Abstract
The scaffold molecule POSH is crucial for the regulation of proliferation and effector function in CD8+ T cells. However, its role in CD4+ T cells is not known. Here we found that, disruption of the POSH scaffold complex established a transcriptional profile that strongly skewed differentiation towards TH2, led to decreased survival and had no affect on cell cycle entry. This is in stark contrast to CD8+ T cells where POSH regulates cell cycle and does not affect survival. Disruption of POSH in CD4+ T cells resulted in the loss of Tak1 dependent activation of JNK1/2 and Tak1 mediated survival. However, in CD8+ T cells, POSH regulates only JNK1. Remarkably, each type of T cell had a unique composition of the POSH scaffold complex and distinct post-translational modifications of POSH. These data indicate that the mechanism that regulates POSH function in CD4+ T cells is different from CD8+ T cells. All together, these data strongly suggest that POSH is essential for the integration of cell-type specific signals that regulate the differentiation, survival and function of T cells.
Introduction
CD4+ T cell activation leads to the acquisition of a unique set of effector functions designed to clear specific types of pathogens. TCR signals ‘set the stage’ and allow for the integration of inflammatory signals to direct CD4+ T cell differentiation into one of several T helper subsets (1–4). Scaffold molecules have the potential to provide an essential function in the regulation of this process by assembling signaling modules from individual components of a given pathway and by organizing nodes of crosstalk between multiple signaling pathways (5–8). Furthermore, scaffold proteins can modulate the nature (quality) of signal output by targeting kinases to particular micro- and nano- domains within a cell or by regulating the composition of a signaling complex (9, 10). Finally, they can modify the amplitude (quantity) of signal by recruiting cofactors that can either amplify or inhibit signaling output (8). Therefore, gaining understanding in the nature and function of scaffolds in T lymphocytes will provide us with the ability to target them to improve immune-based therapeutic interventions.
Numerous scaffold proteins critical for normal T cell function have been identified downstream of the TCR, for example: LAT and SLP76 assemble components of the proximal TCR-signaling complex (11, 12); the Carma1/Bcl10/Malt1 complex regulates NF-κB activation (13), KSR1 aids in the activation of ERK (14, 15) and Carma1/Bcl10 is specific for JNK2 activation (16). More recently, we have identified the molecule Plenty of SH3 domains (POSH) as a scaffold protein that facilitates JNK1 activation in CD8+ T cells (17).
POSH is a multi-domain scaffold protein that was initially shown to be critical for Rac1 dependent activation of JNK and NF-κB (18). Structurally, POSH contains four SH3 domains, a non-canonical Rac binding domain, as well as an NH2-terminal RING finger domain (see Figure 7). POSH regulates several cell functions depending on which molecules bind to the different domains of POSH. For example, components of the JNK signaling pathway have been found in shared complexes with POSH (MAP3Ks, MKK7, JNK1 and JNK2) (17, 19–21). Functionally, POSH cooperates with JIP-1 to regulate JNK-dependent apoptosis in mature sympathetic neurons (19, 20). On the other hand, POSH directs the Rac1 dependent radial migration of neocortical neurons in the developing brain (22). POSH also has a role in the regulation of Siah and Tak1 based survival in drosophila and neurons as well as in leukemia and lung cancer (23–25). Furthermore, POSH is involved in mediating AKT dependent survival signals in breast and lung cancer (26, 27). Whether POSH also regulates these diverse functions in T cells is unclear. We were the first to identify a functional role of POSH in CD8+ T cells (17). However, the role of POSH in CD4+ T cells remains unknown.
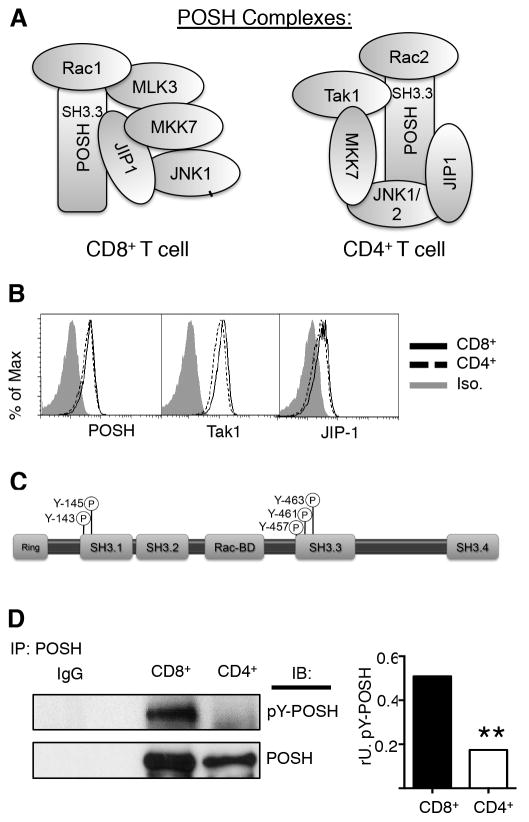
Figure 7. Post-translational modification of POSH is associated with differential scaffold composition between CD4+ and CD8+ T cells.
(A) Model representing the composition and arrangement of POSH complexes in CD4+ and CD8+ T cells. (B) Naïve polyclonal CD4+ and polyclonal CD8+ T cells were stimulated with α-CD3 and α-CD28 in the presence of IL-2 for 4 days and the levels of POSH, Tak1 and JIP-1 were assessed by flow cytometry. (C) Model depicts the organization of the functional domains in POSH and shows the location of modifiable tyrosines. (D) T cells were treated as in (B) and lysates were subjected to IP with POSH and the levels of p-tyrosine (p-Y) was determined by immunoblot. Graph shows the relative units (rU) of p-Y-POSH calculated as the ratio to POSH for each cell. All experiments are representative of n>3 independent experiments. ** indicates p < 0.01.
JNK and Tak1 have important roles in many aspects of both innate and adaptive immune responses (28–31). For T cells in general, the JNK family of MAPKs regulates activation, differentiation, and survival in both a maturation state and cell type dependent manner. For example, JNK activation is important for apoptosis of developing thymocytes, while it is essential for effector function and survival in mature T cells (29, 32, 33). JNK1 and JNK2 have unique functions and the outcome of their activation is very different between CD8+ and CD4+ T cells. In CD4+ T cells, JNK2 facilitates TH1 polarization by inducing IL-12Rβ2 following activation, which in turn enhances the expression of interferon-gamma (IFNγ). By contrast, JNK1 represses TH2 polarization by inhibiting nuclear factor of activated T cells c1 (NFATc1) and promoting the degradation of JunB (34). JNK has also been implicated in the generation of TH17 cells (35). The MAP3K, Tak1, is upstream of JNK and downstream of IL-7 and IL-15 (36, 37) and plays a significant role in T cell development, activation, differentiation and survival. Together, the potential connection between POSH, JNK and Tak1 and the complexity of their involvement in CD4+ T cell differentiation and survival, strongly suggests POSH has an important role in CD4+ T cell biology.
Here we found that POSH has a role in Tak1 dependent activation of JNK1 and JNK2 in CD4+ T cells. This has important consequences for CD4+ T cells. Disruption of POSH function had no affect on cell cycle entry, however it led to a decrease in survival, along with inhibition of TH1 and skewing towards a TH2 phenotype. Importantly, these data are in stark contrast to CD8+ T cells where disruption of POSH led to defects in cell cycle entry while survival remained intact. Most remarkably, these changes are associated with distinct phosphorylation-based modifications of POSH and differential composition of the POSH scaffold complex in CD8+ versus CD4+ T cells. These data suggest a mechanism that explains the distinct T cell-type dependent roles of JNK activation.
Materials and Methods
Mice
C57BL/6 and C57BL/6 Rag-/- mice were maintained in our animal facilities at the University of Missouri. Animal procedures were in accordance with Intuitional Animal Care and Use Committee regulations.
Antibodies and Reagents
POSH, JIP-1, JNK1, JNK2, Rac1, Noxa, MKK7, MLK3, MEKK1, and p-NFATc1 were purchased from Santa Cruz Biotechnology. Tak1, Puma, pSAPK/JNK, pMKK7, p-p38, pIκBα, NFATc1, JunB, Bcl2 and Bim were purchased from Cell Signaling. CD25 APC, T-bet APC, GATA3 PE, IL-4 PE, IFN-γ APC, IL-2 APC, and IL-17A APC were purchased from eBioscience. β-actin was purchased from Sigma. 4G10, Rac1 and Rac2 were purchased from Millipore. Mcl-1 was purchased from Rockland. IL-12Rβ2 PE was purchased from R&D. 7-AAD was purchased from BD.
