Abstract
The neutralization of alpha-4 integrin (Itga4) is currently used as treatment in multiple sclerosis. While most studies focused on its function on lymphocyte migration to the central nervous system, we have uncovered the importance of Itga4 expression on B cells for the generation of regulatory B cells in peripheral immune organs and their control of pathogenic T cell response and CNS pathology. Our study underscores the importance of looking at the dual role of B cells in CNS autoimmunity and provides important perspectives regarding the efficacy and side effects associated with Itga4 neutralization and other B cell targeting therapies.
Introduction
Multiple sclerosis (MS) is an immune-mediated disorder of the central nervous system (CNS) characterized by multifocal areas of leukocyte infiltration, demyelination, and axonal damage (1). Effector CD4+ T cells play an important role in the initiation and progression of the disease. CD4+ T cells provide help to B cells for their maturation into high affinity self-reactive B cells (2). Autoreactive B cells also promote the expansion of autoreactive CD4+ T cells through efficient antigen presentation and release of pro-inflammatory cytokines (2). In addition to disease-promoting activities, emerging evidence support the notion that B cells can have regulatory functions (3–6). Pathogenic T cells are controlled by different regulatory mechanisms, which include, regulatory T cells (Treg) and B cells (Breg) (7, 8). Bregs produce regulatory cytokines and express inhibitory molecules that suppress pathogenic T cells and autoreactive B cells (3, 5, 6, 9). Recently, IL-10- and IL-35-producing Bregs have been shown to control the development MS and its animal model experimental autoimmune encephalomyelitis (EAE) (5, 10), as well as establishing of chronic infections (5). However, little is known about the factors, which are necessary for the generation and stability of these Bregs. A humanized monoclonal antibody against the Itga4 subunit of the Very Late Antigen 4 (VLA-4, Natalizumab) is currently used as MS-modifying therapy (1). Although studies have focused primarily upon its capacity to prevent the migration of lymphocytes into the CNS during the progression of CNS autoimmunity (1), it can also affect the homing of lymphocytes to lymphoid organs (11). In addition, despite its efficacy and overall safety profile, it has been associated with the development of progressive multifocal leukoencephalopathy (PML), a severe disorder caused by JC virus (JCV) infection of the CNS (12). To improve our understanding of the mechanism of action of Natalizumab and the possible risk factors for PML development, we have addressed the role of Itga4 neutralization on Breg functions during the course of EAE.
Materials and methods
Mice
Mice used in this study were all on the C57BL/6 background. Itga4fl/fl mice were crossed with CD19Cre mice obtained from the Jackson Laboratories. Heterozygous CD19Cre were used in this study. Deletion of Itga4 in Itga4fl/fl CD19Cre mice was efficient and equivalent in all B cells subsets in the spleen (Transitional, Follicular, and marginal zone) as determined by flow cytometry. All animals were bred and maintained under specific pathogen-free conditions at the Benaroya Research Institute (Seattle, WA). All experiments have been approved and were performed in accordance with the guidelines of the Benaroya Research Institute Animal Care and Use Committee.
Immunization and EAE induction
EAE was induced by subcutaneous immunization with an emulsion of 150μg of MOG35-55 peptide in CFA supplemented with 4mg/ml of M. tuberculosis extract H37 Ra (Difco). In addition, the animals received 200ng of pertussis toxin intraperitoneally on day 0 and 2 after immunization. In B cell transfer experiments, mice were sublethally irradiated (400rad) and injected intravenously at day −1 before immunization with 10×106 untouched B cells (Stem Cell). Animals were monitored daily for development of EAE with a 0- to 6-point scoring system, as follows: 0, normal; 1, flaccid tail; 2, impaired righting reflex and/or gait; 3, partial hind limb paralysis; 4, total hind limb paralysis; 5, hind limb paralysis with partial fore limb paralysis; 6, moribund state.
