Abstract
Aging is associated with an increase in basal inflammation in the central nervous system (CNS) and an overall decline in cognitive function and poorer recovery following injury. Growing evidence suggests that leukocyte recruitment to the CNS is also increased with normal aging, but to date, no systematic evaluation of these “age-associated” leukocytes have been performed. In this work the effect of aging on CNS leukocyte recruitment was examined. Aging was associated with an increased number of CD45hi leukocytes, primarily composed of conventional CD8+ T cells. These results were strain-independent and seen in both sexes. Intravascular labeling and immunohistology revealed the presence of parenchymal CD8+ T cells in several regions of the brain including the choroid plexus and meninges. These cells had effector memory (CD44+CD62L−) and tissue-resident phenotypes and expressed markers associated with T cell receptor (TCR) activation. Analysis of TCRvβ repertoire usage suggested that entry into the CNS is likely stochastic rather than antigen-driven. Correlational analyses revealed a positive association between CD8 T cell numbers and decreased pro-inflammatory function of microglia. However, the effects of cerebral ischemia and ex-vivo stimulation of these cells dramatically increased production of tumor necrosis factor (TNF), interferon gamma (IFNγ), and monocyte-chemotactic protein-1 (MCP-1/CCL2). Taken together, we identified a novel population of resident memory, immunosurveillant CD8 T cells that represent a hallmark of CNS aging and appear to modify microglia homeostasis under normal conditions, but are primed to potentiate inflammation and leukocyte recruitment following ischemic injury.
Keywords: trafficking, aging, surveillance, stroke, mice
Introduction
Aging is associated with cognitive decline, a heightened risk of neurodegenerative disease, and poorer recovery after injury. People aged 80 and over are now the fastest growing segment of the population, projected to reach 19.4 million in 2030 (1). These statistics highlight growing concerns over the burden to the health services. The aging process exerts profound changes on the nervous and immune systems, which extend into nearly all organ systems (2–4). Indeed, the relationship between these two systems is bidirectional, and the concept of the CNS as an immune privileged organ is evolving (5). It is now recognized that the brain is under constant immune surveillance by resident microglia and trafficking systemic T lymphocytes recruited by the choroid plexus (6–8). These generally serve a protective role as a rapid response system to detect damaging agents that disrupt homeostasis. Although T lymphocytes transit the CNS at low numbers in healthy brain, the role of these cells and how they change with age is not well understood (9). Recent work has shown that CD4 effector memory (TEM) cells with a TCR repertoire specific to CNS antigen are constitutively present in the epithelial layers of the choroid plexus where, at this interface between brain and blood, they are poised to act as immunomodulators of aging and senescence (6, 10). Like neurons, polarized T cells have a multitude of unique functions that serve important and distinct roles in immunity. For example, Scid mice, which lack functional T cells, have cognitive deficits compared to wild-type mice, implying that T cells can influence CNS function (11–13). However little is known regarding the role and identity of the lymphocytes that traffic throughout the CNS under normal conditions and with aging.
Surveillance is the process whereby the immune system identifies and eliminates transformed or infected cells before they become cancerous or cause harm (14). Although little is known concerning the role of aging on immune surveillance in the CNS, the increase in basal inflammatory levels indicative of the phenomenon known as “inflammaging” support the notion that the necessity for surveillance may be heightened (15). The low-grade inflammatory status of the aged CNS may favor immune cell recruitment, but the critical mediators of this phenomenon and the functional role of the specific cell subtypes entering the aged brain in steady state remain to be established. Stichel and Luebbert(16) showed a significant increase in both CD11c+ dendritic cells and CD3+ T cells with wide distribution throughout the brain beginning at middle age and increasing with advanced age. Studies assessing donor human brain tissue have identified the presence of resident CD8+ T cells enriched in white matter and regions of blood-brain barrier leakiness (17, 18). These findings indicate that the observed changes in leukocyte recruitment in the aging mouse brain likely have translational relevance as immune surveillance also occurs in the human CNS.
In the present study the presence of peripheral leukocytes in the aging CNS was assessed to determine the functional relevance of these cells to age-related inflammatory signaling. We evaluated lymphocyte recruitment guidance and response cues and performed a comprehensive phenotypic analysis of cell subsets found in normal CNS. Lastly, to determine if there was a functional consequence of these CNS-resident leukocytes we assessed production of inflammatory mediators after ex-vivo stimulation and in an age-relevant model of brain injury, experimental stroke.
Materials and Methods
Mice/Animals
C57BL/6J mice of 8–12 wks (young adult) and 18–22 months (aged) of age were pair-housed on sawdust bedding in a specific pathogen free facility (light cycle 12/12 h light/dark). The average weight of the naïve, young mice was 29.6 ± 2.3 grams and that of aging mice was 35.7 ± 3.2 grams before sacrifice. All experiments were performed using male C57BL/6J mice unless otherwise stated. Several experiments were performed using BALB/cByJ mice of 11 wks (young adult) and 21 months (aged) of age to determine strain-dependence. A cohort of aged C57BL/6J and BALB/cByJ females was also included. All animals had access to chow and water ad libitium. Animal procedures were performed in accordance with NIH guidelines for the care and use of laboratory animals and approved by the Animal Care Committee of the University of Connecticut Health Center. Ex-vivo studies were performed by an investigator blinded to age.
Tissue Harvesting
Mice were euthanized, transcardially perfused with 60mL cold, sterile PBS, and the brains were harvested. The olfactory bulb, brainstem, and cerebellum were removed. The brain was then divided along the interhemispheric fissure into two hemispheres and then subsequently rinsed with PBS to remove contaminant cells.
Flow cytometry
Blood was drawn by cardiac puncture with heparinized needles. Spleens were removed and processed by mechanical disruption on a 70um filter screen. Red blood cell lysis was achieved by three consecutive 10-minute incubations with Tris-ammonium chloride (Stem Cell Technologies). CNS tissue to be analyzed by flow cytometry was placed in RPMI (Lonza) medium and mechanically and enzymatically digested in collagenase/dispase (1 mg/mL) and DNAse (10mg/mL; both Roche Diagnostics). The cell suspension was filtered through a 70um filter. Leukocytes were harvested from the interphase of a 70%/30% Percoll gradient. Blood and brain leukocytes were washed and blocked with mouse Fc Block (eBioscience) prior to staining with primary antibody-conjugated flourophores (CD45-eF450, CD11b-APCeF780, MHCII-FITC, Ly6C-PerCP-Cy5.5, CD3e-APC, and CD4-PE-Cy7 were purchased from eBioscience), whereas CD8-Bv510, CD69-APC, CD103-PerCP-Cy5.5, PD-1-PE, CD11a-PE-Cy7, and CD49d-PerCP-Cy5.5 were purchased from Biolegend. For live/dead cell discrimination, a fixable viability dye, CASE-AF350 (Invitrogen), was diluted at 1:300 from a working stock of 0.3mg/mL. Data were acquired on a LSRII using FACsDiva 6.0 (BD Biosciences) and analyzed using FlowJo (Treestar Inc.). No less than 100,000 events were recorded for each sample. Resident microglia were identified as the CD45int CD11b+Ly6C− population, whereas bone marrow-derived leukocytes were identified as CD45hi. Cell-specific fluorescence minus one (FMO) controls were used to determine the positivity of each antibody.
For intracellular cytokine staining, a stock solution of brefeldin A (Sigma) was prepared at 20mg/mL in DMSO, and diluted with PBS to obtain a working solution of 0.5mg/mL. Mice were euthanized 8 hours after intravenous injection of brefeldin A (250ul). Leukocytes were collected as described above, and 1ul of GolgiPlug containing brefeldin A (BD Biosciences) was added to 800ul complete RPMI. Cells were subsequently stimulated with PBS or Cell Stimulation Cocktail (eBioscience) containing PMA/ionomycin and incubated for 4 hours at 37ºC (5% CO2). Afterward, cells were resuspended in Fc Block, stained for surface antigens and washed in 100ul of fixation/permeabilization solution (BD Biosciences) for 20 minutes. Cells were then washed twice in 300ul Permeabilization/Wash buffer (BD Biosciences) and resuspended in an intracellular antibody cocktail containing TNF-PE-Cy7, CCL2-PE, and IFNγ-PerCP-Cy5.5 and fixed.
For detection of reactive oxygen species, leukocytes were incubated with redox-sensitive CM-H2DCFDA (5uM; Ex/Em: 495/520) fluorogenic cell-permeant dye (Life Technologies, Invitrogen). Cells were incubated for 20min at 37ºC, washed three times with FACS buffer (without NaAz), and then stained for surface markers including CASE.
To determine TCRvβ usage, we used a mouse vβ TCR screening panel (BD Pharmingen) according to the manufacturer’s instructions. Briefly, after collecting blood and tissue leukocytes each sample was divided into 15 separate FACS tubes and stained for the antibody cocktail including one of 15 respective TCRvβ FITC-conjugated monoclonal antibodies.
Phagocytic activity of microglia was performed as described by Ritzel et al (2015). Briefly, fluorescent latex beads (Fluoresbrite Yellow Green (YG) carboxylate microspheres; 1um diameter; Polysciences) were added to sorted microglia in a final dilution of 1:100 as described (19). After 1-h incubation at 37ºC, the cells were washed three times with FACS buffer, re-suspended in FACS buffer, stained for surface markers, and fixed in PFA.
ELISA cytokine measurement
Plasma and whole tissue brain chemokine levels were determined by ELISA (Milliplex Cytokine/Chemokine Assay, EMD Millipore). In brief, mice were euthanized by avertin injection and blood was collected by cardiac puncture into heparin-coated syringes. Samples were centrifuged (13,000g for 10min at 4C) and plasma was collected and stored frozen (−80C) until assaying. Brain hemispheres were collected and homogenized in ice-cold lysis buffer containing a protease inhibitor cocktail (Roche Diagnositics). Homogenates were sonicated and centrifuged at 4C for 15 min at 14,000 rpm, and supernatants were assayed for total protein using a BCA protein assay kit (Pierce, Thermo scientific). Using a standard curve, protein concentration was determined and 100ug of each sample was loaded into each well in duplicate. Samples were assayed according to the manufacturer’s instructions using a Luminex 200 (Luminex Corporation, Austin, TX, USA) magnetic bead array platform. Inter-and intra-assay coefficients of variation were than less than 10%.
