Abstract
While the incidence of antibody-mediated kidney graft rejection has increased, the key cellular and molecular participants underlying this graft injury remain unclear. Rejection of kidney allografts in mice lacking the chemokine receptor CCR5 is dependent on production of donor-specific antibody. Here we determine if cells expressing cytotoxic function contributed to antibody-mediated kidney allograft rejection in these recipients. Wild type C57BL/6, B6.CCR5−/− and B6.CD8−/−/CCR5−/− mice were transplanted with complete MHC mismatched A/J kidney grafts and intra-graft inflammatory components were followed to rejection. B6.CCR5−/− and B6.CD8−/−/CCR5−/− recipients rejected kidney allografts by day 35 whereas 65% of allografts in wild type recipients survived past day 80 post-transplant. Rejected allografts in wild-type C57BL/6, B6.CCR5−/− and B6.CD8−/−/CCR5−/− recipients expressed high levels of VCAM-1 and MMP7 mRNA that was associated with high serum titers of donor-specific antibody. High levels of perforin and granzyme B mRNA expression peaked on day 6 post-transplant in allografts in all recipients, but were absent in isografts. Depletion of natural killer cells in B6.CD8−/−/CCR5−/− recipients reduced this expression to background levels and promoted the long-term survival of 40% of the kidney allografts. Thus, natural killer cells have a role in increased inflammation during antibody-mediated kidney allograft injury and in rejection of the grafts.
Keywords: kidney allograft, antibody-mediated rejection, NK cells
Whereas the advent of calcineurin inhibitor-based immunosuppression has decreased T cell mediated graft rejection, the incidence of antibody-mediated graft injury and rejection has increased (1–4). Antibodies are reported to be the cause of up to 50% of acute rejection episodes in kidney transplants and more than 60% of late graft failure (3–5). The increased incidence and difficulty in treating antibody-mediating rejection has underscored the need to develop new strategies to identify the cellular and molecular components underlying antibody-mediated kidney graft rejection. Antibody mediated rejection of kidney allografts has distinct histopathologic features from T cell mediated graft injury (6–8). These include neutrophil and macrophage margination in peritubular capillaries and often within the glomeruli. In many cases donor-specific antibody (DSA) doesn’t mediate rejection as a solitary event, but induces the activation of other components of innate and adaptive immunity to contribute to the graft tissue injury. The presence of T cell infiltration and tubulitis is often observed in for-cause biopsies with diagnosis of antibody-mediated rejection (5, 8–10). Such mixed T cell-antibody mediated histopathology may occur in 10–90% of kidney graft biopsies that fulfill the Banff criteria for antibody-mediated rejection (5, 9). In addition, the presence of transcripts associated with NK cell activation in kidney graft biopsies during ABMR is often observed, suggesting a role for NK cells in the pathology (11–13). The contribution of T cells and/or NK cells to kidney graft injury during antibody-mediated rejection, however, remains poorly defined.
We have developed a unique mouse model of antibody-mediated rejection of kidney allografts (14, 15). CCR5-deficient allograft recipients have dysregulated DSA responses where high titers are generated within two weeks of transplantation. In contrast to wild type C57BL/6 recipients of complete MHC-mismatched kidney allografts, all CCR5−/− recipients reject the allografts between days 17 and 30 post- transplant and this rejection is dependent on the production of DSA. We have recently reported the activation of CD8 T cells in CCR5−/− recipients of kidney allografts (16), but the contributions of CD8 T cells as well as NK cells during antibody mediated rejection in these recipients has not been directly tested. In light of clinical studies suggesting a potential impact of donor-reactive T cells and/or NK cells in antibody-mediated rejection of renal allografts, we generated CD8−/−/CCR5−/− mice to test the role of donor-reactive CD8 T cells in antibody-mediated kidney allograft rejection. The goal of the current study was to compare the cellular and molecular events occurring in a temporal manner during the rejection of kidney allografts with vs. without CCR5−/− recipient CD8 T cells and/or NK cells. The results indicate that CD8 T cells are not required during antibody-mediated rejection of kidney allografts but that NK cells mediate intense intra-graft inflammation and play a critical role in rejection.
RESULTS
Renal allograft survival in wild type, CCR5−/− and CD8−/−CCR5−/− recipients
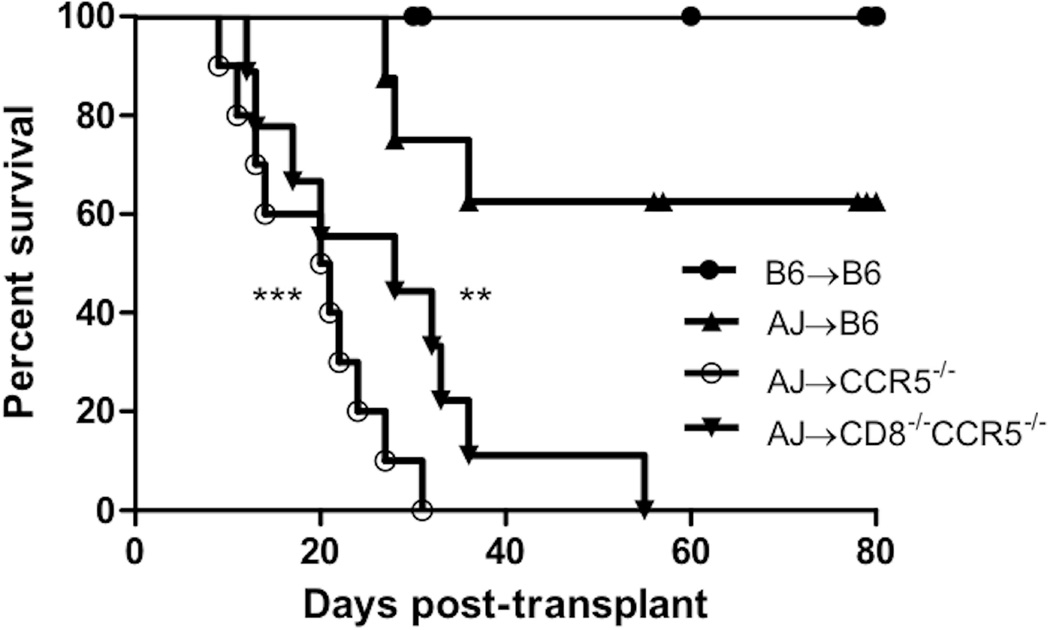
A potential role for donor-reactive CD8 T cells during antibody-mediated rejection to renal allografts in CCR5-deficient recipients was investigated by comparing survival of complete MHC mismatched A/J renal allografts in B6.CCR5−/− vs. B6.CD8−/−CCR5−/− recipients. Groups of wild type C57BL/6 mice received A/J allografts or C57BL/6 isografts. C57BL/6 isografts were maintained long-term (> 60 days) in wild type C57BL/6 recipients without any incidence of graft failure (Figure 1a) as well as in B6.CCR5−/− and B6.CD8−/−CCR5−/− recipients (data not shown). Consistent with our previous studies, renal allografts were maintained in wild type C57BL/6 recipients until day 30 post-transplant when 40% were rejected by day 40 post transplant, but the remaining 60% of the allografts survived with good function beyond day 70 post-transplant. B6.CCR5−/− recipients began rejecting A/J allografts on day 15 post-transplant and all allografts were rejected by day 35 with a mean time survival (MTS) of 16.75 ± 5.70 days. B6.CD8−/−CCR5−/− recipients rejected the renal allografts with slightly delayed kinetics with a MTS of 27.33 ± 13.67 days that was not significantly different from allograft survival in B6.CCR5−/− recipients. Extensive histopathological evidence of antibody-mediated rejection was already obvious by day 6 post-transplant in B6.CD8−/−CCR5−/− and B6.CCR5−/− recipients that included marked capillary dilatation with leukocyte margination and C4d deposition (Figure 1b and Supplemental Figure 1), typical features of antibody-mediated kidney graft rejection (17–19).
Figure 1. Survival of complete MHC-mismatched renal allografts in wild type C57BL/6, B6.CCR5−/− and B6.CD8−/−CCR5−/− recipients.