Cell isolation and flow cytometry
CNS mononuclear cells were isolated as previously described (7). Intracellular cytokine staining was performed as described previously (7). Cells were acquired on LSRII (BD Biosciences), and data were analyzed with FlowJo software. Antibodies were purchased from eBioscience and Biolegend.
T-cell proliferation
Mice were immunized subcutaneously with 150μg of MOG35-55 emulsified in CFA without PT. Draining lymph node cells were collected 8 days after immunization. Cells were cultured at 5×106 cells/ml in RPMI in the presence of increasing concentrations of MOG35-55 for 72h. During the last 16h, cells were pulsed with 1μCi of [3H] thymidine. [3H] thymidine incorporation was measured using a β-counter.
Statistical analysis
The Two-Way Anova was used for statistical comparison of clinical EAE scores. The One-Way Anova was applied for statistical analysis of all the other experiments (*p<0.05; **p<0.01; #<0.005).
Results
To assess the importance of Itga4 blockage on Breg functions, we used myelin oligodendrocyte glycoprotein peptide (MOG35-55)-induced EAE. In this model, pathogenic Th1 and Th17 cells are generated and regulatory B cells are controlling disease severity(3, 13). We immunized mice with specific deletion of Itga4 on B cells (CD19Cre Itga4fl/fl mice) with MOG35-55 and followed clinical signs of EAE. The majority of control mice developed a monophasic disease with ascending paralysis between days 12 and 16 after immunization but their clinical signs improved after day 21 (Fig. 1A). In contrast, CD19Cre Itga4fl/fl mice developed more severe EAE than control mice between day 12 and 21 and maintained higher clinical scores than control mice during recovery (1.94 ± 0.24 for CD19Cre vs 2.84 ± 0.21 for CD19Cre Itga4fl/fl mice at day 26; Fig. 1A).
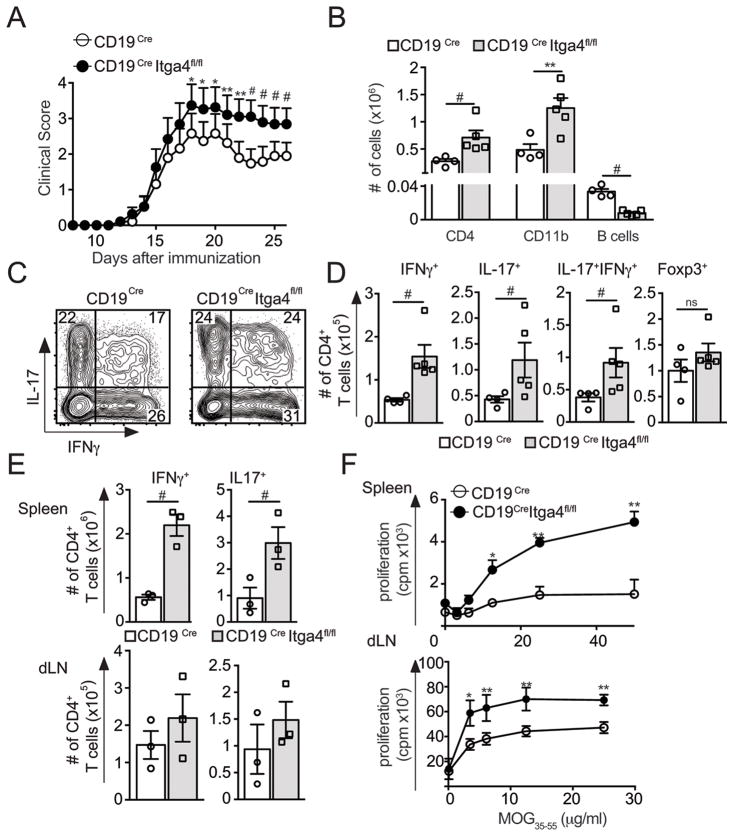
Figure 1. Deletion of Itga4 in B cells leads to EAE exacerbation.
