Abstract
Background
Older individuals with inflammatory bowel disease (IBD) require ongoing medications. We aimed to describe 1) medication use in older and younger IBD patients and 2) medication associations with patient reported outcomes (PRO’s) in older patients.
Methods
We conducted cross-sectional and longitudinal analyses within CCFA Partners internet-based cohort of patients with self-reported IBD. We assessed medication use by disease sub-type and age. We used bivariate analyses to 1) compare medication use in older and younger patients and 2) determine associations between continued steroid use and PRO’s in older patients.
Results
We included 5382 participants with IBD; 1004 were older (≥ age 60). Older patients with Crohn’s disease (CD) had lower anti-tumor necrosis factor alpha (anti-TNF) use at baseline (29.1% vs 44.3%, p<0.001), comparable steroid use (16.0% vs. 16.5%, p=0.77), and higher aminosalicylate use (40.3% vs. 33.9%, p=0.003) versus younger patients. Older ulcerative colitis (UC) patients had similar anti-TNF use (16.0% vs. 19.2%, p=0.16), lower steroid use (9.6% vs. 15.4%, p=0.004) and higher aminosalicylate use (73.8% vs. 68.2%, p=0.04) at baseline. In longitudinal analyses, older CD patients had higher continued steroid use (11.6% vs. 7.8%, p=0.002); which was associated with worsened anxiety (p=0.02), sleep (p=0.01), and fatigue (p=0.001) versus non-use. Older CD patients on steroids, versus anti-TNF or immunomodulators, had increased depression (p=0.04) and anxiety (p=0.03).
Conclusions
Medication utilization differs in older patients with IBD. Older CD patients have higher continued steroid use; associated with worsened PRO’s. As in younger IBD populations; continued steroid use should be limited in older patients.
Keywords: Older, inflammatory bowel disease, Crohn’s disease, ulcerative colitis
Introduction
The populations of developed countries, such as the United States (US), are aging due to low fertility and mortality rates.1, 2 The 65+ age group is the fastest growing in the US with an estimated 31% increase over the past decade.3 In addition to an aging US population, inflammatory bowel disease (IBD) has a bimodal incidence distribution with 15% of cases occurring in the second peak, after 65 years of age.4 Due to these factors, the number of older patients living with IBD is expected to rise—including older persons newly diagnosed with IBD. Older-onset IBD may be associated with a milder disease phenotype with decreased progression to more severe stages such as stricturing or penetrating disease.5, 6 Despite a milder phenotype, older IBD patients, regardless of age of onset, have higher resource utilization with increased rates of in-hospital morbidity and mortality compared to younger IBD patients.4 Therefore, optimizing the management of IBD in the older is increasingly important.7
Prior investigations have shown that the medical treatment strategies used in older IBD patients may be different compared to younger IBD populations, with an increased reliance on corticosteroids and 5-aminosalicylates (5-ASA) as maintenance therapies.8, 9,10 Steroid-sparing strategies such as immunomodulators or biologic agents are used less frequently in older IBD patients in spite of current guidelines that support their use in this population for moderate to severe disease activity.9, 11, 12 Factors such as adverse effects from prolonged or repeated corticosteroid use and delay in the use of appropriate steroid sparing therapies related to disease activity may contribute to the lower short-term therapeutic efficacy and increased rates of adverse events seen in older IBD patients.4, 13, 14 The adverse effects of corticosteroid use among IBD patients have been well-established15, 16 including increased risks of serious infections, mortality, and accelerated bone loss, which are further potentiated when factoring in the independent risk factor of advanced age. However, it is unknown how ongoing continued steroid use affects patient reported outcomes (PROs) such as anxiety, depression, sleep and fatigue in older IBD patients.
We used data from a large Internet-based study of IBD to describe current medical therapies in older IBD patients, rates of continued steroid use, and associations between continued steroid use and patient reported outcomes (PROs). Better information on the treatment experience of older IBD patients might aid in optimizing treatment strategies for this growing population.