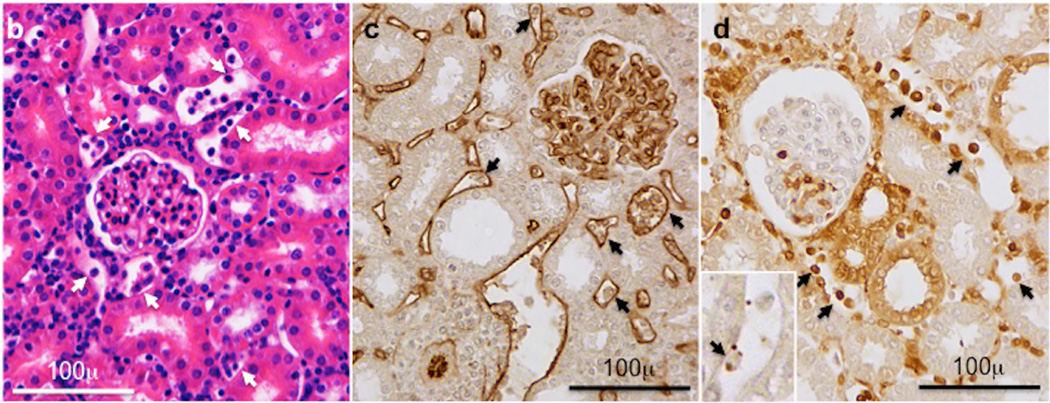
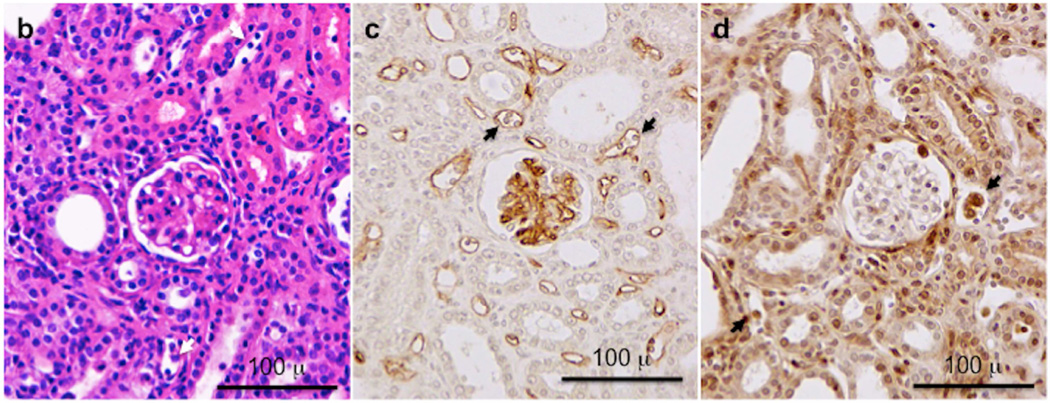
Groups of wild type C57BL/6 (n = 9), B6.CCR5−/− (n = 10) and B6.CD8−/−CCR5−/− (n = 9) mice received renal allografts from A/J donors. A group of wild type C57BL/6 mice (n = 5) also received isografts. (a) Nephrectomy of the remaining native kidney was performed on day 5 post-transplant and graft survival was followed by daily examination of overall animal health. **P<0.01; ***P<0.005; when compared to survival of allografts in wild type C57BL/6 recipients. (b–d) Histological evaluation of kidney allografts from B6.CCR5−/− recipients on day 6 post-transplant stained with: (b) hematoxylin and eosin; (c) anti-C4d antibody; and (d) anti-Mac2 antibody. Arrows indicate marginating leukocytes. Images shown are representative of 3 individual allografts in the group.
Production of DSA in response to renal allografts
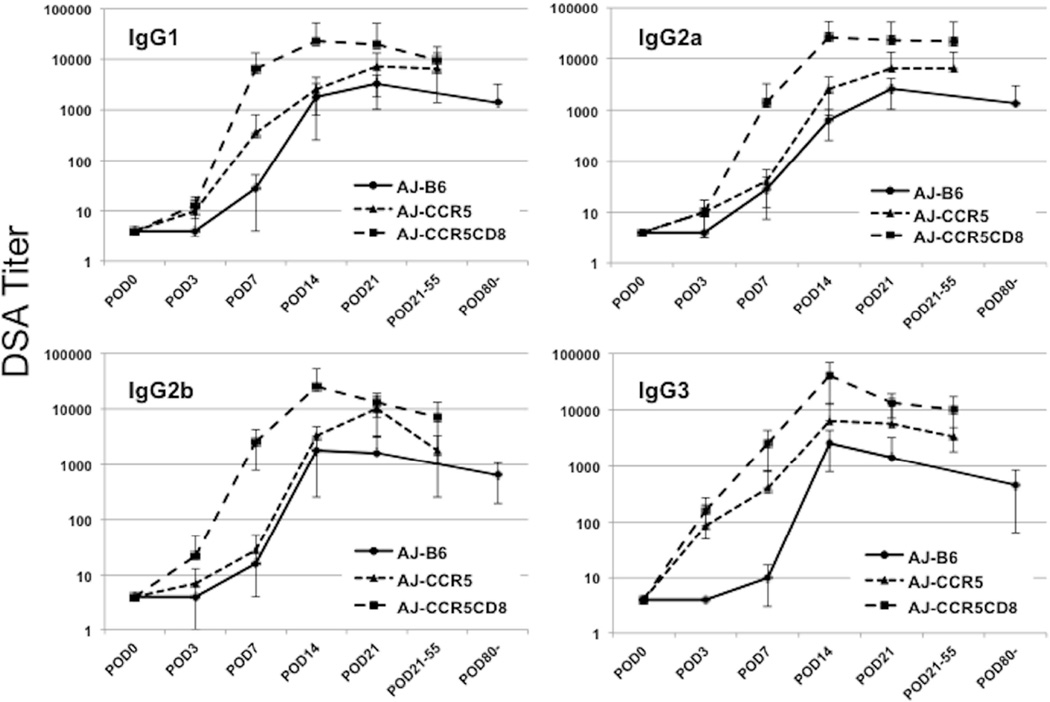
Consistent with previous results indicating induction of high levels of donor-reactive antibody to complete MHC-mismatched renal allografts in B6.CCR5−/− recipients (14, 15), DSA of all IgG isotypes were first detectable in B6.CCR5−/− renal allograft recipient serum by day 7 post-transplant and rose to high titers between days 14–21 that were maintained until allograft rejection (Figure 2). Serum titers of all DSA IgG isotypes were markedly higher at day 7 post-transplant in B6.CD8−/−CCR5−/− recipients than those observed in B6.CCR5−/− recipients and reached peak titers by day 14 post-transplant. In wild type C57BL/7 allograft recipients, DSA levels were low until day 14 post-transplant and all DSA IgG isotypes were 4–10 fold lower than those observed in the B6.CCR5−/− recipients at the time the allografts were rejected in the latter.
Figure 2. Production of donor-reactive IgG antibody in wild type C57BL/6, B6.CCR5−/− and B6.CD8−/−CCR5−/− kidney allograft recipients.
Serum was collected from individual wild type C57BL/6, B6.CCR5−/− and B6.CD8−/−CCR5−/− recipients of A/J kidney allografts on days 3, 7, and 14 post-transplant and at the time of rejection. The sera were tested for reactivity to A/J thymocytes using a flow cytometry-based approach to determine the titer of donor-reactive antibody for each of the IgG subclasses. Data indicate mean titer for each recipient group ± SEM.
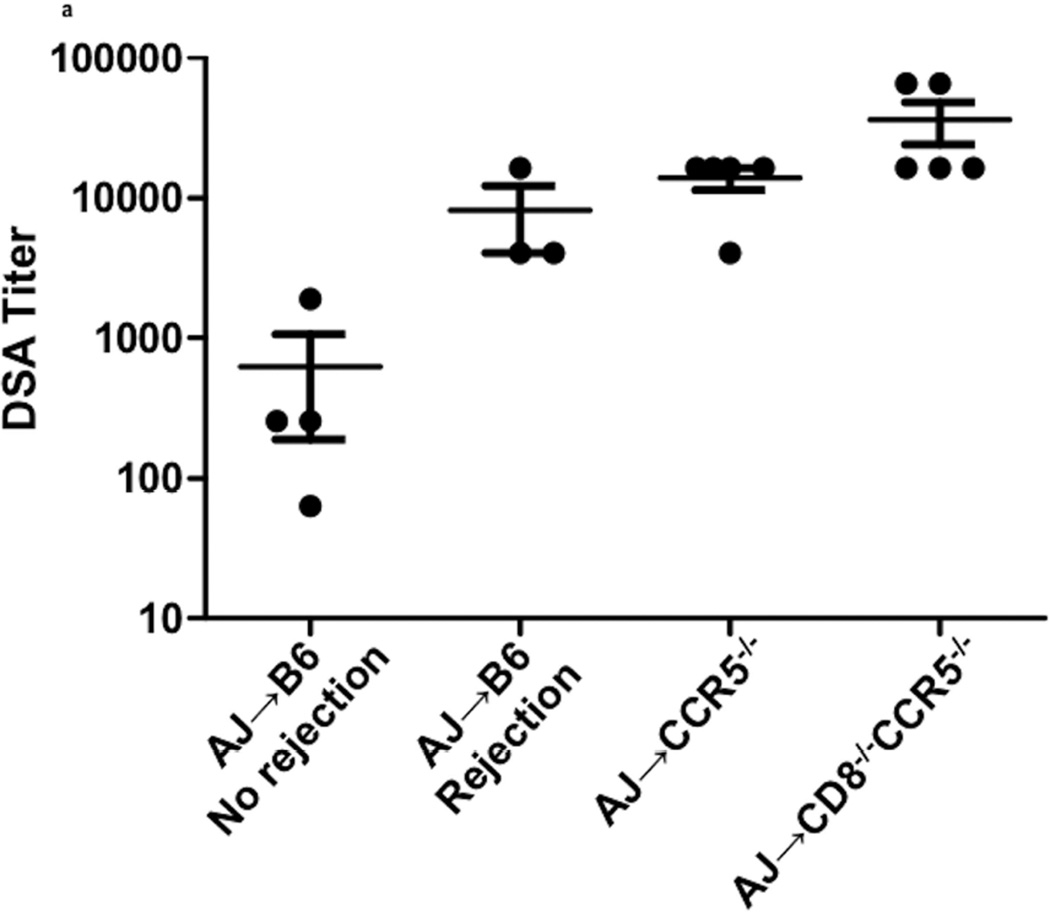
To more closely assess the impact of DSA titer on kidney allograft rejection, the serum titers of IgG DSA in each of the renal allograft recipient groups were assessed at the time of rejection (Figure 3a). DSA titers in B6.CCR5−/− recipients were high (13926 ± 2458) and those in B6.CD8−/−CCR5−/− recipients were approximately 4–5 fold higher (36045 ± 12040). While DSA titers in wild type C57BL/6 allograft recipients with surviving allografts were low on day 40 post-transplant (625 ± 435), DSA titers in wild type recipients that rejected the allografts were more than 10-fold higher (8192 ± 4092) and near the titers observed in B6.CCR5−/− recipients at allograft rejection. Similar to rejecting allografts in CCR5-deficient recipients, rejecting allografts in wild type C57BL/6 recipients had dilated peritubular capillaries with marginated leukocytes, particularly Mac2+ cells, and C4d deposition in the capillaries and in the glomeruli (Figure 3b).
