14. Perceived Stress | - | |||||||||||||
|
| ||||||||||||||
| Mean | 1.46 | .57 | .53 | .59 | 2.67 | 1.48 | 1.44 | 1.37 | .64 | 1.63 | 1.51 | 48.79 | 18.25 | 6.95 |
| Minimum | 0 | 0 | 0 | 0 | .10 | .10 | .10 | .10 | 0 | 0 | 1 | 17 | 0 | 0 |
| Maximum | 4 | 3.83 | 3.40 | 3.17 | 13.42 | 11.83 | 12.45 | 14.00 | 1 | 3 | 2 | 64 | 57 | 16 |
| Variance | .89 | .48 | .52 | .58 | 10.01 | 4.56 | 5.38 | 5.21 | .23 | 1.33 | .25 | 75.87 | 136.81 | 11.70 |
| Standard Deviation | .94 | .69 | .72 | .76 | 3.16 | 2.13 | 2.32 | 2.28 | .48 | 1.15 | .50 | 8.71 | 11.70 | 3.42 |
Notes: n's range from 231-334.
Phi or Cramer's V statistic.
Eta statistic. The means (standard deviations) for the social support subscales were as follows: appraisal- 12.51 (2.76), belonging- 12.32 (2.89), tangible- 12.48 (2.95), and self-esteem- 11.43 (2.63). Correlations between the subscales and AFM and SHSe were higher for the social support total score.
Table 3.
Results from the Multivariate Autoregressive Models, Latent Growth Curve Models (LGCM), and Autoregressive Latent Trajectory (ALT) Models.
| Models | χ2 (df) | CM | Δχ2 (df) | CFI | TLI | RMSEA | SRMR | BIC |
|---|---|---|---|---|---|---|---|---|
| 1. Autoregressive model with cross-lagged paths, full model | 76.09 (15)*** | --- | --- | .87 | .61 | .12 | .06 | 5042.91 |
| 2. LGCM, full model a | 45.66 (16)*** | --- | --- | .93 | .78 | .07 | .04 | 7666.11 |
| 3. ALT, full modelb | --- | --- | --- | --- | --- | --- | --- | --- |
| 4. ALT, no slope variancesb | --- | --- | --- | --- | --- | --- | --- | --- |
| 5. ALT, no quadratic variancesb | --- | --- | --- | --- | --- | --- | --- | --- |
| 6. ALT-5 + no slope variancesc | 19.03 (11) | --- | --- | .98 | .91 | .05 | .02 | 7663.52 |
| 7. ALT-6 + no intercept variance for SHSe | 18.66 (15) | 6 | 2.55 (4) | .99 | .97 | .03 | .02 | 7644.72 |
| 8. ALT-6 + no time specific correlations | 34.51 (14)** | 6 | 24.43 (3)*** | .95 | .83 | .07 | .02 | 7655.11 |
| 9. ALT-6 + time specific correlations equalc | 16.41 (13) | 6 | .65 (2) | .99 | .97 | .03 | .02 | 7653.29 |
| 10. ALT-7 + time specific correlations equal | 20.91 (17) | 7 | 2.18 (2) | .99 | .97 | .03 | .02 | 7634.80 |
| 11. ALT-6 + autoregressive paths equal | 16.80 (13) | 6 | 1.09 (2) | .99 | .97 | .03 | .02 | 7654.64 |
| 12. ALT-11 + time specific correlations equal | 19.06 (17) | 11 | 2.46 (4) | 1.00 | .99 | .02 | .03 | 7617.14 |
|
| ||||||||
| ALT-12 model with Predictors | ||||||||
|
| ||||||||
| ALT-12 + Social Support subscales | 25.87 (25) | --- | --- | 1.00 | .99 | .01 | .02 | 7659.68 |
| ALT-12 + Depressed Mood | 19.74 (19) | --- | --- | 1.00 | 1.00 | .01 | .02 | 7626.32 |
| ALT-12 + Perceived Stress | 23.71 (19) | --- | --- | .99 | .97 | .03 | .03 | 7651.74 |
Notes: CM: comparison model in the Δχ2. Δχ2= Satorra-Bentler scaled χ2. Model modifications were made for both SHSe and AFM unless otherwise noted. All models controlled for study group, seasonality, and baseline home smoking ban status. A non-significant χ2 and the following values were indicative of good model fit: > .95 for CFI and TLI, < .05 for RMSEA and SRMR (Geiser, 2013). A BIC change of 10 or more was interpreted as strong evidence for improved model fit (Raftery, 1995). Nested ALT models were compared using the Satorra-Bentler scaled chi-square difference test (Δχ2; Satorra & Bentler, 2001).