Methods
CCFA Partners is an Internet-based prospective cohort study of over 14,000 adults living with self-reported IBD, including both CD and UC. The cohort initially began recruitment in 2011. The details of cohort development have been previously described.17 Briefly, individuals with IBD are recruited to join CCFA Partners through social media, emails, advertising from the Crohn’s and Colitis Foundation of America (CCFA), CCFA events, and through physicians’ offices. Participants complete surveys every 6 months providing data on disease type, activity, course, medications, and selected PROs. Diagnoses in a randomly selected sample of the cohort have been validated, with over 96% of this sample having IBD confirmed by their physician.18 The surveys include a number of indices previously validated for self-report, including the short Crohn’s disease activity index (sCDAI),19 simple clinical colitis activity index (SCCAI),20, 21 and short IBD questionnaire (SIBDQ).22
For the current analysis, we included all individuals who had completed at least 2 surveys over the course of a 12 month period (baseline and at least 1 follow-up) and whose disease type (CD or UC) had not changed over the course of the study time period. Those with indeterminate colitis (IC) were analyzed in the UC group. We used data from the baseline surveys to conduct a cross-sectional analysis of medication use, disease activity and other characteristics comparing older individuals (age ≥ 60 years) to younger individuals (age 18–59 years). We then conducted two separate longitudinal analyses: (1) Comparisons of rates of long-term continued steroid use (defined as steroid use on 2 consecutive surveys at least 6 months apart) in older versus younger individuals and (2) associations of continued versus non-continued steroid use with various health and quality related PRO’s among only older participants. Sensitivity analyses included an analysis of PRO’s in older IBD patients on continued steroid monotherapy as compared to biologic and/or anti-TNF therapy. To eliminate confounding by disease activity, a second sensitivity analysis evaluated the effects of continued steroid use on PRO’s restricted to those individuals who met criteria for remission at follow up. Remission was defined using a score of <150 on the sCDAI22 for patients with CD or a score of ≤2 on the SCCAI for patients with UC.20, 21 PRO’s were measured using five domains (anxiety, sleep disturbance, pain interference, fatigue, and depression) from the patient reported outcomes measurement information system (PROMIS) as previously described for this cohort.23 Briefly, all PROMIS measures have undergone rigorous development and validation in both general and chronically ill populations. Items are calibrated using a T-score metric with the mean of the US population equal to 50 and standard deviation (SD) of 10. Higher scores indicate “more” of the domain being measured, that is, worse anxiety, sleep disturbance, pain interference, fatigue, and depression. Emerging data suggest than minimally important differences (MIDs) in PROMIS measures are in the range of 2 to 6,24 as suggested by our prior work in this cohort.23
Statistical Analysis
All analyses were performed using STATA 12.0 (College Station, TX). The population was characterized using descriptive statistics, including proportions, means, standard deviations, stratified by CD and UC. Outcomes were compared used bivariate statistics as appropriate. Confidence intervals were 95% and p<0.01 was considered statistically significant. The Institutional Review Board at the University of North Carolina at Chapel Hill approved the study protocol.
Results
Cross-Sectional Analyses of Baseline Surveys
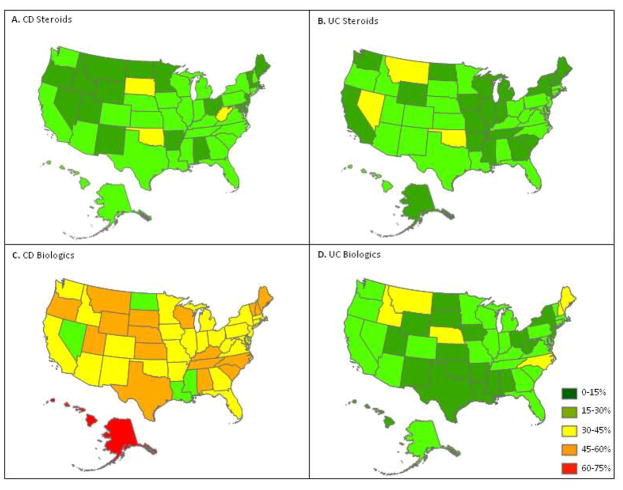
A total of 5382 individuals with self-reported IBD were included. Of these, 3392 had CD and 1873 had UC/IC. Figure 1 shows geographic variation in corticosteroid use and biologic use for the entire cohort, with the highest overall use of biologics in patients with CD. There were 1004 older participants (≥60 years of age), 636 individuals with CD and 368 with UC. The median age for both the older CD and UC populations was 65 years. Characteristics of the CD and UC populations by age group are shown in Table 1 and Table 2 respectively.