Intravascular staining and cell isolation
A total of 7ug anti-CD3e-PE was injected i.v. At 4 minutes after injection, the animals were sacrificed, and perfused with 40mL PBS. Blood was taken by cardiac puncture prior to perfusion to confirm injection efficiency. The brain hemispheres were harvested within 3 minutes, washed with PBS to remove free antibody, and leukocytes were isolated as described above. Leukocytes were then stained with CD45-eF450, CD11b-AF700, CD3e-APCeF780, CD4-APC, CD8-PerCPCy5.5 and CASE. Co-labeling was used determine the percentage of peri-vascular/parenchymal (CD3e-PE−/CD3e-APCeF780+) versus intravascular cells (CD3e-PE+/CD3e-APCeF780+).
Immunohistochemistry
Following PBS perfusion brains were harvested and embedded in an optimal cutting temperature (OCT) embedding solution and subsequently frozen on dry ice and stored at −80ºC. Brains were sectioned on a cryostat at a thickness of 10 μm at a temperature of 17ºC. These sections were mounted on charged slides and stored at −20ºC until use. After being brought to room temperature the sections were fixed with 4% paraformaldehyde (PFA) for 10 minutes, washed and then blocked in 0.1 mol/L phosphate buffer with 0.3% Triton X-100 (Sigma) and 10% donkey serum for one hour. Primary antibodies rat anti-CD8 (Abcam, 1:200), rabbit anti-myelin basic protein (Abcam, 1:200), mouse anti-NeuN (Millipore, 1:200) and rabbit anti-CD3 (Abcam, 1:100) were added and incubated overnight at 4ºC. These sections were washed and secondary antibodies Alexa Flour 488 anti-rat (Invitrogen, 1:1000), Alexa Flour 594 anti-rabbit (Life Technologies, 1:1000), and Alexa Flour 594 anti-mouse (Invitrogen, 1:1000) as well as conjugated antibodies Texas Red Lycopersicon Esculentum (Tomato) Lectin (Vector, 1:200), mouse anti-glial fibrillary acidic protein (GFAP)-Cy3 (Sigma, 1:200) and 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI, Invitrogen, 1:1000) were added and incubated at room temperature for one hour, washed and coverslipped for viewing. Images were acquired with a Zeiss Axiovert microscope (Carl Zeiss, Oberkochen, Germany) using a X-Cite 120Q fluorescence illumination system (Lumen Dynamics Group Inc, Mississauga, ON, Canada) and Zeiss image acquisition software (Zeiss LSM 510).
Middle Cerebral Artery Occlusion (MCAO) model of ischemic stroke
Cerebral ischemia was induced in aged mice (20 months) by 90 min of reversible middle cerebral artery occlusion under isoflurane anesthesia as previously described (20). Rectal temperatures were maintained at approximately 37 ºC during surgery and ischemia with an automated temperature control feedback system. A midline ventral neck incision was made, and unilateral MCAO was performed by inserting a 0.23mm silicone-coated suture (Doccol Corp, Redlands, CA, USA) into the right internal carotid artery 6 mm from the internal carotid/pterygopalatine artery bifurcation via an external carotid artery stump. Mice were euthanized at 4 hours after reperfusion and cells were isolated from contralateral (control) and ipsilateral (ischemic) hemispheres as above. ROS and cytokine production was measured as above following 2 hours of PMA/ionomycin stimulation at 37C.
Statistical Analyses
Data represents averages of cell counts obtained from one hemisphere of individual mice within a group. Data from individual experiments are presented as mean ± SEM and compared with Student’s t-test where appropriate (GraphPad Prism Software Inc, San Diego, CA, USA). Multiple comparisons were assessed by one-way or two-way ANOVA analysis with Tukey post-hoc correction. Significant differences between paired comparisons were conducted with the Holm-Sidak test. Correlation analyses were performed using Pearson’s correlation. p < 0.05 was considered statistically significant.
Results
Aging increases the number of peripheral leukocytes in the CNS
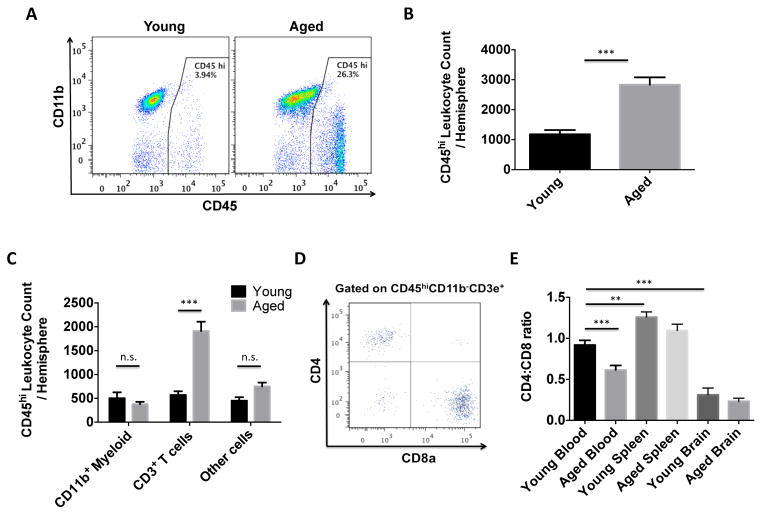
To confirm that aging increases leukocyte trafficking in the CNS we quantified the absolute number of bone marrow-derived cells present in each tissue using the gating strategy shown in Supplemental Figure 1A. The number of CD45hi leukocytes was significantly increased in the aged brain (Figure 1A). Absolute cell counts revealed a nearly three-fold increase in peripheral cells in normal aged brain (Figure 1B, p<0.001, N=13/group). Similar increases were also observed in the aged spinal cord (Supplemental Figure 1B). T cells composed the majority of these age-associated leukocytes, wherein the CD8 subset was more numerous than the CD4 subset (Figures 1C–E, p<0.001). These were identified as conventional TCR α/β, CD8 α/β T cells (data not shown). CD4:CD8 ratios of circulating T cells were decreased with age (p<0.001) and significantly lower in the brain compared to blood and spleen regardless or age (Figure 1E). To determine whether our findings were strain-dependent, we validated our results in the BALB/cByJ strain, which also demonstrated greater numbers of CD8 T cells in their brains with age, with older females exhibiting significantly higher numbers compared to their age-matched male counterparts (Supplemental Figure 1C and Supplemental Figures 2A–D). The results show that the CNS is more permissive to CD8 T lymphocyte trafficking with age.
Figure 1. CNS recruitment of CD8 T cells increases with age.
A representative dot plot depicts an increase in CD45hi peripheral leukocytes in one aged brain hemisphere compared to young (A). The absolute number of CD45hi peripheral leukocytes present in the young and aged brain is quantified (B; N=13/group). The number of cells within the CD45hi population is displayed by subset (C; N=13/group). A representative dot plot shows T cell subsets in the aged brain (D). The CD4:CD8 ratio of T cells is quantified for blood, spleen, and brain in young and aged mice (E; N=10–26/group). Data is representative of the average of individual mice within a group. Error bars show mean SEM. Abbreviation: SEM standard error of mean. *p<0.05; **p<0.01; ***p<0.001
Age-related chemokine production is associated with enhanced recruitment of CD8 T cells into the aging brain
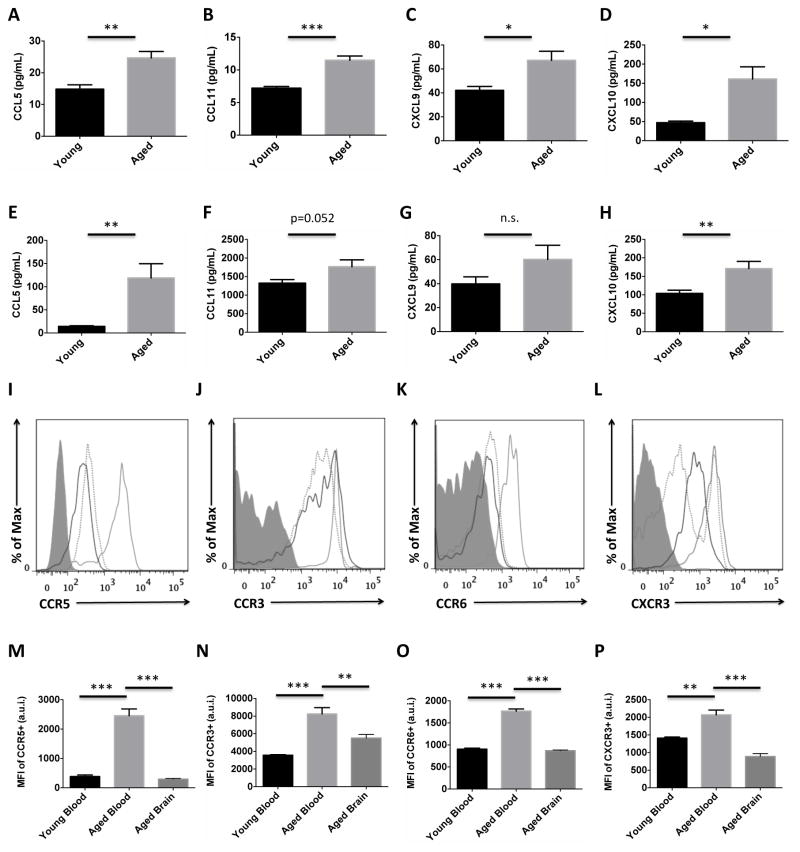
To determine whether CD8 T cell recruitment to the aging brain could result from a directed, homeostatic process rather than a random dispersion into tissues, we examined whole brain protein expression of chemokines known to be involved T cell migration. CCL5 (RANTES), CCL11 (Eotaxin), CXCL9 (MIG), and CXCL10 (IP-10) concentrations were all significantly upregulated in the brain with age by ELISA (Figures 2A–D). Levels of these chemokines were similarly increased in aged plasma (Figures 2E–H), suggesting that these gradient levels are accessible to circulating T cells. Surface expression of chemokine receptors to these respective chemokine ligands was then assessed on both blood and brain CD8 T cells. An increase in expression of these chemokine receptors was found on blood CD8 T cells with age (Figures 2I–L). Interestingly, CD8 T cells in the aged brain expressed lower levels of these receptors relative to those in circulation, and levels were comparable to that found in young blood (Figures 2M–P). These results suggest that the aged brain is a rich source of chemokine cues that are generally required for active T cell migration. After reaching the CNS, CD8 T cells down-regulate expression of their chemokine receptors in order to establish residence.
