Abstract
Illicit drug users are a high risk population for infection with the Human Immunodeficiency Virus (HIV). A strong correlation exists between prohibited drugs use and an increase rate of HIV transmission. Cocaine stands out as one of the most frequently abused illicit drugs and its use is correlated with HIV infection and disease progression. The central nervous system (CNS) is a common target for both drugs of abuse and HIV, and cocaine intake further accelerates neuronal injury in HIV patients. Although the high incidence of HIV infection in illicit drug abusers is primarily due to high risk activities such as needle sharing and unprotected sex, several studies have demonstrated that cocaine enhances the rate of HIV gene expression and replication by activating various signal transduction pathways and downstream transcription factors. In order to generate mature HIV genomic transcript, HIV gene expression has to pass through both the initiation and elongation phases of transcription, which requires discrete transcription factors. In this review, we will provide a detailed analysis of the molecular mechanisms that regulate HIV transcription and discuss how cocaine modulates those mechanisms to upregulate HIV transcription and eventually HIV replication.
Keywords: HIV, Transcription, Replication, Latency, Epigenetic, Cocaine
HIV infection and neurological disorders
According to a recent estimate, approximately 40 million people worldwide are infected with the human immunodeficiency virus (HIV) [1, 2]. Anti-HIV therapy, commonly known as either highly active antiretroviral therapy (HAART) or combined antiretroviral therapy (cART), has been proven to be extremely successful in controlling viral replication and prolonging the life span of HIV infected individuals. Unfortunately, HAART is not able to fully restore the health of HIV patients, even in those with a sustained undetectable viral load. Nearly one third of all HIV infected patients develop neuropathies, collectively known as HIV-associated neurocognitive disorders (HAND) [3, 4]. Neurocognitive impairment, even in milder forms, impacts on general health, adds financial burdens and leads to a deterioration in quality of life [5, 6]. Numerous mechanisms have been proposed to explain these neurocognitive disorders, including neurotoxicity and glial cell activation by viral proteins such as Gp120 and Tat, immune activation, oxidative stress, BBB deterioration, immune cell infiltration, and increased proinflammatory cytokines [7–10]. The suboptimal penetration of HAART in the brain permits sustained low levels of viral replication and brain adaptive HIV evolution, which eventually contributes to the occasional systemic viral blips [11–14]. Moreover, recently we have proposed that owing to the lack of any transcriptional inhibitor, HAART regimens are unable to restrict HIV transcription [15]. Consequently, viral proteins are produced unchecked even in the presence of effective HAART [16]. The immune response against HIV virions and its proteins further contributes to the persistent immune activation and the deterioration of the central nervous system (CNS) in HIV patients [6, 17–26].
CNS is the common target for cocaine and HIV
Injection and non-injection illicit drug use and abuse contribute significantly to HIV infection and transmission [2, 4, 25]. In the United States, HIV infection in drug addicts accounts for one-third of new cases of HIV [2, 27]. Cocaine, one of the most frequent drugs of abuse has been implicated as a major contributing factor for HIV infection, transmission and AIDS progression [28–30]. Both cocaine and HIV target the CNS. Besides promoting HIV replication, cocaine primes the cells to become more susceptible for HIV infection [31–33]. Within a few days of infection, HIV establishes a reservoir in the CNS by infecting different types of brain cells including resident microglial cells and astrocytes, along with visiting macrophages, and lymphocytes [31, 32, 34–37]. Due to suboptimal presence of HAART in brain, it has been proposed that residual viral replication and the presence of viral proteins (e.g. Tat and gp120); the CNS impair the functioning of different kinds of brain cells, including neurons, which results in the overall deterioration of the immune and nervous system [6, 17–26, 38]. Cocaine further accelerates this deleterious process by promoting HIV replication and enhancing HIV gene expression. Thus, cocaine intake significantly augments HIV-associated neurotoxicity (neuro-AIDS) in HIV patients, which is clinically recognized as HAND [4, 6, 39–41]. Despite the success of HAART in controlling circulating HIV, HAND remains a significant co-morbidity responsible for deterioration in quality of life and enhanced mortality of HIV-infected patients [4, 6, 42]. Poor adherence to HAART treatment by cocaine users further contributes to HIV disease progression [43–45]. It is noteworthy that cocaine using HIV patients, despite adherence to HAART, frequently have higher viral loads and exhibit faster viral rebound following HAART interruption, further indicating that besides promoting HIV replication, cocaine enhances the susceptibility of cells to HIV [46–48]. Thus, illicit drug using HIV patients are more prone to the HIV associated comorbidities. Furthermore, the direct correlation of cocaine use with enhanced HIV replications has been well documented by several investigations using HIV infected humanized mouse models [32, 33, 49]. Hence, drug addiction extraordinarily contributes to the health and financial burden on HIV patients and the society as a whole [5, 6].
HIV transcription
HIV replication primarily relies on efficient transcription to generate full genomic HIV transcripts. HIV performs its transcription mainly by using the host cell transcription machinery, with the help of its own master transactivator protein, transactivator of transcription (Tat). In order to generate complete HIV genomic transcripts, transcription requires successful progression through both the initiation and elongation phases (Figure 1). In a recent publication, we have revealed some of the underlying molecular mechanisms that cocaine utilizes to promote both the initiation and elongation phases of HIV transcription in order to enhance the overall rate of HIV gene expression ( [15] Figure 2).
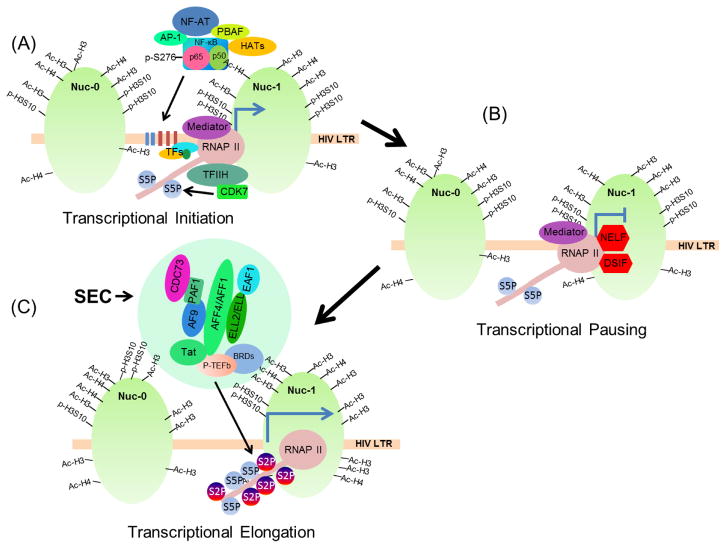
Figure 1. Different phases of HIV transcription.
A), The binding of NF-κB and NF-AT to the enhancer region of HIV LTR strongly upregulates the initiation phase of HIV transcription. The histone acetyl transferases (HATs) recruited by these factors, catalyze the acetylation of core histones and establish the transcriptionally active chromatin (euchromatin) structures. The establishment of euchromatin structures at and around LTR promoter promotes efficient access of LTR to transcription machinery. Transcriptional initiation is characterized by the phosphorylation of serine residues at position 5 (S5) in the heptapeptide (YSPTSPS)52 repeats of the C-terminal domain (CTD) of RNAPII by TFIIH/Cdk7. B), HIV transcription halts after passing through the TAR elements due to the binding of negative transcriptional elongation factors, mainly NELF and DSIF. C), Tat protein of HIV brings the super elongation complex (SEC), containing P-TEFb at HIV LTR along with it. The CDK9 subunit of P-TEFb subsequently catalyzes the phosphorylation of negative elongation factors DSIF and NELF, which leads to either their dissociation from LTR or reverse their function from negative to positive elongation factor. CDK9 also catalyzes the phosphorylation of CTD of RNAPII, primarily at serine 2 residues. This event makes RNAPII highly processive or transcription elongation proficient that result in the generation of complete and properly processed HIV transcripts.
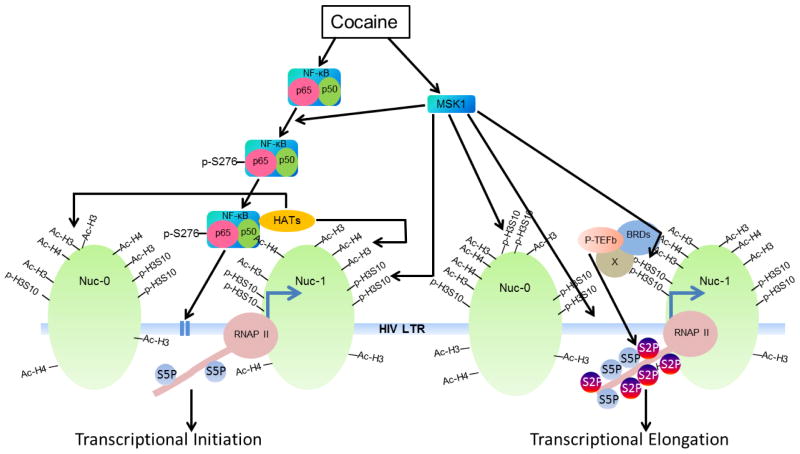
Figure 2. Schematic diagram showing cocaine accelerates HIV transcription by promoting both initiation and elongation phases of HIV transcription.