Figure 3. Titers of donor-reactive IgG antibody at the time of kidney allograft rejection in wild type C57BL/6, B6.CCR5−/− and B6.CD8−/−CCR5−/− recipients.
(a) Titers of donor-reactive IgG antibody were determined in serum collected from allograft recipients at the time of graft rejection or from C57BL/7 recipients with long-term surviving allografts collected on day 40 post-transplant. Data indicate individual titers within each recipient group and the mean titer for each recipient group ± SEM. (b–d) Histological evaluation of a rejecting kidney allograft from a wild C57BL/6 recipient on day 36 post-transplant stained with: (b) hematoxylin and eosin; (c) anti-C4d antibody; and (d) anti-Mac2 antibody. Arrows indicate marginating leukocytes. Images shown are representative of 2 individual allografts in the group.
Histological evaluation of renal allografts in wild type C57BL/6, B6.CCR5−/− and B6.CD8−/−CCR5−/− recipients during development of antibody-mediated injury
On day 3 all grafts, including isografts, had evidence of ischemia-reperfusion induced injury including proteinaceous casts and peritubular neutrophil infiltration at the cortico-medullary border (Supplemental Figure 1). By day 6 post-transplant, the ischemia-reperfusion induced histopathology attenuated. However, allografts from wild type C57BL/6, B6.CCR5−/− and B6.CD8−/−CCR5−/− recipients contained diffuse interstitial CD3+ infiltrates at day 3 that increased on days 6 and 14 and then slowly decreased with time (Supplemental Figure 1a and data not shown). CD3+ cells infiltrating allografts from wild type C57BL/6 and B6.CCR5−/− recipients were primarily localized in the peritubular capillaries whereas large numbers of CD3+ cells infiltrated tubules in allografts from B6.CD8−/−CCR5−/− recipients. This pattern of staining was also observed when the allograft sections were stained to detect granzyme B expressing cells; granzyme B+ cells in allografts from wild type C57BL/6 and B6.CCR5−/− recipients were primarily localized in the peritubular capillaries on day 6 post-transplant and although the numbers decreased thereafter, these cells infiltrated the tubules by day 14 (Supplemental Figure 1b). In contrast, granzyme B+ cells in allografts from B6.CD8−/−CCR5−/− recipients were primarily present in the tubules on day 6 post-transplant and their presence remained in this location when examined on day 14 post-transplant. Beginning on day 6 scattered FoxP3+ cells were present in all of the allografts, but on day 14 post-transplant were more abundant in allografts in wild type recipients (data not shown).
All of the allografts had moderate diffuse C4d deposits in peritubular capillaries on day 6 (data not shown) that increased to strong diffuse C4d deposits by day 14 (Supplemental Figure 2a). C4d deposits were accompanied by intravascular accumulation of Mac2+ macrophages and small platelet aggregates (Supplemental Figure 2b). The macrophages were more numerous in allografts from the B6.CCR5−/− and B6.CD8−/−/CCR5−/− recipients than in the wild type C57BL/6 recipients. The macrophage infiltrates expanded the interstitial compartment of the allograft and the overall cross-sectional dimensions of the allografts were markedly greater than the isografts. In contrast to allografts, isografts had scattered macrophages and T cells in the interstitium. At later time points post-transplant, interstitial fibrosis and glomerulosclerosis increased in the allografts (data not shown).
Intragraft cytokine and chemokine mRNA expression in renal allografts
Differences in inflammation within the allografts from the different recipient groups were also assessed during the course of antibody-mediated rejection by measuring the levels of mRNA encoding inflammatory mediators on days 3, 6, and 14 post-transplant and at the time of rejection from groups of wild type C57BL/6, B6.CCR5−/− and B6.CD8−/−CCR5−/− recipients and isografts from wild type C57BL/6 recipients.
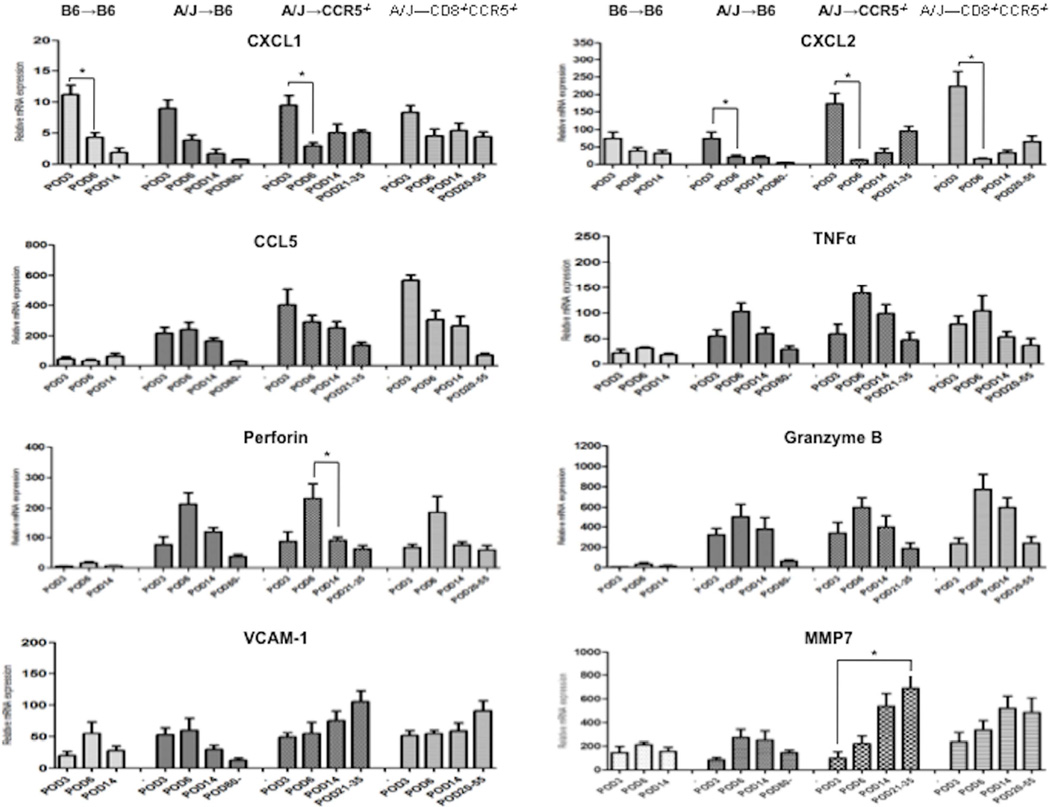
The expression levels of the inflammatory mediators in the allografts fell into three general patterns (Figure 4): 1) the neutrophil chemoattractants CXCL1 and CXCL2 were expressed at peak levels by day 3 post-transplant in the isografts and allografts from all recipients groups and quickly fell, but began to rise again during antibody-mediated rejection of the allografts in the B6.CCR5−/− and B6.CD8−/−CCR5−/− recipients; 2) expression levels of CCL5, TNFα, perforin, and granzyme B were low in isografts in wild type C57BL/6 recipients, but expressed at high levels in allografts from the 3 recipient groups by day 3 post-transplant, rising to peak levels on day 6 post-transplant, and then decreasing with time to renal allograft failure in the CCR5−/− and CD8−/−CCR5−/− recipients; and, 3) expression of VCAM-1 and MMP7 appeared in isografts and allografts at low levels on day 3 post-transplant and decreased with time post-transplant in isografts and allografts in wild type C57BL/6 recipients, but continued to increase to high levels in allografts in B6.CCR5−/− and B6.CD8−/−CCR5−/− recipients to the time of allograft failure.