Inhibitors
Tat-POSH (NH2-GRKKRRQRRRPPRPRKEDELELRKGEMFLVFER-amide) 5-FamTat-POSH (5-carboxyfluorescein-GRKKRRQRRRPPRPRKEDELELRKGEMFLVFER-amide) and Tat-cont. (NH2-GRKKRRQRRRPP-amide) peptides were synthesized by New England Peptides to a purity of >90%, respectively. Peptides were used at 20 μM (except where noted). None of the peptides exhibited nonspecific toxicity at any concentration tested. SP600125 (used at 33 μM) and Tat-JIP1 (used at 20 μM) were purchased from Calbiochem. All inhibitors were added 30 min before stimulation and maintained in culture with media + fresh inhibitor throughout the entire stimulation.
Immunoprecipitations
IP-FCM was performed using α-POSH (YY-5) or α-JIP-1 (2J8) CML beads as previously described (17, 38). In brief, >1,500 bead events were collected for each experiment and data was analyzed using FlowJo (TreeStar). Graphs depicting relative secondary analyte levels were generated by normalizing the geometric MFI of the secondary analyte to the geometric MFI of the primary analyte (to control for potential variations in IP efficiency (loading control)) to Tat-cont.-treated cells. Primary and secondary analyte-binding specificity and significance were determined by comparing their fluorescence to negative controls that included both isotype controls and antibodies to proteins that are known not to be in the complex being analyzed. IP-Westerns were performed using α-POSH (YY-5) antibodies and Protein A/G beads (Santa Cruz Biotech).
In vitro Stimulations
CD4+ T cells were isolated using a mouse CD4+ T cell enrichment kit (Stem Cell Tech) according to the manufacturers instructions. Cells maintained in RPMI 1640 media with 10% FCS and stimulated with 1μg/mL α-CD3 (2C11) and 1 μg/mL α-CD28 (37.51) or 50 ng/mL PMA and 500 ng/mL Ionomycin (Sigma). IL-2 was used at 50 u/mL. Where indicated, cells were labeled with 10 μM CFSE. Golgi Plug (Brefeldin A; BD) was used at 1 μL/mL.
Statistical Analysis
Probability (p) values were calculated with paired two-tailed Student’s t-test or Anova. All analyses were performed with Prism 6 software (Graphpad Inc.).
Results
POSH regulates survival and differentiation of CD4+ T cells
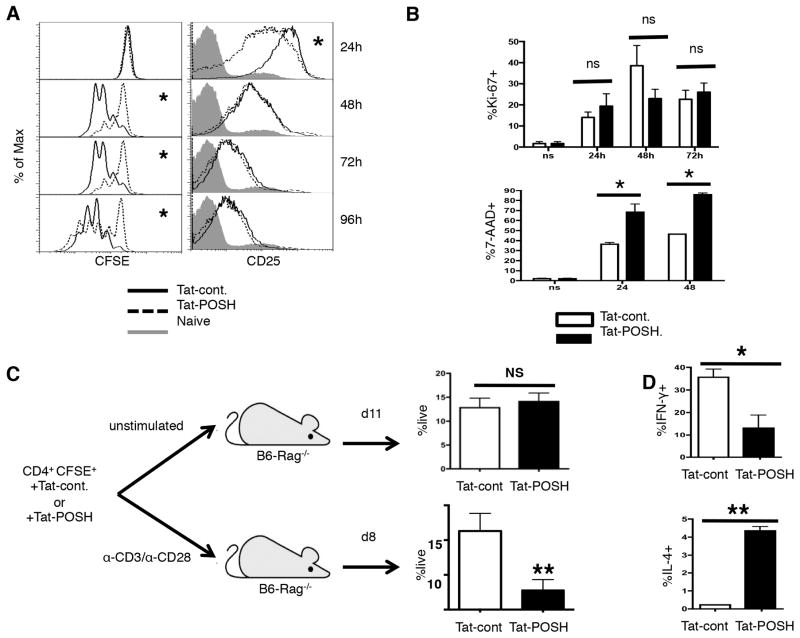
The POSH scaffold complex regulates JNK1 dependent programs of effector function and proliferation in CD8+ T cells (17). JNK activity contributes to proliferation and survival of CD4+ T cells (32, 34, 37, 39, 40). Thus, we determined the role of POSH in directing these processes in CD4+ T cells. Global deletion of POSH would lead to pleiotropic effects that make it difficult to draw clear conclusions. To overcome this, we disrupted the function of POSH with a cell permeable competitive peptide inhibitor comprised of an HIV Tat domain fused to a truncated form of the SH3 domain 3 (SH3.3) of POSH (referred to throughout the text as Tat-POSH) (Sup Figure 1A). We have previously shown that treatment of CD8+ T cells with this inhibitor blocks association of members of the JNK pathway to the SH3.3 domain of POSH, impairing POSH function (17). Therefore, CFSE labeled CD4+ T cells were treated with Tat-POSH or Tat-cont. followed by stimulation with α-CD3 and α-CD28 in the presence of exogenous IL-2. Tat-POSH treated cells proliferated slightly less than Tat-cont. treated cells (Figure 1A). IL-2 is a potent mitogen that drives the proliferation and differentiation of CD4+ T cells through the high affinity IL-2 receptor, CD25. Disruption of POSH function led to only a minor reduction in the expression of CD25 at 24 hours post-stimulation that was recovered by 48 hours (Figure 1A). Similar results were obtained when CD4+ T cells were stimulated in a mixed culture system (data not shown). Interestingly, disruption of the POSH complex had no effect on the expression of IL-2 or IL-17 (Sup Figure 1B). To determine if the failure of Tat-POSH treated cells to accumulate was caused by reduced cell cycle entry or increased cell death, the percent of Ki-67+ and 7-AAD+ cells was determined. Despite the slight trend for fewer cycling cells at 48 hours, there was no significant difference in the percent of cells that entered cell cycle (Ki-67+), suggesting, along with normal IL-2 and CD25 expression, that POSH is dispensable for proliferation of CD4+ T cells (Figure 1B). By contrast, there was a significant increase in the percent of 7-AAD+ cells with Tat-POSH treatment (Figure 1B). Interestingly, this defect only appears when CD4+ T cells undergo strong stimulation. That is, unstimulated CFSE labeled CD4+ T cells treated with Tat-POSH were transferred into lymphopenic hosts (where they primarily encounter lower affinity self antigen) and to allow for significant expansion they were harvested on Day 11. We found that Tat-POSH and control treated cells demonstrated similar proliferation (Sup Figure 1C) and were recovered at a similar frequency (Figure 1C). On the contrary, Tat-POSH treated cells that were stimulated with α-CD3/α-CD28, adoptively transferred into B6-Rag−/− hosts and collected on Day 8 (the peak/effector phase of the response) were recovered at a much lower frequency (Figure 1C). However, those that were recovered had proliferated equivalently to control treated cells (Sup Figure 1D). Together, these data provide strong evidence that POSH is dispensable for proliferation but makes a significant contribution to the survival of strongly activated CD4+ T cells. This is in stark contrast to CD8+ T cells where POSH is important for entry into cell cycle and dispensable for survival (17).
Figure 1. POSH regulates CD4+ T helper differentiation and survival.
(A) Naïve CD4+ T cells were labeled with CFSE, treated with Tat-POSH or Tat-cont. and stimulated with α-CD3 and α-CD28 in the presence of IL-2 and Tat-POSH or cont. for 4 days. Cell division was analyzed by dilution of the CFSE dye (left) and the level of CD25 (right) was determined by flow cytometry. (B) Naïve CD4+ T cells were treated with Tat-POSH or Tat-cont. and stimulated for 24, 48 and 72 hours with exogenous IL-2 and the percent of Ki-67+ cells (top) and 7-AAD+ (bottom) cells was determined. All experiments are representative of n>4 independent experiments. (C) Naïve CD4+ T cells were labeled with CFSE and treated with Tat-POSH or Tat-cont. for 30 minutes. 1×106 cells were either un-stimulated or stimulated with α-CD3 and α-CD28 then adoptively transferred into Rag−/− mice. Cell numbers and division status were assessed in the spleen and lymph nodes at day 11 and day 8. n=3 independent experiments with cohorts of 5 mice per condition per experiment. (D) Naïve CD4+ T cells were treated as in (A) and re-stimulated for 5 hours with PMA/Iono in the presence of BFA n=4. The percent of IL-4+ and IFN-γ+ cells were then determined by ICCS. All graphs show +/− SD * indicates p < 0.05; ** p < 0.01.