Due to error message, residual variance for the observed 12 month SHSe was constrained to 0.
Error message: No convergence.
Error message: PSI not positive definite/negative variance or residual variance.
Baseline levels of AFM were significantly different from zero (M = 2.63, p <001) and there was significant variability among children's initial AFM levels (σ = .96, p <001). There was a trend for linear decreases in AFM over time (M = -11.62, p = 08) followed by significant increases in AFM over time (M = 10.02, p = .04). Significant negative autoregressive paths were detected across adjacent AFM time points, p's < .05, such that higher AFM scores at a previous time point predicted lower AFM scores at the subsequent time point.
Baseline SHSe levels were significantly different from zero (M = 2.23, p <001) with significant variability among individuals (σ = .51, p <.001). SHSe significantly decreased over time (M = -3.59, p < .001), but subsequently increased across later time points (M = 3.77, p < .001). No significant autoregressive paths between adjacent SHSe assessments were detected indicating that previous assessments of SHSe were not predictors of subsequent SHSe assessments. The nonsignificant autoregressive paths for SHSe suggest that there were stronger predictors contributing to the variance in SHSe besides previous SHSe assessments. SHSe at the 4-month follow-up predicted AFM at the 6-month follow-up (β= .14, p = .02), and SHSe at the 6-month follow-up predicted AFM at 12-month follow-up (β = .23, p < .001). No other cross-lagged paths were significant.
Step 2: Addition of Baseline Predictors (Caregiver Social Support, Depressed Mood, and Perceived Stress)
2a. Social support
The subscales (appraisal, belonging, tangible, and self-esteem social support) from the social support measure were added as predictors to the final ALT model. The model fit remained good (Table 3). The results indicated that caregivers with higher baseline self-esteem social support had children with lower baseline AFM levels and fewer increases in AFM over time (Table 4). No other social support subscales predicted AFM or SHSe growth factors.
Table 4.
Standardized Results from the Conditional Multivariate Autoregressive Latent Trajectory (ALT) Models.
| AFM Intercept | AFM Linear | AFM Quadratic | SHSe Intercept | SHSe Linear | SHSe Quadratic | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Models | Beta (S.E.) | p | Beta (S.E.) | p | Beta (S.E.) | p | Beta (S.E.) | p | Beta (S.E.) | p | Beta (S.E.) | p |
| ALT 12 +SS subscales | ||||||||||||
|
| ||||||||||||
| Appraisal SS | .05 (.12) | .70 | -.15 (.48) | .75 | .17 (.42) | .68 | .03 (.09) | .72 | -.23 (.25) | .34 | .31 (.28) | .27 |
| Belonging SS | .01 (.12) | .94 | -.41 (.52) | .43 | .54 (.42) | .20 | -.09 (.10) | .36 | -.19 (.29) | .52 | .32 (.35) | .37 |
| Tangible SS | -.13 (.12) | .30 | .63 (.46) | .17 | -.60 (.38) | .12 | -.003 (.10) | .98 | .25 (.27) | .35 | -.39 (.31) | .21 |
| Self-esteem SS | -.23 (.11) | .04 | .58 (.42) | .17 | -.71 (.32) | .03 | -.006 (.10) | .95 | -.05 (.26) | .85 | .10 (.30) | .75 |
|
| ||||||||||||
| ALT 12 +Depressed Mood | ||||||||||||
|
| ||||||||||||
| Depressed Mood | .40 (.09) | <.001 | -.34 (.60) | .57 | .44 (.53) | .40 | .12 (.08) | .14 | .02 (.23) | .94 | -.15 (.27) | .60 |
|
| ||||||||||||
| ALT 12 +PS | ||||||||||||
|
| ||||||||||||
| PS | .28 (.09) | .002 | -.46 (.52) | .37 | .50 (.49) | .31 | .14 (.08) | .08 | -.18 (.23) | .42 | .17 (.31) | .58 |
Notes: All models controlled for study group, seasonality, and baseline home smoking ban status. SS= social support. AFM= asthma functional morbidity. SHSe= secondhand smoke exposure. S.E.= standard error.