Figure 1.
Baseline medication use by state within CCFA Partners by Crohn’s disease and ulcerative colitis
Table 1.
Characteristics of CCFA Partner’s Crohn’s disease patients by age at baseline
| Characteristics | Older (age ≥ 60) (n=636) Median (IQR) or % |
Younger (age 18–59) (n=2756) Median (IQR) or % |
|---|---|---|
| Age (years) | 65 (62–70) | 39 (29–50) |
| Gender (% female) | 62.9 | 74.9 |
| Education (% >high school) | 90.9 | 91.7 |
| Race (%) | ||
| Caucasian | 97.8 | 94.5 |
| African American | 1.0 | 1.7 |
| Other | 1.2 | 3.8 |
| Current smoking (% yes) | 6.2 | 7.8 |
| BMI | 25.1 (22.2–28.6) | 23.9 (21.2–27.9) |
| Disease duration (years) | 31 (13–42) | 10 (4–21) |
| Ever GI surgery (% yes) | 65.2 | 48.1 |
| Ever GI hospitalization (% yes) | 81.3 | 71.4 |
| Number hospitalizations | 4 (2–6) | 3 (2–6) |
| Current medications (%) | ||
| Biologic Anti-TNF | 29.1 | 44.3 |
| Immunomodulator* | 27.6 | 32.9 |
| Corticosteroids | 16.5 | 16.0 |
| 5-ASA& | 40.3 | 33.9 |
| Remission (sCDAI^ <150) (% yes) | 64.2 | 59.7 |
| sCDAI | 114 (72–184) | 128 (72–198) |
| SIBDQ** | 5.3 (4.5–6) | 4.9 (3.9–5.7) |
Immunomodulator defined as 6-mercaptopurine, azathioprine, or methotrexate
5-aminosalicylate
Short Crohn’s disease activity index
Short inflammatory bowel diseases questionnaire
Table 2.
Characteristics of CCFA Partner’s Ulcerative Colitis patients by age at baseline
| Characteristics | Older (age ≥60) (n=368) Median (IQR) or % |
Younger (age 18–59) (n=1622) Median (IQR) or % |
|---|---|---|
| Age (years) | 65 (63–70) | 38 (29–48) |
| Gender (% female) | 59.0 | 73.2 |
| Education (% >high school) | 90.9 | 93.9 |
| Race | ||
| Caucasian | 97.3 | 91.9 |
| African American | 1.2 | 1.2 |
| Other | 1.5 | 6.8 |
| Current smoking (% yes) | 2.2 | 3.3 |
| BMI | 26.0 (23.3–29.4) | 23.7 (21.3–27.4) |
| Disease duration (years) | 16 (6–31) | 7 (3–14) |
| Ever GI surgery (% yes) | 13.6 | 11.8 |
| Ever hospitalization (% yes) | 39.7 | 47.4 |
| Number hospitalizations | 2 (1–3) | 2(1–3) |
| Current medications | ||
| Biologic Anti-TNF | 16.0 | 19.2 |
| Immunomodulator* | 18.5 | 24.0 |
| Corticosteroids | 9.6 | 15.4 |
| 5-ASA& | 73.8 | 68.2 |
| Remission (SCCAI^ ≤2) (% yes) | 45.9 | 43.9 |
| SCCAI | 3 (1–4) | 3 (1–5) |
| SIBDQ** | 5.5 (4.6–6) | 5 (4–5.8) |
Immunomodulator defined as 6-mercaptopurine, azathioprine, or methotrexate
5-aminosalicylate
Simple clinical colitis activity index
Short inflammatory bowel diseases questionnaire
Crohn’s disease
Older individuals with CD reported a higher rate of remission (64.2% vs 59.7%, p=0.05) determined by short Crohn’s Disease Activity Index (sCDAI) score < 150. Older CD individuals also reported a better health-related quality of life: Short Inflammatory Bowel Disease Questionnaire (SIBDQ) scores were 5.3 (IQR 4.5–6.0) versus 4.9 (IQR 3.9–5.7), p<0.001) at baseline. For CD patients, older participants had markedly higher rates of prior surgery (65.2% vs. 48.1%, p<0.001) compared to the younger participants. Within the CD population, there was no significant difference between use of corticosteroids between the older and younger populations (16.0% vs 16.5%, p=0.77). However, significantly fewer older CD individuals reported biologic anti-tumor necrosis factor-alpha (anti-TNF) use compared to younger CD patients (29.1% vs 44.3%, p<0.001). Aminosalicylate (5-ASA) use was higher amongst older CD patients as compared to younger (40.3% vs 33.9%, p<0.001).