Since POSH is important for JNK activation and JNK are implicated in CD4+ T cell differentiation, we disrupted POSH function in CD4+ T cells and determined their T helper polarization. Naïve CD4+ T cells were isolated and stimulated in un-biasing conditions (41) for three days in the presence of Tat-cont. or Tat-POSH. The surviving cells were then re-stimulated with PMA and Ionomycin for 5 hours and cytokine production was determined by intracellular cytokine staining (ICCS). Tat-POSH treated CD4+ T cells generated markedly fewer IFN-γ+ cells and significantly more cells became IL-4+ (Figure 1D). Tat-POSH treated cells stimulated in the presence of blocking α-IL-4 antibodies and exogenous INF-γ do become TH1 (data not shown). These data are similar to findings in JNK deficient T cells and suggests that the POSH scaffold complex regulates the JNK dependent TH1/TH2 polarization decision in CD4+ T cells.
POSH regulates both JNK1 and JNK2 activation in CD4+ T cells
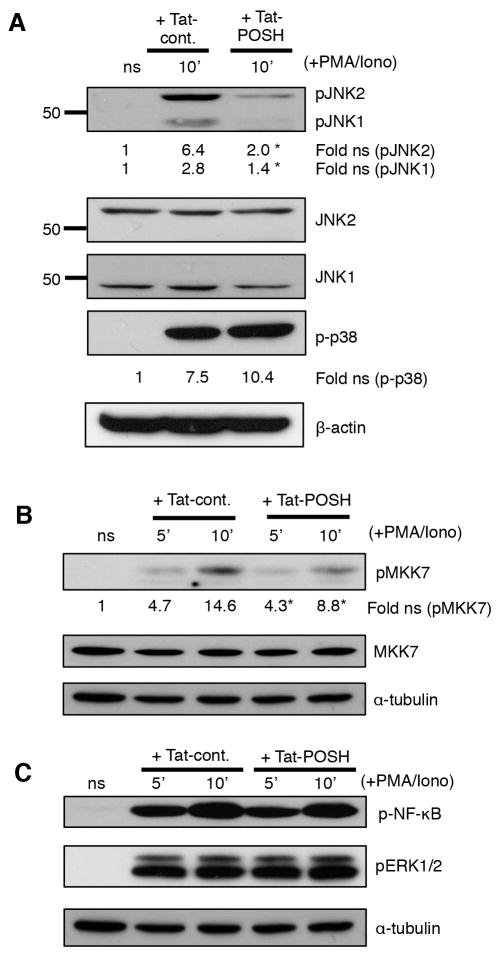
Numerous signaling pathways cooperate to regulate CD4+ T cell differentiation and commitment to a TH1 or TH2 lineage. To determine the POSH-based ‘targets’ involved in this decision, polyclonal CD4+ T cells were purified and stimulated with α-CD3/α-CD28 plus IL-2 for 4 days and re-stimulated with PMA/ionomycin, all in the continuous presence of Tat-POSH or Tat-cont. Disruption of POSH led to a profound loss in the induction of both phospho-JNK1 and phospho-JNK2 (Figure 2A) as well as a loss in the activation of the upstream JNK specific MAP2K, MKK7 (Figure 2B). Importantly, disruption of POSH was highly specific for the JNK pathway in CD4+ T cells, as signaling of other MAPK p38 and ERK; AKT, as well as IκBα and phospho-NFκB induction remained unchanged (Figure 2C, data not shown) (17). Therefore, these data show that POSH regulates both JNK1 and JNK2 activation in CD4+ T cells.
Figure 2. The POSH regulates the activation of both JNK1 and JNK2 in activated CD4+ T cells.
(A–B) Naïve CD4+ T cells were treated with Tat-POSH or Tat-cont. and then stimulated with PMA/Iono for the times indicated and then levels of pJNK1/2, p-p38 (A) and pMKK7 (B) were determined by immunoblotting (C) Cells were treated as in (A) and the levels of p-NF-κB and p-ERK were analyzed by immunoblotting. All experiments are representative of n>3 independent experiments. * indicates p < 0.05.
POSH promotes TH1 differentiation
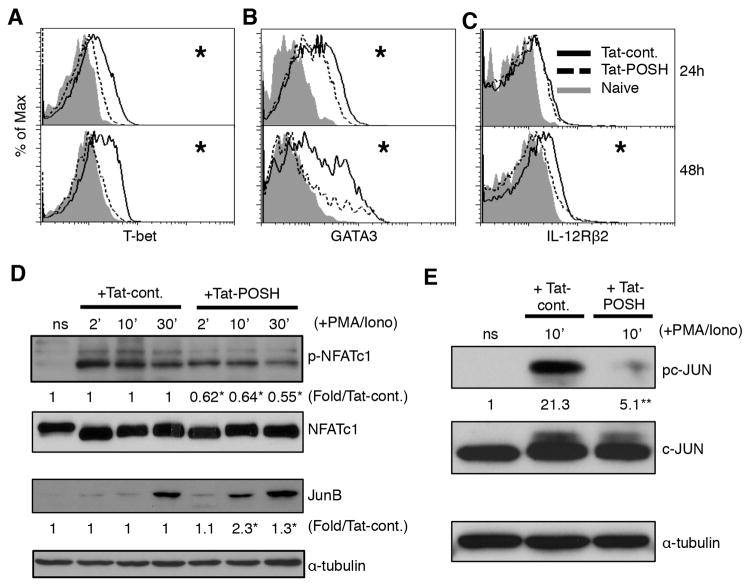
Next we identified the POSH-based contribution to the transcriptional regulation of CD4+ T cell differentiation. T-bet is considered to be a JNK-dependent regulator of TH1 polarization in CD4+ T cells (42). In addition, POSH and JNK1 are required for T-bet expression in CD8+ T cells (17, 43). To determine if the POSH scaffold complex was required for T-bet expression, CD4+ T cells were treated with Tat-POSH or Tat-cont. and stimulated with α-CD3/α-CD28. Then the level of T-bet was determined by flow cytometry. Cells treated with Tat-POSH failed to induce T-bet over the level of naïve control at 24 and 48 hours post stimulation (Figure 3A). On the other hand, expression of the TH2 transcription factor GATA3 (22) was only slightly reduced at 24 hours and while expressed at low levels at 48 hours, it was still significantly higher than non-stimulated cells (Figure 3B). These results suggest that the enhanced TH2 skewing upon loss of POSH function is a consequence of GATA3 expression in the absence of T-bet (44) (Figure 1D). We confirmed that POSH mediated loss of JNK activity enhances TH2 skewing, as the reduction of T-bet and GATA3 expression induced by the JNK kinase inhibitor SP600125 matched levels seen in cells treated with Tat-POSH (Sup Figure 2). JNK2 dependent expression of IL-12Rβ2 is critical for IL-12 mediated TH1 polarization (40). In agreement with this, upon disruption of POSH IL-12Rβ2 expression was significantly reduced 48 hours post stimulation (Figure 3C), suggesting a block in the ability of IL-12 to drive TH1.
Figure 3. Disruption of POSH leads to a transcriptional profile that favors TH2.
(AC) Naïve CD4+ T cells treated with Tat-POSH or Tat-cont. and stimulated with α-CD3 and α-CD28 in the presence of IL-2 for 24 or 48 hours and the levels of T-bet (A), GATA3 (B) and IL-12Rβ2 (C) were determined by flow cytometry. (D) Naïve CD4+ T cells were treated with Tat-POSH or Tat-cont. and stimulated with PMA/Iono and the level of p-NFATc1, NFATc1, JunB and β-actin were determined by immunoblotting. (E) Cells were stimulated as in (D) and pc-JUN and c-JUN were measured by immunoblot. All experiments are representative of n>3 independent experiments. * indicates p < 0.05. ** indicates p < 0.01.