The intercept (M = 3.78, p < .001), the linear factor (M = -9.23, p = .006), and the quadratic factor were significant for AFM (M = 7.38, p = .002). The intercept variability was also significant (σ = .89, p <.001). Baseline levels of SHSe were significantly different from zero (M = 2.51, p < .001) with significant variability (M = .51, p < .001). Unlike the original ALT model, the linear and quadratic growth factors for SHSe were non-significant (M = -2.31, p = .13 and M = 1.71, p = .37, respectively). In other words, when social support is included in the model, the decreases in SHSe (linear factor) and subsequent increases in SHSe (quadratic factor) were non-significant. The autoregressive paths for AFM were consistently significant and negative (i.e., stable decreases in AFM over time) while the paths for SHSe were not (i.e., previous assessments of SHSe were not significant predictors of subsequent SHSe assessments when social support was included in the model). SHSe at 4-months predicted AFM at 6-months (β = .12, p = .03) and SHSe at 6-months predicted AFM at 12-months (β = .22, p < .001).
2b. Depressed Mood
The model fit remained good when depressed mood was included as a predictor and results showed that caregivers with higher levels of depressed mood had children with higher baseline AFM (Table 3 and 4). The AFM intercept was significant (M = 1.94 and σ = .80, p < .001) and there were trends for linear decreases in AFM followed by increases AFM (M = -10.42, p = .10 and M = 8.54, p = .07, respectively). Previous assessments of AFM consistently predicted lower subsequent AFM assessments. Baseline SHSe was different from zero with variability (M = 2.03 and σ = .50, p's < .001). Significant linear decreases in SHSe (M = -3.58, p < .001) were followed by increases (M = 3.88, p < .001). No autoregressive paths for SHSe were detected. SHSe at 4-months and 6-months predicted AFM at 6-months and 12-months, respectively.
2c. Perceived Stress
When perceived stress was added as a predictor, higher caregiver stress was associated with a higher child AFM intercept (Table 3 and 4). The AFM intercept was significant (M = 2.07 and σ = .89, p's < .001) and there were trends for linear decreases in AFM followed by increases (M = -9.39, p = .09 and M = 7.79, p = .08, respectively). Baseline SHSe was different from zero with variability (M = 1.94 and σ = .50, p's < .001). Significant linear decreases in SHSe (M = -3.17, p = .002) were followed by increases in SHSe (M = 3.37, p = .006). The autoregressive paths remained consistent with previous results. SHSe at 4-months (β = .14, p = .02) and 6-months (β = .22, p = .001) predicted AFM at 6-months and 12-months, respectively.
Step 3: Refitting of the ALT Model in the Derived Predictor Subgroups
3a. Self-esteem social support multiple group analysis
A median split on the baseline levels of self-esteem social support was used to divide the sample into low and high social support groups. Median splits were used to create the groups as there are no established cut-off points. The models were re-run within subgroups and the parameter estimates are included in Figure 1. Caregivers with lower self-esteem social support had children with lower baseline levels of AFM and SHSe compared to those with high baseline levels of social support. In the low self-esteem social support group, there were significant linear decreases in SHSe but no significant growth for AFM, while in the high self-esteem social support group, only the quadratic factor for SHSe was significant, suggesting that among caregivers with lower social support, reductions in SHSe were not sufficient to have corresponding reductions in AFM. Further, for those with high social support, there were no significant decreases in SHSe or AFM. When examining the cross-lagged effects, in the low self-esteem social support group, SHSe at 4 and 6-months predicted AFM at 6 and 12-months, respectively, but in the high social support group, only SHSe at 6-months predicted later AFM. The autoregressive paths remained similar to the model without predictors.
Figure 1.
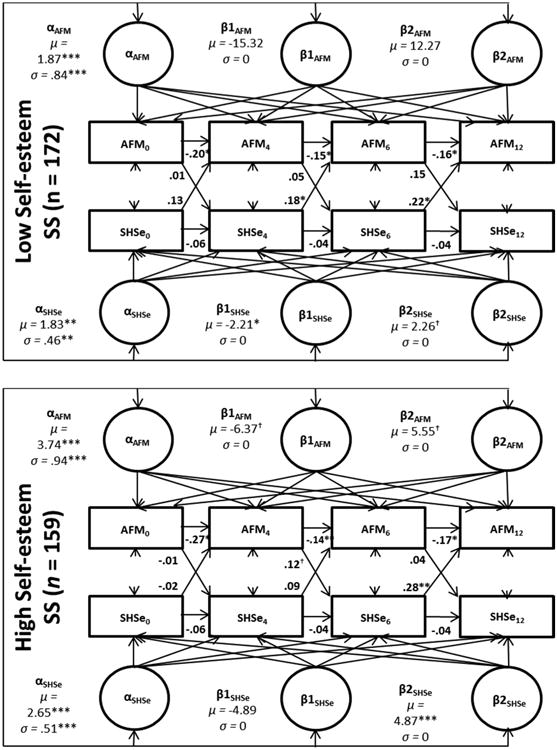
Standardized results from self-esteem social support multiple group ALT 12 model.
