Abstract
Objective
Surgical patients often receive routine postoperative mechanical ventilation with excellent outcomes. However, older patients who receive prolonged mechanical ventilation may have a significantly different long-term trajectory not fully captured in 30-day postoperative metrics. The objective of this study is to describe patterns of mortality and hospitalization for Medicare beneficiaries 66 and older who have major surgery with and without prolonged mechanical ventilation.
Design
Retrospective cohort study
Setting
Hospitals throughout the United States
Patients
5% random national sample of elderly Medicare beneficiaries (age >= 66 years) who underwent 1 of 227 operations previously defined as high-risk during an inpatient stay at an acute care hospital between January 1, 2005 and November 30, 2009.
Interventions
None
Measurements and Main Results
We identified 117,917 episodes for older patients who had high-risk surgery; 4% received prolonged mechanical ventilation during the hospitalization. Patients who received prolonged mechanical ventilation had higher one-year mortality than patients who did not have prolonged ventilation (64% [95% CI, 62-65%] versus 17% [95% CI, 16.4-16.9%]). Thirty-day survivors who received prolonged mechanical ventilation had a one-year mortality of 47% [95% CI, 45-48%]. Thirty-day survivors who did not receive prolonged ventilation were more likely to be discharged home than patients who received prolonged ventilation 71% vs. 10%. Patients who received prolonged ventilation and were not discharged by postoperative day 30 had a substantially increased 1-year mortality (adjusted hazard ratio [AHR]: 4.39 [95% CI, 3.29-5.85]) compared to patients discharged home by day 30. Hospitalized 30-day survivors who received prolonged mechanical ventilation and died within 6 months of their index procedure spent the majority of their remaining days hospitalized.
Conclusions
Older patients who require prolonged mechanical ventilation after high-risk surgery and survive 30 days have a significant 1-year risk of mortality and high burdens of treatment. This difficult trajectory should be considered in surgical decision-making and has important implications for surgeons, intensivists and patients.
Keywords: prolonged mechanical ventilation, surgical outcomes, decision-making, ethics, high-risk surgery, prognosis
Introduction
The measurement and reporting of 30-day mortality rates for surgery has become commonplace, but little is known about longer-term mortality. Longer-term outcomes are meaningful to patients and their families and should be considered in decision making, particularly for patients with a complicated postoperative recovery that necessitates prolonged life-supporting treatment in the intensive care unit (ICU). Decisions about withdrawal of postoperative life-supporting treatments contribute to conflict between and among surgeons and intensivists,1,2 nurses3 and patients' families4 and are possibly linked to this 30-day horizon, specifically public operative mortality reporting.5-7 Furthermore, acutely ill patients can transition to “chronic critical illness,”8 and suffer physical and psychological burdens of continued treatment.9 Information about longer-term outcomes may be especially important for older patients who commonly value quality of life over life prolongation10,11 and may help them to avoid postoperative conflict and unwanted health states.12
Older patients who survive intensive care and receive mechanical ventilation have significant mortality at six months and one year.13,14 Although surgical patients as a group fare better than medical patients, these analyses include surgical patients who routinely require short-term intensive care in the postoperative setting and therefore overestimate survival for postoperative patients who need prolonged life-support.15-17 Most of what is known about longer-term survival after an unwanted surgical outcome is ascribed to specific, isolated postoperative complications.18 But patients requiring life support in the ICU rarely have just one surgical complication19 and family discussions about life-supporting treatments often focus on withdrawal of mechanical ventilation. Survival beyond the conventional 30-day postoperative horizon for older surgical patients who require prolonged life support remains unknown making it difficult to counsel patients and their families about the value of these burdensome treatments.
The aim of this paper is to examine the outcome of prolonged life-supporting treatment for older patients beyond the standard postoperative 30-day metric. We used a longitudinal Medicare claims database to describe the use of prolonged mechanical ventilation and the longer-term patterns of hospitalization and mortality among patients age 66 and older who have had major surgery. We then investigated the association between prolonged mechanical ventilation and one-year survival after major surgery.
Materials and Methods
Cohort
We used a 5% random sample of all fee-for-service claims for Medicare beneficiaries from the Centers of Medicare & Medicaid Services from January 1, 2004 through December 31, 2009. This longitudinal dataset included hospital inpatient, hospital outpatient, skilled nursing facility, hospice and carrier claims.
