Abstract
Apoptotic debris, autoantibody, and IgG-immune complexes (ICs) have long been implicated in the inflammation associated with systemic lupus erythematosus (SLE); however, it remains unclear whether they initiate immune-mediated events that promote disease. In this study, we show that peripheral blood mononuclear cells from SLE patients experiencing active disease, and hematopoietic cells from lupus-prone MRL/lpr and NZM2410 mice accumulate markedly elevated levels of surface-bound nuclear self-antigens. On dendritic cells (DCs) and macrophages (MFs), the self-antigens are part of IgG-ICs that promote FcγRI-mediated signal transduction. Accumulation of IgG-ICs is evident on ex vivo myeloid cells from MRL/lpr mice by 10 weeks of age, and steadily increases prior to lupus nephritis. IgG and FcγRI play a critical role in disease pathology. Passive transfer of pathogenic IgG into IgG-deficient MRL/lpr mice promotes the accumulation of IgG-ICs prior to significant B cell expansion, BAFF secretion, and lupus nephritis. In contrast, diminishing the burden IgG-ICs in MRL/lpr mice through deficiency in FcγRI markedly improves these lupus pathologies. Together, our findings reveal a previously unappreciated role for the cell surface accumulation of IgG-ICs in human and murine lupus.
Introduction
Systemic lupus erythematosus (SLE) is a multi-systemic autoimmune disease with genetic and environmental components that lead to autoimmunity and tissue-damaging inflammation (1, 2). There has long been an association between elevated levels of apoptotic debris and immune complexes (ICs), and their decreased clearance in SLE (3). Defects in opsonins such as mannose binding protein, complement components, and C-reactive protein reduce the clearance of apoptotic debris (4), and deficiency in DNase or RNase leads to poor lysosomal degradation (5). Although these defects heighten the burden of apoptotic debris and promote some of the phenotypes associated with lupus, ablation of opsonins or their receptors is insufficient to promote severe disease (6, 7). One consequence of heightened apoptotic debris is the exposure of the immune system to normally privileged nuclear self-antigens (8, 9). Cell-derived autoantigens exposed on apoptotic debris form immune complexes (ICs) when bound by autoreactive IgG (henceforth referred to as IgG-ICs). Upon binding FcγRs or B cell receptors (BCRs), they promote immune activation of B cells, macrophages (MFs) and dendritic cells (DCs) in part by delivering ligands to TLR7 and TLR9 (10, 11).
Activating FcγRs on human (FcγRI/IIa/IIc/IIIa) and murine (FcγRI/III/IV) phagocytic cells contain ITAM motifs that recruit Syk and activate the PI3k pathway (12). Activation of FcγRs (FcγRI, III, and IV in mouse) is regulated by co-ligation with ITIM-containing inhibitory (FcγRIIB) receptors. In mouse, FcγRIIB represses ITAM-containing FcγRs by recruiting SHIP to dephosphorylate PI(3,4,5)P3 thereby limiting downstream signal propagation (13, 14) and by SHP-1 through inhibitory signaling conditions called ITAMi that desensitizes receptor signal transduction (15).
Studies have identified FcγR polymorphisms as genetic factors influencing susceptibility to SLE and other autoimmune diseases (16, 17). Promoter polymorphisms that reduce FcγRIIB expression on germinal centers (GCs) and activated B cells are associated with murine and human SLE (18, 19). In addition, mice lacking FcγRIIB (20) develop lupus-like disease. Other functional polymorphisms in human FcγRIIa (R/H131) and FcγRIIIa (158V/F) decrease binding to IgG and are thought to diminish clearance of apoptotic debris, yet they are associated with lupus nephritis (21). Thus, polymorphisms in both activating and inhibitory FcγRs are associated with disease.
The pathogenic role of IgG-ICs in lupus has long been associated with their deposits in the kidneys and their ability to activate complement (C3) in lupus nephritis (22, 23). However, later studies showed that deposits of IgG and complement persist in the kidneys of lupus-prone mice when proteinuria and morbidity were diminished by blockade or genetic ablation of BAFF (24, 25). This indicates that IgG and complement deposits are not sufficient to induce lupus nephritis. Further studies using bone marrow chimeras showed that expression of FcγR on hematopoietic cells, rather than kidney mesangial cells, is required for lupus nephritis (26). This indicates that activation of the immune system through FcγRs on hematopoietic cells, rather than the deposits of IgG-IC in the kidney, is important in lupus nephritis. Studies also show that IgG-ICs promote autoantibody secretion in a TLR-dependent manner, and they contribute to immune responses associated with SLE in a TLR-independent manner (10, 11). However, it remains unclear how IgG-ICs plays a role in the pathogenic processes of SLE beyond internalizing TLR ligands to activate B cells.
In this manuscript we show that IgG and apoptotic antigens (as IgG-ICs) accumulate on the surface of myeloid cells prior to the onset of SLE. In lupus-prone mice, nuclear self-antigens were displayed on hematopoietic cells, and in SLE patients, 67–75% with active disease accumulated nuclear self-antigens on peripheral blood B and T cells, and 10–40% displayed nuclear self-antigens on monocytes. On murine DCs and MFs, the antigens were contained within IgG-ICs bound to the activating Fc receptors, FcγRI and FcγRIV. In MRL/lpr mice, accumulation of IgG-ICs on FcγRI induced activation of the PI3k pathway and preceded lupus nephritis, whereas MRL/lpr mice lacking FcγRI (FcγRI−/−MRL/lpr) were protected. In contrast, inducing the accumulation of IgG-IC on the cell surface by passive transfer of anti-nucleosome IgG into AID−/−MRL/lpr mice promoted serological autoimmunity, BAFF secretion, and lupus nephritis. Together, these data identify that the accumulation of IgG-ICs on hematopoietic cells occurs during human and murine SLE and is associated with autoimmunity and disease pathogenesis.
Methods
Animals
C57B6 (B6) and MRL/MpJ-Tnfrs6lpr/J (MRL/lpr; JAX Stock # 000485) colonies were maintained in an accredited animal facility at University of North Carolina at Chapel Hill (UNC-CH). FcγRI−/− (27, 28) and FcγRIII−/− mice (29) were obtained from Dr. Anne Sperling, FcγRIIB−/− (30), FcγRIV−/− (31) from Dr. Charles Jennette, FcRγc−/− (32) from Dr. Alex Szalai. NZM2410 mice (33) were from Dr. Gary Gilkeson. MRL/MpJ mice were purchased from JAX (Stock # 000486). AID−/−MRL/lpr mice were obtained from Dr. Marilyn Diaz at NIEHS (34). FcγRI−/−C57B6 mice were crossed with MRL/lpr mice for 12 generations, followed by an intercross of FcγRI+/−MRL/lpr for 2 generations to produce FcγRI−/−MRL/lpr mice. Tail DNAs were analyzed by PCR (27).
Human samples
Patients who showed SLEDAI scores ≥6, as defined by the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), were selected for enrollment after informed consent in accordance with the UNC institutional internal review board. Peripheral blood samples were collected with sodium heparin (BD Biosciences) during routine clinic visits.
Passive administration of anti-nucleosome antibody
AID−/−MRL/lpr, FcγRI−/−MRL/lpr, or B6 mice (18 to 21 weeks old) were injected with 500 μg of PL2-3 (anti-nucleosome, IgG2a) (35), F(ab′)2 of PL2-3, or Hy1.2 (isotype control IgG2a, anti-TNP) intravenously (i.v.) per mouse every week for 2 or 5 weeks. Five days after the 5th injection, mice were euthanized.
Cells
Splenocytes or total kidney cells were prepared into a single cell suspension. Splenic DCs and MFs were isolated using MicroBeads for CD11c+ cells (DCs) and CD11b+ cells (MFs) following the manufacturer’s instructions (Miltenyi Biotec).
ELISA
For serum anti-nucleosome and anti-dsDNA IgM or IgG levels, plates were coated with dsDNA (10 μg/ml, Sigma) in the presence or absence of histone (40 μg/ml, Sigma). Total serum IgM or IgG levels were measured on a plate coated with anti-IgM or anti-IgG antibodies. For serum BAFF, anti-mouse BAFF (5A8, Enzo) and biotinylated anti-mouse BAFF (1C9, Enzo) were used. Duplicates of serially diluted serum samples were tested.
ELISpot
To analyze the numbers of BAFF secreting cells, isolated splenic DCs or MFs (1x106 and 0.5x106 cells per well) were incubated for 60 hours at 37°C on a ELISpot plates (Millipore) coated with 5A8, then detected using biotinylated 1C9.