Cocaine treatment results in the activation and recruitment of NF-κB and MSK1 at HIV LTR. MSK1 subsequently catalyze the phosphorylation of NF-κB at its serine residue 276. Phosphorylation of NF-κB at 276th serine residue enhances the functional capability of NF-κB by augmenting the interaction of NF-κB with HATs. HATs such as p300 in turn catalyze the acetylation of core histones (mainly H3 and H4) at HIV LTR and facilitate the establishment of transcriptionally active chromatin structures, which boost the access of transcription machinery to HIV LTR and thus facilitate the initiation phase of HIV transcription. MSK1 in addition to phosphorylating NF-κB catalyzes the phosphorylation of histone H3 at serine 10 (H3S10). Phosphorylated histone H3 at 10th residue contributes to the establishment of transcriptionally active chromatin structure at HIV LTR. Moreover, phosphorylated H3S10 also facilitates the recruitment of P-TEFb at HIV LTR through a mechanism that is not yet fully defined; usually with the help of BRD proteins which bind specifically to acetylated histones through their bromo domain. P-TEFb, an established elongation factor, subsequently catalyzes the phosphorylation of several proteins, including RNA Polymerase II (RNAP II) and negative elongation factors. These modifications enhance the processivity of RNAP II and nullify the impact of negative elongation factors, and thus promote the elongation phase of HIV transcription.
HIV transcriptional initiation
The initiation phase of HIV transcription involves the binding of transcription factors such as SP1, TATA-box-binding protein (TBP), and TBP-associated factors (TAFs) which are recruited to the core long terminal repeats (LTR) promoter, an essential component sufficient to sustain basal transcription. HIV LTR core promoter consists of TATA box, initiator sequence and three SP1 binding sites [50–54]. However, efficient initiation of HIV transcription occurs only after the binding of transcriptional activators, primarily NF-kB, NF-AT, AP-1 and STAT5 to the enhancer sequences of HIV LTR [55–58]. These factors, after binding to HIV LTR, promote efficient HIV transcription mainly by recruiting histone acetyl transferases (HATs) and through cooperation with SP1 proteins [59–63] (Figure 1).
Cocaine and HIV transcriptional initiation
The role of cocaine in promoting HIV replication is well established. However very little is known about the involved molecular mechanisms that cocaine utilizes to enhance HIV gene expression. Recently, we have investigated some of the molecular mechanisms that are modulated by cocaine in order to enhance HIV transcription [15].
NF-κB plays a critical role during HIV transcription
The NF-κB/Rel proteins are transcription factors that induce expression of several cellular genes. Many of these genes regulate host immunity and inflammatory responses [64, 65]. Transcription factor NF-κB consists of either homo- or heterodimers of five related proteins, p65 (Rel A), Rel B, c-Rel, p50/p105 (NF-κB) and p52/p100 (NF-κB2) [66]. Of these, one of the best-characterized and functionally most active NF-κB protein is the heterodimer which comprises of Rel A (p65) and p50, which is widely expressed and heavily involved in NF-κB -regulated transactivation [6, 67]. In its inactive state, the NF-κB complexes are sequestered in the cytoplasm through their interaction with inhibitory IκB proteins [66, 68]. However, upon cell activation through a wide array of stimuli serine residues 32 and 36 of IκB become phosphorylated by several kinases, including IκB kinase (IKK) complex, which is composed of IKKα, IKKβ and IKKγ [66, 69–71] and Ribosomal S6 kinase1 (RSK1). This event induces the ubiquitination of IκB at lysine residues 21 and 22 and leads to the 26S proteasome degradation. Due to the dissociation of IκB, the nuclear localization signal (NLS) of NF-κB proteins become exposed and consequently NF-κB translocates into the nucleus [69, 72]. Once in the nucleus, NF-κB binds to the cognate binding sites at the promoter and enhancer regions of the genes and activates their transcription including the HIV LTR enhancer region (Figure 1). The HIV enhancer region is one of the best studied elements which usually contains two functional NF-kB binding motifs [55, 59].
Cocaine accelerates HIV transcriptional initiation by both activating and enhancing the functional activity of NF-kB
Several groups including ours have demonstrated that cocaine promotes both the activation of NF-κB and HIV replication [15, 73–76]. To extend those studies further, using monocytic cell lines (THP1 and U937) and primary monocytes derived macrophages (MDMs) [15], we found that during both acute (one time) and chronic (multiple time for 3 days) treatment, cocaine efficiently activates NF-κB. Interestingly, cocaine not only promotes the activation (nuclear translocation) of NF-κB, but also enhances the functional activity of NF-κB by augmenting the ability of NF-κB to interact with histone acetyltransferases (HATs) (Detailed below and Figure 2). Cocaine induced NF-κB activation is primarily mediated via RSK1 activation, instead of IKKβ, a kinase specifically activated by TNF-α, which is one of the strongest and most specific activator of NF-κB [15, 64, 77]. RSK1 is a downstream kinase of the extracellular signal-regulated kinase (ERK) pathway, one of the main pathways through which cocaine exerts many of its effects [78–80]. Cocaine activated RSK1 primarily phosphorylates IκBβ at Ser19 and Ser23 (our unpublished data), instead of IκBα, which is mainly phosphorylated by IKKβ [81]. NF-κB activation in addition to activating the transcription of other genes, promotes the transcription of its own inhibitor IκBα. The newly formed IκBα interacts with NF-κB and takes it to the cytoplasm and thus negatively regulates expression of NF-κB dependent genes. Interestingly, unlike IκBα, the IκBβ promoter does not have NF-κB binding sites. Consequently, cocaine induced NF-κB activation does not directly lead to the synthesis of its inhibitor. Hence, RSK1 induced NF-κB activation lasts longer and eventually contributes to the persistence of a cocaine effect (our unpublished data and [82]).
In addition to the activation of NF-κB, cocaine exposure enhances the functional activity of NF-κB primarily by activating mitogen- and stress-activated kinase 1 (MSK1). MSK1 is another kinase that becomes activated upon the stimulation of MAPK/extracellular-signal regulated kinase (ERK) cascade [83, 84]. MSK1 subsequently phosphorylates the p65 subunit of NF-κB at serine residue 276 (p65S276). This post-translational modification boosts the interaction of NF-κB with histone acetyltransferases (HATs) [15, 85–87]. Enhanced interaction translates into higher recruitment of HATs at HIV LTR through NF-κB binding to its consensus binding sequences at HIV LTR. HATs in turn acetylate the core histones, which reduce the electrostatic interactions between the histones and DNA. Less interaction between DNA and histones results in more relaxed/open chromatin structures near the LTR promoter. These relaxed transcriptionally active chromatin structures consequently further promote access to all the remaining components of the transcription machinery (Holo-transcription machinery) and even augment the overall flow of transcription machinery at the HIV LTR promoter. These events eventually enhance the rate of transcriptional initiation from the LTR promoter [15].
HIV transcriptional elongation
The efficiency of the elongation phase of HIV transcription is predominantly dependent on the HIV protein Tat. However, prior to sufficient Tat protein generation, the elongation phase of HIV transcription proceeds very slowly. The Tat-independent inefficient phase of HIV transcription is primarily attributed to the presence of two elongation inhibitory factors; negative elongation factor (NELF) and DRB sensitivity-inducing factor (DSIF) at HIV LTR (for recent reviews refer to [88–93]). In addition to restricting HIV transcription, these negative elongation factors ubiquitously inhibit the transcription of numerous cellular genes. Moreover, in brain resident macrophages, microglia, HIV transcription is further repressed by the chicken ovalbumin upstream promoter transcription factor (COUP-TF) interacting protein 2 (CTIP2), an inhibitor of P-TEFb predominantly expressed in microglial cells [94–96]. In addition to using the cellular machinery to relieve the restriction posed by NELF and DSIF, HIV utilizes its specialized protein, Tat to facilitate this function. In the absence of Tat, most of the HIV transcripts halt at approximately 60 nucleotides due to the presence of NELF and DSIF along with non-processive or elongation defective RNA polymerase II (RNAP II) at HIV LTR. Once Tat is synthesized and accumulated in the cell beyond a certain threshold, Tat positively feedbacks the entire system. To perform its functions Tat requires binding to an RNA stem loop structure called the trans-activation response (TAR) element which is present at the 5′ extremity of all HIV transcripts. Along with it Tat brings positive transcription elongation factor b (P-TEFb) protein, which mediates most of the transcriptional functions of Tat [97–99]. The cyclin dependent kinase 9 (CDK9) subunit of P-TEFb subsequently hyper-phosphorylates the C-terminal domain (CTD) of RNA polymerase II (RNAPII) and converts the pausing RNAPII into a processive or elongation proficient polymerase [100, 101]. Besides RNAPII, P-TEFb also phosphorylates inhibitory factors DSIF and NELF. These modifications either dissociate the negative factors or convert them into a positive transcription factor [102–104]. P-TEFb recruitment at LTR is also essential to reactivate latent provirus in primary T cells [105, 106]. Besides recruiting P-TEFb, Tat also brings additional elongation factors, such as ELL2, AFF4, ENL and AF9; together they form a Super Elongation Complex (SEC), at HIV-1 LTR (Figure 1) [68, 107, 108]. ELL2 and the scaffold protein AFF4 have been shown to be the critical components of the SEC, as their knockdown or removal strongly inhibits Tat-dependent LTR-driven reporter gene expression [68, 109]. The binding of AFF4 in the presence of Tat modulates the Tat–TAR recognition motif of Cyclin T1 and increases the affinity of Tat-P-TEFb complex for TAR several folds [110, 111]. The enhanced rate of HIV transcription results in generating more Tat protein and higher Tat levels further accelerate HIV transcription. Hence, HIV transcription enters into the fast Tat-dependent phase, that eventually accelerates HIV transcription several hundred fold [97, 112]. HIV transcription thus differs from normal transcription of cellular genes as it is auto-regulated by Tat protein. These events subsequently relieve all restrictions to HIV transcription, and consequently efficient HIV transcription enhances the rate of generation of full-length mature transcripts. This includes production of unspliced HIV genomic transcripts which get packaged and lead to the generation of new viral particles, indicated as an enhanced rate of HIV replication[113, 114].