NFATc1 and JunB are two additional transcription factors that promote TH2 polarization and are inhibited by JNK1 activation (34). We found that CD4+ T cells had a significant increase in NFATc1 dephosphorylation (activation) in Tat-POSH treated cells (Figure 3D). Furthermore, degradation of JunB was also diminished upon disruption of POSH function (Figure 3D). Additionally, a marked and significant reduction in the activation of the transcription factor c-JUN was observed (Figure. 3E). Together, these data recapitulate the defects in T helper differentiation found in JNK deficient T cells (45). In summary, the data shows that POSH mediated JNK activation is required for proper TH1/TH2 lineage decisions by regulating T-bet, GATA3, IL-12Rβ2, NFATc1, JunB and c-Jun.
POSH regulates intrinsic and extrinsic apoptotic pathways in activated CD4+ T cells
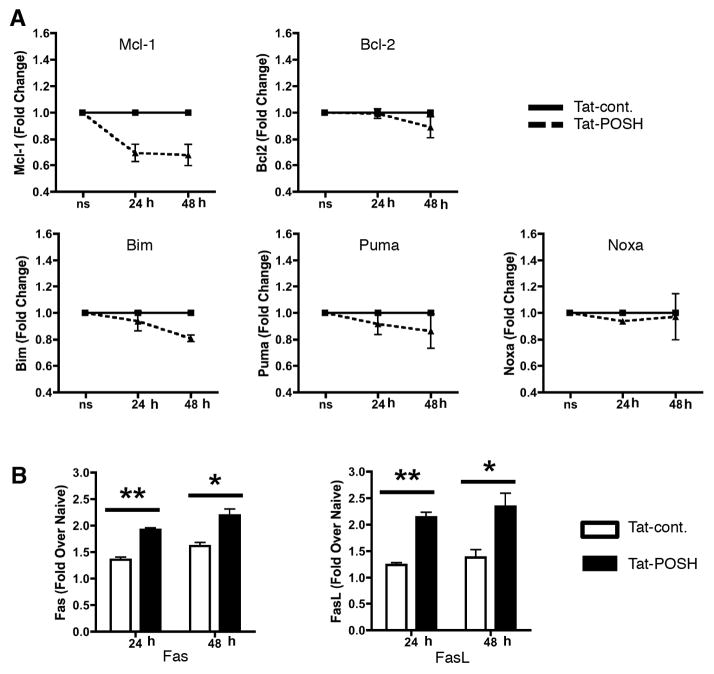
We next investigated how POSH promotes CD4+ T cell survival (Figure 1B). The intrinsic apoptotic pathway is tightly regulated through the balance of expression and activity of pro-apoptotic (Bim/Puma/Noxa) and anti-apoptotic molecules (Mcl-1/Bcl2/Bcl-xL) (46). To determine the role of POSH in this process, purified CD4+ T cells were stimulated in the presence of Tat-cont. or Tat-POSH and levels of pro- and anti-apoptotic molecules were determined by flow cytometry. Tat-POSH treated cells expressed significantly less Mcl-1 than those treated with Tat-cont. at both 24 and 48 hours post-stimulation (Figure 4A). However, Bcl2 expression was unaffected by Tat-POSH treatment (Figure 4A). The level of the pro-apoptotic molecule Bim was slightly reduced only after 48 hours, while there was no effect on Puma or Noxa expression (Figure 4A). To confirm that POSH mediated JNK activity was required for induction of Mcl-1, the experiments were repeated using the JNK kinase inhibitor SP600125 (47). Confirming previous results, CD4+ T cells treated with SP600 and stimulated for 24 or 48 hours had reduced expression of Mcl-1 and increased cell death as measured by 7-AAD staining (Sup Figure 2). These results suggest that POSH is required for survival in activated CD4+ T cells through JNK dependent regulation of Mcl-1. Importantly, these results are in line with a recent report that connected Tak1 mediated JNK activation with Mcl-1 dependent survival of activated CD4+ T cells (37, 48).
Figure 4. POSH modulates intrinsic and extrinsic apoptotic pathways in CD4+ T cells.
(A) Naïve CD4+ T cells were treated with Tat-POSH or Tat-cont. and stimulated with α-CD3 and α-CD28 in the presence of IL-2 for 24 or 48 hours and the levels of anti-apoptotic Mcl-1, Bcl2, and pro-apoptotic Bim, Puma, and Noxa were determined by immunoblot. Data represented as fold change where Tat-cont. is set to 1. (B) Cells were stimulated as in (A) and the levels of Fas (CD95) and FasL (CD178) were determined at 24 and 48 hours post-stimulation. All experiments are representative of >3 independent experiments. * indicates p < 0.05. ** indicates p < 0.01
In activated CD4+ T cells, the extrinsic death receptor pathway consisting of Fas (CD178) and FasL (CD95) can also induce apoptosis (49). The expression of FasL is controlled by TCR-generated signals, through the transcription factors NFAT, NF-κB, Egr-1, Egr-2, and Egr-3, Sp-1, AP-1, ATF-2, c-Myc or FKHRL1 (50–52). Since disruption of POSH led to the defects in both the activation of c-Jun and the de-activation of NFAT, we also assessed the expression of FasL on Tat-POSH or control treated activated CD4+ T cells. Interestingly, unlike reports from JNK deficient T cells (53), this resulted in increased levels of both Fas and FasL expression on days 1 and 2 (Figure 4B). This suggests that a POSH independent pool of JNK is responsible for the regulation of FasL or alternatively, NFAT and NF-κB may be sufficient to maintain FasL expression in these cells. Overall, these results suggest that POSH contributes to the survival of activated CD4+ T cells by regulating both the intrinsic pathway (via Mcl-1) and the extrinsic (Fas and FasL) apoptotic pathways.
The composition of the POSH scaffold complex in CD4+ T cells is distinct from CD8+ T cells
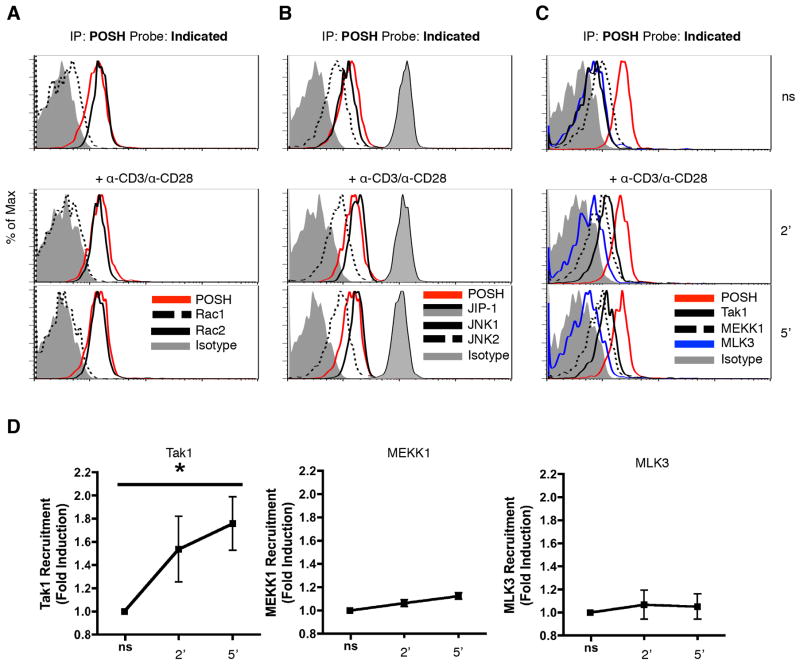
Disruption of the POSH scaffold complex in CD4+ T cells led to impaired JNK1 and JNK 2 activation (Figure 2). This was strikingly different from CD8+ T cells where POSH regulated JNK1 and not JNK2. Therefore, we hypothesized that the composition of the POSH complex was different in these two cell types. POSH functions as a scaffold molecule to facilitate optimal JNK1 activation in CD8+ T cells by recruiting and assembling MLK3, MKK7, and JIP-1 into a functional scaffold complex (17). As the same JNK signaling components are expressed in CD4+ T cells (29), we determined whether they were present in the CD4+ T cell conformation of the POSH complex. To assess this, CD4+ T cells were stimulated with α-CD3 and α-CD28 and subjected to immunoprecipitation by flow cytometry, termed IP-FCM (17, 38, 54) using α-POSH carboxylate-modified latex (CML) beads. As expected, the POSH complex contained the small G protein (GTPase) Rac2 but very little Rac1, in line with the expression pattern of Rac isoforms in CD4+ T cells (Figure 5A) (55). Additionally, MKK7 and JIP-1 were also found in the POSH complex (Figure 5B, Sup Figure 3). Interestingly, both JNK1 and JNK2 were also detected in shared complexes with POSH (Figure 5B). This was consistent with results from Figure 2 where disruption of POSH resulted in the inhibition of both JNK1 and JNK2 phosphorylation. Most interestingly, Tak1 was the predominant MAP3K found in association with POSH in CD4+ T cells. We also observed significant levels of MEKK1 but very little MLK3 (Figure 5C). Intriguingly, upon stimulation with α-CD3/α-CD28, there was a significant increase in the amount of Tak1 found in complex POSH while the expression of MEKK1 and MLK3 remained constant (Figure 5D). This was very different from CD8+ T cells where MLK3 was the predominant MAP3K while Tak1 was not present in the POSH scaffold complex (17). Collectively, these data provide the first indication that the POSH scaffold complex has a unique composition in CD4+ T cells.