We used a previously developed method for identifying beneficiaries undergoing high risk operations.20 Briefly, we defined high-risk surgery as any major operation, e.g. coronary artery bypass grafting, pancreaticoduodenectomy, pneumonectomy and esophagectomy, associated with an inpatient mortality of at least 1% for patients age 65 and older, excluding high mortality procedures that were more likely markers of medical illness, such as tracheostomy, central line and IVC filter placement. This included 227 unique International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes.20,21 When an admission had more than one high-risk operation ICD-9 code, we used the date of the first operation to identify this episode, and did not assign or analyze further operations in the same hospitalization as a new episode. To ensure complete capture of 12 contiguous months of claims for evaluation of co morbidity prior to the index operation, we excluded patients who were less than 66 years of age and those who did not have 12 months of continuous Medicare Part A & B enrollment prior to surgery. Each inpatient admission with a high-risk operation was captured as a separate observation to provide episodic level data, 9,865 such patients contributed to this cohort more than once. This study was reviewed by the University of Wisconsin Institutional Review Board which provided a waiver of consent.
Outcomes
Our primary outcome measure was time to death, censored at 1 year postoperatively.
Exposure variable
We used a broad definition of prolonged mechanical ventilation as our primary predictor variable and as a marker for prolonged life-supporting treatment. We chose this variable because it is a reliable indicator of ventilatory support22-24 and other variables, for example intensive care unit utilization, have significant inter-hospital variability.25We defined prolonged mechanical ventilation using ICD-9-CM code 96.72 for mechanical ventilation of at least 96 hours' duration and/or initial placement of tracheostomy (ICD-9-CM codes 31.1x, 31.2x and/or Current Procedural Terminology (CPT) codes 31600, 31603, 31605, 31610). We excluded tracheostomy placement on the day of index surgery until postoperative day 4 to ensure the tracheostomy was not associated with the index procedure, e.g. laryngectomy. Patients who died within 4 days of surgery (n= 86) were excluded from analysis.
Covariates
We collected clinical and demographic variables in the Medicare administrative files. We collapsed race and ethnicity data, voluntarily provided to the Social Security Administration26, into three groups (white, black, and other) given low number and concern about accurate coding of nonwhite, nonblack subjects in Medicare data.27 We used the Charlson-Deyo score, a measure of co-morbid disease burden validated for administrative databases collected in the year prior to the index operation from ICD-9 diagnosis codes to classify subjects into three groups, low (score 0), medium (score 1-2), and high disease burden (score 3+).28 Among patients surviving to hospital discharge, we generated six categories for patients' discharge destination prior to or at thirty days: discharged to home, inpatient rehabilitation (rehab), skilled nursing facility (SNF), long-term acute care hospital (LTACH), still hospitalized on postoperative day 30 and other. We utilized a prior definition of severe sepsis as the presence of an ICD-9-CM code for bacterial or fungal infection plus diagnosis of acute organ dysfunction.29 New onset dialysis was defined by an ICD-9-CM code for acute renal failure (584.5, 584.6, 584.7, 584.8, or 584.9) along with the procedure ICD-9-CM code for dialysis 39.95 to exclude patients on chronic dialysis.30
Statistical Analysis
For reference, we compared the crude mortality for patients who had prolonged mechanical ventilation to those who did not using Kaplan-Meier survival estimators, and tested for between-group differences with log-rank tests. To describe longer-term mortality after surgery and to mitigate the problem of multiple competing risks, we also estimated mortality conditioned on survival to postoperative day 30.
We used a Cox proportional hazards model conditioned on survival to 30 day post-operation to assess the adjusted effect of inherent patient characteristics along with other life-supporting treatments, severe sepsis, and discharge destination status before or at 30 days on one-year mortality among patients who received prolonged mechanical ventilation. We censored survival time for all subjects alive on December 31, 2009.
We described the discharge destination for all patients in our cohort who were alive on postoperative day 30. We then calculated the proportion of days hospitalized up to 1 year of survival for patients who received prolonged mechanical ventilation and were not discharged 30 days after their index operation. We included all days spent in an acute care hospital in the year following the index operation during both the index admission and all subsequent re-admissions.
Because the date associated with ICD-9-CM code 96.72 may not be accurate, it is possible that prolonged mechanical ventilation may have preceded surgery for some patients. To test the impact of potential misclassification of the timing of prolonged mechanical ventilation, we performed a sensitivity analysis restricted to patients whose date of surgery was the date of hospital admission. To confirm that patients who received a tracheostomy but did not have the concomitant ICD-9-CM code 96.72 still likely received prolonged mechanical ventilation, we performed a sensitivity analysis between those who had ICD-9-CM code 96.72 with or without tracheostomy versus tracheostomy (alone).
