Abstract
Astrocytes play a critical role in supporting the normal physiological function of neurons in the central nervous system (CNS). Astrocyte transplantation can potentially promote axonal regeneration and functional recovery after spinal cord injury (SCI). Fibrin and collagen hydrogels provide a growth-permissive substrate and serve as carriers for therapeutic cell transplantation into an injured spinal cord. However, the application of fibrin and collagen may be limited due to its relatively rapid degradation rate in vivo. In this study, immature astrocytes isolated from neonatal rats were grown into fibrin hydrogel containing aprotinin and collagen hydrogel crosslinked with poly(ethylene glycol) ether tetrasuccinimidyl glutarate (4S-StarPEG), and the cell behavior in these hydrogels was studied. The cell viability of astrocytes in the hydrogels was tested using the LIVE/DEAD® assay and the AlamarBlue® assay, and this study showed that astrocytes maintained good viability in these hydrogels. The cell migration study showed that astrocytes migrated in the fibrin and collagen hydrogels, and the migration speed is similar in these hydrogels. The crosslinking of collagen hydrogel with 4S-StarPEG did not change the astrocyte migration speed. However, the addition of aprotinin in the fibrin hydrogel inhibited astrocyte migration. The expression of chondroitin sulfate proteoglycan (CSPG), including NG2, neurocan, and versican, by astrocytes grown in the hydrogels was analyzed by quantitative RT-PCR. The expression of NG2, neurocan, and versican by the cells in these hydrogels was not significantly different.
Introduction
Astrocytes are the most abundant glial cells in the central nervous system (CNS). They play a critical role in supporting the normal physiological function of neurons in the spinal cord. Astrocytes can balance the concentration of the ions, metabolites, and neurotransmitters in the extracellular matrix. They are associated with neural synapses and mediate connectivity of the neuronal circuit. Astrocytes generate multiple neurotrophic factors that regulate survival, proliferation, and differentiation of neurons. 1-3 Astrocytes are a major source of extracellular proteins and adhesion molecules such as laminin, fibronectin, and neural cell adhesion molecules (NCAMs) in the CNS. 4, 5 Gliotransmitters released from glial cells can facilitate neuronal communication between neurons and other glial cells. 6, 7
Reactive astrocytes are recruited to the lesion after spinal cord injury (SCI). Reactive astrocytes exert structural and metabolic function in neural tissue regeneration by uptaking the excitotoxic glutamate and removing oxidative stress in order to protect the neural tissue. 8 Astrocytes proliferate and migrate to the lesion to fill the space and replace the lost neural cells. The reactive astrocytes could decrease cellular degeneration and minimize the inflammation response, thereby promoting axon growth. 9, 10 It has been identified that astrocytes are a population necessary in the repair of SCI and are crucial for axonal regrowth. Recent studies revealed that astrocyte transplantation can potentially promote axonal regeneration and functional recovery after SCI. 11-17 In one previous study, immature astrocytes isolated from neonatal rats were grafted into the hemisected adult rat spinal cord. 11 The study showed that the grafted cells survived and migrated from the original implantation site. Transplantation of the astrocytes reduced the scar volume. 11
Though cell transplantation can potentially replace lost neural cells and restore biological function, the microenvironment that grafted cells will encounter is unfavorable for their survival. Recent studies have shown that biomaterial scaffolds can be designed as a growth-permissive substrate and serve as a carrier for therapeutic cell transplantation into the injured spinal cord. Due to its soft and flexible mechanical properties, which are similar to those of the spinal cord, the implanted hydrogel causes little mechanical stress to the surrounding tissue of an injured spinal cord. Fibrin and collagen-based hydrogel have specific advantages in tissue regeneration because cells can bind directly to the materials via cell surface integrin receptors, 18-20 and the degradation of collagen and fibrin are natural protease-dependent process in vivo. However, their application may be limited due to their relatively rapid degradation rate in vivo. In this study, we crosslinked collagen hydrogel with poly(ethylene glycol) ether tetrasuccinimidyl glutarate (4S-StarPEG). The biological and material properties of hydrogel generated by collagen can be enhanced by crosslinking. We also fabricated fibrin hydrogel containing aprotinin, which is a competitive serine protease inhibitor used to reduce the biodegradation rate of the fibrin hydrogel. Astrocytes were grown in these hydrogels, and the cell viability and expression of chondroitin sulfate proteoglycan (CSPG) by the astrocytes in the hydrogels were analyzed.
Cell migration is an active cellular process, and it is critical for the endogenous and grafted cells to establish functional connections in the CNS lesion. The implanted hydrogel can mediate cell migration that is critical in the neural regeneration process. A previous study showed that the implantation of fibrin hydrogel into the injured spinal cord can decrease the density of astrocytes surrounding the injury site and promote the migration of astrocytes from the lesion border into the lesion. The lesion border in the fibrin-treated spinal cord lesion exhibited continuity of tissue across the lesion site as a result of the migration of cells into the lesion and thereby reduced the barrier for axonal growth cross the border. 21 In this study, we recorded the migration of astrocytes isolated from neonatal rat brain in the crosslinked collagen hydrogels and fibrin hydrogel containing aprotinin using time-lapse imaging, and we analyzed cell migration speed and distance. The outcome of this study provides direct evidence for the potential application of chemically modified collagen and fibrin hydrogels in neural regeneration.
Results
Astrocyte-induced degradation of fibrin hydrogels was inhibited by aprotinin
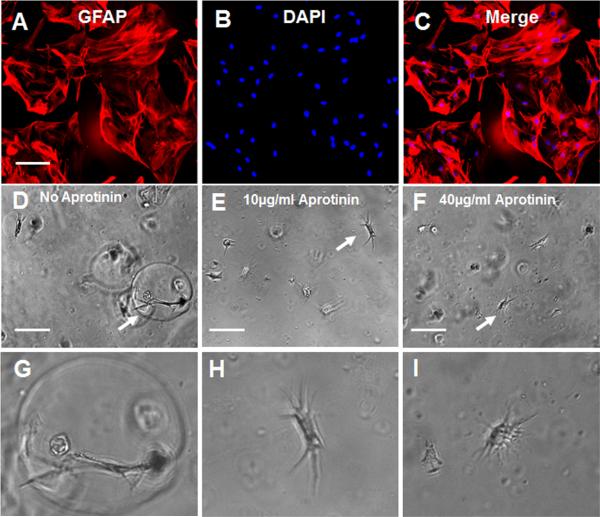
In this study, we labeled the cultured astrocytes with anti-glial fibrillary acidic protein (GFAP) antibody. Many astrocytes showed a star-like appearance with multiple processes originating from the soma. Astrocytes developed multiple processes after the cells were cultured in the fibrin alone hydrogel. In the fibrin hydrogel, hollow circles were seen around astrocytes grown in the hydrogel (Figures 1D, 1G). Observation suggests that the fibrin hydrogel surrounding the cells was digested by the enzyme generated by the astrocytes. However, no hollow circles were observed around astrocytes grown in the fibrin hydrogel containing aprotinin (Figures 1E, 1F, 1H, and 1I).