Figure 5. Composition of the POSH scaffold complex in unique in CD4+ T cells.
(A–D) Naïve CD4+ T cells were stimulated with cross-linked α-CD3 and α-CD28 for 2 or 5 min. Lysates were subjected to IP-FCM using α-POSH CML beads and probed with Rac1 and Rac2 (A), JIP-1, JNK1 and JNK2 (B), or Tak1, MEKK1, and MLK3 (C). Probing with α-POSH was used to confirm pull-down and serve as a ‘loading control’ and an isotype is used as a negative control. (D) The fold induction of MAP3K-POSH interactions over non-stimulated (ns) was calculated. All experiments are representative of >3 independent experiments. * indicates p < 0.05.
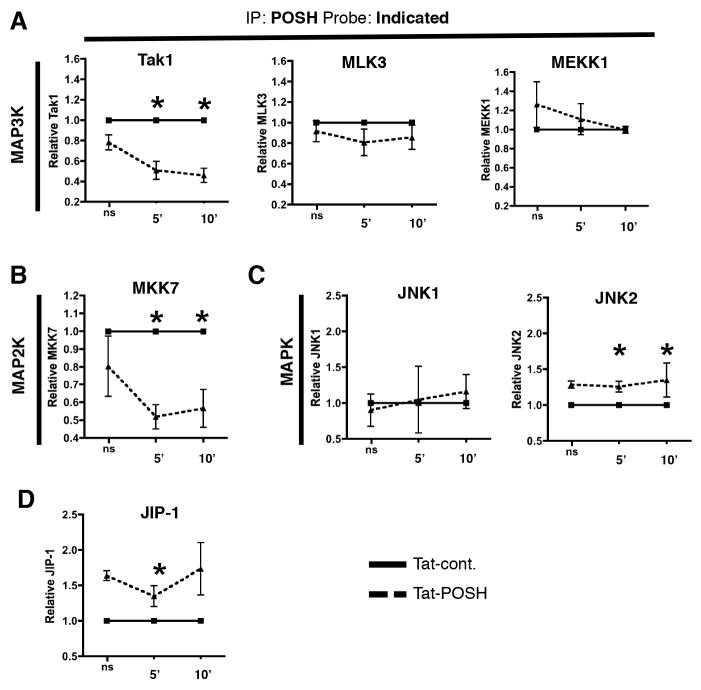
These results led to the question as to how the individual components of the CD4+ T cell POSH complex were arranged. To determine the contribution of POSH SH3.3 in the assembly of the CD4+ T cell POSH scaffold complex, cells were treated with Tat-POSH or Tat-cont. for 30 minutes and then stimulated with α-CD3/α-CD28 and IP-FCM was performed using α-POSH CML beads. Strikingly, we observed a significant reduction in the amount of Tak1 that was bound to POSH in the presence of Tat-POSH compared to Tat-cont. (Figure 6A). This effect was specific to Tak1 as there were no differences in the amount of MLK3 or MEKK1 bound to POSH in the presence of Tat-POSH or Tat-cont. We also observed a significant reduction in the amount of MKK7 recruited to POSH in the presence of Tat-POSH compared to Tat-cont. (Figure 6B). POSH cannot directly bind MKK7 (19) and therefore this result is most likely due to the ability of MKK7 to bind Tak1 (see Figure 7) (56, 57). Next, we examined the role of the POSH SH3.3 domain on the interactions of POSH with JNK1 and JNK2. Surprisingly, there was no difference in the binding of JIP-1, JNK1 or JNK2 to POSH in the presence of Tat-POSH compared to Tat-cont. (Figure 6C). Since, JIP1 is important for JNK recruitment in CD8+ T cells (17, 19) we performed reciprocal IP of JIP-1 in the presence of Tat-POSH or Tat-cont. with similar results (data not shown). This suggests that, unlike CD8+ T cells, the POSH SH3.3 domain is dispensable for POSH/JIP-1 binding in CD4+ T cells (Figure 6D). Therefore, to determine whether the JNK1 and JNK2 binding to POSH were still dependent on JIP-1, we utilized the commercially available Tat-JIP1 inhibitor, which efficiently uncouples the interaction of JIP-1 to JNK1 and JNK2 (58, 59). Intriguingly, while the inhibitor disrupted the association of JIP-1 with JNK1 and JNK2, there was no difference in the binding of POSH to JNK1 and JNK2 in the presence of Tat-JIP1 (Sup Figure 4). This suggests that, at least in the presence of Tat-JIP1, POSH/JNK binding can occur independent of JIP-1 in CD4+ T cells. Taken together, these data provide strong evidence that the POSH scaffold complex has a unique composition in CD4+ and CD8+ T cells. Furthermore, this suggests a possible mechanism by which JNK activation has such distinct outcomes between these two cell types.
Figure 6. Tak1 interacts with POSH SH3.3 domain in CD4+ T cells.
(A–D) Naïve CD4+ T cells were treated with Tat-POSH or Tat-cont. and stimulated with α-CD3 and α-CD28 for the time indicated before being subjected to IP-FCM using α-POSH CML beads. The levels of Tak1, MLK3, MEKK1 (A), MKK7 (B), JNK1 and JNK2 (C), or JIP-1 (D) bound to POSH were then determined. Data is represented as fold change where Tat-cont. is set to 1 for each time-point. All experiments are representative of >3 independent experiments. * indicates p < 0.05.
Unique pattern of post-translational modifications to POSH in CD4+ versus CD8+ T cells
Collectively, these results showed that the POSH complex in CD4+ T cells contains Rac2, Tak1 (binding via the SH3.3 of POSH), MKK7, JIP-1 and JNK1/2 (Figure 7A). On the other hand, the primary POSH complex in CD8+ T cells contains Rac1, MLK3, JIP1, MKK7 and JNK1 (17). Tat-POSH based competitive inhibition of binding to POSH SH3.3 domain disrupted the binding of Tak1 to the POSH complex in CD4+ T cells, whereas in CD8+ T cells this resulted in the loss of JIP-1 and suggests the arrangement shown in (Figure 7A) (17). These findings beg the question as to how composition of the POSH scaffold complex is different in these two types of T cells. To explain this, we performed two tests. First, a change in the stoichiometric ratio of the binding partners could easily influence the composition of the complex. In support of this, the level of Rac1 was extremely low in CD4+ T cells most likely leading to the predominant presence of Rac2 in the POSH complex (Figure 5A, 7A). Remarkably, there was no difference in the levels of expression of POSH, MKK7, Tak1 and JIP-1 in naïve and stimulated CD4+ and CD8+ T cells (Figure 7B, data not shown). This rules out the stoichiometric ratio as the determining factor for the composition of the POSH complex.