Notes: AFM: asthma functional morbidity. SHSe: secondhand smoke exposure.All models controlled for study group, seasonality, and baseline home smoking ban status. *<.05,**<.01,***<.001. †= <.10. ‡ =.05.
The overall model fit was good (x2 = 45.59, p = .22; CFI: .99; TLI: .96; RMSEA: .03; SRMR: .04; BIC: 7,771.68). The x2 contribution for the low self-esteem SS group was 26.18 and the high group was 19.41.
3b. Depressed mood multiple group analysis
Caregivers with lower depressive symptoms had children with higher initial levels of AFM and similar levels of SHSe when compared to caregivers with higher levels. Among those with higher levels of depressed mood, there were significant decreases in SHSe (and significant subsequent increases) but no accompanied decreases in AFM. Amongst those with low levels of depressed mood, there were significant decreases for both SHSe and AFM. For AFM in the low depressed mood group, there were significant increases (quadratic factor) following the initial decreases (linear factor). For the low depressed mood group, SHSe at 4 and 6-months predicted AFM at subsequent follow-ups while the only significant cross-lagged effect for the high depressed mood group was SHSe at 6-months predicting AFM at 12-months (Figure 2).
Figure 2.
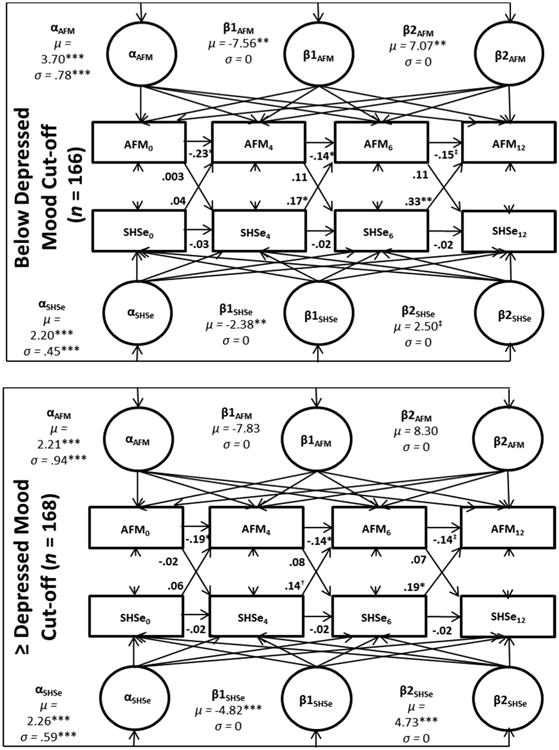
Standardized results from depressed mood multiple group ALT 12 model.
Notes: AFM: asthma functional morbidity. SHSe: secondhand smoke exposure. All models controlled for study group, seasonality, and baseline home smoking ban status.*<.05,**<.01,***<.001. †= <.10. ‡ =.05.
The overall model fit was adequate (x2 = 52.77, p = .07; CFI: .97; TLI: .92; RMSEA: .05; SRMR: .05; BIC: 7,467.15). The x2 contribution was as follows: low group was 17.75 and the high group was 35.02.
3c. Perceived stress multiple group analysis
Caregivers with lower perceived stress had children with higher AFM and SHSe intercepts as compared to caregivers in the high stress group. Across both groups, there were significant decreases in SHSe but no significant reductions in AFM. There were significant increases in SHSe in the high stress group but no such increases in caregivers with lower stress. Cross-lagged effects were similar across groups with SHSe at 6-months predicting AFM at 12-months (Figure 3).
Figure 3.