Cocaine and HIV transcriptional elongation
It is well known that cocaine augments the rate of HIV replication and that cocaine is able to induce the generation of full-length HIV genomic transcripts. As aforementioned, in order to generate the full genomic transcript, HIV transcription has to pass through both the initiation and elongation phase of transcription. Thus, in addition to NF-κB activation, this implies that cocaine activates P-TEFb, an essential factor required for the elongation phase of HIV transcription (Figure 1).
Cocaine promotes HIV transcriptional elongation primarily by activating MSK1
Cocaine activates MSK1 which, besides catalyzing the phosphorylation of the p65 subunit of NF-κB at serine 276, also catalyzes the phosphorylation of histone H3 at serine 10 (P-H3S10) at the HIV LTR promoter [15]. Notably, cocaine also enhances the phosphorylation of p65 and of histone H3 locally at HIV LTR by augmenting the recruitment of MSK1 at LTR [15]. Comparable observations have also been reported for cellular promoters following cocaine exposure [115]. Although several other enzymes besides MSK1 are known to catalyze H3S10 phosphorylation, the predominant role of MSK1 during cocaine exposure has been well established [116–118]. P-H3S10 is a euchromatic mark which facilitates the establishment of transcriptionally active chromatin structure [115, 119]. Accordingly, MSK1 induced H3S10 phosphorylation promotes the establishment of transcriptionally active chromatin structures at HIV-1 LTR following cocaine treatment [15].
In addition to facilitating the establishment of transcriptionally active chromatin structures, P-H3S10 promotes the recruitment of P-TEFb to HIV LTR analogous to other gene promoters [15, 120, 121]. As noted above, P-TEFb plays an essential role in supporting the elongation phase of HIV-1 transcription by catalyzing several above mentioned phosphorylation events (detailed in HIV transcriptional elongation section, Figures 1 and 2) [99–104, 119, 122]. The resultant, complete unspliced HIV transcripts are then packaged and lead to the generation of new viral particles which are represented as enhanced HIV replication. Thus, cocaine boosts HIV-1 gene expression by inducing both the initiation and elongation phases of HIV-1 transcription.
Role of epigenetics in controlling HIV transcription and latency
Like other retroviruses, HIV integrates itself into the host cell genome, preferentially within the intronic regions of actively transcribing genes. This inclination is due to the selective binding preference of lens epithelium-derived growth factor (LEDGF), a protein that plays an important role during HIV integration. LEDGF shows specificity towards transcriptionally active open chromatin structures [123–128]. Analogous to cellular genes, the expression of integrated HIV genome is facilitated by the formation of transcriptionally active chromatin structures around the LTR promoter [129].
The chromatin structures are characterized by their fundamental subunits, nucleosomes. A nucleosome comprises of an octamer, a pair of four core histones (H3, H4, H2A and H2B), which are wrapped around by 147 base pairs of DNA. These core histones undergo various kinds of post-translational modifications such as acetylation, methylation (mono-, di-, or tri-methylation), sumoylation, phosphorylation, ubiquitinylation, etc. [130]. These modifications are called epigenetic modifications, as they can be passed through to the next generation. These epigenetic modifications eventually define the specific nature of the chromatin structures. Chromatin structures, especially in the vicinity of the promoter region of a gene, regulate its expression. Thus, the type and amount of epigenetic modifications harbored play a decisive role in defining specific chromatin structure and subsequent regulation of gene expression [131–133]. The open or relaxed chromatin structure, which promotes access to the transcription machinery at the promoter region of a gene, is called transcriptionally active or euchromatin structure. On the other hand, the closed or compact chromatin structure, which inhibits the access of transcription machinery to the promoter region of a gene is called transcriptionally repressive or heterochromatin structure [130, 132].
The HIV LTR promoter is precisely flanked by two well-placed nucleosomes, independent of the site of integration in the cellular genome The nucleosome-0 (Nuc-0) is located upstream of the LTR promoter and nucleosome-1 (Nuc-1) is assembled downstream from the LTR promoter [129, 134], Figure 1. The epigenetic modifications of these two nucleosomes (Nuc-0 and Nuc-1) play major role in defining the overall chromatin structure near LTR promoter and consequently controlling the initiation of HIV transcription [129, 135, 136].
The chromatin structures at HIV LTR are remodeled mainly by two kinds of protein complexes. One of these protein complexes modifies chromatin structures by inducing various post-translational epigenetic modifications at the N-terminal tails of histones. It is noteworthy that the binding of the basal transcription factors to the chromatin-free promoter region of LTR is not hindered by the surrounding higher order epigenetic nucleosomal structures. The major chromatin reorganization at HIV LTR begins after the binding of factors, such as NF-κB to the enhancer region of the HIV LTR. NF-κB subsequently recruits histone acetyltransferases (HATs), HATs in turn greatly induce remodeling of the surrounding chromatin structures at HIV LTR. Moreover, Tat also brings HATs containing complexes at HIV LTR. Together recruited HATs successively promote the establishment of transcriptionally active chromatin structures at HIV LTR, which, in turn, support Tat-mediated induction of HIV transcription [137–140]. The second group of chromatin-modifying complexes uses energy to change the structures of nucleosomes in order to opening-up or relax nucleosomal structure. The SWI/SNF is one of such complex that changes the location and reorganization of nucleosomes via an ATP dependent mechanism. The SWI/SNF remodeling complexes facilitates the opening of the nucleosomal structures and promotes access of the LTR promoter to transcription factors (for detail, please refer to [141–149]. These events eventually accelerate the overall rate of HIV transcription.
In addition to the post-translational modification of nucleosomal histones, other epigenetic modifications, such as DNA methylation at the CpG islands flanking the transcription start site have also been implicated in regulating the HIV gene expression [150–152]. Even the role of certain noncoding RNA, primarily microRNA (miRNA) has been implicated in HIV transcription and latency [153, 154]. The miRNAs are single-stranded small RNA molecules that bind to specific complementary sequences on the target mRNA and usually inhibit its translation. However miRNA binding occasionally also results in the degradation of specific mRNA [155]. The vital role of viral and cellular miRNAs has been demonstrated in regulating HIV gene expression and latency. In particular, cellular miR-28, miR-125b, miR-150, miR-223 and miR-382, which are enriched in metabolically silent, resting CD4+ T lymphocytes, suppress HIV translation by targeting its mRNA [156–158]. A number of recent papers provide further details of miRNA-mediated regulation of HIV latency [159–168]. Cocaine has been reported to up- and downregulate multiple miRNAs, such as upregulation of miR-181a and downregulation of miR-124 and let-7d [169, 170]. Accordingly, cocaine has been shown to enhance HIV-1 replication in CD4+ T cells by down-regulating miR-125b [36]. Therefore, innovative methodologies designed to manipulate the action of involved miRNAs could be proved useful in regulating HIV gene expression, latency and modulating impact of drugs of abuse.
It is worthy to note that most of the enzymes that catalyze epigenetic modifications do no bind directly to DNA. As a result, the epigenetic enzymes must be recruited to HIV LTR by a variety of DNA binding proteins. HIV transcriptional repressors such as CBF-1, YY1/LSF1, P50 homodimer, AP4, CTIP2, and thyroid hormone receptor recruit chromatin modifying enzymes to the LTR along with several other proteins as multiprotein complexes, which subsequently induce transcriptionally repressive, heterochromatin structures, at HIV LTR [95, 171–175]. We have demonstrated that CBF-1-induced repressive chromatin structures play an important role in restricting HIV transcription and promote HIV latency in primary CD4+ T cells [105]. The role of repressive epigenetic modifications in restricting HIV transcription during latency is quite evident due to the fact that their removal or inhibition leads to the reactivation of latent proviruses [90, 91, 144, 176–178].
It has been well established that epigenetic modifications play an important role in regulating HIV transcription. However, the precise nature of the underlying molecular mechanisms that regulate these specific epigenetic changes at HIV LTR are still not fully defined. Understanding the precise role of epigenetic modifications and the mechanisms involved could be of therapeutic importance in reactivating latent proviruses which are mainly silent due to the lack of productive HIV transcription. Thus, there is an enormous potential for drugs that could manipulate chromatin structures at HIV LTR and regulate HIV gene expression. Different drugs of abuse including cocaine also induce chromatin structure reorganization at numerous cellular genes and HIV LTR (detailed in following section). However, a huge body of work is still required to precisely characterize and define the vital role of epigenetic modifications induced by cocaine. Thus, there is pressing need to examine the molecular mechanisms involved during interactions between the virus and drugs of abuse at the gene expression level to determine how they may be affecting HIV replication.
Impact of cocaine induced epigenetic changes on HIV transcription
The important role for cocaine induced epigenetic modifications in altering the expression of several genes in the central nervous system has been clearly demonstrated [179–181]. Different types and levels of epigenetic modifications of the nucleosomal core histones eventually define the nature of chromatin structure at and around gene promoters. Transcriptionally active, open/euchromatin structures facilitates transcription, whereas transcriptionally repressive, closed/heterochromatin structures suppress transcription by inhibiting the access of transcription machinery to the genetic elements responsible for transcription. Moreover, the long lasting effects of different stimuli on the brain, including cocaine induced plasticity which can persist even after its removal, can be better explained by the concept of relatively stable specific epigenetic modifications and resulting persistent long term expression of selected CNS genes even after removal of stimuli. Epigenetic dysregulations and the resultant changes in the responsible genes have been implicated in several neuronal dysfunctions, including Huntington’s disease, Friedreich ataxia and Rett syndrome [118, 182]. These observations further strengthen the concept that cocaine induced epigenetic modifications may be involved in the exaggeration of neurodegeneration seen in HIV-infected drug abusers. The better understanding of these mechanisms will reveal new drug targets and open up new avenue for better pharmaceutical interventions in drug addict HIV patients.