Figure 2. Age-related changes in chemokine production and chemokine receptor expression in the brain.
Whole brain protein concentrations of CCL5 (A), CCL11 (B), CXCL9 (C), and CXCL10 (D) are quantified in young and aged mice. Plasma concentrations of these respective chemokines (E, F, G, and H) are quantified. Representative histograms show higher expression of CCR5 (I), CCR3 (J), CCR6 (K), and CXCR3 (L) on circulating CD8 T cells with age, and down-regulation on CD8 T cells present in the aged brain. Young blood (dotted gray), aged blood (solid gray), and aged brain (solid black) CD8 T cells are depicted. The mean fluorescence intensity for each of the respective chemokine receptors was quantified (M, N, O, and P; N=5/group). Cell-specific FMO controls were used to determine positive gating (shaded gray). Error bars show mean SEM. Abbreviation: SEM standard error of mean, MFI mean fluorescence intensity. *p<0.05; **p<0.01; ***p<0.001
CD8 T cells are present in perivascular and parenchymal regions throughout the aging CNS
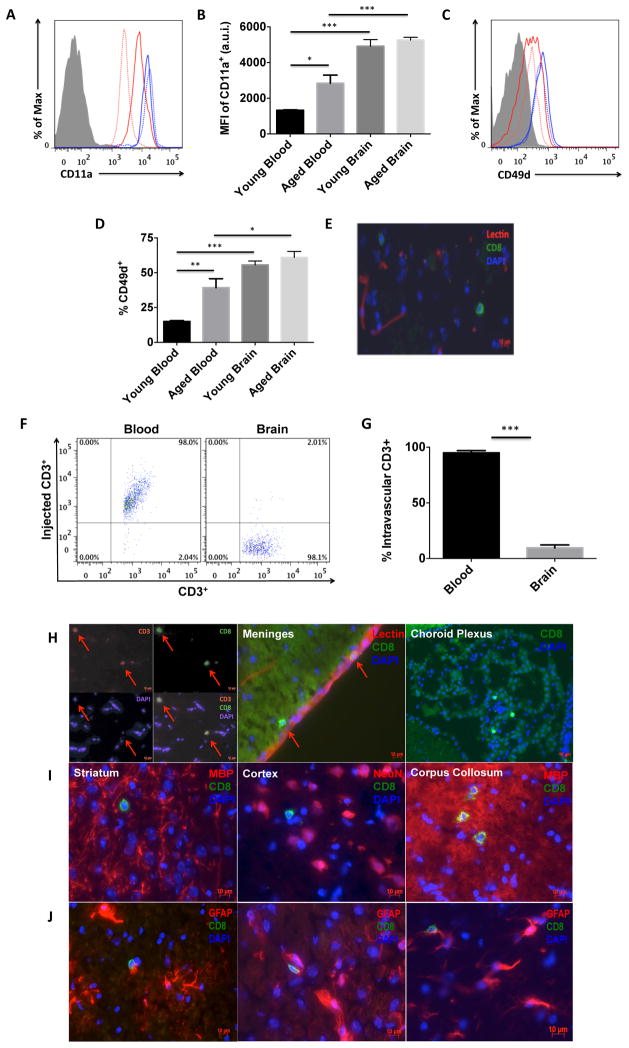
T cell extravasation across the endothelium involves a series of adhesion events that include attachment, rolling, and transmigration. Because ICAM-1 expression is reported to be increased in aged brain vasculature (21, 22), we examined CD8 T cell expression of CD11a (LFA1) and CD49d (VLA4), adhesion molecules required for T cell entry into peripheral tissues. Figures 3A–D show that CD11a and CD49d expression were significantly increased on circulating CD8 T cells with age (p<0.05 and p<0.01, respectively), and further elevated on those present in the brain at either age (p<0.001 and p<0.05, respectively). In both tissues, CD49d-positive CD8 T cells were CD11ahi. The vessel capture of T cells implies subsequent extravasation into perivascular and parenchymal CNS tissue. Using tomato-lectin immunohistochemistry, we identified the putative presence of CD8 T cells outside of lectin-positive lumen of vessels (Figure 3E). To confirm that T cells resided in the parenchymal tissue, and were not adherent to the brain endothelium, we performed an injection labeling experiment as previously described (23, 24). All circulating and vessel-associated cells bound to transcardially injected PE-conjugated CD3e antibody, processed for leukocyte collection, and subsequently stained with APCeF780-conjugated CD3e antibody. Compared to an injection labeling efficiency of ~98% for all blood CD3e-injected+CD8 T cells, only 7.7±5% of aged brain CD8 T cells were positive for CD3e-injected antibody, suggesting these cells are located outside of the vascular network and in the parenchyma or perivascular spaces (Figures 3F–G). Immunohistology revealed the presence of CD3+CD8+ T cells in circumventricular organs (choroid plexus and meninges) and most gray and white matter regions (cortex, striatum, corpus collosum) throughout the anterior-posterior axis of the brain (Figures 3H–I). Further, we found that these cells were often associated with astrocytes, although the precise nature of this association is unknown (Figure 3J).
Figure 3. Extravasation of CD8 T cells is higher into the aged brain.
Representative histograms depict the relative expression of the adhesion molecules CD11a (A) and CD49d (C) on CD8 T cells from the blood (red) and brain (blue) of young (dotted) and aged (solid) mice. Quantification of the mean fluorescence intensity of CD11a+ (B; N=5–6/group) and percentage of CD49d+ (D; N=9–10/group) CD8 T cells is shown. Immunohistochemistry shows the presence of CD8-positive cells (green) outside of lectin-positive (red) blood vessels (E). A representative dot plot from the Intravascular labeling experiment shows the absence of injected-CD3+ antibody on CD8 T cells in the aged brain (F; N= 8/group). The percentage of intravascular-labeled CD3+ CD8 T cells was quantified (G) in the aged brain and blood (for positive control). Representative 10um-thick brain tissue sections illustrating the presence of CD3+CD8+ T cells in the aged brain, meninges, and choroid plexus (H; scale bar = 10μm). CD8+ T cells were found throughout the anterior-posterior axis of the brain, including the striatum, cortex, and corpus collosum (I) and in association with activated astrocytes (J). Cell-specific FMO controls were used to determine positive gating (shaded gray). Error bars show mean SEM. Abbreviation: SEM standard error of mean, DAPI 4′,6-diamidino-2-phenylindole, MBP myelin basic protein, NeuN neuronal nuclei, GFAP glial fibrillary acidic protein. *p<0.05; **p<0.01; ***p<0.001
Age-related, CNS-resident CD8 T cells have memory/effector phenotype and expression markers of T cell-receptor activation
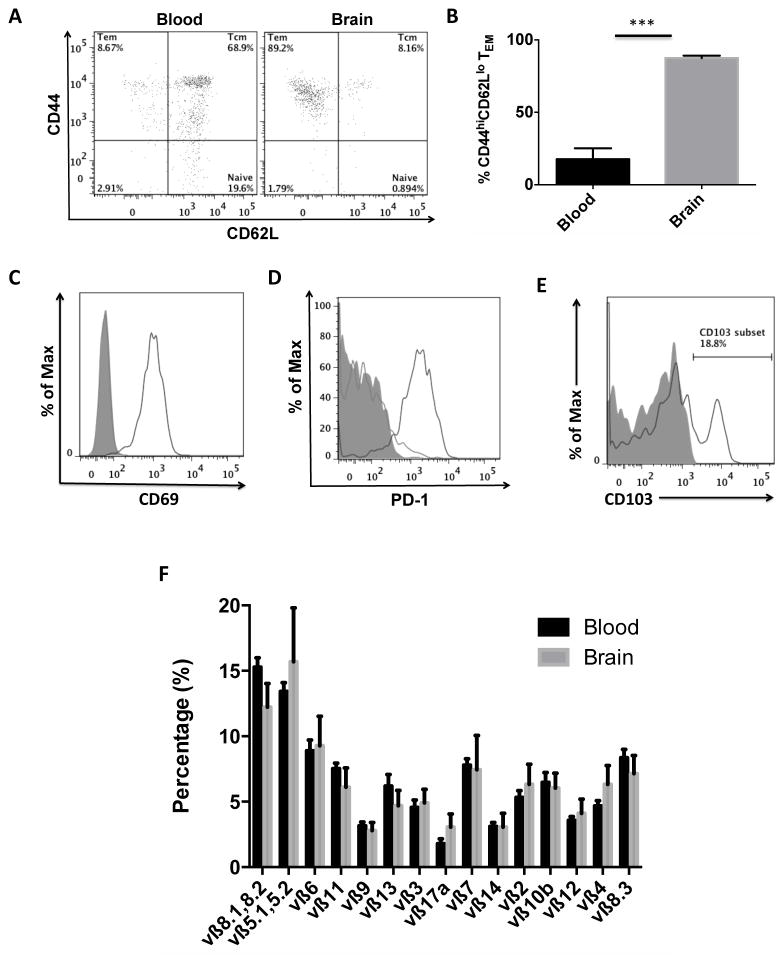
The trafficking of T cells into CNS tissues is thought to occur following antigenic presentation in draining lymph nodes by professional antigen-presenting cells. Antigen-experienced memory T cell subsets are defined by CD44+CD62L+ (Central Memory, TCM) and CD44+CD62L− (Effector Memory, TEM) expression, whereas naïve T cells are largely negative for the activation marker CD44 (25). Applying this paradigm to CD8 T cells in the aging brain we found that the vast majority (87±4%) of all CNS-resident CD8 T cells have an effector memory phenotype, compared to just 9±4% of CD8 T cells in blood (Figures 4A–B). A similar percentage of T cells with effector memory phenotype were observed in young brains, despite the fact that T cells were found in significantly lower abundance. To determine the general activation state of CNS-resident CD8 TEM cells we probed these cells with known markers of T cell activation. The early activation and tissue-retention marker, CD69, was highly upregulated on CNS-resident CD8 T cells compared to those in the blood (Figure 4C, N=5/group, p<0.001). Expression of programmed death-1 (PD-1) receptor, which is upregulated on activated T cells, was also dramatically increased on nearly all CNS-resident T cells compared CD8 T cells in aged blood (Figure 4D, N=5/group, p<0.0001), implying the presence of widespread T cell activation in the aged brain. Expression of the resident memory marker alpha E integrin CD103 was also found on a significant fraction of these cells (Figure 4E). To determine if CNS-resident memory CD8 T cells were responding to antigenic signals present in the aging CNS, CD8 T cells in the blood and brain were screened for TCRvβ usage to determine T cell clonality in tertiary tissues. No differences in TCRvβ usage between CNS-resident CD8 T cells and their blood counterparts were found for any of the 15 TCRvβ families (Figure 4F). Based on the absence of major clonal expansions in the CNS, these results suggest that CD8 T cell entry into the aged brain is largely stochastic, rather than antigen-driven. The following data suggest that the increased trafficking of these cells into the CNS is a stochastic process, whereby a pool of activated, antigen-experienced memory effector CD8 T cells are constitutively present throughout adult life in the CNS.