Figure 1.
Growth of astrocytes in fibrin hydrogels: (A) immnuostaining of astrocytes with anti-GFAP antibody; (B) nuclei of astrocytes are labeled with DAPI; (C) merging of images (A) and (B); (D)–(F) growth of astrocytes in fibrin hydrogels; (D) fibrin hydrogel without aprotinin; (E) fibrin hydrogel with aprotinin (10 μg/ml); (F) fibrin hydrogel with aprotinin (40 μg/ml); (G)–(I) magnified images of cells indicated by arrows in (D)–(F), respectively. Scale bar: 100 μm
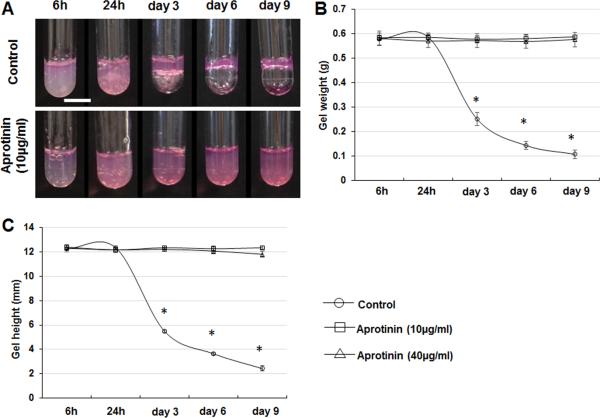
In the degradation study, the height and the weight of the fibrin hydrogels in the plastic tubes were measured after the culture medium was removed (Figure 2). The height and weight of the fibrin hydrogels decreased significantly after the cells were cultured for 3 days. However, the weight and height of fibrin hydrogels with aprotinin did not change significantly after 9 days.
Figure 2.
Degradation of fibrin hydrogel by cultured astrocytes. (A) Images of fibrin hydrogels with cultured astrocytes after the removal of culture medium. (B) The weight of fibrin hydrogels without aprotinin decreased significantly after 3days. The weight of fibrin hydrogels with aprotinin did not change significantly. (C) The height of fibrin hydrogels without aprotinin decreased significantly after 3days. The weight of fibrin hydrogels with aprotinin did not change significantly. *p < 0.05, compared with fibrin hydrogels containing aprotinin at the same time points. Scale bar: 10 mm.
Collagen hydrogels crosslinked with 4S-StarPEG
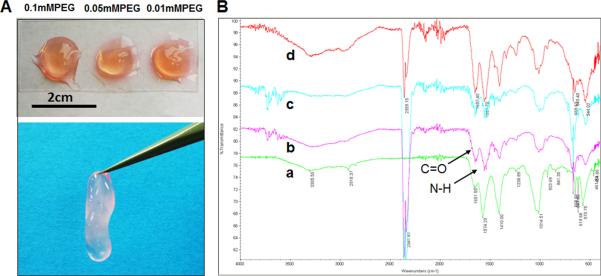
After neutralization, 4S-StarPEG was added into the collagen solution. Cell culture medium was added into the well containing a hydrogel after the collagen solution was incubated for 20 minutes in the 37°C incubator. Stable collagen hydrogels were observed after incubation with the cell culture medium (Figure 3A). FTIR was employed to determine the functional group of type I collagen (Figure 3B). Results show the spectra obtained from FTIR for the different crosslinked and non-crosslinked type I collagen hydrogels. The formation of amide bonds was observed after the hydrogel was crosslinked with 4S-StarPEG.
Figure 3.
Hydrogel formation for type I collagen crosslinked with 4S-StarPEG: (A) crosslinked type I collagen-formed hydrogels; (B) FTIR spectra of type I collagen hydrogels: A, non-crosslinked hydrogel; B, hydrogel crosslinked with 0.01 mM 4S-StarPEG; C, hydrogel crosslinked with 0.05 mM 4S-StarPEG; D, hydrogel crosslinked with 0.1 mM 4S-StarPEG.
Cell viability of astrocytes in hydrogel
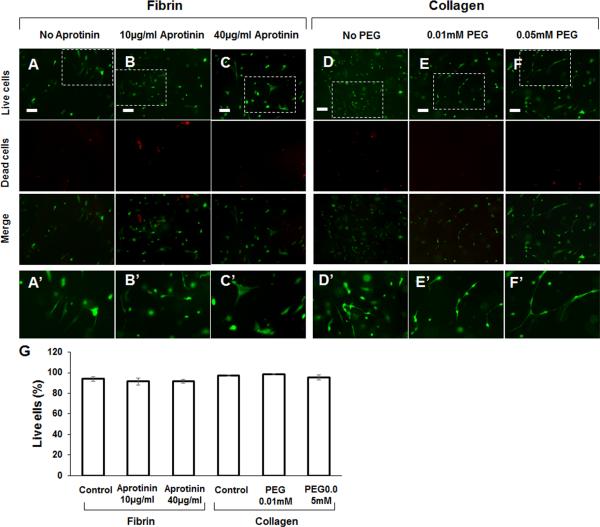
Cell viability of astrocytes in the fibrin hydrogels and collagen hydrogels was determined by a LIVE/DEAD® Cell Vitality Assay kit and viewed under a fluorescent microscope. After the cells were cultured for 6 days, most cells survived in the hydrogels (Figures 4A–4F). Magnified images show that astrocytes extended multiple processes in the fibrin or collagen hydrogels (Figures 4A′–F′). Quantification of live cells and dead cells in the hydrogels showed that the ratio of live astrocytes was 93.8 ± 2.0% in fibrin hydrogel, 91.6 ± 3.3% in fibrin hydrogel containing 10 μg/ml aprotinin, and 91.5 ± 1.7% in fibrin hydrogel containing 40 μg/ml aprotinin. The ratio of live astrocytes to total cells was 97.5 ± 0.6% in collagen hydrogel, 98.5 ± 0.4% in collagen crosslinked with 0.01 mM 4S-StarPEG, and 95.4 ± 2.7% in collagen crosslinked with 0.05 mM 4S-StarPEG. (Figure 4G).
Figure 4.
LIVE/DEAD® cell viability assay for astrocytes grown in hydrogel: (A) fibrin hydrogel without aprotinin; (B) fibrin hydrogel containing aprotinin (10 μm/ml); (C) fibrin hydrogel containing aprotinin (40 μm/ml); (D) non-crosslinked collagen hydrogel; (E) hydrogel crosslinked with 0.01 mM 4S-StarPEG; (F) hydrogel crosslinked with 0.05 mM 4S-StarPEG; (A’–F’) magnified images of insets indicated in (A–F); (G) Percentage of live cells in collagen and fibrin hydrogels as determined by LIVE/DEAD® cell assay. Scale bar: 100 μm.