Cocaine induced Histone modifications at HIV LTR
Interestingly, the mode of cocaine exposure (acute or chronic) also regulates the activation and duration of gene expression by inducing a selective set of epigenetic modifications [181, 183–188]. Genome wide ChIP analysis following cocaine exposure results in both subtle and persistent induction of genes due to selective histone H3 and H4 acetylation [181, 184, 189]. Interestingly, on a number of genes enhanced acetylation of histone H3 was found to be associated with chronic cocaine treatment, while histone H4 hyper-acetylation was found to occur specifically during acute cocaine treatment. However, a lot of genes did not follow this pattern. These facts further validate that histone code for gene regulation is a complex phenomenon. Histone acetylation, although a vital epigenetic modification, still not sufficient to dictate the expression of a gene and contribution of different epigenetic modifications on a particular gene defines its eventual expression [181, 185, 186, 188–190]. Another important finding from these analyses was that cocaine usually promotes gene expression by inducing both phosphorylation and acetylation of core histones. However, cocaine has also been found to suppress a small subset of genes by inducing histone H3 methylation at lysine 9 [130, 191–194]. To further complicate this scenario, recent studies have confirmed that cocaine specifically inhibits class II HDACs, mainly HDAC-4 and -5, but activates Class III HDACs, SIRT1 and SIRT2 [179, 183, 184]. Moreover, cocaine also promotes gene expression by downregulating histone metyltransferses, including G9a and GLP (G9a-like protein). These enzymes catalyze the methylation of histone H3 at lysine 9, a transcriptionally repressive heterochromatic mark [195]. These observations suggest that besides histones, the post-translational modification of several non-histone proteins by these enzymes is responsible for deciding the expression of cellular genes following cocaine exposure.
In the context of HIV infection, we have investigated the role of cocaine induced epigenetic modifications on HIV gene expression [15]. Our results demonstrate that cocaine treatment greatly enhances the recruitment of histone acetyltransferase (HAT), p300, at HIV LTR and leads to the dissociation of histone deacetylase, HDAC1 and HDAC3, from LTR. These events result in hyper-acetylation of core histones both H3 and H4 [15]. Thus, we found that cocaine induced epigenetic modifications at HIV LTR do not follow any strict core histone acetylation pattern. In addition to histone acetylation, cocaine treatment induces phosphorylation of histone H3 at serine 10 (p-H3S10). Acetylation and phosphorylation of core histones partially neutralizes the positive ionic charge of histones and thus both of these epigenetic modifications contribute to the establishment of relaxed transcription-promoting euchromatin structures at HIV LTR. Interestingly, upon cocaine treatment, we also observed the loss of heterochromatic epigenetic modifications such as trimethylation of histone H3 at lysine 9 (H3K9me3) and lysine 27(H3K27me3) from HIV LTR. As indicated in Figure 2, our results demonstrated that cocaine exposure converts the transcriptionally repressive heterochromatin structures into transcriptionally active euchromatic structures at HIV LTR [15]. Accordingly, we found higher recruitment of RNA polymerase II at HIV LTR; further validating the fact that the euchromatin structure promotes the access to transcription machinery at gene promoters and facilitates HIV transcription.
Therapeutic implications to counter cocaine impact
Development of HAART regimens that penetrate better blood brain barrier (BBB)
Current HAART regimens may result in the establishment of several anatomical sanctuary sites such as the CNS because of poor tissue penetration. In these sanctuary sites, a perpetual low level of viral replication has been documented by several studies [196, 197]. Eradication of HIV from sanctuary sites holds the key to HIV cure. Cocaine use further burdens HIV patients by accelerating ongoing HIV replication, primarily in the CNS of HIV patients [6, 198–201]. Hence, better penetrating HAART regimens into HIV sanctuary sites, including the CNS will improve cognitive performance, as has been demonstrated during HAART intensification with better BBB crossing HAART regimens [202, 203].
Inclusion of drugs in HAART regimens that restrict HIV transcription
While HAART efficiently restrict the HIV replication and infection of new cells, it is unable to prevent the transcription of viral proteins. The viral proteins that are produced induce several side effects, especially chronic inflammation, by entering and interacting with cells and cellular proteins, respectively. The CNS is particularly sensitive to the inflammation induced by viral proteins, such as GP120, Tat and Nef. The produced inflammatory cytokines and cytotoxic products secreted by brain cells, especially microglia and perivascular macrophages and to a lesser extent astrocytes subsequently induce neuropathy [7, 204]. Therefore, it implies that cocaine-mediated enhanced HIV transcription elevate the levels of viral proteins even in the presence of HAART regimens and contribute to chronic brain inflammation and deterioration of CNS functioning [15]. Although some HAART agents have shown clear benefit in protecting against neurocognitive impairments, yet higher penetration of HAART drugs may be associated with neurotoxicity [205–208]. Hence, there is an urgent need to perform systematic studies to settle these confounding results.
Conclusion and perspectives
Studies of the effects of cocaine on HIV gene expression are still in their infancy. Our work was the first to describe the salient underlying molecular mechanisms that cocaine utilizes in order to enhance HIV transcription. The transcription factors discussed above are just a few of many that affect HIV gene expression. The main goal of future research is to obtain a comprehensive view of the transcription factors induced by cocaine and their effect on the initiation and elongation phase of expression of relevant genes, especially those that affect HIV gene expression and brain function.
We found that cocaine induces the chromatin remodeling at HIV LTR via MSK1 and histone H3 phosphorylation. Similar epigenetic modifications at various gene promoters, especially in brain cells have been implicated in some of the long-lasting behavioral consequences of cocaine. In order to better understand the cocaine-induced epigenetic modifications at HIV LTR, it will be necessary to involve high throughput approaches, such as ChIP-Seq, to identify numerous other post-translational modifications in both histone and non-histone proteins.
There remains a huge body of work necessary to precisely characterize and define the vital role of epigenetic modifications induced by cocaine. There is a pressing need to examine the molecular mechanisms that are involved during the interactions between the virus and drugs of abuse at the level of gene expression in order to determine how they may be affecting HIV replication. A better understanding of these mechanisms may reveal new drug targets and open up new avenues for better pharmaceutical interventions in HIV-infected drug abusers.
Acknowledgments
The research in Tyagi laboratory is partially funded by National Institute on Drug Abuse (NIDA), NIH Grants, 5R21DA033924-02, 5R03DA033900-02 to MT. This work is also supported by grants of the District of Columbia Center for AIDS Research (DC-CFAR), a NIH-funded program P30AI117970 and startup funds from the George Washington University to MT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Due to page limitation, we could not cite all the relevant literature and we apologize to authors whose papers were not cited.
Footnotes
Conflict of interest:
The authors declare that they have no conflict of interest.