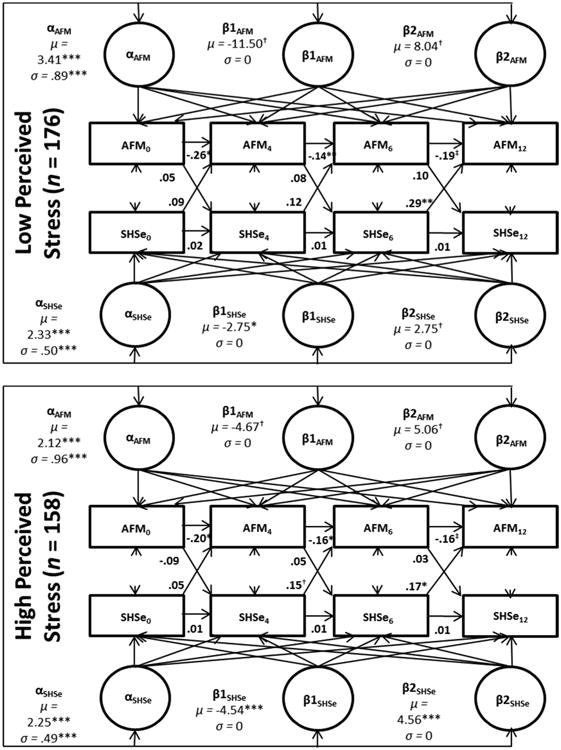
Standardized results from perceived stress multiple group ALT 12 model.
Notes: AFM: asthma functional morbidity. SHSe: secondhand smoke exposure. All models controlled for study group, seasonality, and baseline home smoking ban status.*<.05,**<.01,***<.001. †= <.10. ‡ =.05.
The overall model fit was adequate (x2 = 48.17, p = .15; CFI: .98; TLI: .95; RMSEA: .04; SRMR: .04; BIC: 7,797.99). The x2 contribution was 10.68 for the low group and 37.49 for the high group.
Discussion
SHSe remains a critical problem and almost 60% of children with asthma in the US are exposed to SHS (Quinto et al., 2013). Unfortunately, there is mixed evidence for the efficacy of SHSe interventions for families of children with asthma (Baxi et al., 2014). The present study was the first to examine concurrent and prospective changes in SHSe and AFM after an asthma exacerbation and factors that may impact these changes. A better understanding of how caregiver risk and resource factors are associated with pediatric outcomes after an asthma exacerbation is needed to foster the development of comprehensive interventions for children with asthma that will reduce asthma and tobacco-related morbidity and mortality.
One important finding from this study is that after an asthma exacerbation, children demonstrated significant decreases in either AFM or SHSe, depending on which risk/resource variables were included in the model, but these decreases were always followed by significant increases in AFM or SHSe over time. Specifically, there were initial decreases in AFM (followed by subsequent increases in AFM) when social support was in the model and initial decreases in SHSe (followed by subsequent increases in SHSe) when either depressed mood or perceived stress were included in the model. Thus, after initial decreases in SHSe or AFM, we observed a rebound back to levels that are indicative of poorer asthma control (i.e., more morbidity, more exposure to SHS). Both groups received active smoking/asthma intervention components and thus it makes sense that AFM and SHSe decreased during the active portion of the intervention and then increased post-treatment. While six counseling calls were provided to caregivers over four months, it seems that even longer-term support is needed during the maintenance phase of behavior change in order to prevent significant increases of SHSe and AFM.
As hypothesized, SHSe predicted subsequent AFM at all time points, except during the treatment period (baseline SHSe was not predictive of 4-month AFM). Thus, SHSe may have less predictive power when AFM is very elevated. AFM was likely more elevated at study entry (baseline) because it was the time period closest to the time of the asthma exacerbation. This finding is consistent with previous literature linking SHSe to asthma onset, symptom severity, and AFM among youth and highlights the need for comprehensive asthma management interventions that help families decrease all asthma related triggers and increase asthma control (National Asthma Education and Prevention Program, 2007; U.S. Department of Health and Human Services, 2006).
Importantly, baseline levels of caregiver depressed mood and perceived stress impacted child AFM over time. Consistent with our hypotheses, higher levels of baseline caregiver depressed mood and perceived stress were associated with higher levels of child baseline AFM. Contrary to our hypothesis, baseline caregiver depressed mood and perceived stress were not associated with the baseline levels of SHSe or changes in SHSe over time. Exploratory analyses were conducted to further examine how changes in SHSe and AFM differed among participants with different levels of baseline depressed mood and perceived stress. Results indicated that participants with higher levels of risk factors had less favorable changes in SHSe and AFM. For example, caregivers with high levels of depressed mood at baseline had significant decreases in SHSe over time but no accompanied changes in AFM, while caregivers with fewer depressive symptoms at baseline had significant decreases in both SHSe and AFM over time. Similarly, caregivers with higher baseline perceived stress had a significant rebound of SHSe after initial decreases in SHSe, but caregivers with lower baseline stress had significant decreases in SHSe but no significant rebound.
