Abstract
Heart failure (HF) is a rapidly growing public health issue with an estimated prevalence of >37.7 million individuals globally. HF is a shared chronic phase of cardiac functional impairment secondary to many aetiologies, and patients with HF experience numerous symptoms that affect their quality of life, including dyspnoea, fatigue, poor exercise tolerance, and fluid retention. Although the underlying causes of HF vary according to sex, age, ethnicity, comorbidities, and environment, the majority of cases remain preventable. HF is associated with increased morbidity and mortality, and confers a substantial burden to the health-care system. HF is a leading cause of hospitalization among adults and the elderly. In the USA, the total medical costs for patients with HF are expected to rise from US$20.9 billion in 2012 to $53.1 billion by 2030. Improvements in the medical management of risk factors and HF have stabilized the incidence of this disease in many countries. In this Review, we provide an overview of the latest epidemiological data on HF, and propose future directions for reducing the ever-increasing HF burden.
Heart failure (HF) remains a rising global epidemic with an estimated prevalence of >37.7 million individuals globally1,2. As of 2011, within the USA alone, an estimated 5.7 million individuals live with HF and 870,000 new cases are diagnosed every year3. Many developing nations are in the midst of an epidemiological transition as the disease burden rapidly shifts from diseases related to nutritional deficiencies and infections to degenerative chronic diseases observed in the older population4. Excluding sub-Saharan Africa, the rates of death from noncommunicable diseases such as HF are increasing worldwide5. Data from the Global Burden of Disease Study5 indicate that approximately 17.3 million people died from cardiovascular causes in 2013, which is a 41% increase from the number of deaths attributed to cardiovascular disease in 1990 (REF. 5). The increase in cardiovascular disease burden is primarily due to demographic shifts, namely an expanding and ageing global population6. The ever-increasing incidence of HF in the USA since the 1970s has been described as an epidemic7,8. The number of hospitalizations that included HF as a diagnosis in the USA tripled from 1.27 million in 1979 to 3.86 million in 2004 (REF. 9), signifying a substantial economic burden on the health-care system. Although a diagnosis of HF portends increased mortality and loss of quality-adjusted life years, advances in evidence-based therapies and the quality of care in the modern era have substantially improved outcomes for patients. Between 1979 and 2000 in the USA, the absolute 5-year survival rate for HF increased by 9%10. In this Review, we provide an up-to-date overview of the epidemiology of HF, risk factors and aetiologies contributing to the disease burden, and its effect on health-service utilization and health-care expenditures.
Definition
Diseases of the heart muscle were widely documented in Rudolf Virchow’s publication of Die Cellularpathologie in 1858, in which he defined nonvalvular heart disease as ‘chronic myocarditis’, owing to his observation of inflammation on histological sections of diseased hearts11. Although the classification terminology for HF has evolved considerably since Virchow’s original description, controversies and ambiguities remain. HF is a shared chronic phase of a multitude of cardiac diseases. The ACC Foundation and AHA define HF as “a complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood” (REF. 12). According to their joint guidelines12, HF with reduced ejection fraction (HFrEF) is defined as an ejection fraction ≤40%, whereas HF with preserved ejection fraction (HFpEF) is defined as an ejection fraction ≥50%. Patients with an ejection fraction that falls between this range are considered to have borderline HFpEF. Given that this recommendation was published only 3 years ago in 2013, the literature on HFpEF involving the use of echocardiographic imaging data has varied thresholds for left ventricular ejection fraction between 40% and 55%12. In 2012, the ESC acknowledged the challenges and uncertainties in defining and diagnosing HFpEF, even when holistically evaluating patient symptoms, signs, imaging data, and biomarker studies13. The ESC guidelines integrate Doppler parameters and biomarker data into the HFpEF diagnostic criteria, and mention the potential of strain and speckle tracking as well as diastolic stress testing to improve categorization of patients with HFpEF13.
Although the term ‘cardiomyopathy’ is frequently used in clinical settings to describe ischaemic, valvular, and hypertensive disease, it should be reserved for disease of the myocardium with known genetic or phenotypic patterns. The WHO was among the first to direct a task force to clarify the definition of cardiomyopathies as the scientific understanding of HF aetiologies progressed14,15. The term ‘ischaemic cardiomyopathy’ continues to be used instead of the preferred term ‘ischaemic heart disease’ in various guidelines12,16,17. In 2006, the AHA published a scientific statement to classify cardiomyopathies as distinct diseases of the myocardium that are predominately genetic and associated with mechanical or electrical dysfunction that frequently progress to HF. Primary cardiomyopathies are generally confined to the heart muscle, whereas secondary cardiomyopathies have both myocardial and systemic multiorgan involvement18 (BOX 1). In 2008, the ESC provided their own classification of cardiomyopathies — with acknowledged differences from the AHA 2006 statement — based on their morphological and functional phenotypes19. In 2013, the World Heart Federation proposed a more specific MOGE(S) nosology system for cardiomyopathies, which describes the morphofunctional phenotype (M), organ involvement (O), genetic inheritance pattern (G), aetiological annotation (E), and functional status (S) of the disease. This classification system allows greater flexibility in categorizing overlapping genetic and phenotypic syndromes20. The redefinition of HF taxonomies will continue to evolve concurrently with our understanding of the molecular and genetic pathophysiology of HF.
Box 1. Taxonomy of heart failure aetiologies18,132 Ischaemic.
Coronary artery disease
Coronary dissection
Coronary embolism
Valvular
Rheumatic heart disease
Degenerative valvular disease
Hypertensive (both HFrEF and HFpEF)
Primary cardiomyopathies
Genetic
Hypertrophic cardiomyopathy
Arrhythmogenic cardiomyopathy
Left ventricular noncompaction
Mitochondrial myopathies
Ion-channel disorders (long QT syndrome, Brugada, etc.) Acquired
Tachycardia-induced
Peripartum
Stress-induced (Takotsubo)
Substance-abuse-induced (e.g. alcohol, cocaine)
Toxin-related (e.g. anthracycline)
Myocarditis (inflammatory)
Chagas
HIV
Viral
Giant cell myocarditis
Secondary cardiomyopathies
Amyloidosis
Sarcoidosis
Storage disease (e.g. haemochromatosis, Fabry disease)
Connective tissue disorder (e.g. scleroderma)
Thyroid disease
Endomyocardial fibrosis
Nutritional deficiencies (e.g. selenium, beriberi, kwashiorkor)
Anaemia
Arteriovenous fistula
Congenital heart disease
Pericardial disease
Other
HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Clinical and research definitions of HF are wide-ranging and prone to misclassification. Diagnostic criteria for HF can include physician assessment, laboratory tests, advanced cardiovascular imaging, or invasive haemodynamic catheterization. In 1971, the Framingham Heart Study provided clinical criteria for HF diagnosis based on physical examination and physician adjudication21. Framingham researchers utilized major and minor criteria to establish definite, probable, or questionable diagnoses for congestive HF22. The Carlson criteria, published in 1985, uses a points system based on patient history, physical exam, and chest radiography to determine the certainty of HF, mainly for research purposes23,24. Investigators in other studies, such as the Cardiovascular Health Study25, have diagnosed HF on the basis of a physician panel review of all pertinent medical records, including chest x-ray and echocardiograms25. The latest guidelines on the echocardiographic assessment of left ventricular function from the American Society of Echocardiography, published in 2015, recommend that ejection fraction should be considered abnormal when it is ≤52% for men and ≤54% for women26. Validation studies to assess the sensitivity and specificity of several HF criteria have yielded variable results27,28.
Health-service utilization and hospitalization rates associated with HF frequently depend on medical provider billing information in the community. The limitations on available administrative data have been described, with concerns for the underestimation of HF admissions, misspecification of diagnoses, unbundling of medical conditions, and upcoding29–31, leading some to question the accuracy of International Classification of Disease, Ninth Revision (ICD-9) codes, designed to facilitate international comparability in the collection and presentation of mortality statistics. An estimated one-third of patients hospitalized with HF lack relevant ICD-9 codes as a primary or secondary diagnosis for acute exacerbations29. ICD-10 codes were found to be highly predictive of acute HF compared with physician adjudication (using the Carlson criteria), and were similar to ICD-9 coding reliability with a positive predictive value of 90.2%, negative predictive value of 97.2%, sensitivity of 68.6%, and specificity of 99.3%31. Over time, upcoding and misclassification might substantially misrepresent trends in incidence and prevalence. Improved diagnostic accuracy might confound outcomes such as hospitalization rates and mortality8. More routine utilization of echocardiography and advancements in echocardiographic imaging have led to increased diagnosis of both HFrEF and HFpEF in hospitalized patients, which makes comparing cohorts between eras increasingly challenging.