References
- 1.Tanne JH. Nearly 40 million people worldwide are infected with HIV. British Medical Journal. 2006;332(7553):1289–1289. doi: 10.1136/bmj.332.7553.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buch S, et al. Cocaine and HIV-1 interplay: molecular mechanisms of action and addiction. J Neuroimmune Pharmacol. 2011;6(4):503–15. doi: 10.1007/s11481-011-9297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heaton RK, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of Neurovirology. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alfahad TB, Nath A. Update on HIV-associated neurocognitive disorders. Curr Neurol Neurosci Rep. 2013;13(10):387. doi: 10.1007/s11910-013-0387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook JA, et al. Crack cocaine, disease progression, and mortality in a multicenter cohort of HIV-1 positive women. AIDS. 2008;22(11):1355–63. doi: 10.1097/QAD.0b013e32830507f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nath A, et al. Acceleration of HIV dementia with methamphetamine and cocaine. J Neurovirol. 2001;7(1):66–71. doi: 10.1080/135502801300069737. [DOI] [PubMed] [Google Scholar]
- 7.Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(Suppl 2):S193–8. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- 8.Dohgu S, Fleegal-DeMotta MA, Banks WA. Lipopolysaccharide-enhanced transcellular transport of HIV-1 across the blood-brain barrier is mediated by luminal microvessel IL-6 and GM-CSF. J Neuroinflammation. 2011;8:167. doi: 10.1186/1742-2094-8-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831):988–94. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 10.Nath A. Eradication of human immunodeficiency virus from brain reservoirs. J Neurovirol. 2015;21(3):227–34. doi: 10.1007/s13365-014-0291-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson KA, et al. Astrocyte specific viral strains in HIV dementia. Ann Neurol. 2004;56(6):873–7. doi: 10.1002/ana.20304. [DOI] [PubMed] [Google Scholar]
- 12.Dunfee RL, et al. The HIV Env variant N283 enhances macrophage tropism and is associated with brain infection and dementia. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(41):15160–15165. doi: 10.1073/pnas.0605513103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamers SL, et al. Human immunodeficiency virus-1 evolutionary patterns associated with pathogenic processes in the brain. J Neurovirol. 2010;16(3):230–41. doi: 10.3109/13550281003735709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sturdevant CB, et al. Compartmentalized replication of R5 T cell-tropic HIV-1 in the central nervous system early in the course of infection. PLoS Pathog. 2015;11(3):e1004720. doi: 10.1371/journal.ppat.1004720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahu G, et al. Cocaine promotes both initiation and elongation phase of HIV-1 transcription by activating NF-kappaB and MSK1 and inducing selective epigenetic modifications at HIV-1 LTR. Virology. 2015;483:185–202. doi: 10.1016/j.virol.2015.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rychert J, et al. Detection of HIV gp120 in Plasma During Early HIV Infection Is Associated with Increased Proinflammatory and Immunoregulatory Cytokines. Aids Research and Human Retroviruses. 2010;26(10):1139–1145. doi: 10.1089/aid.2009.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein TW, et al. Cocaine suppresses proliferation of phytohemagglutinin-activated human peripheral blood T-cells. Int J Immunopharmacol. 1993;15(1):77–86. doi: 10.1016/0192-0561(93)90033-u. [DOI] [PubMed] [Google Scholar]
- 18.Mao JT, et al. Cocaine down-regulates IL-2-induced peripheral blood lymphocyte IL-8 and IFN-gamma production. Cell Immunol. 1996;172(2):217–23. doi: 10.1006/cimm.1996.0235. [DOI] [PubMed] [Google Scholar]
- 19.Giunta B, et al. EGCG mitigates neurotoxicity mediated by HIV-1 proteins gp120 and Tat in the presence of IFN-gamma: role of JAK/STAT1 signaling and implications for HIV-associated dementia. Brain Res. 2006;1123(1):216–25. doi: 10.1016/j.brainres.2006.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bansal AK, et al. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. 2000;879(1–2):42–9. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- 21.Eisenstein TK, Hilburger ME. Opioid modulation of immune responses: effects on phagocyte and lymphoid cell populations. J Neuroimmunol. 1998;83(1–2):36–44. doi: 10.1016/s0165-5728(97)00219-1. [DOI] [PubMed] [Google Scholar]
- 22.Peterson PK, et al. Enhancement of HIV-1 replication by opiates and cocaine: the cytokine connection. Adv Exp Med Biol. 1993;335:181–8. doi: 10.1007/978-1-4615-2980-4_26. [DOI] [PubMed] [Google Scholar]
- 23.Bagasra O, Pomerantz RJ. Human immunodeficiency virus type 1 replication in peripheral blood mononuclear cells in the presence of cocaine. J Infect Dis. 1993;168(5):1157–64. doi: 10.1093/infdis/168.5.1157. [DOI] [PubMed] [Google Scholar]
- 24.Nair MP, et al. Cocaine modulates dendritic cell-specific C type intercellular adhesion molecule-3-grabbing nonintegrin expression by dendritic cells in HIV-1 patients. J Immunol. 2005;174(11):6617–26. doi: 10.4049/jimmunol.174.11.6617. [DOI] [PubMed] [Google Scholar]
- 25.Nath A, et al. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S62–9. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- 26.Kaul M, Lipton SA. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res. 2006;4(3):307–18. doi: 10.2174/157016206777709384. [DOI] [PubMed] [Google Scholar]
- 27.Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDS. Neurosci Biobehav Rev. 2008;32(5):883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larrat EP, Zierler S. Entangled Epidemics - Cocaine Use and Hiv Disease. Journal of Psychoactive Drugs. 1993;25(3):207–221. doi: 10.1080/02791072.1993.10472272. [DOI] [PubMed] [Google Scholar]
- 29.Fiala M, et al. Cocaine enhances monocyte migration across the blood-brain barrier - Cocaine’s connection to AIDS dementia and vasculitis? Drugs of Abuse, Immunomodulation, and Aids. 1998;437:199–205. doi: 10.1007/978-1-4615-5347-2_22. [DOI] [PubMed] [Google Scholar]
- 30.Webber MP, et al. A prospective study of HIV disease progression in female and male drug users. Aids. 1999;13(2):257–262. doi: 10.1097/00002030-199902040-00014. [DOI] [PubMed] [Google Scholar]
- 31.Kim SG, et al. Cocaine exposure enhances permissiveness of quiescent T cells to HIV infection. J Leukoc Biol. 2013;94(4):835–43. doi: 10.1189/jlb.1112566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roth MD, et al. Cocaine enhances human immunodeficiency virus replication in a model of severe combined immunodeficient mice implanted with human peripheral blood leukocytes. J Infect Dis. 2002;185(5):701–5. doi: 10.1086/339012. [DOI] [PubMed] [Google Scholar]
- 33.Kim SG, et al. Cocaine-mediated impact on HIV infection in humanized BLT mice. Sci Rep. 2015;5:10010. doi: 10.1038/srep10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nath A, Clements JE. Eradication of HIV from the brain: reasons for pause. AIDS. 2011;25(5):577–80. doi: 10.1097/QAD.0b013e3283437d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Napuri J, et al. Cocaine enhances HIV-1 infectivity in monocyte derived dendritic cells by suppressing microRNA-155. PLoS One. 2013;8(12):e83682. doi: 10.1371/journal.pone.0083682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mantri CK, et al. Cocaine enhances HIV-1 replication in CD4+ T cells by down-regulating MiR-125b. PLoS One. 2012;7(12):e51387. doi: 10.1371/journal.pone.0051387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gekker G, et al. Cocaine-induced HIV-1 expression in microglia involves sigma-1 receptors and transforming growth factor-beta1. Int Immunopharmacol. 2006;6(6):1029–33. doi: 10.1016/j.intimp.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Peterson PK, et al. Cocaine potentiates HIV-1 replication in human peripheral blood mononuclear cell cocultures. Involvement of transforming growth factor-beta. J Immunol. 1991;146(1):81–4. [PubMed] [Google Scholar]
- 39.Baldwin GC, Roth MD, Tashkin DP. Acute and chronic effects of cocaine on the immune system and the possible link to AIDS. J Neuroimmunol. 1998;83(1–2):133–8. doi: 10.1016/s0165-5728(97)00229-4. [DOI] [PubMed] [Google Scholar]
- 40.Antinori A, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hauser KF, et al. Impact of opiate-HIV-1 interactions on neurotoxic signaling. J Neuroimmune Pharmacol. 2006;1(1):98–105. doi: 10.1007/s11481-005-9000-4. [DOI] [PubMed] [Google Scholar]
- 42.Pandya R, et al. HIV-related neurological syndromes reduce health-related quality of life. Can J Neurol Sci. 2005;32(2):201–4. doi: 10.1017/s0317167100003978. [DOI] [PubMed] [Google Scholar]
- 43.Robison LS, et al. Short-term discontinuation of HAART regimens more common in vulnerable patient populations. AIDS Res Hum Retroviruses. 2008;24(11):1347–55. doi: 10.1089/aid.2008.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arnsten JH, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. Journal of General Internal Medicine. 2002;17(5):377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wood E, et al. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. Canadian Medical Association Journal. 2003;169(7):656–661. [PMC free article] [PubMed] [Google Scholar]
- 46.Carrico AW, et al. Affect regulation, stimulant use, and viral load among HIV-Positive persons on anti-retroviral therapy. Psychosomatic Medicine. 2007;69(8):785–792. doi: 10.1097/PSY.0b013e318157b142. [DOI] [PubMed] [Google Scholar]
- 47.Carrico AW. Substance use and HIV disease progression in the HAART era: Implications for the primary prevention of HIV. Life Sciences. 2011;88(21–22):940–947. doi: 10.1016/j.lfs.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Moore RD, Keruly JC, Chaisson RE. Differences in HIV disease progression by injecting drug use in HIV-infected persons in care. Jaids-Journal of Acquired Immune Deficiency Syndromes. 2004;35(1):46–51. doi: 10.1097/00126334-200401010-00006. [DOI] [PubMed] [Google Scholar]
- 49.Roth MD, et al. Cocaine and sigma-1 receptors modulate HIV infection, chemokine receptors, and the HPA axis in the huPBL-SCID model. J Leukoc Biol. 2005;78(6):1198–203. doi: 10.1189/jlb.0405219. [DOI] [PubMed] [Google Scholar]
- 50.Ross EK, et al. Contribution of NF-κB and Sp1 binding motifs to the replicative capacity of human immunodeficiency virus type 1: Distinct patterns of viral growth are determined by T-cell types. J Virol. 1991;65:4350–4358. doi: 10.1128/jvi.65.8.4350-4358.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones K, et al. Activation of the AIDS retrovirus promoter by the cellular transcription factor, Sp1. Science. 1986;232:755–759. doi: 10.1126/science.3008338. [DOI] [PubMed] [Google Scholar]