Ulcerative colitis
The older versus younger UC populations reported similar rates of remission (45.9% vs 43.9%, p=0.53) as determined by a SCCAI score of 2 or lower. Even with similar reported disease activity, older UC patients scored higher on the SIBDQ (5.5 (IQR 4.6–6.0) vs 5.0 (IQR 4.0–5.8), p<0.001). Furthermore, surgery rates in the older population were similar to the younger population for UC (13.6% vs 11.8%, p=.35). Additionally, the older population reported lower corticosteroid use at baseline than younger patients (9.6% vs 15.4%, p=0.004). Differing from the CD population, the older UC patients had similar biologic anti-TNF usage when compared to the younger UC patients (16.0% vs 19.2%, p=0.16). There was a small significant difference between rates of 5-ASA use among the older and younger UC populations (73.8% vs 68.2%, p=0.04).
Longitudinal Analyses
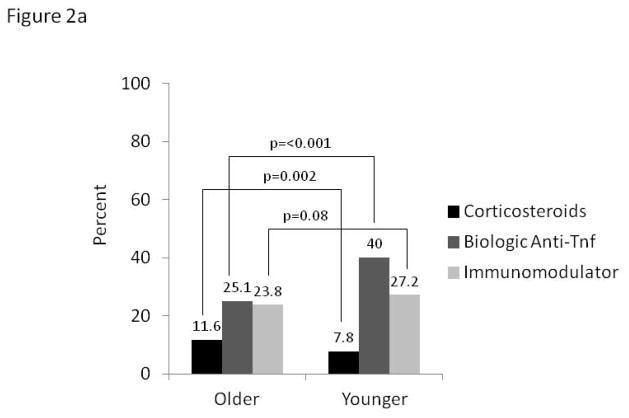
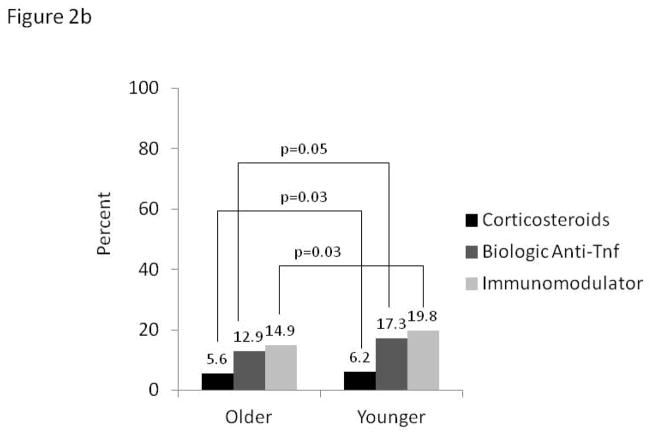
In the longitudinal analyses, using data from both baseline and follow-up visits 6–12 months apart, prevalence of continued steroid use (current use reported at both time points) was 48% higher along older versus younger CD patients (11.6% vs. 7.8%, p=0.002). In contrast, continued use of biologic anti-TNF agents over this same time period was 60% lower in older CD participants, whereas prevalence of immunomodulator use was similar (Figure 2a). For UC patients, continued steroids, biologic anti-TNF agents and immunomodulators were all used less frequently by the older (Figure 2b).
Figure 2.