Histology
Formalin fixed (10%), paraffin-embedded kidneys were stained with H&E (8 micron sections). Glomerular changes and tubulointerstitial inflammation were assessed by a pathologist blinded to the experimental groups. The following criteria were used; Glomerular lesions: (0) no H&E changes, (1) minimal mesangial hypercellularity without visualized immune deposits, (2) focal immune deposits, (3) diffuse glomerulonephritis with widespread subendothelial immune deposits, (4) global immune deposits with associated sclerosis. Interstitial inflammation was scored 0–3 based on the degree of tubulointerstitial involvement: (0) no infiltrate or inflammation, (1) less than 10%, (2) between 10% and 50%, (3) >50% of the tubulointerstitium.
Proteinuria scoring
Urine protein was measured using Uristix strip following the manufacturers instruction (Siemens).
Immunofluorescence
Snap-frozen kidney sections (8 micron) were stained with goat anti-mouse IgG-Fc conjugated with DyLight 488 (Jackson Immunoresearch), and PE-conjugated anti-mouse complement component 3 (C3) (Cedarlane), and then visualized on LSM 710 confocal microscope (20x magnification lens, Carl Zeiss).
Flow Cytometry
Total splenocytes were stained with biotinylated 2.12.3 (mouse anti-mouse Sm, IgG2a) (36, 37), or goat anti-mouse IgG-Fc (Jackson Immunoresearch). Biotinylated anti-canine distemper virus (CDV, IgG2a), or goat anti-rabbit IgG were used as isotype control staining antibodies (ISO). Biotinylated antibodies were detected using streptavidin-Alexa Fluor 647 (Invitrogen). The mean fluorescent intensity (MFI) of surface bound antigen or IgG staining was divided by the MFI values of ISO. For intracellular staining, fixed and permeabilized cells were stained with anti-mouse total Syk (Santa Cruz), pSyk (BD Biosciences), total Akt, pAkt-Threonine (Thr 308), total S6, or pS6 (Cell Signaling). The expression levels of intracellular kinases were calculated as follows; (MFI of phosphorylated signaling molecule/MFI of ISO)/(MFI of total signaling molecule/MFI of ISO). The fold changes over average values of control mice from each group are graphed. For FcγRI staining, cells were blocked with rat serum and stained with biotinylated X54-5/7.1. Whole blood cells from healthy donors or SLE patients were stained for surface DNA (anti-human DNA, 33H11, T. Winkler; University of Erlangen, Germany) (38), Sm (2.12.3), or IgG (anti-human IgG Fc-PE, Jackson Immunoresearch). 33H11 and 2.12.3 were conjugated to Alexa Fluor 647 following manufacturer’s instruction (Invitrogen). Samples were acquired on Cyan flow cytometer (Beckman). Cells were defined as follows; human and mouse B cells (CD19+) and T cell (CD3+), mouse DCs (CD11chiCD11b+), mouse MFs (CD11c−CD11bhi), and human monocytes (CD14+). Murine non-leukocytes are gated as CD45neg population.
Microscopy
Isolated murine splenic DCs or MFs, or whole blood cells from human samples, were stained for IgG, Sm (2.12.3) or DNA (33H11). Colocalization was quantified by calculating the Mander’s coefficient using Image J. For some experiments, cells were incubated with trypsin or DNase for 20 min (37°C) prior to the staining for surface bound antigens.
Real time PCR
Splenic MFs were isolated by incubation on a glass dishes for 2 hours at 37°C. RNA was extracted using RNeasy Mini Kit (Qiagen). cDNA was generated using iScript cDNA synthesis kit (BioRad). The following PCR primers were used: GAPDH, forward 5′-GGC-AAA-TTC-AAC-GGC-ACA-3′ and reverse 5′-GTT-AGT-GGG-GTC-TCG-CTC-CTG-3′; for FcγRI, forward 5′-ACA-CAA-TGG-TTT-ATC-AAC-GGA-ACA-3′ and reverse 5′-TGG-CCT-CTG-GGA-TGC-TAT-AAC-T-3′. Quantitative real-time PCR was performed on ABI Prism 7500 Sequence Detection System (Applied Biosystems).
Statistics
The Mann-Whitney test was used to analyze human data. Kruskal-Wallis test (>3 groups) or Mann-Whitney test (≤3 groups) was used to compare changes over control group. One-way ANOVA was used to compare changes between different conditions. Statistical analysis was performed using GraphPad Prism (GraphPad Software, Inc.).
Results
Nuclear self-antigens are displayed on hematopoietic cells
The clearance of apoptotic debris is critical in maintaining immune homeostasis; however in autoimmune-prone mice, continuous cell turnover can expose the immune system to self-antigens on apoptotic debris. To assess whether apoptotic debris was present on cells from non-autoimmune C57BL/6 (B6) mice, we stained splenocytes with antibodies specific for Smith (Sm), a nuclear self-antigen evident on apoptotic debris (9). Although Sm was not detected on B6 T cells, the levels of Sm were heightened 7–10 fold on splenic DCs, MFs, and B cells when compared to isotype control antibody (Figure 1A). Staining was not unique to anti-Sm as antibodies specific for DNA (33H11), and nucleosomes (PL2-3) showed a similar staining pattern (Supplemental Figure 1A), that by microscopy appeared punctate (Figure 1B). To further characterize the antigens, we treated B6 splenocytes with trypsin (Figure 1B) or DNase (Figure 1C), prior to surface staining for DNA, nucleosome, or Sm. DNase treatment specifically removed DNA, while nucleosomes remained intact. As expected, all nuclear self-antigens were removed by trypsin in agreement with their display on the cell surface. These data indicate that nuclear self-antigens contained within apoptotic debris are displayed on the surface of hematopoietic cells.
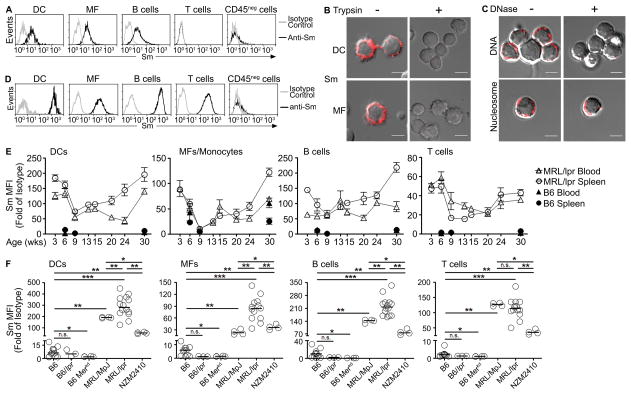
Figure 1. Nuclear self-antigens are displayed on the surface of hematopoietic cells.
(A) Splenic DCs, MFs, B cells, T cells, and CD45neg cells from B6 mice were stained with anti-Sm (2.12.3, black line) or isotype control antibody (gray line) and analyzed by flow cytometry. Representative histograms from >5 experiments (n = >20 mice). (B) Splenic DCs or MFs untreated or treated with trypsin and stained for Sm (2.12.3, red). (C) Splenocytes untreated or treated with DNase (100 μg/ml) were stained for surface DNA (33H11, red) or nucleosome (PL2-3, red). Representative images from 6 experiments (n = 7 mice, 10–15 cells per mouse). Scale bar = 3.5μm. (D) Splenic DCs, MFs, B cells, T cells, and CD45neg cells from MRL/lpr mice (16–28 weeks old) were stained for Sm and analyzed by flow cytometry. Representative data from >5 experiments (n = >20 mice). (E) Splenocytes (circle) or blood cells (triangle) from B6 (black) or MRL/lpr (white) at different ages were stained for Sm and analyzed by flow cytometry. (n = 4–5 mice per age group, 2 experiments). (F) Surface Sm levels were quantitated on splenocytes from different mouse models. (n = 3–14 mice). In (E) results are mean ± SEM. In (F), bars represent median. *p<0.05, **p<0.01, ***p<0.001, n.s. = not significant by Mann-Whitney test. MFI = Median fluorescence intensity.