These findings are congruent with previous research among families with a child with asthma that document that higher rates of stress and depressed mood are associated with higher child AFM (Chen & Schreier, 2008; Pak & Allen, 2012) and SHSe (Butz et al., 2011). Importantly, this is the first study to highlight how these caregiver risk factors are associated with changes in SHSe and AFM over time after an asthma exacerbation. Taken together, these findings indicate a strong need for addressing caregiver stress and depressed mood in interventions focused on reducing SHSe and AFM. A Cochrane systematic review found that adding psychosocial mood management components to smoking cessation interventions increases quit rates (van der Meer, Willemsen, Smit, & Cuijpers, 2013). However, to our knowledge, there are no SHSe interventions that address caregiver depressed mood and/or stress and thus seems to be a ripe area for future research.
Consistent with hypotheses, higher baseline caregiver self-esteem social support, a resource factor, was associated with lower baseline AFM and fewer increases in AFM across time. On the other hand, social support was not associated with baseline levels of SHSe or changes in SHSe over time. The exploratory analyses suggested that among caregivers with low baseline self-esteem social support, decreases in SHSe were not sufficient to result in accompanied changes in AFM. However, the results from this analysis were somewhat murky as there were no significant decreases in either SHSe or AFM for caregivers with high baseline social support. Even though there were no significant changes in AFM to accompany the changes in SHSe for caregivers with low baseline social support, their involvement with an intervention may have been more effective at reducing SHSe because of the embedded social support provided during treatment. Caregivers who already had high levels of social support may have benefitted less from the embedded support. Incorporating mechanisms for social support into asthma/SHSe interventions may help deter increases of AFM back to levels associated with poor asthma control. Notably, Krieger and colleagues found that an asthma management intervention with a social support component significantly increased child symptom free days and parental quality of life (Krieger, Takaro, Song, Beaudet, & Edwards, 2009).
There are several limitations to this study. First, in order to model nonlinear models of change, self-reported SHSe was utilized rather than objective SHSe because there were only two objective SHSe assessments in the parent study. However, baseline self-reported and objective SHSe were significantly correlated. In addition, our models did not control for other potential confounders for AFM in the home environment, e.g., dust, animal dander, cockroaches, mold, or within the asthma regimen, medication use, which may have influenced changes in AFM over time. While our exploratory analyses allowed a more nuanced examination of the changes in SHSe and AFM in different subgroups of the risk and resource factors, it remains preliminary. Future studies should explore these relationships statistically.
Despite the limitations, our data suggest that caregiver depressed mood, stress, and social support are important factors to consider when trying to reduce pediatric SHSe and AFM. More support is also needed for caregivers after treatment ends to prevent increases in AFM and SHSe back to levels indicative of poor asthma control. Facilitating home and/or community-based behavioral and social support may be a mechanism for creating sustainable behavior change support that addresses multiple factors contributing to smoking lapses and relapses, such as ongoing stress, depressed mood, and lack of adequate support. Effective SHSe interventions are critically needed to reduce the disproportionate amount of disease burden and disability-adjusted life-years lost children incur because of SHSe, especially children with asthma (Öberg, Jaakkola, Woodward, Peruga, & Prüss-Ustün, 2011). Future studies should examine the efficacy of including strategies for addressing caregiver risk and resource factors within asthma/SHSe interventions.
Acknowledgments
This study was funded by NIH 5 R01 HL062165-09 (B. Borrelli, PI). The study was conducted at The Miriam Hospital, when Dr. Borrelli was employed there. Dr. Clawson's time was funded by 5 T32 HL076134-09 (R. Wing, PI).