All analyses were performed using Stata version 13 (Stata Corp, College Station, TX) by the first author (MJN) and independently validated in SAS version 9.3(SAS Institute, Cary, NC) by a second investigator (QZ).
Results
Over the 5 year study period, we identified 117,917 admissions during which the beneficiary underwent at least one high-risk operation, representing 106,322 unique Medicare beneficiaries. Of these admissions, 4,944 (4%) required prolonged mechanical ventilation. Patients who had urgent surgery and more comorbid conditions were more likely to have prolonged mechanical ventilation. (Table 1)
Table 1. Characteristics of Medicare beneficiaries who had high-risk surgery between January 1, 2005 and November 30, 2009.
| n=117,917 | PMV | No PMV | ||
|---|---|---|---|---|
|
| ||||
| N | % | N | % | |
| Cohort | 4,944 | 4.2 | 112,973 | 96 |
| Female sex | 2,634 | 53 | 57,692 | 51 |
| age, mean, y | 77.0 | 6.7 (SD) | 76.2 | 6.6 (SD) |
| Race | ||||
| White | 4,279 | 87 | 100,989 | 89 |
| Black | 441 | 8.9 | 7,915 | 7.0 |
| Other | 224 | 4.5 | 4,069 | 3.6 |
| Charlson-Deyo score | ||||
| 0 | 1,176 | 24 | 33,438 | 30 |
| 1-2 | 1,917 | 39 | 44,315 | 39 |
| 3+ | 1,851 | 37 | 35,220 | 31 |
| Urgent/Emergent Surgery | 3,992 | 81 | 64,869 | 57 |
Chi-squared tests of independence used when comparing proportions and categorical outcomes and 2-sample t-tests for means.
All differences were statistically significant at α<.002.
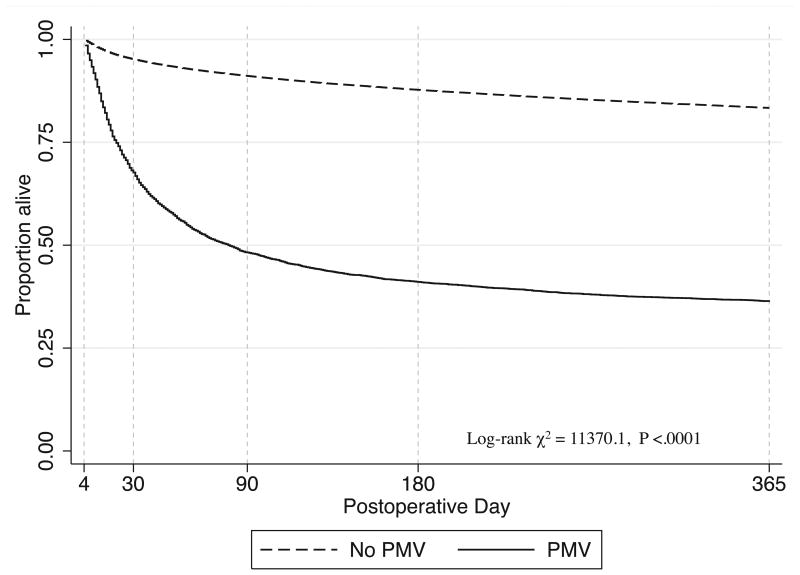
Unadjusted thirty-day postoperative and one-year mortality for patients who received prolonged mechanical ventilationwas32% [95% CI, 31-34] and 64% [95% CI, 62-65] as compared to 4.8% [95% CI,4.7-5.0] and 16.6% [95% CI, 16.4-16.9] for those who did not have prolonged mechanical ventilation. (Figure 1) The survival curves sharply diverge early in the postoperative period and continue to widen until approximately postoperative day 180. Among those surviving at least 30 days after surgery, crude one-year mortality was 47% [95% CI, 45-48%] for patients who received prolonged mechanical ventilation. (Table 2)
Figure 1. One-year survival after high-risk surgery for Medicare beneficiaries who do and do not receive prolonged postoperative mechanical ventilation.
