Abstract
Leukocyte recruitment to inflammation sites progresses in a multistep cascade. Chemokines regulate multiple steps of the cascade including arrest, transmigration and chemotaxis. The most important chemokine receptor in mouse neutrophils is CXCR2, which couples through Gαi2 and Gαi3-containing heterotrimeric G proteins. Neutrophils arrest in response to CXCR2 stimulation. This is defective in Gαi2 deficient neutrophils. Here, we show that Gαi3 deficient neutrophils showed reduced transmigration but normal arrest in mice. We also tested Gαi2 or Gαi3 deficient neutrophils in a CXCL1 gradient generated by a microfluidic device. Gαi3, but not Gαi2, deficient neutrophils showed significantly reduced migration and directionality. This was confirmed in a model of sterile inflammation in vivo. Gαi2, but not Gαi3, deficient neutrophils showed decreased Ca2+ flux in response to CXCR2 stimulation. Conversely, Gαi3, but not Gαi2, deficient neutrophils exhibited reduced AKT phosphorylation upon CXCR2 stimulation. We conclude that Gαi2 controls arrest and Gαi3 controls transmigration and chemotaxis in response to chemokine stimulation of neutrophils.
Introduction
Leukocyte recruitment to inflammation sites progresses in a multistep cascade including capture, slow rolling, rolling, arrest, adhesion strengthening, transmigration and chemotaxis (1–3). Those processes occur as a consequence of the interaction between leukocytes and endothelial cells and the stimulation of the leukocytes. Among various stimuli including selectins (2, 4–9), chemokine are the best known and probably most significant activators of arrest, transmigration and chemotaxis (2, 10, 11). The interaction of CXCL1 with its receptor CXCR2 on leukocytes induces neutrophil arrest (12, 13) and chemotaxis (14) in vitro and in vivo.
When rolling neutrophils encounter immobilized or soluble CXCL1, they rapidly arrest (15). Arrest is dependent on activation of the αLβ2 integrin LFA-1 (2, 5). When CXCL1 is injected into mice, the number of rolling neutrophils drops precipitously to almost zero, but rolling recovers within a few minutes (15). Conversely, the number of adherent neutrophils increases. For stable neutrophil adhesion, additional signaling steps are needed that depend on integrin outside-in signaling (16, 17), provided by both αMβ2 (Mac-1) and αLβ2 (LFA-1).
CXCR2 is a Gαi-coupled receptor (18). The activation of CXCR2 causes the of the Gαi subunit from β and γ subunits of the heterotrimeric G protein. This leads to activation of subsequent downstream signaling pathways including phospholipase C (PLC) -β and PI3Kγ. The βγ-subunits released from Gαi protein rapidly activate PLCβ and the activation of PLCβ elicits calcium mobilization and diacylglycerol production, leading to the activation of the Rap1 guanine nucleotide-exchange factor (GEF) CalDAG-GEFI (19–21). Rap1-GTP mediates rapid integrin activation via talin-1 and kindlin-3 (11, 22, 23). The βγ-subunits also activate PI3Kγ (24). The interaction of the PI3K subunits p101 and p84 with Gβγ subunits induces phosphatidylinositol (3,4,5)-trisphosphate production by p110γ. This signaling pathway is required for chemotaxis. Neutrophils, macrophages and T cells of mice that lack PI3Kγ respond poorly to chemokines (21, 25, 26).
The Gαi family consists of Gα0, Gαi1, Gαi2 and Gαi3, which are blocked by pertussis toxin and Gαz, which is not (27). Gαi2 and Gαi3 are abundantly expressed in leukocytes, and Gαi1 is expressed at low levels (15). Although Gαi2 and Gαi3 share about 85% protein sequence identity, the requirement for Gαi2 and Gαi3 is different among chemokine receptors and cells (28). Gαi2 is required for transendothelial migration of B cells in response to CXCL12, CXCL13 and CCL19 (29). Thymocyte emigration is controlled by both Gαi2 and Gαi3 (28, 30, 31). In neutrophils, Gαi2 is required for the arrest with CXCL1 (15). Gαi2 signaling in lung cells is necessary for eosinophil recruitment in a model of allergic airway inflammation (32). These data may reflect each chemokine receptor’s preferences for Gαi subunits. However, CXCR2 can clearly couple to Gαi2 and Gαi3 (18, 33). It is not known whether the same CXCR2 molecules can associate with Gαi2 and Gαi3, or whether subsets of Gαi2-coupled and Gαi3-coupled CXCR2 exist in neutrophils. Since the roles of Gαi subunits in downstream signaling have not been resolved, the functional consequences of chemokine receptors having a preference for Gαi subunits are poorly understood.
In this study, we tested whether Gαi subunits have preferential downstream signaling pathways and preferential functional consequences. We focused on arrest and chemotaxis of neutrophils of Gαi2 or Gαi3 deficient mice in vitro and in vivo.
Materials and Methods
Animals and cells
We used 8- to 12-week-old Gnai2-deficient mice, Gnai3-deficient mice and littermate control mice on the 129/Sv background (34). Mice were housed in a barrier facility under specific pathogen free conditions. Mice were handled according to the guidelines set by the Department of Laboratory Animal Care at LIAI and all surgical procedures were done as per the guidelines in the protocol approved by the Animal Care Committee of LIAI. Mouse bone marrow PMNs were isolated from femurs and tibias. Marrow cells were flushed from the bones using HBSS (137mM NaCl, 0.53mM KCl, 0.033mM Na2HPO4, 0.4mM NaHCO3, 0.044mM KH2PO4, and 2mM HEPES (pH 7.4)) without Ca2+ and Mg2+, and containing 0.1% BSA. Cells were centrifuged and, after hypotonic lysis of erythrocytes, mixed with antibodies of neutrophil negative selection kit (STEMCELL technologies, Vancouver, BC, Canada). After 30 min, the cells were washed with PBS and neutrophils were negatively selected with RoboSep (STEMCELL technologies). After an additional wash, PMNs were resuspended in HBSS.