Figure 2a: Continued* medication use in Crohn’s disease by age (n=3392)
*Defined by use at baseline and follow-up at least 6 months apart
Figure 2b: Continued* medication use in ulcerative colitis by age (n=1990)
*Defined by use at baseline and follow-up at least 6 months apart
Patient Reported Outcomes in Older Patients
We evaluated affects of continued medication use over 6–12 months on PROs measured at follow up. Among older patients, those with continued steroid use had significantly worsened anxiety (mean 52.5), sleep (mean 52.4) and fatigue (mean 55.3) as compared to non-steroid use. Pain (mean 53.9) and depression (mean 50.5) were also higher, albeit not statistically significantly when compared to non-steroid users. Similar effects were observed within strata of CD and UC participants. Differences in PRO’s overall and by disease subtype are shown in table 3. All comparisons that met statistical significant also met the threshold of ≥2, associated with a minimally important difference clinically. In a sensitivity analysis of only participants in remission at baseline, older patients with continued steroid use still had poorer health related quality of life scores in all 5 PROMIS domains when compared to non-users, although these differences were only significant for the fatigue domain (mean 52.2 vs. mean 48.2, p=0.02). In a separate analysis comparing older IBD patients on continued steroid monotherapy to older IBD patients on immunomodulators or biologic anti-TNF agents without steroids, those on continued steroids had significantly worsened depression (mean 50.8 vs. mean 48.2, p=0.03) and anxiety (mean 52.6 vs. mean 49.8, p=0.04) at follow up.
Table 3.
Patient reported outcomes measured by PROMIS in older patients with IBD
| Patient Reported Outcome | Continued Steroid Use | No Continued Steroid Use | p value* |
|---|---|---|---|
| IBD overall | (n=90) | (n=871) | |
| Anxiety | 52.5 | 50.3 | 0.02 |
| Sleep | 52.4 | 50.1 | 0.01 |
| Pain | 53.9 | 50.9 | 0.07 |
| Fatigue | 55.3 | 51.6 | 0.001 |
| Depression | 50.5 | 48.8 | 0.07 |
| Crohn’s disease | (n=70) | (n=535) | |
| Anxiety | 52.4 | 50.0 | 0.03 |
| Sleep | 52.3 | 50.6 | 0.11 |
| Pain | 53.1 | 50.9 | 0.25 |
| Fatigue | 55.7 | 52.6 | 0.02 |
| Depression | 50.8 | 48.7 | 0.05 |
| Ulcerative colitis | (n=20) | (n=336) | |
| Anxiety | 52.9 | 50.7 | 0.31 |
| Sleep | 52.9 | 49.5 | 0.08 |
| Pain | 56.3 | 50.9 | 0.11 |
| Fatigue | 54.2 | 50.2 | 0.08 |
| Depression | 49.6 | 49.0 | 0.79 |
By student’s t-test
Discussion
In a cross-sectional analysis, we found geographic differences in baseline medication utilization as well as differences between the older and younger CD and UC populations. Steroid use at baseline was similar between age groups for both CD and UC; however, biologic use was markedly lower for older patients with CD as compared to the younger population.
Lower rates of biologic use and higher 5-ASA use in older CD patients may suggest milder disease activity. This is supported by the higher rates of remission and better SIBDQ scores seen in this older CD population. However, despite these indicators supporting a milder disease activity, the reported GI surgery rates for both the older CD and UC populations suggest that disease activity may not be milder in the older. It is possible that providers are reluctant to prescribe biologic agents in this older population. Although biologic agents are highly effective, costs to the patient must be taken into account, especially for older patient populations on a fixed income. Per-patient yearly expenditure is estimated around $8,265–$11,129 for CD, more costly than diabetes, stroke, coronary artery disease, chronic obstructive pulmonary disease, or multiple sclerosis.25–27 The lower rates of biologic use in the older may be partially influenced by patients’ out of pocket expenses for these medications. It is also possible that concerns about safety of these agents in older patients may impact prescribing patterns.14, 28 Interestingly, older patients with CD had significantly higher rates of continued steroid use than younger populations in our longitudinal analysis—perhaps related to these same factors of cost and perceived safety. In fact, corticosteroids, especially when used for more than 3 months duration, are considered potentially inappropriate medications (PIM) by the Beers Criteria. PIMs are associated with increased hospitalizations, costs of care, and mortality among older persons.29 Furthermore, it is important to note that steroids, not biologic anti-TNF agents or immunomodulators, have been associated with increased mortality in patients with IBD.15
Prior studies of medication utilization amongst the older IBD cohort indicate greater reliance on corticosteroids and 5-aminosalicylates as maintenance therapies.8, 9, 30 Biologic agents are used less frequently, in spite of literature supporting efficacy in moderate-to-severe CD and UC. Delays in starting appropriate steroid-sparing therapies may contribute to the potentially lower therapeutic efficacy seen in the few studies available, as well as the increased rates of adverse events.14 Higher rates of discontinuation associated with these medications in older patients may be due to poorer response rates but also may be due to evolving disease activity and declines in physical reserve. Consequences of prolonged disease activity (anemia, malnutrition, dehydration, etc) can be associated with increased infection risk and increased