Legend: PMV – prolonged mechanical ventilation
Table 2. Overall mortality after high-risk surgery and mortality conditional on survival to postoperative day 30, 60, 90 and 180.
| Overall Mortality (Percent) | Mortality if alive on postoperative day 30 (Percent) | Mortality if alive on postoperative day 60 (Percent) | Mortality if alive on postoperative day 90 (Percent) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Days from Surgery | PMV | No PMV | PMV | No PMV | PMV | No PMV | PMV | No PMV |
| 30 | 32 [31-34] | 4.8 [4.7-5.0] | _ | _ | _ | _ | _ | _ |
| 60 | 46 [44-47] | 7.3 [7.1-7.4] | 20 [19-22] | 2.7 [2.5-2.7] | _ | _ | _ | _ |
| 90 | 52 [50-53] | 8.9 [8.7-9.1] | 29 [28-31] | 4.4 [4.2-4.4] | 12 [11-13] | 1.8 [1.8-1.9] | _ | _ |
| 180 | 59 [58-60] | 12.2 [12.1-12.5] | 40 [38-41] | 7.9 [7.7-8.0] | 25 [23-27] | 5.5 [5.3-5.6] | 15 [14-16] | 3.8 [3.7-3.9] |
| 365 | 64 [62-65] | 16.6 [16.4-16.9] | 47 [45-48] | 12.5 [12.2-12.6] | 33 [32-35] | 10.2 [10.0-10.4] | 25 [23-26] | 8.6 [8.4-8.8] |
For patients who received prolonged mechanical ventilation, adjusted analysis showed that those with tracheostomy, new hemodialysis, older age and high comorbidity were more likely to die in one year. Among those surviving to 30 days, discharge destination was highly associated with one-year mortality as patients who were discharged to a SNF or LTACH were significantly more likely to die in one year (both with a 17% one year mortality) than patients discharged to home or rehab (10% and 8% respectively).Patients remaining at an acute care hospital 30 days after surgery had the highest risk of mortality, 43% at one year. (Table 3)
Table 3. Predictors of one-year mortality after prolonged postoperative mechanical ventilation among beneficiaries still alive on postoperative day 30.
| N=3,370 | N | Adjusted HR | P value |
|---|---|---|---|
| Tracheostomy placement* | 1,722 | 1.35 [1.19-1.52] | < .001 |
| Severe Sepsis† | 2,753 | 1.14 [0.99-1.32] | .06 |
| Hemodialysis† | 369 | 1.19 [1.03-1.37] | .015 |
| Discharge Destination by Postoperative Day 30‡ | |||
| Home | 345 | [Reference] | – |
| Rehab | 272 | 1.13 [0.77-1.66] | .53 |
| SNF | 589 | 2.10 [1.55-2.84] | < .001 |
| LTAC | 585 | 3.26 [2.42-4.40] | < .001 |
| Still Hospitalized | 1,459 | 4.39 [3.29-5.85] | < .001 |
| Age at Hospital Admission | |||
| 66-75 | 1,605 | [Reference] | – |
| 76-85 | 1,474 | 1.27 [1.14-1.41] | <.001 |
| 86+ | 291 | 1.65 [1.38-1.97] | <.001 |
| Female Sex | 1,750 | 0.92 [0.83-1.02] | 0.10 |
| Race | |||
| White | 2,902 | [Reference] | – |
| Black | 319 | 1.13 [0.96-1.34] | .13 |
| Other | 149 | 0.92 [0.73-1.16] | .46 |
| Urgent/Emergent Surgery Status | 2,608 | 1.13 [0.99-1.28] | .06 |
| Preoperative Charlson Comorbidity Score | |||
| 0 | 838 | [Reference] | – |
| 1-2 | 1,326 | 1.36 [1.18-1.57] | <.001 |
| 3+ | 1,206 | 1.73 [1.51-1.99] | <.001 |
–performed between postoperative day 4 and 30 of index hospitalization
–during index hospitalization
–Other group not reported due to heteregenous make-up
Among 30-day survivors who had high-risk surgery but did not receive prolonged mechanical ventilation, unadjusted analysis showed that 71% were discharged home while 1.6% remained hospitalized at day 30. In contrast, 10% of 30-day survivors who received prolonged mechanical ventilation were discharged home while 43% were still hospitalized on day 30. While just over 20% of 30-day survivors who did not receive prolonged mechanical ventilation needed longer-term nursing care, nearly 80% of survivors who received prolonged mechanical ventilation were either still in the hospital or had been discharged to a SNF or LTACH. (Table 4)
Table 4. Discharge destination for patients who had high risk surgery and were alive on postoperative day 30.