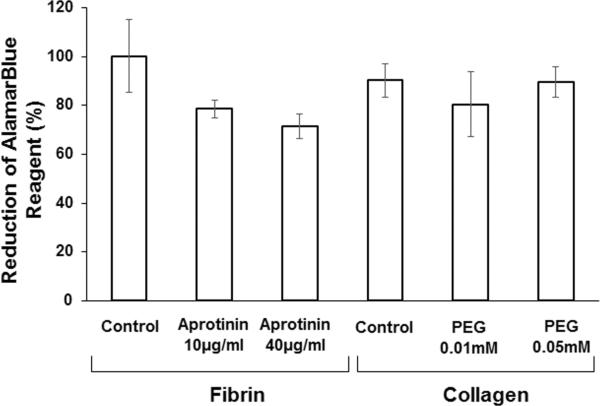
The AlamarBlue® assay showed the high viability of astrocytes in collagen and fibrin hydrogels (Figure 5). The reduction of AlamarBlue® reagent for astrocytes in collagen (90.2 ± 6.8%), collagen hydrogel crosslinked with 4S-StarPEG (0.01mM) (80.4 ± 13.3%), and collagen hydrogel crosslinked with 4S-StarPEG (0.05mM) (89.4 ± 6.2%) was higher than that for astrocytes in fibrin hydrogel with aprotinin (78.6 ± 3.5%) and fibrin hydrogel with aprotinin and fibronectin (71.4 ± 4.9%). However, the difference is not statistically different.
Figure 5.
AlamarBlue® cell viability assay for astrocytes grown in collagen and fibrin hydrogels.
Astrocyte migration in hydrogels
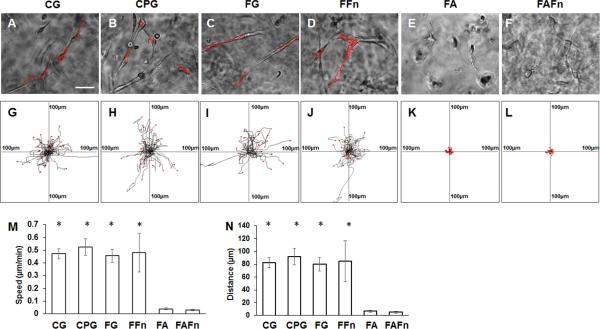
In this study, we investigated the migration of astrocytes in collagen hydrogel and fibrin hydrogel. Because the high concentration of 4S-StarPEG (0.05 mM) for collagen hydrogel crosslinking and aprotinin (40 μg/ml) in fibrin hydrogel did not significantly reduce the viability of astrocytes, we investigated the migration of astrocytes in these hydrogels. We observed that astrocytes migrated in the collagen hydrogel (Figure 6A) (see also supplemental material video 1) and the collagen gel crosslinked with 4S-StarPEG (0.05 mM) (Figure 6B) (see also supplemental material video 2). Fibronectin plays an important role in cell adhesion and migration. In this study, we also investigated the migration of astrocytes in the fibrin hydrogel containing fibronectin and fibrin hydrogel containing both fibronectin and aprotinin. Astrocytes migrated in the fibrin hydrogel (Figure 6C) (see also supplemental material video 3) and the fibrin hydrogel supplemented with fibronectin (25 μg/ml) (Figure 6D) (see also supplemental material video 4). The migration of astrocytes was inhibited in fibrin hydrogel containing aprotinin (40 μg/ml) (see also supplemental material video 5) and fibrin hydrogel containing both fibronectin (25 μg/ml) and aprotinin (40 μg/ml). (Figures 6E, 6F) (see also supplemental material video 6).
Figure 6.
Migration of astrocytes in hydrogels: (A–F) astrocytes migrated in hydrogels (red lines indicate tracks of migration of astrocytes in hydrogels). Scale bar: 50 μm; (G–L) cell migration paths determined by video monitor tracings (position of all cells at t = 0 min represented by origin position (center of frame), with migratory track of each cell at 3 hours plotted as single line on graph; each arm of axes represents 100 μm of translocation distance); (A, G) collagen hydrogel without crosslinking; (B, H) collagen hydrogel crosslinked with 4S-StarPEG (0.05 mM); (C, I) fibrin hydrogel without aprotinin; (D, J) fibrin hydrogel with fibronectin (25 μg/ml); (E, K) fibrin hydrogel with aprotinin (40 μg/ml); (F, L) fibrin hydrogel with fibronectin (25 μg/ml) and aprotinin (40 μg/ml); (M) quantification of cell migration speed in hydrogels; (N) quantification of cell migration distance in hydrogels. *p < 0.05, compared with astrocyte migration in FA or FAFn. CG = collagen hydrogel; CPG = crosslinked collagen hydrogel; FG = fibrin hydrogel; FFn = fibrin hydrogel containing fibronectin; FA = fibrin hydrogel containing aprotinin; FAFn = fibrin hydrogel containing aprotinin and fibronectin.
In Figures 5G–5L, each frame shows the superimposed migration tracks between 28 and 35 astrocytes. The position of all cells at t = 0 minute is represented by the origin (0, 0). Each line represents the migration track of one single cell over a 3-hour period.
The migration speed and distance of astrocytes in different types of hydrogels were quantified. Cell migration velocity was not significantly different for astrocytes in the collagen hydrogel (0.47 ± 0.03 μm/min), crosslinked collagen hydrogel (0.52 ± 0.06 μm/min), fibrin hydrogel (0.45 ± 0.05 μm/min), and fibrin hydrogel incorporated with fibronectin (0.48 ± 0.15 μm/min). The migration distances of astrocytes in the collagen hydrogel, crosslinked collagen hydrogel, fibrin hydrogel, and fibrin hydrogel containing fibronectin are not significantly different. However, the migration of astrocytes in the fibrin hydrogel containing aprotinin (0.03 ± 0.01 μm/min) and fibrin hydrogel containing both aprotinin and fibronectin (0.02 ± 0.01 μm/min) was inhibited.
Expression of CSPGs by astrocytes cultured in hydrogels
Similar to the migration study, we investigated the CSPG gene expression by astrocytes grown in non-crosslinked collagen hydrogel, collagen hydrogel crosslinked with 0.05 mM 4S-StarPEG, and fibrin hydrogel containing 40 μg/ml aprotinin and 25 μg/ml fibronectin. Because the fibrin hydrogel disappeared after the astrocytes (100,000 cells in fibrin hydrogel) grew in it for six days, the CGSP gene expression in this hydrogel was not studied. The expressions of neurocan, NG2, and versican by astrocytes grown on a cell culture plate and in hydrogels were studied after being cultured for 6 days (Figure 7). Expression of the neurocan gene by astrocytes cultured for 6 days on a cell culture plate (0.33 ± 0.05), in collagen hydrogel (0.26 ± 0.03), and crosslinked collagen hydrogel (0.28 ± 0.01) is lower than that expressed by astrocytes in the fibrin hydrogel (0.87 ± 0.23) (Figure 7A). However, the difference is not statistically significant. The expression of versican and NG2 in astrocytes cultured in different hydrogels was not significantly different (Figures 7B, 7C).