Epidemiology
Incidence
Estimates of HF incidence and trends in the global population are scarce and unreliable32. Most of the literature on HF epidemiology and management is derived from high-income, developed nations. In these countries, HF incidence in particular populations has shown signs of stabilization and possible reduction. Improvements in the primary prevention of cardiovascular diseases and the treatment of ischaemic heart disease are the primary drivers of this trend33,34. In general, the global incidence of HF ranges from 100 to 900 cases per 100,000 person-years depending on the diagnostic criteria used and population studied8. Investigators in the Atherosclerosis Risk in Communities Study3, who assessed trends in hospitalization and case fatality rates, estimated 915,000 new cases of HF in the USA in 2012.
Community-based cohorts, such as Framingham and Olmsted County, might provide more reliable information on the incidence and prevalence of HF through their use of case validation sampling strategies. Cases can be confirmed by reviewing medical record, findings on physical exams, and physician panel review. For >65 years, the Framingham Heart Study has reported on risk factors, prevalence, and trajectories of numerous cardiovascular diseases35. Importantly, their diagnostic criteria and methods have remained uniform over time, rendering their reported trends for risk factors and cardiovascular diseases more consistent. However, as both the Framingham and Olmsted County cohorts are predominately made up of white individuals, the trends measured using these cohorts cannot be generalized to more ethnically diverse populations32,35.
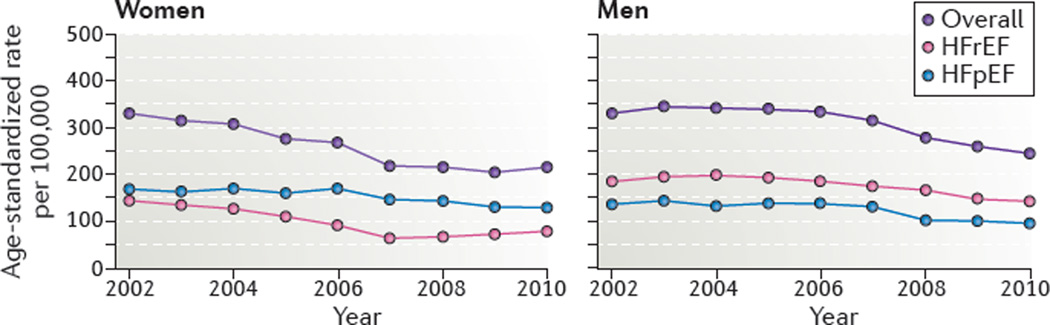
Over the past 60 years, the incidence of HF in the USA has stabilized, and standardized age-adjusted rates are thought to be decreasing. Between 1950 and 1999, the incidence of HF in women from the Framingham cohort reduced from 420 to 327 cases per 100,000 person-years35. However, this reduction was not observed for men, whose HF incidence remained at approximately 564 cases per 100,000 person-years35. Between 2000 and 2010, the age-adjusted and sex-adjusted incidence of HF declined from 315.8 to 219.3 per 100,000 residents in the Olmsted County cohort, a 37.5% decline over the decade32 (FIG. 1). A greater decline in HF incidence was observed in women (43%) than in men (29%)32. The incidence of HF varies between ethnic groups in the USA. The Multi-Ethnic Study of Atherosclerosis36 reported the highest incident rates of HF among African–American individuals, intermediate rates among white and Hispanic individuals, and the lowest rates among Chinese–American individuals. Differences in risk factors (including hypertension and diabetes mellitus), as well as socioeconomic status, contribute to this ethnic disparity in the incidence of HF36.
Figure 1. Incidence of heart failure in Olmsted County between 2000 and 2010.
A decrease in the age-adjusted rate of HF for both men and women was observed between 2000 and 2010. This decrease is most notable for HRrEF. HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction. Modified from Gerber, Y. et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern. Med. 175, 996–1004 (2015), with permission from the American Medical Association.
Similar reductions in HF incidence have been observed in Europe. More than 8,000 participants from the community-based PREVEND cohort study34 from Groningen, the Netherlands recruited in 1997–1998 were followed up for 11 years for assessment of cardiovascular risk factors and disease epidemiology. Using the ESC criteria to diagnose HF, the incident rate of HF was 387.4 cases per 100,000 person-years, with 34% of all cases categorized as HFpEF37,38. A Swedish study using data from an administrative health register in Stockholm comprising 2.1 million inhabitants reported 380 new cases of HF per 100,000 person-years in 2010, with an absolute reduction of 90 cases compared with their 2006 national health data39.
Approximately 80% of the global cardiovascular disease burden occurs in middle-income and low-income countries5,40,41. Projects such as the INTERnational Congestive Heart Failure study38, the PURE study39, and the Global Burden of Disease Study40 aim to measure the burden of HF in these countries5,40,41. The PURE study involves a cohort of 156,424 individuals from three high-income, 10 middle-income, and four low-income nations, and is designed to assess factors related to the development of chronic conditions. After a mean follow-up of 4.1 years, the PURE investigators reported 271 new cases of HF per 100,000 person-years41. Among African patients hospitalized for cardiovascular disease, acute decompensated HF was the most common diagnosis42. Improvements in public-health infrastructure and further research are needed to improve monitoring of the cardiovascular disease burden in these middle-income and low-income nations.
Prevalence
An estimated 37.7 million people are living with HF globally2. The estimates of HF prevalence in developed countries generally range from 1–2% of the adult population43. Although the age-adjusted incidence and prevalence of HF are decreasing, the absolute number of patients with HF has drastically increased, secondary to shifts in the global age distribution, as well as general population growth6.
Before the 1970s, the prevalence of HF in the USA was estimated using hospital records or death certificates with limited reliability, owing to a large proportion of care being provided on an outpatient basis. The development of the first National Health and Nutritional Examination Survey (NHANES) facilitated a more accurate estimation of HF prevalence nationally44. The prevalence of self-reported and clinically defined HF in 1971–1975 was estimated to be 1.1% and 2.0%, respectively44. Investigators in the NHANES study compiled self-reported HF data between 2009 and 2012, and estimated that approximately 5.7 million adults (2.2%) in the USA live with the chronic condition3. Consistent with this figure, the prevalence of HF in Sweden was estimated to be 2.2% for both men and women39. By 2030, the prevalence of HF (not adjusted for age) in the USA is projected to increase by 46% to >8 million people (2.97%)45. An ageing national population is the primary driver for the mounting HF burden.
The HF burden is disproportionately distributed among the elderly. Over half of patients hospitalized with HF are aged >75 years46. The prevalence of HF generally doubles for each decade of life. The prevalence is <1% for those aged <40 years, and >10% for those aged >80 years3. The lifetime risk of developing HF is approximately 20% between the ages of 40 and 80 years for both men and women47. Improvements in medical management have delayed the onset of HF, and prolonged the lives of those who develop the condition. These shifting demographics highlight the importance of integrating geriatric medicine into HF management. Furthermore, as trials have tended to excluded or under-represent older populations, further research on improving outcomes in this age cohort is needed48.
In the USA, the prevalence of HF varies considerably by ethnicity, socioeconomic status, and geographical region. Lower socioeconomic status is associated with higher rates of HF when controlling for known cardiovascular risk factors49,50. African–American individuals have a threefold increased risk of developing dilated cardiomyopathy when controlling for socioeconomic factors and comorbidities3,12. The unadjusted prevalence of HF is similar among white individuals and Hispanic individuals in the USA, and is lowest among Chinese–American individuals51. The southeastern region of the USA, spanning from Georgia in the east to Oklahoma in the west — commonly referred to as the ‘stroke belt’ owing to elevated rates of cerebrovascular events in this region — has a 69% higher age-adjusted mortality from HF than the national average52. Such large variations in disease rates by ethnicity, region, and socioeconomic status suggest that targeting health services might improve the prevention and management of cardiovascular diseases in high-risk communities53.
Cross-sectional and cohort studies conducted in developing countries have identified risk factors and aetiologies for HF, but more detailed epidemiological data are unavailable54–60. Although reliable estimates for middle-income and low-income nations are lacking, evidence from the current literature suggests that HF is the fastest growing cardiovascular condition globally61,62. Of the 1.63 billion people who live in South Asia, 30 million would have had HF in 2011, if the prevalence of HF in South Asia is assumed to be the same as that in the USA63. However, this assumption is purely hypothetical and requires validation.