| PMV | No PMV | |||
|---|---|---|---|---|
|
| ||||
| Discharge Destination | N (%) | N (%) | ||
| Home | 345 | 10% | 76,123 | 71% |
| Inpatient Rehab | 272 | 8.1% | 6,522 | 6.1% |
| Skilled Nursing Facility | 589 | 17% | 20,591 | 19% |
| Long-Term Acute Care Hospital | 585 | 17% | 1,481 | 1.4% |
| Still Hospitalized | 1,459 | 43% | 1,933 | 1.8% |
| Other | 120 | 3.6% | 990 | 0.92% |
Table 5 presents longitudinal outcomes of those patients who had prolonged mechanical ventilation and remained hospitalized on postoperative day 30. Of these 1,459 patients, 1,171(80%) experienced a transition in care before day 60, including 320 (27%) patients who died in the hospital. At postoperative day 60, 288 (20%) patients were still hospitalized and 194 (13%) transitioned from the hospital setting before postoperative day 90. Hospitalized thirty-day survivors who died within 6 months of their index procedure spent the majority of their remaining days hospitalized For example, the median percent of postoperative time spent in the hospital for patients who died between 91-180 days was 57% [IQR 38-84], approximately 3.4 months, with an average of 0.93 readmissions. In comparison, the median percent of time hospitalized for patients who survived to postoperative day 180 (n=668) was 27% [IQR 22-38%], approximately 1.6 months, with an average of 1.47 readmissions.
Table 5. Longitudinal outcomes for patients who received prolonged mechanical ventilation and were still hospitalized on postoperative day 30 based on time of discharge and interval survival.
| Patients hospitalized on: | Day 30 | Day 60 | Day 90 | Day 180 |
|---|---|---|---|---|
| N=1,459 | N=288 | N=90 | N<11* | |
| Disposition prior to next interval: | ||||
| Still Hospitalized | 288 (20%) | 90 (31%) | –* | –* |
| In-Hospital Death | 320 (22%) | 65 (23%) | 28 (31%) | –* |
| Discharged to: | ||||
| Home/Rehab | 216 (15%) | 43 (15%) | –* | –* |
| SNF | 287 (20%) | 52 (18%) | 31 (34%) | –* |
| LTACH | 275 (19%) | 28 (10%) | –* | –* |
| Other | 73 (5%) | 10 (3%) | –* | –* |
|
| ||||
| For those who died between: | 31-60 days | 61-90 days | 91-180 days | 181-365 days |
|
| ||||
| N=423 | N=180 | N=188 | N=110 | |
| Proportion of time hospitalized† | ||||
| Median (IQR) | 100% (100-100) | 85% (62-100) | 57% (38-84) | 32% (22-48) |
| Mean (SD) | 96% (8.6) | 80% (20) | 61% (26) | 39% (22) |
| Number of Readmissions† | ||||
| Mean (SD) | 0.28 (0.49) | 0.60 (0.74) | 0.93 (1.00) | 2.18 (1.59) |
|
| ||||
| For those alive on day: | Day 60 | Day 90 | Day 180 | Day 365 |
| N=1,036 | N=856 | N=668 | N=558 | |
| Proportion of time hospitalized‡ | ||||
| Median (IQR) | 77% (62-100) | 52% (42-72) | 27% (22-38) | 14% (11-19) |
| Mean (SD) | 78% (18) | 59% (21) | 33% (16) | 17% (9) |
| Number of Readmissions‡ | ||||
| Mean (SD) | 1.27 (1.50) | 1.36 (1.56) | 1.47 (1.65) | 1.33 (1.62) |
Exact cell sizes <11 are suppressed due to CMS cell size requirement.
Date of index operation until death.
Date of index operation until end of reference interval.
Sensitivity Analysis
We found no appreciable differences when we restricted the analysis to patients who had surgery on the day of admission. We found no differences in the observed postoperative trajectories whether we constructed our prolonged mechanical ventilation variable with the tracheostomy procedure codes alone or used the ICD-9-CM code 96.72 with or without tracheostomy.
Discussion
Our data suggest that of the roughly half a million elderly Medicare beneficiaries who undergo a high-risk operation annually, nearly 4% will receive prolonged mechanical ventilation. The need for mechanical ventilation for 96 hours or more is associated with a marked increase in postoperative mortality both at 30 days and 1 year. Furthermore, 30-day survivors have substantial need for skilled care as more than half of this group have been transferred to an LTACH or remain hospitalized on postoperative day 30. Burdens of treatment are considerable for six-month and one-year survivors who spend a significant portion of this postoperative time hospitalized.
While many patients who have major surgery receive mechanical ventilation during routine postoperative recovery, older patients who receive postoperative mechanical ventilation for 96 hours or more appear to be a distinct group with a markedly different trajectory. Not surprisingly these patients were more likely to have urgent surgery and a higher burden of comorbid conditions. Survivors to postoperative day 30 have a one-year survival that is remarkably similar to medical ICU patients who receive mechanical ventilation and survive to discharge.13 The high longer-term mortality and considerable use of skilled care question the use of 30-day operative survival as a suitable standalone marker of successful surgery, particularly in this demographic. These findings have important implications for surgeons, intensivists and patients.