Transwell migration assays
Transwell migration assays were performed as described previously with minor modification (35). PMN migration was assessed using Transwell filters (3 μm pores, Corning, Corning, NY), inserted in 24-well plates. The bottom chamber was filled with 0.7 ml of RPMI 1640 containing different concentrations of recombinant mouse CXCL1 (Peprotech, Rocky Hill, NJ)(see Results), and the top chamber was filled with 105 neutrophils in 0.2ml of RPMI 1640. The plates were incubated at 37°C/5% CO2 for 60 min. The number of migrated cells was counted using a hemocytometer.
Calcium flux in suspension assay
Calcium flux assays were performed as described previously (36). Isolated neutrophils were suspended at 5 × 106 cells/ml in RPMI/5% FCS/5 mM Hepes (pH7.4) then incubated with 5 μM fluo-3 (Molecular Probes, Eugene, OR) in the presence of F-127 detergent (0.02% -- Molecular Probes) in the dark at 37°C for 30 min with intermittent mixing every 5 min. The cells were washed twice with Ca2+- and Mg2+-free HBSS supplemented with 20 mM Hepes (pH 7.4) then diluted to 5×106 cells/ml and kept at 4°C in the dark until used. Prior to analysis, the cells were aliquoted into FACS tubes, warmed to RT, then analyzed by flow cytometry (FACScan, Becton Dickenson, San Jose, CA) for 40 seconds to establish a baseline reading. Chemokines were then added and the samples analyzed continuously for 3 min. The data were analyzed by averaging fluorescence per 20 second intervals. Peak Ca2+ flux values were calculated by subtracting baseline readings.
Immunoblotting
Immunoprecipitations and immunoblotting were performed as described previously (37). Cells were lysed in a buffer containing 1% Nonidet P-40, 150 mM NaCl, 50mM Tris-HCl (pH 8.0), 1mM Na orthovanadate, 2mM EDTA, 50mM NaF, and protease inhibitors (Sigma-Aldrich, St. Louis, MO). Cell lysates were subjected to SDS-PAGE and transferred onto nitrocellulose membranes for immunoblotting. The membranes were then incubated with anti-phospho-AKT antibodies (Cell Signaling Technology, Danvers, MA), followed by incubation with HRP-conjugated anti-rabbit IgG antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). The blots were developed using GE Healthcare’s ECL system. They were stripped and reprobed with anti-AKT antibodies (Cell Signaling Technology) to verify equivalent amounts of protein in each lane. Band intensity was quantified using ImageJ (National Institutes of Health).
Gradient maker assay
The microfluidic gradient maker was made and assembled as previously described (38). Gradient maker devices were coated with mouse recombinant ICAM-1-Fc (R&D Systems, Minneapolis, MN) (20 μg/ml) for 20 min and blocked with 1% casein in PBS (Thermo Fisher Scientific, Rockford, IL) for 20min. Bone marrow neutrophils were then loaded into the gradient maker. After 10 min, CXCL1 gradient were established (concentration 62.5nM–0.24nM; exponential). Cell migrations were recorded for 40 min using a digital camera (Sensicam QE, Cooke Corporation, Germany).
Intravital microscopy
Mice were anesthetized with an i.p. injection of 125 mg/kg ketamine hydrochloride (Pfizer, New York, NY), 0.025 mg/kg atropine sulfate (American Regent, Inc, Shirley, NY), and 12.5 mg/kg xylazine (TranquiVed; Phoenix Scientific, St Joseph, MO) and placed on a heating pad. The cremaster muscle was prepared as previously described (39). Microinjection of CXCL1 were performed as previously described (40). Cell migration was recorded using an intravital microscope (Axioskop, Carl Zeiss, Inc.) through the lens of a digital camera (Sensicam QE).
Statistics
Statistical analysis was performed with Prism, and included one-way analysis of variance and t test where appropriate. All data are presented as mean ± SEM. p < 0.05 was considered significant.
Results
Gαi3 is important for transmigration to CXCL1
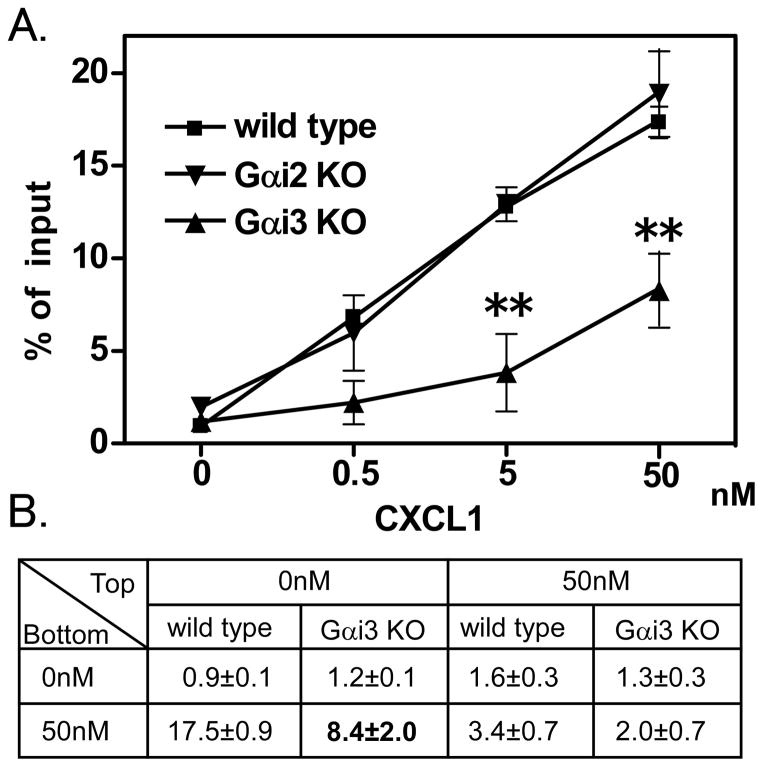
To examine whether Gαi2 and Gαi3 are necessary for leukocyte transmigration, Gαi2 and Gαi3 deficient neutrophils were tested in transwell migration assays. In transmigration to 0.5, 5 or 50nM CXCL, Gαi2 deficient neutrophils did not show any difference compared with wild type neutrophils (Figure 1A). In contrast, Gαi3-deficient neutrophils showed significantly decreased migration (Figure 1A). To test whether transmigration was chemotactic, we performed a checker board assay (Figure 1B). The chemotaxis of Gαi3 deficient neutrophils was reduced by almost 50% (P<0.01 compared to wild type), whereas random migration did not show significant differences between Gαi3 deficient neutrophils and wild type neutrophils..