hospitalizations.4 Biologic agents have recently been associated with increased risks of adverse events in older patients with IBD, but this may be confounded by complications of disease activity.14 In contrast to data from IBD, the rheumatology literature does not describe differing rates of biologic anti-TNF use by age. Data from the anti-rheumatic drug intervention and utilization study (RADIUS) study, a real-world prospective observational program of rheumatoid arthritis patients aimed at assessing prescribing patterns, safety and effectiveness of disease modifying anti-rheumatic drugs (DMARDs) and biologics, showed similar rates of biologic prescribing for the older (≥ 65 years) and younger (<65 years) patients.31 Extrapolations from rheumatoid arthritis-based studies focusing on the older population indicate an increased risk of serious infection and hospitalization with immunosuppression, but the safety signals are greater with corticosteroids and non-biologic DMARDs compared to anti-TNF based therapies.32, 33
Importantly, our study assessed the association of continued steroid use on important PROs in the older population. Given the effects we found on depressive symptoms, anxiety, fatigue, and sleep, continued steroid use should not be considered a “milder” treatment when compared to other forms of immunosuppression. These therapies directly impact a patient’s quality of life. These effects can have a greater impact even than well-known complications of corticosteroids including weight gain, bone health, metabolism, diabetes, and cataracts. These untoward effects can be particularly debilitating in an older population. Factors such as anxiety, fatigue, and depression negatively impact functional status. Declines in functional status in the older may result in increased disability, morbidity and cognitive impairment.34
The strengths of this cohort study include the geographically diverse and large sample size of participants in CCFA Partners, with members in every US state. As older patients are not well-represented in randomized controlled trials and are not necessarily seen in large numbers at individual centers, this unique cohort allows for comparisons of important PROs in a large sample of older IBD patients. Additionally, within our cohort we are able to capture outcome data using validated instruments for self-report. There are also several limitations to this study. First, the patient volunteers who make up the CCFA Partners cohort are not necessarily representative of the IBD population of the US. Therefore, these findings may have limited external generalizability. As internet capability is required to participate, the CCFA Partners members may have higher education levels and socioeconomic status when compared to the IBD population in general. We also do not have access to pharmacy data or records of all prior medications and reasons for discontinuation. We did not collect data on steroid-specific physical manifestations such as striae, fluid retention, cataracts or fractures. We chose to focus on PRO’s instead, such as mood effects, sleep and fatigue, as these manifestations have not been systematically evaluated in a population of older patients on continued corticosteroids. We also do not have validation of all IBD diagnoses from within our cohort. However, in a validation study of a sample within CCFA Partners, over 97% of individuals accurately reported their disease type when compared to records from their treating physician.35 Longitudinal data from within this cohort does have internal validity and the study design allows for optimal collection of PRO data.
In conclusion, older patients with IBD have different medication utilization patterns when compared to those of younger patients. There were large differences in biologic anti-TNF utilization in older versus younger CD patients. Continued steroid use was significantly higher in older CD patients and was negatively associated with important PROs. A better understanding of the complications of continued steroid use in this population will help drive age-specific guidelines for medication use. Ultimately, quality of life measures for older patients with IBD will be improved if continued steroid use can be minimized. With the aging of the IBD population, we need to understand the impact of our various therapies on both IBD activity and patient related outcomes.
Acknowledgments
Grant support: This work was supported by the Crohn’s and Colitis Foundation of America, the Patient Centered Outcomes Research Institute, NIH P30 DK34987 (RSS), and NIH 1K08DK088957-01 (MDK), NIDDK T35 DK07386 (MG)
Footnotes
Author contribution: All authors have made substantial contributions to this work. MDL and MG participated in all of the following: (1) the conception and design of the study, acquisition of data, analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted. MDK and CH participated in 1) the conception and design of the study, (2) final approval of the version to be submitted. CFM participated in 1) the conception and design of the study, acquisition of data, analysis and 2) final approval of the version to be submitted. WC participated in 1) acquisition of data, 2) final approval of the version to be submitted. KS participated in 1) acquisition of data, 2) final approval of the version to be submitted. RSS participated in 1) the conception and design of the study, (2) final approval of the version to be submitted.