For surgeons, our data question deep notions of rescue and responsibility.31,32 By convention, patients who survive 30 days with prolonged mechanical ventilation and other life-supporting treatment have been rescued from their postoperative surgical complication.33,34 As many older patients would forgo even minor interventions resulting in functional impairment,10 rescue from surgical complications to a state of chronic critical illness or other dependency may not be an outcome patients value. Requests by patients and/or their family members to withdraw life-supporting treatments in the postoperative period should be considered in light of this information. Given this marker of a poor outcome is highly recognizable and may occur early in the postoperative course, these data can inform patients and/or their family about longer-term outcomes in the days and weeks following surgery.
For intensivists, who experience conflict with surgeons about withdrawal of postoperative life supporting treatments1,2,4 these data may inform and potentially mitigate conflict about the value of continued life-supporting treatment. As these conflicts stem from disagreement about goals of care, specifically about patient survival and burdens of treatment, information about longer-term survival can contextualize the patient's overall trajectory and add clarity about the value of continued life-supporting treatments. The traditional 30-day horizon, a standard surgical metric, likely contributes to the prognostic optimism endorsed by surgeons during these conflicts,5 whereas these longer-term data cast a much less optimistic shadow.
For older patients who require prolonged postoperative life support and survive 30 days, their course is arduous. They face considerable mortality and heavy burdens of treatment including many inpatient days for both those who survive and those who do not. While there is real variability in the range of possible outcomes, this information is likely valuable to patients and their families as they make decisions about additional interventions. Unfortunately, little is currently known about older patients' goals and preferences in this specific setting. Clarification of treatment goals and unacceptable postoperative outcomes should ideally be discussed between surgeons, patients and their family members preoperatively.
These results question 30-day survival as a measure of surgical success as death on or before 30 days fails to capture much of the impact of major surgery for older patients. Alternative markers using outcomes that are meaningful to patients such as extended ICU stays, prolonged mechanical ventilation and discharge status on postoperative day 30 might provide a more valuable measure for both patients and surgeons. Our data show a step-wise increase in long-term mortality based on discharge status by day 30, suggesting this is both a measurable and potentially meaningful predictor of surgical outcomes. Future research will need to focus on the assessment of functional and cognitive outcomes after high-risk surgery and development of strategies to elicit patients' goals and preferences before the occurrence of a major surgical complication.
Our study has both strengths and limitations. We have a large and representative sample of the United States population age 66 and older and we used an inclusive list of high-risk operations to generate our cohort. The date of death in the Medicare files is considered an accurate method of ascertaining if and when death occurred.35 Billing codes are vulnerable to administrative coding errors. Since ICD-9-CM code 96.72 mandates greater than 96 hours of continuous mechanical ventilation, our methods fail to capture some patients who have had more than 96 nonconsecutive hours of mechanical ventilation during their index hospitalization. Although we would have like to examine how the duration of mechanical ventilation is associated with long-term outcomes more precisely, this is not possible with the information available in Medicare claims data. Furthermore, this administrative data set lacks information about functional status and quality of life that are important for families considering choices about additional treatment. Finally, these data cannot tell us about patient wishes, choices or how they value specific outcomes after high-risk surgery.
Conclusions
Older patients who require prolonged mechanical ventilation after high-risk surgery face high one-year mortality, protracted periods of hospitalization and a significant need for skilled care. This difficult trajectory should be considered during decisions to continue prolonged postoperative life-supporting treatment as some patients may not value such outcomes.
Acknowledgments
Funding: Dr. Schwarze is supported by a training award (KL2TR000428) from the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant (UL1 TR000427) and the Greenwall Foundation (Greenwall Faculty Scholars Program). Dr. Kind was supported by a National Institute on Aging Beeson Career Development Award (K23AG034551), National Institute on Aging, The American Federation for Aging Research, The John A. Hartford Foundation, The Atlantic Philanthropies and The Starr Foundation). Dr. Ehlenbach was supported by a Paul Beeson Career Development Award in Aging Research Program (NIA K23AG038352) funded by the National Institute on Aging, The Atlantic Philanthropies (USA), The John A. Hartford Foundation, the Starr Foundation and an anonymous donor.
These funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; and preparation, review, or approval of the manuscript for publication. No other financial support was declared for the remaining authors.