Figure 1. Role of Gαi2 and Gαi3 in transmigration.
A. Transmigration of Gαi2 deficient or Gαi3 deficient neutrophils in response to CXCL1 was measured by transwell migration assays. CXCL1 was added to the lower chamber only. Data are presented as mean ± SEM. ** indicates P<0.01. B. Checkerboard transwell migration assays of neutrophil chemotaxis to CXCL1. Bold indicates significant difference (P<0.05) from wild type. Data are representative of at least three independent experiments.
Gαi3 is dispensable for neutrophil arrest in vivo
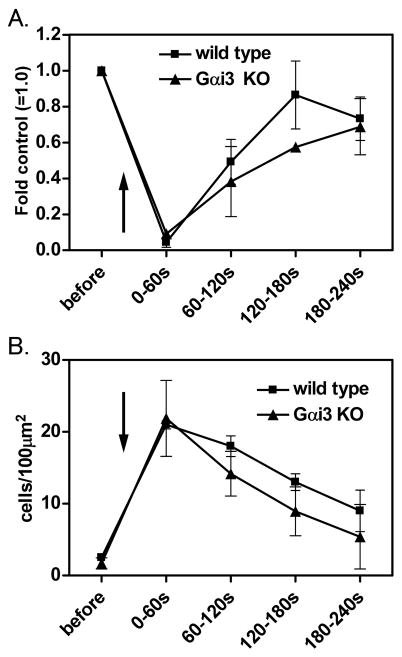
To investigate the role of Gαi3 deficiency on neutrophil arrest, we examined rolling neutrophils in venules of the cremaster muscle using intravital microscopy. When the mouse cremaster muscle is exteriorized, neutrophils roll along the endothelium of venules mediated by P-selectin (39). As expected, injection of CXCL1 evoked an immediate drop in rolling (Figure 2A) and immediate arrest of wild type neutrophils (Figure 2B). There was no significant difference between Gαi3 deficient and wild type neutrophils in rolling or arrest (Figure 2A, 2B).
Figure 2. Rolling flux and neutrophil arrest analyzed by intravital microscopy.
600ng CXCL1 was injected and A. Rolling flux and B. Number of adherent cells were measured by intravital microscopy in cremaster venules. Arrow indicates injection of CXCL1. Data are presented as mean ± SEM. ** indicates P<0.01 from wild type neutrophils. Data are representative of at least three independent experiments.
Gαi3 is required for chemotactic directionality
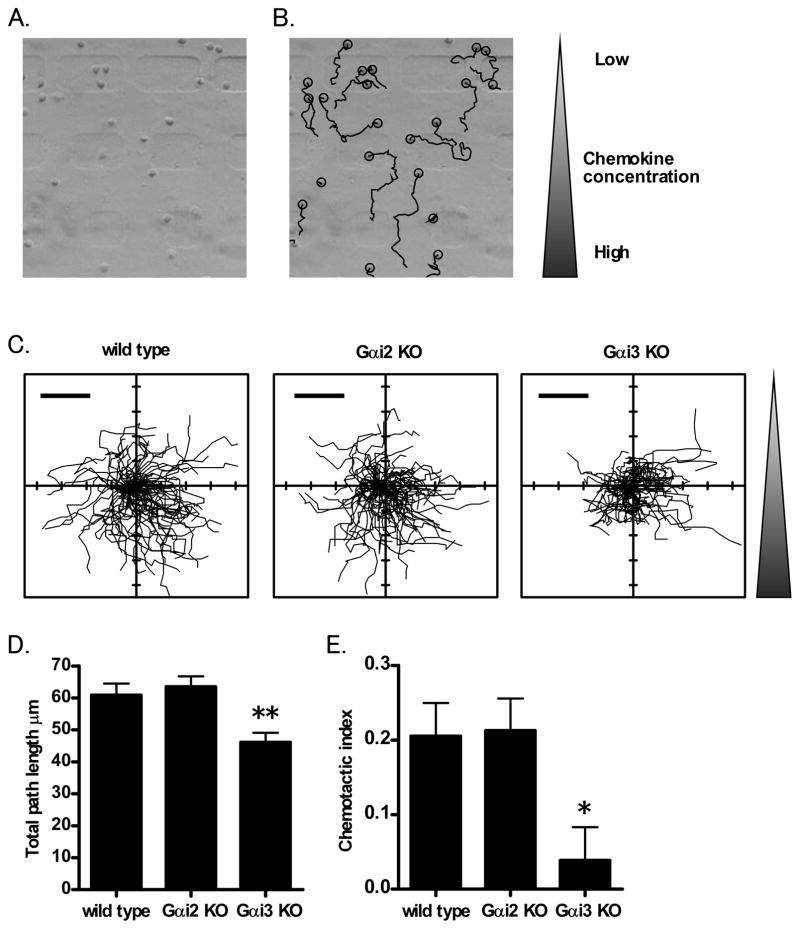
Since the transwell assay suggested a defect in chemotaxis, we tested migration of Gαi3 deficient neutrophils in an array that allows single-cell assessment of migration and directionality. We adapted the microfluidic gradient maker device originally developed for HL-60 cells (38). For neutrophils, we coated the glass surface with ICAM-1 and blocked with casein. After the neutrophils were loaded and attached to the ICAM-1 coated glass surface in the gradient maker, they were exposed to an exponential CXCL1 gradient and migration was observed by CCD camera for 40 min (Figure 3A, 3B). Gαi2 or Gαi3 deficient neutrophils attached to the ICAM-1 polarized and spread normally. Path length and tortuosity by wild type, Gαi2 deficient neutrophils, and Gαi3 deficient neutrophils were then measured (Figure 3C). Although Gαi2 deficient neutrophils did not show any defects compared with wild type neutrophils, Gαi3 deficient neutrophils migrated a shorter way (Figure 3D). The directionality of migration was eliminated in Gαi3 deficient neutrophils (chemotactic index was not significantly different from 0) (Figure 3E). These data show that Gαi3 deficiency leads to a loss of chemotaxis and a decrease in migration.