- 52.Olsen HS, Rosen CA. Contribution of the TATA motif to Tat-mediated transcriptional activation of the human immunodeficiency virus gene expression. J Virol. 1992;66:5594–5597. doi: 10.1128/jvi.66.9.5594-5597.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rittner K, et al. The human immunodeficiency virus long terminal repeat includes a specialised initiator element which is required for Tat-responsive transcription. J Mol Biol. 1995;248:562–580. doi: 10.1006/jmbi.1995.0243. [DOI] [PubMed] [Google Scholar]
- 54.Garcia JA, et al. Human immunodeficiency virus type 1 LTR TATA and TAR region sequences required for transcriptional regulation. EMBO J. 1989;8:765–778. doi: 10.1002/j.1460-2075.1989.tb03437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nabel G, Baltimore DA. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 56.Kinoshita S, et al. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity. 1997;6:235–244. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- 57.Selliah N, et al. The gamma-cytokine regulated transcription factor, STAT5, increases HIV-1 production in primary CD4 T cells. Virology. 2006;344(2):283–91. doi: 10.1016/j.virol.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 58.Yang X, Chen Y, Gabuzda D. ERK MAP kinase links cytokine signals to activation of latent HIV-1 infection by stimulating a cooperative interaction of AP-1 and NF-κB. J Biol Chem. 1999;274:27981–8. doi: 10.1074/jbc.274.39.27981. [DOI] [PubMed] [Google Scholar]
- 59.Perkins ND, et al. A cooperative interaction between NF-κB and Sp1 is required for HIV-1 enhancer activation. EMBO J. 1993;12:3551–3558. doi: 10.1002/j.1460-2075.1993.tb06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gerritsen ME, et al. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc Natl Acad Sci U S A. 1997;94:2927–2932. doi: 10.1073/pnas.94.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia-Rodriguez C, Rao A. Nuclear factor of activated T cells (NFAT)-dependent transactivation regulated by the coactivators p300/CREB-binding protein (CBP) J Exp Med. 1998;187(12):2031–6. doi: 10.1084/jem.187.12.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alcami J, et al. Absolute dependence on κB responsive elements for initiation and Tat-mediated amplification of HIV transcription in blood CD4 T lymphocytes. EMBO J. 1995;14:1552–1560. doi: 10.1002/j.1460-2075.1995.tb07141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bosque A, Planelles V. Induction of HIV-1 latency and reactivation in primary memory CD4+ T cells. Blood. 2008 doi: 10.1182/blood-2008-07-168393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 65.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 66.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18(18):2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 67.Schmitz ML, Baeuerle PA. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO J. 1991;10(12):3805–17. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sobhian B, et al. HIV-1 Tat assembles a multifunctional transcription elongation complex and stably associates with the 7SK snRNP. Mol Cell. 2010;38(3):439–51. doi: 10.1016/j.molcel.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown K, et al. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267(5203):1485–8. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 70.Bonizzi G, et al. Activation of IKKalpha target genes depends on recognition of specific kappaB binding sites by RelB:p52 dimers. EMBO J. 2004;23(21):4202–10. doi: 10.1038/sj.emboj.7600391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25(6):280–8. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 72.Kerr LD, et al. The rel-associated pp40 protein prevents DNA binding of Rel and NF-kappa B: relationship with I kappa B beta and regulation by phosphorylation. Genes Dev. 1991;5(8):1464–76. doi: 10.1101/gad.5.8.1464. [DOI] [PubMed] [Google Scholar]
- 73.Ang E, et al. Induction of nuclear factor-kappaB in nucleus accumbens by chronic cocaine administration. J Neurochem. 2001;79(1):221–4. doi: 10.1046/j.1471-4159.2001.00563.x. [DOI] [PubMed] [Google Scholar]
- 74.Hou YN, et al. A mu-receptor opioid agonist induces AP-1 and NF-kappa B transcription factor activity in primary cultures of rat cortical neurons. Neurosci Lett. 1996;212(3):159–62. doi: 10.1016/0304-3940(96)12799-3. [DOI] [PubMed] [Google Scholar]
- 75.Yao HH, et al. Molecular mechanisms involving sigma receptor-mediated induction of MCP-1: implication for increased monocyte transmigration. Blood. 2010;115(23):4951–4962. doi: 10.1182/blood-2010-01-266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dhillon NK, et al. Cocaine-mediated enhancement of virus replication in macrophages: implications for human immunodeficiency virus-associated dementia. J Neurovirol. 2007;13(6):483–95. doi: 10.1080/13550280701528684. [DOI] [PubMed] [Google Scholar]
- 77.Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;8(11):837–48. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 78.Valjent E, et al. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20(23):8701–9. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Valjent E, et al. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19(7):1826–36. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- 80.Berhow MT, Hiroi N, Nestler EJ. Regulation of ERK (extracellular signal regulated kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J Neurosci. 1996;16(15):4707–15. doi: 10.1523/JNEUROSCI.16-15-04707.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ghoda L, Lin X, Greene WC. The 90-kDa ribosomal S6 kinase (pp90rsk) phosphorylates the N-terminal regulatory domain of IkappaBalpha and stimulates its degradation in vitro. J Biol Chem. 1997;272(34):21281–8. doi: 10.1074/jbc.272.34.21281. [DOI] [PubMed] [Google Scholar]
- 82.Thompson JE, et al. I kappa B-beta regulates the persistent response in a biphasic activation of NF-kappa B. Cell. 1995;80(4):573–82. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 83.Lu L, et al. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29(12):695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 84.Zhai H, et al. Drug-induced alterations in the extracellular signal-regulated kinase (ERK) signalling pathway: implications for reinforcement and reinstatement. Cell Mol Neurobiol. 2008;28(2):157–72. doi: 10.1007/s10571-007-9240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhong H, et al. The phosphorylation status of nuclear NF-κB determines its association with CBP/p300 or HDAC-1. Mol Cell. 2002;9(3):625–636. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]
- 86.Vermeulen L, et al. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1) EMBO J. 2003;22(6):1313–24. doi: 10.1093/emboj/cdg139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Perkins ND, et al. Regulation of NF-kB by cyclin-dependent kinases associated with the p300 coactivator. Science. 1997;275:523–527. doi: 10.1126/science.275.5299.523. [DOI] [PubMed] [Google Scholar]
- 88.Nechaev S, Adelman K. Pol II waiting in the starting gates: Regulating the transition from transcription initiation into productive elongation. Biochimica Et Biophysica Acta-Gene Regulatory Mechanisms. 2011;1809(1):34–45. doi: 10.1016/j.bbagrm.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cho S, Schroeder S, Ott M. CYCLINg through transcription Posttranslational modifications of P-TEFb regulate transcription elongation. Cell Cycle. 2010;9(9):1697–1705. doi: 10.4161/cc.9.9.11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tyagi M, Bukrinsky M. Human immunodeficiency virus (HIV) latency: the major hurdle in HIV eradication. Mol Med. 2012;18:1096–108. doi: 10.2119/molmed.2012.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mbonye U, Karn J. Transcriptional control of HIV latency: cellular signaling pathways, epigenetics, happenstance and the hope for a cure. Virology. 2014;454–455:328–39. doi: 10.1016/j.virol.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taube R, Peterlin BM. Lost in Transcription: Molecular Mechanisms that Control HIV Latency. Viruses-Basel. 2013;5(3):902–U157. doi: 10.3390/v5030902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jadlowsky JK, et al. Negative Elongation Factor Is Required for the Maintenance of Proviral Latency but Does Not Induce Promoter-Proximal Pausing of RNA Polymerase II on the HIV Long Terminal Repeat. Molecular and Cellular Biology. 2014;34(11):1911–1928. doi: 10.1128/MCB.01013-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marban C, et al. COUP-TF interacting protein 2 represses the initial phase of HIV-1 gene transcription in human microglial cells. Nucleic Acids Res. 2005;33(7):2318–31. doi: 10.1093/nar/gki529. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 95.Marban C, et al. Recruitment of chromatin-modifying enzymes by CTIP2 promotes HIV-1 transcriptional silencing. EMBO J. 2007;26(2):412–23. doi: 10.1038/sj.emboj.7601516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eilebrecht S, et al. HMGA1 recruits CTIP2-repressed P-TEFb to the HIV-1 and cellular target promoters. Nucleic Acids Res. 2014;42(8):4962–71. doi: 10.1093/nar/gku168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karn J. Tackling Tat. J Mol Biol. 1999;293:235–254. doi: 10.1006/jmbi.1999.3060. [DOI] [PubMed] [Google Scholar]
- 98.Herrmann CH, Rice AP. Lentivirus Tat proteins specifically associate with a cellular protein kinase, TAK, that hyperphosphorylates the carboxyl-terminal domain of the large subunit of RNA polymerase II: Candidate for a Tat cofactor. J Virol. 1995;69:1612–1620. doi: 10.1128/jvi.69.3.1612-1620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peterlin BM, Price DH. Controlling the elongation phase of transcription with P-TEFb. Mol Cell. 2006;23(3):297–305. doi: 10.1016/j.molcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 100.Parada CA, Roeder RG. Enhanced processivity of RNA polymerase II triggered by Tat-induced phosphorylation of its carboxy-terminal domain. Nature. 1996;384:375–378. doi: 10.1038/384375a0. [DOI] [PubMed] [Google Scholar]