(A–D) EAE was induced in CD19Cre Itga4fl/fl mice and CD19Cre mice. The mean clinical score is shown for each group over time (± SEM). (B) At the peak of the disease, CNS cells were collected. Surface and intracellular cytokine staining of CNS-infiltrating CD4+ T cells were performed and absolute numbers of CNS-infiltrating CD4+ T cells, CD11b+ cells and CD19+ B cells were determined. (C) The plots show the percentages of IL17+, IFNγ+ cells gated on CD4+ T cells. (D) Absolute numbers of Th1 (IFNγ+IL17−), Th17 (IFNγ−IL17+), Th1-17 (IFNγ+IL17+) and Tregs (CD4+ Foxp3+) in the CNS of CD19Cre Itga4fl/fl mice (grey) and CD19Cre mice (white) were determined from 2 independent experiments with 5 mice per group. (E–F) Spleen and draining lymph node (dLN) cells were collected from MOG35-55-immunized mice 8 days after immunization and an intracellular cytokine staining on CD4+ T cells was performed. (E) Mean absolute numbers of IL17+and IFNγ+ producing CD4+ T cells in the spleen (top) and dLN (bottom) for both groups. (F) Proliferative response after MOG restimulation of splenic and dLN cells isolated from MOG35-55-immunized CD19Cre Itga4fl/fl mice and CD19Cre mice was measured by [3H] thymidine incorporation. Results are representative of 2 independent experiments. Statistical significance was designated as follows: * p<0.05 **; p<0.01 and # p<0.005.
To determine the immunological mechanism leading to the increased severity of EAE observed in CD19Cre Itga4fl/fl mice, we investigated the composition of CNS-infiltrating immune cells. Consistent with the enhanced severity of the disease in CD19Cre Itga4fl/fl mice, we observed an increase in the number of CNS-infiltrating CD4+ T cells and CD11b+ macrophages in those mice compared to control mice (Fig. 1B). The increased number of CD4+ T cells reflected an overall increase in all subsets of effector T cells: IL-17+, IFNγ+, and IL-17+ IFNγ+ T cells (Fig. 1C and D). The absolute number of CNS infiltrating Foxp3+ Treg cells was similar between CD19Cre Itga4fl/fl mice and CD19Cre mice, excluding the possibility that the enhanced disease susceptibility in CD19Cre Itga4fl/fl mice was due to a decrease number of Tregs infiltrating the CNS (Fig. 1D). In contrast to an increase in pathogenic CD4+ T cells number, we observed a significant decrease in the number of CNS-infiltrating B cells in CD19Cre Itga4fl/fl mice compared to control mice (Fig. 1B).
This suggests that the presence of B cells in the CNS might be important for the control of pathogenic T cell responses in the target tissue. In this case, pathogenic T cells should be generated at the same frequency in the periphery but their number would be increased in the CNS where Bregs are lacking. Alternatively, the absence of B cells in the CNS might reflect a defect in a subset of B cells that exhibit regulatory functions in the periphery. In this case, we should see an enhanced pathogenic T cell response generated in the periphery. To determine which one of these possibilities was at play, we immunized CD19Cre and CD19Cre Itga4fl/fl mice with MOG35-55 and evaluated MOG-specific CD4+ T cell proliferation and cytokine production 8 days after immunization. We found increased frequencies and absolute numbers of IL-17 and IFNγ producing CD4+ T cells in the spleen and LNs of CD19Cre Itga4fl/fl mice compared to control CD19Cre mice (Fig. 1E). Furthermore, proliferation of splenic and dLN cells from CD19Cre Itga4fl/fl mice in response to MOG35-55 was significantly increased compared to control CD19Cre mice (Fig. 1F). Collectively, these data show that the enhanced frequency of pathogenic IL-17+ and IFNγ+ T cells found in the CNS of CD19Cre Itga4fl/fl mice, and associated with enhanced disease development, originates in the secondary lymphoid organs. It further suggests that Itga4 could interfere with the development and/or function of B cells in secondary lymphoid organs.