Elevated levels of apoptotic debris have been associated with autoimmunity in murine and human SLE (39). To assess whether hematopoietic cells displayed elevated levels of nuclear self-antigens, we quantified the levels of Sm on cells from MRL/lpr mice aged 18–26 weeks (Figure 1D and 1F). Relative to isotype control, Sm levels increased 84- to 260-fold on DCs, MFs and B cells. Surprisingly, T cells also displayed a 109-fold increase. The staining was specific, as T cells from B6 mice did not display Sm (Figure 1A). Further, Sm was not evident on splenic non-leukocyte populations (CD45neg) from B6 or MRL/lpr mice (Figure 1D), indicating that the accumulation of nuclear self-antigens is restricted to hematopoietic cells. No differences between genders were observed. To further assess whether nuclear self-antigens on splenic hematopoietic cells and peripheral blood mononuclear cells from B6 and MRL/lpr mice accumulated over time, we quantitated the levels of Sm over 30 weeks (Figure 1E). Surprisingly, by 3 weeks of age, DCs, monocytes/MFs, B cells, and T cells from blood and spleen of MRL/lpr mice showed high levels in surface Sm (10-, 3-, 5-, and 30-fold on DCs, MFs, B cells, and T cells), compared to peripheral blood cells or splenocytes from B6 mice (single data points at weeks 3, 6, and 30). These levels declined during the next 6 weeks. After week 9, the levels of Sm on splenic MFs and B cells steadily increased, reaching a maximal level at 30 weeks when urine protein levels are high (score >2) (Supplemental Figure 1B). Similarly, the Sm levels on blood and splenic DCs and T cells, and blood monocytes showed steady increase after 9 weeks, returning to levels found at week 3.
To assess whether accumulation of nuclear self-antigens was unique to the MRL/lpr background, we examined other models. In NZM2410 mice, Sm levels were heightened on the surface of all hematopoietic cells, reaching levels that were 35–78 fold higher than isotype control (Figure 1F). Similarly, Sm levels were elevated on cells from MRL/MpJ mice, but not on cells from B6/lpr or B6/Merkd (Figure 1F), suggesting that accumulation of nuclear self-antigens is associated with the lupus-prone background.
SLE patients with active disease exhibit heightened levels of surface bound nuclear antigens
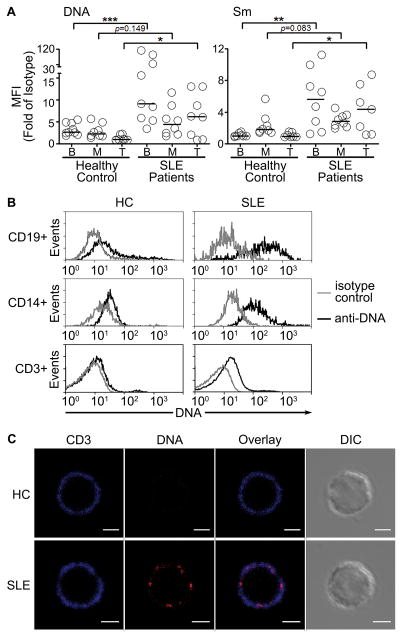
The accumulation of nuclear self-antigens in two spontaneous models of murine SLE suggests an inherent defect in immune cells that could be present in human SLE. To assess this, we analyzed circulating mononuclear cells from healthy controls and SLE patients for surface Sm and DNA (Figure 2). We chose SLE patients experiencing active disease (SLEDAI > 6), since active disease in humans might be most like disease in MRL/lpr mice (Supplemental Table 1). Similar to non-autoimmune mice (Figure 1A), healthy controls showed low levels of Sm and DNA on B cells and monocyte, but not T cells (Figure 2A, B). In contrast, 67–75% of SLE patients showed more than a 2-fold increase of Sm and DNA on B and T cells compared to healthy controls. Among the patients with active disease, there was significant variation in the levels of Sm and DNA on the B cells. In the case of DNA, two SLE patients showed 30- to 40-fold increases, four showed 2- to 7-fold increases, and the rest showed less than a 2-fold increase. On blood monocytes, 10–40% of SLE patients showed a 2- to 3-fold increase of Sm and DNA (Figure 2A). The findings that blood monocytes from SLE patients accumulate less nuclear self-antigens compared to healthy controls is consistent with the murine data where blood monocytes showed similar levels of surface Sm as B6 blood monocytes (Figure 1E). In the patients whose monocytes, B and T cells showed an accumulation of nuclear self-antigens (Figure 2A), microscopy showed punctate staining of DNA (Figure 2C; T cell example) similar to that seen in lupus-prone mice, suggesting receptor aggregation. Thus, the data show that nuclear self-antigens accumulate on hematopoietic cells in human and murine lupus.
Figure 2. Peripheral blood mononuclear cells from SLE patients accumulate surface nuclear self-antigens.
(A) Whole blood cells from healthy controls (HC) or SLE patients (SLE) with SLEDAI score > 6 were analyzed for surface DNA (33H11) or Sm (2.12.3) by flow cytometry. (B) Representative histograms are shown for each cell type (isotype antibody: gray, anti-DNA: black). n = 8–9 from >3 separate experiments. (C) Peripheral blood T cells from HC (upper panels) or SLE patients (lower panels) were stained for CD3 (blue) and DNA (red). Scale bar = 3 μm. (n = 3, 10 cells per sample). In (A), bars represent median. *p<0.05, **p<0.01, ***p<0.001, n.s. = not significant by Mann-Whitney test. MFI = median fluorescence intensity.
Nuclear self-antigens are bound to FcγRs as IgG-immune complexes
Multiple cell surface receptors clear apoptotic debris, including FcγRs. One possibility is that the nuclear self-antigens displayed on myeloid cells represent immune complexes (ICs) bound to FcγRs. To test this idea, we quantified the levels of Sm on immune cells from B6 mice that were deficient in individual FcγRs (Figure 3A). We found that loss of activating receptor FcγRI or FcγRIV reduced Sm levels 40% or 45% on B6 DCs, and 50% on B6 MFs. The receptor(s) displaying self-antigen on B and T cells remains unclear; however, consistent with the limited expression of FcγRs on B and T cells, loss of any FcγR did not reduce the levels of Sm by more than 10% (data not shown).
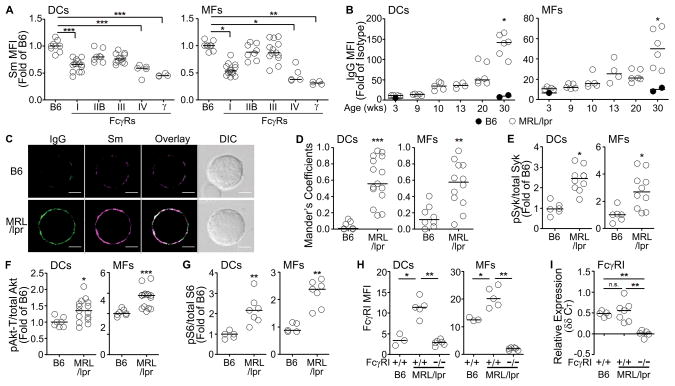
Figure 3. Nuclear self-antigens bind FcγRs as IgG-ICs.
(A) Surface Sm was stained on splenic DCs and MFs from B6 mice deficient of individual FcγR (FcγRI, IIB, III, or IV) or Fc-common-gamma-chain (γ). (n = 4–14 mice, 5 experiments). (B) Surface IgG levels on splenic DCs and MFs from B6 and MRL/lpr mice at different ages were analyzed by flow cytometry. (n = 2–6 mice per age group, 2 experiments). (C) Purified splenic DCs were stained for surface Sm (magenta) and IgG (green). Representative images from >3 experiments. Scale bar = 2.5μm. (n = 5–7 mice, 5–15 cells per mouse). (D) Colocalization of Sm with IgG on DCs and MFs was analyzed using Mander’s Coefficient and ImageJ. Each circle represents a cell (n = 7–15 cells from 2–3 mice, 4 experiments). Expression levels of phosphorylated (E) Syk, (F) Akt-Threonine308 (Akt-T), and (G) S6 in splenic DCs and MFs from B6 and MRL/lpr mice were analyzed by flow cytometry. (n = 5–15 mice, 2–3 experiments). Levels of FcγRI (H) surface, or (I) gene expression, on splenic MFs and DCs from B6, MRL/lpr, and FcγRI−/−MRL/lpr mice were analyzed by flow cytometry or qPCR (relative expression over FcγRI−/−MRL/lpr mice, I). (n = 3–7 mice, 2 experiments). In (A, B, and D–I) bars represent median. *p<0.05, **p<0.01, ***p<0.001 by Kruskal-Wallis test (A) or Mann-Whitney test (B and D–I).
We reasoned that if accumulated nuclear self-antigens were part of immune complexes (ICs) bound to FcγRI/FcγRIV, surface IgG levels would be elevated on myeloid cells. In B6 mice, DCs and MFs consistently showed low levels of IgG (Figure 3B) and Sm (Figure 1A, 1E) over 30 weeks, and they failed to develop lupus nephritis (Supplemental Figure 1B). In MRL/lpr mice, the levels of IgG remained low (at levels of B6 mice) until 10 weeks of age, then steadily increased until 20 weeks, rising sharply between weeks 20 and 30 (14-fold on DCs, 5-fold on MFs compared to B6). This was consistent with the changes in the levels of Sm between 10 to 30 weeks of age (Figure 1E). On DCs and MFs, 70% of the surface IgG was IgG2a and IgG2b (Supplemental Figure 1C) that appeared prior to high levels of proteinuria (score >2; Supplemental Figure 1B). In addition, SLE patients that accumulated Sm and DNA on blood monocytes also expressed surface IgG (Supplemental Figure 1D). Display of nuclear self-antigens on myeloid cells did not involve FcμR because IgM was not found on DCs or MFs from B6 or MRL/lpr mice (Supplemental Figure 1E).