Mortality
Estimating the number of deaths attributable to HF alone is challenging, because HF is commonly categorized as an intermediate stage of an underlying condition such as coronary artery disease, and not the actual cause of death. The Global Burden of Disease Study5 defines the causes of death using ICD-9 and ICD-10 codes. However, the code for HF is treated as a ‘garbage code’ — an ambiguous or vague code associated with a nonspecific cause of death. Death caused by HF would often be reassigned to the most likely underlying cause, such as ischaemic heart disease. Death certificate documentations have the same limitations and might also be subject to inaccuracies. For HF of unknown aetiology, the cause of death is frequently reassigned to coronary artery disease64. Using all-cause cardiovascular mortality as a surrogate for HF mortality trends, the Global Burden of Disease Study estimates that the age-standardized cardiovascular rate of death was reduced by 22% between 1990 (375.5 deaths per 100,000 person-years) and 2013 (293.2 deaths per 100,000 person-years)5. This reduction in overall cardiovascular mortality might also indicate a decrease in global age-adjusted mortality for HF.
In the USA in 2011, one in nine death certificates (n = 300,122) listed HF as a cause of death3. A diagnosis of HF has previously been described as more ‘malignant’ than cancer, given the comparatively low 5-year survival rates65. In 1991, 5-year mortality from HF was 11% higher than the corresponding rates for gastrointestinal cancers65. Comparative 5-year age-adjusted and sex-adjusted survival rates for cancer, stroke, and HF were found to be generally similar in a systematic review, with observed improvements in survival for all three diseases over the past decade66.
Despite the high fatality rates for patients with HF, survival rates have increased remarkably with treatment advances in the developed world. The Framingham Heart Study reported a decline in 5-year mortality from 70% between 1950 and 1969 to 59% between 1990 and 1999 for men, and a reduction from 57% to 45% for women during the respective periods35. The community-based cohort from Olmsted County reported a 20.2% age-adjusted mortality for incident HF at 1-year and 56.2% at 5 years; these rates did not change significantly between 2000 and 2010. More than half of the HF cohort died from noncardiovascular causes; 14.2% of deaths were related to respiratory disease, 12.6% to neoplasm, and 7.1% to mental health32. Earlier analyses from Olmsted also noted a temporal improvement in overall 5-year mortality in the time period since 1979 (REF. 10). Likewise, in England between 1981 and 2010, age-standardized death rates for HF decreased from 130.6 to 51.8 per 100,000 people67. The steepest declines were noted among middle-aged individuals between 55 and 64 years67. National estimates for Sweden in 2010 reported the 5-year HF survival rate as 48%, with 320 deaths per 100,000 person-years for women and 300 deaths per 100,000 person-years for men39. In Germany, the unadjusted 1-year mortality for patients first hospitalized with HF was 23% in 2006 (REF. 68).
Mortality for hospitalized HF has also improved over the past decade. Between 1999 and 2011, in-hospital mortality decreased by 38%, 30-day mortality by 16.4%, and 1-year mortality by 13.0% for Medicare patients with HF in the USA69. Similarly, in Ontario, Canada, 1-year risk-adjusted mortality for outpatients decreased from 17.7% to 16.2% between 1997 and 2007; a nonsignificant decline was also noted for inpatients during the same period70. This increase in mortality might be attributable to several factors. The quality of care and expanded use of evidence-based medical therapies has increased survival for high-risk hospitalized patients. Hospital systems now regularly report HF outcomes and improved care processes71. The rates of smoking have declined, and hypertension control has improved marginally72,73. Furthermore, the improvements in observed outcomes might reflect greater diagnostic sensitivity, increased recognition of HFpEF by clinicians, and shifts in coding practices that create a healthier pool of patients with HF in the current era compared with previous decades74,75.
Estimates of HF mortality in the developing world are limited. Fatality rates for HF are estimated to be 3.72 times higher in low-income countries and 2.61 times higher in middle-income countries than in high-income countries after adjusting for age and sex41. A higher threshold for case definitions, greater disease severity, and limited availability of evidence-based therapies might explain these higher fatality rates in developing nations.
HFpEF
Patients with HFpEF comprise a large and important and subpopulation of individuals with HF. Patients with HFpEF frequently experience delayed diagnosis and limited treatment options. As this population presents with a unique phenotype that is distinct from HFrEF, special attention will be given to the known epidemiology of HFpEF in the following section.
HFpEF is a clinical syndrome defined as HF with normal ejection fraction and impaired diastolic function on objective imaging. For an accurate diagnosis of HFpEF, the HF symptoms in these patients must not be secondary to another condition. HFpEF has received increased attention in the literature as patients diagnosed with this clinical syndrome have unique trajectories and management challenges. The greatest risk factor for HFpEF is hypertension12,76, and additional risk factors include older age, female sex, and diabetes. Rare aetiologies for HFpEF include hypertrophic cardiomyopathy, infiltrative cardiomyopathies such as amyloidosis, and iron-storage diseases such as haemochromatosis. Medical interventions specifically targeting the disease course of HFpEF are lacking37,77.
HFpEF is estimated to comprise between 44% and 72% of all cases of HF78. Differences in case definitions over time have contributed to variations in the reported incidence and prevalence of HFpEF. The prevalence of HFpEF in middle-income and low-income countries is not well characterized76. International trial data that include patients from North America, Europe, and Russia suggest that the prevalence of HFpEF is highest in the USA and Canada, intermediate in Western Europe, and lowest in Eastern Europe and Russia79. In the Olmsted County cohort, the proportion of HFpEF among incident cases of HF increased from 38.0% in 1986 to 47.8% in 2000, and to 56.9% in 2010. Additionally, the decline in the incidence of HFpEF was less than the decline in the incidence of HFrEF32,80. Using data from the AHA’s Get With The Guidelines — Heart Failure project, investigators found that the proportion of patients hospitalized with HFpEF increased from 33% in 2005 to 39% in 2010 (REF. 75). Mortality for HFpEF is slightly lower than that for HFrEF (121 deaths versus 141 deaths per 1,000 patient-years) after adjusting for age, sex, and comorbidities81; however, observed readmission rates are higher for HFpEF than for HFrEF82. To date, no effective therapies are available to improve survival of patients with HFpEF.
Aetiologies
A wide range of cardiac conditions, hereditary defects, and systemic diseases can result in HF (BOX 1). Patients with HF can have mixed aetiologies, which are not mutually exclusive, and HF aetiologies vary considerably between high-income and developing countries41,83. HF has an estimated 17 primary aetiologies, as determined by the Global Burden of Disease Study84. More than two-thirds of all cases of HF can be attributed to four underlying conditions: ischaemic heart disease, chronic obstructive pulmonary disease, hypertensive heart disease, and rheumatic heart disease. Although the Global Burden of Disease Study aims to approximate the burden of right-sided HF from chronic obstructive pulmonary disease, studies estimating the prevalence of right-sided HF are limited and require further study84. High-income regions are disproportionally affected by ischaemic heart disease and chronic obstructive pulmonary disease compared with low-income regions, which in turn are primarily affected by hypertensive heart disease, rheumatic heart disease, cardiomyopathy, and myocarditis2. The assessment and management of cardiovascular risk around the world requires the tailoring of policies to population-specific risks and underlying aetiologies33.
Ischaemic heart disease
Medical descriptions of angina pectoris date back to 1772, but understanding of the pathophysiology underlying the syndrome did not progress until the late 19th century, when calcification and thrombosis of the coronary arteries were first described85,86. Early studies, such as the Framingham Heart Study, identified the risk factors for coronary artery disease, including hypertension, hyperlipidaemia, diabetes, and smoking. The rate of death from cardiovascular causes has steadily declined since the 1960s with increased recognition of cardiovascular risk factors and greater attention to primary prevention87. The incidence of ischaemic heart disease, hypertension, diabetes, and other chronic diseases tends to parallel the increased consumption of food high in fat and sugar, and sedentary behaviour. The incidence and prevalence of these chronic diseases tend to increase unless preventive health strategies are implemented4. This pattern of shifting disease prevalence is described as an epidemiological transition that is strongly associated with the economic development of a country or region.