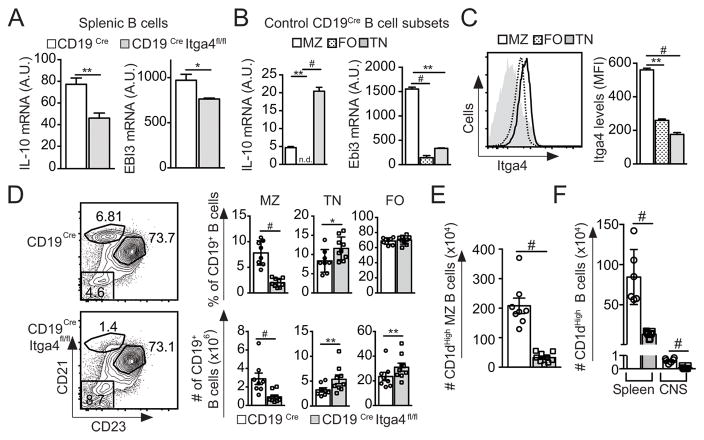
Populations of Bregs that have a protective role during EAE were shown to produce either IL-10 or IL-35 (3, 5, 6). Therefore, we determined whether B cells from CD19Cre Itga4fl/fl mice could produce these regulatory cytokines. Upon analysis, Itga4-deficient splenic B cells produce significantly less IL-10 and Ebi3 (a subunit of IL35) than control B cells (Fig. 2A). In the spleen of naive WT mice, marginal zone (MZ), transitional (TN) and follicular (FO) B cells differ in their ability to produce inflammatory and regulatory cytokines (14). Splenic B cell subsets were isolated from naive control CD19Cre mice to determine which ones produced immunosuppressive cytokines. TN and MZ B cells in naive mice were the main producers of IL-10 and Ebi3 (Fig. 2B). In addition, we compared Itga4 expression on B cell subsets in naive mice and observed that MZ B cells express the highest levels of Itga4 compared to FO and TN B cells (Fig. 2C). This differential expression of regulatory cytokine production by splenic B cells and enhanced Itga4 expression by MZ B cells prompted us to evaluate the distribution of B cell subsets in the spleen of CD19Cre Itga4fl/fl mice. Comparative analysis of B cell distribution in the spleen revealed that the B cell MZ compartment (B220+ CD21High CD23Low/neg) was severely compromised in CD19Cre Itga4fl/fl mice compared to CD19Cre control mice (Fig. 2D). This drastic reduction of MZ B cell number was associated with an increased frequency of TN B cells (B220+ CD21Low CD23neg) and a slight increased frequency of FO B cells (B220+ CD21+ CD23+) (Fig. 2D). Previous analysis demonstrated that Bregs and in particular IL-10-producing MZ B cells express high levels of CD1d (10). Therefore, we analyzed the proportion of B220+ CD21+ CD23− CD1dHigh B cells in CD19Cre Itga4fl/fl mice and their control. As shown in Fig. 2E, naive CD19Cre Itga4fl/fl mice had fewer CD1dHigh MZ B cells compared to CD19Cre control mice. Accordingly, during EAE, splenic B cells expressing high levels of CD1d were significantly decreased in the spleen of CD19Cre Itga4fl/fl mice compared to CD19Cre control mice (Fig. 2F). Furthermore, phenotypical analysis of CNS-infiltrating B cells in control CD19Cre mice showed that these CD1dhi Breg cells were 100 times less numerous in the CNS than in the spleen of control mice with EAE (Fig. 2F).
Figure 2. Itga4 expression by B cells is crucial for the presence of regulatory B cell in the spleen.