Figure 4. Expression of inflammatory mediator mRNA in kidney isografts and allografts in wild type C57BL/6, B6.CCR5−/− and B6.CD8−/−CCR5−/− recipients.
Groups of wild type C57BL/6, B6.CCR5−/− and B6.CD8−/−CCR5−/− mice (n = 4) received renal allografts from A/J donors and a group of wild type C57BL/6 mice received isografts (n = 4). Grafts were harvested on days 3, 7, and 14 post-transplant and at the time of the rejection. On the indicated day post-transplant, kidneys were harvested and whole cell RNA was prepared and analyzed by qRT/PCR for expression of the indicated neutrophil chemoattractant CXCL1 and CXCL2, T cell chemoattractant RANTES, and inflammatory mediator genes. Data indicate the mean expression levels for each group on the indicated day ± SEM. All significant differences are indicated by *P<0.05.
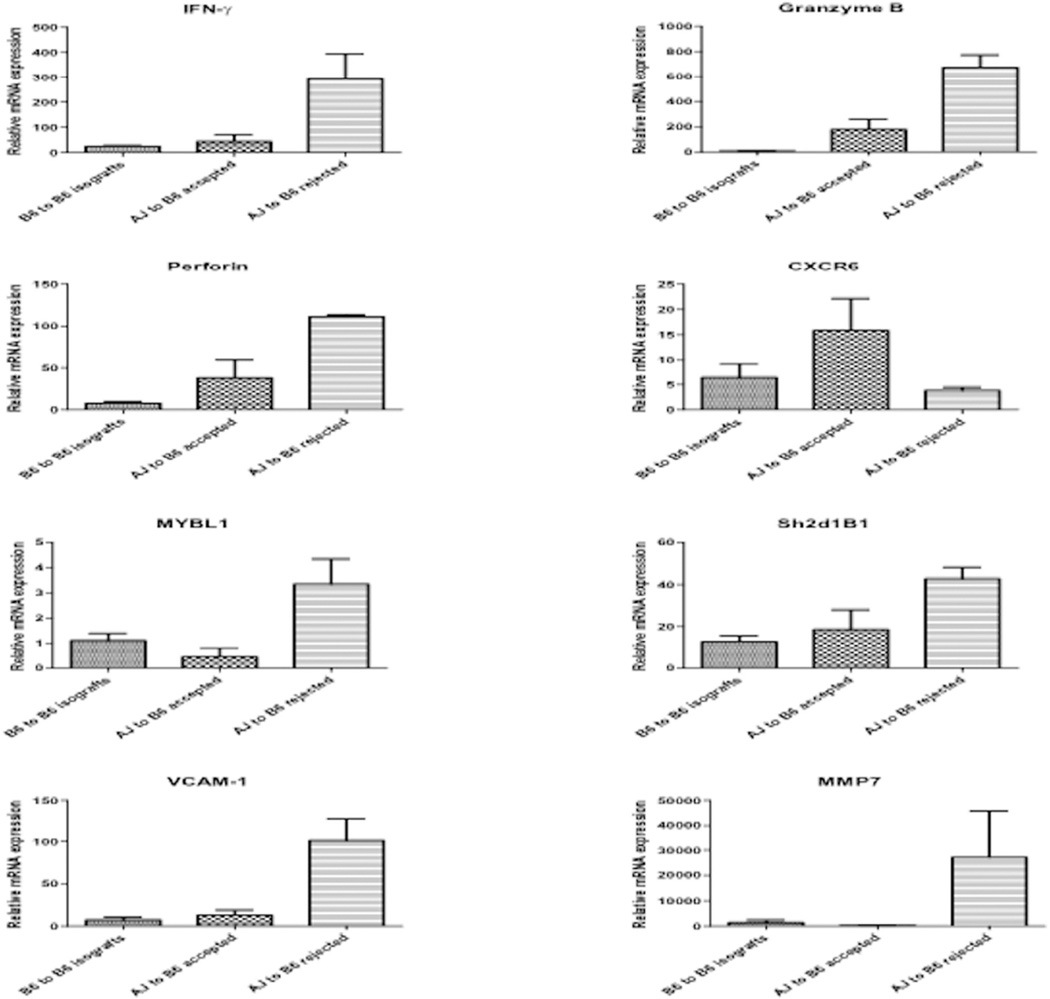
The rejection of 40% of the kidney allografts in wild type recipients with high titers of DSA allowed us to more precisely compare the expression levels of the genes associated with antibody-mediated rejection during allograft rejection vs. long-term survival in wild type recipients. In the rejecting allografts expression of IFN-γ, VCAM-1 and MMP7 mRNA was markedly higher than the expression of these genes in isografts and in the long-term surviving allografts harvested on day 40 from recipients with low DSA titers in wild type recipients (Figure 5). Expression of perforin and granzyme B mRNA was expressed at higher levels in long-term surviving allografts when compared to isografts, but was still 3–4 fold lower than the levels expressed in rejecting allografts. In clinical kidney transplants CXCR6 mRNA is expressed at high levels during acute T cell mediated rejection, but at low levels during antibody-mediated rejection; and, MYBL1 and Sh2d1B1 mRNA are expressed at high levels during antibody-mediated rejection but at low levels during T cell mediated rejection (11). In the long-term surviving kidney allografts in wild type recipients, CXCR6 mRNA expression was 3–4-fold higher than in rejecting allografts (Figure 5). In contrast, MYBL1 and Sh2d1B1 mRNA was expressed at lower levels in the long-term surviving allografts when compared to the rejecting allografts.
Figure 5. Expression of inflammatory mediator mRNA in renal isografts and allografts in wild type C57BL/6 recipients with long-term surviving or rejecting grafts.
Kidney allografts from wild type C57BL/6 recipients were retrieved on day 80 (long-term surviving allografts n = 3) or at the time of rejection (n = 3). C57BL/6 isografts were isolated from the wild type recipients on day 30 post-transplant (n = 4). Whole cell RNA was prepared and analyzed by qRT/PCR for expression of the indicated cytokine and inflammatory mediator genes. Data indicate the mean expression levels for each group ± SEM.
Role of NK cells in early inflammation in renal allografts in CD8−/−CCR5−/− recipients
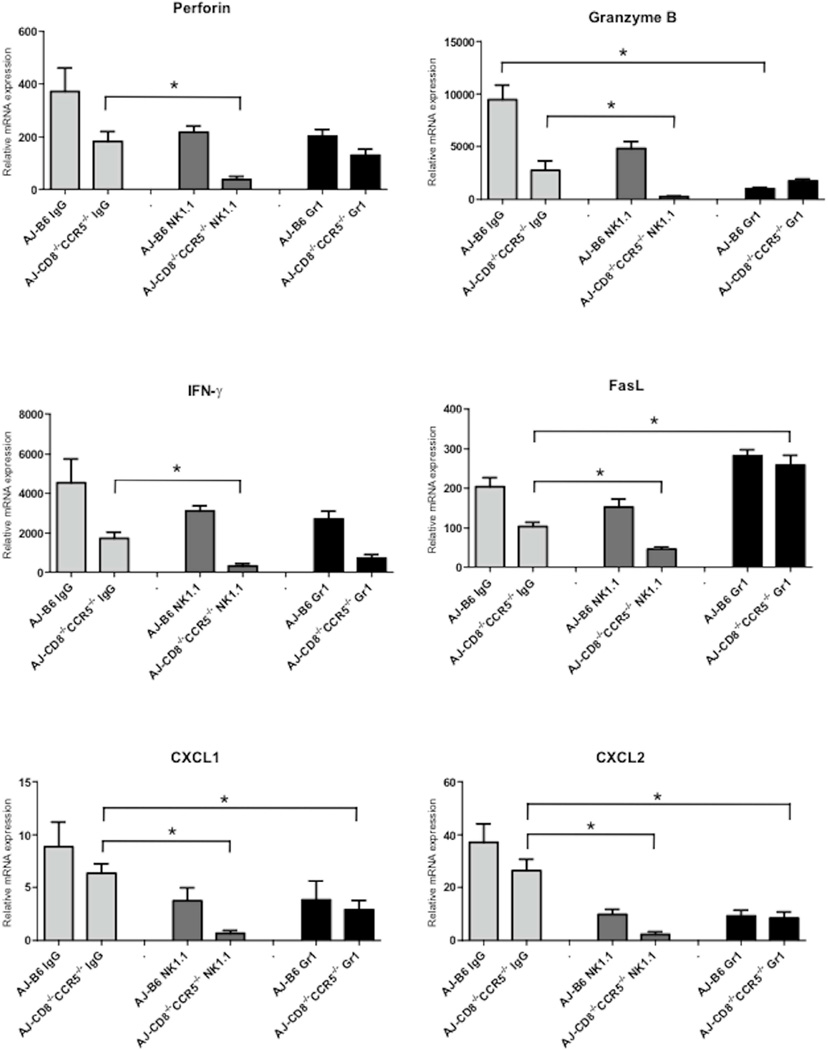
The perforin and granzyme B mRNA expression levels that peaked at day 6 post-transplant in renal allografts in B6.CD8−/−CCR5−/− recipients was somewhat surprising and suggested a non-CD8 T cell mechanism during antibody-mediated rejection. Antibody mediated rejection is associated with neutrophil and macrophage margination in the peritubular capillaries and NK cells have been proposed to play a role in graft injury during antibody-mediated rejection (11, 12, 20, 21). On days 5 and 6 post-transplant, groups of wild type C57BL/6 and CD8−/−CCR5−/− allograft recipients were treated anti-NK1.1 or anti-Gr-1 mAb to deplete NK cells or neutrophils and Gr-1 expressing myeloid cells, respectively, and grafts were harvested on day 7 and analyzed for expression of proinflammatory genes. In CD8−/−CCR5−/− recipients, depletion of NK cells almost completely abrogated IFN-γ, perforin, granzyme B, CXCL1 and CXCL2 expression and markedly decreased FasL expression in the kidney allografts, indicating a role for NK cells in early allograft inflammation at a time point when donor-specific antibody is first detectable in the recipient serum (Figure 6). Treatment of CD8−/−CCR5−/− allograft recipients with anti-Gr-1 mAb also decreased expression of IFN-γ, CXCL1, CXCL2 and granzyme B, but not any of the other tested markers. Treatment of wild type allograft recipients with either anti-NK1.1 or Gr-1 mAb reduced allograft expression of granzyme B, CXCL1 and CXCL2 mRNA but had more modest effects on expression of the other test genes.