Copyright form disclosures: Dr. Schwarze received funding from the Greenwall Foundation and received support for article research from the National Institutes of Health (NIH). Dr. Nabozny received support for article research from the NIH. Dr. Barnato's institution received funding from the National Palliative Care Research Center and the Donaghue Foundation. Dr. Kind received support for article research from the NIH and received funding from the State of Maryland. Her institution received funding from the National Institute on Aging Beeson Career Development Award (K23AG034551 [PI Kind], National Institute on Aging, The American Federation for Aging Research, The John A. Hartford Foundation, The Atlantic Philanthropies and The Starr Foundation), Madison VA Geriatrics Research, Education and Clinical Center, and the University of Wisconsin School of Medicine and Public Health from the Wisconsin Partnership Program. Dr. Ehlenbach received support for article research from the NIH. His institution received funding from the NIH (NIA)-K23. Dr. Smith received support for article research from the NIH. Dr. Greenberg's institution received funding from Covidien.
Footnotes
The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Paul Olson TJ, Brasel KJ, Redmann AJ, Alexander GC, Schwarze ML. Surgeon-reported conflict with intensivists about postoperative goals of care. JAMA Surg. 2013;148(1):29–35. doi: 10.1001/jamasurgery.2013.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danjoux Meth N, Lawless B, Hawryluck L. Conflicts in the ICU: perspectives of administrators and clinicians. Intensive Care Med. 2009;35(12):2068–2077. doi: 10.1007/s00134-009-1639-5. [DOI] [PubMed] [Google Scholar]
- 3.Azoulay E, Timsit JF, Sprung CL, et al. Prevalence and factors of intensive care unit conflicts: the conflicus study. American journal of respiratory and critical care medicine. 2009;180(9):853–860. doi: 10.1164/rccm.200810-1614OC. [DOI] [PubMed] [Google Scholar]
- 4.Cassell J, Buchman TG, Streat S, Stewart RM. Surgeons, intensivists, and the covenant of care: administrative models and values affecting care at the end of life--Updated. Crit Care Med. 2003;31(5):1551–1557. [PubMed] [Google Scholar]
- 5.Schwarze ML, Redmann AJ, Brasel KJ, Alexander GC. The role of surgeon error in withdrawal of postoperative life support. Ann Surg. 2012;256(1):10–15. doi: 10.1097/SLA.0b013e3182580de5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maxwell BG, Wong JK, Miller DC, Lobato RL. Temporal changes in survival after cardiac surgery are associated with the thirty-day mortality benchmark. Health services research. 2014;49(5):1659–1669. doi: 10.1111/1475-6773.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wicclair MR, White DB. Surgeons, intensivists, and discretion to refuse requested treatments. The Hastings Center report. 2014;44(5):33–42. doi: 10.1002/hast.356. [DOI] [PubMed] [Google Scholar]
- 8.Lamas D. Chronic critical illness. N Engl J Med. 2014;370(2):175–177. doi: 10.1056/NEJMms1310675. [DOI] [PubMed] [Google Scholar]
- 9.Nelson JE, Meier DE, Litke A, Natale DA, Siegel RE, Morrison RS. The symptom burden of chronic critical illness. Crit Care Med. 2004;32(7):1527–1534. doi: 10.1097/01.ccm.0000129485.08835.5a. [DOI] [PubMed] [Google Scholar]
- 10.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill. N Engl J Med. 2002;346(14):1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 11.Fried TR, Van Ness PH, Byers AL, Towle VR, O'Leary JR, Dubin JA. Changes in preferences for life-sustaining treatment among older persons with advanced illness. J Gen Intern Med. 2007;22(4):495–501. doi: 10.1007/s11606-007-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patrick DL, Starks HE, Cain KC, Uhlmann RF, Pearlman RA. Measuring preferences for health states worse than death. Med Decis Making. 1994;14(1):9–18. doi: 10.1177/0272989X9401400102. [DOI] [PubMed] [Google Scholar]
- 13.Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble WT. Three-year outcomes for Medicare beneficiaries who survive intensive care. Jama. 2010;303(9):849–856. doi: 10.1001/jama.2010.216. [DOI] [PubMed] [Google Scholar]
- 14.Damuth E, Mitchell JA, Bartock JL, Roberts BW, Trzeciak S. Long-term survival of critically ill patients treated with prolonged mechanical ventilation: a systematic review and meta-analysis. The Lancet Respiratory medicine. 2015 doi: 10.1016/S2213-2600(15)00150-2. [DOI] [PubMed] [Google Scholar]
- 15.Kaarlola A, Tallgren M, Pettila V. Long-term survival, quality of life, and quality-adjusted life-years among critically ill elderly patients. Crit Care Med. 2006;34(8):2120–2126. doi: 10.1097/01.CCM.0000227656.31911.2E. [DOI] [PubMed] [Google Scholar]
- 16.Trouillet JL, Combes A, Vaissier E, et al. Prolonged mechanical ventilation after cardiac surgery: outcome and predictors. The Journal of thoracic and cardiovascular surgery. 2009;138(4):948–953. doi: 10.1016/j.jtcvs.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 17.Esteban A, Anzueto A, Frutos F, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. Jama. 2002;287(3):345–355. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 18.Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242(3):326–341. doi: 10.1097/01.sla.0000179621.33268.83. discussion 341-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferraris VA, Bolanos M, Martin JT, Mahan A, Saha SP. Identification of patients with postoperative complications who are at risk for failure to rescue. JAMA Surg. 2014;149(11):1103–1108. doi: 10.1001/jamasurg.2014.1338. [DOI] [PubMed] [Google Scholar]
- 20.Schwarze ML, Barnato AE, Rathouz PJ, et al. Development of a List of High-Risk Operations for Patients 65 Years and Older. JAMA Surg. 2015 doi: 10.1001/jamasurg.2014.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarze ML. List of High Risk Operations for Patients Over Age 65. Available at: http://www.hipxchange.org/HighRiskSurgery.