Splenocytes were collected from naïve CD19Cre Itga4fl/fl mice and CD19Cre mice. (A) B cells were isolated and IL-10 and Ebi3 mRNA relative expression were determined by quantitative PCR. (*p<0.05; **p<0.01 and #p<0.005). (B) IL-10 and Ebi3 mRNA relative expression on sorted marginal zone (MZ: B220+CD21HighCD23Low), follicular (FO: B220+CD21+CD23+) and transitional (TN: B220+CD21−CD23−) B cells from CD19Cre mice. (C) Expression of Itga4 expression on MZ (black line), FO (dotted line) and TN B cells (grey histogram) from CD19Cre mice as identified in B. (D) Representative CD21 and CD23 expression gated on CD19+ B cells from CD19Cre Itga4fl/fl mice (bottom) and CD19Cre mice (top) to define three B cell subsets: MZ (CD21HighCD23−), TN (CD21negCD23+) and FO (CD21+CD23+). Mean frequency (top) and absolute numbers (bottom) of each B cell subsets from CD19Cre Itga4fl/fl mice (grey) and CD19Cre mice (white) determined from 3 independent experiments with 7–10 mice per group. (E) Absolute numbers of CD1d+ MZ B cells from naive CD19Cre Itga4fl/fl mice and CD19Cre mice. (F) Absolute numbers of CD1d+ among B cells in the spleen and CNS of CD19Cre Itga4fl/fl mice and CD19Cre mice at the peak of EAE. Statistical significance was designated as follows: * p<0.05 **; p<0.01 and # p<0.005.
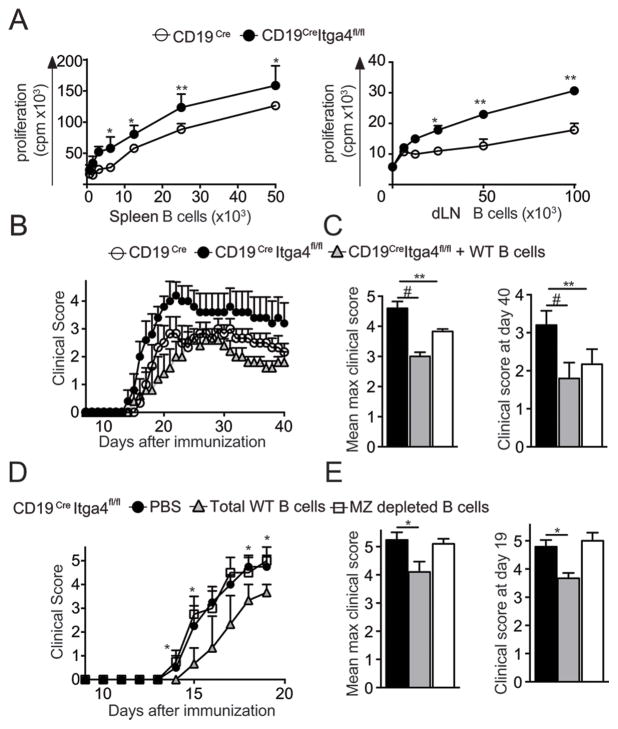
Therefore, our data suggest that a significant portion of regulatory B cell activity might occur in the periphery and might be compromised in CD19Cre Itga4fl/fl mice. To test this hypothesis, we isolated B cells from the spleen of MOG35-55-immunized CD19Cre Itga4fl/fl or control mice and cultured them with naive MOG-specific CD4+ T cells (2D2) in vitro. We found that Itga4-deficient B cells, which did not contain MZ B cells, induced a stronger proliferation of MOG-specific CD4+ T cells than Itga4-competent B cells which had MZ B cells (Fig. 3A). In the LN, plasmablasts have been shown to regulate EAE severity through the control of T cell responses (4). Thus, we have determined how B cells isolated from the dLN influence CD4 T cell responses. We observed a similar increased proliferation of 2D2 CD4+ T cells after culture with Itga4 deficient B cells isolated from dLN of immunized CD19Cre Itga4fl/fl mice compared to CD19Cre mice (Fig. 3A). This was associated with a decrease frequency and absolute number of plasmablast CD138+ cells in the LN of CD19Cre Itga4fl/fl mice compared to control (data not shown). To address if the modulation in B cell populations in CD19Cre Itga4fl/fl mice could directly control EAE, we adoptively transferred WT splenic B cells into CD19Cre Itga4fl/fl mice before immunization. While CD19Cre Itga4fl/fl mice develop exacerbated EAE compared to control CD19Cre mice, the transfer of MZ containing WT B cells into CD19Cre Itga4fl/fl mice was able to reduce EAE severity to the level observed in CD19Cre mice which did not receive B cells (Fig. 3B, C). To confirm that MZ B cells were responsible for this beneficial effect, we transferred total WT splenic B cells or MZ depleted B cells into CD19Cre Itga4fl/fl mice immunized for the development of EAE. While the transfer of splenic B cells reduced EAE severity (Fig. 3D) as seen earlier (Fig. 3B, C), the transfer of MZ depleted B cells did not control EAE progression and severity (Fig. 3D, E). Therefore, these data highlight the crucial role of Itga4 expression for the regulatory function initiated by MZ B cells during CNS autoimmunity.