References
- Baxi R, Sharma M, Roseby R, Polnay A, Priest N, Waters E, et al. Webster P. Family and carer smoking control programmes for reducing children' s exposure to environmental tobacco smoke (Review) Cochrane Database of Systematic Reviews. 2014;3 doi: 10.1002/14651858.CD001746.pub3. Art. No.: CD001746. http://doi.org/10.1002/14651858.CD001746.pub3. [DOI] [PubMed] [Google Scholar]
- Berz J, Carter A, Wagmiller R, Horwitz S, Murdock K, Briggs-Gowan M. Prevalence and correlates of early onset asthma and wheezing in a healthy birth cohort of 2- to 3-year olds. Journal of Pediatric Psychology. 2007;32(2):154–166. doi: 10.1093/jpepsy/jsj123. [DOI] [PubMed] [Google Scholar]
- Bloomberg GR, Chen E. The relationship of psychologic stress with childhood asthma. Immunology and Allergy Clinics of North America. 2005;25(1):83–105. doi: 10.1016/j.iac.2004.09.001. http://doi.org/10.1016/j.iac.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Bollen Ka, Curran PJ. Autoregressive Latent Trajectory (ALT) Models A Synthesis of Two Traditions. Sociological Methods & Research. 2004;32(3):336–383. http://doi.org/10.1177/0049124103260222. [Google Scholar]
- Borrelli B, McQuaid E, Dunsiger S, Hammond K, Novak S, Becker B, Busch A. Smoking cessation in Parents of Children with Asthma and Parents of Healthy Children: Randomized Clinical Trial. 2015 Under Review. [Google Scholar]
- Butz AM, Halterman JS, Bellin M, Tsoukleris M, Donithan M, Kub J, et al. Bollinger ME. Factors associated with second-hand smoke exposure in young inner-city children with asthma. The Journal of Asthma : Official Journal of the Association for the Care of Asthma. 2011;48(5):449–57. doi: 10.3109/02770903.2011.576742. http://doi.org/10.3109/02770903.2011.576742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caka F, Eatough D, Lewis E, Tang H, Hammond S, Leaderer B, et al. Lewtas J. An intercomparison of sampling techniques for nicotine in indoor environments. Environmental Science and Technology. 1990;24(8):1196–1203. [Google Scholar]
- Chen E, Schreier HMC. Does the social environment contribute to asthma? Immunology and Allergy Clinics of North America. 2008;28(3):649–64, x. doi: 10.1016/j.iac.2008.03.007. http://doi.org/10.1016/j.iac.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24(4):385–396. Retrieved from http://www.jstor.org/stable/2136404. [PubMed] [Google Scholar]
- Cohen S, Mermelstein R, Kamarck T, Hoberman H. Measuring the functional components of social support. In: Sarason I, Sarason B, editors. Social support: Theory, research and applications. The Hague, The Netherlands: Martinus Nijhoff; 1985. pp. 73–94. http://doi.org/10.1007/978-94-009-5115-0_5. [Google Scholar]
- Ding D, Wahlgren D, Liles S, Jones J, Hughes S, Hovell M. Secondhand smoke avoidance by preteens living with smokers: To leave or stay? Addictive Behaviors. 2010;35(11):989–994. doi: 10.1016/j.addbeh.2010.06.016. http://doi.org/10.1016/j.addbeh.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser C. Data Analysis with Mplus. New York: The Guilford Press; 2013. [Google Scholar]
- Gerald LB, Gerald JK, Gibson L, Patel K, Zhang S, Mcclure LA. Changes in environmental tobacco smoke exposure and asthma morbidity among urban school children. Chest. 2009;135(4):911–916. doi: 10.1378/chest.08-1869. http://doi.org/10.1378/chest.08-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. http://doi.org/10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Homa D, Neff L, King B, Caraballo R, Bennell R, Babb S, et al. Wang L. Vital Signs: Disparities in Nonsmokers' Exposure to Secondhand Smoke—United States, 1999–2012. MMWR. 2015;64(4):103–108. [PMC free article] [PubMed] [Google Scholar]
- Kingsbury AM, Hayatbakhsh R, Mamun AM, Clavarino AM, Williams G, Najman JM. Trajectories and Predictors of Women's Depression Following the Birth of an Infant to 21 Years: A Longitudinal Study. Maternal and Child Health Journal. 2014 doi: 10.1007/s10995-014-1589-6. http://doi.org/10.1007/s10995-014-1589-6. [DOI] [PubMed]
- Koinis-Mitchell D, Kopel S, Salcedo L, McCue C, McQuaid E. Asthma indicators and neighborhood and family stressors related to urban living in children. American Journal of Health Behavior. 2014;38(1):22–30. doi: 10.5993/AJHB.38.1.3. http://doi.org/http://dx.doi.org/10.5993/AJHB.38.1.3. [DOI] [PubMed] [Google Scholar]