Figure 3. Neutrophil chemotaxis.
A. Neutrophils were injected into the gradient maker coated with ICAM-1. B. and tracked (black lines) chemotaxis of the neutrophils was observed by CCD camera. C. The migration paths of 80–110 wild type, Gαi2 deficient or Gαi3 deficient neutrophils in the gradient maker. Scale bar = 40 μm. D. The total path length migrated by wild type, Gαi2 deficient or Gαi3 deficient neutrophils in the gradient maker during 40 min. E. The chemotactic index (upgradient/total path length) of wild type, Gαi2 deficient or Gαi3 deficient neutrophils. Data are presented as mean ± SEM. ** or * indicates P<0.01 or P<0.05 from wild type neutrophils respectively. Data are representative of at least three independent experiments.
Calcium signaling upon CXCL1 stimulation requires Gαi2 in neutrophils
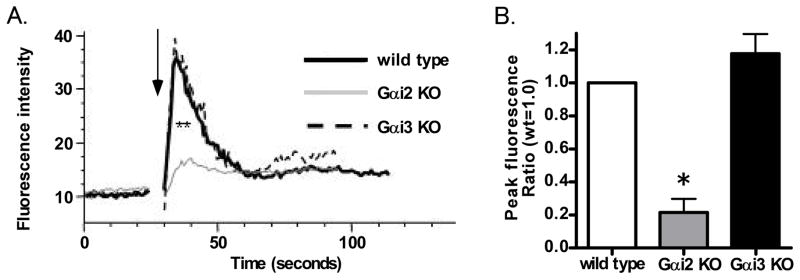
Since Gαi2 deficiency inhibited arrest and Gαi3 deficiency abolished chemotaxis, respectively, we speculated that after CXCR2 stimulation, Gαi2 activation may induce PLCβ2/PLCβ3 activation and calcium flux. To investigate this hypothesis further, we examined intracellular calcium concentration over time. Whereas Gαi3 deficient neutrophils did not show any difference compared to wild type neutrophils, Gαi2 deficient neutrophils exhibited decreased up-regulation of intracellular calcium concentration after CXCR2 stimulation (Figure 4A, 4B).
Figure 4. Gαi2 regulates intracellular Ca2+ in response to CXCL1.
A. Wild type, Gαi2 deficient or Gαi3 deficient neutrophils were loaded with the Ca2+ sensitive dye fluo-3 and stimulated with CXCL1 (arrow). Fluorescence reflecting calcium flux was measured by flow cytometry and the signal was analyzed for 3 min. B. Peak levels of intracellular Ca2+ flux were determined by flow cytometry. Each bar shows the peak fluorescence level of intracellular Ca2+ minus the baseline fluorescence. Data are representative of at least two independent experiments.
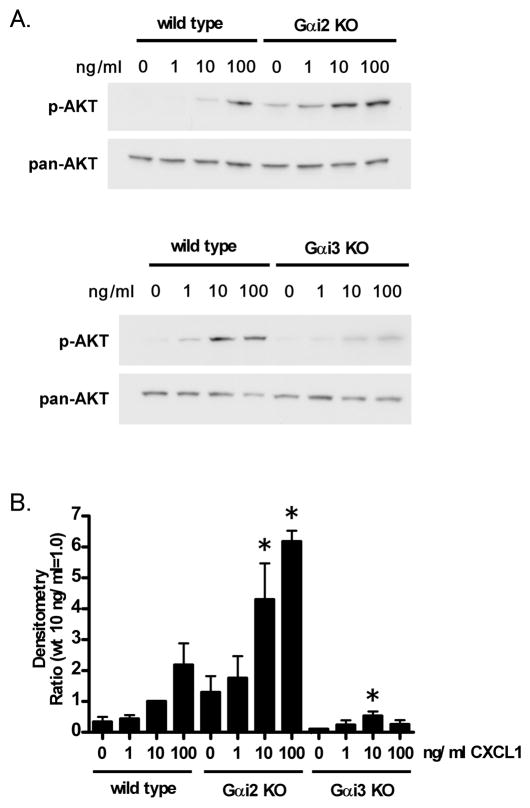
Decreased AKT phosphorylation in response to CXCL1 in Gαi3 deficient neutrophils
Chemotaxis is known to require PI3Kγ (21, 25, 26). To examine PI3Kγ activation, we investigated phosphorylation of AKT, a substrate of PI3Kγ (41), in response to CXCL1. After stimulation with CXCL1, neutrophils were lysed and AKT phosphorylation was detected with western blotting using a phospho-AKT specific antibody. AKT phosphorylation after CXCL1 showed a significant decrease in Gαi3 deficient neutrophils (Figure 5A, 5B). In contrast to Gαi3, AKT phosphorylation was increased in Gαi2 deficient neutrophils compared with wild type neutrophils. The data suggest that Gαi3 is necessary for PI3K activation that leads to chemotaxis.
Figure 5. Gαi3 regulates PI3K activation after CXCL1 stimulation in neutrophils.