Figure 4. CNS-resident CD8 T cells stochastically migrate into the brain and have tissue-resident and effector memory phenotypes.
A representative dot plot shows effector memory phenotype of CD8 T cells in the aged brain and central memory phenotype of CD8 T cells in the aged blood (A). The percentage of CD8 T cells with effector memory phenotype (CD44hiCD62lo) was quantified (B). Representative histograms (N= 5–6/group) depict significant expression of the activation markers CD69 (C) and PD-1 (D) in the brain (solid black) relative to blood (solid gray), as well as the resident memory marker CD103 (E). A mouse vβ TCR screening panel containing 15 FITC-conjugated monoclonal antibodies was used to assess TCR vβ usage in CD8 T cells from the blood and brain of aged mice. The mean percentage for each of the 15 subfamilies of T cell receptor vβ is displayed (F). No differences were found between groups (N= 11/group). Cell-specific FMO controls were used to determine positive gating (shaded gray). Error bars show mean SEM. Abbreviation: SEM standard error of mean. *p<0.05; **p<0.01; ***p<0.001
Increased immune surveillance by CD8 T cells in the aging brain is associated with microglial function
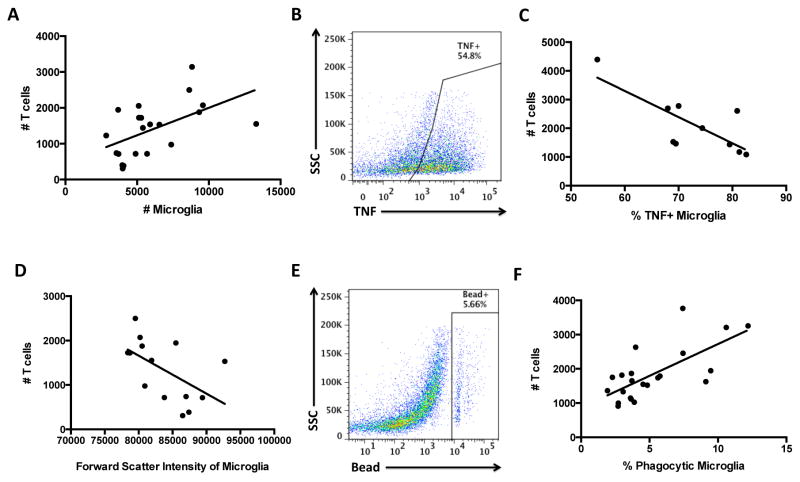
We investigated the functional role of CNS-resident CD8 T cells by correlating the absolute number in each brain with the activation status of microglia in that brain. Correlation analyses revealed that aged brains with greater numbers of CNS-resident CD8 T cells had significantly more microglia (Figure 5A, p<0.05), indicating that these cells may either promote microglia survival or alternatively, increase activation-induced proliferation. Brains that contained large numbers of CNS-resident CD8 T cells, however, were also associated with smaller sized microglia relative to brains with fewer CD8 T cells (Figure 5D, p<0.05), indicative of a more ramified, resting morphology. Functionally, higher numbers of T cells were associated with increased microglia phagocytosis (Figures 5E–F, p<0.001) and decreased TNF production (Figures 5B–C, p<0.05). These data suggest that the increased presence of CD8 T cells in the aged CNS is positively associated with an anti-inflammatory microglia phenotype in the aged brain.
Figure 5. Positive correlation between the number of CNS-resident CD8 T cells and healthy microglia functions in the aged brain.
Correlation analysis between CD8 T cell count and microglia (CD45intCD11b+Ly6C−) count (A; p=0.0125 and r=0.534), percentage TNF-positive (C, p=0.0105 and r=−0.7616), forward scatter/cell size (D; p=0.0421 and r=−0.5488), and percentage bead-positive phagocytic (F; p=0.0003 and r=0.6945). Representative dot plots are shown for microglia TNF production (B) and bead assay (E). For all experiments, N= 10–22/group. Age-matched, cell-specific FMO controls were used to determine positive gating. Abbreviation: TNF tumor necrosis factor.
Basal and stimulus-driven production of cytokines and chemokines following ischemic stroke
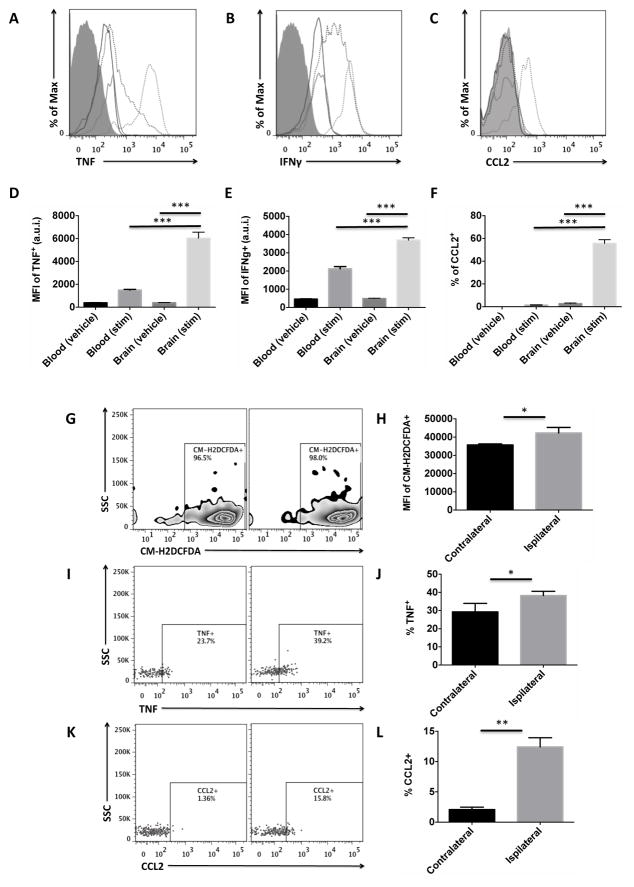
T cells are polarized to effector memory cells following TCR reactivation to selected antigenic stimuli. The effector function of these cells is largely specified by cytokine production. An in vivo brefeldin protocol was used to measure cytokine production in CNS-specific CD8 T cells as in (26). Cytokines commonly associated with CD8 T cell pro-inflammatory responses (TNF and IFNγ) were basally produced at significant levels (Figures 6A–C), whereas granzyme B or perforin were not expressed under any conditions (data not shown). Both the number and relative expression level of TNF and IFNγ were significantly increased after stimulation in CNS-resident CD8 T cells compared to those in blood (Figures 6D–E, p<0.001). CCL2 production by CNS-resident CD8 T cells was significantly higher after stimulation compared to those in the blood (Figure 6F, p<0.001). These data suggest that CNS-resident T cells in the aged CNS are a rich source of pro-inflammatory cytokines, likely contributing to the steady increase in overall levels of inflammation that occurs with advanced age. Next we determined whether these cells were similarly responsive to stimuli in an age-relevant model of brain injury. We tested this in an experimental model of ischemic stroke, as this is a disease that mainly affects the aging population. Cytokine production was assessed at 4 hours post-reperfusion following 90 minutes of occlusion. Reactive oxygen species generation was significantly increased in CD8 T cells subjected to ischemia compared to those in the control contralateral hemisphere, as evidenced by CM-H2DCFDA staining (Figures 6G–H, p<0.05). The percentage of TNF- and CCL2-positive CD8 T cells was also increased in ischemic hemisphere compared to control (Figures 6I–L, p<0.05 and p<0.01, respectively). These data suggest that activated CNS-resident CD8 T cells contribute to the age-related exacerbation of acute ischemic brain injury by amplifying production of pro-inflammatory cytokines and promoting recruitment of peripheral leukocytes.
Figure 6. Acute stimulation of CD8 T cells by PMA/ionomycin and in an age-relevant model of cerebral ischemia.