Thus, we hypothesized that post-translational modifications to POSH account for differences in the POSH scaffold complex between CD4+ and CD8+ T cells. Along these lines, AKT-based phosphorylation, within the POSH Rac binding domain blocks Rac binding (26, 27). Intriguingly, there are five tyrosine residues within POSH/JIP-1 binding sites (19) that can be subjected to phosphorylation as predicted by in silico sequence analyses (phoshoELM, Scansite3 and PhosphositePLus) and shown by mass spectrometry (60). To identify the extent of phospho-tyrosine (p-Tyr) based modifications to POSH, CD4+ and CD8+ T cell blasts were lysed and precipitated with an α-POSH antibody and the tyrosine phosphorylation status of POSH was determined by immunoblotting with α-pTyr antibody, 4G10. Strikingly, we observed significant p-Tyr-based modification of POSH in CD8+ but not in CD4+ T cells (Figure 7C). Thus, these data suggest that post-translational changes at the level of POSH as a potential mechanism to differentially regulate the composition of the POSH complex and activation of JNK to produce an outcome that is unique depending on the T cell type.
Discussion
The POSH scaffold complex directs unique JNK signals in a number of cells types. In this work we have found that POSH is required for Tak1 mediated JNK1 and JNK2 activation downstream of the TCR in CD4+ T cells. Most importantly, POSH mediated JNK1 and JNK2 activation is necessary for cell survival and plays a critical role in directing TH1 differentiation. While portions of these data resemble observations reported for Tak1 and JNK deficient T cells (32, 34, 37, 40), others do not. This suggests that POSH only regulates the subset of JNK and Tak1 functions responsible for effector function and survival in CD4+ T cells. Finally, the composition and function of the POSH complex is remarkably different in CD4+ and CD8+ T cells and correlates with differential post-translational modification of POSH.
The composition of the CD4+ T cell POSH scaffold complex differs significantly from CD8+ T cells. Rac binding to the complex follows the expression of the most prevalent isoform within each cell, which are Rac1 in CD8+ and Rac2 in CD4+ T cells (55). However, functional redundancy between these two Rac isoforms precludes this as a factor in regulating the unique function of POSH (61, 62). In CD8+ T cells the composition of the POSH complex requires the association of two scaffold modules. The POSH half of the scaffold complex binds upstream components of the JNK signal cascade, Rac1 and MLK3, while the JIP-1 portion binds the downstream components MKK7 and JNK1. The two are bound together through POSH SH3.3 domain (17, 19). However, in CD4+ T cells, disruption of POSH SH3.3 domain led to the loss of Tak1 and MKK7. Since, MKK7 cannot bind directly to POSH (19) and Tak1 binds to MKK7 (56, 63), this indicates that Tak1 associates with the SH3.3 domain and is responsible for recruiting MKK7 to the POSH complex. Interestingly, in CD4+ T cells JIP-1 appears to associate with POSH outside of the SH3.3 domain. In support of this, Kukekov et al, showed in both a cell free system and neuronal cell lines that POSH could also directly bind JIP-1 through SH3 domain 4 and a region that lies between the RING domain and SH3 domain 1 (19).
Our data suggests the mechanism behind the unique composition of the POSH complex could be based on phosphorylation of specific tyrosine residues at or near the beginning of POSH SH3.1 and POSH SH3.3. In support of this, these types of modifications are known to regulate the binding specificity of SH3 domains (64, 65). Furthermore, phosphorylation-based modification of these specific residues of POSH has been identified in neurons and T cell leukemias (60). It is important to note that there were no differences in the expression level or modifications to Tak1 or JIP-1 when comparing CD4+ and CD8+ T cells, precluding these arguments as alternative explanations for the differential composition of the POSH complex. Thus, the data suggests that lower levels of phospho-tyrosine modifications to POSH in CD4+ T cells favors Tak1 binding while higher levels of phosphorylation favors JIP-1 binding to SH3.3. In support of this, JIP-1 contains a phospho-tyrosine binding domain (PTB) that is predicted to bind to these types of phospho-tyrosine residues (19). It is unclear what mediates these changes in the modification of POSH and additional studies are needed to establish the causal relationship between phosphorylation and complex composition to address this important question. Regardless, the unique composition of the POSH complex suggests a mechanism for the differential regulation of JNK function in CD4+ and CD8+ T cells.
POSH-dependent JNK activation is only responsible for a subset of CD4+ T cell functions. Disruption of POSH function led to a defect in TH1/TH2 differentiation similar to what has been described for JNK deficient T cells (32, 40, 45, 66–68). On the other hand, however, multiple models of JNK deficiency lead to increased survival, while disruption of POSH function in our system led to a marked increase in T cell apoptosis. These conflicting results suggest there is POSH-independent regulation of JNK or POSH also controls a JNK-independent mechanism of survival. As such, POSH-independent Carma/Bcl10-dependent activation of JNK2 (16) may contribute to some of the differences we observed between disruption of POSH function and JNK deficiencies (for example, normal versus increased IL-2 expression respectively). However, the increased cell death that we found upon disruption of POSH function more closely mimics Tak1 deficiency (37, 48). Consistent with this, CD4+ T cell activation led to increased levels of Tak1 in the POSH complex. Furthermore, inhibition of POSH function led to defects in Mcl-1 expression similar to results from Tak1 inhibition (48). While we have not entirely ruled out the possibility that Tat-POSH could function through the sequestration of JIP-1 or Tak1, independent of their association with POSH, we would argue that this is unlikely when considering the following points. First, we still find JIP-1 in the POSH complex in CD4+ T cells in the presence of Tat-POSH. Second, NK-κB signaling downstream of Tak1 is not affected by the inhibitor in both CD4+ and CD8+ T cells (17). In addition, the inhibitor did not block Tak1 regulation of IL-7 dependent T cell survival in conditions of lymphopenia (37). Thus, these data argue against a global inhibition of Tak1 and Jip-1 function. Furthermore, they suggest that these modes of Tak1 function are POSH independent.
Increased FasL expression upon disruption of POSH function suggests that POSH has a role in the extrinsic pathway of apoptosis and AICD. This contradicts findings where FasL expression, apoptosis and AICD were decreased in the absence of JNK (53). However, this may be compensated for by NFAT, p38 and NF-κB dependent induction of FasL in our system (50–52). Intriguingly, POSH and the E3 ubiquitin ligase Siah have been shown to work together to inhibit death receptor signaling through degradation of signaling intermediates and surface expression of receptors as shown in leukemia, prostate and lung cancer (25, 69). Siah also provides negative feedback of p53 downstream of MDM2 suggesting a point of crosstalk between TCR and POSH and the p53 pathway in CD4+ T cells (70). Interestingly, the E3 ubiquitin ligase activity of Siah has been linked to the activity of MAP3K components of the JNK pathway. Furthermore, a region in close proximity to POSH SH3.3 domain is responsible for binding to Siah (23). Thus, it is possible that in CD4+ T cells POSH mediates crosstalk between Tak1/Siah to regulate the extrinsic apoptosis pathway. Whether this is a direct consequence of the disruption of the POSH complex, change in the activity or location of Siah or a secondary effect of loss of JNK activity remains to be determined.
This work provides the first description of the function of POSH in CD4+ T cells and shows its importance in the differential regulation of JNK function in CD4+ and CD8+ T cells. The consequence of the disruption of POSH function makes it an attractive candidate for therapeutic intervention in the setting of autoimmune diseases such as type I diabetes and multiple sclerosis where CD4+ T cells play an important role. Furthermore, as POSH does not appear to play a role in naïve CD4+ T cell homeostasis this eliminates concerns associated with non-specific CD4+ T cell depletion for these types of treatments. Given the mode of the contribution of POSH to survival, POSH may also serve as an effective target to improve the treatment of various types of tumors.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by Grants from the University of Missouri Mission Enhancement Fund (M.A.D. and E.T.), The University of Missouri Research Board (E.T. and M.A.D.), MU Ellis Fischel Cancer Research Fund (M.A.D.), NIH AI110420 (E.T) and the University of Missouri Life Sciences Fellowship (L.N.C.).
We would like to thank Carmen Pernas and James Osterberg for helpful discussions. Dedicated to Dr. Alan Hall (1952 –2015).
Footnotes
The authors have no conflict of interest.