A. Gαi2 or Gαi3 deficient neutrophils were stimulated with the indicated concentrations of CXCL1 and lysates were immunoblotted with an antibody to phospho-AKT (p-AKT). The membranes were stripped and immunoblotted with pan-AKT as a loading control. B. The values in the bar graph represent mean ± SEM relative intensities as determined by densitometry (wild type neutrophils with 10 ng/ml CXCL1 stimulation=1). * indicates P<0.05 from wild type neutrophils. Data are representative of at least three independent experiments.
Recduced chemotaxis to CXCL1 in Gαi3 deficient neutrophils in vivo
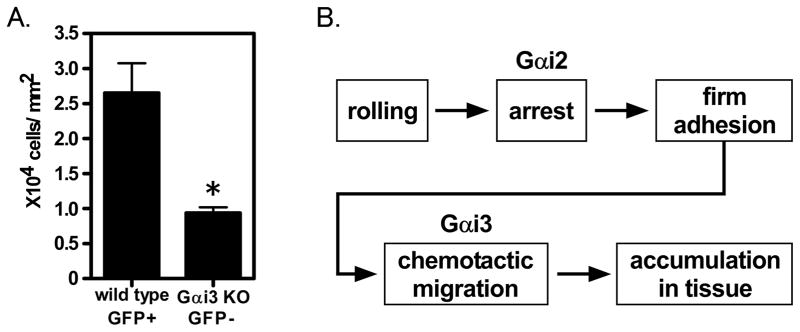
To analyze the net effect of Gαi3 deficiency on neutrophil recruitment, we tested neutrophil accumulation in vivo. We harvested the bone marrow from LysM-GFP wild type mice and Gαi3 deficient mice. Bone marrow cells were mixed at a ratio of 1:1, and injected into lethally irradiated wild type mice. After reconstitution, the cremaster muscle in each of the mixed chimeric mice was exteriorized and CXCL1 was microinjected into the cremaster muscle. After 2 hours, neutrophils migrated to the injection site and the number of migrated cells was counted. The number of GFP positive cells (Gαi3 +/+) and GFP negative cells (Gαi3 −/−) were then compared. Significantly fewer Gαi3 deficient neutrophils migrated compared with Gαi3 wild type neutrophils (Figure 6A). Since the arrest in response to CXCL1 was normal in Gαi3 deficient neutrophils in vivo (Figure 1C, 1D), the data suggest that transendothelial migration and chemotaxis to CXCL1 are defective in Gαi3 deficient neutrophils, and that these defects lead to relevant accumulation defects in vivo.
Figure 6. Defective migration of Gαi3 deficient neutrophils in vivo.
A. The cremaster muscle of mice reconstituted with wild type neutrophils (GFP positive) and Gαi3 deficient neutrophils (GFP negative) was exteriorized. CXCL1 was injected into the cremaster muscle by micropipette. 2 hours later, migrated neutrophils were observed. GFP images were recorded as gray scale by CCD camera and then digitally converted into green color. Number of migrated wild type GFP+ and Gαi3KO GFP- neutrophils were counted. Data are presented as mean ± SEM. * indicates P<0.05. Data are representative of at least two independent experiments. B. In inflammation, neutrophils proceed from rolling to firm adhesion. This arrest process is Gαi2-dependent. Adherent neutrophils show chemotactic migration, which we show to be Gαi3-dependent. Hence, knocking out Gαi2 or Gαi3 both lead to reduced neutrophil accumulation in the tissue.
Discussion
The present data demonstrate that Gαi2 and Gαi3 differentially regulate arrest and chemotaxis in response to CXCR2 in mouse neutrophils. The transmigration, chemotaxis and the AKT phosphorylation of Gαi3 deficient neutrophils were inhibited without defects in the arrest. Conversely, Gαi2 deficient neutrophils have a severe defect in arrest (15) and intracellular calcium mobilization without defects in transmigration and chemotaxis. Since the PLCβ signaling pathway is reflected by intracellular calcium and PI3Kγ signaling is reflected by AKT phosphorylation (11, 21, 22, 25, 26), our data indicate that Gαi2 specifically regulates neutrophil arrest by PLCβ signaling and Gαi3 specifically regulates neutrophil chemotaxis by PI3Kγ signaling.
After dissociation, Gαi proteins inhibit adenylate cyclase (42). Adenylate cyclase is not highly active in resting neutrophils, so we think it is unlikely that inhibition of adenylate cyclase would have major effects on migration, chemotaxis, and arrest. Rather, the free βγ heterodimers are thought to differentially activate PLCβ2 and 3 on the one hand and PI3Kγ on the other (43). It is known that βγ subunits are sufficient to mediate directional neutrophil chemotaxis (44). The specificity of βγ subunits for downstream effectors is an emerging field for investigation (45, 46). Since it is known that different βγ subunits associate with Gαi2 and Gαi3 subunits, and the ability of effectors to be activated by βγ subunits depends on the nature of the dimer, association with the different βγ subunits might be the possible mechanism of the different roles between Gαi2 and Gαi3 subunits(42, 47–49). GPCRs have other signaling pathways, including those initiated by β arrestin and by the various GRKs. Indeed, β arrestin 2 is required for CXCR2-dependent arrest (50). But it is not known whether β arrestin is bound to the same CXCR2 receptor that provides the βγ for PLCβ activation. Whereas the inhibition of adenylate cyclase by Gαi proteins does not seem to have major effects on migration, chemotaxis, and arrest, a role of adenylyl cyclase in the regulation of neutrophil arrest still cannot be ruled out. Calcium flux, which is Gαi2 dependent, activates calcium-dependent soluble adenylyl cyclase resulting in cAMP production, which can lead to activate Rap1a (51). Rap1 can activate LFA-1, which is important for arrest (52). Clearly, much more work is needed to fully elucidate the signaling pathways downstream of GPCRs that differentially regulate LFA-1 activation and arrest on one hand and chemotaxis on the other hand. The present study provides support for the concept that different Gα subunits have different effectors.
