Abstract
Objective
Pulmonary complications are common following hematopoietic stem cell transplantation (HSCT). Numerous idiopathic post-transplantation pulmonary syndromes have been described. Patients at the severe end of this spectrum may present with hypoxemic respiratory failure and pulmonary infiltrates, meeting criteria for Acute Respiratory Distress Syndrome (ARDS). The incidence and outcomes of ARDS in this setting are poorly characterized.
Design
Retrospective cohort study
Setting
Mayo Clinic, Rochester, MN.
Patients
Patients undergoing autologous and allogeneic hematopoietic stem cell transplantation between 1/1/2005 and 12/31/2012.
Interventions
None
Measurements and main results
Patients were screened for ARDS development within one year of HSCT. ARDS adjudication was performed in accordance with the 2012 Berlin criteria. In total, 133 cases of ARDS developed in 2635 patients undergoing HSCT (5.0%). ARDS developed in 75 (15.6%) patients undergoing allogeneic HSCT and 58 (2.7%) patients undergoing autologous HSCT. Median time to ARDS development was 55.4 days (IQR: 15.1 to 139 days) in allogeneic HSCT and 14.2 days (IQR: 10.5 to 124 days) in autologous HSCT. 28-day mortality was 46.6%. At 12 months following HSCT, 89 (66.9%) patients who developed ARDS had died. Only 7 of 133 ARDS cases met criteria for engraftment syndrome, and 15 for diffuse alveolar hemorrhage.
Conclusions
ARDS is a frequent complication following HSCT, dramatically influencing patient-important outcomes. Most cases of ARDS following HSCT do not meet criteria for a more specific post-transplantation pulmonary syndrome. These findings highlight the need to better understand the risk factors underlying ARDS in this population, thereby facilitating the development of effective prevention strategies.
Keywords: ARDS, bone marrow transplant, BMT, hematopoietic stem cell transplantation, engraftment syndrome, respiratory failure
Introduction
More than 50,000 hematopoietic stem cell transplantations (HSCTs) are performed worldwide annually, primarily for the treatment of hematologic malignancies (1). HSCT is the process by which a recipient’s bone marrow is reconstituted with hematopoietic progenitor cells following pre-transplant ‘conditioning’ designed to deplete the existing bone marrow through a combination of high-dose chemotherapy and/or radiation. Progenitor cells may be from the patient (autologous) or from an HLA-matched donor (allogeneic). Over the past 20 years, there has been a steady improvement in morbidity and mortality following HSCT, attributed to advances in post-transplant supportive care, organ support, HLA-matching and treatment of infectious diseases (2). However, the risks of HSCT remain considerable, and with the steady increase in the number of HSCTs performed, the public health impact is substantial (3).
Pulmonary complications are a major source of morbidity and mortality following HSCT (4–6). These complications may be both infectious and noninfectious, often presenting with nonspecific diffuse lung infiltrates and hypoxemic respiratory failure. To better characterize these nonspecific presentations, a multitude of idiopathic and clinically overlapping lung injury syndromes have been described in the post-transplant period, including diffuse alveolar hemorrhage (7–9), engraftment syndrome (10, 11) and idiopathic pneumonia syndrome (5, 12, 13). Crucially, in the absence of well-defined pathological and diagnostic criteria, these syndromes often remain arbitrary and retrospective diagnoses of exclusion with limited clinical relevance at the time of initial hospital admission. Therefore, it may be more meaningful clinically to consider the severe end of the clinical spectrum of post-HSCT hypoxemic respiratory failure in the context of acute respiratory distress syndrome (ARDS), which has well characterized and clinical and pathophysiologic criteria, and may facilitate better understanding of the potentially devastating impact of respiratory failure following HSCT. To date, little data exist on the incidence and impact of developing ARDS in the post-transplant setting. In this retrospective cohort series, we evaluated the incidence of ARDS in all HSCT’s performed between 2005 and 2012 at a single academic center.
Materials and Methods
This investigation utilized a retrospective observational cohort study design. The study was approved by the Mayo Clinic Institutional Review Board (Rochester, Minnesota) prior to its initiation. The requirement for written informed consent was waived by the institutional review. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were used in the conduct of this study as well as in the reporting of our results (14).
Study Population
The study population was retrieved from a list of all patients who underwent autologous or allogeneic HSCT at Mayo Clinic Rochester, Minnesota between January 1 2005 and December 31 2012. All patients aged 18 years or greater and undergoing HSCT for any indication were included. For patients who underwent repeat HSCT, the first HSCT was used as the index event. Patients were excluded if they denied the use of their medical records for research, or had a diagnosis of acute lung injury or ARDS at the time of HSCT (i.e. ARDS was diagnosed prior to the day donor cells were given to the recipient). Outcomes of patients with ARDS were compared to those patients who underwent HSCT but did not develop ARDS. An additional analysis was performed comparing outcomes of patients with ARDS compared to patients admitted to an ICU without ARDS. To define this latter cohort, the first ICU admission greater than 24 hours in the year following HSCT was included for analysis. The study population is outlined in Figure 1.
Figure 1.