- 101.Kim YK, et al. Phosphorylation of the RNA polymerase II carboxyl-terminal domain by CDK9 is directly responsible for human immunodeficiency virus type 1 Tat-activated transcriptional elongation. Mol Cell Biol. 2002;22(13):4622–4637. doi: 10.1128/MCB.22.13.4622-4637.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fujinaga K, et al. Dynamics of human immunodeficiency virus transcription: P-TEFb phosphorylates RD and dissociates negative effectors from the transactivation response element. Mol Cell Biol. 2004;24:787–795. doi: 10.1128/MCB.24.2.787-795.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bourgeois CF, et al. Spt5 cooperates with Tat by preventing premature RNA release at terminator sequences. Mol Cell Biol. 2002;22:1079–1093. doi: 10.1128/MCB.22.4.1079-1093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ivanov D, et al. Domains in the SPT5 protein that modulate its transcriptional regulatory properties. Mol Cell Biol. 2000;20:2970–2983. doi: 10.1128/mcb.20.9.2970-2983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tyagi M, Pearson RJ, Karn J. Establishment of HIV latency in primary CD4+ cells is due to epigenetic transcriptional silencing and P-TEFb restriction. J Virol. 2010;84(13):6425–37. doi: 10.1128/JVI.01519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Budhiraja S, et al. Cyclin T1 and CDK9 T-loop phosphorylation are downregulated during establishment of HIV-1 latency in primary resting memory CD4+ T cells. J Virol. 2013;87(2):1211–20. doi: 10.1128/JVI.02413-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.He N, et al. Human Polymerase-Associated Factor complex (PAFc) connects the Super Elongation Complex (SEC) to RNA polymerase II on chromatin. Proc Natl Acad Sci U S A. 2011;108(36):E636–45. doi: 10.1073/pnas.1107107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chou S, et al. HIV-1 Tat recruits transcription elongation factors dispersed along a flexible AFF4 scaffold. Proc Natl Acad Sci U S A. 2013;110(2):E123–31. doi: 10.1073/pnas.1216971110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.He NH, et al. HIV-1 Tat and Host AFF4 Recruit Two Transcription Elongation Factors into a Bifunctional Complex for Coordinated Activation of HIV-1 Transcription. Molecular Cell. 2010;38(3):428–438. doi: 10.1016/j.molcel.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schulze-Gahmen U, et al. AFF4 binding to Tat-P-TEFb indirectly stimulates TAR recognition of super elongation complexes at the HIV promoter. Elife. 2014;3 doi: 10.7554/eLife.02375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gu J, et al. Crystal structure of HIV-1 Tat complexed with human P-TEFb and AFF4. Cell Cycle. 2014;13(11):1788–97. doi: 10.4161/cc.28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Taube R, et al. Tat transactivation: a model for the regulation of eukaryotic transcriptional elongation. Virology. 1999;264:245–253. doi: 10.1006/viro.1999.9944. [DOI] [PubMed] [Google Scholar]
- 113.Pomerantz RJ, et al. Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: A molecular model for latency. Cell. 1990;61:1271–1276. doi: 10.1016/0092-8674(90)90691-7. [DOI] [PubMed] [Google Scholar]
- 114.Kim S, et al. Temporal aspects of DNA and RNA synthesis during human immunodeficiency virus infection: Evidence for differential gene expression. J Virol. 1989;63:3708–3713. doi: 10.1128/jvi.63.9.3708-3713.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Soloaga A, et al. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. EMBO J. 2003;22(11):2788–97. doi: 10.1093/emboj/cdg273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nestler EJ. Transcriptional mechanisms of drug addiction. Clin Psychopharmacol Neurosci. 2012;10(3):136–43. doi: 10.9758/cpn.2012.10.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Walker DM, et al. Regulation of chromatin states by drugs of abuse. Curr Opin Neurobiol. 2014;30C:112–121. doi: 10.1016/j.conb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brami-Cherrier K, et al. Role of the ERK/MSK1 signalling pathway in chromatin remodelling and brain responses to drugs of abuse. J Neurochem. 2009;108(6):1323–35. doi: 10.1111/j.1471-4159.2009.05879.x. [DOI] [PubMed] [Google Scholar]
- 119.Wei P, et al. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell. 1998;92(4):451–62. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 120.Ivaldi MS, Karam CS, Corces VG. Phosphorylation of histone H3 at Ser10 facilitates RNA polymerase II release from promoter-proximal pausing in Drosophila. Genes Dev. 2007;21(21):2818–31. doi: 10.1101/gad.1604007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hu X, et al. Histone cross-talk connects protein phosphatase 1alpha (PP1alpha) and histone deacetylase (HDAC) pathways to regulate the functional transition of bromodomain-containing 4 (BRD4) for inducible gene expression. J Biol Chem. 2014;289(33):23154–67. doi: 10.1074/jbc.M114.570812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Karn J. The molecular biology of HIV latency: breaking and restoring the Tat-dependent transcriptional circuit. Curr Opin HIV AIDS. 2011;6(1):4–11. doi: 10.1097/COH.0b013e328340ffbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schroder AR, et al. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110(4):521–9. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 124.Han Y, et al. Resting CD4+ T cells from human immunodeficiency virus type 1 (HIV-1)-infected individuals carry integrated HIV-1 genomes within actively transcribed host genes. J Virol. 2004;78(12):6122–33. doi: 10.1128/JVI.78.12.6122-6133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Meehan AM, et al. LEDGF/p75 proteins with alternative chromatin tethers are functional HIV-1 cofactors. PLoS Pathog. 2009;5(7):e1000522. doi: 10.1371/journal.ppat.1000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lewinski MK, et al. Retroviral DNA integration: viral and cellular determinants of target-site selection. PLoS Pathog. 2006;2(6):e60. doi: 10.1371/journal.ppat.0020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vatakis DN, et al. Human Immunodeficiency Virus Integration Efficiency and Site Selection in Quiescent CD4(+) T Cells. Journal of Virology. 2009;83(12):6222–6233. doi: 10.1128/JVI.00356-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brady T, et al. HIV integration site distributions in resting and activated CD4(+) T cells infected in culture. AIDS. 2009;23(12):1461–1471. doi: 10.1097/QAD.0b013e32832caf28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Verdin E, Paras PJ, Van Lint C. Chromatin disruption in the promoter of human immunodeficiency virus type 1 during transcriptional activation. EMBO J. 1993;12:3249–3259. doi: 10.1002/j.1460-2075.1993.tb05994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 131.Wolffe AP. Nucleosome Positioning and Modification - Chromatin Structures That Potentiate Transcription. Trends in Biochemical Sciences. 1994;19(6):240–244. doi: 10.1016/0968-0004(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 132.Narlikar GJ, Fan HY, Kingston RE. Cooperation between complexes that regulate chromatin structure and transcription. Cell. 2002;108(4):475–487. doi: 10.1016/s0092-8674(02)00654-2. [DOI] [PubMed] [Google Scholar]
- 133.Felsenfeld G, Groudine M. Controlling the double helix. Nature. 2003;421(6921):448–453. doi: 10.1038/nature01411. [DOI] [PubMed] [Google Scholar]
- 134.Verdin E. DNase I-hypersensitive sites are associated with both long terminal repeats and with the intragenic enhances of integrated human immunodeficiency virus type 1. J Virol. 1991;65:6790–6799. doi: 10.1128/jvi.65.12.6790-6799.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jordan A, Defechereux P, Verdin E. The site of HIV-1 integration in the human genome determines basal transcriptional activity and response to Tat transactivation. EMBO J. 2001;20(7):1726–1738. doi: 10.1093/emboj/20.7.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jordan A, Bisgrove D, Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 2003;22:1868–1877. doi: 10.1093/emboj/cdg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Marzio G, et al. HIV-1 Tat transactivator recruits p300 and CREB-binding protein histone acetyltransferases to the viral promoter. Proc Natl Acad Sci U S A. 1998;95:13519–13524. doi: 10.1073/pnas.95.23.13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Benkirane M, et al. Activation of integrated provirus requires histone acetyltransferase: p300 and p/CAF are coactivators for HIV-1 Tat. J Biol Chem. 1998;273:24989–24905. doi: 10.1074/jbc.273.38.24898. [DOI] [PubMed] [Google Scholar]
- 139.Hottiger MO, Nabel GJ. Interaction of human immunodeficiency virus type 1 Tat with the transcriptional coactivators p300 and CREB binding protein. J Virol. 1998;72:8252–8256. doi: 10.1128/jvi.72.10.8252-8256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ott M, et al. Acetylation of the HIV-1 Tat protein by p300 is important for its transcriptional activity. Curr Biol. 1999;9:1489–1492. doi: 10.1016/s0960-9822(00)80120-7. [DOI] [PubMed] [Google Scholar]
- 141.Hargreaves DC, Crabtree GR. ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res. 2011;21(3):396–420. doi: 10.1038/cr.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu N, Balliano A, Hayes JJ. Mechanism(s) of SWI/SNF-induced nucleosome mobilization. Chembiochem. 2011;12(2):196–204. doi: 10.1002/cbic.201000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rafati H, et al. Repressive LTR nucleosome positioning by the BAF complex is required for HIV latency. PLoS Biol. 2011;9(11):e1001206. doi: 10.1371/journal.pbio.1001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hakre S, et al. Epigenetic regulation of HIV latency. Curr Opin HIV AIDS. 2011;6(1):19–24. doi: 10.1097/COH.0b013e3283412384. [DOI] [PubMed] [Google Scholar]
- 145.Agbottah E, et al. Effect of SWI/SNF chromatin remodeling complex on HIV-1 Tat activated transcription. Retrovirology. 2006;3:48. doi: 10.1186/1742-4690-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Van Duyne R, et al. Varying modulation of HIV-1 LTR activity by Baf complexes. Journal of Molecular Biology. 2011;411(3):581–96. doi: 10.1016/j.jmb.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mahmoudi T, et al. The SWI/SNF chromatin-remodeling complex is a cofactor for tat transactivation of the HIV promoter. J Biol Chem. 2006;281(29):19960–8. doi: 10.1074/jbc.M603336200. [DOI] [PubMed] [Google Scholar]
- 148.Henderson A, et al. Recruitment of SWI/SNF to the human immunodeficiency virus type 1 promoter. Mol Cell Biol. 2004;24:389–97. doi: 10.1128/MCB.24.1.389-397.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Treand C, et al. Requirement for SWI/SNF chromatin-remodeling complex in Tat-mediated activation of the HIV-1 promoter. EMBO J. 2006;25(8):1690–9. doi: 10.1038/sj.emboj.7601074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kauder SE, et al. Epigenetic regulation of HIV-1 latency by cytosine methylation. PLoS Pathog. 2009;5(6):e1000495. doi: 10.1371/journal.ppat.1000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Blazkova J, et al. CpG methylation controls reactivation of HIV from latency. PLoS Pathog. 2009;5(8):e1000554. doi: 10.1371/journal.ppat.1000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chavez L, Kauder S, Verdin E. In vivo, in vitro, and in silico analysis of methylation of the HIV-1 provirus. Methods. 2011;53(1):47–53. doi: 10.1016/j.ymeth.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Suzuki K, et al. Promoter Targeting RNAs: Unexpected Contributors to the Control of HIV-1 Transcription. Mol Ther Nucleic Acids. 2015;4:e222. doi: 10.1038/mtna.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Budhiraja S, Rice AP. Reactivation of latent HIV: do all roads go through P-TEFb? Future Virology. 2013;8(7):649–659. doi: 10.2217/fvl.13.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 156.Pomerantz RJ, et al. The long terminal repeat is not a major determinant of the cellular tropism of human immunodeficiency virus type 1. J Virol. 1991;65(2):1041–1045. doi: 10.1128/jvi.65.2.1041-1045.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lassen KG, et al. Nuclear retention of multiply spliced HIV-1 RNA in resting CD4+ T cells. PLoS Pathog. 2006;2(7):e68. doi: 10.1371/journal.ppat.0020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Huang J, et al. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat Med. 2007;13(10):1241–7. doi: 10.1038/nm1639. [DOI] [PubMed] [Google Scholar]