To further define the role of ICs in the surface display of self-antigen, we assessed whether Sm and IgG colocalized and showed punctate staining. We found a 6-fold and 4-fold increase in the colocalization of Sm and IgG on MRL/lpr DCs and MFs that exhibited punctate staining consistent with receptor aggregation (Figure 3C–D). To assess whether cell signaling was occurring, we measured the activation state of kinases coupled to FcγRs. We found that the levels of pSyk were increased 2–3 fold, pAkt-Thr308 1.4-fold, and pS6 2-fold in both MRL/lpr DCs and MFs, suggesting chronic activation of the PI3k pathway (Figure 3E–G). Thus, myeloid cells from MRL/lpr mice accumulate IgG-ICs containing nuclear antigens that are bound in part by FcγRI and FcγRIV.
One possibility was that accumulation of IgG-ICs on MRL/lpr DCs and MFs might reflect increased expression of FcγR. We found that DCs and MFs from MRL/lpr mice showed 3-fold and 2-fold increases in FcγRI compared to B6 mice (Figure 3H). Since the elevated levels of FcγRI could reflect an increased rate of transcription, we quantitated FcγR message levels by pPCR. Surprisingly, we found that the levels of FcγRI mRNA from MRL/lpr mice were comparable to those in B6 mice (Figure 3I). This indicates that accumulation of IgG-ICs on the cell surface is associated with increased surface expression of FcγRI; however, these increased levels do not reflect increased transcription, suggesting that the FcγRs might be recycled.
FcγRI−/−MRL/lpr mice show reduced levels of IgG-ICs and diminished autoantibody levels
Our findings indicate that IgG-ICs accumulate on hematopoietic cells in human and murine SLE and induce signal transduction induced by activating FcγRs. To define whether FcγRI contributes to disease, we used MRL/lpr mice deficient in FcγRI (FcγRI−/−MRL/lpr). Previous studies have found that MRL/lpr mice have elevated splenic and lymph node cellularity due to enhanced lymphoproliferation (40). Loss of FcγRI in MRL/lpr mice reduced the numbers of splenic DCs and MFs to levels found in B6 mice, and partially reduced the numbers of T and B cells (Figure 4A). This suggests that FcγRI plays a significant role in the expansion of myeloid cells in MRL/lpr mice. Although B and T cells typically do not express FcγRI, their numbers were reduced in FcγRI−/−MRL/lpr mice, suggesting that loss of FcγRI may indirectly impact lymphocytes.
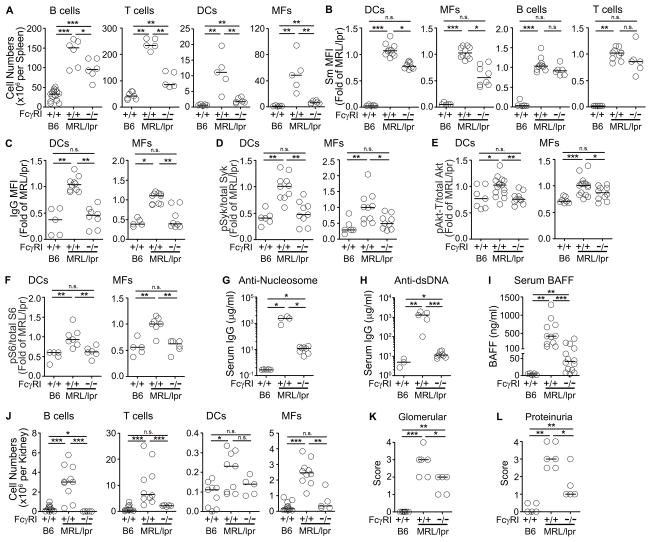
Figure 4. Lack of FcγRI in MRL/lpr mice reduces the levels of surface IgG-IC and lupus-related pathologies.
(A) Numbers of splenic B cells, T cells, DCs, and MFs from age matched B6, MRL/lpr, or FcγRI−/−MRL/lpr mice (20 weeks old) were enumerated by flow cytometry analysis. (n = 5–7 mice, 2 experiments). (B) Surface Sm levels on splenic DCs, MFs, B cells, and T cells. (C) IgG levels on splenic DCs and MFs from B6, MRL/lpr, or FcγRI−/−MRL/lpr mice (>20 weeks old) were analyzed by flow cytometry. (n = 5–8 mice, 3 experiments). The expression of intracellular phosphorylated (D) Syk, (E) Akt-Threonine308, and (F) S6 levels in splenic DCs and MFs were analyzed by flow cytometry. The data for B6 and MRL/lpr includes data from Figure 3E–G. (n = 5–15, 3 experiments). (G) Anti-nucleosome IgG, (H) anti-dsDNA IgG, or (I) BAFF levels in the sera collected from B6, MRL/lpr, or FcγRI−/−MRL/lpr (>20 weeks old) were measured by ELISA. (n = 4–8 mice from 2 experiments for G and H, n=6–15 mice from 5 experiments for I). (J) Number of B cells, T cells, DCs, and MFs infiltrating the kidneys were enumerated by flow cytometry. (n = 5–11 mice, 2 experiments). (K) Levels of glomerular inflammation were scored using H&E stained kidney sections. (n = 5–6, 2 experiments). (L) Urine samples were analyzed for protein levels. Bars represent median. *p<0.05, **p<0.01, ***p<0.001, n.s. = not significant, by Mann-Whitney test (A and D–L) or by Kruskal-Wallis test (B, C).
To define whether FcγRI contributes to the accumulation of ICs in vivo, we quantitated the levels of surface Sm and IgG on DCs and MFs from FcγRI−/−MRL/lpr mice. Compared to MRL/lpr mice, the levels of Sm were reduced 30–40% on DCs and MFs (Figure 4B), and the levels of IgG were decreased to levels found on B6 cells (Figure 4C). This reduction was similar to the contribution of FcγRI in the low level of IgG-ICs displayed on DCs and MFs from B6 mice (Figure 3A). The levels of Sm on B and T cells were not altered in the absence of FcγRI, consistent with the lack of FcγRI expression on these cells (Figure 4B) and the idea that other receptors are involved in the display of self-antigens on lymphocytes.
To establish whether the accumulation of IgG-ICs contributes to the autoimmune phenotype of MRL/lpr mice, we quantitated the levels of intracellular pSyk, pAkt, pS6, serum BAFF, and autoantibody levels in FcγRI−/−MRL/lpr mice. In these mice, the levels of pSyk and pS6 in MFs and DCs, and the levels of pAkt-Thr308 in DCs were diminished to the levels found in B6 mice, while the levels of pAkt-Thr308 in MFs were only reduced 15% (Figure 4D–F). Further, FcγRI−/−MRL/lpr mice showed a 200-fold decrease in serum anti-nucleosome antibody (Figure 4G), and a 95-fold decrease in serum anti-dsDNA antibodies (Figure 4H) when compared to MRL/lpr (FcγRI+/+) mice. Similarly, FcγRI−/−MRL/lpr mice showed a 4-fold decrease in serum BAFF (Figure 4I). Despite these significant improvements in the serological phenotype associated with autoimmunity, the levels of autoantibody and BAFF remained elevated compared to B6 mice. This is consistent with the idea that other receptors display self-antigens on lymphocytes, and the findings that loss of FcγRI alone does not ablate the accumulation of IgG-ICs on myeloid cells. Despite the multiple ways IgG-ICs accumulate on myeloid cells, the data show that FcγRI plays a significant role in the disease of MRL/lpr mice coincident with defects that lead to accumulation of IgG-ICs and chronic FcγR activation.
FcγRI−/−MRL/lpr mice fail to develop nephritis
To assess whether FcγRI is important in lupus nephritis, we quantitated the numbers of renal hematopoietic cells and assessed renal pathology in FcγRI−/−MRL/lpr mice. We found that compared to MRL/lpr mice, the number of T cells were reduced 6-fold, DCs 2-fold and MFs 4-fold. Interestingly, the number of B cells that infiltrated the kidney was less than B6 controls making the reduction 89-fold (Figure 4J). This indicates that migration of DC, MF, T and B cells to the kidney is dependent on FcγRI. In addition, the kidneys showed significantly reduced glomerular inflammation (Figure 4K) and urine protein levels (Figure 4L), although levels remained higher than B6 controls, consistent with a partial role of FcγRI in kidney disease.