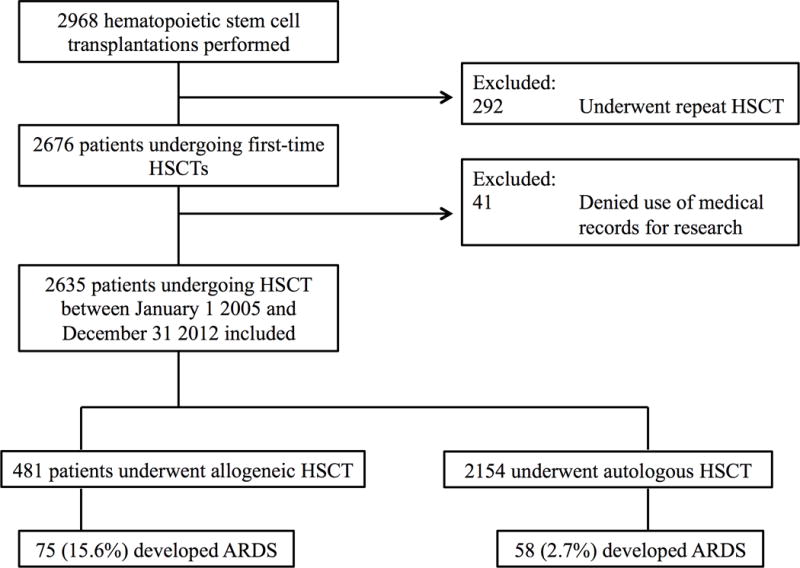
Study flow diagram.
Outcome variable
The primary outcome measure was the presence of ARDS within one year of transplant. ARDS was defined in accordance with the 2012 Berlin Criteria as acute (within one week of a known clinical insult) hypoxemic respiratory failure (arterial oxygen tension/fraction of inspired oxygen less than 300 mm Hg) requiring positive end expiratory pressure of 5cm H20 or greater with bilateral opacities on chest imaging that are not fully explained by cardiac failure or fluid overload (15).
The presence of ARDS was first identified by cross-referencing the database containing patients who have undergone HSCT with a second comprehensive database containing all patients who have screened positive for possible ARDS via a validated electronic syndrome surveillance tool known as the “ARDS sniffer.” This automated system identifies patients that within a 24-hour window have a) an arterial oxygen tension/fraction of inspired oxygen less than 300 mm Hg on arterial blood gas, and b) a chest radiograph report that contains the free text Boolean terms “edema” or “bilateral” and “infiltrate” (16). Where multiple arterial blood gas values exist, the worst value during the 24-hour window was selected. The ARDS sniffer has a negative predictive value of 99.6% (95% CI, 99.3 to 99.8) for identifying ARDS. Each screen-positive patient was independently reviewed by trained study investigators (HY, MEN, JKB). Investigators underwent a structured ARDS tutorial prior to reviewing any data the electronic medical record (EMR). All disagreements were referred to the senior investigator with substantial prior experience in ARDS adjudication (DJK). Secondary outcome measures included in-hospital, 28-day, and 12-month mortality, as well hospital and ICU lengths of stay.
Data retrieval
Data retrieval was performed with the help of a comprehensive institutional clinical research database, the Mayo Clinic Life Sciences System (MCLSS). MCLSS is a clinical data warehouse, developed in collaboration with Mayo Clinic and IBM that allows automated clinical data extraction through a query tool called the Data Discovery and Query Builder (DDQB). Data was also retrieved through an institutional Microsoft SQL-based database that retrieves variables for all ICU patients in near real-time (the ‘ICU datamart’). We have previously validated these data extraction techniques against manual data extraction (16–20). The majority of these variables were obtained using the validated search strategies described above. For variables lacking validated web-based extraction techniques, manual chart review was performed.
Statistical analysis
Categorical variables were summarized as frequency (%) and compared using chi squared tests or Fisher’s exact test, as appropriate. Continuous variables were expressed as mean ± standard deviation when normally distributed or median with interquartile range (IQR) when non-parametric. Continuous variables were compared using Student’s t-tests for parametric data or Wilcoxon analysis for nonparametric data. Time-to-event data were analyzed with the Kaplan–Meier method, and the groups were compared with the use of the log-rank test. Trend analysis was performed by linear regression, with statistical significance determined by t-statistic. In all final analyses, statistical significance was considered present when the two-sided hypothesis test p-value was less than 0.05. All statistical analyses were performed using JMP version 10 (Cary, NC).
Results
A total of 2635 patients underwent first-time HSCT between January 1, 2005 and December 31st 2012 (Figure 1). Of these, 481 patients underwent allogeneic HSCT and 2154 underwent autologous HSCT. The most common indication for allogeneic transplant was acute myeloid leukemia (180/481) and the most common indication for autologous HSCT was multiple myeloma (975/2154) (Table 1).
Table 1.