Ischaemic heart disease was the leading underlying cause of death globally in 2013, accounting for 15.7% of all age-standardized deaths, equating to a total of 8,139,900 deaths5. The Global Burden of Disease Study estimates that the prevalence of ischaemic HF between 1990 and 2010 increased from 240 to 270 per 100,000 person-years in men, and was stable at 190 per 100,000 in women88. Using the estimated rate of myocardial infarctions as a surrogate for the incidence of ischaemic heart disease, there is evidence for epidemiological transitions among both industrialized and developing nations. Age-standardized rates of myocardial infarction decreased between 1990 and 2010 in high-income countries in Australasia, Europe, and North America88. Within these regions, the greatest increase in the number of myocardial infarctions was in Eastern Europe88. In Sweden, the prevalence of ischaemic heart disease among patients with HF declined between 2006 and 2010 by approximately 7–8%39. In the Olmsted County cohort, the proportion of ischaemic HFrEF declined from 39.8% to 29.4% between 2000 and 2010, whereas the proportion of ischaemic HFpEF increased from 29.0% to 32.6% in this same time period32. The decrease in the proportion of patients with HFrEF is likely to be attributable to a reduction in myocardial infarction through both primary and secondary prevention strategies. Furthermore, patients with active myocardial infarction are more swiftly treated with percutaneous coronary intervention and other medical therapies89. With respect to the global burden of ischaemic heart disease, the incidence of acute myocardial infarction worldwide is highest in Eastern Europe and Central Asia, with >340 cases per 100,000 person-years for men and 180 cases per 100,000 person-years for women88. The lowest rates of acute myocardial infarction were observed in high-income nations in Asia88.
Hypertensive heart disease
An increase in blood pressure exposes cardiac myocytes to elevated mechanical stress and neurohormones, which increase myocardial mass and result in left ventricular hypertrophy. These cardiac changes can further progress to HFpEF or HFrEF, even in the absence of obstructive epicardial coronary arteries and myocardial infarction90. NHANES data from the USA show that, between 1999 and 2012, the proportion of patients treated for hypertension improved from 59.8% to 74.7%, and the proportion of patients with hypertension and adequately controlled blood pressure improved from 53.3% to 68.9%73. This improvement in blood pressure control is likely to have contributed to the decline in the incidence of HF. In the Olmsted County cohort, 73.6% of patients with HFrEF had hypertension compared with 89.3% of patients with HFpEF32. The lifetime risk of HF for individuals with blood pressure >160/90 mmHg is double that of those with blood pressure <140/90 mmHg47. Early randomized trials on hypertension control reported that effective treatment of moderate (≥140/90 mmHg) and severe hypertension (≥180/110 mmHg) reduces the risk of HF by 87%91. In the USA, antihypertensive treatment has reduced the incidence of HF by approximately 50%35.
Although hypertension has been recognized as a potent cardiovascular risk factor for many decades, gaps in the treatment of high blood pressure still remain. Hypertension affects all socioeconomic classes. Clinical evaluation of 1,515 consecutive cardiac referrals in Nigeria resulted in a diagnosis of hypertensive HF in 61% of patients92. However, this study was limited in that patients did not receive advanced diagnostic imaging or invasive angiography to confirm the low prevalence of ischaemic heart disease. Investigators in the PURE study93 found that, within a sample of patients with hypertension from high-income countries, only 49% of patients with blood pressure >140/90 mmHg were aware of their diagnosis, while 46.7% received treatment, and 19.0% had adequate control of their hypertension. In low-income countries, the rate of awareness was 40.8%, 31.7% received treatment, and 12.7% had adequate control93. These rates reflect the opportunity for improved management of hypertension globally in reducing preventable cardiovascular diseases.
Valvular and rheumatic HF
In developed countries, most cases of valvular heart disease are degenerative in nature, and the incidence of rheumatic heart disease is exceedingly low. The prevalence of any valve disease diagnosed using echocardiography is estimated to be 2.5% in the USA; prevalence increases substantially with age to 11.7% in individuals aged >75 years94. The prevalence of clinically diagnosed valvular disease is 1.8%94. In 2010, approximately 106,000 valve surgeries were performed in the USA3.
Data on the prevalence of HF among patients with clinical or echocardiographic diagnosis of valvular disease is scarce. Globally, the greatest burden of valvular disease is valvular HF that is secondary to rheumatic heart disease. In high-income nations, the substantial decline in prevalence of valvular disease is attributable to improvements in living conditions and availability of antibiotic therapy. The incidence of rheumatic fever has fallen below 1 case per 100,000 person-years in developed nations95. In developing countries, however, valvular HF resulting from rheumatic disease contributes substantially to morbidity and mortality. In Sudan, the incidence of rheumatic fever is >100 cases per 100,000 person-years95. A conservatively estimated 15.6 million people have rheumatic heart disease globally, with 470,000 new cases and 233,000 deaths per year96. When echocardiography is used to screen affected populations in developing countries, the prevalence of rheumatic heart disease increases tenfold97. Despite the high prevalence of rheumatic heart disease in low-income countries, the global age-standardized mortality has decreased 22% from 375.5 to 293.2 deaths per 100,000 between 1990 and 2013 (REF. 5).
Cardiomyopathies
Quantifying the global burden of cardiomyopathy is difficult given variations in diagnostic capabilities and coding practices5. A study of an Italian outpatient cohort estimated that the distribution of HF aetiologies was 36.0% dilated cardiomyopathy, 45.6% ischaemic cardiomyopathy, 12.9% hypertensive cardiomyopathy, and 5.5% from other causes83. The prevalence of cardiomyopathy in low-income countries is also poorly understood, and larger epidemiological studies are required98. The available data suggest that infectious, inflammatory, and nutritional deficiencies cause HF more commonly in sub-Saharan Africa than in middle-income and high-income countries99. An estimated 26.0% of the HF cases in sub-Saharan Africa have been attributed to the cardiomyopathies, specifically idiopathic dilated cardiomyopathy, HIV-related cardiomyopathy, hypertrophic cardiomyopathy, and endomyocardial fibrosis. Endomyocardial fibrosis is an endemic cardiomyopathy primarily described in Uganda and other regions with profound malnutrition from low protein and high cassava diets. An estimated 24% of dilated cardiomyopathies observed in sub-Saharan Africa might be secondary to myocarditis and autoimmune disorders60,100. Myocarditis has an estimated prevalence of 0.5% to 4.0% globally, and aetiologies vary depending on the region101.
Chagas cardiomyopathy
Chagas disease is an endemic parasitic disease caused by the protozoan Trypanosoma cruzi, and was estimated to affect 5.7 million people worldwide in 2010, mostly in Latin America. The use of insecticides to reduce the numbers of the insect that spreads the parasite (Triatoma, commonly referred to as the kissing bug) since 1990 has reduced the prevalence of Chagas disease from a peak of 15–30 million102,103. The Global Burden of Disease Study estimates that the age-standardized mortality for Chagas disease decreased by 51.7% between 1990 and 2013 (REF. 5). In 2005, an estimated 300,000 immigrants were living with Chagas disease in the USA104.
Chagas disease remains the most common cause of nonischaemic cardiomyopathy in Latin America104. A cohort study observed that 38.3% of patients with Chagas disease progressed to being diagnosed with HF after a 10-year follow-up period105. In the early stages of the disease, patients are likely to develop abnormalities of the cardiac conduction system that can be asymptomatic or detected after reported palpitations or syncopal events. HF symptoms are typically caused by biventricular dysfunction with a greater prominence of right-sided HF symptoms. Chagas cardiomyopathy has a higher mortality than other nonischaemic cardiomyopathies, and patients are at greater risk of sudden cardiac death and malignant arrhythmias than other patients with HF. Deaths from cardiovascular causes account for nearly two-thirds of all Chagas-related deaths106. Antiparasitic treatment with benznidazole in patients with Chagas cardiomyopathy did not improve clinical outcomes or 5-year mortality107. The recommended management for Chagas cardiomyopathy is based on strategies for HFrEF107.
Congenital heart disease
Global estimates of the prevalence of congenital heart disease range from 0.4% to 5.0% of all births108. In the USA, the prevalence of congenital heart disease is estimated to be approximately 1%108. Patients with congenital heart disease tend to under-report their HF symptoms compared with patients with noncongenital HF109. Exercise testing is usually required to understand functional limitations in patients with congenital heart disease, because echocardiographic criteria for HFrEF and HFpEF might not be relevant in this patient cohort. Among adult patients with congenital heart disease, 51% reported NYHA class II to III HF symptoms, and exercise testing revealed further functional limitations109. HF is seen more commonly in patients with congenital heart disease and single or systemic right ventricles108. Adults with six major categories of congenital heart defects (atrial septal defects, congenitally corrected transposition of the great vessels, tetraology of Fallot, transposition of the great arteritis, Ebstein anomaly, and Fontan circulation) all had markedly reduced exercise capacity on cardiopulmonary exercise testing110.
The global burden of congenital heart disease has been underappreciated in low-income countries. Most congenital lesions are not diagnosed at birth and might present only after progression to severe symptoms, and the limited availability of imaging technologies in the developing world further restricts diagnostic capabilities111. Echocardiographic studies in school-aged children in Africa reported high rates of rheumatic and congenital heart disease111. Another small study, in which echocardiography was used to diagnose congenital heart disease in Mozambique, reported a prevalence of 230 cases per 100,000 in school-aged children, with 80% of cases being newly diagnosed112.