Figure 6. Expression of inflammatory mediator mRNA in kidney allografts in B6.CD8−/−CCR5−/− recipients treated with mAb depleting NK or Gr-1+ cells.
Wild type C57BL/6, B6.CCR5−/− and B6.CD8−/−CCR5−/− received renal allografts from A/J donors and a group of wild type C57BL/6 mice received isografts. Groups of 3–5 recipients were treated with 250 µg anti-Gr-1 (RB6.8C5) or anti-NK 1.1 (PK136) mAb to deplete Gr-1+ or NK cells or rat IgG as a control on days 5 and 6 post-transplant. Grafts were harvested on day 7 post-transplant and whole cell RNA was prepared and analyzed by qRT/PCR for expression of the indicated cytokine, chemokine and inflammatory mediator genes. Data indicate the mean expression levels of each test gene in allografts for each group. *P<0.05.
NK cells promote kidney allograft rejection in CD8−/−CCR5−/− recipients
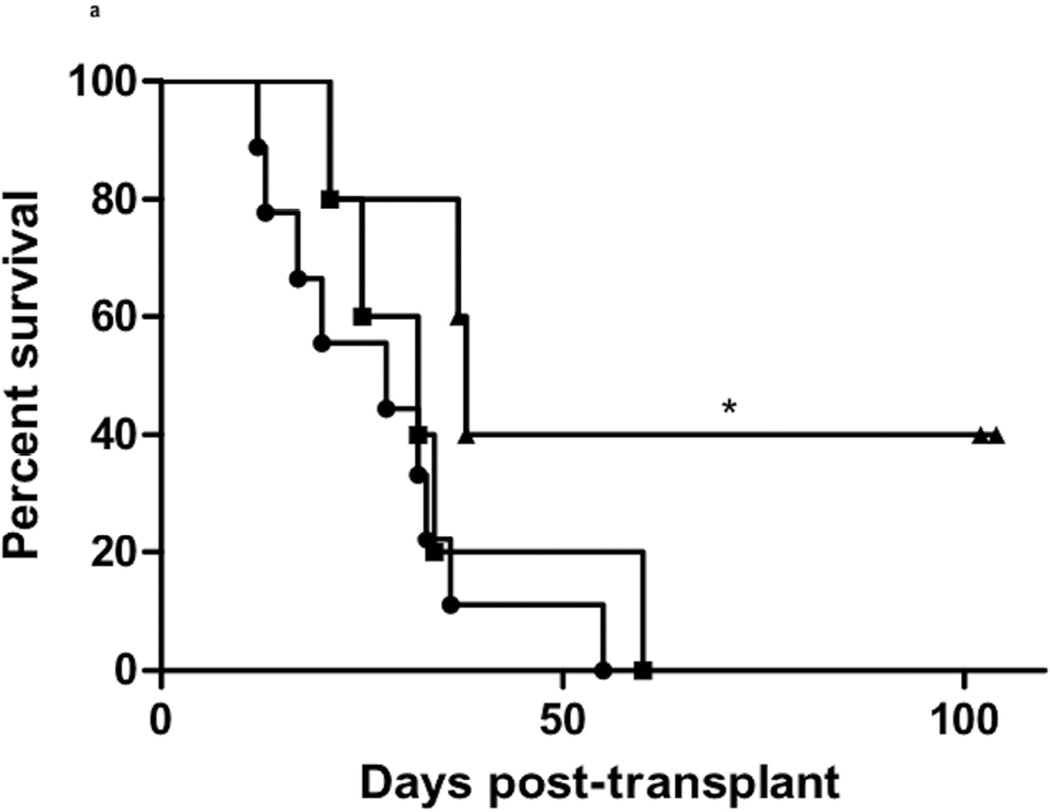
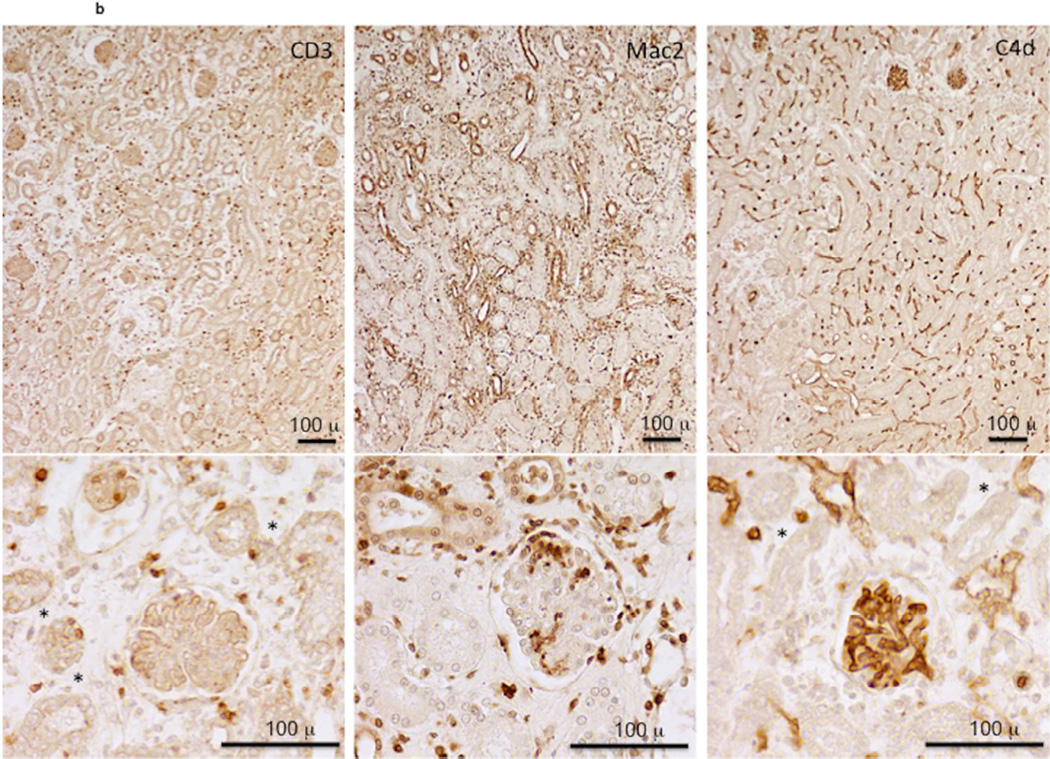
Since the results suggested that NK cell activation increased intra-allograft inflammation at early times post-transplant, the impact of NK cell depletion on kidney allograft survival in B6.CD8−/−CCR5−/− allograft recipients was tested. As an initial test strategy recipients were treated with anti-NK1.1 antibody on days 3, 8 and 13 post-transplant, but this brief course of treatment did not improve survival of the allografts (Figure 7a). However, giving additional weekly doses of the NK cell-depleting antibody resulted in long-term survival of 40% of the kidney allografts beyond day 100. Those allografts that did reject in anti-NK1.1 mAb treated B6.CD8−/−CCR5−/− recipients had substantial edema indicated by tubular separation, infiltrates of CD3+ and Mac2+ cells into the kidney allograft interstitium, and glomeruli with diffuse C4d deposition on the peritubular capillaries and glomeruli (Figure 7b), features similar to those observed during antibody-mediated rejection of allografts in untreated CCR5-deficient recipients.
Figure 7. Depletion of NK cells promotes long-term kidney allograft survival in B6.CD8−/−CCR5−/− recipients.