Baseline demographics of patients undergoing hematopoietic stem cell transplantation. ARDS = Acute Respiratory Distress Syndrome.
| ARDS | No ARDS | ||
|---|---|---|---|
| Baseline | Total | 133 | 2502 |
| characteristics | Male | 74 (55.6%) | 1506 (60.2%) |
| Age at transplant | 52.4 (12.0) | 55.7 (12.0) | |
| Type of transplant | Autologous | 58 (43.6%) | 2096 (83.7%) |
| Allogeneic | 75 (56.4%) | 406 (16.2%) | |
| Diagnoses | Acute lymphoblastic leukemia | 9 (6.8%) | 63 (2.5%) |
| Acute myeloid leukemia | 29 (21.8%) | 170 (6.8%) | |
| Amyloidosis | 12 (9%) | 287 (11.5%) | |
| Chronic lymphocytic leukemia | 5 (3.8%) | 45 (1.8%) | |
| Chronic myeloid leukemia | 6 (4.5%) | 21 (0.8%) | |
| Germ Cell Tumor | 0 (0%) | 13 (0.5%) | |
| Hodgkin’s Lymphoma | 3 (2.3%) | 122 (4.9%) | |
| Light Chain Deposition Disease | 0 (0%) | 8 (0.3%) | |
| Myelodysplastic Syndrome | 12 (9%) | 70 (2.8%) | |
| Multiple Myeloma | 28 (21.1%) | 964 (38.5%) | |
| Myelofibrosis | 0 (0%) | 15 (0.6%) | |
| Non-Hodgkins Lymphoma | 21 (15.8%) | 622 (24.9%) | |
| Other | 4 (3%) | 25 (1%) | |
| Plasma Cell Leukemia | 0 (0%) | 9 (0.4%) | |
| Primitive neuroectodermal tumor | 0 (0%) | 9 (0.4%) | |
| POEMS | 1 (0.8%) | 44 (1.8%) | |
| Severe Aplastic Anemia | 2 (1.5%) | 13 (0.5%) | |
| Testicular Cancer | 1 (0.8%) | 2 (0.1%) |
ARDS incidence and outcomes following HSCT
133 of the 2635 patients undergoing HSCT developed ARDS within 1 year of transplant, with a cumulative incidence of 5.0%. ARDS was more common following allogeneic HSCT, developing in 75 patients (15.6%) compared to 58 patients undergoing autologous HSCT (2.7%) (p < 0.001). The median time to ARDS development was 28.5 days (IQR: 11.9 to 133 days) following HSCT (Figure 2). The median time to ARDS development was significantly longer for those undergoing allogeneic HSCT (55.4 days, IQR: 15.1 to 139 days) compared to those undergoing autologous HSCT (14.2 days, IQR: 10.5 to 124 days, p = 0.03). For ARDS adjudication, inter-observer κ was 0.66 (95% CI: 0.55 – 0.76).
Figure 2.
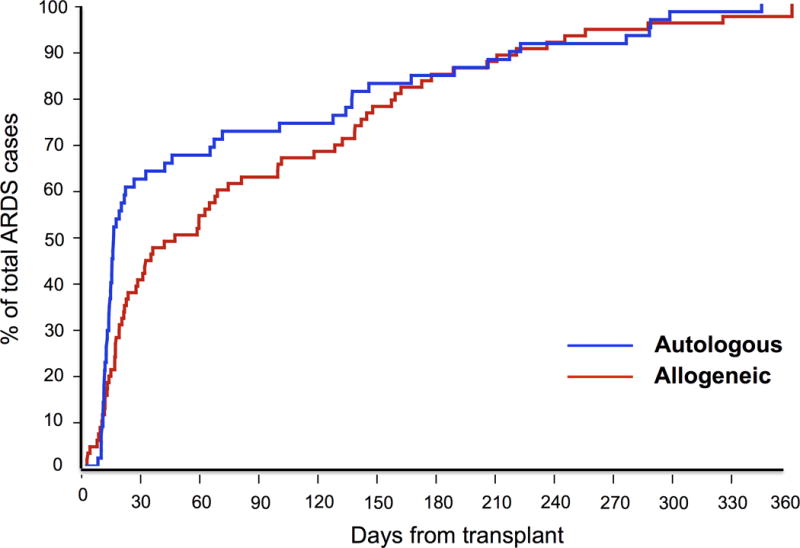
Time to Acute Respiratory Distress Syndrome development following hematopoietic stem cell transplantation for allogeneic and autologous transplants. Patients with autologous stem cell transplantation developed ARDS sooner than allogeneic stem cell transplantation.
Compared to those who did not develop ARDS following HSCT, the mortality of those developing ARDS was significantly higher at 28 days, 90 days and 1 year (Figure 3, Table 2). In patients who developed ARDS following HSCT, the mortality following transplant was generally higher for those undergoing allogeneic HSCT compared to those undergoing autologous HSCT. Specifically, mortality did not differ significantly at 28 days post-transplant but was significantly higher at 90 days post-transplant and at 1 year post-transplant.
Figure 3.
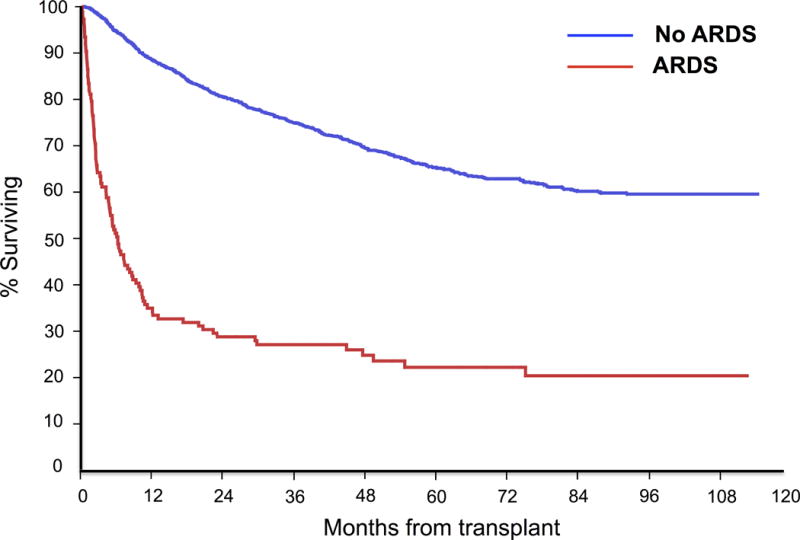
Overall survival following hematopoietic stem cell transplantation in patients who developed Acute Respiratory Distress Syndrome versus those who did not.