Figure 3. Itga4 sufficient B cells can control EAE severity in CD19Cre Itga4fl/fl mice.
(A) Proliferative response of 2D2 Tg CD4+ T cells (MOG35-55 TCR Tg mice) stimulated with MOG35-55 and increasing numbers of B cells isolated from spleen (left) or dLN (right) of MOG35-55-immunized CD19Cre Itga4fl/fl mice (filled circles) and CD19Cre mice (open circles) was measured by [3H] thymidine incorporation. Results are representative of 2 independent experiments with 4 mice. (B) One group of CD19Cre Itga4fl/fl mice was injected intravenously one day prior to immunization with 10×106 untouched B cells isolated from spleen of WT mice (grey triangles). Another group of CD19Cre Itga4fl/fl mice (closed circles) and a group of CD19Cre mice (open circles) received PBS. The next day, all mice were immunized for the development of EAE. The mean clinical score is shown for each group over time (± SEM). (C) Mean maximum clinical score and mean clinical score at the end of the experiment (day 40) were calculated for each group (n= 7 mice per group). (D) CD19Cre Itga4fl/fl mice received PBS (black circle) or 10×106 total WT B cells (grey triangles), or MZ depleted B cells (clear rectangle) as described in (B) and were immunized for EAE development. The mean clinical score is shown for each group over time (± SEM). (E) Mean maximum clinical score and mean clinical score at the day 19 were calculated for each group. Statistical significance was designated as follows: * p<0.05 **; p<0.01 and # p<0.005.
Discussion
A dual role for B cells in EAE and MS is now emerging (2, 8). On one hand, B cells contribute to disease pathogenesis through anti-myelin antibodies production that can induce demyelination, presentation of myelin antigen and production of pro-inflammatory cytokines (2). On the other hand, B cells can control disease progression and severity through the production of immune-regulatory cytokines and/or the induction of other regulatory cells (13, 15). Since the role of B cells in CNS autoimmunity is complex and not all B cell subsets fulfill similar functions, it becomes important to address how current MS-modifying therapies affect B cell populations.