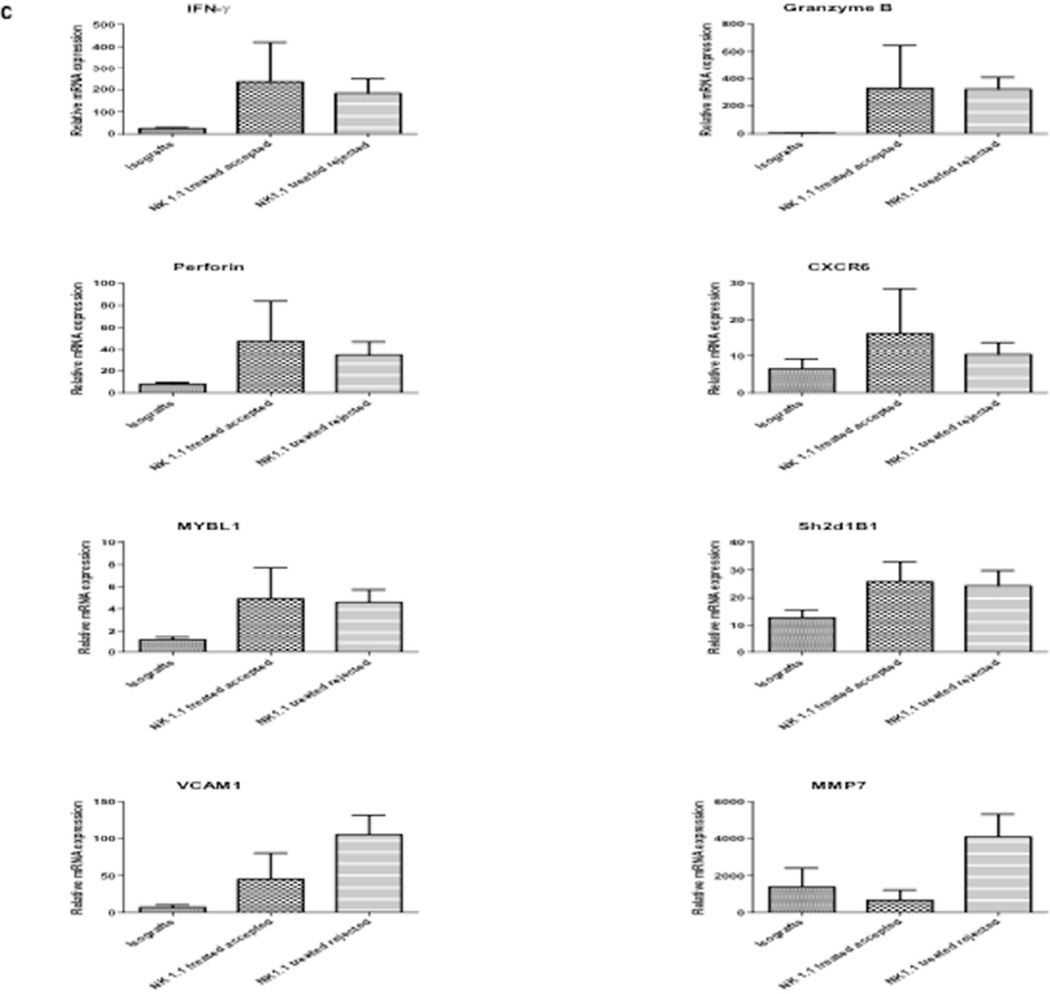
Groups of B6.CD8−/−CCR5−/− mice (n = 5 per group) received renal allografts from A/J donors or isografts and nephrectomy of the remaining native kidney was performed on day 5 post-transplant. The indicated groups of allograft recipients were treated with 200 µg of control rat IgG (-●-) or with anti-NK1.1 mAb either on days 3, 8 and 13 post-transplant (-■-) or on days 3, 8 and 13 post-transplant and then weekly to day 41 post-transplant (-▲-). (a) Allograft survival was followed by daily examination of overall animal health. *P<0.05 when compared to allograft survival in control IgG-treated recipients. (b) Histological evaluation of a rejected kidney allograft on day 16 post-transplant from a B6.CD8−/−CCR5−/− recipient treated with anti-NK1.1 mAb. The graft sections are stained with anti-C3 mAb, anti-Mac2 mAb and C4d antibody as indicated. Images shown are representative of 2 individual allografts that rejected in the group. (c) Grafts were harvested at the time of the rejection or after day 100 post-transplant and whole cell RNA was prepared and analyzed by qRT/PCR for expression of the indicated cytokine, chemokine and inflammatory mediator genes. Data indicate mean expression levels of test genes in 4 individual isografts, 4 individual rejecting allografts and 2 individual accepted allografts harvested on day 100 post-transplant ± SEM.
Finally, the expression levels of the genes associated with antibody-mediated rejection were compared in rejecting vs. long-term surviving kidney allografts in the anti-NK1.1 mAb-treated CD8−/−CCR5−/− allograft recipients. Expression of IFN-γ, perforin and granzyme B mRNA was similar in rejecting and long-term surviving allografts (Figure 7c). There was also no difference in expression of CXCR6, CX3CR1, MYBL1, and Sh2d1B1 between the rejecting and long-term surviving kidney allografts in the NK cell depleted CD8−/−CCR5−/− allograft recipients (Figure 7c and data not shown). In contrast, rejecting allografts expressed high levels of MMP-7 and VCAM-1 mRNA whereas this expression was low/undetectable in long-term surviving allografts.
DISCUSSION
These studies utilized the CCR5−/− mouse model to extend investigation into mechanisms underlying antibody-mediated rejection of kidney allografts. De novo donor-specific antibody responses are rapidly induced in CCR5−/− kidney allograft recipients leading to graft failure with the same histopathologic features observed during antibody-mediated rejection of clinical kidney grafts (14, 15). Results from our previous studies had indicated infiltration with CD8 T cells and high expression of perforin/granzyme B mRNA in rejecting kidney allografts in CCR5−/− recipients (15). The results of the current study indicate that removal of recipient CD8 T cells has little impact on the level of intra-allograft inflammation and rejection and that NK cells play an important role in promoting antibody-mediated kidney allograft inflammation and graft failure in these recipients.
High levels of perforin, granzyme B and IFN-γ mRNA expressing appeared within kidney allografts in wild type and CCR5-deficient recipients 3 days after reperfusion, suggesting effector T cell and/or NK cell activation at this early time. The expression of perforin, granzyme B and IFN-γ are equivalent in wild type, CCR5−/− and CD8−/−CCR5−/− recipients and DSA is not detectable at this time suggesting that ischemia-reperfusion injury and not DSA instigates the early activation of effector T cells or NK cells in the allograft. Correlating with the low titers of serum DSA, C4d deposition appears by day 6 post-transplant in the allograft peritubular capillaries that could augment neutrophil, CD8 T cell and NK cell recruitment and their activation to express functions mediating graft injury. Depletion of recipient NK cells decreases the expression of these genes in kidney allografts in both wild type and CD8−/−CCR5−/− recipients implicating NK cell activation in early allograft tissue inflammation. In addition, recipient treatment with anti-Gr-1 mAb, which depletes neutrophils as well as Gr-1 expressing myeloid and dendritic cells populations, also decreased expression of these genes in kidney allografts in wild type and CD8−/−CCR5−/− recipients. This could indicate that innate Gr-1+ cells increase the inflammation that directs recruitment of effector T cells and/or NK cells to the graft site and would be consistent with studies from this and other laboratories reporting that attenuation of early innate mediated inflammation delays adaptive immune mediated allograft injury (22, 23). Expression of two genes, VCAM-1 and MMP7, were expressed at low levels in isografts and allografts after reperfusion, but in allografts that went on to reject VCAM-1 and MMP7 expression began to increase at the time DSA was detectable in the serum and was at high levels at the time of graft failure. The activities of VCAM-1 and MMP7 are critical components of tissue remodeling (24–26), and their expression in rejecting allografts may indicate antibody-induced initiation of this process.
There has been intense effort in identifying gene signatures that distinguish acute T cell mediated and antibody-mediated rejection of kidney grafts. Recent studies from the Halloran group have demonstrated that during early rejection episodes expression of genes associated with T cell activities are observed in kidney grafts, but later injury of kidney grafts raises a distinct set of genes associated with antibody-mediated rejection (27–29). Whereas T cell mediated rejection is indicated by kidney graft expression of TcRα, CXCR6, GPR171 and NELL2, ongoing antibody-mediated rejection is indicated by expression of NK cell associated genes, CX3CR1, MYBL1, and Sh2D1B (11). When expression of these T cell vs. NK cell gene signatures was tested in accepted vs. rejecting kidney allografts in wild type recipients or in CD8−/−CCR5−/− recipients treated with NK cell depleting antibody, CXCR6 expression was decreased in rejecting grafts but CX3CR1 expression did not correlate with acceptance or rejection in either recipient group. Furthermore, high expression of MYBL1 and Sh2d1B1 was observed in rejecting allografts in wild type recipients that had high DSA titers and expression was low in the accepted grafts. However, there was no difference in this expression in accepted vs. rejecting allografts in CD8−/−CCR5−/− recipients treated with NK cell depleting antibody where the distinction in expression of these genes would be expected.