Table 2.
Mortality of patients undergoing HSCT and developing ARDS, compared to those who did not develop ARDS.
| Time | ARDS (n = 133) | No ARDS (n = 2505) | p | |
|---|---|---|---|---|
| Mortality | 28 days | 16 (12%) | 14 (0.6%) | < 0.001 |
| (following transplant) | 90 days | 48 (36.1%) | 57 (2.3%) | < 0.001 |
| 1 year | 89 (66.9%) | 295 (11.8%) | < 0.001 | |
| 5 years | 101 (75.9%) | 754 (30.1%) | < 0.001 |
Following development of ARDS, ICU mortality was 38.3% and hospital mortality was 57.1%. 28-day mortality following ARDS diagnosis was 46.6% (Table 3). ICU length of stay was 7.9 days (IQR: 2.2 to 13.2 days) and hospital length of stay was 25.3 days (IQR: 11.0 to 44.1 days).
Table 3.
Outcomes of patients developing Acute Respiratory Distress Syndrome, grouped by type of transplant. ARDS = Acute Respiratory Distress Syndrome. HSCT = Hematopoietic Stem Cell Transplant. SOFA = Sequential Organ Failure Assessment score. APACHE = Acute Physiology and Chronic Health Evaluation.
| Patients with ARDS following HSCT | p | ||
|---|---|---|---|
| Allogeneic HSCT (n = 481) |
Autologous HSCT (n = 2154) |
||
| Total number | 75 (15.6%) | 58 (2.7%) | < 0.001 |
| Age | 48.3 ± 11.8 | 58.0 ± 9.9 | < 0.001 |
| Male | 38 (52.3%) | 35 (60.3%) | 0.39 |
| Time to ARDS following HSCT (days) | 55.4 (15.1 – 139) | 14.2 (10.5 – 124) | 0.03 |
| ARDS Severity | |||
| Mild | 2 (2.7%) | 7 (12.1%) | 0.03 |
| Moderate | 14 (18.7%) | 16 (27.6%) | |
| Severe | 59 (78.7%) | 35 (60.0%) | |
| ARDS Risk Factors | |||
| Aspiration | 4 (5.3%) | 9 (15.5%) | 0.05 |
| Emergency / High Risk Surgery | 2 (2.7%) | 1 (1.7%) | 0.72 |
| Pancreatitis | 0 (0.0%) | 0 (0.0%) | N/A |
| Pneumonia | 60 (80%) | 45 (77.6%) | 0.74 |
| Sepsis | 53 (70.7%) | 49 (84.5%) | 0.06 |
| Shock | 45 (60%) | 44 (75.9%) | 0.06 |
| Trauma | 0 (0.0%) | 1 (1.7%) | 0.43 |
| Outcomes | |||
| ICU mortality | 36 (48.0%) | 15 (25.9%) | 0.009 |
| Hospital mortality | 54 (72.0%) | 22 (37.9%) | < 0.001 |
| 28-day mortality | 43 (57.3%) | 19 (32.8%) | 0.005 |
| ICU length of stay | 9.0 (3.3 – 15.0) | 6.8 (2.0 – 12.5) | 0.02 |
| ICU length of stay (survivors) | 8.0 (4.6 – 13.5) | 7.0 (2.0 – 11.2) | 0.35 |
| Hospital length of stay | 26.1 (11.0 – 47.2) | 23.9 (10.7 – 38.3) | 0.18 |
| Hospital length of stay (survivors) | 20.0 (9.6 – 57.2) | 24.7 (14.3 – 36.6) | 0.93 |
| APACHE-III score | 99.4 ± 29.4 | 95.8 ± 28.3 | 0.47 |
| SOFA score | 11.3 ± 4.1 | 10.0 ± 3.7 | 0.07 |
Outcomes were generally worse for patients following allogeneic transplantation compared to autologous transplantation (Table 3). Compared to patients developing ARDS following autologous HSCT, patients developing ARDS following allogeneic HSCT were more likely to have more severe ARDS (p = 0.03), had longer ICU length of stays [allogeneic: 9 days (IQR: 3.3 – 15.0) versus autologous: 6.8 days (2.0 – 12.5), p = 0.02], and had higher mortality in the ICU, hospital and at 28 days (Table 3). There were no significant differences in hospital length of stay, ICU admission APACHE-III or SOFA scores between the two groups. Among the 481 patients undergoing allogeneic transplantation, 215 (44.7%) received reduced intensity conditioning (RIC). Of those receiving RIC, 26 (12.1%) developed ARDS compared to 49 (18.4%) of patients who received regular conditioning regimens (p = 0.06).