Representative histograms show CD8 T cell expression of TNF (A), IFNγ (B), and CCL2 (C) in blood (solid black) and brain (solid gray) and after stimulation (dotted). The respective mean fluorescence intensities (D, E, F) were quantified (N= 4–10/group). Aged mice were subject to 90 minutes of occlusion followed by 2 hours of reperfusion and 1 hour ex-vivo stimulation by PMA/ionomycin. CD8 T cells from the ischemic hemisphere (ipsilateral) were compared to those in the contralateral hemisphere for internal control (N= 4–6/group). A representative zebra plot shows reactive oxygen species levels in CD8 T cells after stroke as measured by CM-H2DCFDA (G) and quantification of mean fluorescence intensity (H). A representative dot plot shows intracellular production of TNF (I) and the percentages quantified (J). A representative dot plot depicts a stroke-induced increase in CCL2 production by CD8 T cells in the aged brain (K). The percentages were quantified (L). Cell-specific FMO controls were used to determine positive gating (shaded gray). Error bars show mean SEM. Abbreviation: SEM standard error of mean, SSC side scatter intensity, MFI mean fluorescence intensity, a.u.i. arbitrary units of intensity CM-H2DCFDA 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester, TNF tumor necrosis factor, IFNγ interferon gamma, CCL2 monocyte chemoattractant protein-1. *p<0.05; **p<0.01; ***p<0.001
Discussion
This work has identified a novel population of immune surveillant CD8 T cells in the aging CNS that may have opposing roles in health and disease. This adds to previous studies demonstrating that immune privilege in the aging CNS is compromised. The aged brain is more permissive to T cell entry, which has functional consequences, especially after injury. We base this observation on several criteria: 1) increased brain production of T cell chemokine cues, 2) enhanced expression of adhesion molecules, 3) higher levels of extravasation into perivascular and parenchymal regions, 4) activated tissue-resident and effector memory phenotypes, 5) presence alters microglia function, and 6) primed to elicit strong cytokine and chemokine responses after sterile brain injury. These cells have a phenotype indicative of resident memory cells (CD44hi CD62L− CD11a+ CD69+ CD103+/−). This is the first study to identify this specific population of “age-associated” CNS-resident memory CD8 T cells and evaluate their influence on the neuroimmunological landscape.
For an organ that has long been described as immune-privileged and devoid of any blood-borne leukocytes, the brain’s relationship to the immune system is now being revised with the advent of a meningeal-lymphatic system and growing recognition that T cells constitutively traffic throughout the CNS (27). Compared to many widely used experimental models of CNS injury, the number of the T cells in the normal aging brain is relatively small, yet emerging data suggests that interfering with this steady-state trafficking of T cells through the meningeal-lymphatic pathway results in significant cognitive impairment (12). Moreover, recent findings suggest that the recovery of resident memory CD8 T cells from non-lymphoid tissues is significantly underestimated as many cells are either killed or discarded during the lengthy tissue processing procedure (28). The nearly three-fold increase in CNS-resident CD8 T cells of old mice highlights the role of aging on neuroinflammatory activity and the greater need for immune surveillance.
Despite the fact the other CD45hi leukocyte populations do not prominently increase in the brain with age, the question arises as to whether T cell recruitment is gradually increased over time, or if there is a critical period in which the brain is more permissive to these lymphocytes. While the recent identification of a CNS lymphatic route implies that T cell trafficking is a constitutive, homeostatic process, the presence of CD8 T cells in the young brain suggests that this process begins early. Although our study was limited to two timepoints, earlier work by Stichel and Luebertt demonstrated that CD3+ T cells accumulate in the several CNS regions with chronological age (16), consistent with the age-related increase of these cells in other peripheral tissues (29–33). These findings support the notion of ‘inflammaging’, which describes the gradual increase in basal inflammatory processes with age (15). In addition to the elevated production of inflammatory mediators, this definition may be extended to account for age-related increases in thymic atrophy, memory conversion, TCR repertoire shrinkage, and migration into non-lymphoid tissues.
Our decision to refer to these cells as ‘CNS-resident’ CD8 T cells is based on several criteria; including the simple fact these cells are present (or reside) in CNS tissue and have a phenotype resembling that of known resident memory T cell populations (34). Among these, they exhibit diminished expression of chemokine receptors such as CCR7 required for lymph node homing (18). Because CD8 T cells of similar phenotype and function are rarely found in blood circulation or layover lymphoid sites, we assume these to be features of those present in non-lymphoid tissues. The distinction between ‘resident’ and ‘trafficking’ T cells is an important one and some have attempted to address this question using parabiosis and adoptive transfer experiments following the generation of memory in infection models, but studies on normal aging are lacking (35–38). Nonetheless, these studies suggest that egress from the CNS is minimal or slow, supporting the idea of long-term retention, or residency. Although the initial migration and entry into tissues may be antigen-driven, resident memory CD8 T cells can persist in the absence of antigens whilst maintaining impressive effector functions (36, 39–44). Seminal work by the Lefrancois group has demonstrated that newly generated memory CD8 T cells migrate to non-lymphoid tissues regardless of site of activation or tissue of origin (28, 35, 45). Regardless of antigen-specificity, the CNS niche provides signals that are likely distinct from all other tissues (e.g., adipose, liver, lung, etc.), priming them to respond and function in ways that are adapted (or specific) to this unique environment. In support of this notion, the Bevan laboratory has shown that CNS-resident CD8 T cells require local niche signals for their maintenance and survival (36). However, residence may only be temporary, and we cannot rule out the possibility that these cells shift their phenotype as they transit out of the CNS and back into circulation. Future studies employing cell-tracking approaches might serve to elucidate the migratory path of this rather reclusive lymphocyte population.
While T cell trafficking through the healthy CNS likely reflects a constitutive requirement for immune surveillance, factors associated with chronic stress may modulate this regulated process. Our observation that brain-related chemokine production is increased with age suggests possible involvement in facilitating T cell migration to the CNS. Moreover, the age-related increase in chemokine receptor expression on circulating CD8 T cells as noted in this study may further enhance this migration. Although speculative, this thinking is premised on several studies in which blocking these particular chemokines (i.e., CCL5, CCL11, CXCL9, and CXCL10) interfered with the normal migration of T cells into the injured CNS (46–55). The cellular source of these chemokines was not assessed in this study, although glial cells are known to be major producers of these signals. For example, astrocyte-secreted CXCL10 promotes the recruitment and entry of lymphocytes into the CNS, and worsens acute spinal cord demyelination in EAE (56, 57). Indeed, there is a growing appreciation for astrocyte-T cell interactions (58–60). The impact of CNS-resident CD8 T cells on astrocyte functions in aged brains remains to be shown. Although aged astrocytes exhibit signs of a senescence-associated phenotype (SASP) with age (61–63) and produce chemokines in response to amyloid-β peptides (64), the role of astrocytes as mediators of T cell recruitment in the aging brain is not understood.
The inverted CD4:CD8 ratios in the young and aged brain implied there was a CD8 T cell-specific pathway in the CNS that warranted further investigation. These findings stand in stark contrast to that found in the choroid plexus, meninges, and cerebrospinal fluid, which suggest CD4 T cells are the predominate subset (6, 10, 65). The relatively low number of CD4 T cells residing in brain parenchyma hindered our attempts to make reliable quantitative assessments of this population, yet much of our unpublished findings suggest that these cells exhibit many of the same features as CNS-resident CD8 T cells, despite having a markedly diminished capacity for stimulus-driven cytokine production. The general absence of CD4 T cells from the CNS parenchyma and preferential localization in portal sites of CNS entry and egress is likely indicative of their homeostatic role, yet our current understanding of these cells is still in its infancy.
CD8 T cells have received comparably less attention than their CD4 counterparts with respect to aging and CNS disease. This is partly due to their narrowly defined role in anti-viral responses. Yet, emerging data suggests that CD8 T cells may play more diverse roles in disease settings. Mounting evidence supports a role for CD8-mediated immune surveillance in peripheral tissues as part of the body’s normal defense against cancer and viral infection (14). The prevalence of pathogenic and oncogenic insults increases with age, triggering the induction of intrinsic defense mechanisms such as cell death signaling and senescence. Naturally extrinsic mechanisms evolved that alert the immune system to eliminate these aberrant cells to prevent further bystander damage. One mechanism through which this occurs is via immune surveillance by tissue-patrolling CD8 T cells. These cells eliminate unhealthy cells that increase presentation of non-self peptide. Although expression of MHC class I protein increases in the brain with age, the role of immune surveillance in aging individuals and like-wise the effect of aging on the efficacy of immune surveillance is understudied and warrants further investigation.
Because endothelial-ICAM expression has been shown to be elevated with age (22), we evaluated if these cells were simply adherent to the vasculature. The majority of CNS-resident CD8 T cells were not associated with the vascular lumen as these cells were largely found extravasated beyond the vascular lumen into many parenchymal regions across the CNS. CD8 T cell-astrocyte interactions have also received growing attention (60, 66). Functional evidence that CD8 T cells are prime candidates for a role in CNS immune surveillance is based on the exquisitely specific destruction of transgenic astrocytes expressing MHCI-restricted hemagglutinin (67). OVA-expressing oligodendrocytes elicit the proliferation and effector memory conversion of responding OT-1 T cells in healthy mice without infiltrating the CNS, indicating that antigen sampling is performed by CD8 T cells under steady state conditions (68). Interestingly, the authors found that neuroinflammation augmented the level of antigen sampling and led to the accumulation of OT-1 T cells in the inflamed brain. Thus, it is increasingly evident that CD8 T cells have the capacity to mediate responses against CNS antigens (69). While the age-related increase in neuroinflammation likely promotes the recruitment of immune surveillant CD8 T cells, how they impact CNS function is poorly understood. Tissue-resident memory CD8 T cells (TRM) provide superior protection against viral reactivation compared to the circulating memory T cell pool, but become dependent on the local milieu for function and survival (36, 42, 70). The increased presence of these cells in the aged CNS may reflect an evolutionary need to generate a rapid response to control senescent transformation of cells and/or prevent reactivation of latent neurotropic viruses.
The role of immunosurveillance as a defense mechanism that safeguards the functional integrity of tissues implies that it is an adaptive response to an environment under constant insult. Normal aging is associated with deficits in microglia phagocytosis and increased expression pro-inflammatory mediators (19). We demonstrated that the increased presence of CNS-resident CD8 T cells in older brains was associated with an attenuation of TNF production and enhanced phagocytic activity by microglia. Although causality cannot be proven, the effects of CD8 T cells on microglia need not occur via direct cell-cell interaction. While T cells are generally associated with CNS injury, other work has shown that T cells can enter the brain and not cause glial pathology (71), with T regulatory subsets showing potent neuroprotection and anti-inflammatory activity (72–75). Furthermore, emerging data indicates that T cells may function to support learning and memory under normal physiological conditions (11). It is possible that by eliminating nearby senescent cells CD8 T cells serve to attenuate age-related neuroinflammation, thereby indirectly preventing the chronic activation of microglia.