Treatment with NK cell depleting antibody did promote the long-term survival of almost half of the kidney allografts in CD8−/−CCR5−/− recipients and the other 60% of the allografts had a delay in time of rejection when compared to control treated CD8−/−CCR5−/− allograft recipients. Together these results support a role for NK cells in contributing to kidney graft failure during antibody-mediated rejection. In addition to the expression of NK cell transcripts in kidney grafts during antibody-mediated rejection, results from a recent animal model have implicated NK cell activity in promoting the chronic injury leading to transplant associated vasculopathy in cardiac allografts in RAG-1−/− recipients infused with DSA (21).
Consistent with our previous reports (14, 15), the DSA in CCR5−/− recipients of complete MHC-mismatched kidney allografts is first detectable at low levels on day 5–7 post-transplant and then increases quickly to peak titers about day 21 post-transplant. The titers of DSA of all IgG isotypes increase more rapidly in CD8−/−CCR5−/− recipients with peak titers observed by day 17 post-transplant. These differences in the kinetics of DSA IgG production suggest that the activation of donor-reactive CD8 T cells restricts the initiation and magnitude of the ongoing DSA response in CCR5-deficient recipients. Such a regulatory effect could be mediated indirectly by competition for space or cytokines in secondary lymphoid organs during the ongoing antibody response. Alternatively, the CD8 T cells could directly restrict the magnitude of the antibody response at early times post-transplant by interfering with the activities of helper T cells or B cells or eliminating these cell populations, possibly through cytotoxic mechanisms. Many studies have demonstrated the CD8 T cell mediated regulation of antibody responses to various antigens, including to allogeneic hepatocytes transplanted within the recipient spleen through cytolytic elimination of recipient B cells (30–33).
Studies from this and several other laboratories have indicated the long-term survival of complete MHC mismatched kidney allografts in wild type recipients (14, 34, 35). One factor underlying this enhanced survival is the rapid infiltration of CD4+FoxP3+ Tregs into the kidney allograft as depletion of Tregs is followed by rapid graft rejection (36). We also observed FoxP3+ cell infiltration into kidney allografts in wild type C57BL/6 recipients as well as in B6.CCR5−/− and B6.CD8−/−CCR5−/− recipients when examined on day 6 post-transplant. Whereas this allograft infiltration was sustained in wild type recipients on day 14, it was markedly decreased in the CCR5−/− and CD8−/−CCR5−/− recipients (data not shown). Consistent with previous studies, 40% of the kidney allografts were rejected by wild type recipients in the current report. We observed low serum DSA titers in wild type recipients with long term surviving kidney allografts whereas high DSA titers were observed in wild type recipients with rejecting allografts and these were close to those observed in B6.CCR5−/− and CD8−/−CCR5−/− recipients at the time of allograft rejection. Furthermore, rejecting kidney allografts in wild type recipients expressed high levels of gene transcripts associated with antibody-mediated rejection in the B6.CCR5−/− and CD8−/−CCR5−/− recipient groups, including VCAM-1 and MMP7, supporting the proposal that DSA activates the expression of these genes as part of the tissue remodeling process. Importantly, these results suggest that in unsensitized wild type mouse recipients DSA is an important factor promoting kidney allograft rejection. Mechanisms that dictate the production of high DSA vs. low DSA titers in wild type recipients remain unknown at this time, but certainly warrant further investigation.
Overall, the results of this study have shown the important role of DSA in mediating injury in kidney allografts leading to graft failure and key transcripts that are consistently expressed during the development of antibody-mediated allograft injury and expressed at high levels at the time of graft failure. NK cells contribute to this rejection and the CCR5−/− kidney allograft model of antibody-mediated rejection should be useful in identifying mechanisms of NK cell mediated graft injury during antibody-mediated rejection.
MATERIALS AND METHODS
Mice
C57BL/6 (H-2b) mice and A/J (H-2a) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). B6.CCR5−/− mice were maintained in the Biological Resources Unit of the Cleveland Clinic. B6.CCR5−/− and B6.CD8α−/− mice were crossed to generate mice with homozygous disruptions in both genes, B6.CD8−/−CCR5−/− mice. All experiments used 8–12 week old male mice and all procedures involving animals were approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic.
Murine kidney transplantation
The left kidney from C57BL/6 or A/J mice was transplanted into C57BL/6, B6.CCR5−/− or B6.CD8−/−CCR5−/− mice with anastomosis to the recipient abdominal aorta and inferior vena cava and ureter anastomosis to the bladder as previously described (37, 38). On day 5 post-transplant the remaining native left kidney was removed so that recipient survival was dependent on kidney graft function. The operative success rate of these transplants was > 85% as determined by recipient survival beyond 7 days post-transplantation with no visual evidence of graft necrosis and hydronephrosis at the time of native nephrectomy. Allograft recipients were treated with anti-Gr-1 (RB6.8C5) or anti-NK 1.1 (PK136) mAb (both from BioXCell, Lebanon, NH) to deplete Gr-1+ or NK cells by i.p. injections of 250 µg aliquots of the mAb, or rat IgG as a control.
Histologic examination of renal tissue
Renal allograft tissues containing full cross-sections were immediately fixed in acid methanol (60% methanol and 10% acetic acid. Paraffin-embedded sections (5µm) were subjected to high-temperature antigen retrieval and paraffin removal in Trilogy (Cell Marque, Hot Springs, AR) in a pressure cooker. Endogenous peroxidase activity was blocked by incubation with 0.3% H2O2, and nonspecific protein interactions were blocked by incubation with a serum free protein block (DAKO, Carpinteria, CA). Slides were incubated with one of the following primary antibodies: monoclonal rat antibody to mouse Mac2, a marker of inflammatory macrophages (Cedarlane Laboratories, Burlington, NC), rat anti-human/mouse CD3 mAb (AbD Serotec, Raleigh, NC) or rabbit polyclonal antiserum to mouse C4d for 60 minutes at room temperature. Staining antibodies were visualized using rat or goat on mouse HRP-Polymer Kits (Biocare Medical, Concord, CA) followed by diaminobenzidine and counterstained with hematoxylin. Images were captured with a 12-megapixel DS-Ri1 Digital Camera using NIS-Elements software (Nikon, Melville, NY).
Determination of DSA titers
The presence and titers of DSA were assessed as previously reported (14, 39, 40). Briefly, aliquots of kidney donor A/J thymocyte suspensions were incubated with serial dilutions of recipient sera taken on days 3, 6, and 14 and at the time of allograft rejection. After washing, the cells were resuspended in staining buffer (Dulbecco’s PBS with 2% FCS/0.02% NaN3) containing FITC-conjugated rat anti-mouse IgG1, IgG2a, IgG2b or IgG3 mAb (Pharmingen, San Diego, CA) for 30 min on ice. The cells were then washed, fixed and analyzed by flow cytometry. The mean channel fluorescence (MCF) of each dilution of each serum sample was determined and the dilution that returned the MCF to the level observed when A/J thymocytes were stained with a 1:4 dilution of normal wild-type serum was divided by two and reported as the titer. Antibody titers are expressed as mean ± SEM and differences were determined using Mann–Whitney U-test and analyzed using GraphPad prism (GraphPad Software, San Diego, CA).
RNA extraction and quantitative analysis of chemokines
Total RNA was extracted from kidney grafts using the RNeasy TM Mini Kit (QIAGEN, Valencia, CA) and reverse transcribed using the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Reverse transcription and Real-Time PCR were performed using commercially available reagents and probes on a 7500 Fast Real-Time Thermocycler, all from Applied Biosystems. For quantification of mRNA expression, target gene expression was normalized to Mrpl32 gene expression. Individual graft samples were plated in duplicate and the data for each group are expressed as mean test cytokine expression level ± SEM.
Statistical analysis
All data were analyzed using GraphPad Prism Pro (GraphPad Software, Inc., San Diego, CA). Analysis of renal allograft survival was plotted using Kaplan-Meier cumulative survival curves and differences in survival between groups determined using the Log-rank (Mantel-Cox) test. For other experiments statistical analyses were performed using the Mann–Whitney nonparametric test to analyze differences between experimental groups. P <0.05 was considered significant. Error bars reflect SEM for each group.
Supplementary Material
Acknowledgments
This work was supported by grant NIH PO1 AI087506 from the NIAID/NIH to AV, WMB, and RLF
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
All of the authors declare that they have no competing interests with the reported work.