FcγRI−/−MRL/lpr mice failed to develop lupus nephritis coincident with significantly reduced serological phenotype. This could reflect reduced autoantibody production that diminishes the formation of IgG-ICs, hence the accumulation on the cell surface and heightened signal transduction. Alternatively, lupus nephritis might develop independent of FcγRI. To sort out these possibilities, we passively transferred pathogenic anti-nucleosome IgG2a (PL2-3) into FcγRI−/−MRL/lpr mice. We found that 5-weeks of PL2-3 injection did not change the levels of Sm and IgG on the surface of DCs and MFs, or induce glomerular inflammation or proteinuria in FcγRI−/−MRL/lpr mice compared to PBS treated control mice (Supplemental Figure 2A–E). However, deposits of IgG and C3 remain evident in the kidney of MRL/lpr mice regardless of disease pathology or the presence of FcγRI (Supplemental Figure 2F). Collectively, the data show that FcγRI plays a major role in many of the serological and cellular phenotypes associated with autoimmunity (Figure 4A–I). It also partially contributes to kidney disease (Figure 4J–L). This suggests that FcγRIV may also play a role in kidney pathology (41). We are currently backcrossing FcγRIV−/− mice to the MRL/lpr background to assess this possibility.
Passive transfer of IgG heightens surface ICs coincident with early autoantibody secretion
Our results show that loss of FcγRI markedly reduces the accumulation of IgG-ICs on myeloid cells and the pathologies associated with SLE (Figure 4). To begin to understand their role in disease, we defined the temporal order of events leading to lupus-related pathologies. For this, we developed an in vivo model where we passively transferred pathogenic anti-nucleosome IgG2a into IgG-deficient MRL/lpr mice. Our selection of anti-nucleosome IgG2a (PL2-3) was based on in vitro experiments showing that co-culturing PL2-3, but not anti-TNP (Hy1.2; IgG2a), during the derivation of MRL/lpr bone marrow-derived DCs promoted the accumulation of IgG and secretion of BAFF (Supplemental Figure 1F and G). BMDCs from B6 mice treated with PL2-3 failed to secrete BAFF or increase the level of surface IgG supporting the findings that accumulation is unique to mice genetically-prone to lupus (Figure 3B–D). In addition, anti-nucleosome (PL2-3) is pathogenic, and the IgG2a isotype binds to FcγRI and FcγRIV and is a highly displayed isotype on the surface of myeloid cells from MRL/lpr mice (Supplemental Figure 1C).
Our model uses AID−/−MRL/lpr mice since deficiency in activation-induced cytidine deaminase (AID) prevents class switch. These mice also fail to develop serological phenotypes of autoimmunity or lupus nephritis (34). Thus, passive transfer of anti-nucleosome IgG2a (PL2-3) would induce disease pathology and allow us to order the events that occur during the onset of autoimmune disease. AID−/−MRL/lpr mice were intravenously treated with PL2-3 weekly for 2 or 5 weeks (Figure 5A). Separate cohorts were treated with PBS, isotype control antibody, or F(ab′)2 of PL2-3. We found that after 2 weeks of PL2-3 treatment, the levels of surface IgG increased 2-fold on DCs and MFs and showed a punctate staining similar to that found on MRL/lpr mice (Figure 5B and 3C). By 5 weeks, the levels of IgG on DCs increase to 3-fold, while on MFs the levels remained comparable to those at 2 weeks. Surface accumulation of IgG was not evident when PL2-3 was injected into B6 mice (Supplemental Figure 2B), or when AID−/−MRL/lpr mice were treated with F(ab′)2 of PL2-3, or an isotype control antibody (anti-TNP; Hy1.2; IgG2a) (Figure 5B). To assess whether the treatment of PL2-3 prolonged or enhanced signaling from FcγRs, we quantitated the levels of pSyk in ex vivo DCs and MFs (Figure 5C). After 5 weeks of PL2-3 treatment, pSyk levels in DCs were increased 1.8-fold, and in MFs the levels were increased 1.5-fold compared to PBS treated mice. This suggests that PL2-3 treatment promotes the surface accumulation of IgG-ICs, and activates FcγRs on myeloid cells.
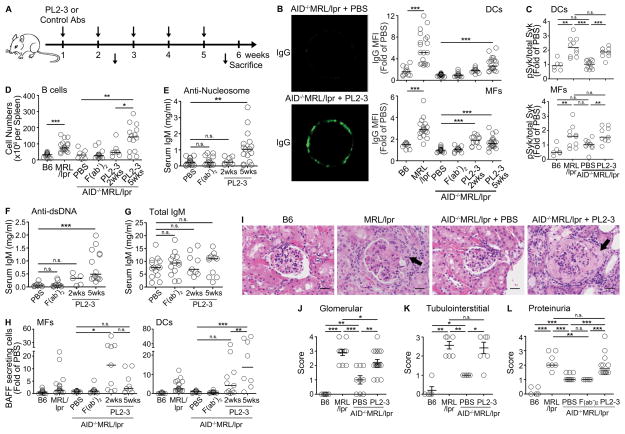
Figure 5. Anti-nucleosome IgG induces accumulation of IgG-ICs prior to appearance of lupus-related pathologies.
(A) AID−/−MRL/lpr mice were treated (i.v.) with PL2-3 (500 μg/mouse) or control antibodies once a week for 2 or 5 weeks. Untreated, age matched B6 and MRL/lpr mice were used as controls. (B) Surface bound IgG (green) on purified splenic MFs from PBS (upper left) or PL2-3 (lower left) treated mice for 2 weeks. Representative images from 3 experiments (10–15 cells/mouse). Surface IgG on splenic DCs (upper right) and MFs (lower right) analyzed by flow cytometry. (C) The expression of intracellular phosphorylated Syk (pSyk) levels in splenic DCs and MFs analyzed by flow cytometry. The data for B6 and MRL/lpr control mice includes data from Figure 3E. (D) Splenic B cells enumerated by flow cytometry. (E) Levels of anti-nucleosome, (F) anti-dsDNA, or (G) total IgM in sera were analyzed by ELISA. (H) BAFF secreting splenic DCs or MFs enumerated by ELISPOT. (I) H&E stained kidney sections. Arrows indicate fibrocellular crescents. Representative images from >3 experiments. Scale bar = 1μm. Scores of (J) glomerular and (K) tubulointerstital inflammation of the kidneys. (L) Proteinuria scores. In (B–L), n = 2–5 mice per treatment per experiment, >3 experiments. In (B–H, J–L) bars represent median. *p<0.05, **p<0.01, ***p<0.001, n.s.= not significant by Kruskal-Wallis test (B) or Mann-Whitney test (C–H, and J–L).
To define whether the accumulation of IgG-ICs was associated with autoantibody titers in PL2-3 treated AID−/−MRL/lpr mice, we enumerated B cells and measured serum autoantibody levels after 2 and 5 weeks of PL2-3 treatment. We found that by 2 weeks, there was a 1.5-fold increase in the number of splenic B cells that reached 3.6-fold after 5-weeks of injection (Figure 5D). The numbers of DCs, MFs, and T cells were not different at 2 or 5 weeks post injection (data not shown). Initially, the expanded B cells secreted low levels of anti-dsDNA IgM (2 weeks); however, by 5 weeks of treatment, the levels of anti-nucleosome and anti-dsDNA IgM were increased 6- and 7-fold respectively (Figure 5E, F). Since the levels of total IgM were not affected (Figure 5G), the data suggest that the increase in autoantibody titers and the number of B cells was due to activation and expansion of autoreactive B cells. Thus, accumulation of IgG-ICs on the surface of myeloid cells is concurrent with B cell expansion and the initial production of autoantibody.
Heightened levels of BAFF allow autoreactive B cells to survive during the transitional stage of development (42). To determine whether accumulation of IgG-ICs promotes BAFF secretion in vivo, we enumerated BAFF secreting MFs and DCs in AID−/−MRL/lpr mice after 2 and 5 weeks of PL2-3 injection (Figure 5H). We found that production of BAFF in MRL/lpr MFs reached a maximal 11-fold increase at 2 weeks, the time point correlated with the maximal accumulation of IgG-ICs on MFs (Figure 5B), then declined to 4-fold over PBS or F(ab′)2 controls. In contrast, BAFF secretion in DCs increased 4-fold at 2 weeks and an additional 10-fold by 5 weeks post PL2-3 injection. Thus, surface IgG-ICs accumulate rapidly on MFs concurrent with early BAFF secretion, but preceding the significant increases in the number of B cells and the levels of autoantibodies. In contrast, the accumulation of IgG-ICs on DCs and their secretion of BAFF are delayed, occurring concomitantly with B cell expansion and heightened autoantibody.