Natalizumab, a humanized monoclonal antibody against the integrin alpha 4 subunit of the Very Late Antigen 4 (VLA-4), is currently used as a disease-modifying therapy for MS (1). Most studies have focused on the importance of Itga4 for the trafficking and migration of lymphocytes to the CNS (7, 16). In this study, we have addressed the role of Itga4 neutralization on the generation and function of Bregs. The balance between pathogenic B cells and Bregs in EAE can be modulated by the immunization regimen. Indeed, immunization of C57BL/6 mice with recombinant human MOG (rhMOG) protein induces B cell activation and production of anti-MOG antibodies, which are pathogenic in association with T cell–mediated CNS inflammation (17, 18). Consistent with a pathogenic role of B cells after rhMOG immunization and the capacity of Itga4 neutralization to block the entry of lymphocytes in the CNS, a recent report in this journal showed that CD19Cre Itga4fl/fl mice immunized with rhMOG have a modest reduction in EAE susceptibility (16). In our study, we have immunized mice with MOG35-55 peptide, which does not elicit a strong humoral response and does not generate pathogenic B cells since EAE development is not abrogated in B cell deficient animals (3, 13). In contrast to CD19Cre Itga4fl/fl mice immunized with rhMOG (16), CD19Cre Itga4fl/fl mice immunized with MOG35-55 peptide develop more severe and sustained EAE than control mice. Similarly to the previous study, we observed a requirement of Itga4 for the entry of B cells in the CNS. However, our data suggest that Itga4 is important to maintain regulatory B cell activity and that a significant portion of this Breg activity is occurring in the periphery. Indeed, enhanced CD4+ T cell proliferation and pathogenic cytokine production was observed in the peripheral lymphoid organs of CD19Cre Itga4fl/fl mice immunized with MOG35-55 and was associated with a decrease in MZ and MZ CD1dhi B cells in the spleen (Fig. 2A, B, E, F). During EAE, the number of CNS-infiltrating CD1dhi B cells was also reduced in the CNS of CD19Cre Itga4fl/fl mice compared to CD19Cre control mice (Fig. 2F) consistent with a general decrease in CNS infiltrating B cells (Fig. 1B). However, the CD1dhi B cell population was 100 times less abundant in the CNS than in the spleen of CD19Cre mice (Fig. 1F). Therefore, although we cannot completely rule out regulatory B cell activity in the CNS, we propose that an important part of the Breg immunoregulatory effects takes place in peripheral lymphoid organs. This would explain why some studies have failed to detect numerous Breg in the CNS (2). On the other hand, we have established a clear requirement for Itga4 expression on B cells for MZ (especially CD1dhi) B cell and IL-10 and IL-35-producing B cell presence in the spleen since these populations are dramatically compromised in the spleen of CD19Cre Itga4fl/fl mice. Furthermore, we have established that the regulatory cytokines IL-10 and IL-35 are preferentially expressed in the MZ B cell subset. Although MZ B cells are mostly spleen resident, they have been shown to recirculate (19). Upon immunization, a significant proportion of regulatory B cells nodes express CD138 in association or not with BLIMP1 (4, 5) in the spleen and LN where they can limit T cell responses. In support to this hypothesis, the frequencies of CD138+ (BLIMP1+ and BLIMP1−) B cells present in the LN and spleen of CD19Cre Itga4fl/fl mice after immunization with MOG35-55 was significantly decreased compared to CD19Cre Het mice (data not shown). Therefore, the lack of B cell subsets with regulatory potential in the lymphoid organs of CD19Cre Itga4fl/fl mice together with the increase proliferative response of LN and spleen cells to MOG35-55 peptide indicate that, both lymphoid organs can be the site of active control of these responses by regulatory B cells. They further indicate that the regulation of the immune response requires properly organized lymphoid tissues and/or the maturation of MZ B cells in the spleen.
Despite its efficacy and overall good safety profile, Natalizumab therapy has been associated with the development of PML, a severe disorder caused by JC virus infection of the CNS (20). MZ B cells are often considered innate immune cells capable of mounting a quick and efficient T cell independent response against blood-born pathogens (19). Although our study does not address this point directly, it is possible that the retention of MZ B cells in the spleen through the expression of Itga4 might be required for efficient JC virus containment and latency. This will be an important area of investigation and may shed light on the complex dissemination of this virus in the body of immunocompromised individuals.
Importantly, our study, which links Itga4 to MZ and Bregs should promote further investigation on the role of MZ B cells in the ontogeny of Bregs during CNS autoimmunity and infection to further improve current MS-modifying therapies.
Acknowledgments
SG received a fellowship grant from the National Multiple Sclerosis Society (NMSS) (FG 2019). EB received research grant support from the NMSS (RG 4486) and National Institutes of Health (R21 NS077116, R01 NS081687).
We Thank Dr. T. Papayannopoulou and Dr. Oukka for providing the CD19Cre Itga4fl/fl mice.
Footnotes
Competing financial interests
The authors declare no competing financial interest.