Figure 7.
Expression of neurocan, NG2, and versican by astrocytes grown in hydrogels. Expressed as fold change compared with gene expression by astrocytes in fibrin hydrogel containing aprotinin. Each target gene is normalized to GAPDH. CG = collagen hydrogel; CPG = crosslinked collagen hydrogel; FA = fibrin hydrogel containing aprotinin; FAFn = fibrin hydrogel containing aprotinin and fibronectin.
Discussion
The repair of an injured spinal cord is a significant scientific challenge because the injured spinal cord has limited capacity to regenerate and restore its functional connections. Recent advances in the research of cell therapy and application of biomaterials offers more approaches for the cure of an injured spinal cord. The implantation of biomaterial can generate a permissive environment for the growth of grafted cells and conduct axonal growth. Collagen and fibrin are biocompatible and biodegradable and are widely used natural biomaterial in tissue engineering. Previous studies have shown that the implantation of collagen hydrogel containing growth factors improved regeneration and functional recovery of an injured spinal cord. 22-24 Fibrin scaffolds delivering therapeutic cells 25 and growth factors 26, 27 were implanted into an injured spinal cord and improved axonal regeneration.
Type I collagen is a major component of the extracellular matrix and possesses cell-adhesive and signaling domains that are critical for axonal growth. Fibrin is a fibrous and non-globular protein that helps in blood clotting and has been used extensively as a biopolymer scaffold in neural repair. Implantation of fibrin scaffolds fabricated with neural stem cells into an injured rat spinal cord resulted in functional recovery. 28 Type I collagen and fibrin have been approved by the Food and Drug Administration (FDA) for implantable medical materials. However, fibrin is sensitive to proteolytic degradation by enzymes, such as matrix metalloproteinases and plasmin. 29 Aprotinin is a competitive serine protease inhibitor that forms stable complexes with and blocks the active sites of a few proteases such as plasmin, trypsin, chymotrypsin, and kallikrein. 30 Previous studies have demonstrated that the addition of aprotinin in the fibrin hydrogel can reduce its degradation rate. 31, 32 It was reported that crosslinking collagen with 4S-StarPEG can efficiently reduce the degradation rate of collagen without significant toxicity to cells grown in the hydrogel. 33 Though chemically treated collagen and fibrin hydrogels extended the longevity of biomaterials, the cellular behavior of astrocytes in hydrogel has not been reported. In this study, we characterized the major cellular behaviors including cell viability, cell migration, and the expression of CSPG in these hydrogels.
In this study, we showed that the crosslinking of collagen with 4S-StarPEG was confirmed by FTIR. The astrocytes in fibrin hydrogel degraded the hydrogel in the cells. However, the addition of aprotinin in the hydrogel prevented the astrocyte-induced hydrogel degradation. A previous study has demonstrated that 4S-StarPEG can effectively crosslink collagen hydrogel and that cells can grow in 4S-StarPEG crosslinked collagen hydrogel. 33, 34 In this study, we studied cell viability using the LIVE/DEAD® assay and the AlamarBlue® assay. In the LIVE/DEAD® assay, we found that the rate of live astrocytes in fibrin gel and collagen is similar. Crosslinking of collagen with 4S-StarPEG and treatment of fibrin with aprotinin did not significantly affect cell viability.
SCI can cause a complex cascade of molecular and cellular events that result in the loss of neurons, demyelination, and formation of a glial scar. The death of oligodendrocytes after SCI causes demyelination and the loss of appropriate axonal function. In response to demyelination, oligodendrocyte precursor cells migrate from the gray and white matter of the spinal cord to the lesion to myelinate the axons. It has been reported that Schwann cells participate in the regeneration process post-SCI. Schwann cells can regenerate and migrate to the injury site to myelinate axons.35 Transplantation of OPCs and Schwann cells enhanced the myelination of axons and improved their functional recovery.35-40 Schwann cells are an important source of cells to facilitate myelination in SCI because they can be isolated from the peripheral nerve and cultured in vitro. Several studies reported the migration of stem cells, 41, 42 oligodendrocyte precursors, 43 and Schwann cells after transplantation into the adult spinal cord. 44, 45 One study showed that after the neuronal restricted precursors (NRPs) and glial-restricted precursors (GRPs) were transplanted into a hemisected rat spinal cord, excellent survival was observed, and they migrated out of the injury site and differentiated into mature CNS phenotypes, including many neurons. 46 Astrocytes play an important role in the repair of SCI by acting as a trophic support for growth axons. The implantation of conductive biomaterials may mediate the migration of astrocytes into the SCI lesion. A prior in vitro study showed that astrocytes can migrate in collagen hydrogel crosslinked with genipin. 47 Fibrin hydrogel can provide a permissive environment, promoting the migration of support cells into a lesion to enhance axonal regeneration. In this study, the cell migration assay showed that astrocytes migrated randomly in the fibrin and collagen hydrogels. The migration speed of astrocytes in these hydrogels is similar. The crosslinking of collagen with 4S-StarPEG did not change the cell migration speed in this hydrogel. However, the addition of aprotinin in the fibrin hydrogel inhibited the migration of astrocytes.
Serine proteases, including tissue plasminogen activator (tPA) and urokinase plasminogen activator (uPA) and their high-affinity cell surface receptors, play an important role in regulating the cell-extracellular matrix interaction and cell migration by localizing fibrinolysis and inducing intracellular signaling pathways. The binding of uPA to the uPA receptor (uPAR) at the cell surface can induce cell adhesion and migration.48, 49 It was reported that RhoA, Rac 1, Rho kinase can mediate the uPA/uPAR-induced migration of human vascular smooth muscle cells via the Tyk2/PI-3K signaling complex.50 Downregulation of uPA in glioblastoma cells inhibited the PI3k/Akt signaling pathway and cell migration.51 Astrocytes can synthesize both uPA and tPA, and their expression is regulated developmentally.52-55 It was reported that plasminogen activator activity can regulate astrocyte migration.52 Aprotinin, a serine protease inhibitor, can inhibit the function of plasminogen activator. In this study, the addition of aprotinin in fibrin hydrogel slowed down the astrocyte-induced fibrinolysis and inhibited astrocyte migration. This observation suggested that the migration of astrocyte in fibrin hydrogel is in a plasminogen activator activity-dependent fashion. A previous study reported the influence of the mechanical properties of materials on cell attachment and proliferation.56 In our study, we found that 4S-StarPEG was not toxic to astrocytes and did not affect cell motility. Stiffness of the hydrogel may also affect cell motility for cells grown in the hydrogel. In a future study, we will modulate the stiffness of the hydrogel and determine the effect of mechanical properties on cell migration.