Passive transfer of anti-nucleosome IgG promotes renal disease
Renal failure is one of the leading causes of mortality in human and murine SLE. To understand whether the accumulation of IgG-ICs on the surface of cells precedes lupus nephritis, we assessed renal pathology after 2 and 5 weeks of PL2-3 treatment. At 2 weeks, H&E stained kidney sections from PL2-3 treated mice did not show morphologic changes despite the accumulation of IgG on DCs and MFs (data not shown, Figure 5B). After 5 weeks of PL2-3 treatment, extensive tubular and glomerular inflammation, including the formation of fibrocellular crescents was evident (Figure 5I). These changes were much like those found in MRL/lpr mice. In accordance, scores for glomerular and tubulointerstitial inflammation, and proteinuria were increased in MRL/lpr mice and in PL2-3 treated AID−/−MRL/lpr mice, but not in B6, PBS or F(ab′)2 treated AID−/−MRL/lpr mice (Figure 5J-L). The ability of PL2-3 to induce kidney pathology was specific to lupus-prone mice, as B6 mice treated with PL2-3 did not develop renal pathology (Supplemental Figure 2C–F). Our data in the passive antibody transfer model shows that the accumulation of IgG-ICs on the cell surface precedes glomerulonephritis. This is much like the timeline of the accumulation of surface IgG in MRL/lpr mice where IgG-ICs increase at 10 weeks of age prior to high levels of proteinuria (score >2) (Figure 3B, Supplemental Figure 1B). Collectively, our data show that passive transfer of anti-nucleosome IgG into AID−/−MRL/lpr mice induces the surface accumulation of IgG-ICs on myeloid cells as the early antibody response begins, promoting chronic FcγRI signaling and BAFF secretion that leads to extensive B cell expansion and lupus nephritis.
Discussion
Our findings identify a previously unidentified defect that promotes the accumulation of nuclear antigens (Sm, DNA, and nucleosomes) on the surface of hematopoietic cells. This defect was evident in two genetically unrelated strains of lupus-prone mice, and on peripheral blood mononuclear cells from SLE patients. On myeloid cells, the self-antigens were part of IgG-ICs bound by the activating receptors FcγRI and FcγRIV. B and T cells also accumulated self-antigens; however, the receptors involved remain unknown. We used several mouse models to define whether accumulation of IgG-ICs on the surface of myeloid cells contributes to the pathogenesis of SLE. In the AID−/−MRL/lpr model treated with anti-nucleosome, we showed that IgG-ICs accumulated concomitantly with the activation of Syk and secretion of BAFF, but prior to the significant expansion of autoreactive B cells and lupus nephritis. F(ab′)2 of anti-nucleosome IgG did not elicit changes in the serological phenotype or renal disease, indicating that the autoimmune phenotype required IgG-ICs/FcγR interactions. In SLE patients experiencing active disease (SLEDAI >6), nuclear self-antigens were displayed on the surface of peripheral blood B cells, and to a lesser extent on T cells and monocytes. In addition, loss of FcγRI (FcγRI −/−MRL/lpr) reduced surface IgG, decreased signal transduction (pSyk, pAkt, and pS6), diminished serum BAFF, and reduced kidney disease. Together, the data show that after the early autoantibody response, chronic interaction of IgG-ICs and activating FcγRs amplifies the disease process. It also provides insight into how FcγRs on circulating myeloid cells, rather than kidney mesangial cells, might contribute to renal pathology in SLE (26, 43).
Autoreactive B cells are normally maintained in an unresponsive state as a result of chronic engagement of the B cell receptor by self-antigens (44). The findings that membrane-bound apoptotic self-antigens are present at low levels on the surface of DCs, MFs and B cells from B6 mice (Figure 1A) raises the possibility that they deliver tolerogenic signals to B cells (45). This is supported by previous studies in the hen egg lysosome (HEL) model of tolerance, showing that membrane-bound HEL induces a stronger BCR signal than soluble HEL (45), and in the Sm model of tolerance, showing that soluble Sm or snRNPs fail to maintain the unresponsive state associated with anergy (46). Thus, in non-autoimmune mice, the low levels of nuclear self-antigens on DCs, MFs, and B cells may tolerize low-affinity autoreactive B cells. However, when a high burden of nuclear antigens accumulate on hematopoietic cells in MLR/lpr mice, they could promote a break in tolerance (44) by providing a source of TLR agonists that chronically stimulate TLR7 and TLR9 (47), or by providing a source of membrane-bound nuclear antigens that activate autoreactive BCRs “in trans”. Similarly, accumulated IgG-ICs on myeloid cells constitutively crosslink activating FcγRs and heighten cytokine secretion (Figures 3A–G, 4B–F, 5B, 5C, and 5H, and Supplemental Figure 1F–G).
Our data show that hematopoietic cells from both B6 and MRL/lpr mice display nuclear self-antigens; however, only MRL/lpr mice develop disease. The low levels of self-antigens displayed on B6 hematopoietic cells, and their lack of autoimmune disease, suggest the need for a threshold level of activation to promote the autoimmune pathology. Another possibility is that a protective signal is conferred by opsonins other than IgG that coat apoptotic debris (48). We found that B6 cells displayed low levels of Sm; however, IgG was barely detectable (Figure 1A, 1E, 1F, 3B, 3C, and 3D). Similarly, disease-free MRL/MpJ, or 3–9 weeks old MRL/lpr mice showed high levels of surface bound nuclear antigens despite low levels of surface IgG (Figure 1E, 1F and 3B). This might reflect the binding of C-reactive protein (CRP), an acute phase protein that opsonizes apoptotic debris and binds to activating FcγRI and inhibitory FcγRIIB (49). Whether or how the exchange of CRP for IgG could promote disease remains unclear; however, CRP might confer unique downstream signals that are protective (50) since CRPTg(NZB/NZW)F1 mice and CRP-treated MRL/lpr mice show delayed disease (51, 52). Although CRP bound apoptotic debris may be protective in a non-autoimmune-prone environment, in MRL/lpr mice the high burden of apoptotic debris could activate intracellular TLRs, triggering a break in tolerance and the production of autoreactive antibodies at early ages. This would increase the production of IgG-ICs, thus amplifying the downstream effects of FcγRI. It is also supported by the data in Figure 4B-C showing that in FcγRI−/−MRL/lpr mice, the levels of Sm were moderately decreased (30–40%), while the decrease in IgG were more significant (50–60%) and coincident with the lack of disease pathology. The data are consistent with the idea that binding of pathogenic IgG to FcγRI gives rise to lupus pathology.
It was striking that AID−/−MRL/lpr mice passively administered pathogenic IgG2a developed fulminant lupus nephritis, while AID−/−MRL/lpr mice treated with F(ab′)2 of IgG2a or FcγRI−/−MRL/lpr mice treated or untreated with intact IgG2a were void of severe renal disease. The lack of severe disease in the latter mice was observed in spite of the presence of renal IgG and C3 deposits (Supplemental Figure 2F). Together, these findings indicate that the interaction of IgG-ICs and FcγRI plays crucial roles in the pathogenesis of lupus nephritis, but deposits of IgG/C3 in the kidney appears not to be sufficient to induce fulminant disease. It is possible that passive transfer of anti-nucleosome IgG2a into AID−/−MRL/lpr mice induces deposits in the kidney independent of FcγRs by forming ICs with nuclear antigens that deposit on the glomerular basement membrane (53). However, since resident renal cells such as mesangial cells, podocytes, and renal endothelial cells do not express FcγRI (43), our data showed that lupus nephritis is dependent on constant binding of IgG-ICs to FcγRI expressed on hematopoietic cells. Whether disease depends on the secretion of FcγR-dependent cytokines, or signals that promote the migration of cells to the kidney is under investigation. It is noteworthy that although a previous study showed lupus nephritis in MRL/lpr mice lacking the Fc common gamma chain (FcRγc, a subunit of murine FcγRI, III, and IV) (54), another study had found that FcRγc -deficient mice maintain a partially functional FcγRI (28). Coupled with our data, it raises the possibility that FcγRI contributed to disease in the MRL/lpr model.
An interesting observation in autoimmune MRL/lpr mice is that the burden of IgG-ICs varies depending on the source of cells. All hematopoietic splenocytes from MRL/lpr mice accumulate ICs (Figure 1D–F); however, the levels of surface IgG-ICs on blood monocytes (wk30) were comparable to those from B6 mice (Figure 1E). This was also evident in human SLE where the levels of surface bound nuclear antigens on circulating monocytes were comparable to those from healthy donors (Figure 2A), despite reports that human blood monocytes express elevated levels of FcγRI (55). Circulating monocytes activate and differentiate into MFs upon migration to tissues. Thus, it is possible that accumulation of IgG-ICs on blood monocytes promotes their migration to the tissues leaving only blood monocytes that display low levels of nuclear self-antigens. Given that activating FcγRs account for 50–60% of the display of nuclear self-antigens on myeloid cells (Figure 3A, 4C), another possibility is that MFs utilize receptors that are not highly expressed on blood monocytes for the display of IgG-opsonized apoptotic debris.