- 22.Cox CE, Carson SS, Holmes GM, Howard A, Carey TS. Increase in tracheostomy for prolonged mechanical ventilation in North Carolina, 1993-2002. Crit Care Med. 2004;32(11):2219–2226. doi: 10.1097/01.ccm.0000145232.46143.40. [DOI] [PubMed] [Google Scholar]
- 23.Dewar DM, Kurek CJ, Lambrinos J, Cohen IL, Zhong Y. Patterns in costs and outcomes for patients with prolonged mechanical ventilation undergoing tracheostomy: an analysis of discharges under diagnosis-related group 483 in New York State from 1992 to 1996. Crit Care Med. 1999;27(12):2640–2647. doi: 10.1097/00003246-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Zilberberg MD, Luippold RS, Sulsky S, Shorr AF. Prolonged acute mechanical ventilation, hospital resource utilization, and mortality in the United States. Crit Care Med. 2008;36(3):724–730. doi: 10.1097/CCM.0B013E31816536F7. [DOI] [PubMed] [Google Scholar]
- 25.Boumendil A, Angus DC, Guitonneau AL, et al. Variability of intensive care admission decisions for the very elderly. PLoS One. 2012;7(4):e34387. doi: 10.1371/journal.pone.0034387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waldo DR. Accuracy and Bias of Race/Ethnicity Codes in the Medicare Enrollment Database. Health care financing review. 2004;26(2):61–72. [PMC free article] [PubMed] [Google Scholar]
- 27.Arday SL, Arday DR, Monroe S, Zhang J. HCFA's racial and ethnic data: current accuracy and recent improvements. Health care financing review. 2000;21(4):107–116. [PMC free article] [PubMed] [Google Scholar]
- 28.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 29.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification Codes for Acute Renal Failure. Journal of the American Society of Nephrology : JASN. 2006;17(6):1688–1694. doi: 10.1681/ASN.2006010073. [DOI] [PubMed] [Google Scholar]
- 31.Schwarze ML, Bradley CT, Brasel KJ. Surgical “buy-in”: the contractual relationship between surgeons and patients that influences decisions regarding life-supporting therapy. Crit Care Med. 2010;38(3):843–848. doi: 10.1097/CCM.0b013e3181cc466b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cassell J, Buchman TG, Streat S, Stewart RM. Surgeons, intensivists, and the covenant of care: administrative models and values affecting care at the end of life--Updated. Crit Care Med. 2003;31(5):1551–1557. discussion 1557-1559. [PubMed] [Google Scholar]
- 33.Sheetz KH, Dimick JB, Ghaferi AA. The association between hospital care intensity and surgical outcomes in medicare patients. JAMA Surg. 2014;149(12):1254–1259. doi: 10.1001/jamasurg.2014.552. [DOI] [PubMed] [Google Scholar]
- 34.Silber JH, Kaestner R, Even-Shoshan O, Wang Y, Bressler LJ. Aggressive treatment style and surgical outcomes. Health services research. 2010;45(6 Pt 2):1872–1892. doi: 10.1111/j.1475-6773.2010.01180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. [March 11, 2015];Strengths and Limitations of CMS Administrative Data in Research. Available at: http://www.resdac.org/resconnect/articles/156.