- Krieger J, Takaro T, Song L, Beaudet N, Edwards K. The Seattle–King County Healthy Homes II Project: a randomized controlled trial of asthma self-management support comparing clinic-based nurses and in- Archives of Pediatrics & Adolescent Medicine. 2009;163(2):141–149. doi: 10.1001/archpediatrics.2008.532. http://doi.org/10.1001/archpediatrics.2008.532.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaderer B, Hammond S. Evaluation of vapor-phase nicotine and respirable suspended particle mass as markers for environmental tobacco smoke. Environmental Science and Technology. 1991;25:770–777. http://doi.org/10.1021/es00016a023. [Google Scholar]
- Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiological Studies-Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychology and Aging. 1997;12:277–287. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- Matt GE, Hovell MF, Zakarian JM, Bernert JT, Pirkle JL, Hammond SK. Measuring secondhand smoke exposure in babies: The reliability and validity of mother reports in a sample of low-income families. Health Psychology. 2000;19(3):232–241. doi: 10.1037//0278-6133.19.3.232. http://doi.org/10.1037//0278-6133.19.3.232. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User's Guide. Seventh. Los Angeles: Muthén & Muthén; n.d. [Google Scholar]
- National Asthma Education and Prevention Program. Guidelines for the diagnosis and management of asthma. National Asthma Education and Prevention Program Expert Panel Report 3. Bethesda, MD: 2007. [Google Scholar]
- Öberg M, Jaakkola MS, Woodward A, Peruga A, Prüss-Ustün A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. The Lancet. 2011;377(9760):139–146. doi: 10.1016/S0140-6736(10)61388-8. http://doi.org/10.1016/S0140-6736(10)61388-8. [DOI] [PubMed] [Google Scholar]
- Otsuki M, Rand CS, Butz AM, Riekert KA. B103 Practice Variation, Patient Behavior And Differences In Disease Susceptibility As Sources Of Health Outcome Disparities. American Thoracic Society; 2009. Environmental Tobacco Smoke Exposure and the Longitudinal Trajectories of Asthma Morbidity among Inner-City Children; p. A3757. http://doi.org/doi:10.1164/ajrccm-conference.2009.179.1_MeetingAbstracts.A3757. [Google Scholar]
- Pak L, Allen PJ. The Impact of Maternal Depression On Children with Asthma. 2012;38(1):11–20. [PubMed] [Google Scholar]
- Payne TJ, Andrew M, Butler KR, Wyatt SB, Dubbert PM, Mosley TH. Psychometric Evaluation of the Interpersonal Support Evaluation List-Short Form in the ARIC Study Cohort. SAGE Open. 2012;2(3) http://doi.org/10.1177/2158244012461923. [Google Scholar]
- Quinto K, Kit B, Lukacs S, Akinbami L. Environmental Tobacco Smoke Exposure in Children Aged 3‒19 Years With and Without Asthma in the United States, 1999‒2010 NCHS data brief. Hyattsville, MD: National Center for Health Statistics; 2013. [PubMed] [Google Scholar]
- Radloff L. The CES-D scale a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Raftery A. Bayesian model selection in social research. Sociological Methodology. 1995;25:111–163. [Google Scholar]
- Rand C, Butz A, Kolodner K, Huss K, Eggleston P, Malveaux F. Emergency department visits by urban African American children with asthma. Journal of Allergy and Clinical Immunology. 2000;105:83–90. doi: 10.1016/s0091-6749(00)90182-9. [DOI] [PubMed] [Google Scholar]
- Rosier MJ, Bishop J, Nolan T, Robertson CF, Carlin JB, Phelan PD. Measurement of functional severity of asthma in children. American Journal of Respiratory and Critical Care Medicine. 1994;149(6):1434–1441. doi: 10.1164/ajrccm.149.6.8004295. [DOI] [PubMed] [Google Scholar]
- Satorra A, Bentler PM. A scaled difference chi-square test statistic for moment structure analysis. Psychometrika. 2001;66(4):507–514. doi: 10.1007/s11336-009-9135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheckner B, Arcoleo K, Feldman J. The effect of parental social support and acculturation on childhood asthma control. Journal of Asthma. 2015;6:1–8. doi: 10.3109/02770903.2014.991969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley EM, Busch A, McQuaid EL, Borrelli B. Structural and Functional Support in the Prediction of Smoking Cessation in Caregivers of Children with Asthma. Behavioral Medicine. 2014:1–8. doi: 10.1080/08964289.2014.931274. http://doi.org/10.1080/08964289.2014.931274. [DOI] [PMC free article] [PubMed]
- U.S. Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon. General, Atlanta, GA: US …. Atlanta, Georgia: 2006. [PubMed] [Google Scholar]
- Van der Meer R, Willemsen M, Smit F, Cuijpers P. Smoking cessation interventions for smokers with current or past depression (Review) Cochrane Database of Systematic Reviews. 2013;8 doi: 10.1002/14651858.CD006102.pub2. Art No.: CD006102. [DOI] [PubMed] [Google Scholar]