It has been reported that Gαi2 deficiency can inhibit transmigration in B lymphocytes (29). However, in neutrophils, Gαi2 did not show any effect in the transmigration assay. This cell context-dependent difference might be explained by the existence of neutrophil or B cells specific scaffold proteins for Gαi2 and downstream molecules. This is supported by the fact that scaffold proteins are important for the function of CXCR2 in neutrophils (53). Alternatively, there may be different expression levels of the Gαi subunits among these cell types. Although Gαi3 was detected in lymphocytes, protein levels of Gαi3 were not compared between lymphocytes and neutrophils (54). In B cells, p110δ PI3K, rather than p110γ PI3K, is more important for chemotaxis (55, 56). This difference could be another possible reason for the difference of the Gαi2 requirement in chemotaxis between B cells and neutrophils.
p-AKT levels are significantly increased in the Gαi2-deficient mice (Figure 5). A previous study by Thompson et al showed that Gαi3 mediated blockade of Gαi2 activation in lymphocyte evoked by the competition or steric hindrance of Gαi2 interaction with the CXCR3 receptor (28). Therefore, the increase of p-AKT in the Gαi2-deficient neutrophils could be caused by the increased activation of Gαi3 signaling by the loss of the blockade by Gαi2 competition or steric hindrance. In addition to this, since Wiege K et al. showed compensatory up-regulation of Gαi subunits or Gβ subunits in the absence of the other Gαi protein in neutrophils (57), the compensatory up-regulation might also be the cause of increased p-AKT. This suggests that Gαi2 and Gαi3 have different roles in chemokine signaling.
Whereas p-AKT was increased in the Gαi2-deficient neutrophils compared with wild type neutrophils (Figure 5), transmigration and chemotaxis were not significantly increased (Figure 1, 3). More activation of p-AKT than is necessary might show no significant results. Alternatively, the compensatory regulation by other molecules might mask a possible effect.
In earlier studies of thioglycollate peritonitis and LPS-induced lung inflammation, Gαi2-deficient mice showed defective accumulation of neutrophils in vivo, presumably because of the arrest defect (15). Our new data suggest that whereas Gαi2 and Gαi3 subunits have the different roles, both can play important roles in the neutrophil recruitment in vivo (Figure 6B). Differential roles of different Gαi subunits suggest that these molecules could be specifically targeted pharmacologically to achieve differential effects. It might be expected that differential inhibition of chemotaxis but not arrest would lead to accumulation of neutrophils on the vessel wall, adherent but unable to transmigrate. This would be expected to induce inflammation and thrombosis (58). On the other hand, selective blockade of chemotaxis may have better anti-inflammatory effects, for instance, after ischemia and reperfusion.
In conclusion, we demonstrate differential function of Gαi2 and Gαi3 in CXCR2-induced arrest and migration of neutrophils. Gαi2 is required for PLCβ activation, calcium flux and LFA-1 activation (15), whereas Gαi3 is required for transmigration and chemotaxis.
Acknowledgments
This work was supported by grants from NIH (HL 111969 to K.L.). Y.K. was supported by a Postdoctoral Fellowship grant from the JSPS.
References
- 1.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 2.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 3.Zarbock A, Ley K. Neutrophil adhesion and activation under flow. Microcirculation. 2009;16:31–42. doi: 10.1080/10739680802350104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abram CL, Lowell CA. The ins and outs of leukocyte integrin signaling. Annu Rev Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuwano Y, Spelten O, Zhang H, Ley K, Zarbock A. Rolling on E- or P-selectin induces the extended but not high-affinity conformation of LFA-1 in neutrophils. Blood. 2010;116:617–624. doi: 10.1182/blood-2010-01-266122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yago T, Shao B, Miner JJ, Yao L, Klopocki AG, Maeda K, Coggeshall KM, McEver RP. E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin alphaLbeta2-mediated slow leukocyte rolling. Blood. 2010;116:485–494. doi: 10.1182/blood-2009-12-259556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stadtmann A, Brinkhaus L, Mueller H, Rossaint J, Bolomini-Vittori M, Bergmeier W, Van Aken H, Wagner DD, Laudanna C, Ley K, Zarbock A. Rap1a activation by CalDAG-GEFI and p38 MAPK is involved in E-selectin-dependent slow leukocyte rolling. Eur J Immunol. 2011 doi: 10.1002/eji.201041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller H, Stadtmann A, Van Aken H, Hirsch E, Wang D, Ley K, Zarbock A. Tyrosine kinase Btk regulates E-selectin-mediated integrin activation and neutrophil recruitment by controlling phospholipase C (PLC) gamma2 and PI3Kgamma pathways. Blood. 2010;115:3118–3127. doi: 10.1182/blood-2009-11-254185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarbock A, Ley K. Mechanisms and consequences of neutrophil interaction with the endothelium. Am J Pathol. 2008;172:1–7. doi: 10.2353/ajpath.2008.070502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 11.Thelen M, Stein JV. How chemokines invite leukocytes to dance. Nat Immunol. 2008;9:953–959. doi: 10.1038/ni.f.207. [DOI] [PubMed] [Google Scholar]
- 12.Seo SM, McIntire LV, Smith CW. Effects of IL-8, Gro-alpha, and LTB(4) on the adhesive kinetics of LFA-1 and Mac-1 on human neutrophils. Am J Physiol Cell Physiol. 2001;281:C1568–1578. doi: 10.1152/ajpcell.2001.281.5.C1568. [DOI] [PubMed] [Google Scholar]