Given the lack of stereotypical anti-viral responses and recent evidence showing clonal expansion of CNS antigen-specific CD4 T cells in the choroid plexus of aged mice, we hypothesized that CNS-resident CD8 T cell activation is similarly induced by CNS antigen. However, we report relatively little clonal expansion of TCRvβ subsets in the aged CNS as evidenced by equilibrium in TCRvβ usage between those in the CNS and blood. Thus, our data argue that their migration into the CNS is stochastic and not antigen-driven. Consistent with our findings, studies using OVA-specific T cells have shown that CNS-irrelevant T cells recognizing non-mammalian antigen also have the capacity to enter the CNS (71). Novel transgenic approaches designed to probe the efficiency of CNS immune surveillance have shown that antigen-containing microglia and astrocytes, which are normally confined within the CNS barrier, are routinely surveyed by naïve and antigen-specific CD8 T cells, resulting in the exquisitely-specific destruction of these cells (38, 67, 76, 77). Interestingly, the elimination of these antigen-containing glia occurred in the absence of any bystander damage, implying that the mechanisms underlying CD8 T cell surveillance are wired to protect the brain from foreign cells in a remarkably effective manner. Thus, it is not unreasonable to assume that the elimination of abnormal or senescent cells is a routine occurrence in the healthy brain, increasing with age and stress. Taken together, the consensus understanding is that the CNS provides a niche that maintains both antigen-specific and non-specific effector memory CD8 T cells (78–80). Further, these works support the notion of an immune surveillant pathway in the CNS that maintains not only the acute steady state trafficking of CD8 T cells, but their long-term maintenance as well. Surveillance also occurs at CNS drainage sites, as intracranial injection of OVA peptide results in T cell priming in peripheral lymphoid organs and the subsequent infiltration and persistence of OT-1 T cells into the brain (36, 80, 81). Because the age-related accumulation of CD8 memory cells described in our study represent a naturally occurring population of CNS-resident T cells, it may stand as a useful model for understanding the biology of tissue-resident memory and immune surveillance. Taken together, the migration of effector memory CD8 T cells into the aged CNS does not require stimulation by CNS antigen, although deeper sequencing of the T cell receptor repertoire might be a useful approach in determining the antigenic specificity of these cells.
We demonstrated that upon activation by either PMA/ionomycin stimulation or ischemic brain injury, CNS-resident CD8 T cells in aged brains are prone to elicit highly volatile responses that include the enhanced production of reactive oxygen species, TNF, and IFNγ, as well several chemokines involved in granulocyte recruitment. These cells may therefore predispose the aged brain to overreact to stimuli, further exaggerating the inflammatory response and altering the context of injury via the recruitment of injury-causing neutrophils. This is in contrast to that of the infiltrating CD8 T cell population, which is involved in the delayed response to ischemia during the recovery period. Indeed, the inflammatory milieu and leukocyte response in the ischemic brain is dramatically different in older mice (82–86). Furthermore, these exaggerated immune responses are tightly linked to worse outcomes in aged mice (33, 87). Whether stroke-induced activation of CNS-resident CD8 T cells results in the biased recruitment of neutrophils in the aged brain by producing elevated levels of chemokines is not known, but would provide an alternative explanation for differences in the kinetics of leukocyte infiltration with age. Moreover, given their role in promoting leukocyte recruitment, CNS-resident CD8 T cells may represent important therapeutic targets in the acute period following stroke.
Although rarely described, age-related CNS-resident CD8 T cells have now been observed in multiple mouse strains and even humans, implying that augmented immune surveillance of the CNS is a conserved adaptation to aging (17, 18, 88). Older BALB/cByJ and C57BL/6J males exhibited similar CNS leukocyte compositions. Interestingly, aged female BALB/cByJ had higher frequencies of CD4 T cells, consistent with the Th2 bias of this particular strain and sex (89, 90). We also noted significant sex differences in the number of CNS-resident T cells in aged mice, with females having roughly twice as many as males. Although sex differences in activation status were not examined, the large number of injury-responsive CNS-resident T cells present in the aged female brain suggests that they are predisposed to greater inflammatory activity and worse outcomes following stroke. Indeed, clinical data and past work from our laboratory has shown that aged females exhibit greater acute injury after ischemic stroke compared to aged males (83, 91). Moreover, this sexually dimorphism in immune surveillance may partially explain why autoimmune and anti-viral responses are ostensibly stronger in females or why the diagnosis of malignant brain tumors is twice as likely in males and results in greater cognitive impairment and mortality compared to females (92–97). Whether ovarian hormones mediate this sex effect is not known, as aged female mice are post-menopausal and young females were not examined in this study. However, our previous work suggests that T cell numbers in the injured brain negatively correlate with circulating estradiol levels in aging females, suggesting that estrogen may normally suppress T cell trafficking into the brain (33). Understanding the biological consequences of these sex differences remains an area rife for exploration.
Although little is known about naturally occurring populations of tissue-resident T cells in non-lymphoid tissues like the CNS, they are believed to be an important part of adaptive immune memory and immune surveillance by rapidly addressing and clearing infected and transformed cells (38). However, the pathological accumulation of these cells can drive disease progression in these tissues, highlighting the dual nature of these cells (98). The absolute number of these cells obtained from the aged brain prohibits their utility in adoptive transfer experiments. Nevertheless, our findings suggest that resident memory CD8 T cells are poised to influence CNS function by direct interaction with aged glial cells. This data suggests that the working definition of ‘inflamm-aging’ in the CNS should include the increased level of immune surveillance by CD8 T cells. These T cells may be included with other cell types known to have the senescence-associated secretory phenotype (61, 99–102). Senescent cells promote tumor progression through the release of pro-inflammatory molecules and are relatively more vulnerable to transformation due to the age-related accumulation of DNA mutations. Thus, senescence surveillance is believed to be critical for tumor suppression(14, 99, 103–105). Resident microglial function is influenced by the increased presence of these cells. However, the beneficial role for CD8 T cells in suppressing the pro-inflammatory activity of microglia under normal conditions is in contrast to their exaggerated response to stimuli resulting in the elevated production of pro-inflammatory cytokines and chemokine signals that favor recruitment of granulocytes. Interestingly, memory T cells are known to accumulate in several tertiary tissues with advanced age. Moreover, the age-related increase in the number of these tissue-resident cells may likely predispose older mice to worse outcomes and account for age-related differences in inflammation following traumatic injuries such as ischemic stroke. Future studies are needed to determine whether these interactions can be experimentally manipulated to better understand their functional role in normal aging.
Although it has long been presumed that T cells visit non-lymphoid tissues like the brain, many believed these cells to circulate back to the lymph nodes. Emerging data suggests that some T cells functionally adapt to these environments and reside in peripheral tissues forever (106, 107). While resident memory CD8 T cells have superior effector functions and provide frontline protection against infection and tumor development, the effects of aging on the distribution and function of these cells in otherwise healthy mice are largely unknown. In this study we have shown that aging increases the recruitment of CD8 T cells to the CNS. Here these effector memory cells establish residence and stand poised to react to inflammatory stimuli in the wake of sterile injury. By potentiating the neuroinflammatory response to stroke, we demonstrated that the age-related increase in the level of immune surveillance by CD8 T cells may have unintended consequences that could profoundly alter the course of injury in aged mice.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants R21 NS076293-01A1 (Louise D. McCullough) and F31 NS083244-01A1 (Rodney M. Ritzel).
We gratefully acknowledge Dr. Robert Clark and Dr. Stefan Brocke for helpful discussions. We also acknowledge Brett Friedler for his technical assistance.