- 159.Yeung ML, Benkirane M, Jeang KT. Small non-coding RNAs, mammalian cells, and viruses: regulatory interactions? Retrovirology. 2007:4. doi: 10.1186/1742-4690-4-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bennasser Y, Yeung ML, Jeang KT. RNAi therapy for HIV infection: principles and practicalities. Biodrugs. 2007;21(1):17–22. doi: 10.2165/00063030-200721010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Triboulet R, Benkirane M. Interplay between HIV-1 replication and the microRNA-silencing pathway. M S-Medecine Sciences. 2007;23(6–7):590–592. doi: 10.1051/medsci/20072367590. [DOI] [PubMed] [Google Scholar]
- 162.Sun GH, Rossi JJ. MicroRNAs and their potential involvement in HIV infection. Trends in Pharmacological Sciences. 2011;32(11):675–681. doi: 10.1016/j.tips.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kumar A, Jeang KT. Insights into cellular microRNAs and human immunodeficiency virus type 1 (HIV-1) Journal of Cellular Physiology. 2008;216(2):327–331. doi: 10.1002/jcp.21488. [DOI] [PubMed] [Google Scholar]
- 164.Huang J, et al. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat Med. 2007;13(10):1241–7. doi: 10.1038/nm1639. [DOI] [PubMed] [Google Scholar]
- 165.Sung TL, Rice AP. miR-198 inhibits HIV-1 gene expression and replication in monocytes and its mechanism of action appears to involve repression of cyclin T1. PLoS Pathog. 2009;5(1):e1000263. doi: 10.1371/journal.ppat.1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Corbeau P. Interfering RNA and HIV: reciprocal interferences. PLoS Pathog. 2008;4(9):e1000162. doi: 10.1371/journal.ppat.1000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Narayanan A, et al. Analysis of the roles of HIV-derived microRNAs. Expert Opin Biol Ther. 2011;11(1):17–29. doi: 10.1517/14712598.2011.540564. [DOI] [PubMed] [Google Scholar]
- 168.Klase Z, et al. HIV-1 TAR element is processed by Dicer to yield a viral micro-RNA involved in chromatin remodeling of the viral LTR. BMC Mol Biol. 2007;8:63. doi: 10.1186/1471-2199-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chandrasekar V, Dreyer JL. microRNAs miR-124, let-7d and miR-181a regulate cocaine-induced plasticity. Mol Cell Neurosci. 2009;42(4):350–62. doi: 10.1016/j.mcn.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 170.Sartor GC, St Laurent G, 3rd, Wahlestedt C. The Emerging Role of Non-Coding RNAs in Drug Addiction. Front Genet. 2012;3:106. doi: 10.3389/fgene.2012.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tyagi M, Karn J. CBF-1 promotes transcriptional silencing during the establishment of HIV-1 latency. EMBO J. 2007;26(24):4985–95. doi: 10.1038/sj.emboj.7601928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Coull JJ, et al. The human factors YY1 and LSF repress the human immunodeficiency virus type 1 long terminal repeat via recruitment of histone deacetylase 1. J Virol. 2000;74(15):6790–9. doi: 10.1128/jvi.74.15.6790-6799.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hsia SC, Shi YB. Chromatin disruption and histone acetylation in regulation of the human immunodeficiency virus type 1 long terminal repeat by thyroid hormone receptor. Mol Cell Biol. 2002;22:4043–52. doi: 10.1128/MCB.22.12.4043-4052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Williams SA, et al. NF-kappaB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation. EMBO J. 2006;25(1):139–149. doi: 10.1038/sj.emboj.7600900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Imai K, Okamoto T. Transcriptional repression of human immunodeficiency virus type 1 by AP-4. J Biol Chem. 2006;281(18):12495–505. doi: 10.1074/jbc.M511773200. [DOI] [PubMed] [Google Scholar]
- 176.Choudhary SK, Margolis DM. Curing HIV: Pharmacologic Approaches to Target HIV-1 Latency. Annual Review of Pharmacology and Toxicology. 2011;5151:397–418. doi: 10.1146/annurev-pharmtox-010510-100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Margolis DM. Histone deacetylase inhibitors and HIV latency. Curr Opin HIV AIDS. 2011;6(1):25–9. doi: 10.1097/COH.0b013e328341242d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mbonye U, Karn J. Control of HIV latency by epigenetic and non-epigenetic mechanisms. Curr HIV Res. 2011 doi: 10.2174/157016211798998736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Renthal W, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56(3):517–29. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 180.Levine AA, et al. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proc Natl Acad Sci U S A. 2005;102(52):19186–91. doi: 10.1073/pnas.0509735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kumar A, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48(2):303–14. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 182.Tsankova N, et al. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8(5):355–67. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 183.Maze I, Nestler EJ. The epigenetic landscape of addiction. Ann N Y Acad Sci. 2011;1216:99–113. doi: 10.1111/j.1749-6632.2010.05893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Renthal W, et al. Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron. 2009;62(3):335–48. doi: 10.1016/j.neuron.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14(8):341–50. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Renthal W, Nestler EJ. Histone acetylation in drug addiction. Semin Cell Dev Biol. 2009;20(4):387–94. doi: 10.1016/j.semcdb.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Feng J, Nestler EJ. Epigenetic mechanisms of drug addiction. Curr Opin Neurobiol. 2013;23(4):521–8. doi: 10.1016/j.conb.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nestler EJ. Epigenetic mechanisms of drug addiction. Neuropharmacology. 2014;76(Pt B):259–68. doi: 10.1016/j.neuropharm.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004;24(24):5603–10. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Brami-Cherrier K, et al. Parsing molecular and behavioral effects of cocaine in mitogen- and stress-activated protein kinase-1-deficient mice. J Neurosci. 2005;25(49):11444–54. doi: 10.1523/JNEUROSCI.1711-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chiappelli F, et al. Molecular epigenetics, chromatin, and NeuroAIDS/HIV: Immunopathological implications. Bioinformation. 2008;3(1):47–52. doi: 10.6026/97320630003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shapshak P, et al. Molecular epigenetics, chromatin, and NeuroAIDS/HIV: Translational implications. Bioinformation. 2008;3(1):53–7. doi: 10.6026/97320630003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Shapshak P, et al. Bioinformatics models in drug abuse and Neuro-AIDS: Using and developing databases. Bioinformation. 2006;1(3):86–8. doi: 10.6026/97320630001086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Black YD, et al. Altered attention and prefrontal cortex gene expression in rats after binge-like exposure to cocaine during adolescence. J Neurosci. 2006;26(38):9656–65. doi: 10.1523/JNEUROSCI.2391-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Maze I, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327(5962):213–6. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cory TJ, et al. Overcoming pharmacologic sanctuaries. Curr Opin HIV AIDS. 2013;8(3):190–5. doi: 10.1097/COH.0b013e32835fc68a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Palmer S, Josefsson L, Coffin JM. HIV reservoirs and the possibility of a cure for HIV infection. J Intern Med. 2011;270(6):550–60. doi: 10.1111/j.1365-2796.2011.02457.x. [DOI] [PubMed] [Google Scholar]
- 198.Parikh N, et al. Cocaine Alters Cytokine Profiles in HIV-1-Infected African American Individuals in the DrexelMed HIV/AIDS Genetic Analysis Cohort. J Acquir Immune Defic Syndr. 2014 doi: 10.1097/QAI.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ioannidis JPA, et al. Dynamics of HIV-1 viral load rebound among patients with previous suppression of viral replication. Aids. 2000;14(11):1481–1488. doi: 10.1097/00002030-200007280-00003. [DOI] [PubMed] [Google Scholar]
- 200.Chiasson MA, et al. Risk factors for human immunodeficiency virus type 1 (HIV-1) infection in patients at a sexually transmitted disease clinic in New York City. Am J Epidemiol. 1990;131(2):208–20. doi: 10.1093/oxfordjournals.aje.a115491. [DOI] [PubMed] [Google Scholar]
- 201.Anthony JC, et al. New evidence on intravenous cocaine use and the risk of infection with human immunodeficiency virus type 1. Am J Epidemiol. 1991;134(10):1175–89. doi: 10.1093/oxfordjournals.aje.a116021. [DOI] [PubMed] [Google Scholar]
- 202.Smurzynski M, et al. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS. 2011;25(3):357–65. doi: 10.1097/QAD.0b013e32834171f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rappaport J, Volsky DJ. Role of the macrophage in HIV-associated neurocognitive disorders and other comorbidities in patients on effective antiretroviral treatment. J Neurovirol. 2015;21(3):235–41. doi: 10.1007/s13365-015-0346-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lipton SA, Gendelman HE. Seminars in medicine of the Beth Israel Hospital, Boston. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995;332(14):934–40. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- 205.Letendre S, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65(1):65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Robertson KR, et al. Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology. 2010;74(16):1260–6. doi: 10.1212/WNL.0b013e3181d9ed09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Marra CM, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23(11):1359–66. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Perez-Valero I, et al. Neurocognitive impairment in patients treated with protease inhibitor monotherapy or triple drug antiretroviral therapy. PLoS One. 2013;8(7):e69493. doi: 10.1371/journal.pone.0069493. [DOI] [PMC free article] [PubMed] [Google Scholar]