CSPGs are extracellular proteoglycans consisting of a protein core and a chondroitin sulfate side chain. In CNS injury, the expression of these molecules is upregulated around the lesion site and contributes to glial scar formation that limits axonal regeneration. In a previous study, fibrin hydrogel was implanted into a hemisected rat spinal cord, and the density of CSPG surrounding the injury site was analyzed. Immunostaining of NG2 revealed that the density of NG2 staining surrounding the injury site in groups treated with fibrin scaffolds was not different from that in control groups. 21 In this study, we analyzed the expression of NG2, neurocan, and versican by astrocytes grown in collagen hydrogel and fibrin hydrogel. The expression of neurocan by astrocytes grown in collagen hydrogel and 4S-StarPEG-crosslinked collagen hydrogels was lower compared with the expression by astrocytes grown in aprotinin-treated fibrin hydrogels, though it was not statistically significant. The expression of NG2 and versican by cells in different hydrogels was not significantly different.
Fibronectin can be recognized by cell surface receptors and is responsible for the attachment of cells to the extracellular matrix. It was reported that during brain development or injury, fibronectin and other matrix proteins may promote the migration of neuronal progenitor cells. 57 Fibrin hydrogel containing fibronectin improved cell attachment and proliferation. 58 Implantation of this mixture into an injured spinal cord showed improved tissue integration and axonal growth. A previous investigation studied the migration of ten cell lines on fibronectin-coated substrata. Among the ten cell lines, the migration of virus-transformed fibroblast WI-38 VA13 cells, the virus-transformed 3T3 cells, and baby hamster kidney cell line (BHK) cells was enhanced on fibronectin-coated substrata. However, it is not clear if fibronectin can enhance the migration of astrocytes.59 In this study, we added fibronectin into the fibrin hydrogel and studied cell migration in the fibrin hydrogel containing fibronectin and the fibrin hydrogel containing both fibronectin and aprotinin. We found that the migration speed of astrocytes in fibrin hydrogels with or without fibronectin was not different significantly. The addition of aprotinin in the fibrin hydrogel and fibrin hydrogel containing fibronectin inhibited astrocyte migration in these hydrogels.
A number of studies have shown that the degradation of fibrin hydrogels was slowed down when aprotinin was added in a cell culture medium60-63 or directly incorporated into fibrin scaffolds.64-66 These studies have suggested that fibrin materials modified with aprotinin can be potentially used in neural tissue engineering. A recent study reported the host tissue response to the fibrin scaffolds containing aprotinin in a subcutaneous implant model. The grafted fibrin scaffolds containing aprotinin resulted in increased vascularization of the scaffold material and improved integration of cell-seeded scaffolds with the host tissue.66 However, it was reported that the application of aprotinin in cardiac surgery increased the risk of complications such as acute renal failure, myocardial infarction and heart failure, and stroke, compared with tranexamic acid (TXA) and ε-aminocaproic acid (ε-ACA).67 In this study, we found that the addition of aprotinin in fibrin hydrogel was not toxic to astrocytes. Because TXA can replace aprotinin in fibrin scaffolds to prevent fibrinolysis, it will be interesting to study astrocyte motility in fibrin hydrogel containing TXA.
Conclusion
In this study, we fabricated 4S-StarPEG-crosslinked collagen hydrogel and fibrin hydrogel containing aprotinin. We grew astrocytes isolated from neonatal rats in these hydrogels and studied the cell behavior. This investigation demonstrated that astrocytes maintained good cell viability in collagen hydrogel crosslinked with 4S-StarPEG or fibrin hydrogels containing aprotinin. Cell migration study showed that astrocytes migrated in the fibrin and collagen hydrogels, and the migration speed was similar in these hydrogels. The crosslinking of collagen hydrogel with 4S-StarPEG did not change the astrocyte migration speed. However, the addition of aprotinin in the fibrin hydrogel inhibited astrocyte migration. A quantitative RT-PCR assay showed that the expression of major CSPGs, including NG2, neurocan, and versican, by the astrocytes in the hydrogels was not significantly different.
Materials and methods
Fabrication of type I collagen and fibrin hydrogels
Type I collagen of rat tail tendon (3.5 mg/ml, Corning, Corning, NY) was used to fabricate the collagen hydrogel. The pH of the collagen solution was adjusted to 7 by adding NaOH aqueous solution (1M) and phosphate-buffered saline (PBS) solution (5×). The hydrogel was then crosslinked with different concentrations of 4S-StarPEG. The final concentration of 4S-StarPEG in collagen solution was either 0.01 mM, 0.05 mM, or 0.1 mM. Collagen hydrogel without any crosslinker was used as the control. To form the fibrin hydrogel, a human fibrinogen (Sigma-Aldrich, St. Louis, MO) solution (20 mg/mL) was dissolved in PBS. Then 25 μl of thrombin (Sigma-Aldrich, St. Louis, MO) solution (10 U/ml) was added into each well to mix with the fibrinogen solution in order to initialize fibrin hydrogel formation. Then the plate was transferred into an incubator (37° C and 5% CO2) to form a stable hydrogel. To strengthen the fibrin hydrogel, aprotinin was added into the fibrinogen solution before the fibrin hydrogel was formed. The final concentration of aprotinin in the fibrin hydrogels was either 10 μg/ml or 40 μg/ml.
Astrocyte culture and cell growth in collagen and fibrin hydrogels
All procedures involving animals in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of Wichita State University. All experiments were performed in compliance with the national guidelines and institutional guidelines of Wichita State University. The cell cultures of astrocytes were prepared from the brains of newborn (postnatal day 1 to day 3) Sprague Dawley rats as previously described. 68 In brief, cerebral cortices were isolated from the brains of neonatal rats after they were sacrificed. The cortex tissues were triturated gently through a 5 ml syringe with needle. The tissue suspension was passed through a 70 mm nylon cell strainer, and the flow-through was collected with a 50 ml conical tube. The isolated cells were cultured for about 7–14 days. After reaching confluency, the cultures were shaken to remove macrophages and progenitor cells. The adherent astrocytes were cultured subsequently for studying the hydrogels. The cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Lifetechnology, Grand Island, NY). The medium was changed every 2–3 days, and the cell culture was incubated with 5% CO2 at 37°C. The cells of passage 2, 3, and 4 were used in these studies. To seed astrocytes into collagen hydrogel, cells were added into the neutralized collagen solution containing a crosslinker. To seed astrocytes into fibrin hydrogel, cells were added into the fibrinogen solution containing aprotinin. Then the thrombin solution was mixed with fibrinogen. Mixtures of either the collagen solution or fibrin solution were maintained at 37 °C in a humidified atmosphere of 5% CO2 for 20 minutes before adding the cell culture medium into the formed hydrogels.