References
- 1.Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. Washington, DC: US Census Bureau; 2014. pp. 25–1140. [Google Scholar]
- 2.Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, Tenner AJ, Cotman CW. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J Neuroinflammation. 2012;9:179. doi: 10.1186/1742-2094-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz M, Kipnis J, Rivest S, Prat A. How do immune cells support and shape the brain in health, disease, and aging? J Neurosci. 2013;33:17587–17596. doi: 10.1523/JNEUROSCI.3241-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wrona D. Neural-immune interactions: an integrative view of the bidirectional relationship between the brain and immune systems. J Neuroimmunol. 2006;172:38–58. doi: 10.1016/j.jneuroim.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Banks WA. The blood-brain barrier in neuroimmunology: Tales of separation and assimilation. Brain Behav Immun. 2015;44:1–8. doi: 10.1016/j.bbi.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baruch K, Ron-Harel N, Gal H, Deczkowska A, Shifrut E, Ndifon W, Mirlas-Neisberg N, Cardon M, Vaknin I, Cahalon L, Berkutzki T, Mattson MP, Gomez-Pinilla F, Friedman N, Schwartz M. CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc Natl Acad Sci U S A. 2013;110:2264–2269. doi: 10.1073/pnas.1211270110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015 doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sierra A, Beccari S, Diaz-Aparicio I, Encinas JM, Comeau S, Tremblay ME. Surveillance, phagocytosis, and inflammation: how never-resting microglia influence adult hippocampal neurogenesis. Neural Plast. 2014;2014:610343. doi: 10.1155/2014/610343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gemechu JM, Bentivoglio M. T Cell Recruitment in the Brain during Normal Aging. Front Cell Neurosci. 2012;6:38. doi: 10.3389/fncel.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kivisakk P, Mahad DJ, Callahan MK, Trebst C, Tucky B, Wei T, Wu L, Baekkevold ES, Lassmann H, Staugaitis SM, Campbell JJ, Ransohoff RM. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A. 2003;100:8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kipnis J, Gadani S, Derecki NC. Pro-cognitive properties of T cells. Nat Rev Immunol. 2012;12:663–669. doi: 10.1038/nri3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radjavi A, Smirnov I, Derecki N, Kipnis J. Dynamics of the meningeal CD4(+) T-cell repertoire are defined by the cervical lymph nodes and facilitate cognitive task performance in mice. Mol Psychiatry. 2014;19:531–533. doi: 10.1038/mp.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radjavi A, Smirnov I, Kipnis J. Brain antigen-reactive CD4+ T cells are sufficient to support learning behavior in mice with limited T cell repertoire. Brain Behav Immun. 2014;35:58–63. doi: 10.1016/j.bbi.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 16.Stichel CC, Luebbert H. Inflammatory processes in the aging mouse brain: participation of dendritic cells and T-cells. Neurobiol Aging. 2007;28:1507–1521. doi: 10.1016/j.neurobiolaging.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Loeffler C, Dietz K, Schleich A, Schlaszus H, Stoll M, Meyermann R, Mittelbronn M. Immune surveillance of the normal human CNS takes place in dependence of the locoregional blood-brain barrier configuration and is mainly performed by CD3(+)/CD8(+) lymphocytes. Neuropathology. 2011;31:230–238. doi: 10.1111/j.1440-1789.2010.01167.x. [DOI] [PubMed] [Google Scholar]
- 18.Smolders J, Remmerswaal EB, Schuurman KG, Melief J, van Eden CG, van Lier RA, Huitinga I, Hamann J. Characteristics of differentiated CD8(+) and CD4 (+) T cells present in the human brain. Acta Neuropathol. 2013;126:525–535. doi: 10.1007/s00401-013-1155-0. [DOI] [PubMed] [Google Scholar]
- 19.Ritzel RM, Patel AR, Pan S, Crapser J, Hammond M, Jellison E, McCullough LD. Age- and location-related changes in microglial function. Neurobiol Aging. 2015;36:2153–2163. doi: 10.1016/j.neurobiolaging.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 20.McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 21.Miguel-Hidalgo JJ, Nithuairisg S, Stockmeier C, Rajkowska G. Distribution of ICAM-1 immunoreactivity during aging in the human orbitofrontal cortex. Brain Behav Immun. 2007;21:100–111. doi: 10.1016/j.bbi.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu YZ, Nygard M, Kristensson K, Bentivoglio M. Regulation of cytokine signaling and T-cell recruitment in the aging mouse brain in response to central inflammatory challenge. Brain Behav Immun. 2010;24:138–152. doi: 10.1016/j.bbi.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, Qunaj L, Griffith TS, Vezys V, Barber DL, Masopust D. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc. 2014;9:209–222. doi: 10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson KG, Sung H, Skon CN, Lefrancois L, Deisinger A, Vezys V, Masopust D. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J Immunol. 2012;189:2702–2706. doi: 10.4049/jimmunol.1201682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts AD, Woodland DL. Cutting edge: effector memory CD8+ T cells play a prominent role in recall responses to secondary viral infection in the lung. J Immunol. 2004;172:6533–6537. doi: 10.4049/jimmunol.172.11.6533. [DOI] [PubMed] [Google Scholar]
- 26.Liu F, Whitton JL. Cutting edge: re-evaluating the in vivo cytokine responses of CD8+ T cells during primary and secondary viral infections. J Immunol. 2005;174:5936–5940. doi: 10.4049/jimmunol.174.10.5936. [DOI] [PubMed] [Google Scholar]
- 27.Ellwardt E, Walsh JT, Kipnis J, Zipp F. Understanding the Role of T Cells in CNS Homeostasis. Trends Immunol. 2016 doi: 10.1016/j.it.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyarto BZ, Southern PJ, Masopust D. Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell. 2015;161:737–749. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinet KZ, Bloquet S, Bourgeois C. Ageing combines CD4 T cell lymphopenia in secondary lymphoid organs and T cell accumulation in gut associated lymphoid tissue. Immun Ageing. 2014;11:8. doi: 10.1186/1742-4933-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh P, Coskun ZZ, Goode C, Dean A, Thompson-Snipes L, Darlington G. Lymphoid neogenesis and immune infiltration in aged liver. Hepatology. 2008;47:1680–1690. doi: 10.1002/hep.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lumeng CN, Liu J, Geletka L, Delaney C, Delproposto J, Desai A, Oatmen K, Martinez-Santibanez G, Julius A, Garg S, Yung RL. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J Immunol. 2011;187:6208–6216. doi: 10.4049/jimmunol.1102188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thome JJ, Yudanin N, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, Kato T, Lerner H, Shen Y, Farber DL. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 2014;159:814–828. doi: 10.1016/j.cell.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manwani B, Liu F, Scranton V, Hammond MD, Sansing LH, McCullough LD. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp Neurol. 2013;249:120–131. doi: 10.1016/j.expneurol.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 35.Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 36.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U S A. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wakim LM, Woodward-Davis A, Liu R, Hu Y, Villadangos J, Smyth G, Bevan MJ. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol. 2012;189:3462–3471. doi: 10.4049/jimmunol.1201305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carbone FR. Tissue-Resident Memory T Cells and Fixed Immune Surveillance in Nonlymphoid Organs. J Immunol. 2015;195:17–22. doi: 10.4049/jimmunol.1500515. [DOI] [PubMed] [Google Scholar]
- 39.Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, Vezys V, Masopust D. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark RA. Resident memory T cells in human health and disease. Sci Transl Med. 2015;7:269rv261. doi: 10.1126/scitranslmed.3010641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 42.Ariotti S, Beltman JB, Chodaczek G, Hoekstra ME, van Beek AE, Gomez-Eerland R, Ritsma L, van Rheenen J, Maree AF, Zal T, de Boer RJ, Haanen JB, Schumacher TN. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc Natl Acad Sci U S A. 2012;109:19739–19744. doi: 10.1073/pnas.1208927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark RA, Watanabe R, Teague JE, Schlapbach C, Tawa MC, Adams N, Dorosario AA, Chaney KS, Cutler CS, Leboeuf NR, Carter JB, Fisher DC, Kupper TS. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci Transl Med. 2012;4:117ra117. doi: 10.1126/scitranslmed.3003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci U S A. 2012;109:7037–7042. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, Woodland DL, Lefrancois L. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- 46.Roy A, Mondal S, Kordower JH, Pahan K. Attenuation of microglial RANTES by NEMO-binding domain peptide inhibits the infiltration of CD8(+) T cells in the nigra of hemiparkinsonian monkey. Neuroscience. 2015;302:36–46. doi: 10.1016/j.neuroscience.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaerve A, Muller HW. Chemokines in CNS injury and repair. Cell Tissue Res. 2012;349:229–248. doi: 10.1007/s00441-012-1427-3. [DOI] [PubMed] [Google Scholar]
- 48.Sporici R, Issekutz TB. CXCR3 blockade inhibits T-cell migration into the CNS during EAE and prevents development of adoptively transferred, but not actively induced, disease. Eur J Immunol. 2010;40:2751–2761. doi: 10.1002/eji.200939975. [DOI] [PubMed] [Google Scholar]
- 49.Denes A, Humphreys N, Lane TE, Grencis R, Rothwell N. Chronic systemic infection exacerbates ischemic brain damage via a CCL5 (regulated on activation, normal T-cell expressed and secreted)-mediated proinflammatory response in mice. J Neurosci. 2010;30:10086–10095. doi: 10.1523/JNEUROSCI.1227-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ransohoff RM. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity. 2009;31:711–721. doi: 10.1016/j.immuni.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nie CQ, Bernard NJ, Norman MU, Amante FH, Lundie RJ, Crabb BS, Heath WR, Engwerda CR, Hickey MJ, Schofield L, Hansen DS. IP-10-mediated T cell homing promotes cerebral inflammation over splenic immunity to malaria infection. PLoS Pathog. 2009;5:e1000369. doi: 10.1371/journal.ppat.1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Byrne FR, Winters A, Brankow D, Hu S, Juan T, Steavenson S, Doellgast G, Kuchimanchi K, Brown H, Anderson S, Smelt S, Sullivan T, Alcorn D, Tocker J, Dean C, Jr, Macmaster J, Kirchner J, Buys J, Manoukian R, Jiao E, Zou X, Campanella GS, Siu G. An antibody to IP-10 is a potent antagonist of cell migration in vitro and in vivo and does not affect disease in several animal models of inflammation. Autoimmunity. 2009;42:171–182. doi: 10.1080/08916930802629547. [DOI] [PubMed] [Google Scholar]