References
- 1.Knudson KM, Goplen NP, Cunningham CA, Daniels MA, Teixeiro E. Low-affinity T cells are programmed to maintain normal primary responses but are impaired in their recall to low-affinity ligands. Cell reports. 2013;4:554–565. doi: 10.1016/j.celrep.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 3.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Panhuys N, Klauschen F, Germain RN. T-cell-receptor-dependent signal intensity dominantly controls CD4(+) T cell polarization In Vivo. Immunity. 2014;41:63–74. doi: 10.1016/j.immuni.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acuto O, V, Bartolo D, Michel F. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nat Rev Immunol. 2008;8:699–712. doi: 10.1038/nri2397. [DOI] [PubMed] [Google Scholar]
- 6.Hancock JF. Ras proteins: different signals from different locations. Nat Rev Mol Cell Biol. 2003;4:373–384. doi: 10.1038/nrm1105. [DOI] [PubMed] [Google Scholar]
- 7.Mor A, Philips MR. Compartmentalized Ras/MAPK signaling. Annu Rev Immunol. 2006;24:771–800. doi: 10.1146/annurev.immunol.24.021605.090723. [DOI] [PubMed] [Google Scholar]
- 8.Shaw AS, Filbert EL. Scaffold proteins and immune-cell signalling. Nat Rev Immunol. 2009;9:47–56. doi: 10.1038/nri2473. [DOI] [PubMed] [Google Scholar]
- 9.Kholodenko BN, Hancock JF, Kolch W. Signalling ballet in space and time. Nat Rev Mol Cell Biol. 2010;11:414–426. doi: 10.1038/nrm2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Hollander GA, Gascoigne NR, Palmer E. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 11.Balagopalan L, Coussens NP, Sherman E, Samelson LE, Sommers CL. The LAT story: a tale of cooperativity, coordination, and choreography. Cold Spring Harb Perspect Biol. 2010;2:a005512. doi: 10.1101/cshperspect.a005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 13.Wang D, You Y, Case SM, McAllister-Lucas LM, Wang L, DiStefano PS, Nunez G, Bertin J, Lin X. A requirement for CARMA1 in TCR-induced NF-kappa B activation. Nature immunology. 2002;3:830–835. doi: 10.1038/ni824. [DOI] [PubMed] [Google Scholar]
- 14.Filbert EL, Nguyen A, Markiewicz MA, Fowlkes BJ, Huang YH, Shaw AS. Kinase suppressor of Ras 1 is required for full ERK activation in thymocytes but not for thymocyte selection. Eur J Immunol. 2010;40:3226–3234. doi: 10.1002/eji.201040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen A, Burack WR, Stock JL, Kortum R, Chaika OV, Afkarian M, Muller WJ, Murphy KM, Morrison DK, Lewis RE, McNeish J, Shaw AS. Kinase suppressor of Ras (KSR) is a scaffold which facilitates mitogen-activated protein kinase activation in vivo. Molecular and Cellular Biology. 2002;22:3035–3045. doi: 10.1128/MCB.22.9.3035-3045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blonska M, Pappu BP, Matsumoto R, Li H, Su B, Wang D, Lin X. The CARMA1-Bcl10 signaling complex selectively regulates JNK2 kinase in the T cell receptor-signaling pathway. Immunity. 2007;26:55–66. doi: 10.1016/j.immuni.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham CA, Knudson KM, Peng BJ, Teixeiro E, Daniels MA. The POSH/JIP-1 scaffold network regulates TCR-mediated JNK1 signals and effector function in CD8 T cells. Eur J Immunol. 2013 doi: 10.1002/eji.201343635. [DOI] [PubMed] [Google Scholar]
- 18.Tapon N, Nagata K, Lamarche N, Hall A. A new rac target POSH is an SH3-containing scaffold protein involved in the JNK and NF-kappaB signalling pathways. EMBO J. 1998;17:1395–1404. doi: 10.1093/emboj/17.5.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kukekov NV, Xu Z, Greene LA. Direct interaction of the molecular scaffolds POSH and JIP is required for apoptotic activation of JNKs. J Biol Chem. 2006;281:15517–15524. doi: 10.1074/jbc.M601056200. [DOI] [PubMed] [Google Scholar]
- 20.Xu Z, Kukekov NV, Greene LA. POSH acts as a scaffold for a multiprotein complex that mediates JNK activation in apoptosis. EMBO J. 2003;22:252–261. doi: 10.1093/emboj/cdg021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Z, Kukekov NV, Greene LA. Regulation of apoptotic c-Jun N-terminal kinase signaling by a stabilization-based feed-forward loop. Molecular and Cellular Biology. 2005;25:9949–9959. doi: 10.1128/MCB.25.22.9949-9959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang T, Sun Y, Zhang F, Zhu Y, Shi L, Li H, Xu Z. POSH localizes activated Rac1 to control the formation of cytoplasmic dilation of the leading process and neuronal migration. Cell reports. 2012;2:640–651. doi: 10.1016/j.celrep.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Xu Z, Sproul A, Wang W, Kukekov N, Greene LA. Siah1 interacts with the scaffold protein POSH to promote JNK activation and apoptosis. J Biol Chem. 2006;281:303–312. doi: 10.1074/jbc.M509060200. [DOI] [PubMed] [Google Scholar]
- 24.Tsuda M, Langmann C, Harden N, Aigaki T. The RING-finger scaffold protein Plenty of SH3s targets TAK1 to control immunity signalling in Drosophila. EMBO Rep. 2005;6:1082–1087. doi: 10.1038/sj.embor.7400537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christian PA, Fiandalo MV, Schwarze SR. Possible role of death receptor-mediated apoptosis by the E3 ubiquitin ligases Siah2 and POSH. Molecular cancer. 2011;10:57. doi: 10.1186/1476-4598-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Figueroa C, Tarras S, Taylor J, Vojtek AB. Akt2 negatively regulates assembly of the POSH-MLK-JNK signaling complex. J Biol Chem. 2003;278:47922–47927. doi: 10.1074/jbc.M307357200. [DOI] [PubMed] [Google Scholar]
- 27.Lyons TR, Thorburn J, Ryan PW, Thorburn A, Anderson SM, Kassenbrock CK. Regulation of the Pro-apoptotic scaffolding protein POSH by Akt. J Biol Chem. 2007;282:21987–21997. doi: 10.1074/jbc.M704321200. [DOI] [PubMed] [Google Scholar]
- 28.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 29.Rincon M, Davis RJ. Regulation of the immune response by stress-activated protein kinases. Immunol Rev. 2009;228:212–224. doi: 10.1111/j.1600-065X.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- 30.Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 32.Dong C, Yang DD, Tournier C, Whitmarsh AJ, Xu J, Davis RJ, Flavell RA. JNK is required for effector T-cell function but not for T-cell activation. Nature. 2000;405:91–94. doi: 10.1038/35011091. [DOI] [PubMed] [Google Scholar]
- 33.Rincon M, Whitmarsh A, Yang DD, Weiss L, Derijard B, Jayaraj P, Davis RJ, Flavell RA. The JNK pathway regulates the In vivo deletion of immature CD4(+)CD8(+) thymocytes. J Exp Med. 1998;188:1817–1830. doi: 10.1084/jem.188.10.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong C, Yang DD, Wysk M, Whitmarsh AJ, Davis RJ, Flavell RA. Defective T cell differentiation in the absence of Jnk1. Science. 1998;282:2092–2095. doi: 10.1126/science.282.5396.2092. [DOI] [PubMed] [Google Scholar]
- 35.Van der Velden J, Janssen-Heininger YM, Mandalapu S, Scheller EV, Kolls JK, Alcorn JF. Differential requirement for c-Jun N-terminal kinase 1 in lung inflammation and host defense. PloS one. 2012;7:e34638. doi: 10.1371/journal.pone.0034638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wan YY, Chi H, Xie M, Schneider MD, Flavell RA. The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat Immunol. 2006;7:851–858. doi: 10.1038/ni1355. [DOI] [PubMed] [Google Scholar]