The incidence of ARDS was stable over time (Table 4). There was no significant change in ICU mortality, in-hospital mortality, or 28-day mortality between 2005 and 2012. Severity of illness, assessed by SOFA and APACHE-3 scores, was also stable over time. While there was no change in ICU length of stay over time (p = 0.54), there was a significant decline in hospital length of stay (p = 0.02 for trend).
Table 4.
Trends over time in Acute Respiratory Distress Syndrome incidence and outcomes following hematopoietic stem cell transplantation. ARDS = Acute Respiratory Distress Syndrome. SOFA = Sequential Organ Failure Assessment score. APACHE = Acute Physiology and Chronic Health Evaluation
| Year of ARDS development | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | p (for trend) |
|---|---|---|---|---|---|---|---|---|---|
| ARDS cases per Year | 20 (6.4%) | 21 (6.9%) | 12 (4.3%) | 12 (4%) | 15 (4.9%) | 13 (3.9%) | 19 (4.6%) | 18 (4.6%) | 0.08 |
| Total number of transplants | 311 | 304 | 277 | 297 | 306 | 334 | 412 | 394 | |
| Mortality at 28 days following ARDS development | 9 (45%) | 10 (47.6%) | 6 (50%) | 6 (50%) | 6 (40%) | 7 (53.8%) | 7 (36.8%) | 10 (55.6%) | 0.82 |
| Death in ICU | 8 (40%) | 8 (38.1%) | 5 (41.7%) | 4 (33.3%) | 4 (26.7%) | 6 (46.2%) | 6 (31.6%) | 9 (50%) | 0.69 |
| Death in Hospital | 12 (60%) | 12 (57.1%) | 7 (58.3%) | 8 (66.7%) | 7 (46.7%) | 7 (53.8%) | 11 (57.9%) | 10 (55.6%) | 0.45 |
| ICU length of stay (median) | 6.6 | 5.9 | 12.5 | 6.8 | 5.4 | 9.9 | 9.1 | 8.6 | 0.54 |
| Hospital length of stay (median) | 33.3 | 27.5 | 29.2 | 20.6 | 21.4 | 25.3 | 25.0 | 15.9 | 0.02 |
| SOFA (mean) | 10.8 | 11.9 | 8.6 | 11.3 | 10.6 | 10.9 | 10.5 | 10.3 | 0.76 |
| APACHE-3 (mean) | 72.2 | 107.5 | 87.0 | 104.1 | 102.7 | 110.1 | 107.8 | 95.3 | 0.19 |
A subgroup of patients developing ARDS met diagnostic criteria for the idiopathic noninfectious pulmonary syndromes following HSCT. Specifically, 7 of 133 ARDS cases (5.3%) met criteria for engraftment syndrome and 15 of 133 (11.3%) for diffuse alveolar hemorrhage. The remainder did not meet diagnostic criteria for one of the defined noninfectious pulmonary syndromes.
Outcomes of patients developing ARDS compared to HSCT patients admitted to ICU without ARDS
Of 2635 patients undergoing HSCT, 540 patients were admitted to the ICU during the first year following transplantation (Table 5). A total of 133 (24.6%) had ARDS during ICU admission, while 407 (75.4%) did not develop ARDS. Compared to those ICU admissions with ARDS, patients admitted to the ICU without ARDS were older (56.6 ± 13.1 years versus 52.5 ± 12.0 years, p = 0.001). In terms of risk factors, patients who developed ARDS were more likely to have pneumonia [105 (78.9%) versus 87 (21.4%), p < 0.001], shock [89 (66.9%) versus 214 (52.6%), p = 0.03], sepsis [102 (76.7%) versus 272 (66.8%), p = 0.004] and an aspiration event [13 (9.8%) versus 4 (1%), p < 0.001]. Other ARDS risk factors (polytrauma, pancreatitis, emergency surgery or high-risk cardiac, aortic vascular or thoracic surgery) were uncommon in both groups. Severity of illness was greater in the group that developed ARDS compared to the ICU admissions who did not. SOFA score on day of admission was 10.7 ± 4.0 in the ARDS cohort compared to 6.0 ± 3.7 in the non-ARDS ICU cohort (p < 0.001). APACHE-3 score was 97.8 ± 28.9 in the ARDS cohort compared to 78.1 ± 24.6 in the non-ARDS ICU cohort (p < 0.001). Outcomes were significantly worse in ICU admissions that developed ARDS compared to those who did not. Specifically, the ARDS cohort had longer ICU length of stays [7.9 (2.2 – 13.2) days versus 1.3 (0.8 – 2.9) days, p < 0.001] and hospital length of stays [25.3 (11.0 – 44.1) days versus 9.0 (4.3 – 17.3) days, p < 0.001] compared to the non-ARDS ICU cohort. Both in-ICU mortality (51 (38.3%) versus 19 (4.7%), p < 0.001) and in-hospital mortality (76 (57.1%) versus 40 (9.8%), p < 0.001) were also higher in the ARDS cohort.
Table 5.