- 13.Smith ML, Olson TS, Ley K. CXCR2- and E-selectin-induced neutrophil arrest during inflammation in vivo. J Exp Med. 2004;200:935–939. doi: 10.1084/jem.20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 15.Zarbock A, Deem TL, Burcin TL, Ley K. Galphai2 is required for chemokine-induced neutrophil arrest. Blood. 2007;110:3773–3779. doi: 10.1182/blood-2007-06-094565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gakidis MA, Cullere X, Olson T, Wilsbacher JL, Zhang B, Moores SL, Ley K, Swat W, Mayadas T, Brugge JS. Vav GEFs are required for beta2 integrin-dependent functions of neutrophils. J Cell Biol. 2004;166:273–282. doi: 10.1083/jcb.200404166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertram A, Zhang H, von Vietinghoff S, de Pablo C, Haller H, Shushakova N, Ley K. Protein kinase C-theta is required for murine neutrophil recruitment and adhesion strengthening under flow. J Immunol. 2012;188:4043–4051. doi: 10.4049/jimmunol.1101651. [DOI] [PubMed] [Google Scholar]
- 18.Wu D, LaRosa GJ, Simon MI. G protein-coupled signal transduction pathways for interleukin-8. Science. 1993;261:101–103. doi: 10.1126/science.8316840. [DOI] [PubMed] [Google Scholar]
- 19.Crittenden JR, Bergmeier W, Zhang Y, Piffath CL, Liang Y, Wagner DD, Housman DE, Graybiel AM. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med. 2004;10:982–986. doi: 10.1038/nm1098. [DOI] [PubMed] [Google Scholar]
- 20.Bergmeier W, Goerge T, Wang HW, Crittenden JR, Baldwin AC, Cifuni SM, Housman DE, Graybiel AM, Wagner DD. Mice lacking the signaling molecule CalDAG-GEFI represent a model for leukocyte adhesion deficiency type III. J Clin Invest. 2007;117:1699–1707. doi: 10.1172/JCI30575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-beta2 and -beta3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science. 2000;287:1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- 22.Kinashi T, Katagiri K. Regulation of immune cell adhesion and migration by regulator of adhesion and cell polarization enriched in lymphoid tissues. Immunology. 2005;116:164–171. doi: 10.1111/j.1365-2567.2005.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefort CT, Rossaint J, Moser M, Petrich BG, Zarbock A, Monkley SJ, Critchley DR, Ginsberg MH, Fassler R, Ley K. Distinct roles for talin-1 and kindlin-3 in LFA-1 extension and affinity regulation. Blood. 2012;119:4275–4282. doi: 10.1182/blood-2011-08-373118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephens LR, Eguinoa A, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, Smrcka AS, Thelen M, Cadwallader K, Tempst P, Hawkins PT. The G beta gamma sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell. 1997;89:105–114. doi: 10.1016/s0092-8674(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 25.Hirsch E, V, Katanaev L, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, Joza N, Mak TW, Ohashi PS, Suzuki A, Penninger JM. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 27.Wilkie TM, Gilbert DJ, Olsen AS, Chen XN, Amatruda TT, Korenberg JR, Trask BJ, de Jong P, Reed RR, Simon MI, Jenkins NA, Copeland NG. Evolution of the mammalian G protein alpha subunit multigene family. Nat Genet. 1992;1:85–91. doi: 10.1038/ng0592-85. [DOI] [PubMed] [Google Scholar]
- 28.Thompson BD, Jin Y, Wu KH, Colvin RA, Luster AD, Birnbaumer L, Wu MX. Inhibition of G alpha i2 activation by G alpha i3 in CXCR3-mediated signaling. J Biol Chem. 2007;282:9547–9555. doi: 10.1074/jbc.M610931200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han SB, Moratz C, Huang NN, Kelsall B, Cho H, Shi CS, Schwartz O, Kehrl JH. Rgs1 and Gnai2 regulate the entrance of B lymphocytes into lymph nodes and B cell motility within lymph node follicles. Immunity. 2005;22:343–354. doi: 10.1016/j.immuni.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 30.Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, Brabet P, Boulay G, Bradley A, Birnbaumer L. Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat Genet. 1995;10:143–150. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- 31.Jiang M, Spicher K, Boulay G, Martin-Requero A, Dye CA, Rudolph U, Birnbaumer L. Mouse gene knockout and knockin strategies in application to alpha subunits of Gi/Go family of G proteins. Methods Enzymol. 2002;344:277–298. doi: 10.1016/s0076-6879(02)44721-0. [DOI] [PubMed] [Google Scholar]
- 32.Pero RS, Borchers MT, Spicher K, Ochkur SI, Sikora L, Rao SP, Abdala-Valencia H, O’Neill KR, Shen H, McGarry MP, Lee NA, Cook-Mills JM, Sriramarao P, Simon MI, Birnbaumer L, Lee JJ. Galphai2-mediated signaling events in the endothelium are involved in controlling leukocyte extravasation. Proc Natl Acad Sci U S A. 2007;104:4371–4376. doi: 10.1073/pnas.0700185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 34.Nagata K, Ye C, Jain M, Milstone DS, Liao R, Mortensen RM. Galpha(i2) but not Galpha(i3) is required for muscarinic inhibition of contractility and calcium currents in adult cardiomyocytes. Circ Res. 2000;87:903–909. doi: 10.1161/01.res.87.10.903. [DOI] [PubMed] [Google Scholar]
- 35.Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–593. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Meng F, Chu CL, Takai T, Lowell CA. The Src family kinases Hck and Fgr negatively regulate neutrophil and dendritic cell chemokine signaling via PIR-B. Immunity. 2005;22:235–246. doi: 10.1016/j.immuni.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Fujimoto M, Kuwano Y, Watanabe R, Asashima N, Nakashima H, Yoshitake S, Okochi H, Tamaki K, Poe JC, Tedder TF, Sato S. B cell antigen receptor and CD40 differentially regulate CD22 tyrosine phosphorylation. J Immunol. 2006;176:873–879. doi: 10.4049/jimmunol.176.2.873. [DOI] [PubMed] [Google Scholar]