We certify that this manuscript, including related data, figures and tables has not been previously published and the manuscript is not under consideration elsewhere.
The authors have no financial disclosures relevant to this work. Dr. Long has previously served as consultant to Abbvie, Salix and NPS Pharmaceuticals. Dr. Kappelman has previously served as a consultant to Abbvie, Janssen, GlasoSmithKline, and Cubist.
References
- 1.Grayson K, Vincent VAV. Bureau USC, editor. THE NEXT FOUR DECADES, The Older Population in the United States: 2010 to 2050. 2010. [Google Scholar]
- 2.Benchimol EI, Manuel DG, Guttmann A, et al. Changing age demographics of inflammatory bowel disease in Ontario, Canada: a population-based cohort study of epidemiology trends. Inflamm Bowel Dis. 2014;20:1761–9. doi: 10.1097/MIB.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 3.http://www.aoa.acl.gov/Aging_Statistics/Profile/index.aspx.
- 4.Ananthakrishnan AN, McGinley EL, Binion DG. Inflammatory bowel disease in the elderly is associated with worse outcomes: a national study of hospitalizations. Inflamm Bowel Dis. 2009;15:182–9. doi: 10.1002/ibd.20628. [DOI] [PubMed] [Google Scholar]
- 5.Charpentier C, Salleron J, Savoye G, et al. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut. 2014;63:423–32. doi: 10.1136/gutjnl-2012-303864. [DOI] [PubMed] [Google Scholar]
- 6.Ha CY, Newberry RD, Stone CD, et al. Patients with late-adult-onset ulcerative colitis have better outcomes than those with early onset disease. Clin Gastroenterol Hepatol. 2010;8:682–687. e1. doi: 10.1016/j.cgh.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ha CY, Katz S. Clinical outcomes and management of inflammatory bowel disease in the older patient. Curr Gastroenterol Rep. 2013;15:310. doi: 10.1007/s11894-012-0310-4. [DOI] [PubMed] [Google Scholar]
- 8.Benchimol EI, Cook SF, Erichsen R, et al. International variation in medication prescription rates among elderly patients with inflammatory bowel disease. J Crohns Colitis. 2013;7:878–89. doi: 10.1016/j.crohns.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Juneja M, Baidoo L, Schwartz MB, et al. Geriatric inflammatory bowel disease: phenotypic presentation, treatment patterns, nutritional status, outcomes, and comorbidity. Dig Dis Sci. 2012;57:2408–15. doi: 10.1007/s10620-012-2083-x. [DOI] [PubMed] [Google Scholar]
- 10.Johnson SL, Bartels CM, Palta M, et al. Biological and steroid use in relationship to quality measures in older patients with inflammatory bowel disease: a US Medicare cohort study. BMJ Open. 2015;5:e008597. doi: 10.1136/bmjopen-2015-008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–23. doi: 10.1038/ajg.2009.727. quiz 524. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenstein GR, Hanauer SB, Sandborn WJ. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465–83. doi: 10.1038/ajg.2008.168. quiz 464, 484. [DOI] [PubMed] [Google Scholar]
- 13.Parian A, Ha CY. Older age and steroid use are associated with increasing polypharmacy and potential medication interactions among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1392–400. doi: 10.1097/MIB.0000000000000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lobaton T, Ferrante M, Rutgeerts P, et al. Efficacy and safety of anti-TNF therapy in elderly patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2015;42:441–51. doi: 10.1111/apt.13294. [DOI] [PubMed] [Google Scholar]
- 15.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4:621–30. doi: 10.1016/j.cgh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Ezzat Y, Hamdy K. The frequency of low bone mineral density and its associated risk factors in patients with inflammatory bowel diseases. Int J Rheum Dis. 2010;13:259–65. doi: 10.1111/j.1756-185X.2010.01542.x. [DOI] [PubMed] [Google Scholar]
- 17.Long MD, Kappelman MD, Martin CF, et al. Development of an internet-based cohort of patients with inflammatory bowel diseases (CCFA Partners): methodology and initial results. Inflamm Bowel Dis. 2012;18:2099–106. doi: 10.1002/ibd.22895. [DOI] [PubMed] [Google Scholar]