Degradation of fibrin hydrogel by astrocytes
To investigate the degradation rate of the fibrin hydrogel by astrocytes, Astrocytes were seeded in fibrin hydrogels (0 μg/ml, 10 μg/ml, 40 μg/ml aprotinin) with the cell density of 40,000 cells/0.5ml hydrogel in plastic tubes. The height and the weight of the hydrogels were measured at different time points of cell culture (6h, 1 day, 3 days, 6days and 9days) after the culture medium was removed.
LIVE/DEAD® cell viability assay
Astrocytes were seeded in various collagen hydrogels (0 mM, 0.01 mM, 0.05 mM 4S-StarPEG) and fibrin hydrogels (0 μg/ml, 10 μg/ml, 40 μg/ml aprotinin). In this assay, 40,000 cells were seeded in 0.5ml collagen or fibrin hydrogels. The LIVE/DEAD® cell vitality assay (Lifetechnology, Grand Island, NY) was performed for the astrocytes cultured in the hydrogels for 6 days. Reagents for the LIVE/DEAD® assay were ethidium homodimer-1 (Ethd-1) with a molecular weight of 856.77, and calcein AM with a molecular weight 994.87. Solutions of the assay were removed from the freezer and allowed to warm to room temperature. EthD-1 stock solution (2 μl, 2 mM) and calcein AM stock solution (0.5 μl of 4 mM) were added to a sterile PBS solution (1 ml) and vortexed. The solution (300 μl) was added directly to each cell culture well and incubated for 30 minutes at room temperature. The cells were then viewed under a fluorescent microscope. At least three independent experiments were performed in this study. Four images of the cells within the hydrogel in each experiment were recorded. The live and dead cells in the images were counted, and the ratio of live cells to total cells was quantified.
AlamarBlue® assay
The viability of astrocytes in the hydrogel was studied by monitoring their metabolic activity using the AlamarBlue® assay (Pierce Biotechnology, Rockford, lL). Astrocytes were seeded in collagen hydrogels (0 mM, 0.01 mM, 0.05 mM 4S-StarPEG) and fibrin hydrogels with aprotinin (10 μg/ml, 40 μg/ml aprotinin). The cell seeding density was 50,000 cells in each collagen (0.5 ml per hydrogel) or fibrin hydrogel (0.5 ml per hydrogel), and the cells were cultured for 3 days. These cells were then incubated with a cell culture medium containing 10% (v/v) AlamarBlue® reagent for 4 hours. Absorbance was measured at wavelengths of 570 nm and 600 nm in a microplate reader (Synergy Mx Monochromator-Based Multi-Mode Microplate Reader, Winooski, VT).
Infrared spectroscopy
Fourier transform infrared spectroscopy (FTIR) was performed to study the chemical bond formation of collagen hydrogel with or without crosslinking. Collagen hydrogels with or without crosslinking were freeze-dried for FTIR testing. Spectra were obtained with a Spectrum 100 FT-IR Spectrometer Perkin Elmer (PerkinElmer, Waltham, MA). To obtain an IR spectrum, the specimen (hydrogel) was loaded onto a salt plate and placed in a mounting plate in the sample loading compartment. The parameters were adjusted. In FTIR, the infrared beam enters the compartment, passes through the sample, and finally enters the detector area, resulting in a spectrum with peaks.
Migration of astrocytes in collagen and fibrin hydrogels
To study the migration of astrocytes in the hydrogel, 30,000 cells were seeded in 150 μl collagen hydrogels containing 4S-StarPEG (0.05 mM), fibrin hydrogels containing aprotinin (40 μg/ml), and fibrin hydrogels containing aprotinin (40 μg/ml) and fibronectin (25 μg/ml). The cells in the non-crosslinked collagen hydrogel and fibrin hydrogel without aprotinin were used as a control. After the cells were cultured for 24 hours, the migration of astrocytes was recorded with a time-lapse microscope (Zeiss Axio Observer microscope) placed in a plastic incubator with 5% CO2 at 37°C. 69, 70 Sterile conditions were maintained throughout. The time-lapse image recording was performed to record cell migration using ZEN 2011 imaging microscope software. Images were captured using a digital camera (AxioCam MRm Rev.3 with FireWire). The migration of cells was recorded for 3 hours. The time-lapse images were analyzed using NIH ImageJ software (National Institutes of Health, Bethesda, MD). The cell migration distance and speed were quantified. The distance is the full length of cell migration. The cell migration speed was calculated from the full distance of cell migration in a given period of time.
Real-time RT-PCR
In order to study the quantitative real-time polymerase chain reaction (qRT-PCR), 100,000 cells were seeded in collagen hydrogels (0 mM, 0.05 mM 4S-StarPEG), fibrin hydrogel containing aprotinin (40 μg/ml aprotinin), and fibrin hydrogel containing aprotinin (40 μg/ml) and fibronectin (25 μg/ml). Total RNA of the astrocytes cultured for 6 days was extracted using an RNeasy Micro Kit (Qiagen, Germantown, MD) according to the supplier's protocol. The amount of RNA was determined using a NanoDrop 2000c (Thermo Scientific, Waltham, MA). The cDNA was reverse-transcribed from the total RNA using a High-Capacity cDNA Reverse Transcription Kit (Lifetechnology, Grand Island, NY) according to the manufacturer's protocol. The qRT-PCR was performed using the Power SYBR® Master Mix by the StepOnePlus™ qRT-PCR System (Lifetechnology, Grand Island, NY) at 95°C for 10 minutes, and then 40 cycles at 95°C for 15 seconds followed by 60°C for 60 seconds. Gene transcription was normalized in relation to transcription of the housekeeping rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The 2−ΔΔCt method was used to calculate the relative gene expression for each target gene. Primers used in the qRT-PCR are provided in Table 1.
Table 1.
Primers for qRT-PCR
| Gene | Oligonucleotide (5’-3’) |
|---|---|
| NG2 | F: AGCAGGCCAAACAGATCATCT |
| R: AGTCACTCAGCACCGTGTCTG | |
| Versican | F: TTGACCAGTGCGATTACGGC |
| R: CAGGGAGAGGGAAGCATGTC | |
| Neurocan | F: TGATGGTGGCGCATGAGAAT |
| R: CCACACAGCACTGTACCCTT | |
| GAPDH | F: GAC ATG CCG CCT GGA GAA AC |
| R: AGC CCA GGA TGC CCT TTA GT |
Statistics
Data were expressed as means ± SD and analyzed by using one-way ANOVA (post-hoc Bonferroni) with SPSS version 17.0 software package (SPSS Inc., Chicago, IL). P-values less than 0.05 were considered statistically significant.
Supplementary Material
Insight, innovation, integration.
Natural biomaterials based-hydrogel can serve as a growth-permissive substrate for therapeutic cell transplantation into the injured spinal cord. To improve their in vivo function, the degradation rate of these materials can be modified by protease inhibitors and crosslinkers. Cell migration is a critical process for the endogenous and grafted neural cells to establish functional connections in central nervous system lesion. In this study, we integrated 3D cell culture and time-lapse microscopy to study the astrocyte motility in chemically modified fibrin and collagen hydrogels. The study provides an insight into the dynamic cellular behaviors including cell viability and motility and the expression of chondroitin sulfate proteoglycans in these hydrogels.