References
- 1.Djamali A, Kaufman DB, Ellis TM, et al. Diagnosis and management of antibody-mediated rejection: current status and novel approaches. Am J Transplant. 2014;14:255–271. doi: 10.1111/ajt.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Einecke G, Sis B, Reeve J, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009;9:2520–2531. doi: 10.1111/j.1600-6143.2009.02799.x. [DOI] [PubMed] [Google Scholar]
- 3.Gosset C, Lefaucheur C, Glotz D. New insights in antibody-mediated rejection. Curr Opin Nephrol Hypertens. 2014;23:597–604. doi: 10.1097/MNH.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 4.Sellares J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:389–399. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]
- 5.Lefaucheur C, Loupy A, Vernerey D, et al. Antibody-mediated vascular rejection of kidney allografts: a population-based study. Lancet. 2013;381:313–319. doi: 10.1016/S0140-6736(12)61265-3. [DOI] [PubMed] [Google Scholar]
- 6.Halloran PF, Wadgymer A, Ritchie S, et al. The significance of the anti-class I antibody response. I. Clinical and pathologic features of anti-class I mediated rejection. Transplantation. 1990;49:85–91. doi: 10.1097/00007890-199001000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Trpkov K, Campbell P, Pazderka F, et al. Pathologic features of acute renal allograft rejection associated with donor-specific antibody. Analysis using the Banff grading schema. Transplantation. 1996;61:1586–1592. doi: 10.1097/00007890-199606150-00007. [DOI] [PubMed] [Google Scholar]
- 8.Nickeleit V, Andreoni K. The classification and treatment of antibody-mediated renal allograft injury: where do we stand? Kidney Int. 2007;71:7–11. doi: 10.1038/sj.ki.5002003. [DOI] [PubMed] [Google Scholar]
- 9.Randhawa P. T-cell-mediated rejection of the kidney in the era of donor-specific antibodies: diagnostic challenges and clinical significance. Curr Opin Organ Transplant. 2015;20:325–332. doi: 10.1097/MOT.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 10.Sun Q, Liu L-H, Cheng Z, et al. Treatment of early mixed cellular and humoral renal allograft rejection with tacrolimus and mycophenolate mofetil. Kidney Int. 2007;71:24–30. doi: 10.1038/sj.ki.5001870. [DOI] [PubMed] [Google Scholar]
- 11.Hidalgo LG, Sellares J, Sis B, et al. Interpreting NK cell transcripts versus T cell transcripts in renal transplant biopsies. Am J Transplant. 2012;12:1180–1191. doi: 10.1111/j.1600-6143.2011.03970.x. [DOI] [PubMed] [Google Scholar]
- 12.Hidalgo LG, Sis B, Sellares J, et al. NK cell transcripts and NK cells in kidney biopsies from patients with donor-specific antibodies: evidence for NK cell involvment in antibody-mediated rejection. Am J Transplant. 2010;10:1812–1822. doi: 10.1111/j.1600-6143.2010.03201.x. [DOI] [PubMed] [Google Scholar]
- 13.Venner JM, Hidalgo LG, Famulski K, et al. The molecular landscape of antibody-mediated kidney transplant rejection: evidence for NK involvement through CD16a Fc receptors. Am J Transplant. 2015;15:1336–1348. doi: 10.1111/ajt.13115. [DOI] [PubMed] [Google Scholar]
- 14.Bickerstaff A, Nozaki T, Wang J-J, et al. Acute humoral rejection of renal allografts in CCR5−/− recipients. Am J Transplant. 2008;8:557–566. doi: 10.1111/j.1600-6143.2007.02125.x. [DOI] [PubMed] [Google Scholar]
- 15.Abe T, Ishii D, Gorbacheva V, et al. Anti-huCD20 antibody therapy for antibody-mediated rejection of renal allografts in a mouse model. Am J Transplant. 2015;15:1192–1204. doi: 10.1111/ajt.13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishii D, Rosenblum JM, Nozaki T, et al. Novel CD8 T cell alloreactiviites in CCR5-deficient recipients of class II MHC disparate kidney grafts. J Immunol. 2014;193:3816–3824. doi: 10.4049/jimmunol.1303256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria-an addition to the Banff '97 classification of renal allograft rejection. Am J Transplant. 2003;3:708–714. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 18.Colvin RB. Antibody-mediated renal allograft rejection: diagnosis and pathogenesis. J Am Soc Nephrol. 2007;18:1046–1056. doi: 10.1681/ASN.2007010073. [DOI] [PubMed] [Google Scholar]
- 19.Lefaucheur C, Nochy D, Hill GS, et al. Determinants of poor graft outcome in patients with antibody-mediated acute rejection . Am J Transplant. 2007;7:832–841. doi: 10.1111/j.1600-6143.2006.01686.x. [DOI] [PubMed] [Google Scholar]
- 20.Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat Rev Immunol. 2005;5:807–817. doi: 10.1038/nri1702. [DOI] [PubMed] [Google Scholar]
- 21.Hirohashi T, Chase CM, Della Pelle P, et al. A novel pathway of chronic allograft rejection mediaed by NK cells and alloantibody. Am J Transplant. 2012;12:313–321. doi: 10.1111/j.1600-6143.2011.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Sawy T, Belperio JA, Strieter RM, et al. Inhibition of polymorphonuclear leukocyte-mediated graft damage synergizes with short-term costimulatory blockade to prevent cardiac allograft rejection. Circulation. 2005;112:320–331. doi: 10.1161/CIRCULATIONAHA.104.516708. [DOI] [PubMed] [Google Scholar]
- 23.Kreisel D, Sugimoto S, Zhu J, et al. Emergency granulopoiesis promotes neutrophil-dendriitc cell encounters that prevent mouse lung allograft acceptance. Blood. 2011;118:6172–6182. doi: 10.1182/blood-2011-04-347823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rohani MG, Parks WC. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015;44–46:113–121. doi: 10.1016/j.matbio.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol. 2008;40:1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnoor M. Endothelial actin-binding proteins and actin dynamics in leukocyte transendothelial migration. J Immunol. 2015;194:3535–3541. doi: 10.4049/jimmunol.1403250. [DOI] [PubMed] [Google Scholar]
- 27.Halloran PF, Chang J, Famulski K, et al. Disappearance of T cell-mediated rejection despite continued antibody-mediated rejection in late kidney transplant recipients. J Am Soc Nephrol. 2014;26:1711–1720. doi: 10.1681/ASN.2014060588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halloran PF, Reeve JP, Pereira AB, et al. Antibody-mediated rejection, T cell-mediated rejection, and the injury-repair response: new insights from the Genome Canada studies of kidney transplant biopsies. Kidney Int. 2013;85:258–264. doi: 10.1038/ki.2013.300. [DOI] [PubMed] [Google Scholar]
- 29.Sellares J, Reeve J, Loupy A, et al. Molecular diagnosis of antibody-mediated rejection in human kidney transplants. Am J Transplant. 2013;13:971–983. doi: 10.1111/ajt.12150. [DOI] [PubMed] [Google Scholar]
- 30.Coutelier JP. Enhancement of IgG production elicited in mice by treatment with anti-CD8 antibody. Eur J Immunol. 1991;21:2617–2620. doi: 10.1002/eji.1830211046. [DOI] [PubMed] [Google Scholar]
- 31.Sayeh E, Sterling K, Speck E, et al. IgG antiplatelet immunity is dependent on an early innate natural killer cell-derived interferon-γ response that is regulated by CD8+ T cells. Blood. 2004;103:2705–2709. doi: 10.1182/blood-2003-10-3552. [DOI] [PubMed] [Google Scholar]
- 32.Zimmerer JM, Pham TA, Sanders VM, Bumgardner GL. CD8+ T cells negatively regulate IL-4-dependent, IgG1-dominant posttransplant alloantibody production. J Immunol. 2010;185:7285–7292. doi: 10.4049/jimmunol.1001655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmerer JM, Pham TA, Wright CL, et al. Alloprimed CD8+ T cells regulate alloantibody and eliminate alloprimed B cells through perforin- and FasL-dependent mechanisms. Am J Transplant. 2014;14:295–2304. doi: 10.1111/ajt.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell PS, Chase CM, Colvin RB, Plate JMD. Kidney transplants in mice. An analysis of the immune status of mice bearing long-term, H-2 incompatible transplants. J Exp Med. 1978;147:1449–1468. doi: 10.1084/jem.147.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bickerstaff AA, Wang JJ, Pelletier RP, Orosz CG. Murine renal allografts: spontaneous acceptance is associated with regulated T cell-mediated immunity. J Immunol. 2001;167:4821–4827. doi: 10.4049/jimmunol.167.9.4821. [DOI] [PubMed] [Google Scholar]
- 36.Miyajima M, Chase CM, Alessandrini A, et al. Early acceptance of renal allografts in mice is dependent on FoxP3+ cells. Am J Pathol. 2011;178:1635–1645. doi: 10.1016/j.ajpath.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z, Schlachta C, Duff J, et al. Improved techniques for kidney transplantation in mice. Microsurgery. 1995;16:103–109. doi: 10.1002/micr.1920160212. [DOI] [PubMed] [Google Scholar]
- 38.Rong S, Lewis AG, Kunter U, et al. A knotless technique for kidney transplantation in the mouse. J Transplant. 2012;2012:127215. doi: 10.1155/2012/127215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hattori Y, Bucy RP, Kubota Y, et al. Antibody-mediated rejection of single class I MHC-disparate cardiac allografts. Am J Transplant. 2012;12:2017–2028. doi: 10.1111/j.1600-6143.2012.04073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nozaki T, Amano H, Bickerstaff A, et al. Antibody-mediated rejection of cardiac allografts in CCR5-deficient recipients. J Immunol. 2007;179:5238–5245. doi: 10.4049/jimmunol.179.8.5238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.