- 53.Campanella GS, Tager AM, El Khoury JK, Thomas SY, Abrazinski TA, Manice LA, Colvin RA, Luster AD. Chemokine receptor CXCR3 and its ligands CXCL9 and CXCL10 are required for the development of murine cerebral malaria. Proc Natl Acad Sci U S A. 2008;105:4814–4819. doi: 10.1073/pnas.0801544105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ubogu EE, Callahan MK, Tucky BH, Ransohoff RM. CCR5 expression on monocytes and T cells: modulation by transmigration across the blood-brain barrier in vitro. Cell Immunol. 2006;243:19–29. doi: 10.1016/j.cellimm.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ochiai E, Sa Q, Brogli M, Kudo T, Wang X, Dubey JP, Suzuki Y. CXCL9 is important for recruiting immune T cells into the brain and inducing an accumulation of the T cells to the areas of tachyzoite proliferation to prevent reactivation of chronic cerebral infection with Toxoplasma gondii. Am J Pathol. 2015;185:314–324. doi: 10.1016/j.ajpath.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Phares TW, Stohlman SA, Hinton DR, Bergmann CC. Astrocyte-derived CXCL10 drives accumulation of antibody-secreting cells in the central nervous system during viral encephalomyelitis. J Virol. 2013;87:3382–3392. doi: 10.1128/JVI.03307-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mills Ko E, Ma JH, Guo F, Miers L, Lee E, Bannerman P, Burns T, Ko D, Sohn J, Soulika AM, Pleasure D. Deletion of astroglial CXCL10 delays clinical onset but does not affect progressive axon loss in a murine autoimmune multiple sclerosis model. J Neuroinflammation. 2014;11:105. doi: 10.1186/1742-2094-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie L, Yang SH. Interaction of astrocytes and T cells in physiological and pathological conditions. Brain Res. 2015;1623:63–73. doi: 10.1016/j.brainres.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huseby ES, Kamimura D, Arima Y, Parello CS, Sasaki K, Murakami M. Role of T cell-glial cell interactions in creating and amplifying central nervous system inflammation and multiple sclerosis disease symptoms. Front Cell Neurosci. 2015;9:295. doi: 10.3389/fncel.2015.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barcia C, Sanderson NS, Barrett RJ, Wawrowsky K, Kroeger KM, Puntel M, Liu C, Castro MG, Lowenstein PR. T cells’ immunological synapses induce polarization of brain astrocytes in vivo and in vitro: a novel astrocyte response mechanism to cellular injury. PLoS One. 2008;3:e2977. doi: 10.1371/journal.pone.0002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur J Neurosci. 2011;34:3–11. doi: 10.1111/j.1460-9568.2011.07738.x. [DOI] [PubMed] [Google Scholar]
- 62.Orre M, Kamphuis W, Osborn LM, Melief J, Kooijman L, Huitinga I, Klooster J, Bossers K, Hol EM. Acute isolation and transcriptome characterization of cortical astrocytes and microglia from young and aged mice. Neurobiol Aging. 2014;35:1–14. doi: 10.1016/j.neurobiolaging.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 63.Rodriguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A. Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 64.Johnstone M, Gearing AJ, Miller KM. A central role for astrocytes in the inflammatory response to beta-amyloid; chemokines, cytokines and reactive oxygen species are produced. J Neuroimmunol. 1999;93:182–193. doi: 10.1016/s0165-5728(98)00226-4. [DOI] [PubMed] [Google Scholar]
- 65.Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sasaki K, Bean A, Shah S, Schutten E, Huseby PG, Peters B, Shen ZT, Vanguri V, Liggitt D, Huseby ES. Relapsing-remitting central nervous system autoimmunity mediated by GFAP-specific CD8 T cells. J Immunol. 2014;192:3029–3042. doi: 10.4049/jimmunol.1302911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cabarrocas J, Bauer J, Piaggio E, Liblau R, Lassmann H. Effective and selective immune surveillance of the brain by MHC class I-restricted cytotoxic T lymphocytes. Eur J Immunol. 2003;33:1174–1182. doi: 10.1002/eji.200323492. [DOI] [PubMed] [Google Scholar]
- 68.Harris MG, Hulseberg P, Ling C, Karman J, Clarkson BD, Harding JS, Zhang M, Sandor A, Christensen K, Nagy A, Sandor M, Fabry Z. Immune privilege of the CNS is not the consequence of limited antigen sampling. Sci Rep. 2014;4:4422. doi: 10.1038/srep04422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun D, Whitaker JN, Huang Z, Liu D, Coleclough C, Wekerle H, Raine CS. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J Immunol. 2001;166:7579–7587. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- 70.Ariotti S, Haanen JB, Schumacher TN. Behavior and function of tissue-resident memory T cells. Adv Immunol. 2012;114:203–216. doi: 10.1016/B978-0-12-396548-6.00008-1. [DOI] [PubMed] [Google Scholar]
- 71.Smorodchenko A, Wuerfel J, Pohl EE, Vogt J, Tysiak E, Glumm R, Hendrix S, Nitsch R, Zipp F, Infante-Duarte C. CNS-irrelevant T-cells enter the brain, cause blood-brain barrier disruption but no glial pathology. Eur J Neurosci. 2007;26:1387–1398. doi: 10.1111/j.1460-9568.2007.05792.x. [DOI] [PubMed] [Google Scholar]
- 72.Li P, Gan Y, Sun BL, Zhang F, Lu B, Gao Y, Liang W, Thomson AW, Chen J, Hu X. Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann Neurol. 2013;74:458–471. doi: 10.1002/ana.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beers DR, Henkel JS, Zhao W, Wang J, Huang A, Wen S, Liao B, Appel SH. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain. 2011;134:1293–1314. doi: 10.1093/brain/awr074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gendelman HE, Appel SH. Neuroprotective activities of regulatory T cells. Trends Mol Med. 2011;17:687–688. doi: 10.1016/j.molmed.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reynolds AD, Banerjee R, Liu J, Gendelman HE, Mosley RL. Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson’s disease. J Leukoc Biol. 2007;82:1083–1094. doi: 10.1189/jlb.0507296. [DOI] [PubMed] [Google Scholar]
- 76.Agudo J, Ruzo A, Park ES, Sweeney R, Kana V, Wu M, Zhao Y, Egli D, Merad M, Brown BD. GFP-specific CD8 T cells enable targeted cell depletion and visualization of T-cell interactions. Nat Biotechnol. 2015;33:1287–1292. doi: 10.1038/nbt.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao J, Zhao J, Perlman S. De novo recruitment of antigen-experienced and naive T cells contributes to the long-term maintenance of antiviral T cell populations in the persistently infected central nervous system. J Immunol. 2009;183:5163–5170. doi: 10.4049/jimmunol.0902164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bergmann CC, Altman JD, Hinton D, Stohlman SA. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J Immunol. 1999;163:3379–3387. [PubMed] [Google Scholar]
- 79.Hawke S, Stevenson PG, Freeman S, Bangham CR. Long-term persistence of activated cytotoxic T lymphocytes after viral infection of the central nervous system. J Exp Med. 1998;187:1575–1582. doi: 10.1084/jem.187.10.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qing Z, Sewell D, Sandor M, Fabry Z. Antigen-specific T cell trafficking into the central nervous system. J Neuroimmunol. 2000;105:169–178. doi: 10.1016/s0165-5728(99)00265-9. [DOI] [PubMed] [Google Scholar]
- 81.Ling C, Sandor M, Fabry Z. In situ processing and distribution of intracerebrally injected OVA in the CNS. J Neuroimmunol. 2003;141:90–98. doi: 10.1016/s0165-5728(03)00249-2. [DOI] [PubMed] [Google Scholar]
- 82.Doyle KP, Cekanaviciute E, Mamer LE, Buckwalter MS. TGFbeta signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. J Neuroinflammation. 2010;7:62. doi: 10.1186/1742-2094-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu F, McCullough LD. Interactions between age, sex, and hormones in experimental ischemic stroke. Neurochem Int. 2012;61:1255–1265. doi: 10.1016/j.neuint.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Popa-Wagner A, Badan I, Walker L, Groppa S, Patrana N, Kessler C. Accelerated infarct development, cytogenesis and apoptosis following transient cerebral ischemia in aged rats. Acta Neuropathol. 2007;113:277–293. doi: 10.1007/s00401-006-0164-7. [DOI] [PubMed] [Google Scholar]
- 85.Sieber MW, Claus RA, Witte OW, Frahm C. Attenuated inflammatory response in aged mice brains following stroke. PLoS One. 2011;6:e26288. doi: 10.1371/journal.pone.0026288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sieber MW, Guenther M, Jaenisch N, Albrecht-Eckardt D, Kohl M, Witte OW, Frahm C. Age-specific transcriptional response to stroke. Neurobiol Aging. 2014;35:1744–1754. doi: 10.1016/j.neurobiolaging.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 87.Shapira S, Sapir M, Wengier A, Grauer E, Kadar T. Aging has a complex effect on a rat model of ischemic stroke. Brain Res. 2002;925:148–158. doi: 10.1016/s0006-8993(01)03270-x. [DOI] [PubMed] [Google Scholar]
- 88.Kleine TO, Benes L. Immune surveillance of the human central nervous system (CNS): different migration pathways of immune cells through the blood-brain barrier and blood-cerebrospinal fluid barrier in healthy persons. Cytometry A. 2006;69:147–151. doi: 10.1002/cyto.a.20225. [DOI] [PubMed] [Google Scholar]
- 89.Sellers RS, Clifford CB, Treuting PM, Brayton C. Immunological variation between inbred laboratory mouse strains: points to consider in phenotyping genetically immunomodified mice. Vet Pathol. 2012;49:32–43. doi: 10.1177/0300985811429314. [DOI] [PubMed] [Google Scholar]
- 90.Pennell LM, Galligan CL, Fish EN. Sex affects immunity. J Autoimmun. 2012;38:J282–291. doi: 10.1016/j.jaut.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 91.Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab. 2009;29:792–802. doi: 10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sun T, Plutynski A, Ward S, Rubin JB. An integrative view on sex differences in brain tumors. Cell Mol Life Sci. 2015;72:3323–3342. doi: 10.1007/s00018-015-1930-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg. 2015;109:9–15. doi: 10.1093/trstmh/tru167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Klein SL. Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. Bioessays. 2012;34:1050–1059. doi: 10.1002/bies.201200099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathological perspective. Am J Pathol. 2008;173:600–609. doi: 10.2353/ajpath.2008.071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barna M, Komatsu T, Bi Z, Reiss CS. Sex differences in susceptibility to viral infection of the central nervous system. J Neuroimmunol. 1996;67:31–39. doi: 10.1016/0165-5728(96)00022-7. [DOI] [PubMed] [Google Scholar]
- 97.Han X, Lundberg P, Tanamachi B, Openshaw H, Longmate J, Cantin E. Gender influences herpes simplex virus type 1 infection in normal and gamma interferon-mutant mice. J Virol. 2001;75:3048–3052. doi: 10.1128/JVI.75.6.3048-3052.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med. 2015;21:688–697. doi: 10.1038/nm.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zender L, Rudolph KL. Keeping your senescent cells under control. Aging (Albany NY) 2009;1:438–441. doi: 10.18632/aging.100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Young AR, Narita M. SASP reflects senescence. EMBO Rep. 2009;10:228–230. doi: 10.1038/embor.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Senovilla L, Galluzzi L, Zitvogel L, Kroemer G. Immunosurveillance as a regulator of tissue homeostasis. Trends Immunol. 2013;34:471–481. doi: 10.1016/j.it.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 102.Salama R, Sadaie M, Hoare M, Narita M. Cellular senescence and its effector programs. Genes Dev. 2014;28:99–114. doi: 10.1101/gad.235184.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sagiv A, Krizhanovsky V. Immunosurveillance of senescent cells: the bright side of the senescence program. Biogerontology. 2013;14:617–628. doi: 10.1007/s10522-013-9473-0. [DOI] [PubMed] [Google Scholar]
- 104.Ovadya Y, Krizhanovsky V. Senescent cells: SASPected drivers of age-related pathologies. Biogerontology. 2014;15:627–642. doi: 10.1007/s10522-014-9529-9. [DOI] [PubMed] [Google Scholar]
- 105.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thome JJ, Farber DL. Emerging concepts in tissue-resident T cells: lessons from humans. Trends Immunol. 2015;36:428–435. doi: 10.1016/j.it.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, Bickham KL, Lerner H, Goldstein M, Sykes M, Kato T, Farber DL. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.