What leads to the accumulation of IgG-ICs on the surface of cells is currently under investigation. Possible defects include failure to internalize IgG-ICs bound by FcγRI, disrupted trafficking to the lysosome, diminished degradation in the lysosome, or aberrant recycling of IgG-ICs (56–58). Our study provides a new insight into how apoptotic debris and IgG-ICs contribute to heightened BAFF secretion and lupus nephritis. Recent advances in treating lupus show that 43–58% of patients treated with anti-BAFF reduce SLEDAI scores more than 4 points compared to 36–46% in control subjects (59). Therefore, understanding the molecular events that lead to the accumulation of nuclear self-antigen might prove fruitful in providing a means to reduce high BAFF and simultaneously other activating FcγR-mediated events that promote autoimmune pathologies.
Supplementary Material
Acknowledgments
We would like to thank Drs. Charles Jennette (University of N. Carolina-Chapel Hill) for FcγRIV−/−B6 mice and FcγRIIB−/− mice. Alex Szalai (University of Alabama-Birmingham) for FcRγc−/− mice, Anne Sperling (University of Chicago) for FcγRI −/− and FcγRIII−/− mice, Gary Gilkeson (Medical University of S. Carolina) for NZM2410 mice, George Tsokos (Beth Israel Deaconess Medical Center) for B6/lpr mice, Mark Hogarth (Burnet Institute, Australia) for anti-FcγRI antibody (X54-5/7.1), and Thomas Winkler and Joachim Kalden (University Erlangen-Nuernberg, Germany) for anti-DNA antibody (33H11). Our thanks to Diane Carnathan and Chris Hilliard for technical assistance in the early study establishing the accumulation of IgG-ICs on hematopoietic cells. We also thank to the Flow Cytometry Core (NCI P30CA016086), the Microscopy Services Laboratory (CA 16086-26), the Histology Core Laboratory, and the Lineberger Comprehensive Cancer Center Biostatistics Core Facility for their support.
Footnotes
This work was supported by NIH R01AI070984, NIH R21AI105613, Alliance for Lupus Research, and the National Center for Advancing Translational Sciences (NCATS; National Institutes of Health) through Grant Award Number 1UL1TR001111. A.J.M. was supported by 5T32AI07273-27.
Author Contributions: S-A. K. directed and performed experiments, analyzed data, and wrote the manuscript; A.J.M. performed experiments and analyzed data; J.L.R performed experiments and analyzed data; S.H.C analyzed data, C.J. and M.D. provided mice; T.K.T, J.L.R and J.S. provided patient samples; Y.F. analyzed kidney pathology; Y.K.T. performed statistical analysis; B.J.V. codirected experiments and wrote the manuscript. The authors declare no competing financial interests.
References
- 1.Deapen D, Escalante A, Weinrib L, Horwitz D, Bachman B, Roy-Burman P, Walker A, Mack TM. A revised estimate of twin concordance in systemic lupus erythematosus. Arthritis Rheum. 1992;35:311–318. doi: 10.1002/art.1780350310. [DOI] [PubMed] [Google Scholar]
- 2.Harley JB, Kelly JA, Kaufman KM. Unraveling the genetics of systemic lupus erythematosus. Springer Semin Immunopathol. 2006;28:119–130. doi: 10.1007/s00281-006-0040-5. [DOI] [PubMed] [Google Scholar]
- 3.Bardana EJ, Jr, Harbeck RJ, Hoffman AA, Pirofsky B, Carr RI. The prognostic and therapeutic implications of DNA:anti-DNA immune complexes in systemic lupus erythematosus (SLE) Am J Med. 1975;59:515–522. doi: 10.1016/0002-9343(75)90259-4. [DOI] [PubMed] [Google Scholar]
- 4.Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14:166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Ramirez-Ortiz ZG, Pendergraft WF, 3rd, Prasad A, Byrne MH, Iram T, Blanchette CJ, Luster AD, Hacohen N, El Khoury J, Means TK. The scavenger receptor SCARF1 mediates the clearance of apoptotic cells and prevents autoimmunity. Nat Immunol. 2013;14:917–926. doi: 10.1038/ni.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RA, Earp HS, Matsushima G, Reap EA. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196:135–140. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radic M, Marion T, Monestier M. Nucleosomes are exposed at the cell surface in apoptosis. J Immunol. 2004;172:6692–6700. doi: 10.4049/jimmunol.172.11.6692. [DOI] [PubMed] [Google Scholar]
- 9.Qian Y, Wang H, Clarke SH. Impaired clearance of apoptotic cells induces the activation of autoreactive anti-Sm marginal zone and B-1 B cells. J Immunol. 2004;172:625–635. doi: 10.4049/jimmunol.172.1.625. [DOI] [PubMed] [Google Scholar]
- 10.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leadbetter EA, I, Rifkin R, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Kimberly RP. Targeting the Fc receptor in autoimmune disease. Expert Opin Ther Targets. 2014;18:335–350. doi: 10.1517/14728222.2014.877891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Rauh MJ, Krystal G. Of mice and men: elucidating the role of SH2-containing inositol 5-phosphatase (SHIP) in human disease. Clin Invest Med. 2002;25:68–70. [PubMed] [Google Scholar]
- 15.Pinheiro da Silva F, Aloulou M, Benhamou M, Monteiro RC. Inhibitory ITAMs: a matter of life and death. Trends Immunol. 2008;29:366–373. doi: 10.1016/j.it.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Duits AJ, Bootsma H, Derksen RH, Spronk PE, Kater L, Kallenberg CG, Capel PJ, Westerdaal NA, Spierenburg GT, Gmelig-Meyling FH, et al. Skewed distribution of IgG Fc receptor IIa (CD32) polymorphism is associated with renal disease in systemic lupus erythematosus patients. Arthritis Rheum. 1995;38:1832–1836. doi: 10.1002/art.1780381217. [DOI] [PubMed] [Google Scholar]
- 17.Alizadeh BZ, Valdigem G, Coenen MJ, Zhernakova A, Franke B, Monsuur A, van Riel PL, Barrera P, Radstake TR, Roep BO, Wijmenga C, Koeleman BP. Association analysis of functional variants of the FcgRIIa and FcgRIIIa genes with type 1 diabetes, celiac disease and rheumatoid arthritis. Hum Mol Genet. 2007;16:2552–2559. doi: 10.1093/hmg/ddm194. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Y, Hirose S, Sanokawa-Akakura R, Abe M, Mi X, Li N, Miura Y, Shirai J, Zhang D, Hamano Y, Shirai T. Genetically determined aberrant down-regulation of FcgammaRIIB1 in germinal center B cells associated with hyper-IgG and IgG autoantibodies in murine systemic lupus erythematosus. Int Immunol. 1999;11:1685–1691. doi: 10.1093/intimm/11.10.1685. [DOI] [PubMed] [Google Scholar]
- 19.Blank MC, Stefanescu RN, Masuda E, Marti F, King PD, Redecha PB, Wurzburger RJ, Peterson MG, Tanaka S, Pricop L. Decreased transcription of the human FCGR2B gene mediated by the -343 G/C promoter polymorphism and association with systemic lupus erythematosus. Hum Genet. 2005;117:220–227. doi: 10.1007/s00439-005-1302-3. [DOI] [PubMed] [Google Scholar]
- 20.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 21.Bredius RG, Fijen CA, De Haas M, Kuijper EJ, Weening RS, Van de Winkel JG, Out TA. Role of neutrophil Fc gamma RIIa (CD32) and Fc gamma RIIIb (CD16) polymorphic forms in phagocytosis of human IgG1- and IgG3-opsonized bacteria and erythrocytes. Immunology. 1994;83:624–630. [PMC free article] [PubMed] [Google Scholar]
- 22.Unanue E, Dixon FJ. Experimental Glomerulonephritis. Iv. Participation of Complement in Nephrotoxic Nephritis. J Exp Med. 1964;119:965–982. doi: 10.1084/jem.119.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madaio MP, Carlson J, Cataldo J, Ucci A, Migliorini P, Pankewycz O. Murine monoclonal anti-DNA antibodies bind directly to glomerular antigens and form immune deposits. J Immunol. 1987;138:2883–2889. [PubMed] [Google Scholar]