- 18.Randell RL, Long MD, Cook SF, et al. Validation of an internet-based cohort of inflammatory bowel disease (CCFA partners) Inflamm Bowel Dis. 2014;20:541–4. doi: 10.1097/01.MIB.0000441348.32570.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thia K, Faubion WA, Jr, Loftus EV, Jr, et al. Short CDAI: development and validation of a shortened and simplified Crohn’s disease activity index. Inflamm Bowel Dis. 2011;17:105–11. doi: 10.1002/ibd.21400. [DOI] [PubMed] [Google Scholar]
- 20.Turner D, Seow CH, Greenberg GR, et al. A systematic prospective comparison of noninvasive disease activity indices in ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7:1081–8. doi: 10.1016/j.cgh.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Jowett SL, Seal CJ, Phillips E, et al. Defining relapse of ulcerative colitis using a symptom-based activity index. Scand J Gastroenterol. 2003;38:164–71. doi: 10.1080/00365520310000654. [DOI] [PubMed] [Google Scholar]
- 22.Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol. 1996;91:1571–8. [PubMed] [Google Scholar]
- 23.Kappelman MD, Long MD, Martin C, et al. Evaluation of the patient-reported outcomes measurement information system in a large cohort of patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2014;12:1315–23. e2. doi: 10.1016/j.cgh.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yost KJ, Eton DT, Garcia SF, et al. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. J Clin Epidemiol. 2011;64:507–16. doi: 10.1016/j.jclinepi.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–9. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Kappelman MD, Rifas–Shiman SL, Porter CQ, et al. Direct Health Care Costs of Crohn’s Disease and Ulcerative Colitis in US Children and Adults. Gastroenterology. 2008;135:1907–1913. doi: 10.1053/j.gastro.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunnarsson C, Chen J, Rizzo JA, et al. Direct health care insurer and out-of-pocket expenditures of inflammatory bowel disease: evidence from a US national survey. Dig Dis Sci. 2012;57:3080–91. doi: 10.1007/s10620-012-2289-y. [DOI] [PubMed] [Google Scholar]
- 28.Ha C, Katz S. Management of inflammatory bowel disease in the elderly: do biologicals offer a better alternative? Drugs Aging. 2013;30:871–6. doi: 10.1007/s40266-013-0120-x. [DOI] [PubMed] [Google Scholar]
- 29.American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616–31. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taleban S. Challenges in the Diagnosis and Management of Inflammatory Bowel Disease in the Elderly. Curr Treat Options Gastroenterol. 2015;13:275–86. doi: 10.1007/s11938-015-0059-6. [DOI] [PubMed] [Google Scholar]
- 31.Gibofsky A, Palmer WR, Goldman JA, et al. Real-world utilization of DMARDs and biologics in rheumatoid arthritis: the RADIUS (Rheumatoid Arthritis Disease-Modifying Anti-Rheumatic Drug Intervention and Utilization Study) study. Curr Med Res Opin. 2006;22:169–83. doi: 10.1185/030079906X80341. [DOI] [PubMed] [Google Scholar]
- 32.Atzeni F, Sarzi-Puttini P, Botsios C, et al. Long-term anti-TNF therapy and the risk of serious infections in a cohort of patients with rheumatoid arthritis: comparison of adalimumab, etanercept and infliximab in the GISEA registry. Autoimmun Rev. 2012;12:225–9. doi: 10.1016/j.autrev.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Widdifield J, Bernatsky S, Paterson JM, et al. Serious infections in a population-based cohort of 86,039 seniors with rheumatoid arthritis. Arthritis Care Res. 2013;65:353–61. doi: 10.1002/acr.21812. [DOI] [PubMed] [Google Scholar]
- 34.Colon-Emeric CS, Whitson HE, Pavon J, et al. Functional decline in older adults. Am Fam Physician. 2013;88:388–94. [PMC free article] [PubMed] [Google Scholar]
- 35.Randell RL. Validation of an internet-based cohort of inflammatory bowel disease (CCFA partners) Inflammatory bowel diseases. 2014;20:541–544. doi: 10.1097/01.MIB.0000441348.32570.34. [DOI] [PMC free article] [PubMed] [Google Scholar]