Burden on health services
Hospitalizations
In high-income countries, HF is the most common diagnosis in hospitalized elderly patients aged >65 years (REF. 113). HF hospitalization represents 1–2% of all hospital admissions114. In the USA, HF is the leading primary diagnosis for hospitalization, with approximately 1 million discharges every year between 2000 and 2010 (REF. 3). The number of hospitalizations that included HF as a reason for admission tripled between 1979 and 2004 (REF. 9). In this time period, age-adjusted hospitalization rates for a primary diagnosis of HF increased from 219 to 390 per 100,000 person-years9. In the Olmsted County cohort, an average 1.34 hospitalizations occurred per person-year among patients with HF, and 63% were secondary to noncardiovascular causes32. Between 1999 and 2011, the HF hospitalization rate among Medicare patients in the USA decreased from 1,390 to 925 per 100,000 person-years69, and the average length of stay shortened considerably from 3.1 days to 1.9 days69.
Similar trends in hospitalization rates for HF have also been observed in Europe. In France, the age-standardized hospitalization rate for HF was 246.2 per 100,000 population in 2012 (REF. 115). Rates of hospitalization were stable between 2000 and 2010, whereas standardized inpatient mortality decreased by 3.3% per year on average115. The number of hospital admissions for HF peaked in the 1990s in the Netherlands, Scotland, and Sweden, followed by a decline114. In the UK, 5% of all admissions from the emergency department to the hospital are for HF116. HF hospitalizations are projected to increase by >50% by the year 2035, owing to an ageing population116.
In the USA, differences in HF hospitalization rates between ethnic groups have been described. The likelihood of hospitalization for HF is 50% higher for African–American individuals, 20% higher for Hispanic individuals, and 50% lower for Asian individuals than for white individuals117. These reported ethnic differences in hospitalization rates were not controlled for socioeconomic factors, which might explain the variations in hospitalization rates.
National registries have provided insight into international practice variations for the treatment of HF and identified areas for quality improvement. Adherence to evidenced-based therapies for HF is highest in North America, Western Europe, and Japan118. However, the use of mineralocorticoid-receptor blockers was found to be lower in North America than in other high-income regions. The lowest rates of adherence to HF therapy were in Eastern Europe and Asia (excluding Japan)118. In the Get With The Guidelines — Heart Failure project, quality of care for all ethnic groups in the USA was similar, but in-hospital mortality was notably lower for African–American and Hispanic individuals119. In 2013, the median risk-standardized 30-day hospital readmission rate for HF was 21.9%, and ranged from 17.0% to 28.2%. The median hospital readmission rate for HF decreased by 1.5% between 2010 and 2013 (REF. 120).
Ambulatory care
In 2011, a total of 553,000 emergency department visits for HF were recorded in the USA3, and in 2012, a primary diagnosis of HF was charted for 1,774,000 outpatient visits. In the UK, the average general practitioner cares for 30 patients with HF and diagnoses HF in an additional 10 patients annually116. The quality of outpatient care for patients with HF has been assessed by studying the frequency of guideline-recommended practices for eligible patients. Guideline-recommended therapy includes use of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers, β-blockers, mineralocorticoid-receptor antagonists, anticoagulant therapy for atrial fibrillation or flutter, cardiac resynchronization therapy, implantable cardioverter–defibrillators, and education on self-management of HF. Treatment compliance with these seven guideline-recommended measures was found to reduce 2-year mortality71.
Financial burden
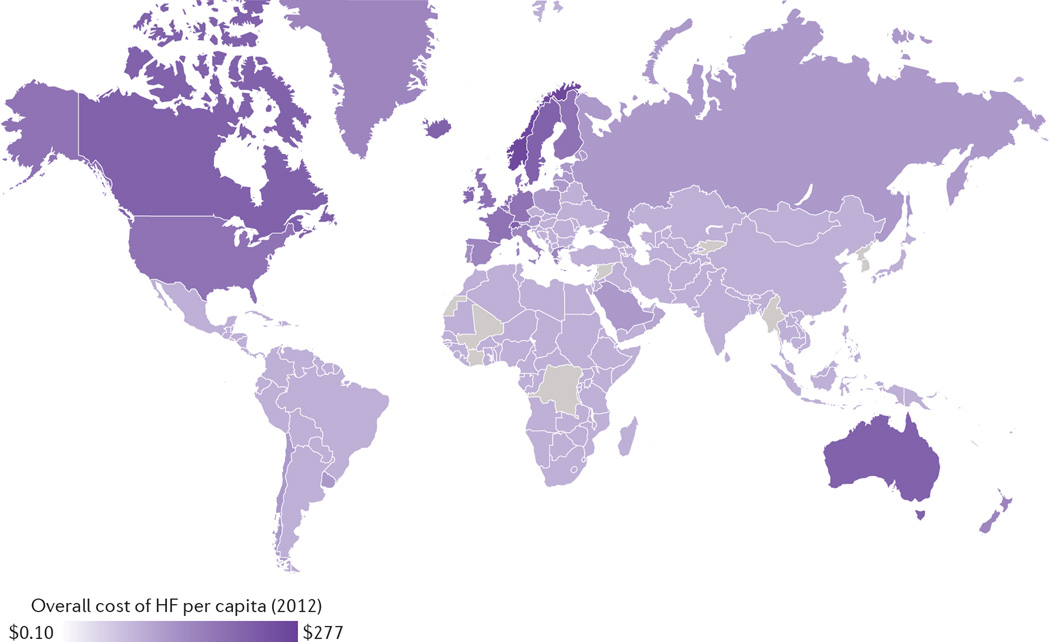
Accurate estimates for the global financial burden of HF are challenging given data limitations. Attempts have been made to calculate the global cost of HF using known national health expenditures. A study published in 2014 estimated that US$108 billion was spent on HF globally in 2012, 60% of which was spent directly on medical costs121. A majority of the worldwide expenditure for HF (86%) was attributed to high-income regions that constitute only 18% of the global population121 (FIG. 2). The prevalence of HF in the USA is projected to increase by 46% between 2012 and 2030, with total medical costs predicted to rise from US$20.9 billion to $53.1 billion. Nearly 80% of these projected expenses are attributed to increased hospitalizations. Indirect costs of lost productivity from morbidity and premature mortality are estimated to increase from US$9.8 billion to $16.6 billion by 2030, and total direct and indirect medical costs will increase from US$30.7 billion to $69.8 billion122. The prevalence of HF among Medicare’s highest-cost patients was 44% in 2010, and HF admissions were the costliest preventable hospitalization for those beneficiaries123. The mean cost per hospital admission for HF in the USA was $10,775 in 2011. The highest-expenditure patients were found to have more comorbidities and higher inpatient mortality124.
Figure 2. Global cost of HF per capita in 2012.
The map shows the estimated per capita cost of HF based on reported national health-care expenditures and expected HF burden. HF, heart failure. Modified from Cook, C. et al. The annual global economic burden of heart failure. Int. J. Cardiol. 171, 368–376 (2014), with permission from Elsevier.
The UK’s Centre for Economics and Business Research estimates that the cost of cardiovascular disease in Europe will increase from €102.1 billion in 2014 to €122.6 billion by 2020 (REF. 125). In Sweden and the UK, 2% of the entire national health-care budget is spent managing HF116,126. In Sweden, 31% of HFrEF medical expenditures were for outpatient care, 29% for primary cardiac hospitalizations, and 40% were for noncardiac hospitalizations127. In the developing world, an estimated US$15.1 billion was spent on HF in 2012.121 A small Nigerian cohort study reported US$2,128 in expenditures per case of HF in 2009. Outpatient costs comprised 56% of total expenditure owing primarily to transportation expenses for monthly visits128.
Future directions
Shifting demographics and epidemiological transitions foretell a rapid rise in the number of patients with HF. Primary prevention of HF is the most effective means of improving quality of life and reducing health-care expenditure41. For both developed and developing nations, improved control of hypertension, reduction in tobacco use, and targeting of lifestyle factors are the most effective means of reducing HF incidence and prevalence. Although evidence indicates that the incidence and prevalence of HF is stabilizing in high-income countries, large gaps remain in what we know about the control of known risk factors for earlier identification of at-risk patients, to allow for expedited intervention that might further reduce the HF burden129. Maximizing adherence to evidence-based practices for outpatients will prove to be the best strategy to improve the management of HF; the potential gains from novel therapeutics will pale in comparison to what can be gained by improving primary prevention and treatment-adherence strategies130. These strategies should be tailored to local populations for maximal benefits131, and more research and resources should be directed towards low-income nations that harbour the largest burden of preventable HF.