References
- 1.Steinman L. Immunology of relapse and remission in multiple sclerosis. Annual review of immunology. 2014;32:257–281. doi: 10.1146/annurev-immunol-032713-120227. [DOI] [PubMed] [Google Scholar]
- 2.Mann MK, Ray A, Basu S, Karp CL, Dittel BN. Pathogenic and regulatory roles for B cells in experimental autoimmune encephalomyelitis. Autoimmunity. 2012;45:388–399. doi: 10.3109/08916934.2012.665523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nature immunology. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto M, Baba A, Yokota T, Nishikawa H, Ohkawa Y, Kayama H, Kallies A, Nutt SL, Sakaguchi S, Takeda K, Kurosaki T, Baba Y. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity. 2014;41:1040–1051. doi: 10.1016/j.immuni.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, Ries S, Dang VD, Jaimes Y, Daridon C, Li R, Jouneau L, Boudinot P, Wilantri S, Sakwa I, Miyazaki Y, Leech MD, McPherson RC, Wirtz S, Neurath M, Hoehlig K, Meinl E, Grutzkau A, Grun JR, Horn K, Kuhl AA, Dorner T, Bar-Or A, Kaufmann SH, Anderton SM, Fillatreau S. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507:366–370. doi: 10.1038/nature12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, Wingfield PT, Kim SH, Egwuagu CE. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nature medicine. 2014;20:633–641. doi: 10.1038/nm.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glatigny S, Duhen R, Arbelaez C, Kumari S, Bettelli E. Integrin alpha L controls the homing of regulatory T cells during CNS autoimmunity in the absence of integrin alpha 4. Scientific reports. 2015;5:7834. doi: 10.1038/srep07834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fillatreau S, Gray D, Anderton SM. Not always the bad guys: B cells as regulators of autoimmune pathology. Nature reviews Immunology. 2008;8:391–397. doi: 10.1038/nri2315. [DOI] [PubMed] [Google Scholar]
- 9.Ray A, Basu S, Williams CB, Salzman NH, Dittel BN. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol. 2012;188:3188–3198. doi: 10.4049/jimmunol.1103354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Lu TT, Cyster JG. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 2002;297:409–412. doi: 10.1126/science.1071632. [DOI] [PubMed] [Google Scholar]
- 12.Tan CS, I, Koralnik J. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. The Lancet Neurology. 2010;9:425–437. doi: 10.1016/S1474-4422(10)70040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185:2240–2252. doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barr TA, Brown S, Ryan G, Zhao J, Gray D. TLR-mediated stimulation of APC: Distinct cytokine responses of B cells and dendritic cells. European journal of immunology. 2007;37:3040–3053. doi: 10.1002/eji.200636483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov EI, Spolski R, Poe JC, Leonard WJ, Tedder TF. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491:264–268. doi: 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehmann-Horn K, Sagan SA, Bernard CC, Sobel RA, Zamvil SS. B-cell very late antigen-4 deficiency reduces leukocyte recruitment and susceptibility to central nervous system autoimmunity. Annals of neurology. 2015;77:902–908. doi: 10.1002/ana.24387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molnarfi N, Schulze-Topphoff U, Weber MS, Patarroyo JC, Prod’homme T, Varrin-Doyer M, Shetty A, Linington C, Slavin AJ, Hidalgo J, Jenne DE, Wekerle H, Sobel RA, Bernard CC, Shlomchik MJ, Zamvil SS. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. The Journal of experimental medicine. 2013;210:2921–2937. doi: 10.1084/jem.20130699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliver AR, Lyon GM, Ruddle NH. Rat and human myelin oligodendrocyte glycoproteins induce experimental autoimmune encephalomyelitis by different mechanisms in C57BL/6 mice. J Immunol. 2003;171:462–468. doi: 10.4049/jimmunol.171.1.462. [DOI] [PubMed] [Google Scholar]
- 19.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annual review of immunology. 2009;27:267–285. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 20.Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, Lee S, Plavina T, Scanlon JV, Sandrock A, Bozic C. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. The New England journal of medicine. 2012;366:1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]