- 38.Schrum AG, Gil D, Dopfer EP, Wiest DL, Turka LA, Schamel WW, Palmer E. High-sensitivity detection and quantitative analysis of native protein-protein interactions and multiprotein complexes by flow cytometry. Science’s STKE : signal transduction knowledge environment. 2007;2007:pl2. doi: 10.1126/stke.3892007pl2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swat W, Fujikawa K, Ganiatsas S, Yang D, Xavier RJ, Harris NL, Davidson L, Ferrini R, Davis RJ, Labow MA, Flavell RA, Zon LI, Alt FW. SEK1/MKK4 is required for maintenance of a normal peripheral lymphoid compartment but not for lymphocyte development. Immunity. 1998;8:625–634. doi: 10.1016/s1074-7613(00)80567-1. [DOI] [PubMed] [Google Scholar]
- 40.Yang DD, Conze D, Whitmarsh AJ, Barrett T, Davis RJ, Rincon M, Flavell RA. Differentiation of CD4+ T cells to Th1 cells requires MAP kinase JNK2. Immunity. 1998;9:575–585. doi: 10.1016/s1074-7613(00)80640-8. [DOI] [PubMed] [Google Scholar]
- 41.Hendricks DW, Fink PJ. Recent thymic emigrants are biased against the T-helper type 1 and toward the T-helper type 2 effector lineage. Blood. 2011;117:1239–1249. doi: 10.1182/blood-2010-07-299263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 43.Gao Y, Tao J, Li MO, Zhang D, Chi H, Henegariu O, Kaech SM, Davis RJ, Flavell RA, Yin Z. JNK1 is essential for CD8+ T cell-mediated tumor immune surveillance. Journal of immunology. 2005;175:5783–5789. doi: 10.4049/jimmunol.175.9.5783. [DOI] [PubMed] [Google Scholar]
- 44.Kanhere A, Hertweck A, Bhatia U, Gokmen MR, Perucha E, Jackson I, Lord GM, Jenner RG. T-bet and GATA3 orchestrate Th1 and Th2 differentiation through lineage-specific targeting of distal regulatory elements. Nature communications. 2012;3:1268. doi: 10.1038/ncomms2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong C, Davis RJ, Flavell RA. Signaling by the JNK group of MAP kinases. c-jun N-terminal Kinase. Journal of clinical immunology. 2001;21:253–257. doi: 10.1023/a:1010975124110. [DOI] [PubMed] [Google Scholar]
- 46.Strasser A, Puthalakath H, O’reilly L, Bouillet P. What do we know about the mechanisms of elimination of autoreactive T and B cells and what challenges remain. Immunol Cell Biol. 2008;86:57–66. doi: 10.1038/sj.icb.7100141. [DOI] [PubMed] [Google Scholar]
- 47.Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirata Y, Sugie A, Matsuda A, Matsuda S, Koyasu S. TAK1-JNK axis mediates survival signal through Mcl1 stabilization in activated T cells. J Immunol. 2013;190:4621–4626. doi: 10.4049/jimmunol.1202809. [DOI] [PubMed] [Google Scholar]
- 49.Siegel RM, Chan FK, Chun HJ, Lenardo MJ. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nat Immunol. 2000;1:469–474. doi: 10.1038/82712. [DOI] [PubMed] [Google Scholar]
- 50.Teixeiro E, Garcia-Sahuquillo A, Alarcon B, Bragado R. Apoptosis-resistant T cells have a deficiency in NF-kappaB-mediated induction of Fas ligand transcription. Eur J Immunol. 1999;29:745–754. doi: 10.1002/(SICI)1521-4141(199903)29:03<745::AID-IMMU745>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 51.Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 52.Kavurma MM, Khachigian LM. Signaling and transcriptional control of Fas ligand gene expression. Cell Death Differ. 2003;10:36–44. doi: 10.1038/sj.cdd.4401179. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J, Gao JX, Salojin K, Shao Q, Grattan M, Meagher C, Laird DW, Delovitch TL. Regulation of fas ligand expression during activation-induced cell death in T cells by p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase. J Exp Med. 2000;191:1017–1030. doi: 10.1084/jem.191.6.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teixeiro E, Daniels M, Hamilton S, Schrum A, Bragado R, Jameson S, Palmer E. Different T Cell Receptor Signals Determine CD8+ Memory Versus Effector Development. Science. 2009;323:502. doi: 10.1126/science.1163612. [DOI] [PubMed] [Google Scholar]
- 55.Avruch J, Tornqvist HE, Gunsalus JR, Price DJ, Kyriakis JM, Yurkow EJ, Ahmad MF, Banerjee P. The role of tyrosine-and serine-threonine-protein phosphorylation in insulin action. Advances in second messenger and phosphoprotein research. 1990;24:295–300. [PubMed] [Google Scholar]
- 56.Whitmarsh AJ. The JIP family of MAPK scaffold proteins. Biochem Soc Trans. 2006;34:828–832. doi: 10.1042/BST0340828. [DOI] [PubMed] [Google Scholar]
- 57.Whitmarsh AJ, Davis RJ. Structural organization of MAP-kinase signaling modules by scaffold proteins in yeast and mammals. Trends in biochemical sciences. 1998;23:481–485. doi: 10.1016/s0968-0004(98)01309-7. [DOI] [PubMed] [Google Scholar]
- 58.Barr RK, Kendrick TS, Bogoyevitch MA. Identification of the critical features of a small peptide inhibitor of JNK activity. J Biol Chem. 2002;277:10987–10997. doi: 10.1074/jbc.M107565200. [DOI] [PubMed] [Google Scholar]
- 59.Melino M, Hii CS, McColl SR, Ferrante A. The effect of the JNK inhibitor, JIP peptide, on human T lymphocyte proliferation and cytokine production. J Immunol. 2008;181:7300–7306. doi: 10.4049/jimmunol.181.10.7300. [DOI] [PubMed] [Google Scholar]
- 60.Bruckner SR, Tammariello SP, Kuan CY, Flavell RA, Rakic P, Estus S. JNK3 contributes to c-Jun activation and apoptosis but not oxidative stress in nerve growth factor-deprived sympathetic neurons. J Neurochem. 2001;78:298–303. doi: 10.1046/j.1471-4159.2001.00400.x. [DOI] [PubMed] [Google Scholar]
- 61.Dumont C, Corsoni-Tadrzak A, Ruf S, de Boer J, Williams A, Turner M, Kioussis D, Tybulewicz VL. Rac GTPases play critical roles in early T-cell development. Blood. 2009;113:3990–3998. doi: 10.1182/blood-2008-09-181180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walmsley MJ, Ooi SK, Reynolds LF, Smith SH, Ruf S, Mathiot A, Vanes L, Williams DA, Cancro MP, Tybulewicz VL. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science. 2003;302:459–462. doi: 10.1126/science.1089709. [DOI] [PubMed] [Google Scholar]
- 63.Mooney LM, Whitmarsh AJ. Docking interactions in the c-Jun N-terminal kinase pathway. J Biol Chem. 2004;279:11843–11852. doi: 10.1074/jbc.M311841200. [DOI] [PubMed] [Google Scholar]
- 64.Mayer BJ, Eck MJ. SH3 domains. Minding your p’s and q’s. Curr Biol. 1995;5:364–367. doi: 10.1016/s0960-9822(95)00073-x. [DOI] [PubMed] [Google Scholar]
- 65.Seet BT, Dikic I, Zhou MM, Pawson T. Reading protein modifications with interaction domains. Nat Rev Mol Cell Biol. 2006;7:473–483. doi: 10.1038/nrm1960. [DOI] [PubMed] [Google Scholar]
- 66.Chow CW, Dong C, Flavell RA, Davis RJ. c-Jun NH(2)-terminal kinase inhibits targeting of the protein phosphatase calcineurin to NFATc1. Mol Cell Biol. 2000;20:5227–5234. doi: 10.1128/mcb.20.14.5227-5234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Constant SL, Dong C, Yang DD, Wysk M, Davis RJ, Flavell RA. JNK1 is required for T cell-mediated immunity against Leishmania major infection. J Immunol. 2000;165:2671–2676. doi: 10.4049/jimmunol.165.5.2671. [DOI] [PubMed] [Google Scholar]
- 68.Weiss L, Whitmarsh AJ, Yang DD, Rincon M, Davis RJ, Flavell RA. Regulation of c-Jun NH(2)-terminal kinase (Jnk) gene expression during T cell activation. J Exp Med. 2000;191:139–146. doi: 10.1084/jem.191.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bursen A, Moritz S, Gaussmann A, Moritz S, Dingermann T, Marschalek R. Interaction of AF4 wild-type and AF4.MLL fusion protein with SIAH proteins: indication for t(4;11) pathobiology? Oncogene. 2004;23:6237–6249. doi: 10.1038/sj.onc.1207837. [DOI] [PubMed] [Google Scholar]
- 70.Watanabe M, Moon KD, Vacchio MS, Hathcock KS, Hodes RJ. Downmodulation of tumor suppressor p53 by T cell receptor signaling is critical for antigen-specific CD4(+) T cell responses. Immunity. 2014;40:681–691. doi: 10.1016/j.immuni.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.