Outcomes of patients admitted to the intensive care unit within 1 year of hematopoietic stem cell transplantation. ARDS = Acute Respiratory Distress Syndrome. HSCT = Hematopoietic Stem Cell Transplant. SOFA = Sequential Organ Failure Assessment score. APACHE = Acute Physiology and Chronic Health Evaluation.
| Patients admitted to the ICU within 1 year following HSCT | |||
|---|---|---|---|
| ARDS | No ARDS | p | |
| Total number | 133 | 407 | |
| Age | 52.5 ± 12.0 | 56.6 ± 13.1 | 0.001 |
| Male | 73 (56.2%) | 237 (58.2%) | 0.6 |
| Time to ARDS following HSCT (days) | 28.5 (11.9 – 133) | n/a | |
| ARDS Severity | |||
| Mild | 9 (6.8%) | n/a | |
| Moderate | 30 (22.6%) | n/a | n/a |
| Severe | 94 (70.7%) | n/a | |
| ARDS Risk Factors | |||
| Aspiration | 13 (9.8%) | 4 (1%) | < 0.001 |
| Emergency / High Risk Surgery | 3 (2.3%) | 5 (1.2%) | 0.41 |
| Pancreatitis | 0 (0%) | 0 (0%) | n/a |
| Pneumonia | 105 (78.9%) | 87 (21.4%) | < 0.001 |
| Sepsis | 102 (76.7%) | 272 (66.8%) | 0.004 |
| Shock | 89 (66.9%) | 214 (52.6%) | 0.03 |
| Trauma | 1 (0.8%) | 0 (0%) | n/a |
| Outcomes | |||
| ICU mortality | 51 (38.3%) | 19 (4.7%) | < 0.001 |
| Hospital mortality | 76 (57.1%) | 40 (9.8%) | < 0.001 |
| 28-day mortality | 62 (46.6%) | 40 (9.8%) | < 0.001 |
| ICU length of stay | 7.9 (2.2 – 13.2) | 1.3 (0.8 – 2.9) | < 0.001 |
| ICU length of stay (survivors) | 7.5 (3.0 – 11.5) | 1.2 (0.8 – 2.8) | < 0.001 |
| Hospital length of stay | 25.3 (11.0 – 44.1) | 9.0 (4.3 – 17.3) | < 0.001 |
| Hospital length of stay (survivors) | 23.6 (13.1 – 38.6) | 8.8 (4.2 – 15.8) | < 0.001 |
| APACHE-III score | 97.8 ± 28.9 | 78.1 ± 24.6 | < 0.001 |
| SOFA score | 10.7 ± 4.0 | 6.0 ± 3.7 | < 0.001 |
Discussion
In this study, we defined the epidemiology and outcomes of ARDS following HSCT. The primary finding of this study is that the overall incidence of ARDS in the year following HSCT is 5.0%, with a higher incidence in those undergoing allogeneic HSCT compared to those undergoing autologous HSCT. The prognosis of those patients who develop ARDS following HSCT is poor, with a 1-year mortality of 66.8% compared to 11.8% in those who did not develop ARDS. A notable finding of our study was that the majority of patients developing ARDS following HSCT had pneumonia, sepsis or shock prior to the development of ARDS, with just 4 of 133 patients with ARDS not having one of these three risk factors. This highlights the importance and ongoing impact of infectious complications following HSCT, despite recent improvements in the management of infectious diseases in the post-HSCT period (2).
Pulmonary complications are a common source of morbidity and mortality in patients with hematological malignancies who undergo HSCT (4, 5). Such complications may be associated with a need for mechanical ventilation, extended ICU and hospital length of stays, concomitant organ failure and death (21). Little data exist regarding the epidemiology and outcomes of ARDS following HSCT. This study addresses that critical knowledge gap, and helps better define the incidence and impact of ARDS in the post-HSCT setting. However, prior studies have provided some data on ARDS in patients with solid or hematological malignancies in general. A post-hoc subgroup analysis of patients in the ARDS Network trials noted that cancer patients with ARDS had a poorer outcome than those without ARDS (22). In another post-hoc retrospective analysis of clinical trials by the French and Belgian critical care trials group from 1990 to 2011, investigators identified over a thousand patients with ARDS and a history of malignancy (23). In keeping with our study, the majority of patients with ARDS and malignancy had either a pulmonary or extrapulmonary infection prior to ARDS development, and prognosis was poor, with an in-hospital mortality of 64%. In a case series of patients with solid and hematological malignancies that developed during a period of neutropenia following chemotherapy administration, mortality was again substantial, with a 28-day mortality of 63% (24). However, just 14 of these patients had undergone prior HSCT at any point, and the timing of HSCT in relation to the development of ARDS was not specified. While these studies highlight the important unmet need in the care of cancer patients with ARDS, our study provides the most rigorous and complete data to date, allowing us to define incidence and outcomes more comprehensively.