- 38.Herzmark P, Campbell K, Wang F, Wong K, El-Samad H, Groisman A, Bourne HR. Bound attractant at the leading vs. the trailing edge determines chemotactic prowess. Proc Natl Acad Sci U S A. 2007;104:13349–13354. doi: 10.1073/pnas.0705889104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ley K, Bullard DC, Arbones ML, Bosse R, Vestweber D, Tedder TF, Beaudet AL. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J Exp Med. 1995;181:669–675. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ley K, Allietta M, Bullard DC, Morgan S. Importance of E-selectin for firm leukocyte adhesion in vivo. Circ Res. 1998;83:287–294. doi: 10.1161/01.res.83.3.287. [DOI] [PubMed] [Google Scholar]
- 41.Hawkins PT, Anderson KE, Davidson K, Stephens LR. Signalling through Class I PI3Ks in mammalian cells. Biochem Soc Trans. 2006;34:647–662. doi: 10.1042/BST0340647. [DOI] [PubMed] [Google Scholar]
- 42.Milligan G, Kostenis E. Heterotrimeric G-proteins: a short history. Br J Pharmacol. 2006;147(Suppl 1):S46–55. doi: 10.1038/sj.bjp.0706405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kehrl JH. Chemoattractant receptor signaling and the control of lymphocyte migration. Immunol Res. 2006;34:211–227. doi: 10.1385/IR:34:3:211. [DOI] [PubMed] [Google Scholar]
- 44.Surve CR, Lehmann D, Smrcka AV. A chemical biology approach demonstrates G protein betagamma subunits are sufficient to mediate directional neutrophil chemotaxis. J Biol Chem. 2014;289:17791–17801. doi: 10.1074/jbc.M114.576827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albert PR, Robillard L. G protein specificity: traffic direction required. Cell Signal. 2002;14:407–418. doi: 10.1016/s0898-6568(01)00259-5. [DOI] [PubMed] [Google Scholar]
- 46.Wolfe JT, Wang H, Howard J, Garrison JC, Barrett PQ. T-type calcium channel regulation by specific G-protein betagamma subunits. Nature. 2003;424:209–213. doi: 10.1038/nature01772. [DOI] [PubMed] [Google Scholar]
- 47.Gilman AG. Nobel Lecture. G proteins and regulation of adenylyl cyclase. Bioscience reports. 1995;15:65–97. doi: 10.1007/BF01200143. [DOI] [PubMed] [Google Scholar]
- 48.Spiegel AM, Weinstein LS, Shenker A, Hermouet S, Merendino JJ., Jr G proteins: from basic to clinical studies. Advances in second messenger and phosphoprotein research. 1993;28:37–46. [PubMed] [Google Scholar]
- 49.Schmidt CJ, Thomas TC, Levine MA, Neer EJ. Specificity of G protein beta and gamma subunit interactions. J Biol Chem. 1992;267:13807–13810. [PubMed] [Google Scholar]
- 50.Molteni R, Crespo CL, Feigelson S, Moser C, Fabbri M, Grabovsky V, Krombach F, Laudanna C, Alon R, Pardi R. Beta-arrestin 2 is required for the induction and strengthening of integrin-mediated leukocyte adhesion during CXCR2-driven extravasation. Blood. 2009;114:1073–1082. doi: 10.1182/blood-2008-10-183699. [DOI] [PubMed] [Google Scholar]
- 51.Han H, Stessin A, Roberts J, Hess K, Gautam N, Kamenetsky M, Lou O, Hyde E, Nathan N, Muller WA, Buck J, Levin LR, Nathan C. Calcium-sensing soluble adenylyl cyclase mediates TNF signal transduction in human neutrophils. J Exp Med. 2005;202:353–361. doi: 10.1084/jem.20050778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katagiri K, Hattori M, Minato N, Irie S, Takatsu K, Kinashi T. Rap1 is a potent activation signal for leukocyte function-associated antigen 1 distinct from protein kinase C and phosphatidylinositol-3-OH kinase. Mol Cell Biol. 2000;20:1956–1969. doi: 10.1128/mcb.20.6.1956-1969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Y, Wang S, Farooq SM, Castelvetere MP, Hou Y, Gao JL, Navarro JV, Oupicky D, Sun F, Li C. A chemokine receptor CXCR2 macromolecular complex regulates neutrophil functions in inflammatory diseases. J Biol Chem. 2012;287:5744–5755. doi: 10.1074/jbc.M111.315762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang TT, Zong Y, Dalwadi H, Chung C, Miceli MC, Spicher K, Birnbaumer L, Braun J, Aranda R. TCR-mediated hyper-responsiveness of autoimmune Galphai2(−/−) mice is an intrinsic naive CD4(+) T cell disorder selective for the Galphai2 subunit. Int Immunol. 2003;15:1359–1367. doi: 10.1093/intimm/dxg135. [DOI] [PubMed] [Google Scholar]
- 55.Reif K, Okkenhaug K, Sasaki T, Penninger JM, Vanhaesebroeck B, Cyster JG. Cutting edge: differential roles for phosphoinositide 3-kinases, p110gamma and p110delta, in lymphocyte chemotaxis and homing. J Immunol. 2004;173:2236–2240. doi: 10.4049/jimmunol.173.4.2236. [DOI] [PubMed] [Google Scholar]
- 56.Ortolano S, I, Hwang Y, Han SB, Kehrl JH. Roles for phosphoinositide 3-kinases, Bruton’s tyrosine kinase, and Jun kinases in B lymphocyte chemotaxis and homing. Eur J Immunol. 2006;36:1285–1295. doi: 10.1002/eji.200535799. [DOI] [PubMed] [Google Scholar]
- 57.Wiege K, Ali SR, Gewecke B, Novakovic A, Konrad FM, Pexa K, Beer-Hammer S, Reutershan J, Piekorz RP, Schmidt RE, Nurnberg B, Gessner JE. Galphai2 is the essential Galphai protein in immune complex-induced lung disease. J Immunol. 2013;190:324–333. doi: 10.4049/jimmunol.1201398. [DOI] [PubMed] [Google Scholar]
- 58.von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Kollnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, Eckart A, Haidari S, Rudelius M, Schulz C, Echtler K, Brinkmann V, Schwaiger M, Preissner KT, Wagner DD, Mackman N, Engelmann B, Massberg S. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]