Conclusions
HF is the most rapidly growing cardiovascular condition globally, conferring a substantial burden on health-care systems worldwide. In high-income nations, improvements in public health have shifted demographics towards an ageing population with a high prevalence of chronic diseases. Elderly individuals are living with HF longer than ever, which necessitates improved systems to manage chronic disease, improve health outcomes, and reduce health-care expenditures. HFpEF is accounting for an increasing share of the prevalence of HF in the developed world, but therapies to reduce mortality in affected patients have not been discovered. Meanwhile, preventable childhood diseases are transitioning to preventable cardiovascular diseases in developing nations. Although more reliable epidemiological data are required, low-income nations have a disproportionately high incidence of preventable causes of HF, such as hypertensive heart disease and rheumatic heart disease. Future research should be aimed at addressing the ever-expanding challenges in HF prevention and management in this new era.
Key points.
Heart failure (HF) is the most rapidly growing cardiovascular condition globally
HF with preserved ejection fraction accounts for an increasing portion of HF in the developed world, and therapies to improve health outcomes are needed
Improvement in the primary prevention of cardiovascular disease and the treatment of ischaemic heart disease have reduced the age-adjusted prevalence of HF in the developed world
Advances in HF treatment and prevention have resulted in a decline in mortality in developed nations
Acknowledgments
B.Z. is supported by the NIH Cardiovascular Scientist Training Program (T32 HL007895).
G.C.F. declares that he has received grants from the AHRQ and NIH, and is a consultant for Amgen, Baxter, Bayer AG, Janssen Pharmaceuticals, Medtronic, and Novartis.
Footnotes
Competing interests statement
B.Z. declares no competing interests.
References
- 1.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat. Rev. Cardiol. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mozaffarian D, et al. Heart disease and stroke statistics — 2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 5.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth Ga, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N. Engl. J. Med. 2015;372:1333–1341. doi: 10.1056/NEJMoa1406656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCullough PA, et al. Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J. Am. Coll. Cardiol. 2002;39:60–69. doi: 10.1016/s0735-1097(01)01700-4. [DOI] [PubMed] [Google Scholar]
- 8.Roger VL. Epidemiology of heart failure. Circ. Res. 2013;113:646–659. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S., 1979 to 2004. J. Am. Coll. Cardiol. 2008;52:428–434. doi: 10.1016/j.jacc.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 10.Roger VL, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 11.Virchow R. Die Cellularpathologie in Ihrer Begründung auf Physiologische und Pathologische Gewebelehre. Hirschwald; 1858. Aug, [Google Scholar]
- 12.Yancy CW, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 13.McMurray JJV. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Eur. Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization & International Society and Federation Cardiology. Report of the WHO/ISFC task force on the definition and classification of cardiomyopathies. Heart. 1980;44:672–673. doi: 10.1136/hrt.44.6.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson P, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 16.McKelvie RS, et al. The 2012 Canadian Cardiovascular Society heart failure management guidelines update: focus on acute and chronic heart failure. Can. J. Cardiol. 2013;29:168–181. doi: 10.1016/j.cjca.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Lindenfeld J, et al. HFSA 2010 comprehensive heart failure practice guideline. J. Card. Fail. 2010;16:e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Maron BJ, et al. Contemporary definitions and classification of the cardiomyopathies. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 19.Elliott P, et al. Classification of the cardiomyopathies: a position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur. Heart J. 2008;29:270–276. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 20.Arbustini E, et al. The MOGE(S) classification for a phenotype-genotype nomenclature of cardiomyopathy: endorsed by the world heart federation. Glob. Heart. 2013;8:355–382. doi: 10.1016/j.gheart.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Mahmood SS, Wang TJ. The epidemiology of congestive heart failure: the Framingham Heart Study perspective. Glob. Heart. 2013;8:77–82. doi: 10.1016/j.gheart.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N. Engl. J. Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 23.Carlson KJ, Lee DC, Goroll AH, Leahy M, Johnson RA. An analysis of physicians’ reasons for prescribing long-term digitalis therapy in outpatients. J. Chron. Dis. 1985;38:733–739. doi: 10.1016/0021-9681(85)90115-8. [DOI] [PubMed] [Google Scholar]
- 24.Lee DS, et al. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med. Care. 2005;43:182–188. doi: 10.1097/00005650-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Schellenbaum GD, et al. Survival associated with two sets of diagnostic criteria for congestive heart failure. Am. J. Epidemiol. 2004;160:628–635. doi: 10.1093/aje/kwh268. [DOI] [PubMed] [Google Scholar]
- 26.Lang RM, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the European association of cardiovascular imaging. J. Am. Soc. Echocardiogr. 2015;28:1.e14–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Mosterd A, et al. Classification of heart failure in population based research: an assessment of six heart failure scores. Eur. J. Epidemiol. 1997;13:491–502. doi: 10.1023/a:1007383914444. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson H, et al. Cardiac and pulmonary causes of dyspnoea — validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. Eur. Heart J. 1987;8:1007–1014. doi: 10.1093/oxfordjournals.eurheartj.a062365. [DOI] [PubMed] [Google Scholar]
- 29.Goff DC, Pandey DK, Chan FA, Ortiz C, Nichaman MZ. Congestive heart failure in the United States: is there more than meets the I(CD code)? The Corpus Christi Heart Project. Arch. Intern. Med. 2000;160:197–202. doi: 10.1001/archinte.160.2.197. [DOI] [PubMed] [Google Scholar]
- 30.O’Malley KJ, et al. Measuring diagnoses: ICD code accuracy. Health Serv. Res. 2005;40:1620–1639. doi: 10.1111/j.1475-6773.2005.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frolova N, et al. Assessing the use of international classification of diseases-10th revision codes from the emergency department for the identification of acute heart failure. JACC Heart Fail. 2015;3:386–391. doi: 10.1016/j.jchf.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Gerber Y, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern. Med. 2015;175:996–1004. doi: 10.1001/jamainternmed.2015.0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao D, Liu J, Xie W, Qi Y. Cardiovascular risk assessment: a global perspective. Nat. Rev. Cardiol. 2015;12:301–311. doi: 10.1038/nrcardio.2015.28. [DOI] [PubMed] [Google Scholar]
- 34.Djoussé L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302:394–400. doi: 10.1001/jama.2009.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy D, et al. Long-term trends in the incidence of and survival with heart failure. N. Engl. J. Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 36.Bahrami H, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch. Intern. Med. 2008;168:2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brouwers FP, et al. Incidence and epidemiology of new onset heart failure with preserved versus reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur. Heart J. 2013;34:1424–1431. doi: 10.1093/eurheartj/eht066. [DOI] [PubMed] [Google Scholar]
- 38.Meyer S, et al. Sex differences in new-onset heart failure. Clin. Res. Cardiol. 2015;104:342–350. doi: 10.1007/s00392-014-0788-x. [DOI] [PubMed] [Google Scholar]
- 39.Zarrinkoub R, et al. The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur. J. Heart Fail. 2013;15:995–1002. doi: 10.1093/eurjhf/hft064. [DOI] [PubMed] [Google Scholar]
- 40.Dokainish H, et al. Heart failure in low- and middle-income countries: background, rationale, and design of the INTERnational Congestive Heart Failure Study (INTER-CHF) Am. Heart J. 2015;170:627.e1–634.e1. doi: 10.1016/j.ahj.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Yusuf S, et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N. Engl. J. Med. 2014;371:818–827. doi: 10.1056/NEJMoa1311890. [DOI] [PubMed] [Google Scholar]