- 24.Jacob CO, Pricop L, Putterman C, Koss MN, Liu Y, Kollaros M, Bixler SA, Ambrose CM, Scott ML, Stohl W. Paucity of clinical disease despite serological autoimmunity and kidney pathology in lupus-prone New Zealand mixed 2328 mice deficient in BAFF. J Immunol. 2006;177:2671–2680. doi: 10.4049/jimmunol.177.4.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramanujam M, Wang X, Huang W, Liu Z, Schiffer L, Tao H, Frank D, Rice J, Diamond B, Yu KO, Porcelli S, Davidson A. Similarities and differences between selective and nonselective BAFF blockade in murine SLE. J Clin Invest. 2006;116:724–734. doi: 10.1172/JCI26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergtold A, Gavhane A, D’Agati V, Madaio M, Clynes R. FcR-bearing myeloid cells are responsible for triggering murine lupus nephritis. J Immunol. 2006;177:7287–7295. doi: 10.4049/jimmunol.177.10.7287. [DOI] [PubMed] [Google Scholar]
- 27.Ioan-Facsinay A, de Kimpe SJ, Hellwig SM, van Lent PL, Hofhuis FM, van Ojik HH, Sedlik C, da Silveira SA, Gerber J, de Jong YF, Roozendaal R, Aarden LA, van den Berg WB, Saito T, Mosser D, Amigorena S, Izui S, van Ommen GJ, van Vugt M, van de Winkel JG, Verbeek JS. FcgammaRI (CD64) contributes substantially to severity of arthritis, hypersensitivity responses, and protection from bacterial infection. Immunity. 2002;16:391–402. doi: 10.1016/s1074-7613(02)00294-7. [DOI] [PubMed] [Google Scholar]
- 28.Barnes N, Gavin AL, Tan PS, Mottram P, Koentgen F, Hogarth PM. FcgammaRI-deficient mice show multiple alterations to inflammatory and immune responses. Immunity. 2002;16:379–389. doi: 10.1016/s1074-7613(02)00287-x. [DOI] [PubMed] [Google Scholar]
- 29.Hazenbos WL, Gessner JE, Hofhuis FM, Kuipers H, Meyer D, Heijnen IA, Schmidt RE, Sandor M, Capel PJ, Daeron M, van de Winkel JG, Verbeek JS. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity. 1996;5:181–188. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 30.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 31.Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005;23:41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 33.Rudofsky UH, Evans BD, Balaban SL, Mottironi VD, Gabrielsen AE. Differences in expression of lupus nephritis in New Zealand mixed H-2z homozygous inbred strains of mice derived from New Zealand black and New Zealand white mice. Origins and initial characterization. Lab Invest. 1993;68:419–426. [PubMed] [Google Scholar]
- 34.Jiang C, Foley J, Clayton N, Kissling G, Jokinen M, Herbert R, Diaz M. Abrogation of lupus nephritis in activation-induced deaminase-deficient MRL/lpr mice. J Immunol. 2007;178:7422–7431. doi: 10.4049/jimmunol.178.11.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Losman MJ, Fasy TM, Novick KE, Monestier M. Monoclonal autoantibodies to subnucleosomes from a MRL/Mp(−)+/+ mouse. Oligoclonality of the antibody response and recognition of a determinant composed of histones H2A, H2B, and DNA. J Immunol. 1992;148:1561–1569. [PubMed] [Google Scholar]
- 36.Retter MW, Eisenberg RA, Cohen PL, Clarke SH. Sm and DNA binding by dual reactive B cells requires distinct VH, V kappa, and VH CDR3 structures. J Immunol. 1995;155:2248–2257. [PubMed] [Google Scholar]
- 37.Bloom DD, Davignon JL, Cohen PL, Eisenberg RA, Clarke SH. Overlap of the anti-Sm and anti-DNA responses of MRL/Mp-lpr/lpr mice. J Immunol. 1993;150:1579–1590. [PubMed] [Google Scholar]
- 38.Winkler TH, Jahn S, Kalden JR. IgG human monoclonal anti-DNA autoantibodies from patients with systemic lupus erythematosus. Clin Exp Immunol. 1991;85:379–385. doi: 10.1111/j.1365-2249.1991.tb05735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colonna L, Lood C, Elkon KB. Beyond apoptosis in lupus. Curr Opin Rheumatol. 2014;26:459–466. doi: 10.1097/BOR.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 41.Giorgini A, Brown HJ, Lock HR, Nimmerjahn F, Ravetch JV, Verbeek JS, Sacks SH, Robson MG. Fc gamma RIII and Fc gamma RIV are indispensable for acute glomerular inflammation induced by switch variant monoclonal antibodies. J Immunol. 2008;181:8745–8752. doi: 10.4049/jimmunol.181.12.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev. 2010;237:205–225. doi: 10.1111/j.1600-065X.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- 43.Suwanichkul A, Wenderfer SE. Differential expression of functional Fc-receptors and additional immune complex receptors on mouse kidney cells. Mol Immunol. 2013;56:369–379. doi: 10.1016/j.molimm.2013.05.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gauld SB, Benschop RJ, Merrell KT, Cambier JC. Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat Immunol. 2005;6:1160–1167. doi: 10.1038/ni1256. [DOI] [PubMed] [Google Scholar]
- 45.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 46.Lee SR, Rutan JA, Monteith AJ, Jones SZ, Kang SA, Krum KN, Kilmon MA, Roques JR, Wagner NJ, Clarke SH, Vilen BJ. Receptor crosstalk spatially restricts p-ERK during TLR4 stimulation of autoreactive B cells. J Immunol. 2012;189:3859–3868. doi: 10.4049/jimmunol.1200940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Viglianti GA, Lau CM, Hanley TM, Miko BA, Shlomchik MJ, Marshak-Rothstein A. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–847. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 48.Gershov D, Kim S, Brot N, Elkon KB. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J Exp Med. 2000;192:1353–1364. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marnell L, Mold C, Du Clos TW. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005;117:104–111. doi: 10.1016/j.clim.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 50.Salmond RJ, Brownlie RJ, Morrison VL, Zamoyska R. The tyrosine phosphatase PTPN22 discriminates weak self peptides from strong agonist TCR signals. Nat Immunol. 2014;15:875–883. doi: 10.1038/ni.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodriguez W, Mold C, Marnell LL, Hutt J, Silverman GJ, Tran D, Du Clos TW. Prevention and reversal of nephritis in MRL/lpr mice with a single injection of C-reactive protein. Arthritis Rheum. 2006;54:325–335. doi: 10.1002/art.21556. [DOI] [PubMed] [Google Scholar]
- 52.Szalai AJ, Weaver CT, McCrory MA, van Ginkel FW, Reiman RM, Kearney JF, Marion TN, Volanakis JE. Delayed lupus onset in (NZB x NZW)F1 mice expressing a human C-reactive protein transgene. Arthritis Rheum. 2003;48:1602–1611. doi: 10.1002/art.11026. [DOI] [PubMed] [Google Scholar]
- 53.van Bavel CC, van der Vlag J, Berden JH. Glomerular binding of anti-dsDNA autoantibodies: the dispute resolved? Kidney Int. 2007;71:600–601. doi: 10.1038/sj.ki.5002126. [DOI] [PubMed] [Google Scholar]
- 54.Matsumoto K, Watanabe N, Akikusa B, Kurasawa K, Matsumura R, Saito Y, Iwamoto I, Saito T. Fc receptor-independent development of autoimmune glomerulonephritis in lupus-prone MRL/lpr mice. Arthritis Rheum. 2003;48:486–494. doi: 10.1002/art.10813. [DOI] [PubMed] [Google Scholar]
- 55.Li Y, Lee PY, Sobel ES, Narain S, Satoh M, Segal MS, Reeves WH, Richards HB. Increased expression of FcgammaRI/CD64 on circulating monocytes parallels ongoing inflammation and nephritis in lupus. Arthritis Res Ther. 2009;11:R6. doi: 10.1186/ar2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salmon JE, Kimberly RP, Gibofsky A, Fotino M. Defective mononuclear phagocyte function in systemic lupus erythematosus: dissociation of Fc receptor-ligand binding and internalization. J Immunol. 1984;133:2525–2531. [PubMed] [Google Scholar]
- 57.Kanno H, Tachiwaki O, Nose M, Kyogoku M. Immune complex-degradation ability of macrophages in MRL/Mp-lpr/lpr lupus mice and its regulation by cytokines. Clin Exp Immunol. 1994;95:115–121. doi: 10.1111/j.1365-2249.1994.tb06024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 59.Dennis GJ. Belimumab: a BLyS-specific inhibitor for the treatment of systemic lupus erythematosus. Clin Pharmacol Ther. 2012;91:143–149. doi: 10.1038/clpt.2011.290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.