Acknowledgement
We acknowledge Li Yao' s start-up funding, Wichita State University and the National Institute of General Medical Sciences (P20 GM103418) from the National Institutes of Health.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Bozoyan L, Khlghatyan J, Saghatelyan A. J. Neurosci. 2012;32:1687–1704. doi: 10.1523/JNEUROSCI.5531-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perea G, Araque A. J. Physiol. Paris. 2006;99:92–97. doi: 10.1016/j.jphysparis.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Powell EM, Geller HM. Glia. 1999;26:73–83. doi: 10.1002/(sici)1098-1136(199903)26:1<73::aid-glia8>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Pindzola RR, Doller C, Silver J. Dev. Biol. 156:34–48. doi: 10.1006/dbio.1993.1057. [DOI] [PubMed] [Google Scholar]
- 5.Powell EM, Meiners S, DiProspero NA, Geller HM. Cell. Tissue. Res. 1997;290:385–393. doi: 10.1007/s004410050945. [DOI] [PubMed] [Google Scholar]
- 6.Araque A, Navarrete M. Philos. Trans. R. Soc. Lond. B. Bio.l Sci. 2010;365:2375–2381. doi: 10.1098/rstb.2009.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagelhus EA, Amiry-Moghaddam M, Bergersen LH, Bjaalie JG, Eriksson J, Gundersen V, Leergaard TB, Morth JP, Storm-Mathisen J, Torp R, Walhovd KB, Tønjum T. Mech. Ageing. Dev. 2013;134:449–459. doi: 10.1016/j.mad.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Toy D, Namgung U. Exp. Neurobiol. 2013;22:68–76. doi: 10.5607/en.2013.22.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yiu G, He Z. Nat. Rev. Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. J. Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang JJ, Chuah ML, Yew DT, Leung PC, Tsang DC. Neuroscience. 1995;65:973–981. doi: 10.1016/0306-4522(94)00519-b. [DOI] [PubMed] [Google Scholar]
- 12.Davies JE, Huang C, Proschel C, Noble M, Mayer-Proschel M, Davies SJA. J. Biol. 2006;5:7. doi: 10.1186/jbiol35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies JE, Proschel C, Zhang N, Noble M, Mayer-Proschel M, Davies SJA. J. Biol. 2008;7:24. doi: 10.1186/jbiol85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies SJA, Shih CH, Noble M, Mayer-Proschel M, Davies JE, Proschel C. PloS. one. 2011;6:e17328. doi: 10.1371/journal.pone.0017328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan C, Zheng Y, Cheng X, Qi X, Bu P, Luo X, Kim DH, Cao Q. Int. J. Biol. Sci. 2013;9:78. doi: 10.7150/ijbs.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas C, Fischer I. J. Neurotrauma. 2013;30:1035–1052. doi: 10.1089/neu.2013.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kliot M, Smith GM, Siegal JD, Silver J. Exp. Neurol. 1990;109:57–69. doi: 10.1016/s0014-4886(05)80008-1. [DOI] [PubMed] [Google Scholar]
- 18.Greiling D, Clark RA. J. Cell. Sci. 1997;110:861–870. doi: 10.1242/jcs.110.7.861. [DOI] [PubMed] [Google Scholar]
- 19.Kim SH, Turnbull J, Guimond S. J. Endocrinol. 2011;209:139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- 20.Feng X, Clark RA, Galanakis D, Tonnesen MG. J. Invest. Dermatol. 1999;113:913–919. doi: 10.1046/j.1523-1747.1999.00786.x. [DOI] [PubMed] [Google Scholar]
- 21.Johnson PJ, Parker SR, Sakiyama-Elbert SE. J. Biomed. Mater. Res. A. 2010;92:152–163. doi: 10.1002/jbm.a.32343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houweling DA, Lankhorst A, Gispen WH, Bar PR, Joosten EA. Exp. Neurol. 1998;153:49–59. doi: 10.1006/exnr.1998.6867. [DOI] [PubMed] [Google Scholar]
- 23.Houweling DA, Van Asseldonk JTH, Lankhorst AJ, Hamers FPT, Martin D, Bar PR, Joosten EAJ. Neurosci. Lett. 1998;251:193–196. doi: 10.1016/s0304-3940(98)00536-9. [DOI] [PubMed] [Google Scholar]
- 24.Jimenez Hamann MC, Tator CH, Shoichet MS. Exp. Neurol. 2005;194:106–119. doi: 10.1016/j.expneurol.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 25.Johnson PJ, Tatara A, McCreedy DA, Shiu A, Sakiyama-Elbert SE. Soft Matter. 2010;6:5127–5137. doi: 10.1039/c0sm00173b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson PJ, Parker SR, Sakiyama-Elbert SE. Biotechnol. Bioeng. 2009;104:1207–1214. doi: 10.1002/bit.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King V, Phillips J, Hunt-Grubbe H, Brown R, Priestley J. Biomaterials. 2006;27:485–496. doi: 10.1016/j.biomaterials.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 28.Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchta C, Hedrich HC, Macher M, Hocker P, Redl H. Biomaterials. 2005;26:6233–6241. doi: 10.1016/j.biomaterials.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Kang HM, Kalnoski MH, Frederick M, Chandler WI. Thromb. Res. 2005;115:327–340. doi: 10.1016/j.thromres.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Smith JD, Chen A, Ernst LA, Waggoner AS, Campbell PG. Bioconjug. Chem. 2007;18:695–701. doi: 10.1021/bc060265o. [DOI] [PubMed] [Google Scholar]
- 32.Lorentz KM, Kontos S, Frey P, Hubbell JA. Biomaterials. 2011;32:430–438. doi: 10.1016/j.biomaterials.2010.08.109. [DOI] [PubMed] [Google Scholar]