- 42.Sliwa K, Mayosi BM. Recent advances in the epidemiology, pathogenesis and prognosis of acute heart failure and cardiomyopathy in Africa. Heart. 2013;99:1317–1322. doi: 10.1136/heartjnl-2013-303592. [DOI] [PubMed] [Google Scholar]
- 43.Cowie MR, et al. The epidemiology of heart failure. Eur. Heart J. 1997;18:208–225. doi: 10.1093/oxfordjournals.eurheartj.a015223. [DOI] [PubMed] [Google Scholar]
- 44.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J. Am. Coll. Cardiol. 1992;20:301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 45.Heidenreich PA, et al. Forecasting the impact of heart failure in the united states a policy statement from the american heart association. Circ. Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J, Dharmarajan K, Wang Y, Krumholz HM. National trends in heart failure hospital stay rates, 2001 to 2009. J. Am. Coll. Cardiol. 2013;61:1078–1088. doi: 10.1016/j.jacc.2012.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lloyd-Jones DM, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 48.Lazzarini V, Mentz RJ, Fiuzat M, Metra M, O’Connor CM. Heart failure in elderly patients: distinctive features and unresolved issues. Eur. J. Heart Fail. 2013;15:717–723. doi: 10.1093/eurjhf/hft028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hawkins NM, Jhund PS, McMurray JJV, Capewell S. Heart failure and socioeconomic status: accumulating evidence of inequality. Eur. J. Heart Fail. 2012;14:138–146. doi: 10.1093/eurjhf/hfr168. [DOI] [PubMed] [Google Scholar]
- 50.Ramsay SE, et al. Inequalities in heart failure in older men: prospective associations between socioeconomic measures and heart failure incidence in a 10-year follow-up study. Eur. Heart J. 2014;35:442–447. doi: 10.1093/eurheartj/eht449. [DOI] [PubMed] [Google Scholar]
- 51.Yeboah J, et al. Prognosis of individuals with asymptomatic left ventricular systolic dysfunction in the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2012;126:2713–2719. doi: 10.1161/CIRCULATIONAHA.112.112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mujib M, Zhang Y, Feller MA, Ahmed A. Evidence of a ‘heart failure belt’ in the southeastern United States. Am. J. Cardiol. 2011;107:935–937. doi: 10.1016/j.amjcard.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cook NL, Lauer MS. The socio-geography of heart failure: why it matters. Circ. Heart Fail. 2011;4:244–245. doi: 10.1161/CIRCHEARTFAILURE.111.962191. [DOI] [PubMed] [Google Scholar]
- 54.Stewart S, et al. Predominance of heart failure in the heart of Soweto study cohort: emerging challenges for urban African communities. Circulation. 2008;118:2360–2367. doi: 10.1161/CIRCULATIONAHA.108.786244. [DOI] [PubMed] [Google Scholar]
- 55.Cotter G, Cotter-Davison B, Ogah OS. The burden of heart failure in Africa. Eur. J. Heart Fail. 2013;15:829–831. doi: 10.1093/eurjhf/hft073. [DOI] [PubMed] [Google Scholar]
- 56.Bocchi EA. Heart failure in South America. Curr. Cardiol. Rev. 2013;9:147–156. doi: 10.2174/1573403X11309020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pillai HS, Ganapathi S. Heart failure in South Asia. Curr. Cardiol. Rev. 2013;9:102–111. doi: 10.2174/1573403X11309020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al-Shamiri MQ. Heart failure in the Middle East. Curr. Cardiol. Rev. 2013;9:174–178. doi: 10.2174/1573403X11309020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo Y, Lip GYH, Banerjee A. Heart failure in East Asia. Curr. Cardiol. Rev. 2013;9:112–122. doi: 10.2174/1573403X11309020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bloomfield GS, Barasa FA, Doll JA, Velazquez EJ. Heart failure in Sub-Saharan Africa. Curr. Cardiol. Rev. 2013;9:157–173. doi: 10.2174/1573403X11309020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bennett DA, et al. Study protocol: systematic review of the burden of heart failure in low- and middle-income countries. Syst. Rev. 2012;1:59. doi: 10.1186/2046-4053-1-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Banerjee A, Mendis S. Heart failure: the need for global health perspective. Curr. Cardiol. Rev. 2013;9:97–98. doi: 10.2174/1573403X11309020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur. J. Heart Fail. 2015;17:884–892. doi: 10.1002/ejhf.319. [DOI] [PubMed] [Google Scholar]
- 64.Snyder ML, et al. Redistribution of heart failure as the cause of death: the Atherosclerosis Risk in Communities Study. Popul. Health Metr. 2014;12:10. doi: 10.1186/1478-7954-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJV. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur. J. Heart Fail. 2001;3:315–322. doi: 10.1016/s1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 66.Askoxylakis V, et al. Long-term survival of cancer patients compared to heart failure and stroke: a systematic review. BMC Cancer. 2010;10:105. doi: 10.1186/1471-2407-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rahimi K, Duncan M, Pitcher A, Emdin CA, Goldacre MJ. Mortality from heart failure, acute myocardial infarction and other ischaemic heart disease in England and Oxford: a trend study of multiple-cause-coded death certification. J. Epidemiol. Commun. Health. 2015;69:1000–1005. doi: 10.1136/jech-2015-205689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ohlmeier C, et al. Incidence, prevalence and 1-year all-cause mortality of heart failure in Germany: a study based on electronic healthcare data of more than six million persons. Clin. Res. Cardiol. 2015;104:688–696. doi: 10.1007/s00392-015-0841-4. [DOI] [PubMed] [Google Scholar]
- 69.Krumholz HM, Normand ST, Wang Y. Trends in hospitalizations and outcomes for acute cardiovascular disease and stroke, 1999–2011. Circulation. 2014;130:966–975. doi: 10.1161/CIRCULATIONAHA.113.007787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yeung DF, et al. Trends in the incidence and outcomes of heart failure in Ontario, Canada: 1997 to 2007. CMAJ. 2012;184:E765–E773. doi: 10.1503/cmaj.111958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fonarow GC, et al. Associations between outpatient heart failure process-of-care measures and mortality. Circulation. 2011;123:1601–1610. doi: 10.1161/CIRCULATIONAHA.110.989632. [DOI] [PubMed] [Google Scholar]
- 72.Thun MJ, et al. 50-year trends in smoking-related mortality in the United States. N. Engl. J. Med. 2013;368:351–364. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Egan BM, Li J, Hutchison FN, Ferdinand KC. Hypertension in the United States, 1999 to 2012: Progress Toward Healthy People 2020 goals. Circulation. 2014;130:1692–1699. doi: 10.1161/CIRCULATIONAHA.114.010676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Psaty BM, Boineau R, Kuller LH, Luepker RV. The potential costs of upcoding for heart failure in the United States. Am. J. Cardiol. 1999;84:108–109. doi: 10.1016/s0002-9149(99)00205-2. [DOI] [PubMed] [Google Scholar]
- 75.Steinberg BA, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 76.Andersson C, Vasan RS. Epidemiology of heart failure with preserved ejection fraction. Heart Fail. Clin. 2014;10:377–388. doi: 10.1016/j.hfc.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Udelson JE. Heart failure with preserved ejection fraction. Circulation. 2011;124:e540–e543. doi: 10.1161/CIRCULATIONAHA.111.071696. [DOI] [PubMed] [Google Scholar]
- 78.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function: epidemiology, clinical characteristics, and prognosis. J. Am. Coll. Cardiol. 2004;43:317–327. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 79.Kristensen SL, et al. International geographic variation in event rates in trials of heart failure with preserved and reduced ejection fraction. Circulation. 2014;131:43–53. doi: 10.1161/CIRCULATIONAHA.114.012284. [DOI] [PubMed] [Google Scholar]
- 80.Owan TE, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N. Engl. J. Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 81.Berry C, et al. The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur. Heart J. 2012;33:1750–1757. doi: 10.1093/eurheartj/ehr254. [DOI] [PubMed] [Google Scholar]
- 82.Cheng RK, et al. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am. Heart J. 2014;168:721–730. doi: 10.1016/j.ahj.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 83.Baldasseroni S, et al. Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: a report from the Italian Network on congestive heart failure. Am. Heart J. 2002;143:398–405. doi: 10.1067/mhj.2002.121264. [DOI] [PubMed] [Google Scholar]
- 84.Hawkins NM, et al. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur. J. Heart Fail. 2009;11:130–139. doi: 10.1093/eurjhf/hfn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heberden W. Some account of a disorder of the breast. Med. Trans. 1772;2:59–67. [Google Scholar]
- 86.Hektoen L. Embolism of the left coronary artery; sudden death. Med. News. 1892;61:1–6. [Google Scholar]
- 87.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N. Engl. J. Med. 2012;366:54–63. doi: 10.1056/NEJMra1112570. [DOI] [PubMed] [Google Scholar]