While the impact of ARDS on patient-important outcomes remains substantial, data from the ARDS Network’s randomized controlled trials suggests that the overall mortality from ARDS is declining (25). This finding was mirrored at the enrolling site of the present investigation where we previously demonstrated a decline in ARDS-associated mortality between 2001 and 2008, despite increases in patient severity of illness and the prevalence of medical comorbidities (19). Moreover, the incidence of ARDS decreased by almost half, primarily the result of a fall in hospital-acquired ARDS. This decline in mortality and incidence of ARDS has been attributed to numerous practice changes, including the institution of low-tidal volume ventilation (26), fluid-conservative resuscitation (27), improved treatment of sepsis (28), restrictive blood transfusion (29) and institution of a male-predominant donor pool for plasma (30). While like-to-like comparisons can be challenging, the mortality following ARDS development in patients who have undergone HSCT in our study remains substantially higher than what has been reported in these other studies (19, 31). Notably, our study did not show improvements in ARDS incidence or mortality over the 8-year study period, highlighting that improvements in ARDS outcomes in other clinical settings have not yet translated to the HSCT population.
Our results suggest that the majority of ARDS develops during or after the engraftment phase following HSCT. Engraftment generally occurs earlier following autologous transplantation than allogeneic, and ARDS development follows a similar time course (Figure 2). In the minority of patients who develop ARDS during the neutropenic phase prior to engraftment, ARDS may occur through activation of alveolar macrophages (32).
Further study is needed to analyze specific risk factors for ARDS development in the HSCT population, including the presence of both known risk factors for ARDS such sepsis, shock, drug-related lung toxicities and pneumonia (18, 33), as well as yet undefined ARDS risk factors potentially unique to this population. For example, our study highlighted a trend towards lower incidence of ARDS in those patients receiving reduced intensity conditioning chemotherapy regimens compared to patients receiving standard myeloablative regimens. Given the growing number of HSCT performed annually, the high morbidity and mortality associated with ARDS development (Figure 3, Table 2) and the substantial healthcare utilization associated with such complications, ARDS in the post-HSCT population has a major public health impact warranting further investigation.
Our study has a number of strengths that deserve mention. It is the first observational study to specifically evaluate the incidence, mortality and other patient-important outcomes for patients who develop ARDS following HSCT. Defining the magnitude of the problem in this vulnerable and critically ill population is the crucial first step to further investigating ARDS in this clinical setting. Patients undergoing HSCT are a potentially fruitful population for ongoing investigation of ARDS prevention strategies as the clinical insult that results in ARDS (stem cell transplantation) is known in advance of developing the clinical syndrome of ARDS. This predictability may facilitate future trials evaluating promising ARDS prevention strategies. Given these patients often receive “multiple hits” in the post-transplant period, including transfusions, sepsis, shock, pneumonia and other infections, future observational studies in this population could potentially identify the differential contribution of each of these risk factors in developing ARDS. This can facilitate the identification of potentially preventable exposures prior to the development of ARDS, with the goal of reducing the incidence and impact of ARDS in the post-HSCT period (34). This study also utilized rigorous methods for ARDS adjudication and risk factor ascertainment when compared to prior studies.
Importantly, our study also has some limitations. The study population for this investigation was from a single academic medical center. This limited the overall sample size, the number of ARDS events and consequently the overall precision of the results. This additionally raises concern for referral and institution-specific bias as well as overall generalizability. This study was retrospective, and consequently we discovered ARDS events only if they occurred at our institution, which would potentially exclude patients who developed ARDS elsewhere. However, most HSCT patients are closely followed at our institution in the year following transplant, and frequently return if any post-transplant complications occur. Additionally, patients typically stay locally for the initial few months to facilitate the close clinical and laboratory monitoring required, and this is the time period where the majority of ARDS cases develop. Consequently, we feel that this study does capture the majority of ARDS cases that develop following HSCT. Nonetheless, since some cases may be missed, our estimated ARDS incidence of 5.0% in the year following HSCT may be conservative.
Conclusion
ARDS remains a frequent complication following HSCT, dramatically influencing patient survival and other patient-important outcomes. Patients undergoing allogeneic HSCT have a higher incidence of ARDS, and ARDS develops later, when compared to those undergoing autologous HSCT. Importantly, most cases of ARDS following HSCT do not meet criteria for a more specific post-transplantation pulmonary syndrome. These findings highlight the urgent need to better understand the risk factors underlying ARDS in this specific population, thereby facilitating the future development and implementation of effective prevention strategies.
Acknowledgments
We would like to posthumously thank Dr. Bekele Afessa for his pioneering work on pulmonary complications following HSCT.
Funding/support: None.
Copyright form disclosures: Dr. Kor received support for article research from the National Institutes of Health (NIH), consulted for NIH/NHLBI, and received royalties from Up-to-Date. His institution received grant support from NIH/NHLBI. Dr. Nolan is employed by the Mayo School of Graduate Medical Education (Residency employer) and received support for travel from the Mayo School of Graduate Medical Education (Support for academic conference attendance unrelated to this paper). Dr. Gajic disclosed work for hire.