- 33.Collin EC, Grad S, Zeugolis DI, Vinatier CS, Clouet JR, Guicheux JJ, Weiss P, Alini M, Pandit AS. Biomaterials. 2011;32:2862–2870. doi: 10.1016/j.biomaterials.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 34.Taguchi T, Xu L, Kobayashi H, Taniguchi A, Kataoka K, Tanaka J. Biomaterials. 2005;26:1247–1252. doi: 10.1016/j.biomaterials.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 35.Guest JD, Hiester ED, Bunge RP. Exp Neurol. 2005;192:384–93. doi: 10.1016/j.expneurol.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 36.Bambakidis NC, Miller RH. Spine J. 2004;4:16–26. doi: 10.1016/j.spinee.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Cao Q, He Q, Wang Y, Cheng X, Howard RM, Zhang Y, DeVries WH, Shields CB, Magnuson DS, Xu XM, Kim DH, Whittemore SR. J Neurosci. 2010;30:2989–3001. doi: 10.1523/JNEUROSCI.3174-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinzon A, Calancie B, Oudega M, Noga BR. J Neurosci Res. 2001;64:533–41. doi: 10.1002/jnr.1105. [DOI] [PubMed] [Google Scholar]
- 39.Pearse DD, Sanchez AR, Pereira FC, Andrade CM, Puzis R, Pressman Y, Golden K, Kitay BM, Blits B, Wood PM, Bunge MB. Glia. 2007;55:976–1000. doi: 10.1002/glia.20490. [DOI] [PubMed] [Google Scholar]
- 40.Bunge MB. J Spinal Cord Med. 2008;31:262–9. doi: 10.1080/10790268.2008.11760720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chow SY, Moul J, Tobias CA, Himes BT, Liu Y, Obrocka M, Hodge L, Tessler A, Fischer I. Brain. Res. 2000;874:87–106. doi: 10.1016/s0006-8993(00)02443-4. [DOI] [PubMed] [Google Scholar]
- 42.Cao QL, Zhang YP, Howard RM, Walters WM, Tsoulfas P, Whittemore SR. Exp. Neurol. 2001;167:48–58. doi: 10.1006/exnr.2000.7536. [DOI] [PubMed] [Google Scholar]
- 43.Franklin RJ, Bayley SA, Blakemore WF. Exp. Neurol. 1996;137:263–276. doi: 10.1006/exnr.1996.0025. [DOI] [PubMed] [Google Scholar]
- 44.Xu XM, Chen A, Guenard V, Kleitman N, Bunge MB. J. Neurocytol., 1997. 26:1–16. doi: 10.1023/a:1018557923309. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Dancausse H, Grijalva I, Oliveira M, Levi AD. J. Neurosci. Methods. 2003;125:83–91. doi: 10.1016/s0165-0270(03)00044-x. [DOI] [PubMed] [Google Scholar]
- 46.Lepore AC, Fischer I. Exp. Neurol. 2005;194:230–242. doi: 10.1016/j.expneurol.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 47.Macaya DJ, Hayakawa K, Arai K, Spector M. Biomaterials. 2013;34:3591–3602. doi: 10.1016/j.biomaterials.2012.12.050. [DOI] [PubMed] [Google Scholar]
- 48.Busso N, Masur SK, Lazega D, Waxman S, Ossowski L. J Cell Biol. 1994;126:259–270. doi: 10.1083/jcb.126.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stahl A, Mueller BM. Cancer Res. 1994;54:3066–3071. [PubMed] [Google Scholar]
- 50.Kiian I, Tkachuk N, Haller H, Dumler I. Thromb Haemost. 2003;89:904–14. [PubMed] [Google Scholar]
- 51.Chandrasekar N, Mohanam S, Gujrati M, Olivero WC, Dinh DH, Rao JS. Oncogene. 2003;22:392–400. doi: 10.1038/sj.onc.1206164. [DOI] [PubMed] [Google Scholar]
- 52.Faber-Elman A, Miskin R, Schwartz M. J Neurochem. 1995;65:1524–35. doi: 10.1046/j.1471-4159.1995.65041524.x. [DOI] [PubMed] [Google Scholar]
- 53.Kalderon N, Ahonen K, Fedoroff S. Glia. 1990;3:413–426. doi: 10.1002/glia.440030513. [DOI] [PubMed] [Google Scholar]
- 54.Moonen G, Grau-Wagemans MP, Selak I, Lefebvre P, Rogister B, Vassalli JD, Belin D. Dev. Brain Res. 1985;20:41–48. doi: 10.1016/0165-3806(85)90085-9. [DOI] [PubMed] [Google Scholar]
- 55.Tang H, Fu WY, Ip NY. Neurosci Lett. 2000;285:143–6. doi: 10.1016/s0304-3940(00)00998-8. [DOI] [PubMed] [Google Scholar]
- 56.Cai L, Lu J, Sheen V, Wang S. Biomacromolecules. 2012;13:342–349. doi: 10.1021/bm201763n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tonge DA, de Burgh HT, Docherty R, Humphries MJ, Craig SE, Pizzey J. Brain. Res. 2012;1453:8–16. doi: 10.1016/j.brainres.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.King VR, Alovskaya A, Wei DY, Brown RA, Priestley JV. Biomaterials. 2010;31:4447–4456. doi: 10.1016/j.biomaterials.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 59.Straus AH, Carter WG, Wayner EA, Hakomori S. Exp Cell Res. 1989;183:26–39. doi: 10.1016/0014-4827(89)90423-0. [DOI] [PubMed] [Google Scholar]
- 60.Willerth SM, Arendas KJ, Gottlieb DI, Sakiyama-Elbert SE. Biomaterials. 2006;27:5990–6003. doi: 10.1016/j.biomaterials.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cholewinski E, Dietrich M, Flanagan TC, Schmitz-Rode T, Jockenhoevel S. Tissue Eng Part A. 2009;15:3645. doi: 10.1089/ten.tea.2009.0235. [DOI] [PubMed] [Google Scholar]
- 62.Ahmed TA, Griffith M, Hincke M. Tissue Eng. 2007;13:1469. doi: 10.1089/ten.2006.0354. [DOI] [PubMed] [Google Scholar]
- 63.Ye Q, Zünd G, Benedikt P, Jockenhoevel S, Hoerstrup SP, Sakyama S, Hubbell JA, Turina M. Eur J Cardiothorac Surg. 2000;17:587. doi: 10.1016/s1010-7940(00)00373-0. [DOI] [PubMed] [Google Scholar]
- 64.Lorentz LM, Kontos S, Frey P, Hubbell JA. Biomaterials. 2011;32:430–438. doi: 10.1016/j.biomaterials.2010.08.109. [DOI] [PubMed] [Google Scholar]
- 65.Smith JD, Chen A, Ernst LA, Waggoner AS, Campbell PG. Bioconjug Chem. 2007;18:695–701. doi: 10.1021/bc060265o. [DOI] [PubMed] [Google Scholar]
- 66.Thomson KK, Dupras SK, Murry CE, Scatena M, Regnier M. Angiogenesis. 2014;17:195–205. doi: 10.1007/s10456-013-9388-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mangano DT, Tudor IC, Dietzel C. N Engl J Med. 2006;354:353–365. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 68.Laabs TL, Wang H, Katagiri Y, McCann T, Fawcett JW, Geller HM. J. Neurosci. 2007;27:14494–14501. doi: 10.1523/JNEUROSCI.2807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Y, Wang PS, Lucas G, Li R, Yao L. Stem. Cell. Res. Ther. 2015;6:1–11. doi: 10.1186/s13287-015-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yao L, Li Y, Knapp J, Smith P. J. Cell. Physiol. 2015;230:1515–1524. doi: 10.1002/jcp.24897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.