- 88.Moran AE, et al. The global burden of ischemic heart disease in 1990 and 2010: the global burden of disease 2010 study. Circulation. 2014;129:1493–1501. doi: 10.1161/CIRCULATIONAHA.113.004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brouwers FP, Van Gilst WH, Van Veldhuisen DJ. The changing face of heart failure: are we really making progress? Eur. J. Heart Fail. 2013;15:960–962. doi: 10.1093/eurjhf/hft126. [DOI] [PubMed] [Google Scholar]
- 90.Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327–334. doi: 10.1161/CIRCULATIONAHA.108.845792. [DOI] [PubMed] [Google Scholar]
- 91.Yusuf S, Thom T, Abbott RD. Changes in hypertension treatment and in congestive heart failure mortality in the United States. Hypertension. 1989;13:I74–I79. doi: 10.1161/01.hyp.13.5_suppl.i74. [DOI] [PubMed] [Google Scholar]
- 92.Ojji D, Stewart S, Ajayi S, Manmak M, Sliwa K. A predominance of hypertensive heart failure in the Abuja Heart Study cohort of urban Nigerians: a prospective clinical registry of 1515 de novo cases. Eur. J. Heart Fail. 2013;15:835–842. doi: 10.1093/eurjhf/hft061. [DOI] [PubMed] [Google Scholar]
- 93.Chow CK, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310:959–968. doi: 10.1001/jama.2013.184182. [DOI] [PubMed] [Google Scholar]
- 94.Nkomo VT, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 95.Essop MR, Nkomo VT. Rheumatic and nonrheumatic valvular heart disease: epidemiology, management, and prevention in Africa. Circulation. 2005;112:3584–3591. doi: 10.1161/CIRCULATIONAHA.105.539775. [DOI] [PubMed] [Google Scholar]
- 96.Carapetis JR. Rheumatic heart disease in developing countries. N. Engl. J. Med. 2007;357:439–441. doi: 10.1056/NEJMp078039. [DOI] [PubMed] [Google Scholar]
- 97.Marijon E, et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. N. Engl. J. Med. 2007;357:470–476. doi: 10.1056/NEJMoa065085. [DOI] [PubMed] [Google Scholar]
- 98.Sliwa K, Damasceno A, Mayosi BM. Epidemiology and etiology of cardiomyopathy in Africa. Circulation. 2005;112:3577–3583. doi: 10.1161/CIRCULATIONAHA.105.542894. [DOI] [PubMed] [Google Scholar]
- 99.Moran A, et al. The epidemiology of cardiovascular diseases in Sub-Saharan Africa: the global burden of diseases, injuries and risk factors 2010 study. Prog. Cardiovasc. Dis. 2013;56:234–239. doi: 10.1016/j.pcad.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mayosi BM. Contemporary trends in the epidemiology and management of cardiomyopathy and pericarditis in Sub-Saharan Africa. Heart. 2007;93:1176–1183. doi: 10.1136/hrt.2007.127746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cooper LT, Keren A, Sliwa K, Matsumori A, Mensah GA. The global burden myocarditis. Glob. Heart. 2014;9:121–129. doi: 10.1016/j.gheart.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 102.Bonney KM. Chagas disease in the 21st century: a public health success or an emerging threat? Parasite. 2014;21:11. doi: 10.1051/parasite/2014012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Longo DL, Bern C. Chagas’ disease. N. Engl. J. Med. 2015;373:456–466. doi: 10.1056/NEJMra1410150. [DOI] [PubMed] [Google Scholar]
- 104.Bern C, Montgomery SP. An estimate of the burden of Chagas disease in the United States. Clin. Infect. Dis. 2009;49:e52–e54. doi: 10.1086/605091. [DOI] [PubMed] [Google Scholar]
- 105.Marin Neto JA, Simões MV, Sarabanda AV. Chagas’ heart disease. Arq. Bras. Cardiol. 1999;72:247–280. doi: 10.1590/s0066-782x1999000300001. [DOI] [PubMed] [Google Scholar]
- 106.Rassi A, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 107.Morillo CA, et al. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N. Engl. J. Med. 2015;373:1295–1306. doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- 108.van der Bom T, et al. The changing epidemiology of congenital heart disease. Nat. Rev. Cardiol. 2011;8:50–60. doi: 10.1038/nrcardio.2010.166. [DOI] [PubMed] [Google Scholar]
- 109.Diller G-P, et al. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation. 2005;112:828–835. doi: 10.1161/CIRCULATIONAHA.104.529800. [DOI] [PubMed] [Google Scholar]
- 110.Fredriksen PM, et al. Aerobic capacity in adults with various congenital heart diseases. Am. J. Cardiol. 2001;87:310–314. doi: 10.1016/s0002-9149(00)01364-3. [DOI] [PubMed] [Google Scholar]
- 111.Zühlke L, Mirabel M, Marijon E. Congenital heart disease and rheumatic heart disease in Africa: recent advances and current priorities. Heart. 2013;99:1554–1561. doi: 10.1136/heartjnl-2013-303896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marijon E, et al. Prevalence of congenital heart disease in schoolchildren of Sub-Saharan Africa, Mozambique. Int. J. Cardiol. 2006;113:440–441. doi: 10.1016/j.ijcard.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 113.Braunwald E. The war against heart failure: the Lancet lecture. Lancet. 2015;385:812–824. doi: 10.1016/S0140-6736(14)61889-4. [DOI] [PubMed] [Google Scholar]
- 114.Zannad F, Agrinier N, Alla F. Heart failure burden and therapy. Europace. 2009;11(Suppl. 5):v1–v9. doi: 10.1093/europace/eup304. [DOI] [PubMed] [Google Scholar]
- 115.Gabet A, Juillière Y, Lamarche-Vadel A, Vernay M, Olié V. National trends in rate of patients hospitalized for heart failure and heart failure mortality in France, 2000–2012. Eur. J. Heart Fail. 2015;17:583–590. doi: 10.1002/ejhf.284. [DOI] [PubMed] [Google Scholar]
- 116.National Clinical Guideline Centre (UK) Chronice heart failure: national clinical guideline for diagnosis and management in primary and secondary care. National Center for Biotechnology Information. 2010 [online], http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0046956/pdf/PubMedHealth_PMH0046956.pdf.
- 117.Brown DW, Haldeman GA, Croft JB, Giles WH, Mensah GA. Racial or ethnic differences in hospitalization for heart failure among elderly adults: Medicare, 1990 to 2000. Am. Heart J. 2005;150:448–454. doi: 10.1016/j.ahj.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 118.Ambrosy AP, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J. Am. Coll. Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 119.Thomas KL, et al. Association of race/ethnicity with clinical risk factors, quality of care, and acute outcomes in patients hospitalized with heart failure. Am. Heart J. 2011;161:746–754. doi: 10.1016/j.ahj.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 120.Schwartz J, et al. Medicare hospital quality chartbook performance report on outcome measures. Centers for Medicare & Medicaid Services. 2014 [online], https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/hospitalqualityinits/downloads/medicare-hospital-quality-chartbook-2014.pdf.
- 121.Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int. J. Cardiol. 2014;171:368–376. doi: 10.1016/j.ijcard.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 122.Heidenreich PA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ. Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Joynt KE, Gawande AA, Orav EJ, Jha AK. Contribution of preventable acute care spending to total spending for high-cost Medicare patients. JAMA. 2013;309:2572–2578. doi: 10.1001/jama.2013.7103. [DOI] [PubMed] [Google Scholar]
- 124.Ziaeian B, Sharma PP, Yu T-C, Johnson KW, Fonarow GC. Factors associated with variations in hospital expenditures for acute heart failure in the United States. Am. Heart J. 2015;169:282.e15–289.e15. doi: 10.1016/j.ahj.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bernick S. The economic cost of cardiovascular disease from 2014–2020 in six European economies. Centre for Economics and Business Research. 2014 [online], http://www.cebr.com/wp-content/uploads/2015/08/Short-Report-18.08.14.pdf. [Google Scholar]
- 126.Rydén-Bergsten T, Andersson F. The health care costs of heart failure in Sweden. J. Intern. Med. 1999;246:275–284. doi: 10.1046/j.1365-2796.1999.00520.x. [DOI] [PubMed] [Google Scholar]
- 127.Mejhert M, et al. Long term health care consumption and cost expenditure in systolic heart failure. Eur. J. Intern. Med. 2013;24:260–265. doi: 10.1016/j.ejim.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 128.Ogah OS, et al. Economic burden of heart failure: investigating outpatient and inpatient costs in Abeokuta, Southwest Nigeria. PLoS ONE. 2014;9:e113032. doi: 10.1371/journal.pone.0113032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hernandez AF. Preventing heart failure. JAMA. 2013;310:44. doi: 10.1001/jama.2013.7589. [DOI] [PubMed] [Google Scholar]
- 130.Cohn JN. Heart failure in 2013: continue what we are doing to treat HF, but do it better. Nat. Rev. Cardiol. 2014;11:69–70. doi: 10.1038/nrcardio.2013.212. [DOI] [PubMed] [Google Scholar]
- 131.McLean RC, Jessup M. The challenge of treating heart failure: a diverse disease affecting diverse populations. JAMA. 2013;310:2033–2034. doi: 10.1001/jama.2013.282773. [DOI] [PubMed] [Google Scholar]
- 132.Maron BJ, Thiene G. Hurst’s Hear. McGraw Hill Medical; 2011. [Google Scholar]