Footnotes
Conflicts of interest: None
The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gratwohl A, Baldomero H, Aljurf M, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;303(16):1617–1624. doi: 10.1001/jama.2010.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afessa B, Abdulai RM, Kremers WK, et al. Risk factors and outcome of pulmonary complications after autologous hematopoietic stem cell transplant. Chest. 2012;141(2):442–450. doi: 10.1378/chest.10-2889. [DOI] [PubMed] [Google Scholar]
- 5.Afessa B, Litzow MR, Tefferi A. Bronchiolitis obliterans and other late onset non-infectious pulmonary complications in hematopoietic stem cell transplantation. Bone marrow transplantation. 2001;28(5):425–434. doi: 10.1038/sj.bmt.1703142. [DOI] [PubMed] [Google Scholar]
- 6.Chi AK, Soubani AO, White AC, et al. An update on pulmonary complications of hematopoietic stem cell transplantation. Chest. 2013;144(6):1913–1922. doi: 10.1378/chest.12-1708. [DOI] [PubMed] [Google Scholar]
- 7.Majhail NS, Parks K, Defor TE, et al. Diffuse alveolar hemorrhage and infection-associated alveolar hemorrhage following hematopoietic stem cell transplantation: related and high-risk clinical syndromes. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2006;12(10):1038–1046. doi: 10.1016/j.bbmt.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Solh M, Oommen S, Vogel RI, et al. A prognostic index for survival among mechanically ventilated hematopoietic cell transplant recipients. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2012;18(9):1378–1384. doi: 10.1016/j.bbmt.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Wanko SO, Broadwater G, Folz RJ, et al. Diffuse alveolar hemorrhage: retrospective review of clinical outcome in allogeneic transplant recipients treated with aminocaproic acid. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2006;12(9):949–953. doi: 10.1016/j.bbmt.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Capizzi SA, Kumar S, Huneke NE, et al. Peri-engraftment respiratory distress syndrome during autologous hematopoietic stem cell transplantation. Bone marrow transplantation. 2001;27(12):1299–1303. doi: 10.1038/sj.bmt.1703075. [DOI] [PubMed] [Google Scholar]
- 11.Marin D, Berrade J, Ferra C, et al. Engraftment syndrome and survival after respiratory failure post-bone marrow transplantation. Intensive Care Med. 1998;24(7):732–735. doi: 10.1007/s001340050653. [DOI] [PubMed] [Google Scholar]
- 12.Panoskaltsis-Mortari A, Griese M, Madtes DK, et al. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med. 2011;183(9):1262–1279. doi: 10.1164/rccm.2007-413ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda T, Hackman RC, Guthrie KA, et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood. 2003;102(8):2777–2785. doi: 10.1182/blood-2003-05-1597. [DOI] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 15.Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 16.Alsara A, Warner DO, Li G, et al. Derivation and validation of automated electronic search strategies to identify pertinent risk factors for postoperative acute lung injury. Mayo Clin Proc. 2011;86(5):382–388. doi: 10.4065/mcp.2010.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herasevich V, Yilmaz M, Khan H, et al. Validation of an electronic surveillance system for acute lung injury. Intensive Care Med. 2009;35(6):1018–1023. doi: 10.1007/s00134-009-1460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gajic O, Dabbagh O, Park PK, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183(4):462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G, Malinchoc M, Cartin-Ceba R, et al. Eight-year trend of acute respiratory distress syndrome: a population-based study in Olmsted County, Minnesota. Am J Respir Crit Care Med. 2011;183(1):59–66. doi: 10.1164/rccm.201003-0436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kor DJ, Warner DO, Alsara A, et al. Derivation and diagnostic accuracy of the surgical lung injury prediction model. Anesthesiology. 2011;115(1):117–128. doi: 10.1097/ALN.0b013e31821b5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groeger JS, Lemeshow S, Price K, et al. Multicenter outcome study of cancer patients admitted to the intensive care unit: a probability of mortality model. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1998;16(2):761–770. doi: 10.1200/JCO.1998.16.2.761. [DOI] [PubMed] [Google Scholar]
- 22.Soubani AO, Shehada E, Chen W, et al. The outcome of cancer patients with acute respiratory distress syndrome. J Crit Care. 2014;29(1):183 e187–183 e112. doi: 10.1016/j.jcrc.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Azoulay E, Lemiale V, Mokart D, et al. Acute respiratory distress syndrome in patients with malignancies. Intensive Care Med. 2014;40(8):1106–1114. doi: 10.1007/s00134-014-3354-0. [DOI] [PubMed] [Google Scholar]
- 24.Mokart D, van Craenenbroeck T, Lambert J, et al. Prognosis of acute respiratory distress syndrome in neutropenic cancer patients. Eur Respir J. 2012;40(1):169–176. doi: 10.1183/09031936.00150611. [DOI] [PubMed] [Google Scholar]
- 25.Erickson SE, Martin GS, Davis JL, et al. Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med. 2009;37(5):1574–1579. doi: 10.1097/CCM.0b013e31819fefdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 27.National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N. Wiedemann HP, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 28.Pro CI, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 30.Gajic O, Rana R, Winters JL, et al. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med. 2007;176(9):886–891. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 32.Azoulay E, Darmon M, Delclaux C, et al. Deterioration of previous acute lung injury during neutropenia recovery. Crit Care Med. 2002;30(4):781–786. doi: 10.1097/00003246-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Trillo-Alvarez C, Cartin-Ceba R, Kor DJ, et al. Acute lung injury prediction score: derivation and validation in a population-based sample. Eur Respir J. 2011;37(3):604–609. doi: 10.1183/09031936.00036810. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed AH, Litell JM, Malinchoc M, et al. The role of potentially preventable hospital exposures in the development of acute respiratory distress syndrome: a population-based study. Crit Care Med. 2014;42(1):31–39. doi: 10.1097/CCM.0b013e318298a6db. [DOI] [PMC free article] [PubMed] [Google Scholar]


